Abstract
Digital health interventions refer to the use of digital technology and connected devices to improve health outcomes and healthcare delivery. This includes telemedicine, electronic health records, wearable devices, mobile health applications, and other forms of digital health technology. To this end, several research and developmental activities in various fields are gaining momentum. For instance, in the medical devices sector, several smart biomedical materials and medical devices that are digitally enabled are rapidly being developed and introduced into clinical settings. In the pharma and allied sectors, digital health-focused technologies are widely being used through various stages of drug development, viz. computer-aided drug design, computational modeling for predictive toxicology, and big data analytics for clinical trial management. In the biotechnology and bioengineering fields, investigations are rapidly growing focus on digital health, such as omics biology, synthetic biology, systems biology, big data and personalized medicine. Though digital health-focused innovations are expanding the horizons of health in diverse ways, here the development in the fields of medical devices, pharmaceutical technologies and biotech sectors, with emphasis on trends, opportunities and challenges are reviewed. A perspective on the use of digital health in the Indian context is also included.
Keywords: Medical devices, Telemedicine, Biomaterials, Drug discovery, Bioengineering
Introduction
Digital health is a rapidly growing field that offers exciting opportunities for innovation and improvement in healthcare delivery. The goal of digital health is to make healthcare more efficient, accessible, and effective, by leveraging the power of digital technology to collect, analyze, store and share health data. Electronic Health Records (EHRs), telemedicine, mobile health apps, wearable devices, the internet of medical things and cutting-edge digital technology constitute digital health. The digital health market has been growing rapidly in recent years and is expected to continue its growth trajectory in the near future. The global digital health market size was valued at approximately US$211 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 18.6% from 2023 to 2030 [1]. In the Indian context, the digital health market is reported to be about US$12.2 billion in 2023 and is projected to reach US$25.64 billion by 2027 with a CAGR of about 20.4% [2]. The digital health market is highly fragmented and is characterized by a large number of small and medium-sized enterprises operating in various segments, such as wearable devices, telemedicine, EHRs, and mobile health apps. Major players in the digital health market include Apple, Google, Philips, Medtronic, and Roche, among others [3].
The growth of the digital health market can be attributed to several factors, including the increasing adoption of smartphones and other digital devices, the growing demand for remote monitoring and telemedicine services, and the increasing focus on the development of digital health solutions to address the challenges posed by the COVID-19 pandemic. Several groups are working on various aspects of digital health, and the number of scientific publications in this area has been growing rapidly in recent years (Fig. 1).
Fig. 1.
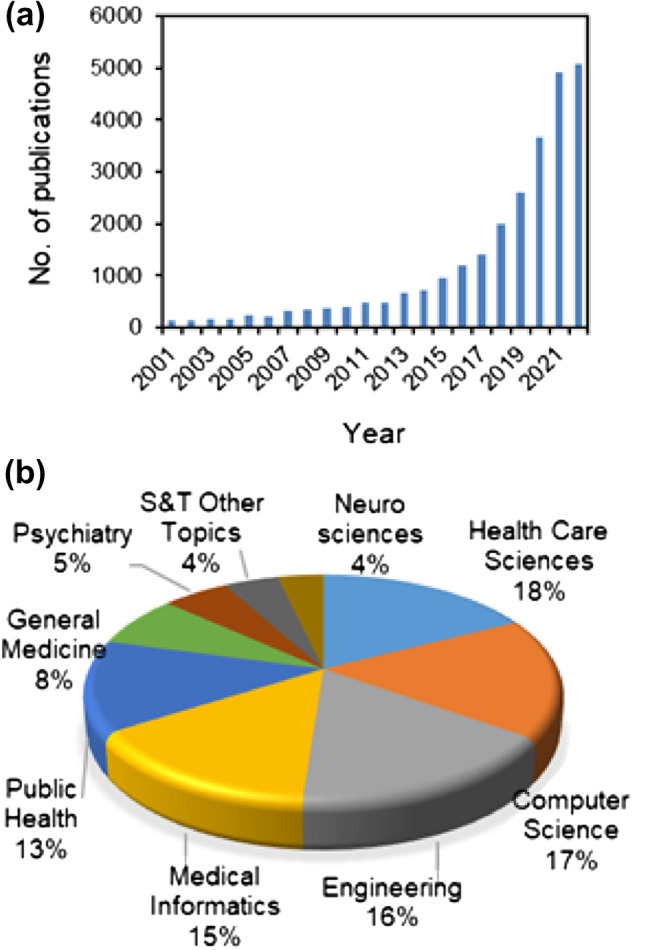
An overview of the publication trends in digital health as available from the web of science (a), and details of sectors where much of the research work is being focused (b)
The medical device sector has seen significant innovations in digital health in recent years. These range from wearable devices, remote monitoring systems, telemedicine devices, and electronic drug dispensing units to smart inhalers. These innovations in the medical device sector have the potential to greatly improve healthcare delivery and patient outcomes by providing more efficient and effective ways to monitor and manage health. Similarly, in the pharma sector, Digital Health Technologies (DHTs) are being used in many ways. Several DHTs are being used to speed up the drug development process through (i) drug design by virtual screening tools, (ii) reducing animal usage by predictive toxicology, and (iii) streamlining clinical trials by digital data management. In the biotechnology and bioengineering front, developments in the field of omics, synthetic and systems biology, big data and precision medicine are leaning towards digital health. This review gives an overview of the trends, opportunities and challenges for digital health innovations in the medical device, pharma and bio-allied fields. Although there are pervious review articles on digital health in general, the current review was the first of its kind covering digital health technologies across the key segments in the health sector i.e. medical devices, pharma and biotechnology.
Developments in medical devices and allied technologies toward digital health
Medical devices
Digital health-focused medical devices are devices that utilize digital technologies to improve health and healthcare. These devices are playing an increasingly important role in improving healthcare delivery by enabling remote patient monitoring, increasing access to medical services, and reducing healthcare costs. They also offer the potential for improved patient outcomes by enabling early detection and intervention in medical conditions. However, there are challenges associated with the use of these devices, such as the need for appropriate regulatory oversight, privacy concerns, and attention to cybersecurity risks. Examples of digital health-focused medical devices are described below.
Wearable devices
Wearable technology has been an active area of research in recent years, with numerous advances. These devices such as smartwatches and fitness trackers monitor various aspects of a person's health, such as heart rate, sleep patterns, and physical activity [4]. Some wearable devices also have features such as ECG monitoring and fall detection. The latest wearable medical devices global market report underlines that the market would grow from US$22.44 billion (2022) to US$27.37 billion by the end of 2023, with a predicted annual growth rate of 21.9%. It further suggests that the trend will continue with the same CAGR to reach $60.48 billion in 2027. Following are some of the latest trends and developments in this area:
(a) Augmented reality (AR) integrated wearable devices It is an area that has seen significant growth in recent years [5, 6]. Researchers are exploring ways to integrate AR technology into wearable devices to create an enhanced user experience. This could include things like displaying information directly on a user's field of vision or providing additional context to the real world.
(b) Artificial Intelligence (AI) integrated wearable devices AI is also being integrated into wearable devices to provide users with more advanced features and capabilities [7]. For example, wearable devices could use AI to analyze data from various sensors and make predictions about a user's health or provide personalized recommendations.
(c) Energy harvesting wearable devices This is another area of research in wearable technology [8]. Researchers are developing devices that can generate their power through movement, body heat, or other sources, which would make them more self-sufficient and reduce the need for charging.
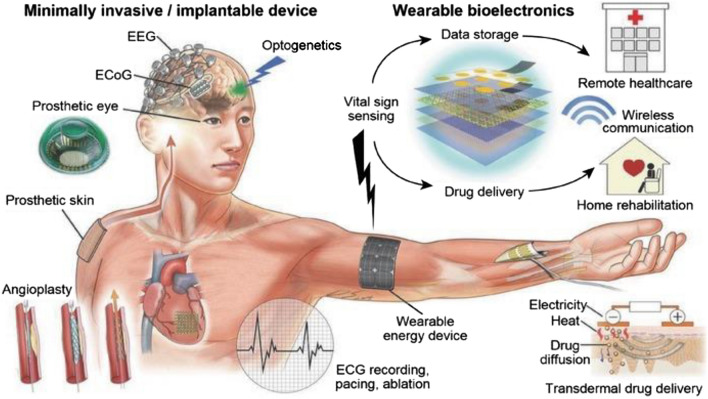
A schematic representation of wearable, minimally invasive/implantable devices integrated with DHTs is presented in Fig. 2.
Fig. 2.
A schematic illustration of an array of wearable, minimally invasive/implantable devices integrated with DHTs. Reproduced with permission from [9]. © 2016 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim
Diagnostic devices
These are devices used for diagnostic purposes, such as glucose meters for diabetic patients, spirometry devices for pulmonary function testing, and portable ultrasound machines. Following are some of the latest trends and developments in this area:
(a) Point-of-care (POC) diagnostics POC testing devices are becoming increasingly popular as they allow for rapid diagnostic testing at the point of care. These devices are designed to be small, portable, and easy to use, making it possible to diagnose a wide range of conditions in a variety of settings [10].
(b) Non-invasive diagnostics Non-invasive diagnostic devices are being developed to provide a more comfortable and convenient testing experience for patients [11]. For example, devices that use breath analysis or skin sensors to diagnose conditions are being developed, eliminating the need for invasive procedures such as blood tests.
(c) Nanotechnology-based diagnostics Nanotechnology is being used to develop new diagnostic devices that are more sensitive and efficient [12]. For example, nanoparticle-based devices are being developed that can detect specific biomarkers in blood and other body fluids, allowing for the early detection of diseases such as cancers.
Therapeutic devices
These are devices used for treatment, such as insulin pumps, implantable cardiac pacemakers, and deep brain stimulation devices. Following are some of the latest trends and developments in this area:
(a) Wearable therapeutics These devices are becoming increasingly popular as they allow for continuous and non-invasive treatment. For example, wearable devices are being developed to deliver electrical stimulation to the brain to treat conditions such as depression or to deliver drugs directly to the site of an injury [13, 14].
(b) Non-invasive stimulators Non-invasive stimulation devices, such as transcranial magnetic or electrical stimulators, are being developed to treat a variety of conditions, including depression, anxiety, and chronic pain. These devices use magnetic or electrical fields to stimulate specific regions of the brain, providing a safe and non-invasive alternative to traditional treatments [15].
(c) Regenerative therapeutics Regenerative medicine is a growing area of research, and therapeutic devices are being developed to support the growth and regeneration of damaged tissue [16]. For example, devices are being developed to deliver growth factors to the site of an injury, promoting tissue repair and regeneration.
Medical imaging devices
These are devices used for imaging the body, such as X-ray machines, CT scanners, and MRI machines. Following are some of the latest trends and developments in this area:
(a) Artificial Intelligence-enabled classification and detection which is being used to improve the accuracy and efficiency of medical imaging devices [17]. For example, AI algorithms can be trained to identify patterns in medical images, such as X-rays or CT scans, helping healthcare providers make more informed diagnoses and prognoses.
(b) Medical imaging-assisted customized 3D printing products medical imaging-assisted 3D printing technology is being used to create customized medical devices, such as surgical models, prosthetics and implantable systems [18, 19]. This can be particularly useful for patients with complex medical conditions, as 3D printing allows for the creation of devices that are tailored to the individual's specific needs.
(c) Non-Invasive Imaging Invasive imaging modalities are followed for diagnosing complicated medical conditions, such as coronary angiography for diagnosing coronary artery stenosis. To this end, non-invasive imaging devices that use ultrasound or optical imaging to visualize internal organs or tissues are being developed for detecting various conditions, such as coronary artery stenosis, myocardial infarction, liver metastasis, and beyond [20].
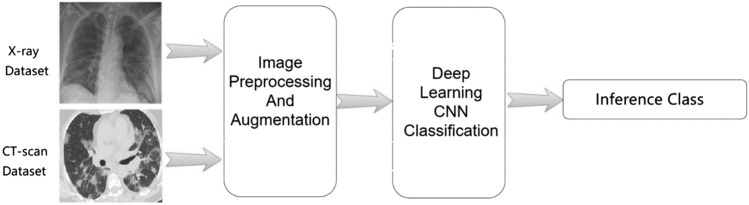
A schematic representation of AI-based medical image detection and analysis in the context of COVID-19 is presented in Fig. 3.
Fig. 3.
A schematic showing deep learning-based medical image detection and analysis in the context of COVID-19. Reproduced from [21]. © The Authors 2021
Telemedicine devices
These are devices that enable remote patient monitoring and teleconsultations, such as remote patient monitoring systems, webcams, and handheld devices with cameras and communication capabilities. Following are some of the latest trends and developments in this area.
(a) Wearable telemedicine devices These devices are becoming increasingly popular, as they allow for continuous monitoring of a patient's health status [22]. These devices can track vital signs, such as heart rate and blood pressure, and transmit the data to healthcare providers for analysis.
(b) Remote diagnostic and intervention tools These tools are being developed to allow healthcare providers to diagnose and attend to conditions remotely [11, 23]. For example, some telemedicine devices are equipped with cameras and other tools that allow healthcare providers to examine and intervene as necessary to attend to a patient remotely.
(c) Integration with Electronic Health Records Telemedicine devices are being integrated with EHRs to provide a more comprehensive view of a patient's health status [24, 25]. This integration can help healthcare providers make more informed decisions about a patient's treatment plan, as they have access to a patient's complete medical history.
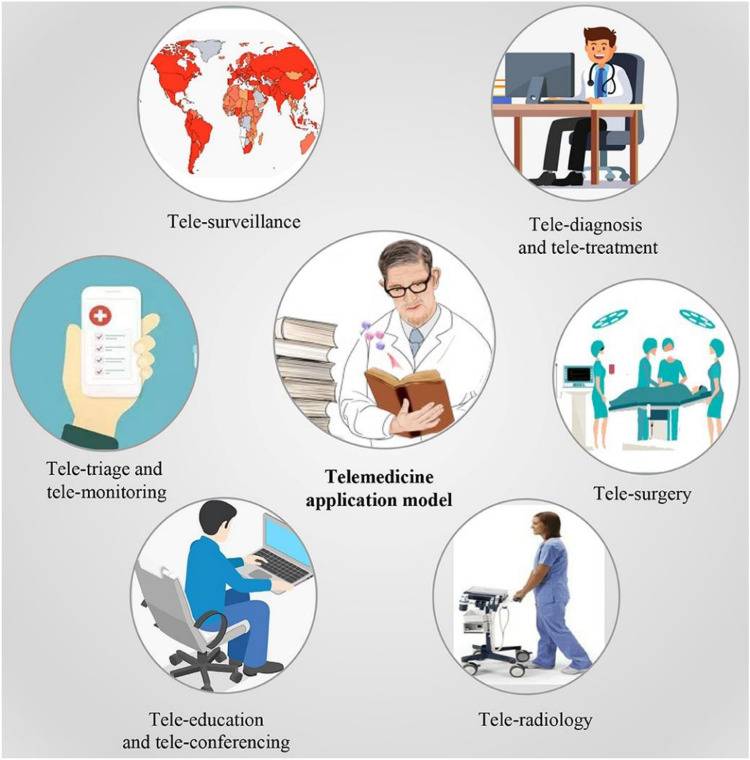
A schematic representation showing various DHT-enabled telemedicine modalities is presented in Fig. 4.
Fig. 4.
A schematic showing various avenues of digital health technology-enabled telemedicine modalities. Reproduced from [26]. © The Authors 2021
Medical materials
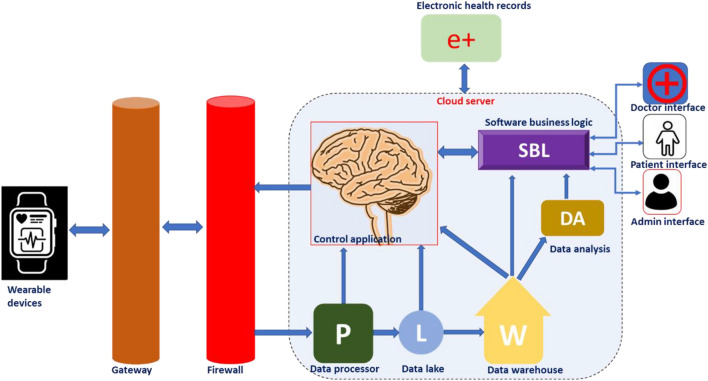
As in many other avenues, the advent of digital health paved the way for tremendous development in the field of material science and related research [27]. Modern material science can contribute smart materials and analytical tools suitable for developing wearable medical devices and sensors required for this purpose. The wearable devices could help in the monitoring of chronic health conditions, therapy, diagnosis, rehabilitation, and tracking of physical activities [28]. Timely interventions supported by the real-time monitoring of health parameters using wearable devices would save the lives of many. The cloud-based operation of wearable medical devices enables medical professionals to monitor real-time vital parameters and to plan the requirement of physical visits, changes in therapy, and modalities for disease management. A schematic representation showing the work-flow behind cloud-based device performance is presented in Fig. 5.
Fig. 5.
Schematic representation of work flow behind a cloud-based wearable device
The materials used for the fabrication of smart wearable medical devices should be biocompatible, flexible/wearable, lightweight, cost-effective, and smart enough to generate transmittable signals in response to changes in physiological parameters such as arterial pulse, body temperature, humidity, motion, and biomarkers in body fluids. Recognition of signals and their transformation are the two fundamental processes associated with any sensors used in the healthcare sector [29]. In wearable medical devices, the sensors respond to various parameters such as pressure, strain, temperature, the concentration of biomarkers, etc., and generate transmittable electronic/optical signals. Pressure/strain sensors, humidity/gas sensors, electrochemical sensors, colorimetric sensors, etc., are the major types of wearable sensors employed in the healthcare sector [30]. Even though many inorganic and metallic materials are available with excellent conductivity and sensing capability, inflexibility hinders their application in wearable devices.
Recently a wide variety of advanced smart materials have been utilized for developing wearable devices for healthcare applications. The most prominent ones are described below.
Ionic liquids
Ionic liquids were frequently used for the development of wearable sensors owing to their flexibility, conductivity, broad electrochemical window, better miscibility, negligible toxicity, and low vapor pressure [31]. Ionic liquid-based smart materials have been reported for a wide range of healthcare applications.
Wearable strain/pressure sensors: Ionic liquid smart devices could convert mechanical strain into processable and transmissible electrical signals in both resistive and capacitive modes. wearable motion sensors could be suitable for monitoring the elderly or rehabilitating population to assess their progress and to provide intervention as and when required [32].
Thermal sensors: As ionic liquids and ionic liquid crystals are capable of thermal transitions of their phases; they could be employed for monitoring the body temperatures of patients [33].
Breathing monitors: Ionic liquid-based wearable strain sensors were also reported to monitor the breathing events of patients with COPD or sleep apnoea. Stomach attachment of IL-based wearable breath rate sensors would provide alarms during dangerous breath variations or apnoea [34].
Sensors for cardiovascular parameters: Ionic liquid-based wearable devices were reported for ECG and EMG recordings [35].
Others: Ionic liquid-based devices were reported for monitoring skin humidity and evolved gases [36], glucose or lactate levels and pH from sweat [37], and for applications in therapeutics and drug delivery [38].
Carbon materials
A wide array of carbon nanomaterials like carbon nanotubes (CNTs), graphene-based materials, and carbon black (CB) were exploited for healthcare applications. Low cost, mass production capability, biocompatibility, and good mechanical and conduction behaviors made them suitable for generating smart medical devices. Carbon-based smart devices could be fabricated by a variety of methods such as chemical vapor deposition, drop casting, spin coating, screen or inkjet printing, and vacuum filtration. Their major applications are:
Wearable sensors for strain, pressure, temperature and humidity [39].
Biosensors for biomarker detection [40].
Others: bone and cartilage regeneration, Bioimaging, and Breath analysis [41].
An overview of various applications of DHT-enabled carbon nanomaterials in medical and other allied fields is presented in Fig. 6.
Fig. 6.
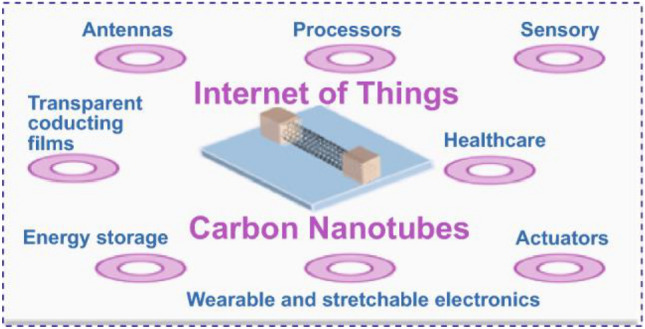
An overview of various applications of DHT-enabled carbon nanomaterials in medical and other allied fields. Reproduced from [42]. © The Authors 2021
Gold nanomaterials
Gold nanomaterials are known to have better electrical conductivity, mechanical flexibility, biocompatibility, and a wide electrochemical sensing window. Their surfaces could be modified by suitable chemical reactions to improve their electrical and optical behaviors to fine-tune sensing capabilities.
Wearable strain/pressure sensor: Mechanical perturbation on the nano-dimensional gold is converted into a readable electrical signal. They mainly follow a resistance-type, capacitance-type, piezoelectric-type, or triboelectric-type transduction mechanism. Strain/pressure sensors could be applied in soft robotics, human–machine interactions, human motion detection systems, and in health monitoring [43].
Humidity sensors for human breath analysis: Humidity sensors function on the variation of the impedance values of the membranes with respect to humidity variations [44].
Others: Gold nanomaterial-based wearable biosensors were reported for various biomarkers, Wearable pH sensors, and bioimaging therapeutics and drug delivery [45].
In addition to these materials other nano materials, conducting polymers and smart materials were reported to be contributing to the area of digital health [46]. Thus, material science research flourished extensively due to the arrival of digital health platforms. In addition to material science research, the analytical modalities were also influenced by the rapid development of digital health. NIR and Raman-based non-invasive disease monitoring strategies were reported for the detection of disease conditions and measuring vital parameters [47, 48]
Developments in pharma and allied areas toward digital health
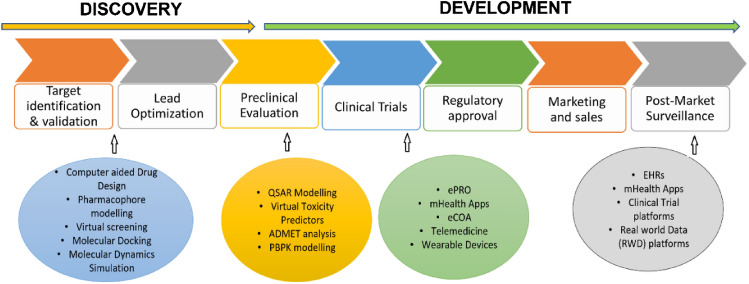
The tremendous expansion of DHTs at both customer and professional levels has opened a better arena for the effective utilization of digital resources for the benefit of human welfare. In this context, DHTs are playing an increasingly important role in the delivery of pharmaceutical care. Despite the widespread acceptance of personalized technologies in pharma health care, the DHT system is not comprehensively reviewed in terms of drug discovery and development. Drug discovery and development is a complex and multi-step process that involves multiple stages generally taking a time frame of 10–12 years [49]. The following is a general flowchart that outlines the process of drug discovery:
-
(i)
Target identification and validation: In this stage, researchers identify and validate a biological target (e.g., a protein, gene, or pathway) that is involved in the disease process. They use various high-throughput screening techniques to identify the small molecule or biological entities (hits) that modulate the activity of the target and have potential therapeutic effects [50].
-
(ii)
Lead optimization: In the optimization stage, the researchers optimize the potency, selectivity, pharmacokinetics, and pharmacodynamics of the lead compounds to produce lead candidates [51].
-
(iii)
Pre-clinical evaluation: This is the stage where the researcher conducts a series of in vitro and in vivo studies to evaluate the safety, efficacy, pharmacokinetics, and pharmacodynamics of the lead candidates.
-
(iv)
Clinical trials: Lead candidates that have passed preclinical testing are then tested in human clinical trials to evaluate their safety and efficacy in a larger population.
-
(v)
Regulatory approval: If the clinical trials are successful, the drug is then submitted to regulatory agencies for approval.
-
(vi)
Marketing and sales: only after the drug gets approved, it can be manufactured and marketed for therapeutic use.
-
(vii)
Post-market surveillance: This will be an indefinite process making the regulators monitor the efficacy and safety of the drug throughout its lifetime.
In this context, digital technologies are playing an increasingly important role in the development of new drugs. Some of the key ways that digital technologies are being used in drug development are presented in Fig. 7 and are detailed in the following sections.
Fig. 7.
An overview of applications of digital health technologies in drug discovery and development
Drug design
Computer-aided drug design (CADD) is the process of creating new drugs based on a thorough understanding of the biological target and its interaction with potential drugs [52]. It is a multi-disciplinary field that combines knowledge from chemistry, biology, pharmacology, and computational modeling to develop new drugs. There are several in silico approaches in practice for computer-aided drug design. This includes,
Pharmacophore modeling It is a computational approach used to predict the molecular features that are responsible for a molecule's biological activity. This is a useful tool for drug discovery and design, as it allows researchers to identify key structural features that are important for a molecule's interaction with its target protein, and to design new molecules that are likely to have similar activities. There are two types of pharmacophore modeling. (a) Ligand-based drug design This approach is based on the structural information of the active ligands that bind to the target. In a study by Kist et al. [53] by employing a ligand-based drug design approach, novel potential inhibitors of the mTor pathway were identified as having comparable or better properties to that of the classic drug rapamycin. (b) Structure-based drug design This strategy uses the three-dimensional structure of the biological target to design drugs that fit into specific pockets or active sites on the target, effectively blocking its activity. An example of a structure-based drug design strategy is reported in the development of 5 LOX inhibitors, a therapeutic target for asthma as well as other inflammatory diseases [54]. Catalyst software package, LigandScout, MOE (Molecular Operating Environment, Schrodingers Maestro, PyRx are some of the software packages used to generate pharmacophore models.
Drug target fishing This is a computational approach that is used to identify potential drug targets for a particular disease or biological process. And the goal of drug target fishing is to find proteins or other molecular targets that are likely to be involved in the disease or process of interest and to design drugs that can interact with these targets in a specific way to produce a therapeutic effect [55]. Different approaches could be employed for drug target identification that includes (i) homology modeling: comparing the structure of a protein of interest to the structures of other related proteins that are already known targets and identifying conserved regions in the protein structure that could serve as a potential target [56], (ii) bioinformatics: analyzing biological data, such as genomic sequences, transcriptomic data, and protein–protein interaction data, to identify potential drug targets [57]. (iii) systems biology: studying the complex interactions between different biological components in a particular disease or process [58] and (iv) High-Throughput screening [59]. The experimental setup in this context is the molecular docking and molecular dynamics (MD) simulation. There are several widely used software programs available for molecular docking, including AutoDock, Glide, Leadit, and eHiTS. There are several widely used software programs available for molecular dynamics simulation, including GROMACS, AMBER, NAMD, CHARMM, and LAMMPS that have wide applications in drug discovery.
Pre-clinical research
Pre-clinical research in drug development is a phase where a variety of experiments are conducted to assess the safety and efficacy of a new drug candidate. These may include toxicity studies to determine the potential for harmful effects, pharmacology studies to evaluate the drug’s interactions with the body, and efficacy studies to determine the potential therapeutic benefits of the drug. They involve animal models and the administration of the drugs to animals to assess any adverse effects and determine the optimal dosing regimen. Animal testing has been used for decades to evaluate the safety and efficacy of new drug candidates, but there is growing concern about the ethical and scientific limitations of this approach [60]. As a result, there is a growing interest in developing alternative methods to animal testing. In silico modeling of biological systems is one such approach that could be employed as an alternative to animal testing. The computer simulations of biological systems predict the behavior of a particular biological system, and hence, are used to evaluate the safety and efficacy of potential new drugs. In silico models can also be used to analyze large amounts of data, such as gene expression data or proteomics data generated following the interaction of the drug with the biological system. By using AI and Machine Learning (ML) algorithms, these models can identify patterns in the data that would not be easily noticeable by a human researcher, providing new insights into the biological mechanisms underlying disease and thereby helping in the appropriate intervention strategies. The following are the commonly used tools in predicting safety and toxicity in pre-clinical research.
QSAR (Quantitative Structure–Activity Relationship) models These are computational tools that are used to predict the biological activity of a chemical compound based on its molecular structure [61]. ML algorithms, such as artificial neural networks, decision trees, and support vector machines, are then used to identify relationships between molecular descriptors and biological activities [62]. Several software programs can be used for QSAR modeling, including KNIME, Pipeline Pilot, MOE (Molecular Operating Environment), OChem and R.
Virtual Toxicity Predictors (VTPs) Unlike QSAR models, virtual toxicity predictor software tools use molecular modeling and simulation to predict the toxicity of a potential new treatment based on its molecular structure. QSAR models typically provide a quantitative prediction of the toxicity of a chemical compound, while VTPs can provide more detailed information about the potential toxicity mechanisms [63]. Some of the popular software programs used for virtual toxicity prediction include ToxCast, Toxtree, VEGA (Virtual Expert System for Toxicity Assessment), OSIRIS, Leadscope and eTOXlab.
ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) analysis It helps in understanding the pharmacokinetics and pharmacodynamics of a drug and is a crucial step in the drug discovery process [64]. There are several software programs available for ADMET analysis, including both commercial and open-source options. Some popular choices include Schrodinger, Simulations Plus, OpenEye, Pipeline Pilot, Molsoft ICM-Pro, SwissADME, DEREK. However, the choice of software will depend on the specific needs of the user and the type of ADMET analysis being performed.
PBPK (Physiologically-Based Pharmacokinetic) modeling This type of modeling takes into account the anatomy and physiology of the body to simulate the distribution and elimination of drugs. The main goal of PBPK modeling is to predict how a drug will behave in the body based on the known physiological properties of the drug and the individual being treated.[65]. Examples of software available for PBPK modeling include SimCyp GastroPlus, PK-Sim, ADAPT II, PKQuest, MCSim.
Clinical trials
The primary goal of a clinical trial is to determine if a new drug is effective in treating a specific medical condition and if it is safe for human use. Clinical trials are usually conducted in three phases, each of which provides increasing amounts of information about the drug's safety and effectiveness. Phase 1 trials typically involve a small number of healthy volunteers and are designed to test the drug's safety and identify any side effects. Phase 2 trials involve a larger number of patients with the specific medical condition the drug is intended to treat. These trials are designed to test the drug's effectiveness and gather additional information about its safety. Phase 3 trials involve an even larger number of patients and are the final stage of testing before a drug is submitted for approval by regulatory agencies [66]. These trials are designed to provide a more complete picture of the drug's benefits and risks and to confirm its effectiveness.
The DHTs are rapidly changing the way clinical trials are conducted. They are being used to streamline the clinical trial process, allowing for faster and more efficient trials[67] Here are some examples of DHTs used in clinical trials:
Electronic Patient-Reported Outcomes (ePRO) ePRO is a digital tool that allows patients to report symptoms, side effects, and other outcomes directly to the study team. This technology can help improve patient compliance and reduce the need for in-person visits [68].
Mobile Health (mHealth) Applications mHealth applications can be used to collect data from patients, provide education and support, and monitor health status. This technology can help increase patient engagement and improve data quality [69].
Electronic Clinical Outcome Assessments (eCOA) eCOA is a digital tool that allows patients to self-report outcomes, such as quality of life, using a smartphone or tablet. This technology can help reduce the burden on patients and improve data quality [70].
Telemedicine Telemedicine technology, such as video conferencing, can be used to conduct virtual visits with patients. This technology can help reduce the need for in-person visits, increase patient convenience, and improve patient engagement [71].
Wearable Devices Wearable devices, such as smartwatches and fitness trackers, can be used to collect data on physical activity, sleep patterns, and other health metrics. This technology can help improve data quality and increase patient engagement [72].
Post-market surveillance
DHTs have revolutionized the way drugs are monitored after they have been approved and entered the market. In the past, post-market surveillance of drugs relied heavily on passive systems, where healthcare professionals and patients reported adverse events or side effects. However, with the advent of DHTs, this process has become more proactive and efficient. Some examples of DHTs used in post-market surveillance of drugs include:
Electronic health records EHRs provide a centralized platform for healthcare professionals to report and track adverse events associated with drugs. This information can then be analyzed to identify potential safety issues with a drug [73].
Mobile health (mHealth) applications mHealth apps allow patients to easily report adverse events or side effects from their smartphones. This provides a more direct and convenient way for patients to report issues, which can lead to quicker identification of safety concerns [74].
Clinical trials platforms Clinical trial platforms have become increasingly digital, allowing for real-time monitoring of drug safety during the clinical trial phase. This information can then be used in post-market surveillance to identify potential safety issues with a drug [75].
Real-world data (RWD) platforms RWD platforms gather and analyze data from a variety of sources, including EHRs, claims data, and patient-generated data, to provide a more complete picture of a drug's safety profile. This information can be used to identify potential safety issues and monitor the effectiveness of drugs in real-world settings [76].
Overall, digital technologies are playing a critical role in drug discovery and development, helping to improve the speed, efficiency, and accuracy of the drug development process. This is leading to the discovery of new treatments for a wide range of diseases and conditions, improving patient outcomes and transforming the healthcare industry.
Developments in bio and allied technologies toward digital health
Digital health in biotechnology and bioengineering refers to the use of digital technologies to develop and improve biotechnology and bioengineering products and applications. Digital health is playing a critical role in these fields by enabling the development of more sophisticated models of biological systems and by facilitating the optimization of bio-based healthcare product design and manufacture. Following are some of the key areas where the innovation-driven science and technological advances can transform digital health worldwide.
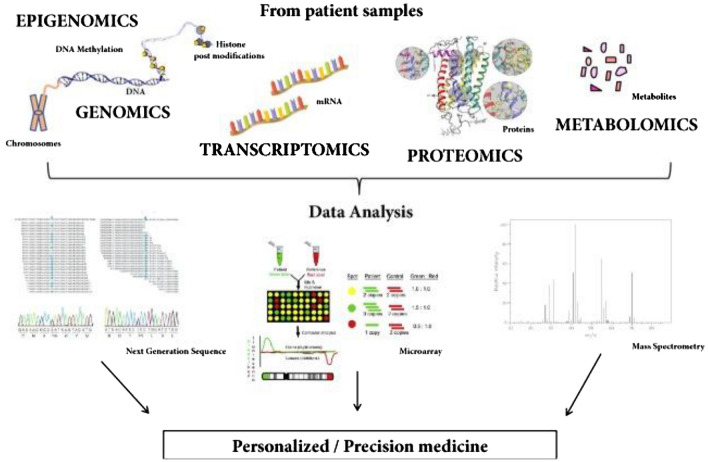
Omics biology
Omics is a field of study in medicine that encompasses various sub-disciplines such as genomics, transcriptomics, proteomics, metabolomics, and epigenomics. The goal of omics is to understand the underlying mechanisms of biological processes and diseases by looking at the collective behavior of all the molecules involved, such as genes, proteins, and metabolites. By combining data from these various omics disciplines, researchers can gain a more comprehensive understanding of the molecular basis of health and disease, which may lead to the development of new diagnostic tools, therapies, and personalized medicine approaches.
Omics technologies are also playing an increasingly important role in digital health, where they can be leveraged to improve the accuracy and precision of health assessments, diagnoses, and treatments. Following are a few examples of how Omics technologies can aid in digital health.
(a) Personalized medicine Omics data can be used to predict an individual's risk for developing certain diseases, monitor their health status over time, and tailor treatments to their unique genetic profile [77].
(b) Predictive analytics Predictive models based on omics data can be used to identify individuals who are at high risk for a disease, such as cancer, and to monitor their health status over time, allowing for early intervention and improved outcomes [78].
(c) Clinical decision support Clinical decision support systems that incorporate omics data can provide healthcare providers with real-time information and recommendations to help them make more informed treatment decisions for their patients [79].
(d) Omics data collecting medical devices medical devices that collect omics data, such as continuous glucose monitoring systems, can be used to monitor a patient's health status and to provide early warning signs of potential health issues.
(e) Omics data integrated telemedicine platforms that incorporate omics data can provide remote healthcare services, such as virtual consultations, to individuals in remote or underserved communities, improving access to care and outcomes [80].
A schematic representation showing omics-based DHTs towards personalized medicine is presented in Fig. 8.
Fig. 8.
A schematic showing potential applications of omics-based digital health technologies toward personalized medicine. Reproduced from [81]. © The Authors 2018
Big data analytics
Big data refers to extremely large and complex data sets that are generated from various sources, including EHRs, medical imaging, genetic sequencing, and other sources. The market size for big data in digital health has been growing rapidly in recent years, driven by the increasing adoption of DHTs and the growing demand for data-driven decision-making in healthcare. According to market research, the global big data in digital health market was valued at approximately US$ 39.7 billion in 2022 and is expected to grow at a CAGR of 19.2% from 2022 to 2032 [82]. However, the use of big data in medicine also raises concerns about privacy, security, and the ethics of data collection and analysis. It is important to address these concerns and ensure that the benefits of big data are maximized while minimizing its risks.
Big data plays a critical role in digital health by providing the vast amounts of information that are needed to drive innovation and improve patient outcomes.
(a) Big electronic health records data analytics One of the main ways that big data is used in digital health is through the analysis of EHRs and other sources of health-related data [83]. EHRs contain a vast amount of patient information, including demographic data, medical history, lab results, and other information. By analyzing this data, healthcare providers can gain insights into patient populations and identify trends and patterns that can inform decision-making and improve patient care.
(b) Big wearable devices data analytics big data is used in digital health through the analysis of data generated by wearable devices and other DHTs [84]. These devices generate vast amounts of data, including information about physical activity, sleep patterns, and other health-related metrics. This data can be used to track health status, monitor disease progression, and inform treatment plans.
(c) Big omics data analytics Another way that big data is used in medicine is through the analysis of genetic data [85]. Advances in genetic sequencing technologies have enabled the rapid and cost-effective generation of large amounts of genetic data, which can be used to identify the genetic basis of diseases and inform the development of personalized medicine.
(d) Big imaging data analytics Big data is also being used in medical imaging to improve diagnosis and treatment [86]. For example, advanced algorithms can be used to analyze medical images to identify patterns and anomalies that may indicate disease. This information can then be used to inform diagnosis and treatment planning.
(e) Big data and predictive analytics Big data is also being used in digital health to develop predictive models and algorithms that can improve health outcomes. For example, ML algorithms can be trained on large data sets to identify patterns and relationships that can inform decision-making and improve disease management [87].
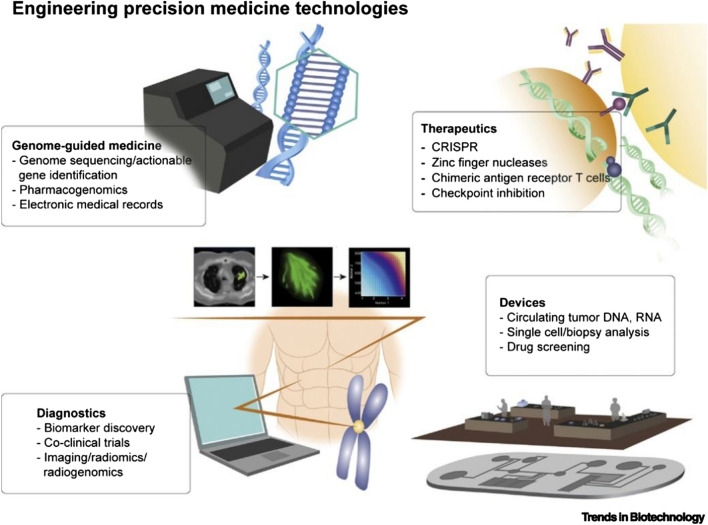
Personalized/precision medicine
Personalized medicine, also known as precision medicine, is a medical approach that takes into account individual differences in genes, environment, and lifestyle to develop a customized approach to healthcare [88]. The goal of personalized medicine is to provide the right treatment, at the right time, for the right patient. In traditional medicine, treatments are often based on a "one-size-fits-all" approach, which does not take into account the unique differences between individuals. However, with the advancement of genomic technologies and the increasing availability of patient data, it is now possible to tailor treatments to the specific needs of each patient. It is therefore important to note that personalized medicine is still in its early stages of development, and more research is needed to fully realize its potential.
Several DHTs are playing a critical role in personalized medicine viz. EHRs, telemedicine tools, wearable devices, big data analytics, additive manufacturing, AI/ML-based algorithms, and several personalized mobile apps. These personalized DHTs are playing an increasingly important role in precision medicine, providing healthcare providers with the tools they need to deliver more effective and efficient care to patients. Examples of personalized medicine include:
(a) Precision or personalized care Precision oncology is a type of personalized medicine that uses genetic information to tailor cancer treatments to the specific needs of each patient [89]. Precision psychiatry is a type of personalized medicine that uses genetic information to tailor psychiatric treatments to the specific needs of each patient [90].
(b) Precision or personalized drug dosage Personalized drug therapy is a type of personalized medicine that uses genetic information to determine the most effective drug for a particular patient [91].
(c) Precision surgical models The use of digital technologies such as computer-aided designing and manufacturing helps in scanning the defect site and manufacturing a surgical model utilizing 3D printing for enabling the surgeons to plan the surgery effectively and efficiently [92].
(d) Personalized regenerative therapies Advanced tissue engineering and regenerative medicine technologies such as 3D bioprinting help in bio-fabricating a tissue that is not only biocompatible but also fits precisely to the defect size of the patient [93].
(e) Precision public health Proactive use of technology brings in new avenues to address many age-old public health issues. Optimal use of geographic information systems (GIS) and other spatiotemporal analysis will help in more precise and timely field-level interventions, which are crucial in the early detection and control of infectious diseases. This is well documented in the control strategies of the recent Zika outbreak in the USA. In Florida, only two small counties were to be put under lockdown, that too for a short while, to arrest the spread of Zika in 2016 [94]. The predict and prevent framework is a futuristic method to address the burgeoning problem of non-communicable diseases through precision public health. The use of Electronic medical Support for Public health (ESP) and its visualization platform Riscape helps in long-term follow-up and real-time intervention in the surveillance of NCDs [95].
A schematic representation showing DHT-enabled personalized medicine is presented in Fig. 9.
Fig. 9.
A schematic showing integration of several technologies toward personalized/precision digital health. Reproduced with permission from [88]. © 2019 Elsevier Ltd
Synthetic biology
Synthetic biology is a multidisciplinary field that combines biology and engineering to design and construct novel biological systems for various applications [96]. It involves the manipulation of genetic material and metabolic pathways in living organisms to create new functions or modify existing ones. In synthetic biology, researchers use a combination of molecular biology techniques, computational modeling, and engineering principles to design, build, and test biological systems. These systems can be used in a wide range of applications, including the creation of new medicines, the development of biosensors, and beyond. One of the key features of synthetic biology is the use of standard biological parts, such as genes and regulatory elements, that can be combined and reused to create complex biological systems. This modular approach allows for rapid design and testing, as well as the potential for large-scale deployment of these systems. Synthetic biology has the potential to revolutionize many areas of biotechnology, medicine, and beyond, but it also requires careful consideration and oversight to ensure its safe and responsible development.
Synthetic biology has the potential to play a significant role in digital health [97]. One example of how synthetic biology can be used in digital health is the development of biosensors [98]. Biosensors are devices that use biological components, such as enzymes or antibodies, to detect specific substances. Synthetic biology can be used to design and construct biosensors that are specific for certain biomarkers, such as glucose or cholesterol, which can be used for continuous monitoring of health status. The results can be transmitted wirelessly to a digital platform for analysis and interpretation, enabling remote monitoring and disease management. Another example is the use of synthetic biology in the development of personalized medicine [99]. By using synthetic biology to design and engineer cells, researchers can create new therapeutic interventions that are tailored to a specific individual's needs [100]. For example, synthetic biology can be used to create cells that produce a specific protein or to correct genetic mutations that cause disease. These cells can then be monitored and controlled using digital technologies, enabling real-time monitoring of treatment efficacy and enabling adjustments to the therapy as needed. Overall, synthetic biology has the potential to revolutionize digital health by enabling the development of new technologies that can be integrated into digital platforms to improve health and healthcare.
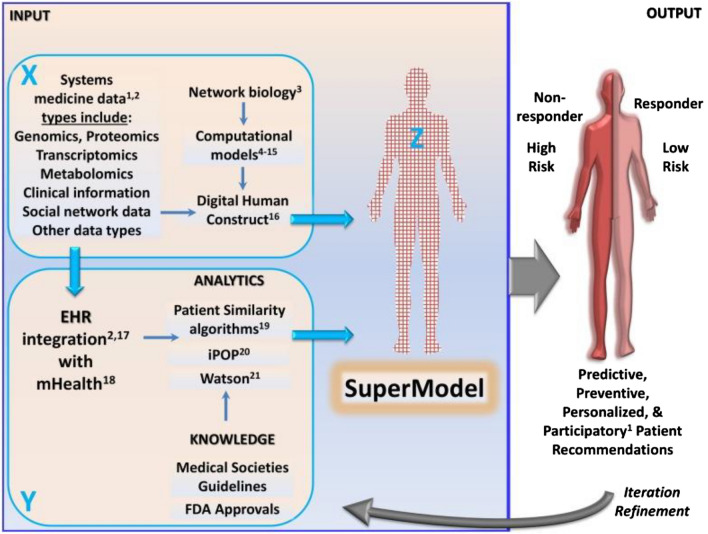
Systems biology
Systems biology is an interdisciplinary field of study that aims to understand the complex relationships between the components of biological systems, such as cells, tissues, and organs [101]. It seeks to understand how these systems interact and function as a whole, rather than simply focusing on individual components in isolation. Systems biology approaches biological systems from a holistic perspective, using computational and mathematical models to simulate the interactions between different components and to predict the behavior of the system as a whole. It also incorporates high-throughput data from various sources, such as genomics, proteomics, and metabolomics, to create a comprehensive view of the system. One of the key goals of systems biology is to understand the underlying mechanisms of disease and to develop new therapeutic strategies that target the root causes of diseases, rather than just their symptoms. It also seeks to improve our understanding of the interactions between different components of the body and how they contribute to health and disease. In addition, systems biology is playing an important role in the development of personalized medicine, as it provides a framework for integrating patient-specific data and generating personalized models of disease.
Systems biology can play a critical role in digital health in several ways as follows. (a) Predictive Modelling: Systems biology can be used to build predictive models of disease, which can help healthcare providers to identify individuals at risk of developing certain conditions and to develop targeted prevention strategies [102]. (b) Clinical Decision Support: Systems biology can be used to develop clinical decision support systems, which can help healthcare providers to make more informed treatment decisions based on the latest scientific evidence and patient-specific data [103]. (c) Clinical Trial Design: Systems biology can be used to inform the design of clinical trials, by helping to identify the most promising therapeutic targets and to predict the outcomes of different treatment strategies [104]. (d) Data Integration and Management: Systems biology can be used to integrate and manage large amounts of patient data, including genomic, proteomic, and clinical data, to create a comprehensive view of the patient and to inform the development of personalized treatment plans [105]. (e) Monitoring and Evaluation: Systems biology can be used to monitor the effectiveness of treatments and to evaluate the impact of treatments on patient outcomes [106]. Overall, systems biology offers a framework for integrating and analyzing large amounts of patient data and for developing personalized models of disease, which can inform the development of more effective and efficient treatment strategies.
A schematic representation showing DHTs integrated systems biology approaches toward effective clinical decisions are presented in Fig. 10.
Fig. 10.
A schematic showing a systems biology approach toward developing super models for effective clinical decisions. Reproduced from [107]. © The Authors 2015
Challenges associated with digital health technologies
With opportunities comes risks, and the same is true for the DHTs that present several challenges along with their opportunities [108].
(a) Privacy, security and ethical concerns With the increasing growth of mobile-based health apps and connected health systems, much the data including the personal information of patients is being collected. For instance, privacy and security are major concerns in digital health, with the sensitive nature of health data, making it a prime target for hackers. Also, DHTs had several ethical issues including the question of who owns the data.
(b) Interoperability This is another major challenge in digital health, with different digital health systems not being able to communicate with each other effectively. This makes it difficult for healthcare providers to access and share patient data, which can negatively impact patient outcomes.
(c) Regulatory framework Regulation is yet another bottleneck in digital health, with different countries having different regulations and guidelines for DHTs.
(d) Public awareness As many healthcare providers and patients are resistant to change and not fully understanding the benefits of DHTs, creating awareness about its benefits and risks is necessary for ensuring that DHTs are accepted by the general population.
(e) Legislative issues The laws of the land should appreciate the newer trends in science and technology for their optimal use. For example, vagueness in the legal validity of digital prescriptions was a major hurdle in the update of telemedicine in India before the hurried enactment of the Telemedicine Practice Guidelines in 2020, in the wake of the Covid-19 pandemic [109, 110].
Digital health—an Indian perspective
Digital health—initiatives from the Government of India
Digital health is a rapidly growing field that involves the use of digital technologies to improve health and healthcare delivery in India [111]. In recent years, there has been a significant investment in digital health infrastructure and initiatives, and a tremendous increase in the use of DHTs by both healthcare providers and patients in India. Examples of digital health initiatives in India include:
(a) Telemedicine Telemedicine services are widely available in India and are being used to provide remote consultations and support to patients in rural and underserved areas [112].
(b) Electronic health records Several hospitals have initiated programs to establish EHRs for all their clients, and even a comprehensive nationwide EHR platform is under consideration for the storage and sharing of patient health information between healthcare providers [113].
(c) mHealth The use of mobile health technologies, such as mobile apps and SMS-based services, is widespread in India and is being used to deliver health information and services to patients [114].
(d) Digital health marketplaces Digital health marketplaces, such as online pharmacies and telemedicine platforms, are becoming increasingly popular in India and are providing patients with access to a wide range of health products and services [115].
(e) Artificial intelligence in healthcare AI is being used in various applications in the Indian healthcare system, such as in radiology, oncology, and cardiology, to improve diagnosis and treatment [116].
Several institutions in India are working on digital health, including (a) Indian Council of Medical Research (ICMR): The ICMR is the main body responsible for promoting and coordinating biomedical research in India and has been involved in several digital health initiatives. (b) Apollo Hospitals: Apollo Hospitals is one of the largest healthcare groups in India and is a pioneer in the use of DHTs, including telemedicine and EHRs. (c) Tata Consultancy Services (TCS): TCS is a leading technology and consulting company in India and is involved in several digital health initiatives, including the development of EHRs and telemedicine solutions. (d) MedTech Zone: MedTech Zone is a digital health accelerator program in India that supports the development of early-stage digital health start-ups. (e) AI in Healthcare India: AI in Healthcare India is a non-profit organization that promotes the use of AI in healthcare in India and provides a platform for the exchange of ideas and knowledge on AI in healthcare. These are just a few of the many institutions in India that are involved in digital health initiatives.
Several companies in India are working on digital health, including (a) Practo and Doctor on Call: Practo and Doctor on Call are prominent digital health companies in India offering online doctor appointments including remote consultations and support to patients. (b) NetMeds, Tata 1 mg, Medlife and PharmEasy: these are prominent online platforms for ordering medicines and booking diagnostic tests. (c) HealthKart and HealthifyMe: these are some prominent digital health platforms in India that provide health supplements, and zpersonalized health and wellness coaching. (d) GoQii: GoQii is a digital health platform in India that provides personalized health and wellness coaching and wearable fitness trackers. (e) Besides, several small to medium scale digital health services offer online diagnostic services. These are just a few of the many companies in India that are involved in digital health initiatives. The Indian digital health market is rapidly growing, and many more companies and start-ups are entering the market.
The National Health Authority of the Government of India supports digital health through several schemes. Some of the prominent schemes include (a) Digital India: Digital India is a government initiative in India that aims to transform India into a digitally empowered society and knowledge economy. The initiative includes several components related to digital health, including CoWin and Arogya Sethu (https://digitalindia.gov.in/). (b) e-Health & Telemedicine: various Information & Communication Technologies (ICT)-enabled initiatives are undertaken for improving the efficiency and effectiveness of the public healthcare system (https://main.mohfw.gov.in/Organisation/departments-health-and-family-welfare/e-Health-Telemedicine). (c) Ayushman Bharat Digital Mission (ABDM): through this mission, the Govt. of India aims to develop the backbone necessary to support the integrated digital health infrastructure of the country (https://abdm.gov.in/). (d) National Digital Health Mission (NDHM): The NDHM is a government-led health mission in India that aims to provide universal health coverage to all the citizens in the country through digital technologies (https://www.makeinindia.com/national-digital-health-mission). (e) National Health Stack (NHS): The program aims to facilitate the collection of comprehensive healthcare data to aid in policymaking, allocation of resources and identification of needy populations for health schemes. These are just a few of the many government schemes and it is seen that the Indian government is committed to promoting and supporting digital health initiatives in the country. A flyer released by Govt. of India on NDHM is presented in Fig. 11.
Fig. 11.

A schematic representation showing various components of the National Digital Health Mission initiative by the Government of India. Adopted with permission from [117]. © 2023 Sanskriti IAS
Despite the significant opportunities and progress in digital health in India, several challenges need to be addressed, including the need for robust privacy and security measures, the need for greater investment in digital health infrastructure, and the need for greater training and capacity building for healthcare providers.
Digital health—initiatives from the state of Kerala (India)
Kerala is at the forefront of implementing EHRs for its population. Well before the era of the Individual Health ID of the Ayushman Digital Health Mission, Govt. of Kerala launched its ambitious eHealth Kerala project to create EHRs for all of its citizens in 2016. Though not perfect, it has added to the impetus of digitalization of the health sector in Kerala, thanks to the earlier implementation of the District Health System software (DHIS2) in all the 1000-plus public health institutions in the state.
The health workers in Kerala are familiar with digital health tools, and many public health centers use electronic medical records. However, the use of data for decision-making is not yet a norm in the health system, nor the public health area. Reasons for this low use of information are many, the lack of a clear data policy on who can have access to the data at various levels is a major one.
The state could take advantage of the e-Sanjeevani telemedicine platform during the Covid-19 pandemic to cater to the healthcare needs of its population. In the current e-Health Kerala project, there is a facility to do telemedicine consultations within the regular consultation hours. A few institutions in Kerala, like the Regional Cancer Centre (RCC) and SCTIMST, are going ahead with the doctor-to-doctor e-Sanjeevani consultations.
Digital health—initiatives from SCTIMST, Trivandrum (India)
Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST) is an Institution of National Importance under the Department of Science and Technology, Govt. of India. The institute is known for its high-quality advanced treatment of cardiac and neurological disorders, indigenous development of technologies for biomedical devices and public health training and research. The Institute has three wings—the Hospital, Biomedical Technology Wing and the Achutha Menon Centre for Health Science Studies (AMCHSS). The institute is proactive in catching up with the latest technologies in the field including DHTs.
In the clinical scenario, the medical wing of the institute has a custom-built electronic medical record system for its clinical services, entirely created by the in-house computer division. In recent years, the institute has incorporated newer standards, including the SNOMED-CT coding for the diagnostic fields. The anonymized data extraction from this system has supported many research initiatives of the institute. Similarly, SCTIMST has created a fully geo-referenced mapping of its field practice area covering around 35,000 households (a population of 1.32 lakhs) with community participation [118]. The experiences from such and similar initiatives have given confidence to the state government to undertake more challenging digital health interventions like the e-Health Kerala project. Besides, the various divisions of the medical wing are actively involved in the development of various DHTs in clinical settings.
The Biomedical Technology Wing of the Institute was instrumental in nurturing the Indian medical device industry through know-how development and transfer, providing internationally accredited testing services and offering technology incubation facilities for young entrepreneurs. The BMT wing is actively involved in the development of DHT-enabled medical devices such as para-corporeal left ventricular assist device, centrifugal blood pump with blood flowmeter, deep brain stimulator system for movement disorders, intracranial electrodes, optical peripheral nerve stimulator, 3D printed liver and skin tissue constructs for regenerative applications, PT/INR sensing devices, loop-mediated isothermal amplification-based diagnostic kits, implantable cardioverter defibrillator, programmable hydrocephalus shunt, implantable micro infusion pump with wireless recharging system, and POC kits for sepsis and chlamydia trachomatis. Besides, there are many innovative projects based on smart biomaterials and combinational medical devices under development.
The AMCHSS, the public health division of SCTIMST, was a partner in the customization of the DHIS-2 software for the Indian context, which was piloted in its field practice area in Athiyannur block in Thiruvananthapuram. This led to many field-based research initiatives on digital health, spanning from its use in infectious diseases [119–122] to non-communicable diseases [123–125]. In recent times AMCHSS is moving ahead with infectious disease modeling and the use of data science approaches to large-scale data [126–130]. Lately, the ICMR has entrusted AMCHSS with the analysis of the COVID-19 test data for the entire country. Besides, the AMCHSS is actively involved in the development of various public-health-focused DHTs.
Conclusions
Digital health technologies (DHTs) aim to improve the healthcare system across the globe. By providing more accurate diagnoses, enabling more effective treatments and improving patient engagement and compliance, DHTs have the potential to significantly improve patient outcomes. By enabling more efficient and effective delivery of healthcare services and by reducing the need for in-person visits, DHTs can reduce healthcare costs. DHTs can increase access to healthcare through means of remote consultations and support to patients in remote and underserved areas. By compiling patient data from several healthcare providers a more comprehensive digital health record of each patient can be created, providing more accurate and comprehensive patient data to healthcare providers. Thus DHTs can significantly improve healthcare delivery by enabling informed clinical decisions. Further big data can be analyzed by AI-clinical decision support systems to aid the healthcare provider.
Digital health is a rapidly developing field and DHTs are providing new opportunities for innovation and growth, and thus are transforming the medical, pharma, biotech and allied fields. In the medical devices sector, innovations in the field of smart materials, wearable devices, and AI/ML-based systems are rapidly being introduced for clinical use. In the pharma sector, the use of digital technologies has widespread use through various stages of drug development viz. drug design, preclinical validation, and clinical trial. In the biotech and bioengineering sector, digital technologies are aiding in the development of precision and personalized medicinal products. This means there is a growing opportunity for start-ups and established companies to develop new and innovative DHTs. However, a word of caution is necessary to beware of the risks associated with DHTs including ethical and technical concerns.
Author contribution
All authors listed have made an equal and substantial contribution to the work.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial conflicts of interest to disclose.
References
- 1.Digital Health Market Size, Share & Trends Report, 2030. https://www.grandviewresearch.com/industry-analysis/digital-health-market
- 2.Digital Health—India. https://www.statista.com/outlook/dmo/digital-health/india
- 3.Smartphone-based patient monitoring global market report 2022. https://www.businesswire.com/news/home/20230104005482/en/Smartphone-Based-Patient-Monitoring-Global-Market-Report-2022-Featuring-Leading-Players---Apple-Boston-Scientific-Cerner-Medtronic-and-Phillips-Healthcare---ResearchAndMarkets.com
- 4.Dunn J, Runge R, Snyder M. Wearables and the medical revolution. Pers Med. 2018;15:429–448. doi: 10.2217/pme-2018-0044. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesan M, Mohan H, Ryan JR, et al. Virtual and augmented reality for biomedical applications. Cell Rep Med. 2021;2:100348. doi: 10.1016/j.xcrm.2021.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinolo Gil MJ, Gonzalez-Medina G, Lucena-Anton D, et al. Augmented reality in physical therapy: systematic review and meta-analysis. JMIR Serious Games. 2021;9:e30985. doi: 10.2196/30985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2020;33:10–18. doi: 10.1177/0840470419873123. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Lou Z, Chen D, et al. Recent advances in flexible/stretchable supercapacitors for wearable electronics. Small. 2018;14:1702829. doi: 10.1002/smll.201702829. [DOI] [PubMed] [Google Scholar]
- 9.Choi S, Lee H, Ghaffari R, et al. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv Mater. 2016;28:4203–4218. doi: 10.1002/adma.201504150. [DOI] [PubMed] [Google Scholar]
- 10.Gous N, Boeras DI, Cheng B, et al. The impact of digital technologies on point-of-care diagnostics in resource-limited settings. Expert Rev Mol Diagn. 2018;18:385–397. doi: 10.1080/14737159.2018.1460205. [DOI] [PubMed] [Google Scholar]
- 11.Salem M, Elkaseer A, El-Maddah IAM, et al. Non-invasive data acquisition and iot solution for human vital signs monitoring: applications. Limit Future Prospects Sens. 2022;22:6625. doi: 10.3390/s22176625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belushkin A, Yesilkoy F, Altug H. Nanoparticle-enhanced plasmonic biosensor for digital biomarker detection in a microarray. ACS Nano. 2018;12:4453–4461. doi: 10.1021/acsnano.8b00519. [DOI] [PubMed] [Google Scholar]
- 13.Kar A, Ahamad N, Dewani M, et al. Wearable and implantable devices for drug delivery: applications and challenges. Biomaterials. 2022;283:121435. doi: 10.1016/j.biomaterials.2022.121435. [DOI] [PubMed] [Google Scholar]
- 14.Long Y, Li J, Yang F, et al. Wearable and implantable electroceuticals for therapeutic electrostimulations. Adv Sci. 2021;8:2004023. doi: 10.1002/advs.202004023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilotto A, Rizzetti MC, Lombardi A, et al. Cerebellar rTMS in PSP: a double-blind sham-controlled study using mobile health technology. Cerebellum. 2021;20:662–666. doi: 10.1007/s12311-021-01239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farahani M, Shafiee A. Wound healing: from passive to smart dressings. Adv Healthc Mater. 2021;10:2100477. doi: 10.1002/adhm.202100477. [DOI] [PubMed] [Google Scholar]
- 17.Gore JC. Artificial intelligence in medical imaging. Magn Reson Imaging. 2020;68:A1–A4. doi: 10.1016/j.mri.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Pugliese L, Marconi S, Negrello E, et al. The clinical use of 3D printing in surgery. Update Surg. 2018;70:381–388. doi: 10.1007/s13304-018-0586-5. [DOI] [PubMed] [Google Scholar]
- 19.Sun L, Wong Y. Personalized three-dimensional printed models in congenital heart disease. J Clin Med. 2019;8:522. doi: 10.3390/jcm8040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirajuddin A, Mirmomen SM, Kligerman SJ, et al. Ischemic heart disease: noninvasive imaging techniques and findings. Radiographics. 2021;41:E990–E1021. doi: 10.1148/rg.2021200125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang D, Martinez C, Visuña L, et al. Detection and analysis of COVID-19 in medical images using deep learning techniques. Sci Rep. 2021;11:19638. doi: 10.1038/s41598-021-99015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shan R, Sarkar S, Martin SS. Digital health technology and mobile devices for the management of diabetes mellitus: state of the art. Diabetologia. 2019;62:877–887. doi: 10.1007/s00125-019-4864-7. [DOI] [PubMed] [Google Scholar]
- 23.Ong DSY, Poljak M. Smartphones as mobile microbiological laboratories. Clin Microbiol Infect. 2020;26:421–424. doi: 10.1016/j.cmi.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Coons SJ, Eremenco S, Lundy JJ, et al. Capturing patient-reported outcome (PRO) data electronically: the past, present, and promise of epro measurement in clinical trials. Patient Cent Outcomes Res. 2015;8:301–309. doi: 10.1007/s40271-014-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinh-Le C, Chuang R, Chokshi S, Mann D. Wearable health technology and electronic health record integration: scoping review and future directions. JMIR MHealth UHealth. 2019;7:e12861. doi: 10.2196/12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Y-T, Chen L, Yue W-W, Xu H-X. Digital technology-based telemedicine for the COVID-19 pandemic. Front Med. 2021;8:646506. doi: 10.3389/fmed.2021.646506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo C, Ashrafian H, Ghafur S, et al. Challenges for the evaluation of digital health solutions—a call for innovative evidence generation approaches. NPJ Digit Med. 2020;3:110. doi: 10.1038/s41746-020-00314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Campbell AS, de Ávila BE-F, Wang J. Wearable biosensors for healthcare monitoring. Nat Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koydemir HC, Ozcan A. Wearable and implantable sensors for biomedical applications. Annu Rev Anal Chem. 2018;11:127–146. doi: 10.1146/annurev-anchem-061417-125956. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Xia K, Wang H, et al. Advanced carbon for flexible and wearable electronics. Adv Mater. 2019;31:1801072. doi: 10.1002/adma.201801072. [DOI] [PubMed] [Google Scholar]
- 31.Correia DM, Fernandes LC, Fernandes MM, et al. Ionic liquid-based materials for biomedical applications. Nanomaterials. 2021;11:2401. doi: 10.3390/nano11092401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi DY, Kim MH, Oh YS, et al. Highly stretchable, hysteresis-free ionic liquid-based strain sensor for precise human motion monitoring. ACS Appl Mater Interfaces. 2017;9:1770–1780. doi: 10.1021/acsami.6b12415. [DOI] [PubMed] [Google Scholar]
- 33.Yamada S, Toshiyoshi H. Temperature sensor with a water-dissolvable ionic gel for ionic skin. ACS Appl Mater Interfaces. 2020;12:36449–36457. doi: 10.1021/acsami.0c10229. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Lowe A, Kalra A, Yu Y. A flexible strain sensor based on embedded ionic liquid. Sensors. 2021;21:5760. doi: 10.3390/s21175760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Z, Wu P. Water-resistant ionogel electrode with tailorable mechanical properties for aquatic ambulatory physiological signal monitoring. Adv Funct Mater. 2021;31:2107226. doi: 10.1002/adfm.202107226. [DOI] [Google Scholar]
- 36.Esteves C, Palma SICJ, Costa HMA, et al. Tackling humidity with designer ionic liquid-based gas sensing soft materials. Adv Mater. 2022;34:2107205. doi: 10.1002/adma.202107205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curto VF, Fay C, Coyle S, et al. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sens Actuators B Chem. 2012;171–172:1327–1334. doi: 10.1016/j.snb.2012.06.048. [DOI] [Google Scholar]
- 38.Zandu SK, Chopra H, Singh I. Ionic liquids for therapeutic and drug delivery applications. Curr Drug Res Rev. 2020;12:26–41. doi: 10.2174/2589977511666191125103338. [DOI] [PubMed] [Google Scholar]
- 39.Jian M, Wang C, Wang Q, et al. Advanced carbon materials for flexible and wearable sensors. Sci China Mater. 2017;60:1026–1062. doi: 10.1007/s40843-017-9077-x. [DOI] [Google Scholar]
- 40.Castro KPR, Colombo RNP, Iost RM, et al. Low-dimensionality carbon-based biosensors: the new era of emerging technologies in bioanalytical chemistry. Anal Bioanal Chem. 2023 doi: 10.1007/s00216-023-04578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das S, Pal M. Review—non-invasive monitoring of human health by exhaled breath analysis: a comprehensive review. J Electrochem Soc. 2020;167:037562. doi: 10.1149/1945-7111/ab67a6. [DOI] [Google Scholar]
- 42.Pang J, Bachmatiuk A, Yang F, et al. Applications of carbon nanotubes in the internet of things era. Nano-Micro Lett. 2021;13:191. doi: 10.1007/s40820-021-00721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi J, Xianyu Y. Gold nanomaterials-implemented wearable sensors for healthcare applications. Adv Funct Mater. 2022;32:2113012. doi: 10.1002/adfm.202113012. [DOI] [Google Scholar]
- 44.Ali I, Chen L, Huang Y, et al. Humidity-responsive gold aerogel for real-time monitoring of human breath. Langmuir. 2018;34:4908–4913. doi: 10.1021/acs.langmuir.8b00472. [DOI] [PubMed] [Google Scholar]
- 45.Haine AT, Niidome T. Gold nanorods as nanodevices for bioimaging, photothermal therapeutics, and drug delivery. Chem Pharm Bull (Tokyo) 2017;65:625–628. doi: 10.1248/cpb.c17-00102. [DOI] [PubMed] [Google Scholar]
- 46.Jin H, Jin Q, Jian J. Smart materials for wearable healthcare devices. In: Ortiz JH, editor. Wearable technologies. InTech; 2018. [Google Scholar]
- 47.Choo-Smith L-P, Edwards HGM, Endtz HP, et al. Medical applications of Raman spectroscopy: from proof of principle to clinical implementation. Biopolymers. 2002;67:1–9. doi: 10.1002/bip.10064. [DOI] [PubMed] [Google Scholar]
- 48.Kothari R, Jones V, Mena D, et al. Raman spectroscopy and artificial intelligence to predict the Bayesian probability of breast cancer. Sci Rep. 2021;11:6482. doi: 10.1038/s41598-021-85758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deore AB, Dhumane JR, Wagh R, Sonawane R. The stages of drug discovery and development process. Asian J Pharm Res Dev. 2019;7:62–67. doi: 10.22270/ajprd.v7i6.616. [DOI] [Google Scholar]
- 50.Hughes J, Rees S, Kalindjian S, Philpott K. Principles of early drug discovery: principles of early drug discovery. Br J Pharmacol. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Showell GA, Mills JS. Chemistry challenges in lead optimization: silicon isosteres in drug discovery. Drug Discov Today. 2003;8:551–556. doi: 10.1016/S1359-6446(03)02726-0. [DOI] [PubMed] [Google Scholar]
- 52.Vemula D, Jayasurya P, Sushmitha V, et al. CADD, AI and ML in drug discovery: a comprehensive review. Eur J Pharm Sci. 2023;181:106324. doi: 10.1016/j.ejps.2022.106324. [DOI] [PubMed] [Google Scholar]
- 53.Kist R, Timmers LFSM, Caceres RA. Searching for potential mTOR inhibitors: ligand-based drug design, docking and molecular dynamics studies of rapamycin binding site. J Mol Graph Model. 2018;80:251–263. doi: 10.1016/j.jmgm.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 54.Aparoy P, Kumar Reddy K, Reddanna P. Structure and ligand based drug design strategies in the development of novel 5- LOX inhibitors. Curr Med Chem. 2012;19:3763–3778. doi: 10.2174/092986712801661112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanakaveti V, Shanmugam A, Ramakrishnan C et al (2020) Computational approaches for identifying potential inhibitors on targeting protein interactions in drug discovery. In: Advances in protein chemistry and structural biology. Elsevier, pp 25–47 [DOI] [PubMed]
- 56.Chikhale RV, Gupta VK, Eldesoky GE, et al. Identification of potential anti-TMPRSS2 natural products through homology modelling, virtual screening and molecular dynamics simulation studies. J Biomol Struct Dyn. 2021;39:6660–6675. doi: 10.1080/07391102.2020.1798813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li K, Du Y, Li L, Wei D-Q. Bioinformatics approaches for anti-cancer drug discovery. Curr Drug Targets. 2019;21:3–17. doi: 10.2174/1389450120666190923162203. [DOI] [PubMed] [Google Scholar]
- 58.Yu T, Cheng L, Yan X, et al. Systems biology approaches based discovery of a small molecule inhibitor targeting both c-Met/PARP-1 and inducing cell death in breast cancer. J Cancer. 2020;11:2656–2666. doi: 10.7150/jca.40758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aldewachi H, Al-Zidan RN, Conner MT, Salman MM. High-throughput screening platforms in the discovery of novel drugs for neurodegenerative diseases. Bioengineering. 2021;8:30. doi: 10.3390/bioengineering8020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferdowsian HR, Beck N. Ethical and scientific considerations regarding animal testing and research. PLoS ONE. 2011;6:e24059. doi: 10.1371/journal.pone.0024059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Achary PGR. Applications of quantitative structure-activity relationships (QSAR) based virtual screening in drug design: a review. Mini-Rev Med Chem. 2020;20:1375–1388. doi: 10.2174/1389557520666200429102334. [DOI] [PubMed] [Google Scholar]
- 62.Staszak M, Staszak K, Wieszczycka K, et al. Machine learning in drug design: Use of artificial intelligence to explore the chemical structure–biological activity relationship. WIREs Comput Mol Sci. 2022 doi: 10.1002/wcms.1568. [DOI] [Google Scholar]
- 63.Raies AB, Bajic VB. In silico toxicology: computational methods for the prediction of chemical toxicity: computational methods for the prediction of chemical toxicity. Wiley Interdiscip Rev Comput Mol Sci. 2016;6:147–172. doi: 10.1002/wcms.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Workman P. How much gets there and what does it do?: The need for better pharmacokinetic and pharmacodynamic endpoints in contemporary drug discovery and development. Curr Pharm Des. 2003;9:891–902. doi: 10.2174/1381612033455279. [DOI] [PubMed] [Google Scholar]
- 65.Bouzom F, Ball K, Perdaems N, Walther B. Physiologically based pharmacokinetic (PBPK) modelling tools: how to fit with our needs?: PBPK MODELLING TOOLS. Biopharm Drug Dispos. 2012;33:55–71. doi: 10.1002/bdd.1767. [DOI] [PubMed] [Google Scholar]
- 66.Mahan VL. Clinical trial phases. Int J Clin Med. 2014;05:1374–1383. doi: 10.4236/ijcm.2014.521175. [DOI] [Google Scholar]
- 67.Gold M, Amatniek J, Carrillo MC, et al. Digital technologies as biomarkers, clinical outcomes assessment, and recruitment tools in Alzheimer’s disease clinical trials. Alzheimers Dement Transl Res Clin Interv. 2018;4:234–242. doi: 10.1016/j.trci.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin. 2012;62:336–347. doi: 10.3322/caac.21150. [DOI] [PubMed] [Google Scholar]
- 69.Gong K, Yan Y-L, Li Y, et al. Mobile health applications for the management of primary hypertension: a multicenter, randomized, controlled trial. Medicine (Baltimore) 2020;99:e19715. doi: 10.1097/MD.0000000000019715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordon S, Crager J, Howry C, et al. Best practice recommendations: user acceptance testing for systems designed to collect clinical outcome assessment data electronically. Ther Innov Regul Sci. 2022;56:442–453. doi: 10.1007/s43441-021-00363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galsky MD, Shahin M, Jia R, et al. Telemedicine-enabled clinical trial of metformin in patients with prostate cancer. JCO Clin Cancer Inform. 2017 doi: 10.1200/CCI.17.00044. [DOI] [PubMed] [Google Scholar]
- 72.Beg S, Handa M, Shukla R, et al. Wearable smart devices in cancer diagnosis and remote clinical trial monitoring: transforming the healthcare applications. Drug Discov Today. 2022;27:103314. doi: 10.1016/j.drudis.2022.06.014. [DOI] [PubMed] [Google Scholar]
- 73.Patel VN, Kaelber DC. Using aggregated, de-identified electronic health record data for multivariate pharmacosurveillance: a case study of azathioprine. J Biomed Inform. 2014;52:36–42. doi: 10.1016/j.jbi.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Needamangalam Balaji J, Prakash S, Park Y, et al. A scoping review on accentuating the pragmatism in the implication of mobile health (mHealth) technology for tuberculosis management in India. J Pers Med. 2022;12:1599. doi: 10.3390/jpm12101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holmén C, Piehl F, Hillert J, et al. A Swedish national post-marketing surveillance study of natalizumab treatment in multiple sclerosis. Mult Scler J. 2011;17:708–719. doi: 10.1177/1352458510394701. [DOI] [PubMed] [Google Scholar]
- 76.Antonijević Z, Beckman RA. Platform trial designs in drug development: umbrella trials and basket trials. Boca Raton: CRC Press; 2019. [Google Scholar]
- 77.Battaglini D, Al-Husinat L, Normando AG, et al. Personalized medicine using omics approaches in acute respiratory distress syndrome to identify biological phenotypes. Respir Res. 2022;23:318. doi: 10.1186/s12931-022-02233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poirion OB, Jing Z, Chaudhary K, et al. DeepProg: an ensemble of deep-learning and machine-learning models for prognosis prediction using multi-omics data. Genome Med. 2021;13:112. doi: 10.1186/s13073-021-00930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tran KA, Kondrashova O, Bradley A, et al. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021;13:152. doi: 10.1186/s13073-021-00968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turek C, Wróbel S, Piwowar M. OmicsON—integration of omics data with molecular networks and statistical procedures. PLoS ONE. 2020;15:e0235398. doi: 10.1371/journal.pone.0235398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galeone C, Scelfo C, Bertolini F, et al. Precision medicine in targeted therapies for severe asthma: is there any place for “omics” technology? BioMed Res Int. 2018;2018:1–15. doi: 10.1155/2018/4617565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Big data analytics in healthcare market. https://www.factmr.com/report/369/big-data-analytics-healthcare-market
- 83.Hemingway H, Asselbergs FW, Danesh J, et al. Big data from electronic health records for early and late translational cardiovascular research: challenges and potential. Eur Heart J. 2018;39:1481–1495. doi: 10.1093/eurheartj/ehx487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Alexander CA. Big data analytics in medical engineering and healthcare: methods, advances and challenges. J Med Eng Technol. 2020;44:267–283. doi: 10.1080/03091902.2020.1769758. [DOI] [PubMed] [Google Scholar]
- 85.Koppad S, B A, Gkoutos GV, Acharjee A, Cloud computing enabled big multi-omics data analytics. Bioinforma Biol Insights. 2021;15:1177932221. doi: 10.1177/11779322211035921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morris MA, Saboury B, Burkett B, et al. Reinventing radiology: big data and the future of medical imaging. J Thorac Imaging. 2018;33:4–16. doi: 10.1097/RTI.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 87.Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019;20:e262–e273. doi: 10.1016/S1470-2045(19)30149-4. [DOI] [PubMed] [Google Scholar]
- 88.Ho D, Quake SR, McCabe ERB, et al. Enabling technologies for personalized and precision medicine. Trends Biotechnol. 2020;38:497–518. doi: 10.1016/j.tibtech.2019.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sadler D, Okwuosa T, Teske AJ, et al. Cardio oncology: Digital innovations, precision medicine and health equity. Front Cardiovasc Med. 2022;9:951551. doi: 10.3389/fcvm.2022.951551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vajawat B, Varshney P, Banerjee D. Digital gaming interventions in psychiatry: evidence. Appl Chall Psych Res. 2021;295:113585. doi: 10.1016/j.psychres.2020.113585. [DOI] [PubMed] [Google Scholar]
- 91.Raijada D, Wac K, Greisen E, et al. Integration of personalized drug delivery systems into digital health. Adv Drug Deliv Rev. 2021;176:113857. doi: 10.1016/j.addr.2021.113857. [DOI] [PubMed] [Google Scholar]
- 92.Montanhesi PK, Coelho G, Curcio SAF, Poffo R. Three-dimensional printing in minimally invasive cardiac surgery: optimizing surgical planning and education with life-like models. Braz J Cardiovasc Surg. 2022 doi: 10.21470/1678-9741-2020-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jamróz W, Szafraniec J, Kurek M, Jachowicz R. 3D printing in pharmaceutical and medical applications—recent achievements and challenges. Pharm Res. 2018;35:176. doi: 10.1007/s11095-018-2454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bedford J, Farrar J, Ihekweazu C, et al. A new twenty-first century science for effective epidemic response. Nature. 2019;575:130–136. doi: 10.1038/s41586-019-1717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Canfell OJ, Davidson K, Woods L, et al. Precision public health for non-communicable diseases: an emerging strategic roadmap and multinational use cases. Front Public Health. 2022;10:256. doi: 10.3389/fpubh.2022.854525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cubillos-Ruiz A, Guo T, Sokolovska A, et al. Engineering living therapeutics with synthetic biology. Nat Rev Drug Discov. 2021;20:941–960. doi: 10.1038/s41573-021-00285-3. [DOI] [PubMed] [Google Scholar]
- 97.Davies JA. Synthetic biology: rational pathway design for regenerative medicine. Gerontology. 2016;62:564–570. doi: 10.1159/000440721. [DOI] [PubMed] [Google Scholar]
- 98.Sridhar S, Ajo-Franklin CM, Masiello CA. A framework for the systematic selection of biosensor chassis for environmental synthetic biology. ACS Synth Biol. 2022;11:2909–2916. doi: 10.1021/acssynbio.2c00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jain KK. Synthetic biology and personalized medicine. Med Princ Pract. 2013;22:209–219. doi: 10.1159/000341794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McNerney MP, Doiron KE, Ng TL, et al. Theranostic cells: emerging clinical applications of synthetic biology. Nat Rev Genet. 2021;22:730–746. doi: 10.1038/s41576-021-00383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zupanic A, Bernstein HC, Heiland I. Systems biology: current status and challenges. Cell Mol Life Sci. 2020;77:379–380. doi: 10.1007/s00018-019-03410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lopatkin AJ, Collins JJ. Predictive biology: modelling, understanding and harnessing microbial complexity. Nat Rev Microbiol. 2020;18:507–520. doi: 10.1038/s41579-020-0372-5. [DOI] [PubMed] [Google Scholar]
- 103.Irmisch A, Bonilla X, Chevrier S, et al. The Tumor Profiler Study: integrated, multi-omic, functional tumor profiling for clinical decision support. Cancer Cell. 2021;39:288–293. doi: 10.1016/j.ccell.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 104.McEwen SC, Merrill DA, Bramen J, et al. A systems-biology clinical trial of a personalized multimodal lifestyle intervention for early Alzheimer’s disease. Alzheimers Dement Transl Res Clin Interv. 2021 doi: 10.1002/trc2.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolstencroft K, Owen S, Krebs O, et al. SEEK: a systems biology data and model management platform. BMC Syst Biol. 2015;9:33. doi: 10.1186/s12918-015-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobeissy FH, Guingab-Cagmat JD, Razafsha M, et al. Leveraging biomarker platforms and systems biology for rehabilomics and biologics effectiveness research. PM&R. 2011;3:S139–S147. doi: 10.1016/j.pmrj.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 107.Brown S-A. Building SuperModels: emerging patient avatars for use in precision and systems medicine. Front Physiol. 2015 doi: 10.3389/fphys.2015.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cummins N, Schuller BW. Five crucial challenges in digital health. Front Digit Health. 2020;2:536203. doi: 10.3389/fdgth.2020.536203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Board of Governors in Supersession of the Medical Council of India (2020) Telemedicine practice guidelines-enabling registered medical practitioners to provide healthcare using telemedicine
- 110.Latifi R, Doarn CR. Perspective on COVID-19: finally, telemedicine at center stage. Telemed E-Health. 2020;26:1106–1109. doi: 10.1089/tmj.2020.0132. [DOI] [PubMed] [Google Scholar]
- 111.Gudi N, Lakiang T, Pattanshetty S, et al. Challenges and prospects in india’s digital health journey. Indian J Public Health. 2021;65:209. doi: 10.4103/ijph.IJPH_1446_20. [DOI] [PubMed] [Google Scholar]
- 112.Dash S, Aarthy R, Mohan V. Telemedicine during COVID-19 in India—a new policy and its challenges. J Public Health Policy. 2021;42:501–509. doi: 10.1057/s41271-021-00287-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srivastava SK. Adoption of electronic health records: a roadmap for India. Healthc Inform Res. 2016;22:261. doi: 10.4258/hir.2016.22.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Madanian S, Parry DT, Airehrour D, Cherrington M. mHealth and big-data integration: promises for healthcare system in India. BMJ Health Care Inform. 2019;26:e100071. doi: 10.1136/bmjhci-2019-100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Al Dahdah M, Mishra RK. Digital health for all: the turn to digitized healthcare in India. Soc Sci Med. 2023;319:114968. doi: 10.1016/j.socscimed.2022.114968. [DOI] [PubMed] [Google Scholar]
- 116.Katyayan A, Katyayan A, Mishra A. Enhancing India’s health care during COVID era: role of artificial intelligence and algorithms. Indian J Otolaryngol Head Neck Surg. 2022;74:2712–2713. doi: 10.1007/s12070-020-02101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.National Digital Health Mission. https://www.sanskritiias.com/current-affairs/national-digital-health-mission
- 118.Soman B. Participatory GIS in action, a public health initiative from Kerala, India. ISPRS Int Arch Photogramm Remote Sens Spat Inf Sci. 2014;XL-8:233–237. doi: 10.5194/isprsarchives-XL-8-233-2014. [DOI] [Google Scholar]
- 119.Babu AN, Niehaus E, Shah S, et al. Smartphone geospatial apps for dengue control, prevention, prediction, and education: MOSapp, DISapp, and the mosquito perception index (MPI) Environ Monit Assess. 2019;191:393. doi: 10.1007/s10661-019-7425-0. [DOI] [PubMed] [Google Scholar]
- 120.Chaudhary S, Soman B. Spatiotemporal analysis of environmental and physiographic factors related to malaria in Bareilly district, India. Osong Public Health Res Perspect. 2022;10:10. doi: 10.24171/j.phrp.2021.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singh G, Mitra A, Soman B. Development and use of a reproducible framework for spatiotemporal climatic risk assessment and its association with decadal trend of dengue in India. Indian J Community Med. 2022;47:50. doi: 10.4103/ijcm.ijcm_862_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valson JS, Soman B. Spatiotemporal clustering of dengue cases in Thiruvananthapuram district. Kerala’ Indian J Public Health. 2017;61:74. doi: 10.4103/ijph.IJPH_26_16. [DOI] [PubMed] [Google Scholar]
- 123.Sarma PS, Sadanandan R, Thulaseedharan JV, et al. Prevalence of risk factors of non-communicable diseases in Kerala, India: results of a cross-sectional study. BMJ Open. 2019 doi: 10.1136/bmjopen-2018-027880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ulahannan SK, Wilson A, Chhetri D, et al. Alarming level of severe acute malnutrition in Indian districts. BMJ Glob Health. 2022;7:e007798. doi: 10.1136/bmjgh-2021-007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Valson JS, Kutty VR, Soman B, Jissa VT. Spatial clusters of diabetes and physical inactivity: do neighborhood characteristics in high and low clusters differ? Asia Pac J Public Health. 2019 doi: 10.1177/1010539519879322. [DOI] [PubMed] [Google Scholar]
- 126.Mitra A, Soman B, Singh G (2021) An interactive dashboard for real-time analytics and monitoring of COVID-19 outbreak in india: a proof of Concept. arXiv:210809937Cs
- 127.Mitra A, Soman B, Gaitonde R et al (2023) Data science approaches to public health: case studies using routine health data from India
- 128.Singh G, Patrikar S, Sarma PS, Soman B (2020) Time-dependent dynamic transmission potential and instantaneous reproduction number of COVID-19 pandemic in India. medRxiv. 10.1101/2020.07.15.20154971
- 129.Singh G, Srinivas G, Jyothi EK, et al. Containing the first outbreak of COVID-19 in a healthcare setting in India: the sree chitra experience. Indian J Public Health. 2020;64:240. doi: 10.4103/ijph.IJPH_483_20. [DOI] [PubMed] [Google Scholar]
- 130.Singh G, Soman B. Spatiotemporal epidemiology and forecasting of dengue in the state of Punjab, India: study protocol. Spat Temp Epidemiol. 2021;39:100444. doi: 10.1016/j.sste.2021.100444. [DOI] [PubMed] [Google Scholar]