Abstract
Objective:
To ensure readers are informed consumers of functional magnetic resonance imaging research in headache, to outline ongoing challenges in this area of research, and to describe potential considerations when asked to collaborate on functional magnetic resonance imaging research in headache as well as to suggest future directions for improvement in the field.
Background:
Functional magnetic resonance imaging has played a key role in understanding headache pathophysiology, and mapping networks involved with headache-related brain activity has the potential to identify intervention targets. Some investigators have also begun to explore its use for diagnosis.
Methods/Results:
The manuscript is a narrative review of the current best practices in functional magnetic resonance imaging in headache research, including guidelines on transparency and reproducibility. It also contains an outline of the fundamentals of magnetic resonance imaging theory, task-related study design, resting-state functional connectivity, relevant statistics and power analysis, image pre-processing, and other considerations essential to the field.
Conclusion:
Best practices to increase reproducibility include methods transparency, eliminating error, using a priori hypotheses and power calculations, using standardized instruments and diagnostic criteria, and developing large-scale, publicly available datasets.
Keywords: fMRI, functional magnetic resonance imaging, migraine, cluster headache
Introduction
Our understanding of headache is constantly evolving and is subject to ongoing investigation and debate. In the 2022 Nature Reviews Disease Primer on Migraine, authors noted that “[a]lthough understanding migraine attack pathophysiology has substantially improved, the pathogenesis of the disease migraine…remains poorly understood”1. The authors go on to outline what is known, including the phenomenon of spreading depolarization1. For several of the agreed-upon aspects of headache pathophysiology, functional magnetic resonance imaging (fMRI) has played a key role2.
Neuroimaging has made these contributions in part because it is a non-invasive method for bridging our understanding between basic science findings in animal models and the human brain. An example is the role of fMRI (which in essence tracks changes in oxygenated blood flow) in our understanding of spreading cortical depolarization3. As with many other avenues for scientific discovery, the most compelling use for fMRI is in parallel with other techniques. In the example of spreading cortical depolarization, this phenomenon was initially observed in animal models using electrophysiologic methods. These observations have since been confirmed and ultimately strengthened with imaging methods, including magnetoencephalography in animal models, positron emission tomography (PET) in humans, and of course, fMRI in humans.2, 4 The use of fMRI has expanded in part because of its ease of use, as it does not require a contrast agent, is non-radioactive, can be performed on most existing MRI (magnetic resonance imaging) scanners, and has finer spatial resolution than PET or electroencephalography (EEG).
The primary use of fMRI in headache remains in the investigation of pathophysiology and not, at this time, individual diagnosis. This is an area under active investigation, and several attempts have been made to use fMRI to develop a biomarker or classification algorithm for migraine, either using fMRI data alone5–7 or in combination with structural MRI data8. Only one of these, by Tu et al., validated study findings against independent datasets. Tu et al. achieved an accuracy comparing migraine without aura to healthy controls of 84.2% and comparing to a combination of healthy controls and those with chronic lower back pain or fibromyalgia of 73.1%5. With a sensitivity of 77.8% and a specificity of 75.9% in this later analysis, for a combined value greater than 1.5, this test could be considered useful.9 However, non-methodological barriers also slow implementation of fMRI biomarker as a diagnostic tool, including concerns about the ethics and liability issues surrounding the use of artificial intelligence in radiology on the part of radiologists and pain clinicians and researchers10, 11, and regulatory issues12.
While understanding migraine pathophysiology is an important goal, one might question fMRI’s importance if it’s not being used for diagnosis. A key contribution is fMRI’s ability to map networks involved with headache-related brain activity, which could potentially identify intervention targets, including targets for neuromodulation using techniques such as transcranial magnetic stimulation13. Given the growth of fMRI for headache research, the objectives of this review are to ensure this journal’s readers are informed consumers of this area of scientific inquiry, to outline fMRI’s ongoing challenges for headache specialists, and to describe potential considerations when asked to collaborate on fMRI research, as well as suggesting future directions for improvement in the field. This review is intended to cover essentials and due to length constraints is not meant to be comprehensive.
Methods
This manuscript is a narrative review of current best practices in using fMRI for headache research, including the application of international guidelines on Best Practices in Data Analysis and Sharing in Neuroimaging using MRI14. Literature reviewed included published primary research and review articles indexed in PubMed, and textbooks and reports, written in English, inclusive of years 1936–2022. Relevant online publications were included when known to the authors and when representing primary source material.
Methods of fMRI Research
The BOLD contrast
Magnetic resonance imaging takes advantage of the differing tissue responses to electromagnetic fields in order to image the body noninvasively and without radiation. It can be used to examine numerous tissue properties, such as structure15, diffusion patterns16, 17, and blood flow18, 19 by varying patterns of electromagnetic field pulses within what is typically a 1.5–7 Tesla magnetic field. In the case of fMRI, pulse sequences allow researchers to indirectly measure activation of small scale neuronal ensembles, such as cortical columns20, 21 or layers22–24 through changes in regional blood flow25.
The main signal of interest in fMRI is referred to as the blood-oxygen-level-dependent (BOLD) contrast, or signal25. This signal depends on the ratio of oxygenated to deoxygenated hemoglobin within the blood26. Oxygenated hemoglobin is lightly repelled by magnetic forces (weakly diamagnetic) while deoxygenated hemoglobin is attracted to magnetic forces (strongly paramagnetic)27, 28. These differences lead to detectable magnetic susceptibility differences when the ratio of oxygenated to deoxygenated hemoglobin changes, leading to the BOLD signal3.
When neurons become active, there are two main phenomena that are relevant to BOLD imaging. First, active neurons uptake oxygen to meet their metabolic needs, which converts nearby oxyhemoglobin to deoxyhemoglobin25. Second, blood flow to the area of neuronal activation is increased in excess of metabolic needs (hemodynamic uncoupling)25, 29. Because the increase in oxygenated blood is greater than metabolic oxygen consumption, the ratio of oxygenated to deoxygenated hemoglobin increases within the area of increased neuronal activity30. These changes in the BOLD response are characterized by the hemodynamic response function31, 32 as seen in Figure 1. The hemodynamic response function often begins with an initial dip in signal, possibly due to initial oxygen uptake to meet metabolic demands33. After 3–6 seconds post-stimulus32, a large peak follows due to regional blood flow increase. Finally, a small potential post-stimulus undershoot may be observed as the ratio of oxygenated to deoxygenated hemoglobin returns to baseline34. Altogether, this type of fMRI utilizes changes in the hemodynamic response function to track regional blood flow differences related to neuronal activity changes. This signal is sensitive to both increases and decreases in neuronal activity35. It is important to recognize that the BOLD signal indirectly measures brain activity through blood flow changes related to metabolism3. Activity is measured at the level of the “voxel,” or three-dimensional version of a pixel, as determined by slice thickness.
Figure 1:
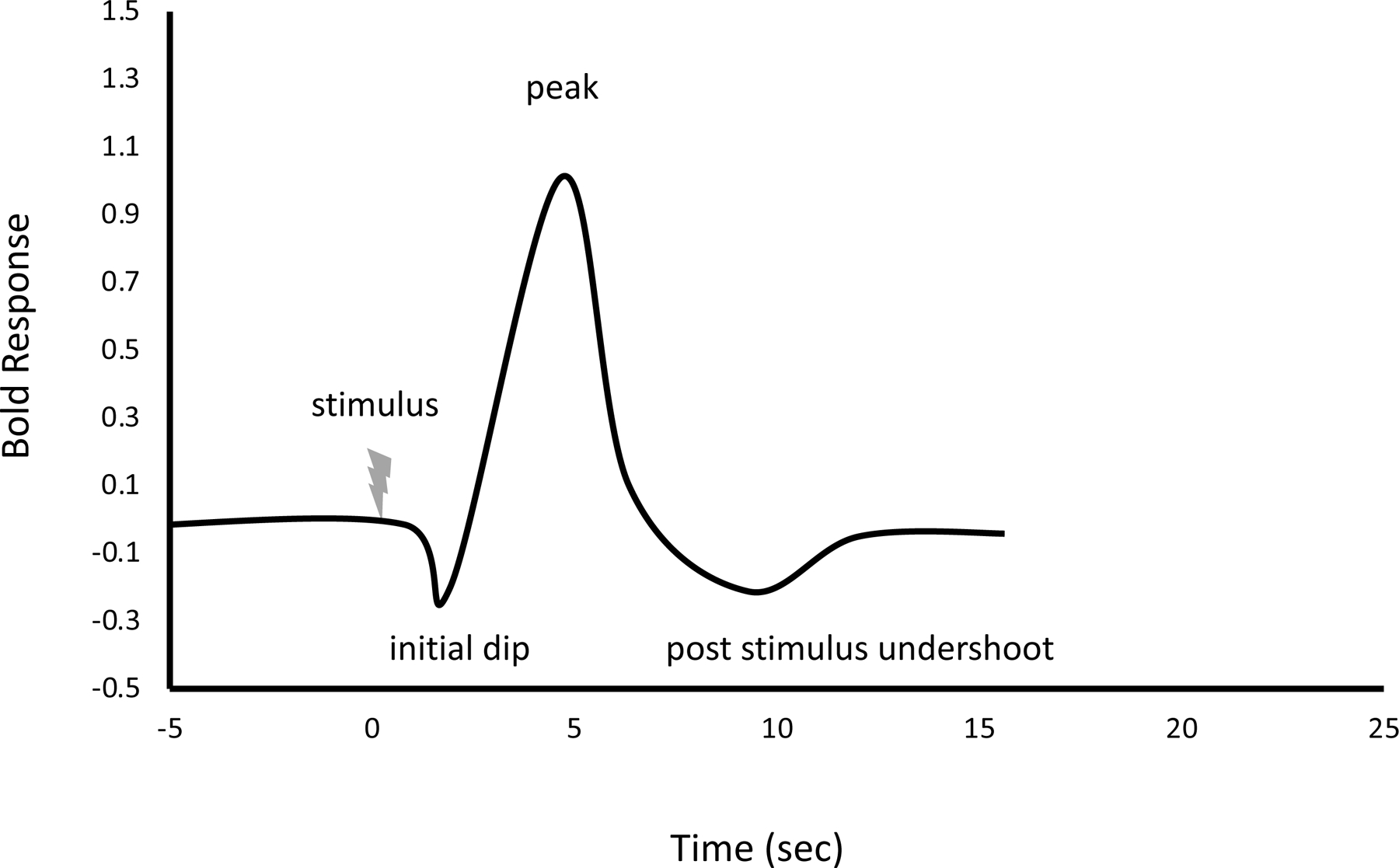
Hemodynamic Response Function
Representative of averaged response after ~1 second stimulus at 7 Tesla strength. Curve normalized by the height of the hemodynamic response function as a function of the time after stimulus.171, 172
Arterial spin labeling
A different type of contrast from BOLD is perfusion-based contrast, focused on intravascular tissue perfusion by tagged blood flow. This is accomplished by using electromagnetic field pulses to label protons in upstream blood and then detecting where it travels in the brain or labeling based on blood flow velocity36. The mechanics of this technique have been summarized for clinicians by Grade et al37 and includes mention of the use of phased array coils. Surface, volume, and phased array coils are additional hardware placed around the head to improve the signaling and detection3 with this method and others. The benefits of this method include changes in blood flow that are quantifiable, e.g. with units such as mL blood/100 g tissue/min38. It was recently used to investigate interictal blood flow differences in a small group of patients with migraine with or without aura in the area of the extrastriate visual cortex and found hyperperfusion in area V5.39
Task-based fMRI
Theory
One of the two primary uses of fMRI is to assess brain activity during the performance of a “task.” Tasks can include sensory, motor, or cognitive activities40, 41. Task-based fMRI studies most commonly compare BOLD signals between two or more different conditions or states42, in which one or more conditions are designated as the control condition. An example is comparing brain responses during painful stimuli to those during nonpainful stimuli. In this case, the task is sensory, and the experimental condition might be painful heat versus a nonpainful warmth control condition43, 44. Differences between these two conditions are thought to represent brain activity specific to the pain stimulus administered.
Task selection
Selecting specific and narrowly defined stimuli is necessary to precisely target brain functions of interest (e.g., mapping thalamic neurons involved in allodynic response to heat or light touch during migraine45). Historically, many if not most task-based fMRI studies in headache have involved sensory stimulus exposure46. Overall, task/stimulus selection should involve careful consideration of factors such as feasibility, external validity, generalizability, and reproducibility. In pain investigations, fMRI studies comparing brain function during painful heat compared to rest allow a fairly well-controlled investigation of the pain response; however, observed effects may not be truly generalizable to the headache-specific pain response. If instead, brain response during periods with headache is compared to periods without, the results may better reflect mechanisms underlying headache pain, but study design becomes much more difficult. Appropriately timing periods of headache during fMRI is substantially more challenging than administering a controlled painful stimulus task.
Study design
Stimulus presentation timing and arrangement are also important study design considerations. The majority of task-based fMRI studies use either “block” or “event-related” designs. In block designs, stimuli belonging to one condition (e.g., visual stimulation) are presented in a group in an alternating fashion with another condition (e.g., rest from visual stimulation), resulting in an additive effect on the BOLD response across stimuli in each block42, 47, 48.
Event-related designs consider individualized trial responses and typically involve more rapid change between conditions compared to block designs42, 47–49. This design was used in a painful trigeminal nerve stimulation study, in which puffs of room air, gaseous ammonia, or rose odor, were presented in random order50. While block designs can provide increased statistical power, higher reliability, and greater observed BOLD signal change, event-related designs can allow analyses to take individual responses to each trial into account (e.g., response speed or accuracy) and can facilitate the use of adaptive paradigms that adjust on a trial-by-trial basis based on individualized performance42, 47, 48, 51–53. It is important to recognize that block designs may increase the likelihood that participants will be able to anticipate, or predict the timing or pattern of stimulus onset, which can in turn influence their pattern of brain activity54, 55. Varying the block sizes, the order of stimuli, and the amount of time between stimuli can help minimize this effect. Event-related designs can further do so, as they allow for more random stimulus presentation, reducing the potential for prediction or habituation52, 56. Event-related designs may also support better estimation of the hemodynamic response compared to block designs56, 57. Mixed block and event-related designs are used to simultaneously assess both event-related brain activity and sustained activity across blocks of stimuli, but this approach presents unique challenges in analysis, such as the potential to misattribute brain activity across conditions51, 52.
Resting-state fMRI
Theory
Resting-state fMRI (rs-fMRI) scans are most often acquired when the brain is in a relaxed and unfocused state, as opposed to performing a focused cognitive task or reacting to external stimuli58, 59. In practice, subjects are instructed to simply let their mind wander freely within the scanner for several minutes59. In this relaxed state, the brain is primarily driven by internal processing demands. Study of the brain at rest allows examination of intrinsic circuitry and connectivity without the confounding effects of a task or external stimuli59.
Resting-state fMRI has greatly advanced the study of the brain’s connectivity and networks. It relies in part on the theory that brain areas that are active at the same time are in some way functionally connected to each other60. The analysis of these dynamics describes what is termed “resting state functional connectivity” (rsFC). This differs from assessing the structural connectivity of white matter tracts, termed tractography. Broadly speaking, tractography investigates the brain’s structural wiring while functional connectivity focuses on neural activity being transmitted along this wiring61. Tractography is performed using a different type of MRI called diffusion tensor imaging (DTI), which examines the direction of water flow in a voxel, and is particularly well-suited to mapping the integrity and direction of myelin sheaths3. In practice, fMRI and DTI are complementary methods that differ in their acquisition of data and can be integrated for analysis and interpretation. For a comparison of roles of various neuroimaging modalities (including fMRI, structural MRI, tractography, magneto-, and electrophysiology) in brain connectivity characterization, see the review by Bassett and Bullmore62.
Hypothesis-driven analysis
Seed-based
In the most straightforward method, a single location or brain region (the “seed region”) is hypothesized to be involved in the clinical symptom of interest, such as processing noxious sensation. The fluctuating BOLD signal in this region is correlated with activity in every voxel within the brain59. The resulting statistics for all voxels are then displayed as a whole-brain spatial map, showing functional connectivity between the seed region and other cortical and subcortical regions. Anatomical boundaries of the seed region or region of interest (ROI) could be delineated using anatomical atlases63–65 such as a Brodmann’s Area, or defined using functional activation coordinates from independent task-based datasets59. This design can examine clinical population connectivity differences, such as patients with migraine with and without aura, or headache treatment intervention effects on connectivity. A limitation of seed-based functional connectivity is the choice of seed region, which must be selected carefully based on study hypotheses. Different seed regions will yield different results for the same study.66 Importantly, using datasets that include the same subjects for both seed region selection and the subsequent connectivity analysis should be avoided, as outcomes and conclusions are limited by the resulting dependencies in the analysis, termed statistical “double-dipping”67.
Data-driven analysis
Independent component analysis
The most widely used data-driven method is independent component analysis (ICA)68–70. This method estimates a set of statistically independent signals, each with an associated spatial map and timing of their spontaneous fluctuations. Each ICA component summarizes a connectivity pattern, often termed an intrinsic connectivity network (ICN). Spatial maps of resting state ICNs can be compared to detect connectivity pattern differences between groups or treatment conditions, or correlated to investigate differences in large-scale coordinated activity patterns throughout the entire brain.
As a part of this analysis, it is necessary to classify the resulting components into different ICNs and noise components71. Identifying individual networks by comparing them to atlases of known networks is a common practice72. Additionally, ICA requires specifying the number of networks and noise components in advance. This number strongly influences which networks are estimated and their spatial extent, with higher numbers potentially resulting in subnetworks of a larger ICN73–75. Even with the specified network number held constant between studies, different noise levels can result in different network sets, potentially limiting replicability.
Local vs. global connectivity
Local connectivity is frequently investigated using a metric termed regional homogeneity (ReHo)76, which indicates whether a voxel is coherent and synchronized with its neighbors and they with it. Limitations of ReHo include its exclusive focus on hyper-local connectivity, connections no more than the width of an fMRI voxel.
Global connectivity, along with local connectivity, is often investigated using measurements provided by the mathematical study of networks, termed graph theory77. To apply graph theory, the brain is first parcellated into individual regions using an ROI atlas, termed nodes. Fluctuating activity at each node is then estimated by averaging encompassed voxels, and nodes’ activities are correlated to estimate the whole-brain network. In contrast to previously described methods, the resulting network encompasses many more connections, potentially only limited by the ROI atlas’ resolution. This whole-brain network is then analyzed by calculating summary metrics, and quantitatively measuring abstract network features. The most widely applied graph theory measures include the following.
Clustering: the tendency of a node’s connected neighbors to themselves share a connection.
Characteristic path length: the number of intermediary connections between nodes.
Degree: the number of a node’s connections.
Modularity: the extent to which the entire network can be partitioned into relatively unconnected parts.
Networks in graph theory are commonly displayed using circles representing nodes, connected by lines. Node arrangement can include ordered circular displays, spring-based layouts grouping similar nodes, or more abstract visualizations. For a sound introduction to graph theory, including an overview of its main concepts and measures, see Sporns’ review on this topic78. For a more detailed but still accessible understanding of the neurobiology and physics of complex networks, see the review by Bullmore and Sporns79.
Measuring low-frequency fluctuations and local activity
Resting-state fMRI does not allow an inherent measure of the “activity” occurring at any voxel within the brain. As a surrogate measure of activity during the resting state, amplitude of low-frequency fluctuations80 (ALFF) or fractional ALFF81 (fALFF) are commonly used. These measures calculate the degree of fluctuations at the individual voxel level with results displayed as spatial maps. This approach was recently used to predict response to transcutaneous auricular vagus nerve stimulation in patients with migraine without aura82. The study’s primary outcome measure was improvement in the visual analog scale score in the fourth week of treatment, and while their patients did achieve this, it was not significantly associated with changes in fALFF, though a secondary outcome of duration of migraine attack was, with change occurring in the bilateral precuneus. For further helpful introductory information on ALFF and ReHO as well as a good primer on neuroimaging, see Lv et al.83
Interpretation of rs-fMRI results
For methods resulting in whole-brain spatial maps, such as seed-based FC, ICA, ReHo, and fALFF, results are interpreted according to relevant neuroanatomical regions highlighted by the analysis. For instance, using a seed in the nociceptive periaqueductal gray (PAG) increased connectivity with somatosensory cortical voxels in subjects with migraine has been interpreted as impairment of the descending pain modularity system centered on the PAG84. Correlations between network time series from ICA, i.e. wide-spread patterns of coordinated neural activity, are interpreted in terms of whole-network effects. An example of analyzing connections between pre-specified ROI is the improvement seen in connectivity between a subset of regions within Executive Control and Salience Networks in patients with chronic migraine after sphenopalatine ganglion block, suggesting the procedure modifies specific circuitry underlying the cognitive and salient aspects of pain85. Graph theory measures are interpreted as rearrangement in the overall network topology, such as widespread disconnections throughout the entire brain network.
Image preprocessing
Two main issues surrounding initial fMRI data collection must be addressed with preprocessing before group analyses can be performed: (1) the shape and position of scanned brain regions are not aligned across scans and patients, and (2) the data comprise both the signal of interest (BOLD) and noise and artifacts. Preprocessing addresses both issues, significantly increasing the signal-to-noise ratio and allowing comparison across patients and scans. Many studies preprocess data with “out-of-the-box” established major software packages, such as the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library (FSL), Analysis of Functional NeuroImages (AFNI), Statistical Parametric Mapping (SPM), or fMRIPrep. This may be completed via a hosted pipeline (e.g., University of Southern California Laboratory of Neuro Imaging – LONI86) or in-house. Numerous studies combine subsets of major packages or develop custom tools to address various preprocessing aspects, which may impact reproducibility.
While there are many methods, theories, and software that can be utilized for preprocessing, there are numerous commonly applied themes, listed below.
Distortion correction
Common fMRI sequences assume homogeneity of the MRI machine’s magnetic field within the scanned region; however, placing a patient’s body within the scanner creates field inhomogeneities, leading to geometric distortions87. Distortion correction addresses this. Commonly, magnetic field inhomogeneities are mapped out with initial scans88. From there, distortion correction methods can utilize this field map to “unwarp” these distortions.
Slice time correction
Magnetic resonance imaging data are typically collected in slices, often one slice at a time, with a delay between each slice. Slice time correction involves using the calculated time offset of each slice to realign the slices in time to each other89.
Motion correction
Patient head motion is the largest contributor to artifacts and noise in MRI. Motion correction involves aligning each collected slice to a reference slice (e.g. the first collected slice)90. This alignment process is iterative and utilizes a cost function to determine which steps in the transformation process should be applied to reach optimal alignment90. Movement confounds are more problematic in rs-fMRI as compared to task-fMRI, requiring more stringent protocols to avoid false positive results.
Normalization to a template
Comparing patient scans to each other is challenging due to differing scanner head positioning, as well as anatomic brain structure variations. Spatial normalization overcomes these issues by aligning scans across patients to the same established template brain. This allows for similar relative regions or stereotaxic coordinates to be compared both within and across studies (e.g., using neurosynth.org). Two of the most commonly utilized coordinate systems include Montreal Neurologic Institute (e.g., MNI152 200991, 92), and Talairach93. Whereas motion correction typically only uses a linear transformation90, normalization often includes a non-linear transformation94, 95.
Coregistration with structural scans
With a typical resolution of 2–4 mm3, functional imaging often lacks the level of detail necessary to identify fine neuroanatomic landmarks. In order to improve visualization of results, higher-resolution structural scans are often obtained along with the functional scans.3 Many image preprocessing protocols also use coregistration, or alignment with a subject’s structural scans in addition to normalization to a template for greater accuracy.3
Spatial smoothing
Spatial smoothing involves averaging signals across small sets of neighboring voxels across the whole image. As the true BOLD signal of closely neighboring brain voxels is inherently similar due to shared blood supply and function, smoothing significantly increases the signal-to-noise ratio96. It is typically performed by choosing a Gaussian kernel with a full width at half maximum (FWHM) that is approximately the same diameter as the expected activated clusters of voxels, perhaps 6–8mm, for group studies. While improving the signal-to-noise ratio and enhancing the validity of common statistical assumptions underlying subsequent analyses, it is useful to note that the size of the smoothing kernel can also bias analysis results – larger kernels will tend to increase sensitivity to detect larger areas of activation, while smaller kernels will have the opposite effect.3
Denoising
Even after the above preprocessing steps, scans will still include non-neuronal noise. The most prominent sources include motion-related and physiologically-induced signal changes such as those from cardiac or respiratory pulsations97. Commonly employed techniques to maximize signal-to-noise ratio include nuisance regression of motion parameters and global signal, filtering out signal not related to the timescale of task-based stimuli in task-based fMRI (temporal filtering), nuisance regression of principal components of regions outside grey matter-related BOLD signal (e.g., cerebral spinal fluid), or use of independent component analysis to identify various noise types97. Physiological artifacts may be corrected by recording cardiac and respiratory fluctuations during scanning then using retrospective denoising algorithms (e.g. RETROICOR98) to remove noise correlated with these. Noise artifacts may be especially challenging to remove when their frequency correlates with the fMRI task99, and the optimal denoising strategy may depend on study design100. For instance, in studies of pain, heart rate may increase with painful stimuli101. Denoising is particularly important when analyzing small ROI, for example, when performing brainstem functional imaging of hypothalamic activation in cluster headache102.
Statistics
General linear model
Functional MRI studies often model BOLD response in two-level designs incorporating subject and group-level information. At the individual subject level, task-related timing is used to predict BOLD activity at each voxel in a multiple regression model. This results in a test statistic, such as the beta value in multiple regression, for each subject and each voxel. Group results are then obtained by combining subject-level results for each voxel in a general linear model (GLM). Associated p-values are calculated for each voxel, and displayed as a whole-brain spatial map, or statistical parametric map103, 104. Importantly, due to the large number of statistical tests involved, false positive results can be found in isolated voxels due to random noise. As such, it is critically important to control for this by correcting for multiple comparisons, as described below.
Multiple comparisons
For typical fMRI analyses, comparisons can be made at each of the ~100,000 voxels in the brain, in each subject, leading to a risk of false positive results. Therefore, failure to adequately correct for multiple comparisons will very likely result in false, or as in the case of a famous fMRI experiment on a dead salmon, deliberately absurd conclusions105. Absence of multiple comparisons correction in an fMRI study is a red flag and resulting conclusions should be treated with appropriate skepticism.
Multiple comparisons correction can be applied either to individual voxels or to clusters of adjacent voxels. Methods include Bonferroni correction to control familywise error rate (FWE), the probability of any false positive result, by dividing the uncorrected p-value significance level by the number of tests in the analysis3. Another commonly-used method is the false discovery rate (FDR), which controls the proportion of false positives by ranking all p-values and applying a sequential cutoff106. In contrast to the stricter Bonferroni correction, FDR has increased sensitivity to true positive results, with the potential tradeoff of including more false positives. Either method is acceptable for fMRI studies and both are preferable to even strict uncorrected p-values.
Cluster-level methods to control for multiple comparisons group neighboring voxels into clusters and then test the overall cluster size. Individual voxel-level p-values are first grouped into clusters by applying a cluster defining threshold (CDT) using a p-value cutoff. As a result, clusters of adjacent voxels with similarly low p-values are grouped together. For fMRI analyses, the recommended CDT is at least p<0.01, and is likely more conservative in many cases107. For example, if the voxel-level test statistic is from the gaussian distribution of z-statistics, or z-scores, applying a CDT of p<0.01 corresponds to thresholding all the significance level of voxels at z>2.05 or z<−2.05. Subsequently, the size of each cluster is tested for significance and reported as a cluster-level p-value that is then corrected for multiple comparisons. When displaying results in figures or tables, clusters or voxels that are non-significant after correcting for multiple comparisons should either not be displayed or clearly marked as non-significant.
Power
While more challenging for fMRI studies, a priori power analyses can help to estimate the necessary sample size for a study108–111, with available analytical toolboxes designed for fMRI power calculations (e.g., Neuropower112). Increasing sample size can improve reliability and reproducibility, but there are often constraints on study sample size due to cost, time, or limitations relating to the participant population. Multi-site studies and data sharing are potential approaches to this in addition to facilitating meta-analyses109, 113. The development of large-scale, publicly available neuroimaging datasets reflects recent efforts across the field to provide larger samples than most investigators could feasibly collect independently (e.g., Human Connectome Project114). Although large samples are advantageous for improving power, smaller-scale datasets still play a key role in neuroimaging research, particularly in studies involving repeated-measures intervention studies, or in assessing novel MRI paradigms115, 116. Repeated measures at the individual level can be powerful in driving data-driven discovery and can address unique research questions117–121. For example, a study by Schulte and May included daily scans of a single patient with migraine for one month to capture fMRI changes preceding migraine attacks122. Increasing the amount of data collected per person (e.g., by increasing the number of trials in task-based fMRI111 or increasing resting-state scan length123–125) can also improve power and reliability126.
Reproducibility concerns
Concerns regarding the reproducibility of neuroimaging research results (i.e., the “reproducibility crisis” or “replication crisis”) have garnered much attention in recent years109, 110, 116, 127–130. Many of these can be addressed through careful attention to approaches outlined elsewhere in this article, such as best-practice pre-processing, appropriately addressing multiple comparisons, and statistical power; however, questionable research practices (e.g., testing different analytical approaches until a significant p-value is found, or “p-hacking”131, 132), suboptimal study design, publication bias favoring positive results, variability in analytical methods, and lack of transparency in methods may also play a role113, 128, 130, 133. Although sometimes termed a “crisis,” the dialogue in this area has actually served to bolster ongoing efforts across the neuroimaging research community to promote the adoption of research practices supporting improved rigor and reproducibility, including a strong momentum towards increasing transparency and open science practices in MRI research109, 134–137. One such practice is pre-registering studies, in which planned hypotheses and analyses are described prior to conducting the study, which can increase confidence in study results by reducing positive publication bias, p-hacking, and instances of hypothesizing after results are known (“HARKing” 138)109, 128, 132, 136. Published manuscripts should also outline which analyses were planned ahead of time and which were exploratory and/or data-driven109, 128.
Putting it all Together: Quality fMRI Headache Research
Given what is described above, one may already discern some of the issues to consider when reviewing this literature. Although there is no reporting guideline checklist, such as STROBE (Strengthening the Reporting of Observational Studies in Epidemiology)139, listed by the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network140 for fMRI studies, there are other resources. The Organization for Human Brain Mapping’s Committee on Best Practices in Data Analysis and Sharing (COBIDAS) issued a report in 2016 on MRI research called the Best Practices in Data Analysis and Sharing in Neuroimaging using MRI14. Its Appendix D, consisting of “Itemized lists of best practices and reporting items,” can serve this purpose.
In addition to subject selection, participant consent, and other reporting criteria common to all clinical research, the following checklist features elements particular to fMRI of a well-performed and well-reported study.
For an excellent review of overall study design considerations in functional connectivity studies specific to migraine, see Maleki and Golub’s review, which discusses questions of task vs resting-state and related concepts for this population13. For a review of pain neuroimaging in general with good attention to task design related to nociceptive stimulation, see Moayedi et al.141 The following sections will focus on some additional items.
Ictal vs interictal
In the Schulte and May study of a single individual’s daily evolution of fMRI over a one-month period and three migraine attacks, the authors found differences between ictal and interictal patterns of activity, with changes even during the prodrome period.122 Other studies have shown differences between ictal and interictal fMRI as well142. As a result, most studies attempt to image patients that are either ictal or inter-ictal, without combining the two as noted in Maleki and Golub’s review13. To accomplish this, studies may require that patients have been migraine-free for 72 hours prior to scanning if they are trying to capture the interictal period.5
Age
Functional MRI study design considerations could include how age is handled in pediatric studies. Of note, there has been a call to use age-specific atlases or templates in neuroimaging, particularly in younger children, given the evolving myelination patterns into adulthood143, 144; however, many important pediatric studies use software packages for analysis such as FreeSurfer that in turn use atlases based on older adults. This includes the enormous and still-ongoing longitudinal Adolescent Brain Cognitive Development Study (ABCD) and its Brain Imaging Data Structure (BIDS) standards-compliant Community Collection145.
Sex and gender
Sex and gender are emerging areas of importance in headache medicine and research146. The NIH has begun to focus on reporting these variables with new requirements for considering sex as a biologic variable147, though there is still progress to be made on reporting gender148. There are known sex differences in post-pubertal brain structure, including the amygdala149, a region shown to have altered connectivity in migraine150. Considering sex and gender separately is also important, as transgender individuals who have undergone gender-affirming hormone therapy have reported alterations in headache with this therapy151. Given findings of sex differences in migraine patients with task-based fMRI152 and resting state fMRI153, it is reasonable to address this difference in study design or analysis in fMRI research, e.g. matching controls and cases for sex, or covarying for sex in statistical analysis.
Handedness
Resting-state functional connectivity patterns have been shown to differ by handedness154. As such, it is reasonable to gather information on handedness and use this as a covariate in fMRI studies.
Scanner
Multisite acquisition
In the case of relatively rare diseases, such as cluster headache, it may be desirable to recruit and scan individuals at multiple sites across a geographic region to gain statistical power for analysis; however, intra-individual differences in rsFC pattern when acquired in different scanners and different sites has been demonstrated.155 The Functional Biomedical Informatics Research Network has published recommendations on how to reduce inter-site variability that should be considered when designing a multi-site study.156
Magnet strength
Although higher magnetic field strength, e.g. 3 Tesla (T) or 7T MRI as compared to 1.5T improves sensitivity to detect smaller areas of functional activation, higher field strengths cause additional artifacts that must be considered. This tradeoff is likely worthwhile, however, for smaller regions of interest such brainstem nuclei, e.g the trigeminal nucleus.157
Safety
Certain subjects may need to be excluded from any MRI study due to risk from exposure to the magnetic field, such as those with certain implanted or onplanted devices, e.g. cardiac pacemakers. For a more complete reference on this topic, see the American College of Radiology’s current ACR Manual on MR Safety.158
Significance
In assessing the significance of findings beyond p-values, some have begun to explore the use of sensitivity and specificity5 and others have suggested that activation clusters of voxels should be reported using a confidence interval159–161 or confidence set162. It can also be helpful to consider whether data from other experimental methods support an fMRI study’s conclusions. For example, altered connectivity in the PAG as a feature of migraine pathophysiology seems reasonable given its demonstrated role in reducing pain when stimulated in vivo163. As with many other avenues for scientific discovery, the strongest use for fMRI is in parallel with other techniques.
Considerations if asked to collaborate on fMRI research
If asked to collaborate on an fMRI study, consider whether the above have been addressed in the study design as well as the degree to which potential imaging collaborators understand headache or how early in the study design headache expertise was included. It is also important to consider the goals of potential collaborators in using fMRI to study headache, namely, the development of new experimental methods vs disease characterization. An example of new methods development is a recent paper demonstrating a novel method of identifying functional networks, dubbed functional areas of unitary pooled activity by the authors, in the visual cortex in migraine patients vs healthy controls164. It is often desirable to demonstrate a new imaging method on a well-characterized disease state such as migraine. In contrast, an example of disease characterization is a recent task-based fMRI study of trigeminonociceptive stimulation with ammonia in cluster headache patients, using established methods for brainstem imaging and an established ammonia stimulation protocol102.
Separate considerations may pertain to studies in special populations, such as children. For instance, braces are commonly found in pediatric subjects. Although braces are MR-safe, they have long been known to cause imaging artifacts, primarily signal loss or frontal lobe distortion in certain imaging sequences165, 166. Most imaging artifacts, including hardware-related, are more noticeable at higher field strengths167, an important consideration for fMRI at 7 Tesla. Expense is also a limiting factor in data collection; at most institutions, hourly scanning costs for investigator-initiated NIH-funded studies are typically $600-$700.
Conclusions
The methods outlined above describe several attributes of research that can be applied to fMRI headache research to increase reproducibility, including methods transparency, eliminating error, using a priori hypotheses, and using power calculations. We could add to this use of standardized instruments such as the National Institute of Neurologic Disorders and Stroke Common Data elements168 for covariates, use of the current International Classification of Headache Disorders169 criteria for diagnosis, and development of large datasets, such as the American Registry for Migraine Research170. The promise of the field for understanding headache and potentially identifying biomarkers for treatment response or diagnosis is high.
Table 1.
Checklist of fMRI study essentials
| Are the Figures thresholded or unthresholded? | |
| Have the authors addressed multiple comparisons in some way? | |
| Have the authors reported areas of peak activation coordinates for task-based fMRI? | |
| Did the authors have pre-specified hypotheses and/or primary outcome(s)? | |
| If multiple scanners and/or image acquisition sites, has this been addressed, particularly if the project is longitudinal? | |
| Have scanner noise and related field inhomogeneities been addressed? | |
| Was handedness reported and addressed? | |
| Has physiologic noise been addressed? | |
| Has subject motion been corrected? |
Acknowledgements:
JAH would like to thank Angeliki Vgontzas, who suggested I write on this topic after Todd Schwedt’s presentation at the Graham Headache Center as Visitans Scolaris Capitis Dolor.
Financial Support:
NIH/NINDS Child Neurologist Career Development Program (CNCDP) grant K12NS098482 subaward to Jennifer Hranilovich
Abbreviations
- fMRI
functional magnetic resonance imaging
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- EEG
lectroencephalography
- BOLD
blood-oxygen-level-dependent
- rsFC
resting-state functional connectivity
- DTI
diffusion tensor imaging
- ROI
region of interest
- ICA
independent components analysis
- ICN
intrinsic connectivity network
- ALFF
amplitude of low frequency fluctuations
- fALFF
fractional amplitude of low frequency fluctuations
- ReHO
regional homogeneity
- FSL
Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library
- AFNI
Analysis of Functional NeuroImages
- SPM
Statistical Parametric Mapping
- LONI
Laboratory of Neuro Imaging
- MNI
Montreal Neurologic Institute
- ANOVA
Analysis of Variance
- FWHM
full width at half maximum
- GLM
general linear model
- FWE
familywise error
- FDR
false discovery rate
- CDT
cluster defining threshold
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- EQUATOR
Enhancing the QUAlity and Transparency Of health Research
- COBIDAS
Committee on Best Practices in Data Analysis and Sharing
- ABCD
Adolescent Brain Cognitive Development
- BIDS
Brain Imaging Data Structure. 2
Footnotes
Conflict of Interest Statement:
Jennifer A. Hranilovich declares no conflicts of interest. Kristina T. Legget declares no conflicts of interest. Keith C. Dodd declares no conflicts of interest. Korey P. Wylie declares no conflicts of interest. Jason R. Tregellas declares no conflicts of interest.
References
- 1.Ferrari MD, Goadsby PJ, Burstein R, et al. Migraine. Nat Rev Dis Primers 2022;8:2. [DOI] [PubMed] [Google Scholar]
- 2.Russo A, Silvestro M, Tessitore A, Tedeschi G. Recent Insights in Migraine With Aura: A Narrative Review of Advanced Neuroimaging. Headache 2019;59:637–649. [DOI] [PubMed] [Google Scholar]
- 3.Huettel SA, Song AW, McCarthy G. Functional magnetic resonance imaging, Third edition. ed. Sunderland, Massachusetts, U.S.A.: Sinauer Associates, Inc., Publishers, 2014. [Google Scholar]
- 4.Eikermann-Haerter KM, Michael. Pathophysiology of Aura. In: Silberstein SD, Lipton RB, Dodick D, Wolff HG, eds. Wolff's headache and other head pain / [edited by] Silberstein Stephen D, Lipton Richard B, Dodick David W, 8th ed. Oxford ; New York: Oxford University Press, 2008: 121–131. [Google Scholar]
- 5.Tu Y, Zeng F, Lan L, et al. An fMRI-based neural marker for migraine without aura. Neurology 2020;94:e741–e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong CD, Gaw N, Fu Y, Li J, Wu T, Schwedt TJ. Migraine classification using magnetic resonance imaging resting-state functional connectivity data. Cephalalgia : an international journal of headache 2017;37:828–844. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Zhang J, Liu Q, Wang Y. Multimodal MRI-based classification of migraine: using deep learning convolutional neural network. Biomed Eng Online 2018;17:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Wu Q, Zhang J, et al. Discriminative Analysis of Migraine without Aura: Using Functional and Structural MRI with a Multi-Feature Classification Approach. PLoS One 2016;11:e0163875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Power M, Fell G, Wright M. Principles for high-quality, high-value testing. Evidence-Based Medicine 2013;18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis KD, Flor H, Greely HT, et al. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nature Reviews Neurology 2017;13:624–638. [DOI] [PubMed] [Google Scholar]
- 11.Geis JR, Brady A, Wu CC, et al. Ethics of artificial intelligence in radiology: summary of the joint European and North American multisociety statement. Insights into Imaging 2019;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.OECD. Policy Issues for the Development and Use of Biomarkers in Health. Paris,: OECD, 2011. [Google Scholar]
- 13.Maleki N, Gollub RL. What Have We Learned From Brain Functional Connectivity Studies in Migraine Headache? Headache 2016;56:453–461. [DOI] [PubMed] [Google Scholar]
- 14.Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M, Kriegeskorte N, Milham MP, Poldrack RA, Poline J-B, Proal E, Thirion B, Van Essen DC, White T, Yeo BTT. Best Practices in Data Analysis and Sharing in Neuroimaging using MRI. bioRxiv 2016. [DOI] [PMC free article] [PubMed]
- 15.Symms M, Jäger HR, Schmierer K, Yousry TA. A review of structural magnetic resonance neuroimaging Neuroscience for Neurologists: PUBLISHED BY IMPERIAL COLLEGE PRESS AND DISTRIBUTED BY WORLD SCIENTIFIC PUBLISHING CO., 2006: 343–375. [Google Scholar]
- 16.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J 1994;66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001;13:534–546. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 1990;14:68–78. [DOI] [PubMed] [Google Scholar]
- 19.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci 2003;23:3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yacoub E, Shmuel A, Logothetis N, Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. NeuroImage 2007;37:1161–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yacoub E, Harel N, Ugurbil K. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci U S A 2008;105:10607–10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goense J, Merkle H, Logothetis NK. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron 2012;76:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koopmans PJ, Barth M, Orzada S, Norris DG. Multi-echo fMRI of the cortical laminae in humans at 7 T. NeuroImage 2011;56:1276–1285. [DOI] [PubMed] [Google Scholar]
- 24.Olman CA, Harel N, Feinberg DA, et al. Layer-specific fMRI reflects different neuronal computations at different depths in human V1. PLoS One 2012;7:e32536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci U S A 1998;95:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown GG, Perthen JE, Liu TT, Buxton RB. A primer on functional magnetic resonance imaging. Neuropsychol Rev 2007;17:107–125. [DOI] [PubMed] [Google Scholar]
- 27.Pauling L, Coryell CD. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A 1936;22:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990;87:9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A 1986;83:1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox PT. The coupling controversy. Neuroimage 2012;62:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med 1992;25:390–397. [DOI] [PubMed] [Google Scholar]
- 32.Buckner RL. Event-related fMRI and the hemodynamic response. Hum Brain Mapp 1998;6:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 1996;272:551–554. [DOI] [PubMed] [Google Scholar]
- 34.Chen JJ, Pike GB. Origins of the BOLD post-stimulus undershoot. Neuroimage 2009;46:559–568. [DOI] [PubMed] [Google Scholar]
- 35.Goense J, Bohraus Y, Logothetis NK. fMRI at High Spatial Resolution: Implications for BOLD-Models. Front Comput Neurosci 2016;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez-Garcia L, Lahiri A, Schollenberger J. Recent progress in ASL. NeuroImage 2019;187:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alarcon A, Martinez-Biarge M, Cabanas F, Hernanz A, Quero J, Garcia-Alix A. Clinical, biochemical, and neuroimaging findings predict long-term neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. The Journal of pediatrics 2013;163:828–834 e821. [DOI] [PubMed] [Google Scholar]
- 38.Loggia ML, Segerdahl AR, Howard MA, Tracey I. Imaging clinically relevant pain states using arterial spin labeling. PAIN Reports 2019;4:e750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michels L, Villanueva J, O'Gorman R, et al. Interictal Hyperperfusion in the Higher Visual Cortex in Patients With Episodic Migraine. Headache 2019;59:1808–1820. [DOI] [PubMed] [Google Scholar]
- 40.Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magnetic resonance in medicine 1992;25:390–397. [DOI] [PubMed] [Google Scholar]
- 41.DeYoe EA, Bandettini P, Neitz J, Miller D, Winans P. Functional magnetic resonance imaging (FMRI) of the human brain. J Neurosci Methods 1994;54:171–187. [DOI] [PubMed] [Google Scholar]
- 42.Glover GH. Overview of functional magnetic resonance imaging. Neurosurg Clin N Am 2011;22:133–139, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med 2013;368:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanasescu R, Cottam WJ, Condon L, Tench CR, Auer DP. Functional reorganisation in chronic pain and neural correlates of pain sensitisation: A coordinate based meta-analysis of 266 cutaneous pain fMRI studies. Neurosci Biobehav Rev 2016;68:120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 2010;68:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwedt TJ, Chiang CC, Chong CD, Dodick DW. Functional MRI of migraine. Lancet Neurol 2015;14:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp 1997;5:329–340. [DOI] [PubMed] [Google Scholar]
- 48.Bandettini PA. fMRI Cambridge, MA: The MIT Press, 2020. [Google Scholar]
- 49.Henson R Efficient experimental design for fMRI. In: Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD, eds. Statistical Parametric Mapping: The Analysis of Functional Brain Images London: Academic Press, 2007: 193–210. [Google Scholar]
- 50.Stankewitz A, Voit HL, Bingel U, Peschke C, May A. A new trigemino-nociceptive stimulation model for event-related fMRI. Cephalalgia : an international journal of headache 2010;30:475–485. [DOI] [PubMed] [Google Scholar]
- 51.Amaro E, Jr., Barker GJ. Study design in fMRI: basic principles. Brain Cogn 2006;60:220–232. [DOI] [PubMed] [Google Scholar]
- 52.Petersen SE, Dubis JW. The mixed block/event-related design. Neuroimage 2012;62:1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bennett CM, Miller MB. fMRI reliability: influences of task and experimental design. Cogn Affect Behav Neurosci 2013;13:690–702. [DOI] [PubMed] [Google Scholar]
- 54.Valente G, Kaas AL, Formisano E, Goebel R. Optimizing fMRI experimental design for MVPA-based BCI control: Combining the strengths of block and event-related designs. Neuroimage 2019;186:369–381. [DOI] [PubMed] [Google Scholar]
- 55.Pilgrim LK, Fadili J, Fletcher P, Tyler LK. Overcoming confounds of stimulus blocking: an event-related fMRI design of semantic processing. NeuroImage 2002;16:713–723. [DOI] [PubMed] [Google Scholar]
- 56.Dale AM. Optimal experimental design for event-related fMRI. Human brain mapping 1999;8:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maus B, van Breukelen GJ, Goebel R, Berger MP. Optimal design for nonlinear estimation of the hemodynamic response function. Human brain mapping 2012;33:1253–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537–541. [DOI] [PubMed] [Google Scholar]
- 59.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci 2010;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8:700–711. [DOI] [PubMed] [Google Scholar]
- 61.Hermundstad AM, Bassett DS, Brown KS, et al. Structural foundations of resting-state and task-based functional connectivity in the human brain. Proc Natl Acad Sci U S A 2013;110:6169–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol 2009;22:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amunts K, Mohlberg H, Bludau S, Zilles K. Julich-Brain: A 3D probabilistic atlas of the human brain's cytoarchitecture. Science 2020;369:988-+. [DOI] [PubMed] [Google Scholar]
- 64.Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human Brain Mapping 2003;19:224–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 66.Cordes D, Haughton VM, Arfanakis K, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 67.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 2009;12:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. NeuroImage 2005;25:294–311. [DOI] [PubMed] [Google Scholar]
- 69.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping 2001;14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hyvarinen A, Oja E. Independent component analysis: algorithms and applications. Neural Networks 2000;13:411–430. [DOI] [PubMed] [Google Scholar]
- 71.Griffanti L, Douaud G, Bijsterbosch J, et al. Hand classification of fMRI ICA noise components. Neuroimage 2017;154:188–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 2012;22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abou-Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V. The effect of model order selection in group PICA. Hum Brain Mapp 2010;31:1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allen EA, Erhardt EB, Damaraju E, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci 2011;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wylie KP, Kronberg E, Legget KT, Sutton B, Tregellas JR. Stable Meta-Networks, Noise, and Artifacts in the Human Connectome: Low- to High-Dimensional Independent Components Analysis as a Hierarchy of Intrinsic Connectivity Networks. Front Neurosci 2021;15:625737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004;22:394–400. [DOI] [PubMed] [Google Scholar]
- 77.Newman MEJ. Networks : an introduction Oxford ; New York: Oxford University Press, 2010. [Google Scholar]
- 78.Sporns O Structure and function of complex brain networks. Dialogues Clin Neurosci 2013;15:247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009;10:186–198. [DOI] [PubMed] [Google Scholar]
- 80.Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 2007;29:83–91. [DOI] [PubMed] [Google Scholar]
- 81.Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 2008;172:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng M, Zhang Y, Wen Z, et al. Early Fractional Amplitude of Low Frequency Fluctuation Can Predict the Efficacy of Transcutaneous Auricular Vagus Nerve Stimulation Treatment for Migraine Without Aura. Front Mol Neurosci 2022;15:778139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lv H, Wang Z, Tong E, et al. Resting-State Functional MRI: Everything That Nonexperts Have Always Wanted to Know. American Journal of Neuroradiology 2018;39:1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 2011;70:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krebs K, Rorden C, Androulakis XM. Resting State Functional Connectivity After Sphenopalatine Ganglion Blocks in Chronic Migraine With Medication Overuse Headache: A Pilot Longitudinal fMRI Study. Headache 2018;58:732–743. [DOI] [PubMed] [Google Scholar]
- 86.Dinov ID, Petrosyan P, Liu Z, et al. High-throughput neuroimaging-genetics computational infrastructure. Front Neuroinform 2014;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen JE, Glover GH. Functional Magnetic Resonance Imaging methods. Neuropsychol Rev 2015;25:289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med 1995;34:65–73. [DOI] [PubMed] [Google Scholar]
- 89.Sladky R, Friston KJ, Tröstl J, Cunnington R, Moser E, Windischberger C. Slice-timing effects and their correction in functional MRI. Neuroimage 2011;58:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ashburner J, Friston K. Rigid Body Registration. Statistical Parametric Mapping: Elsevier, 2007: 49–62.
- 91.Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 2001;356:1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mazziotta J, Toga A, Evans A, et al. A four-dimensional probabilistic atlas of the human brain. J Am Med Inform Assoc 2001;8:401–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Talairach J Co-planar stereotaxic atlas of the human brain-3-dimensional proportional system. An approach to cerebral imaging 1988.
- 94.Ashburner J, Andersson JL, Friston KJ. High-dimensional image registration using symmetric priors. Neuroimage 1999;9:619–628. [DOI] [PubMed] [Google Scholar]
- 95.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 96.Mikl M, Marecek R, Hlustík P, et al. Effects of spatial smoothing on fMRI group inferences. Magn Reson Imaging 2008;26:490–503. [DOI] [PubMed] [Google Scholar]
- 97.Caballero-Gaudes C, Reynolds RC. Methods for cleaning the BOLD fMRI signal. Neuroimage 2017;154:128–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 2000;44:162–167. [DOI] [PubMed] [Google Scholar]
- 99.Birn RM, Murphy K, Handwerker DA, Bandettini PA. fMRI in the presence of task-correlated breathing variations. NeuroImage 2009;47:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mayer AR, Ling JM, Dodd AB, Shaff NA, Wertz CJ, Hanlon FM. A comparison of denoising pipelines in high temporal resolution task-based functional magnetic resonance imaging data. Human brain mapping 2019;40:3843–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tousignant-Laflamme Y, Rainville P, Marchand S. Establishing a link between heart rate and pain in healthy subjects: a gender effect. J Pain 2005;6:341–347. [DOI] [PubMed] [Google Scholar]
- 102.Schulte LH, Haji AA, May A. Phase dependent hypothalamic activation following trigeminal input in cluster headache. The journal of headache and pain 2020;21:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human brain mapping 1994;2:189–210. [Google Scholar]
- 104.Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human brain mapping 1994;1:210–220. [DOI] [PubMed] [Google Scholar]
- 105.Bennett CM, Miller MB, Wolford GL. neural correlates of interspecies perspective taking in the post-mortem atlantic salmon: an argument for multiple comparison correction. Neuroimage 2009;47.19457371
- 106.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B 1995;57:289–300. [Google Scholar]
- 107.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A 2016;113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mumford JA. A power calculation guide for fMRI studies. Soc Cogn Affect Neurosci 2012;7:738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poldrack RA, Baker CI, Durnez J, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 2017;18:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Turner BO, Paul EJ, Miller MB, Barbey AK. Small sample sizes reduce the replicability of task-based fMRI studies. Commun Biol 2018;1:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen G, Pine DS, Brotman MA, et al. Hyperbolic trade-off: The importance of balancing trial and subject sample sizes in neuroimaging. Neuroimage 2022;247:118786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Durnez J, Degryse J, Moerkerke B, et al. Power and sample size calculations for fMRI studies based on the prevalence of active peaks. bioRxiv 2016;049429.
- 113.Bossier H, Roels SP, Seurinck R, et al. The empirical replicability of task-based fMRI as a function of sample size. Neuroimage 2020;212:116601. [DOI] [PubMed] [Google Scholar]
- 114.Van Essen DC, Smith SM, Barch DM, et al. The WU-Minn Human Connectome Project: an overview. Neuroimage 2013;80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosenberg MD, Finn ES. How to establish robust brain-behavior relationships without thousands of individuals. Nat Neurosci 2022;25:835–837. [DOI] [PubMed] [Google Scholar]
- 116.Marek S, Tervo-Clemmens B, Calabro FJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature 2022;603:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Poldrack RA, Laumann TO, Koyejo O, et al. Long-term neural and physiological phenotyping of a single human. Nat Commun 2015;6:8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fedorenko E The early origins and the growing popularity of the individual-subject analytic approach in human neuroscience. Curr Opin Behav Sci 2021;40:105–112. [Google Scholar]
- 119.Naselaris T, Allen E, Kay K. Extensive sampling for complete models of individual brains. Curr Opin Behav Sci 2021;40:45–51. [Google Scholar]
- 120.Poldrack RA. Diving into the deep end: a personal reflection on the MyConnectome study. Curr Opin Behav Sci 2021;40:1–4. [Google Scholar]
- 121.Pritschet L, Taylor CM, Santander T, Jacobs EG. Applying dense-sampling methods to reveal dynamic endocrine modulation of the nervous system. Curr Opin Behav Sci 2021;40:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016;139:1987–1993. [DOI] [PubMed] [Google Scholar]
- 123.Birn RM, Molloy EK, Patriat R, et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage 2013;83:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pannunzi M, Hindriks R, Bettinardi RG, et al. Resting-state fMRI correlations: From link-wise unreliability to whole brain stability. Neuroimage 2017;157:250–262. [DOI] [PubMed] [Google Scholar]
- 125.Elliott ML, Knodt AR, Cooke M, et al. General functional connectivity: Shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks. Neuroimage 2019;189:516–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nee DE. fMRI replicability depends upon sufficient individual-level data. Commun Biol 2019;2:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baker M 1,500 scientists lift the lid on reproducibility. Nature 2016;533:452–454. [DOI] [PubMed] [Google Scholar]
- 128.Munafo MR, Nosek BA, Bishop DVM, et al. A manifesto for reproducible science. Nat Hum Behav 2017;1:0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elliott ML, Knodt AR, Ireland D, et al. What Is the Test-Retest Reliability of Common Task-Functional MRI Measures? New Empirical Evidence and a Meta-Analysis. Psychol Sci 2020;31:792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hensel WM. Double trouble? The communication dimension of the reproducibility crisis in experimental psychology and neuroscience. Eur J Philos Sci 2020;10:44. [Google Scholar]
- 131.Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol Sci 2011;22:1359–1366. [DOI] [PubMed] [Google Scholar]
- 132.Bishop D Rein in the four horsemen of irreproducibility. Nature 2019;568:435. [DOI] [PubMed] [Google Scholar]
- 133.Lewandowsky S, Oberauer K. Low replicability can support robust and efficient science. Nat Commun 2020;11:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE. Guidelines for reporting an fMRI study. Neuroimage 2008;40:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carp J The secret lives of experiments: methods reporting in the fMRI literature. Neuroimage 2012;63:289–300. [DOI] [PubMed] [Google Scholar]
- 136.Gorgolewski KJ, Poldrack RA. A Practical Guide for Improving Transparency and Reproducibility in Neuroimaging Research. PLoS Biol 2016;14:e1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nichols TE, Das S, Eickhoff SB, et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci 2017;20:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kerr NL. HARKing: hypothesizing after the results are known. Pers Soc Psychol Rev 1998;2:196–217. [DOI] [PubMed] [Google Scholar]
- 139.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 140.Pandis N, Fedorowicz Z. The international EQUATOR network: enhancing the quality and transparency of health care research. J Appl Oral Sci 2011;19:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moayedi M, Salomons TV, Atlas LY. Pain Neuroimaging in Humans: A Primer for Beginners and Non-Imagers. J Pain 2018;19:961.e961–961.e921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maleki N, Szabo E, Becerra L, et al. Ictal and interictal brain activation in episodic migraine: Neural basis for extent of allodynia. PLoS One 2021;16:e0244320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sanchez CE, Richards JE, Almli CR. Age-specific MRI templates for pediatric neuroimaging. Dev Neuropsychol 2012;37:379–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci 1999;2:859–861. [DOI] [PubMed] [Google Scholar]
- 145.Feczko E, Conan G, Marek S, et al. Adolescent Brain Cognitive Development (ABCD) Community MRI Collection and Utilities. bioRxiv 2021:2021.2007.2009.451638.
- 146.Ackley E, Halker Singh RB. Sex and gender: Opportunities to expand research and understanding within headache medicine. Headache 2022;62:771–773. [DOI] [PubMed] [Google Scholar]
- 147.Clayton JA. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol Behav 2018;187:2–5. [DOI] [PubMed] [Google Scholar]
- 148.National Academies of Sciences EaM. Measuring Sex, Gender Identity, and Sexual Orientation Washington, DC: National Academies of Sciences, Engineering and Medicine, 2022. [PubMed] [Google Scholar]
- 149.Bramen JE, Hranilovich JA, Dahl RE, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebral cortex (New York, NY : 1991) 2011;21:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chong CD, Schwedt TJ, Hougaard A. Brain functional connectivity in headache disorders: A narrative review of MRI investigations. J Cereb Blood Flow Metab 2019;39:650–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hranilovich JA, Kaiser EA, Pace A, Barber M, Ziplow J. Headache in transgender and gender-diverse patients: A narrative review. Headache 2021;61:1040–1050. [DOI] [PubMed] [Google Scholar]
- 152.Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain 2012;135:2546–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gecse K, Baksa D, Dobos D, et al. Sex Differences of Periaqueductal Grey Matter Functional Connectivity in Migraine. Frontiers in Pain Research 2021;2. [DOI] [PMC free article] [PubMed]
- 154.Joliot M, Tzourio-Mazoyer N, Mazoyer B. Intra-hemispheric intrinsic connectivity asymmetry and its relationships with handedness and language Lateralization. Neuropsychologia 2016;93:437–447. [DOI] [PubMed] [Google Scholar]
- 155.Badhwar A, Collin-Verreault Y, Orban P, et al. Multivariate consistency of resting-state fMRI connectivity maps acquired on a single individual over 2.5 years, 13 sites and 3 vendors. NeuroImage 2020;205:116210. [DOI] [PubMed] [Google Scholar]
- 156.Glover GH, Mueller BA, Turner JA, et al. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J Magn Reson Imaging 2012;36:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Colizoli O, de Gee JW, van der Zwaag W, Donner TH. Functional magnetic resonance imaging responses during perceptual decision-making at 3 and 7 T in human cortex, striatum, and brainstem. Human brain mapping 2022;43:1265–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.ACR Committee on MR Safety. ACR Manual on MR Safety: American College of Radiology, 2020.
- 159.Engel SA, Burton PC. Confidence intervals for fMRI activation maps. PLoS One 2013;8:e82419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hupé JM. Statistical inferences under the Null hypothesis: common mistakes and pitfalls in neuroimaging studies. Front Neurosci 2015;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen G, Taylor PA, Cox RW. Is the statistic value all we should care about in neuroimaging? NeuroImage 2017;147:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bowring A, Telschow F, Schwartzman A, Nichols TE. Spatial confidence sets for raw effect size images. NeuroImage 2019;203:116187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Roizen MF, Newfield P, Eger EI, Hosobuchi Y, Adams JE, Lamb S. Reduced anesthetic requirement after electrical stimulation of periaqueductal gray matter. Anesthesiology 1985;62:120–123. [DOI] [PubMed] [Google Scholar]
- 164.Huang J, Wilkins A. The Functional Network of the Visual Cortex Is Altered in Migraine. Vision (Basel) 2021;5. [DOI] [PMC free article] [PubMed]
- 165.Lissac M, Coudert JL, Briguet A, Amiel M. Disturbances caused by dental materials in magnetic resonance imaging. Int Dent J 1992;42:229–233. [PubMed] [Google Scholar]
- 166.Miao X, Wu Y, Liu D, et al. Whole-Brain Functional and Diffusion Tensor MRI in Human Participants with Metallic Orthodontic Braces. Radiology 2020;294:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bernstein MA, Huston J, Ward HA Imaging artifacts at 3.0T. J Magn Reson Imaging 2006;24:735–746. [DOI] [PubMed] [Google Scholar]
- 168.Grinnon ST, Miller K, Marler JR, et al. National Institute of Neurological Disorders and Stroke Common Data Element Project - approach and methods. Clin Trials 2012;9:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia : an international journal of headache; 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 170.Schwedt TJ, Digre K, Tepper SJ, et al. The American Registry for Migraine Research: Research Methods and Baseline Data for an Initial Patient Cohort. Headache 2020;60:337–347. [DOI] [PubMed] [Google Scholar]
- 171.Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. NeuroImage 1999;9:416–429. [DOI] [PubMed] [Google Scholar]
- 172.Siero JC, Hendrikse J, Hoogduin H, Petridou N, Luijten P, Donahue MJ. Cortical depth dependence of the BOLD initial dip and poststimulus undershoot in human visual cortex at 7 Tesla. Magn Reson Med 2015;73:2283–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]


