Abstract
Introduction
COVID-19 Associated Mucormycosis (CAM), an opportunistic fungal infection, surged during the second wave of SARS Cov-2 pandemic. Since immune responses play an important role in controlling this infection in immunocompetent hosts, it is required to understand immune perturbations associated with this condition for devising immunotherapeutic strategies for its control. We conducted a study to determine different immune parameters altered in CAM cases as compared to COVID-19 patients without CAM.
Methodology
Cytokine levels in serum samples of CAM cases (n = 29) and COVID-19 patients without CAM (n = 20) were determined using luminex assay. Flow cytometric assays were carried out in 20 CAM cases and 10 controls for determination of frequency of NK cells, DCs, phagocytes, T cells and their functionalities. The cytokine levels were analyzed for their association with each other as well as with T cell functionality. The immune parameters were also analyzed with respect to the known risk factors such as diabetes mellitus and steroid treatment.
Results
Significant reduction in frequencies of total and CD56 + CD16 + NK cells (cytotoxic subset) was noted in CAM cases. Degranulation responses indicative of cytotoxicity of T cell were significantly hampered in CAM cases as compared to the controls. Conversely, phagocytic functions showed no difference in CAM cases versus their controls except for migratory potential which was found to be enhanced in CAM cases. Levels of proinflammatory cytokines such as IFN-γ, IL-2, TNF-α, IL-17, IL-1β, IL-18 and MCP-1 were significantly elevated in cases as compared to the control with IFN-γ and IL-18 levels correlating negatively with CD4 T cell cytotoxicity. Steroid administration was associated with higher frequency of CD56 + CD16- NK cells (cytokine producing subset) and higher MCP-1 levels. Whereas diabetic participants had higher phagocytic and chemotactic potential and had higher levels of IL-6, IL-17 and MCP-1.
Conclusion
CAM cases differed from the controls in terms of higher titers of proinflammatory cytokines, reduced frequency of total and cytotoxic CD56 + CD16 + NK cell. They also had reduced T cell cytotoxicity correlating inversely with IFN-γ and IL-18 levels, possibly indicating induction of negative feedback mechanisms while diabetes mellitus or steroid administration did not affect the responses negatively.
Keywords: COVID-19 Associated Mucormycosis (CAM), Proinflammatory cytokines, T cell cytotoxicity, NK cells
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic infected more than 600 million people that resulted in a death toll of about more than 6.5 million people worldwide (WHO, 2022). In addition to acute respiratory disease, the patients suffered from various extrapulmonary complications and also post-COVID long-term consequences (Al-Kuraishy et al., 2022a, Al-Kuraishy et al., 2022e, Batiha et al., 2022b). The second wave of the pandemic faced a sudden emergence of mucormycosis co-infections worldwide, particularly in India. Mucormycosis is a highly invasive opportunistic fungal infection affecting patients with immunocompromised conditions. Mucormycosis is mainly caused by ubiquitous fungi such as Rhizopus, Absidia, Rhizomucor and Mucor belonging to the Order- Mucorales, Class- Zygomycetes (Eucker et al., 2001). Immunity plays an important role in protection against fungal diseases and hence cases of mucormycosis have been reported to be associated with immunosuppressive therapy especially in patients with haematological malignancies and organ transplantation (Lamoth et al., 2017). Patients with neutropenia and uncontrolled diabetes also demonstrate increased susceptibility to acquiring this infection (Khanna et al., 2021, Tansir et al., 2017). Rapid progression and high mortality in mucormycosis may be attributed to its invasiveness, lack of safe and effective treatment alternatives as well as to the failure of the immune system to clear the pathogens.
Protective immune mechanisms in mucormycosis still remain elusive. The innate immune system acts as the first line of defense mechanism against the growth and invasion by mucorales. Innate immune cells, especially macrophages, act as the main effector cells for eliminating fungal spores through phagocytosis (Nicolas et al., 2020). The fungal spores start germinating if they are not contained and eliminated by the innate immune system, leading to the establishment of infection (Hassan and Voigt, 2019). Immunosuppressive therapy especially with corticosteroids as well as uncontrolled diabetes are known to impair phagocytic functions, increasing susceptibility to fungal infections (Lecube et al., 2011, Philippe et al., 2003). Natural killer (NK) cells are known to exert direct antifungal activity through cytotoxicity and indirectly by releasing different cytokines (Schmidt et al., 2017). Conversely, mucorales have shown to exhibit immunosuppressive effects on NK cells (Schmidt et al., 2013). Adaptive immune response has been thought to play a less significant role in protection against mucormycosis (Ghuman and Voelz, 2017). Th1 and Th17 cells are considered to be mediating protection in mucormycosis as against Th2 cells, which might increase susceptibility to fungal infections (Borghi et al., 2014). Cytokines and chemokines secreted by different immune cells also play an important role in mounting protective immune response.
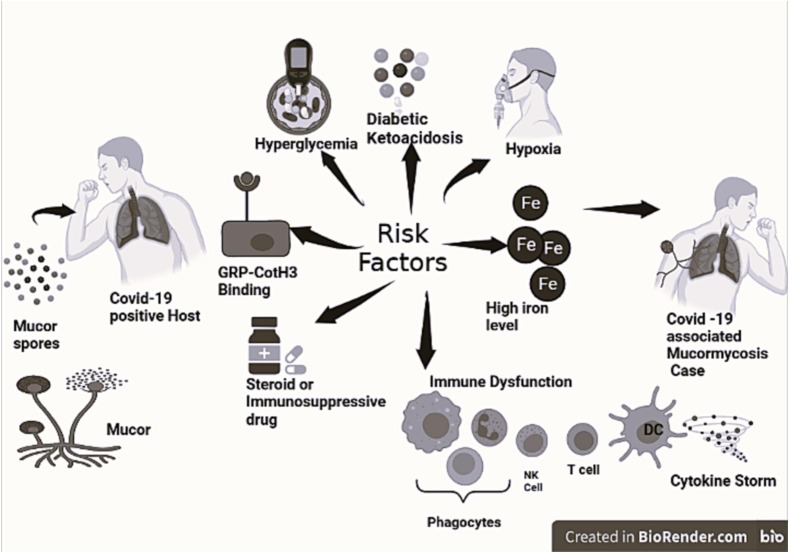
Fig. 1 depicts the possible host factors in COVID-19 patients making them susceptible to superinfection by mucorales. The exact immunopathogenic mechanisms underlying the association between COVID-19 and mucormycosis are not fully understood, but it has been suggested that the dysregulation of the immune response and the overproduction of proinflammatory cytokines may play a role. The COVID-19 disease has been shown to be associated with pronounced perturbations in innate and adaptive immune responses (Babalghith et al., 2022, Kuri-Cervantes et al., 2020). Additionally, preexisting conditions like diabetes mellitus, usage of corticosteroids and other immunotherapeutic agents such as tocilizumab contributed significantly to immune dysregulation in these patients, increasing the risk of acquiring mucormycosis (Bhogireddy et al., 2021). Downregulation of Angiotensin-converting enzyme 2 (ACE2) activity due to SARS CoV2 spike protein with resultant Angiotensin II upregulation also promotes proinflammatory and prothrombotic responses, hyperglycemia as well as Glucose Related Protein 78 (GRP78) and Spore Coat Protein Homologs (CoTH) interaction facilitating superinfection with mucorales (Abassi et al., 2020, Ali and Spinler, 2021, Gumashta and Gumashta, 2021). It is critical to carefully balance the immune responses while treating COVID-19 and other infectious diseases to avoid complications such as secondary infections and to maximize the effectiveness of immune responses against pathogens. It is, therefore, important to determine immune perturbations in patients with COVID-19 associated mucormycosis and compare them against immune profiles of COVID-19 cases who did not have mucormycosis. This might help understand immunopathogenesis of COVID-19 Associated Mucormycosis (CAM) and might also help devise immunotherapeutic or prophylactic strategies. The present investigation was conducted having such perspective in consideration.
Fig. 1.
Risk factors playing role in COVID-19 associated mucormycosis (CAM): Host factors in COVID-19 patients contributing to their increased susceptibility to development of mucormycosis are displayed in the figure.
2. Methods
2.1. Study population
Immunological studies were conducted at ICMR-NARI and ICMR-NIIH as part of a multi-site case control investigation in India (Anand et al., 2022). The study participants were enrolled from D.Y. Patil Medical College and Hospital, Pune and Grant Government Medical College and J J group of Hospitals, Mumbai. Approvals were obtained from the respective institutional ethics committees prior to initiation of the study. Written informed consent was obtained from all participants recruited in the present investigation. A case of CAM was considered as a COVID-19 patient suspected of mucormycosis based on clinical presentation and confirmed microbiologically for the same. Controls were randomly enrolled from COVID-19 patients admitted or discharged within 3–4 weeks of diagnosing a CAM case in the same hospital and without the diagnosis of mucormycosis. To prevent misclassification bias, nasal swabs were collected from the controls at enrolment and subjected to direct microscopy of the wet mount with KOH to rule out the presence of mucormycosis in them. The detailed methodology for selecting cases and controls has been described elsewhere (Anand et al., 2022). Cases and controls for immunological investigations were selected based on the criteria of age more than 18 years and consenting to an approximately 16 ml blood draw. Granulocytes and peripheral blood mononuclear cells (PBMCs) were separated from heparinized blood and serum samples were separated from plain vacutainer for storage in the LN2 cryostorage system and −80 °C till their use, respectively.
2.2. PBMC and granulocyte Isolation, freezing and revival
PBMC and Granulocytes were isolated by density gradient centrifugation using Histopaque 1077 (Sigma, H8889) and GranuloSepTM GSM 1119 (HiMedia), respectively (Slifkin and Cumbie, 1992). They were washed twice, and suspended in RPMI (Sigma) supplemented with 10% FBS and antibiotics (penicillin, 10,000 IU/ml; streptomycin, 10 mg/ml) (Sigma), hereafter referred to as complete medium. The PBMC were stored in a freezing medium consisting of fetal bovine serum with 10% dimethyl sulfoxide, while Granulocytes were stored in a freezing medium containing autologous plasma and 10% dimethyl sulfoxide. The samples were required to be collected on an urgent basis while the patients were still admitted without waiting for availability of all the required consumables. Hence cryopreserved cells were used for the assays after standardizing procedure for cryopreservation and revival of granulocytes to get more than 70% recovery and viability as per the previously published literature (Boonlayangoor et al., 1980, Richman, 1983). The frozen PBMCs and Granulocytes were stored in liquid nitrogen. PBMC and Granulocytes were revived to assess their phenotype and functionality. Granulocytes were quickly thawed at 37 °C, washed with RPMI supplemented with 20% autologous plasma and rested for 2 h. PBMCs were also quickly thawed at 37 °C, washed with RPMI supplemented with 20% fetal bovine serum, rested for 2 h, and then processed further.
2.3. Flow cytometry assays to assess phenotype and functionality of immune cells
NK cell phenotypes were determined using markers such as anti CD3-APC (Clone HIT3a/ SK7), anti CD16-FITC (Clone 3G8), anti CD56-PE (Clone NCAM 16.2) (Clone B27) (All antibodies were procured from BD Biosciences, San Jose, CA) as per a previously published literature (Kulkarni et al., 2014). NK cell functionality was assessed by expression of CD107a-APC Cy-7 and intracellular IFN-γ PECy-7 after stimulating them with PMA (1 ng /ml) and Ionomycin (2 μM) for 4 h.
Frequency and activation status of Dendritic cells were analyzed by using antibodies against the surface markers consisting of Lineage 1 Cocktail-FITC, anti HLA-DR-Percp-Cy5.5 (Clone L243), anti CD11c-APC (Clone S-HCL-3), anti CD123-PECy-7 (Clone 7G3), anti CD86-PECF594 (Clone 2331 FUN-1), and anti CD80-APC Cy-7 (Clone L307.4) (BD Biosciences, San Jose, CA) (Dhamanage et al., 2017, Donaghy et al., 2001).
Phagocytic activity and Respiratory Burst were determined using the Phagocytosis Assay kit and Respiratory Burst Assay Kit (Abcam, Cambridge, UK), respectively, as per the manufacturers’ instructions with the addition of a Live-Dead marker. Respiratory burst was assessed using a positive control included in the kit and incubating the cells at 37 °C for 20 min. The cell Migration/Chemotaxis Assay Kit (Abcam, Cambridge, UK) utilized a Boyden chamber, where the cells migrate through a semipermeable membrane under a migration inducer included in the kit.
For T cell assay, stimulation of PBMCs (1x106 cells per tube) was done with a 16-mer Peptide MGQTNDGAYRDPTDNN from CotH3 (Chibucos et al., 2016) along with co– stimulatory anti-human anti CD28 and CD49d antibodies (eBioscience, San Diego, CA, USA). PMA (1 ng /ml) and Ionomycin (2 μM) were used as a positive control. Anti CD107-APC Cy-7 (Clone H4A3) was added at the time of stimulation for assessing the expression of the degranulation marker. Golgi stop Brefeldin A (10ug/ml- final concentration) (eBioscience, San Diego, CA, USA) was added after 2 h of incubation of the in vitro cell culture. The cells incubated overnight were further stained with antibodies against surface markers for 30 min at room temperature in the dark. The cells were lysed, permeabilized, and stained with intracellular antibody anti IFN-γ-PECy-7 (Clone B27) for 30 min in the dark. Antibody cocktail for T cell surface markers included anti CD3-APC (Clone HIT3a/ SK7), anti CD4-FITC (Clone RPA-T4) and anti CD8-PE (Clone RPA T8) (BD Biosciences, San Jose, CA). After staining, the cells were acquired within 24 h to get 100,000 gated events on FACS Aria Fusion (BD Biosciences). The data was analyzed using FlowJo version 8.0.3.
2.4. Luminex based assays to assess levels of proinflammatory cytokines
Bio-Plex Pro Human Cytokine Screening Panel (Bio-Rad, USA) was used for evaluation of pro inflammatory cytokines (IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-18, IL17A, MCP-1, TNF-α) according to the manufacturer’s instruction. Ready to use reagents are available in the kit that consisted of standards and a 96-well plate. The protocol was designed such that 50 µL of samples/ standards were added to antibody-conjugated magnetic beads and incubated for one hour at room temperature. Biotinylated detection antibody was added to the magnetic beads after a washing step. This resulted in the formation of the sandwich complex. This was followed by the addition of streptavidin –phycoerythrin conjugate to the mixture. Cytokine concentrations were determined using a Bio-Plex 200 system (Bio-Rad, USA). Data acquisition was performed using Bio Plex Manager 6.0 software (Bio-Rad, USA). A standard curve was made using the standards provided by the manufacturer and was used to present the concentration of tested cytokines in terms of picogram per milliliter. Sensitivity of the assay to detect the required cytokines was below 1 pg/ml (Bio-Rad Laboratories, 2019, Bio-Rad Laboratories, 2016).
3. Statistical analysis
Data analysis was done using GraphPad prism version 9.0 software. Data is presented as numbers and percentages or median and interquartile range (IQR). Doses of steroids such as dexamethasone, methyl prednisolone and hydrocortisone were converted to equivalent doses of prednisolone and multiplied with the duration to obtain the cumulative dose administered. Significance between the two groups was determined by the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Responses to stimuli were compared by Wilcoxon signed rank test between unstimulated and treated cells. Correlation analysis was conducted using a non-parametric Spearman test. The differences with p values ≤ 0.05 were considered statistically significant.
4. Results
4.1. Study population
Profiles of the cases and controls enrolled for immunological study are presented in Table 1 , which also summarizes known risk factors for mucormycosis such as steroid treatment, diabetes mellitus, and neutropenia. Cases and controls analyzed for immunological parameters did not differ in their socio-demographic characteristics and presence of risk factors, except for the proportion affected by diabetes mellitus, which was found to be significantly higher in cases than in controls. Cases were also found to present with a more severe form of COVID-19 at the time of diagnosis than the controls as assessed by the World Health Organization (WHO) ordinal scale (Characterisation and Management of, 2020). None of the study participants had neutropenia as suggested by their absolute neutrophil counts being higher than 1500 cells/mm3.
Table 1.
Profiles of cases and controls.
| Cases (n = 29) | Controls (n = 20) | p-value | |
|---|---|---|---|
| Age, years: Median (IQR) | 49 (39–56) | 40.5 (31–58.5) | 0.3315 |
| Males: n (%) | 23 (79.31%) | 15 (75%) | 0.722 |
| Severity score by WHO Clinical Progression Scale: Median (IQR) | 5 (5–7) | 4 (4–6) | 0.0087 |
| Steroid received at the time of admission: n (%) | 7(24.14) | 9 (45.00) | 0.126 |
|
1 (11.11) | 1(14.29) | 0.849 |
|
6 (85.71) | 8 (88.89) | 0.849 |
| Type of steroid: n (%) | 0.066 | ||
|
2 (28.57) | 6 (66.67) | |
|
1 (14.29) | 2 (22.22) | |
|
0 | 1 (11.11) | |
|
4 (57.14) | 0 | |
| Duration of steroid, days: Median (IQR) | 2 (1–6) | 6 (4–7) | 0.1346 |
| Cumulative dose of prednisolone equivalent, mg: Median (IQR) | 37.5 (25–150) | 237.6 (158.4–350) | 0.1252 |
| Number with diabetes mellitus: n (%) | 23 (79.31%) | 2 (10.00%) | <0.001 |
| Absolute Neutrophil count, cells/mm3: Median (IQR) | 4860 (3780–7475) | 5575 (4403–10685) | 0.555 |
| Percent Neutrophil count, %: Median (IQR) | 71.50 (58–79)% | 72 (63–83)% | 0.500 |
4.2. Frequency and functionality of peripheral NK cells and their subsets in COVID-19 patients with and without mucormycosis
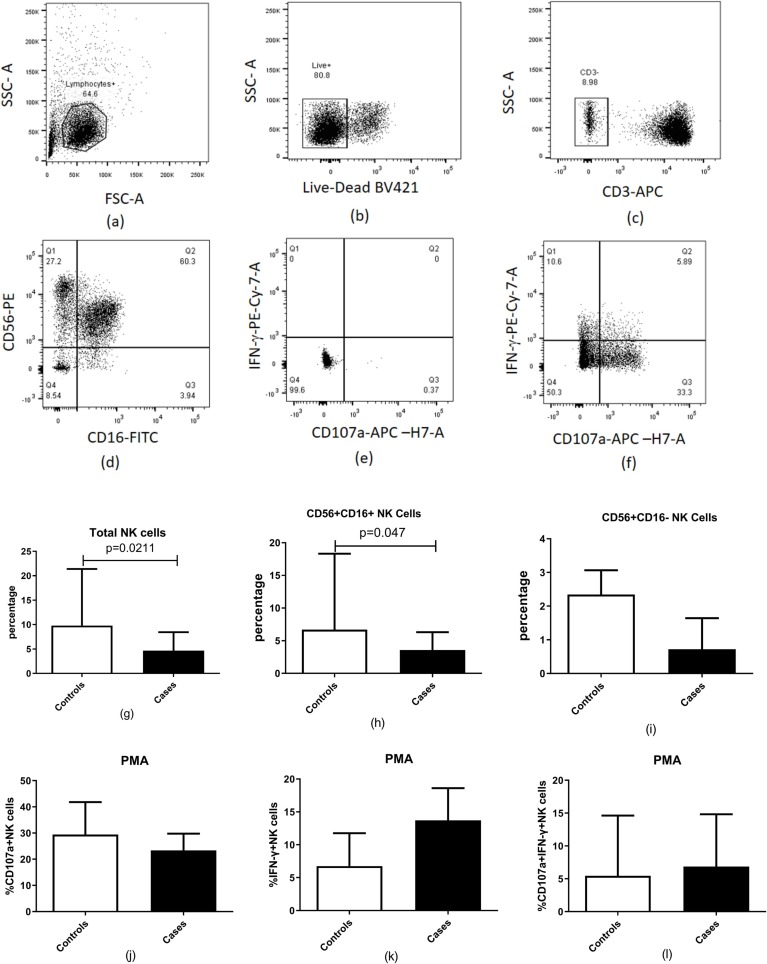
Peripheral NK cell percentages and their subsets were evaluated as per the previously published gating strategy (Kulkarni et al., 2014) in CAM cases and COVID-19 patients (controls). Dot plots showing the gating strategy is presented in Fig. 2 (a–f). Controls showed a significantly higher percentage of both total NK (p = 0.0211) and the CD56 + CD16 + NK cells (p = 0.048) as compared to the CAM cases. There was no significant difference in the functional parameters of NK cells between the cases and the controls as shown in Fig. 2 (g-l).
Fig. 2.
Estimation of NK cell percentages in patients with COVID-19 and mucormycosis (n = 20) compared to patients with COVID-19 (n = 10). (a-f) Representative flow cytometry plots showing gating strategy used for analysis of NK cells are presented. Lymphocytes identified by forward and side scatter were further gated for identification of the NK cells using the markers displayed in the figure. (g) Percentages of total natural killer (NK) cell (h) Percentages of CD56 + CD16 + NK cells (i) Percentage of CD56 + CD16- NK cells, (j-i) NK cell functionality after stimulation with PMA: (j) Percentage of CD 107a + NK cells (k) Percentage of IFN-γ + NK cells (l) Percentage of CD 107a and IFN-γ + NK cells are shown in the figure. Histograms and error bars represent median ± IQR of the percentages.
4.3. Frequency of myeloid dendritic cells (mDCs) and plasmacytoid dendritic cells (pDCs) in COVID-19 patients with and without mucormycosis
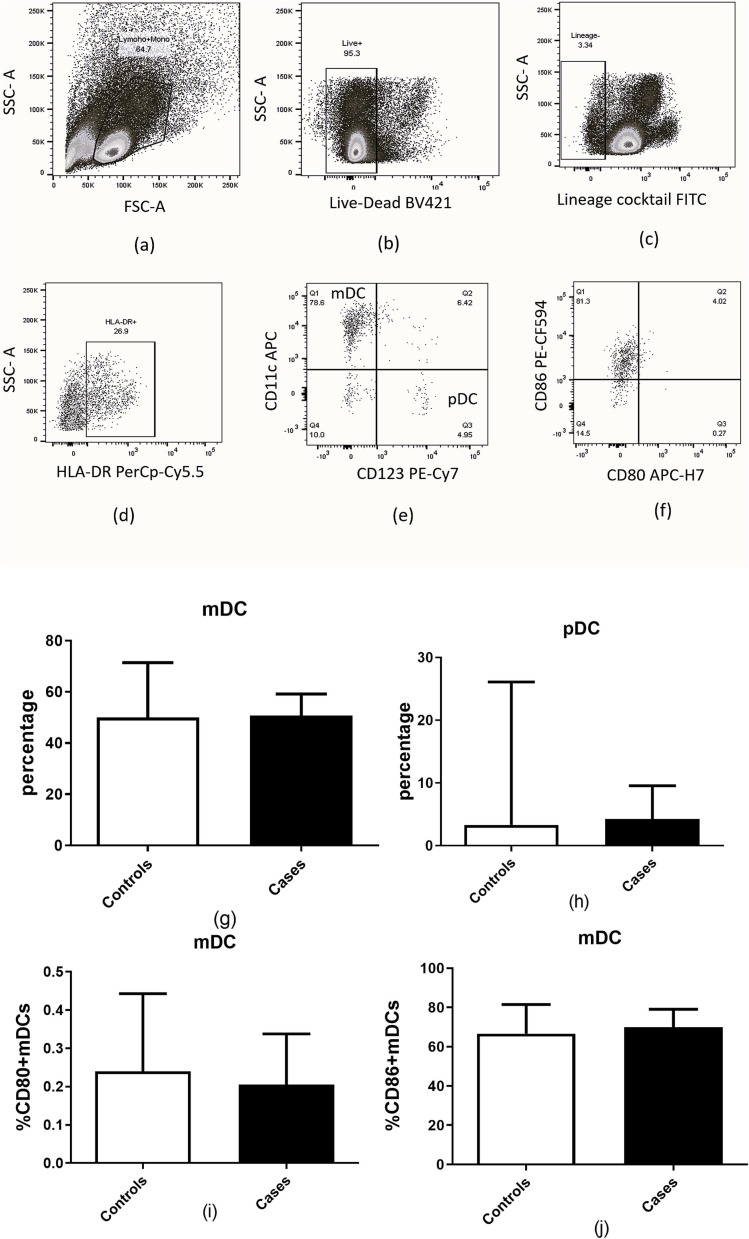
The frequency of myeloid dendritic cells (mDCs) and plasmacytoid dendritic cells (pDCs) assessed using markers selected as per the previously published literature (Dhamanage et al., 2017, Donaghy et al., 2001) did not differ significantly in the present study groups (Fig. 3 ). The functional analytes which included the expression of co-stimulatory molecules, CD80 and CD86 also did not show any significant difference between them. The gating strategy used for identification of the DC subset is presented in Fig. 3(a–f).
Fig. 3.
Estimation of mDCs and pDCs in patients with COVID-19 and mucormycosis (n = 20) compared to patients with COVID-19 (n = 10). (a-f) Representative flow cytometry plots showing gating strategy used for analysis of dendritic cells are presented. Markers used for identification of dendritic cells are displayed in the figure. (g) Percentage of mDCs.(h) Percentage of pDCs (i) Percentage of CD80 + mDCs (j) Percentage of CD86 + mDCs are shown in the figure. Histograms and error bars represent median ± IQR of the percentages.
4.4. Estimation of phagocytic cell functionality in COVID-19 patients with and without mucormycosis
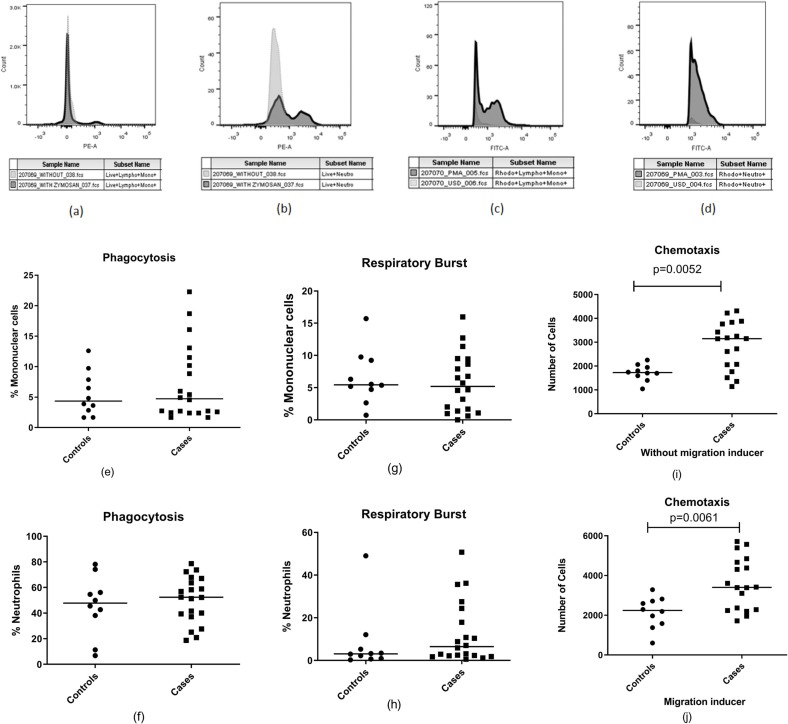
Functionality of mononuclear and polynuclear phagocytes was assessed after reviving the PBMCs and granulocytes. The average viability of granulocytes and mononuclear cells after revival was more than 80% as assessed by flow cytometry using a viability marker. Phagocytic functionalities among cases and controls were assessed by zymosan particle uptake, chemotactic activity and the respiratory burst on PMA stimulation. There was no difference in the phagocytic functionalities when compared between cases and control except for the chemotactic activity (Fig. 4 ). Monocyte in the cases were found to have more migratory potential than the control group in the presence and absence of a migration inducer (p = 0.0068 and 0.0078, respectively).
Fig. 4.
Estimation of phagocytic cell functionality in cases (n = 20) and control (n = 10) groups. (a,b) Histogram showing zymosan particle phagocytosis by monocytes (a) and neutrophils (b), (c,d) Histogram showing respiratory burst activity demonstrated by monocytes (c) and neutrophils (d) as indicated by rhodamine 123 fluoroscence, (e,f) Dot plots showing Phagocytic cell activity of mononuclear cells and neutrophils (g,h) Dot plots showing Respiratory burst activity of mononuclear cells and neutrophils. (i,j) Dot plots showing number of cells exhibiting chemotactic migration without inducer (P = 0.0068) and with inducer (P = 0.0078). Horizontal lines in the dot plots represent median values.
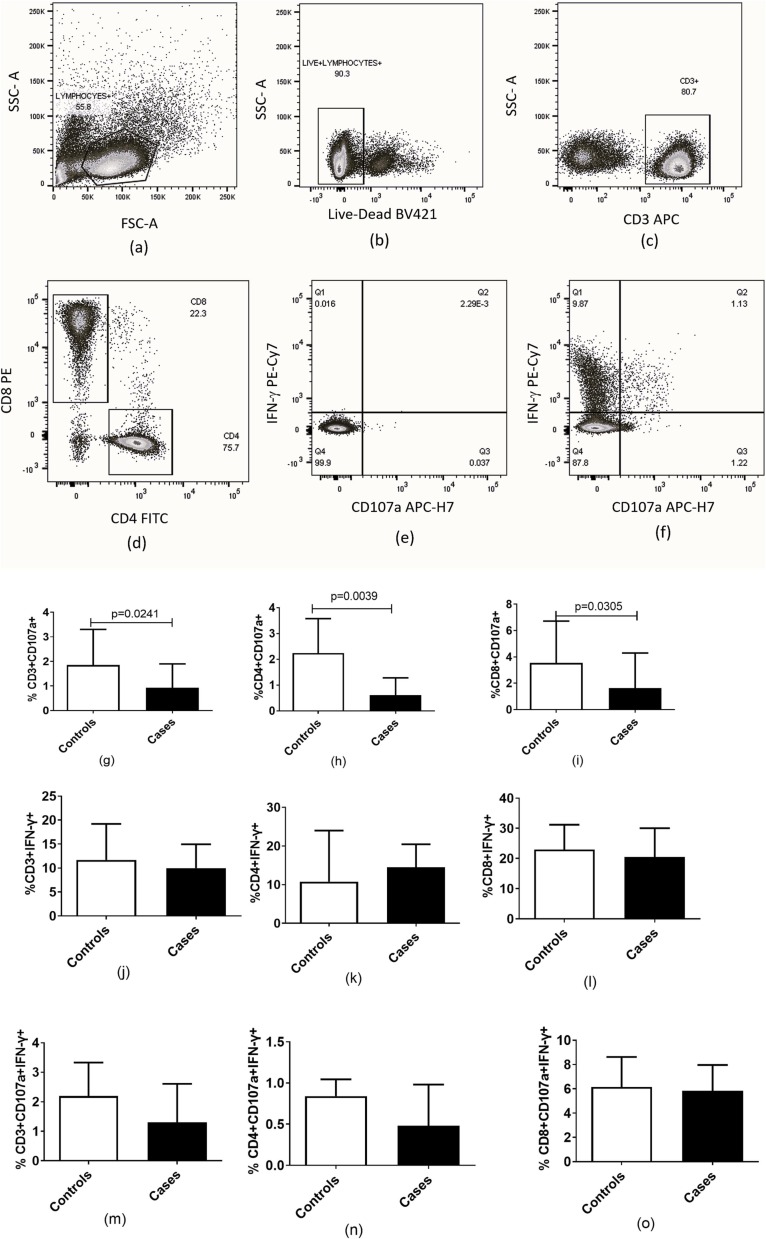
4.5. Estimation of T cell functionality in COVID-19 patients with and without mucormycosis
T cell functionality in the study was assessed by expression of CD107a, a degranulation marker and intracellular IFN-γ expression after stimulation with PMA/Ionomycin and the CotH3 peptide (Fig. 5 ). Controls had higher percentages of CD107a expressing CD3+ (p = 0.0261), CD4+ (p = 0.0055) and CD8+ (p = 0.0329) cells after stimulation with PMA and Ionomycin than in the cases. There were no significant differences in terms of IFN-γ expression as well as polyfunctionality as assessed by combined expression of both the markers among cases and controls. Not much response was detected after stimulation of cells of cases as well as controls by the CotH3 peptide and hence the results are not shown.
Fig. 5.
Estimation of acquired immune response in the patients with COVID-19 associated mucormycosis (n = 20) compared to patients with COVID −19 (n = 10). (a–f) Representative flow cytometry plots showing gating strategy used for analysis of CD4 + and CD8 + T cells are presented. Lymphocytes identified by forward and side scatter were further gated for identification of the T cells using the markers displayed in the figure. (g–i) Percentage of CD107a expressing CD3+, CD4 + and CD8 +. (j–l) Percentage of IFN-γ expressing CD3+, CD4 + and CD8 +. (m–o) Percentage of CD107a and IFN-γ in unison expressing CD3+, CD4 + and CD8 after stimulation with PMA and Ionomycin are shown in the figure. Histograms and error bars represent median ± IQR of the percentages.
4.6. Serum cytokine profile in COVID-19 patients with and without mucormycosis
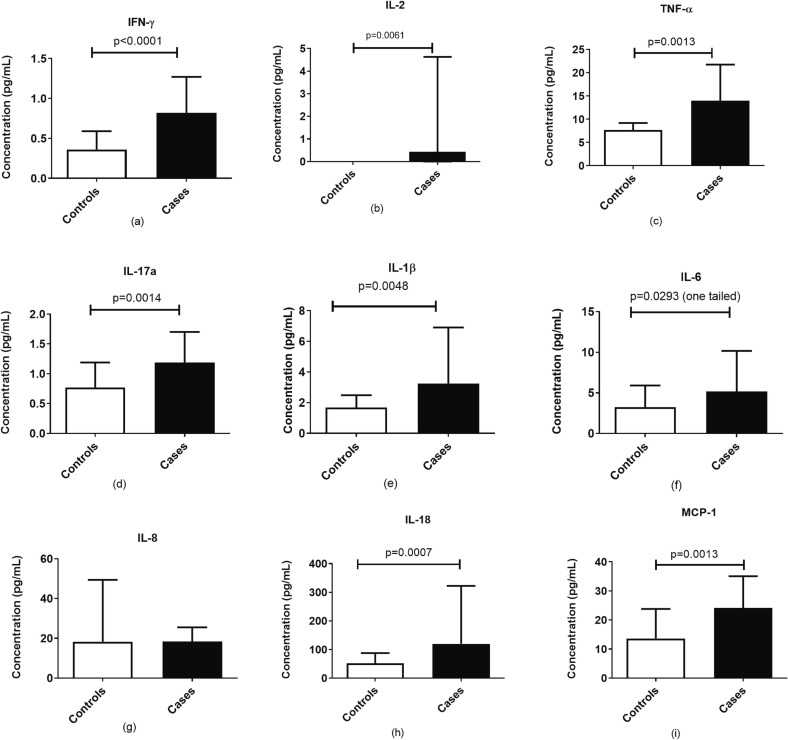
The pro-inflammatory cytokines among the case and control group were analyzed using the Bio-Plex Pro Human Cytokine Panel (Bio-Rad, USA). It was observed that IFN-γ (p < 0.0001), IL-2 (p = 0.0070), TNF-α (p = 0.0018), IL-17 (p = 0.0018), IL-1β (p = 0.0056), IL-18 (p = 0.009) and MCP-1 (p = 0.0017) were significantly elevated in cases in comparison to the controls as shown in Fig. 6 . Also, IL-6 was found to be elevated in the case group as compared to the control group, but the difference was not significant.
Fig. 6.
Concentration of cytokines in serum samples of the patients with COVID-19 and mucormycosis (n = 29) compared to patients with COVID-19 (n = 20). Histograms represent the levels expressed as median ± IQR of (a) IFN-γ, (b) IL-2, (c) TNF-α, (d) IL-17a, (e) IL-1b, (f) IL-6, (g) IL-8, (h) IL-18 and (i) MCP-1.
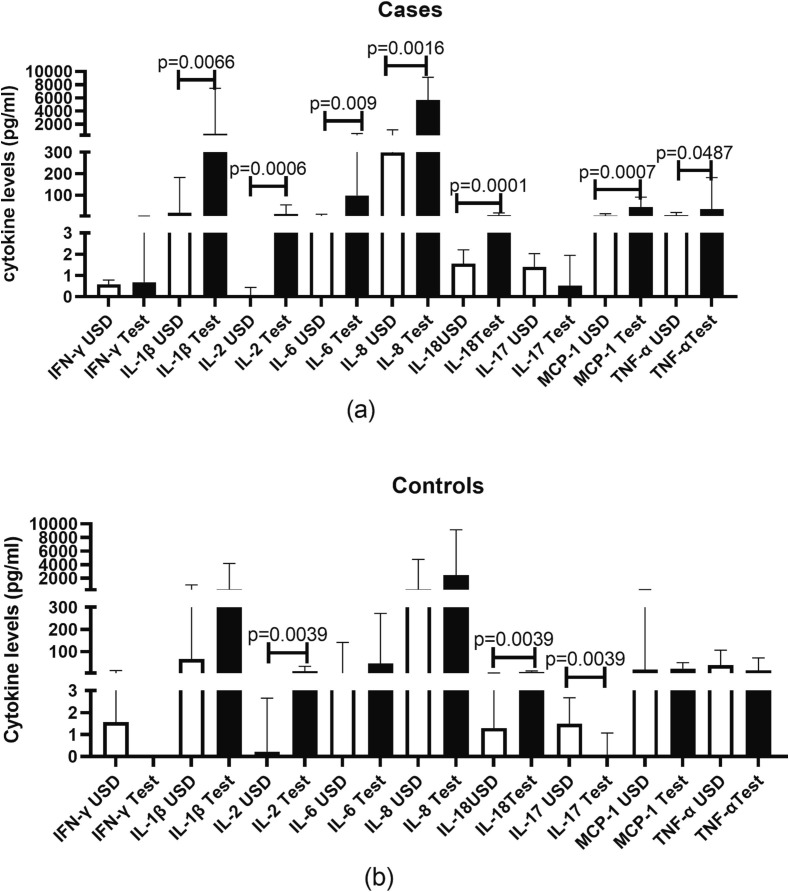
4.7. Cytokine responses to spore protein in COVID-19 patients with and without mucormycosis
Cytokine responses elicited by spore protein in PBMCs from cases and controls are shown in Fig. 7 . Spore protein elicited proinflammatory cytokine responses in cases as evident by significant rise in the levels of IL-1b (p = 0.0066), IL-2 (p = 0.0006), IL-6 (p = 0.009), IL-8 (p = 0.0016), IL-18 (p = 0.0001), MCP-1 (p = 0.0007) and TNF-a (p = 0.0483) in supernatants of the simulated cells versus their unstimulated counterparts. As compared in cases, the proinflammatory cytokine responses were lower in controls as there was significantly higher secretion of only IL-2 (p = 0.0039) and IL-18 (p = 0.0039) after spore protein stimulation than the unstimulated cells. There was significant suppression of IL-17 (p = 0.0039) secretory response after stimulation with the spore protein in controls.
Fig. 7.
Concentration of cytokines in supernatants of PBMCs stimulated with CotH3 peptide. Histograms represent the levels expressed as median ± IQR. Levels of the cytokines mentioned on X-axis in unstimulated (USD) versus peptide stimulated (Test) cells of cases (a) and controls (b) are shown in the figure.
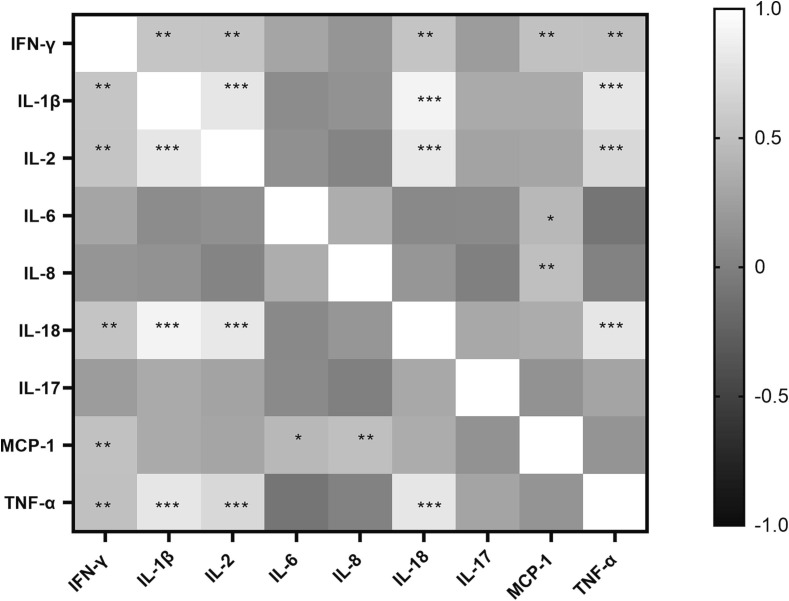
4.8. Correlation of serum cytokines with each other:
Serum cytokines levels were further analysed to determine if they correlate with each other. The Heatmap presented in Fig. 8 illustrates the coefficient of correlation between different cytokine levels and their level of significance. IFN-γ was found to be positively correlated with IL-1β (ρ = 0.541, p = 0.002), IL-2 (p = 0.534, p = 0.003), IL-18 (r = 0.530, p = 0.003), MCP-1 (r = 0.515, p = 0.004) and TNF-α (r = 0.505,p = 0.005). IL-1β additionally correlated with IL-2 (r = 0.804, p < 0.0001), IL-18 (r = 0.905, p < 0.0001) and TNF-α (r = 0.807, p < 0.0001). IL-2 further correlated with IL-18 (r = 0.812, p < 0.0001), TNF-α (r = 0.702, p < 0.0001) and IL-18 with TNF-α (r = 0.808, p < 0.0001). Whereas IL-6 (r = 0.438, p = 0.018) and IL-8 (r = 0.491, p = 0.007) correlated only with MCP-1.
Fig. 8.
Heatmap plotted to show correlation coefficients (r values) as per Spearman correlation analysis of serum levels of different cytokines with each other. Scale for r values is given in the figure. * indicate significant p values for correlation between the cytokines. * - p < 0.05, **- p < 0.01, ***- p < 0.001.
4.9. Correlation of serum cytokines with T cell cytotoxicity
Serum cytokine levels were also correlated with CD4 + and CD8 + T cell cytotoxicity as assessed by CD107a expression and results are presented in Table 2. The CD4 + T cell expressing CD107a showed significant negative correlation with IFN-γ (r = -0.0420, p = 0.0233), IL-8 (r = -0.374, p = 0.0459) and IL-18 (r = -0.447, p = 0.0151). The cytokines failed to show any correlation with CD8 + T cells expressing CD107a.
Table 2.
Correlation between serum levels of proinflammatory cytokines and CD4/CD8 T cells expressing CD107a.
| CD4 + CD107a+ |
CD8 + CD107a+ |
|||
|---|---|---|---|---|
| R value | P value | R value | P value | |
| IFN-γ | −0.4201 | 0.0233 | −0.2243 | 0.2421 |
| IL-1β | −0.3297 | 0.0807 | 0.02195 | 0.91 |
| IL-2 | −0.2378 | 0.2141 | 0.06055 | 0.755 |
| IL-6 | −0.3342 | 0.0764 | −0.1777 | 0.3565 |
| IL-8 | −0.3736 | 0.0459 | −0.0631 | 0.745 |
| IL-18 | −0.4469 | 0.0151 | −0.06947 | 0.7203 |
| IL-17 | −0.128 | 0.5082 | 0.09926 | 0.6084 |
| MCP-1 | −0.2931 | 0.1228 | −0.2385 | 0.2127 |
| TNF-α | −0.204 | 0.2884 | 0.02869 | 0.8825 |
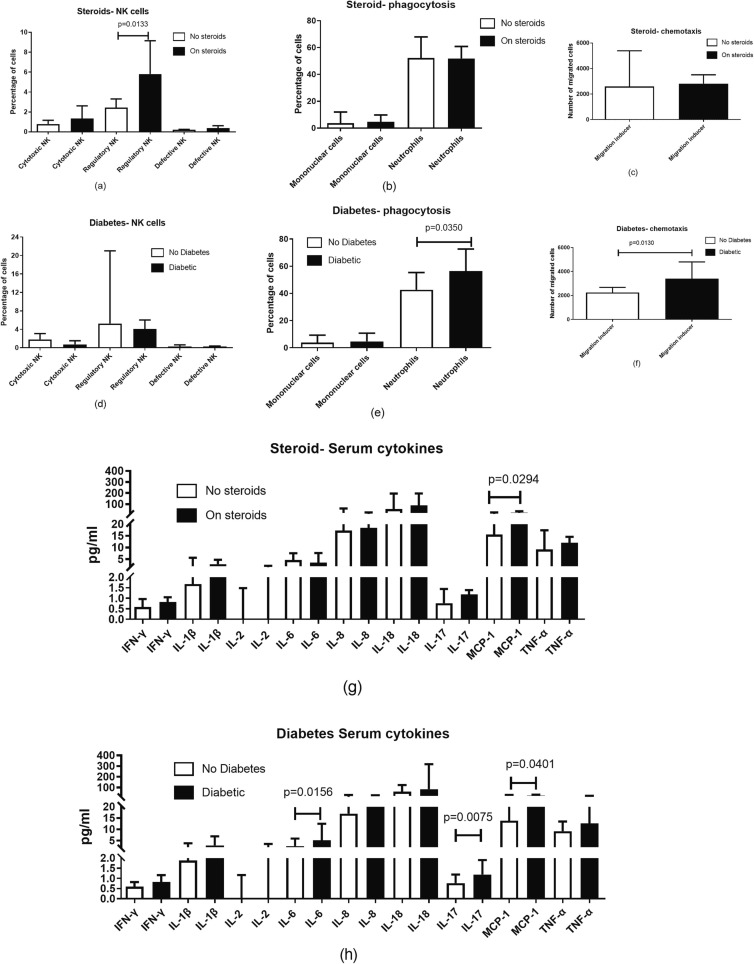
4.10. Association of risk factors with immune perturbations
Immune parameters were compared in patients with and without steroid treatment for COVID-19 as well as among those with and without diabetes to determine if they differed as per risk factors (Fig. 9 ). Steroid treatment influenced frequency of CD56 + CD16- NK cells (p = 0.0157) and MCP-1 (p = 0.0294) levels, both of which were found to be higher in patients on steroids, whereas presence of diabetes was associated with higher neutrophilic phagocytosis (p = 0.0374) and chemotactic migration of phagocytes (p = 0.0130), levels of IL-6 (p = 0.0156), IL-17 (p = 0.0075). MCP-1 (p = 0.0401) was also found to be higher in patients with diabetes compared to those without it. The rest of the parameters did not show any significant difference in the presence or absence of these two risk factors.
Fig. 9.
Comparison of Immune functionalities in participants with and without diabetes mellitus and steroid treatment irrespective of presence or absence of mucormycosis. Histograms represent the levels expressed as median ± IQR.
5. Discussion
Several studies have been conducted to determine risk factors associated with CAM so as to help devise strategies to control it. Mucormycosis being an opportunistic infection, mainly affects immunocompromised hosts, indicating the importance of immune responses in its control. Hence, it is important to focus on different immune responses and deficiencies associated with this condition in addition to studies identifying risk factors associated with it. We enrolled CAM cases and COVID-19 controls to study the immune responses associated with the condition. Our cases differed from the controls in terms of having a higher COVID-19 severity score and a higher proportion suffering from diabetes as also reported earlier (John et al., 2021). Since innate immune mechanisms serve as a first line of defense, they are considered to be of the utmost importance in controlling it. We focused on different innate immune cells such as neutrophils, monocytes, DCs and NK cells to determine if their frequencies and / or functionalities differ between the cases and the controls. NK cells, especially CD56 + CD16 + subpopulation of NK cells which is shown to predominantly mediate cytotoxic functionalities (Cooper et al., 2001), were found to be higher in controls as compared to the cases indicating compromised NK cell responses associated with mucormycosis. Although CD107a expression was marginally higher in controls and IFN-γ secretion was higher in cases following stimulation with PMA/Ionomycin, they failed to show any statistical significance. Noticeably, fungal infections have been shown to suppress NK cell activity (Schmidt et al., 2017, Schmidt et al., 2013) and hence are likely to be associated with reduced NK cell frequency as well as functionality as shown in previous reports (Akiba et al., 2001, Chen et al., 2021).
As against NK cells, myeloid and plasmacytoid dendritic cells failed to show significant difference in frequency as well as expression of CD80 and CD86, costimulatory receptors involved in antigen presentation. Phagocytic functions mediated by neutrophils as well as macrophages have been shown to be the mainstay of protection from mucormycosis (Ibrahim et al., 2012). However, similar to DCs, phagocytic functions did not seem to be adversely affected in CAM cases as against their COVID-19 controls. Functionality of phagocytes was assessed by 3 different assays, such as phagocytic ability, respiratory burst and chemotactic potential. Neutrophils as well as monocytes did not differ between cases and controls in their ability to phagocytize zymosan particles as well as in mounting respiratory burst response to PMA stimulation. However, monocytes in the cases were found to be more responsive to chemotactic stimuli, possibly indicating their increased activation in comparison to controls. Absolute and percentage neutrophil counts were also found to be similar in cases and controls in our study although neutropenia has been shown to act as a risk factor for developing mucormycosis (Spellberg et al., 2005).
Further, innate immune cells after activation, secrete a variety of pro-inflammatory cytokines. COVID-19 is also characterized by hyper-inflammation and raised levels of circulating pro-inflammatory cytokines (Webb et al., 2020). When we assessed the pro-inflammatory cytokine levels to determine if they differed between case and control groups, we found that the levels of all the cytokines except IL-8 were significantly raised in cases as compared to controls. Mucoralean fungi have been shown to induce secretion of pro-inflammatory cytokines from PBMCs (Wurster et al., 2017), which might have caused heightened cytokine responses in our CAM cases. We also assessed cytokine secretory responses of PBMCs from our cases and controls after stimulation with CotH3 spore peptide. Proinflammatory cytokines were mainly released by PBMCs from cases after stimulation with spore peptide. The plasma levels of the cytokines were also checked to determine possible associations between them. IFN-γ, IL-2, TNF-α, IL-18, and IL-1β showed significant positive correlations with each other. While IL-6 and IL-8 mainly correlated with MCP-1. IL-1β and IL-18 belong to the IL-1 cytokine family and are secreted by activated macrophages (Van Den Eeckhout et al., 2020). They have been shown to promote T helper 1 (Th1) cell differentiation (Van Den Eeckhout et al., 2020), which might explain their strong correlations with Th1 cytokines like IFN-γ, IL-2, and TNF-α, indicating their association with adaptive immune responses. Whereas IL-8 and MCP-1 are mainly the components of innate immunity and act by serving as chemoattractants for neutrophils (Henkels et al., 2011) and macrophages (Deshmane et al., 2009), respectively. Both these cytokines were shown to be induced by the IL-6 trans-signaling pathway (Kang et al., 2020).
Although adaptive responses are considered to be of secondary importance in controlling fungal infections, possibly due to their delayed actions, they form a mainstay of specific protective immunity elicited against any pathogen. We assessed the functionality of CD4 and CD8 + T cells against spore peptide as well as PMA/Ionomycin. We did not find much response against spore protein in terms of CD107a and IFN-γ expression when assessed by flow cytometry. However, degranulation response as assessed by CD107a expression after stimulation with PMA/Ionomycin was significantly higher in controls as compared to cases indicating compromised cytotoxic potential of T cells in CAM cases. Severe COVID-19 infections were shown to be associated with impaired cytotoxic responses in PBMCs (Vigon et al., 2021). Further, the impaired cytotoxicity was shown to be promoted by proinflammatory cytokines such as IL-6 (Hou et al., 2014, Mazzoni et al., 2020). Hence, strategies for ameliorating proinflammatory responses would be beneficial (Al-Kuraishy et al., 2022b, Al-Kuraishy et al., 2022c, Al-Kuraishy et al., 2022d, Batiha et al., 2022a, Batiha et al., 2021). We also assessed if this response correlates with IL-6 or other proinflammatory cytokine levels. Since the difference in IL-6 levels between cases and controls was less striking, it might not have affected cytotoxicity in our cases, as evident by the absence of correlation between IL and 6 levels and impaired cytotoxicity. However, CD4+, but not CD8+, T cell degranulation response was found to be negatively correlated with IFN-γ and IL-18 levels with p values<0.05. IFN-γ has been shown to regulate inflammatory responses by initiating negative feedback mechanisms to control Th1 immune responses (Feuerer et al., 2006). Similarly, IL-18 has also shown to suppress cell mediated cytotoxicity by inducing TIM-3 expression (Ndhlovu et al., 2012, Schofield et al., 2017).
We also determined if these immune perturbations were associated with the presence of risk factors viz. steroid treatment or diabetes, irrespective of whether they had mucormycosis or not. Frequency of CD56 + CD16- NK cells, which is shown to be the predominant cytokine producing NK cell subset (Cooper et al., 2001), was found to be higher in patients on steroids. But steroid treatment or the presence of diabetes did not affect the frequency of cytotoxic CD56 + CD16 + NK cells in our patients. Steroids have been reported to suppress cytotoxic NK cell responses in multiple studies, while CD56 + cells were shown to be unaffected during steroid treatment in one of the studies (Gomaa et al., 2014). There is a report showing expansion of CD56 + NK cells with their enhanced potential for cytokine production when the cells were synergistically treated by hydrocortisone and IL-15 (Perez et al., 2005). IL-15 levels have been reported to be elevated in COVID-19 patients correlating with longer duration of hospitalization (Singh et al., 2022). Increased CD56 + CD16- NK cells observed in our study might have been contributed by the synergistic effect between steroids and IL-15.
Levels of many proinflammatory cytokines, including IL-6, IL-17 and MCP-1, which were found to be elevated in our study have been reported to be increased in type 2 diabetes (Randeria et al., 2019). Phagocytic functions have also been shown to be affected by diabetes, corticosteroid therapy as well as by COVID-19 (Ehrchen et al., 2019, Koenis et al., 2021, Lecube et al., 2011, Nomani et al., 2021). Surprisingly, our diabetic patients had higher phagocytic activity displayed by neutrophils as compared to non-diabetic patients, in contrast to previous reports showing lowered phagocytic activity (Lecube et al., 2011). The phagocytic activity in our experiments was assessed using zymosan particles, which are known to interact with TLR2 (Sato et al., 2003) as against bacterial LPS which interacts with TLR-4. Expression of TLR-2 has been shown to be upregulated in type 2 diabetes in multiple studies (Sepehri et al., 2016) including that on phagocytic cells (Morris et al., 2012). One of the limitations of our study was its small sample size. This investigation was conducted during the time between the second and third waves of the COVID-19 outbreak in India, and obtaining consent to collect adequate amounts of blood specimens from in-patients for immunological investigations at times seemed difficult. We therefore undertook this pilot exploratory investigation as part of a larger case-control study (Anand et al., 2022).
6. Conclusion
Immune responses in CAM cases differed from COVID-19 controls in terms of higher titers of proinflammatory cytokines possibly induced in response to fungal antigens as spore protein was found to elicit proinflammatory cytokine responses. The CAM cases also had reduced frequency of total and cytotoxic CD56 + CD16 + NK cells, possibly indicating suppressive responses induced by fungi. Reduced T cell cytotoxicity in cases correlated inversely with IFN-γ and IL-18 levels, possibly indicating negative feedback mechanisms induced for regulating immune responses. Risk factors such as diabetes or steroid administration did not seem to affect these responses negatively in our study, possibly indicating the role of other mechanisms in predisposing these patients to mucormycosis.
Funding
This work was supported by Indian Council of Medical Research.
CRediT authorship contribution statement
Ashwini Shete: Conceptualization, Formal analysis, Supervision, Validation, Writing – original draft. Supriya Deshpande: Formal analysis, Methodology, Writing – review & editing. Jyoti Sawnt: Formal analysis, Methodology, Writing – review & editing. Nidhi Warthe: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Madhuri Thakar: Conceptualization, Writing – review & editing. Manisha Madkaikar: Conceptualization, Writing – review & editing. Vandana Pradhan: Formal analysis, Methodology, Writing – review & editing. Prajwal Rao: Formal analysis, Investigation, Supervision, Writing – review & editing. Shalesh Rohatgi: Formal analysis, Methodology, Project administration, Writing – review & editing. Aparna Mukherjee: Conceptualization, Writing – review & editing. Tanu Anand: Conceptualization, Writing – review & editing. Aanchal Satija: Conceptualization, Writing – review & editing. Poonam Sharma Velamuri: Conceptualization, Writing – review & editing. Madhuchhanda Das: Conceptualization, Writing – review & editing. Nidhi Deasi: Methodology, Writing – review & editing. Alok Kumar Tembhurne: Methodology, Writing – review & editing. Reetika Yadav: Methodology, Writing – review & editing. Swapnal Pawaskar: Methodology, Writing – review & editing. Chhaya Rajguru: Writing – review & editing. Lalitkumar R. Sankhe: Writing – review & editing. Shrinivas S. Chavan: Writing – review & editing. Samiran Panda: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank laboratory staff from ICMR-NARI, Pune and ICMR-NIIH, Mumbai for their help in sample processing and immunological assays. We also thank staff from Dr. D. Y. Patil Medical College, Hospital & Research Centre, Pune and Grant Government Medical College & J J group of Hospitals, Mumbai, for their help in enrolment of study participants. We especially thank the study participant for their time and participation in this study. We thank Indian Council of Medical Research for funding the study (Mucormycosis/13/2021-ECD-II).
Data availability
Data will be made available on request.
References
- Abassi Z., Higazi A.A.R., Kinaneh S., Armaly Z., Skorecki K., Heyman S.N. ACE2, COVID-19 infection, inflammation, and coagulopathy: missing pieces in the puzzle. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.574753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba H., Motoki Y., Satoh M., Iwatsuki K., Kaneko F. Recalcitrant trichophytic granuloma associated with NK-cell deficiency in a SLE patient treated with corticosteroid. Eur. J. Dermatol. 2001;11:58–62. [PubMed] [Google Scholar]
- Ali M.A.M., Spinler S.A. COVID-19 and thrombosis: from bench to bedside. Trends Cardiovasc. Med. 2021;31:143–160. doi: 10.1016/j.tcm.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy H.M., Al-Gareeb A.I., Al-Niemi M.S., Alexiou A., Batiha G.E. Calprotectin: the link between acute lung injury and gastrointestinal injury in Covid-19: Ban or Boon. Curr. Protein Pept. Sci. 2022;23:310–320. doi: 10.2174/1389203723666220610124303. [DOI] [PubMed] [Google Scholar]
- Al-Kuraishy H.M., Al-Gareeb A.I., Alkazmi L., Habotta O.A., Batiha G.E. High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons. Inflammopharmacology. 2022;30:811–820. doi: 10.1007/s10787-022-00988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy H.M., Al-Gareeb A.I., El-Saber Batiha G. The possible role of ursolic acid in Covid-19: a real game changer. Clin. Nutr. ESPEN. 2022;47:414–417. doi: 10.1016/j.clnesp.2021.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy H.M., Al-Gareeb A.I., Elekhnawy E., Batiha G.E. Nitazoxanide and COVID-19: a review. Mol. Biol. Rep. 2022;49:11169–11176. doi: 10.1007/s11033-022-07822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy H.M., Batiha G.E., Faidah H., Al-Gareeb A.I., Saad H.M., Simal-Gandara J. Pirfenidone and post-Covid-19 pulmonary fibrosis: invoked again for realistic goals. Inflammopharmacology. 2022;30:2017–2026. doi: 10.1007/s10787-022-01027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand T., Mukherjee A., Satija A., Velamuri P.S., Singh K.J., Das M., Josten K., Yadav P.D., Sahay R.R., Keche A.Y., et al. A case control investigation of COVID-19 associated mucormycosis in India. BMC Infect. Dis. 2022;22:856. doi: 10.1186/s12879-022-07844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babalghith, A.O., Al-Kuraishy, H.M., Al-Gareeb, A.I., De Waard, M., Sabatier, J.M., Saad, H.M., and Batiha, G.E., 2022. The Potential Role of Growth Differentiation Factor 15 in COVID-19: A Corollary Subjective Effect or Not? Diagnostics (Basel) 12. [DOI] [PMC free article] [PubMed]
- Batiha G.E., Al-Gareeb D.A.I., Qusti S., Alshammari E.M., Rotimi D., Adeyemi O.S., Al-Kuraishy H.M. Common NLRP3 inflammasome inhibitors and Covid-19: Divide and Conquer. Sci. Afr. 2021;e01084 doi: 10.1016/j.sciaf.2021.e01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiha G.E., Al-Gareeb A.I., Elekhnawy E., Al-Kuraishy H.M. Potential role of lipoxin in the management of COVID-19: a narrative review. Inflammopharmacology. 2022;30:1993–2001. doi: 10.1007/s10787-022-01070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiha G.E., Al-Kuraishy H.M., Al-Gareeb A.I., Alruwaili M., AlRuwaili R., Albogami S.M., Alorabi M., Saad H.M., Simal-Gandara J. Targeting of neuroinflammation by glibenclamide in Covid-19: old weapon from arsenal. Inflammopharmacology. 2022:1–7. doi: 10.1007/s10787-022-01087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogireddy R., Krishnamurthy V., Jabaris S.S., Pullaiah C.P., Manohar S. Is Mucormycosis an inevitable complication of Covid-19 in India? Braz. J. Infect. Dis. 2021;25 doi: 10.1016/j.bjid.2021.101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bio-Rad Laboratories, I. (2016). https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6499.pdf. https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6499.pdf.
- Bio-Rad Laboratories, I. (2019). Bio-Plex Pro Human Th17 Cytokine Assays https://www.bio-rad.com/sites/default/files/webroot/web/pdf/lsr/literature/Bulletin_6249.pdf.
- Boonlayangoor P., Telischi M., Boonlayangoor S., Sinclair T.F., Millhouse E.W. Cryopreservation of human granulocytes: study of granulocyte function and ultrastructure. Blood. 1980;56:237–245. [PubMed] [Google Scholar]
- Borghi M., Renga G., Puccetti M., Oikonomou V., Palmieri M., Galosi C., Bartoli A., Romani L. Antifungal Th Immunity: Growing up in Family. Front. Immunol. 2014;5:506. doi: 10.3389/fimmu.2014.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Characterisation, W.H.O.W.G.o.t.C., and Management of, C.-i., 2020. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 20, e192-e197. [DOI] [PMC free article] [PubMed]
- Chen G., Qin C.J., Wu M.Z., Liu F.F., Liu S.S., Liu L. The frequency of natural killer cell subsets in patients with acquired immune deficiency syndrome with deep fungal infections. Infect Drug Resist. 2021;14:467–473. doi: 10.2147/IDR.S269905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibucos M.C., Soliman S., Gebremariam T., Lee H., Daugherty S., Orvis J., Shetty A.C., Crabtree J., Hazen T.H., Etienne K.A., et al. An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat. Commun. 2016;7:12218. doi: 10.1038/ncomms12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M.A., Fehniger T.A., Caligiuri M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamanage A., Thakar M., Paranjape R. Human immunodeficiency virus-1 impairs IFN-alpha production induced by TLR-7 agonist in plasmacytoid dendritic cells. Viral Immunol. 2017;30:28–34. doi: 10.1089/vim.2016.0084. [DOI] [PubMed] [Google Scholar]
- Donaghy H., Pozniak A., Gazzard B., Qazi N., Gilmour J., Gotch F., Patterson S. Loss of blood CD11c(+) myeloid and CD11c(-) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- Ehrchen J.M., Roth J., Barczyk-Kahlert K. More than suppression: glucocorticoid action on monocytes and macrophages. Front. Immunol. 2019;10:2028. doi: 10.3389/fimmu.2019.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eucker J., Sezer O., Graf B., Possinger K. Mucormycoses. Mycoses. 2001;44:253–260. [PubMed] [Google Scholar]
- Feuerer M., Eulenburg K., Loddenkemper C., Hamann A., Huehn J. Self-limitation of Th1-mediated inflammation by IFN-gamma. J. Immunol. 2006;176:2857–2863. doi: 10.4049/jimmunol.176.5.2857. [DOI] [PubMed] [Google Scholar]
- Ghuman H., Voelz K. Innate and adaptive immunity to mucorales. J. Fungi Basel. 2017;3 doi: 10.3390/jof3030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa M.F., Elkholy A.G., El-Said M.M., Abdel-Salam N.E. Combined oral prednisolone and heparin versus heparin: the effect on peripheral NK cells and clinical outcome in patients with unexplained recurrent miscarriage. A double-blind placebo randomized controlled trial. Arch. Gynecol. Obstet. 2014;290:757–762. doi: 10.1007/s00404-014-3262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumashta J., Gumashta R. COVID19 associated mucormycosis: Is GRP78 a possible link? J. Infect. Public Health. 2021;14:1351–1357. doi: 10.1016/j.jiph.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M.I.A., Voigt K. Pathogenicity patterns of mucormycosis: epidemiology, interaction with immune cells and virulence factors. Med. Mycol. 2019;57:S245–S256. doi: 10.1093/mmy/myz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkels K.M., Frondorf K., Gonzalez-Mejia M.E., Doseff A.L., Gomez-Cambronero J. IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3) FEBS Lett. 2011;585:159–166. doi: 10.1016/j.febslet.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W., Jin Y.H., Kang H.S., Kim B.S. Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. J. Virol. 2014;88:8479–8489. doi: 10.1128/JVI.00724-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, T.M., Jacob, C.N., and Kontoyiannis, D.P., 2021. When Uncontrolled Diabetes Mellitus and Severe COVID-19 Converge: The Perfect Storm for Mucormycosis. J Fungi (Basel) 7. [DOI] [PMC free article] [PubMed]
- Kang S., Tanaka T., Inoue H., Ono C., Hashimoto S., Kioi Y., Matsumoto H., Matsuura H., Matsubara T., Shimizu K., et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. PNAS. 2020;117:22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna M., Challa S., Kabeil A.S., Inyang B., Gondal F.J., Abah G.A., Minnal Dhandapani M., Manne M., Mohammed L. Risk of mucormycosis in diabetes mellitus: a systematic review. Cureus. 2021;13:e18827. doi: 10.7759/cureus.18827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenis D.S., Beegun I., Jouvene C.C., Aguirre G.A., Souza P.R., Gonzalez-Nunez M., Ly L., Pistorius K., Kocher H.M., Ricketts W., et al. Disrupted resolution mechanisms favor altered phagocyte responses in COVID-19. Circ. Res. 2021;129:e54–e71. doi: 10.1161/CIRCRESAHA.121.319142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A.G., Paranjape R.S., Thakar M.R. Higher expression of activating receptors on cytotoxic NK cells is associated with early control on HIV-1C multiplication. Front. Immunol. 2014;5:222. doi: 10.3389/fimmu.2014.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri-Cervantes L., Pampena M.B., Meng W., Rosenfeld A.M., Ittner C.A.G., Weisman A.R., Agyekum R.S., Mathew D., Baxter A.E., Vella L.A., et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoth F., Chung S.J., Damonti L., Alexander B.D. Changing epidemiology of invasive mold infections in patients receiving azole prophylaxis. Clin. Infect. Dis. 2017;64:1619–1621. doi: 10.1093/cid/cix130. [DOI] [PubMed] [Google Scholar]
- Lecube A., Pachon G., Petriz J., Hernandez C., Simo R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One. 2011;6:e23366. doi: 10.1371/journal.pone.0023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A., Salvati L., Maggi L., Capone M., Vanni A., Spinicci M., Mencarini J., Caporale R., Peruzzi B., Antonelli A., et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Invest. 2020;130:4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J., Williams N., Rush C., Govan B., Sangla K., Norton R., Ketheesan N. Burkholderia pseudomallei triggers altered inflammatory profiles in a whole-blood model of type 2 diabetes-melioidosis comorbidity. Infect. Immun. 2012;80:2089–2099. doi: 10.1128/IAI.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndhlovu L.C., Lopez-Verges S., Barbour J.D., Jones R.B., Jha A.R., Long B.R., Schoeffler E.C., Fujita T., Nixon D.F., Lanier L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas, F.E., Murcia, L., Navarro, E., Navarro-Mendoza, M.I., Perez-Arques, C., and Garre, V., 2020. Mucorales Species and Macrophages. J Fungi (Basel) 6. [DOI] [PMC free article] [PubMed]
- Nomani M., Varahram M., Tabarsi P., Hashemian S.M., Jamaati H., Malekmohammad M., Ghazi M., Adcock I.M., Mortaz E. Decreased neutrophil-mediated bacterial killing in COVID-19 patients. Scand. J. Immunol. 2021;94:e13083. doi: 10.1111/sji.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S.A., Mahaira L.G., Demirtzoglou F.J., Sotiropoulou P.A., Ioannidis P., Iliopoulou E.G., Gritzapis A.D., Sotiriadou N.N., Baxevanis C.N., Papamichail M. A potential role for hydrocortisone in the positive regulation of IL-15-activated NK-cell proliferation and survival. Blood. 2005;106:158–166. doi: 10.1182/blood-2004-08-3232. [DOI] [PubMed] [Google Scholar]
- Philippe B., Ibrahim-Granet O., Prevost M.C., Gougerot-Pocidalo M.A., Sanchez Perez M., Van der Meeren A., Latge J.P. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 2003;71:3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randeria S.N., Thomson G.J.A., Nell T.A., Roberts T., Pretorius E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc. Diabetol. 2019;18:72. doi: 10.1186/s12933-019-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman C.M. Prolonged cryopreservation of human granulocytes. Transfusion. 1983;23:508–511. doi: 10.1046/j.1537-2995.1983.23684074273.x. [DOI] [PubMed] [Google Scholar]
- Sato M., Sano H., Iwaki D., Kudo K., Konishi M., Takahashi H., Takahashi T., Imaizumi H., Asai Y., Kuroki Y. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 2003;171:417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Tramsen L., Perkhofer S., Lass-Florl C., Hanisch M., Roger F., Klingebiel T., Koehl U., Lehrnbecher T. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology. 2013;218:939–944. doi: 10.1016/j.imbio.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Tramsen L., Lehrnbecher T. Natural killer cells in antifungal immunity. Front. Immunol. 2017;8:1623. doi: 10.3389/fimmu.2017.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Ioannidis L.J., Karl S., Robinson L.J., Tan Q.Y., Poole D.P., Betuela I., Hill D.L., Siba P.M., Hansen D.S., et al. Synergistic effect of IL-12 and IL-18 induces TIM3 regulation of gammadelta T cell function and decreases the risk of clinical malaria in children living in Papua New Guinea. BMC Med. 2017;15:114. doi: 10.1186/s12916-017-0883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehri Z., Kiani Z., Nasiri A.A., Kohan F. Toll-like receptor 2 and type 2 diabetes. Cell. Mol. Biol. Lett. 2016;21:2. doi: 10.1186/s11658-016-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Kasarpalkar N., Bhowmick S., Paradkar G., Talreja M., Shah K., Tiwari A., Palav H., Kaginkar S., Kulkarni R., et al. Opposing roles for sMAdCAM and IL-15 in COVID-19 associated cellular immune pathology. J. Leukoc. Biol. 2022;111:1287–1295. doi: 10.1002/JLB.3COVBCR0621-300R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifkin M., Cumbie R. Comparison of the Histopaque-1119 method with the Plasmagel method for separation of blood leukocytes for cytomegalovirus isolation. J. Clin. Microbiol. 1992;30:2722–2724. doi: 10.1128/jcm.30.10.2722-2724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B., Edwards J., Jr., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansir G., Rastogi N., Ramteke P., Kumar P., Soneja M., Biswas A., Kumar S., Jorwal P., Baitha U. Disseminated mucormycosis: a sinister cause of neutropenic fever syndrome. Intractable Rare Dis Res. 2017;6:310–313. doi: 10.5582/irdr.2017.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Eeckhout B., Tavernier J., Gerlo S. Interleukin-1 as innate mediator of T cell immunity. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.621931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigon L., Fuertes D., Garcia-Perez J., Torres M., Rodriguez-Mora S., Mateos E., Corona M., Saez-Marin A.J., Malo R., Navarro C., et al. Impaired cytotoxic response in PBMCs from patients with COVID-19 admitted to the ICU: biomarkers to predict disease severity. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.665329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B.J., Peltan I.D., Jensen P., Hoda D., Hunter B., Silver A., Starr N., Buckel W., Grisel N., Hummel E., et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2 doi: 10.1016/S2665-9913(20)30343-X. e754–e763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2022). WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/.
- Wurster S., Thielen V., Weis P., Walther P., Elias J., Waaga-Gasser A.M., Dragan M., Dandekar T., Einsele H., Loffler J., Ullmann A.J. Mucorales spores induce a proinflammatory cytokine response in human mononuclear phagocytes and harbor no rodlet hydrophobins. Virulence. 2017;8:1708–1718. doi: 10.1080/21505594.2017.1342920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.