Abstract
OBJECTIVES
Upper gastrointestinal bleeding (UGIB) is a common complication in adults treated with veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for refractory cardiogenic shock or cardiac arrest. We aimed to determine risk factors, prevalence and outcomes associated with VA-ECMO-associated UGIB in adult patients.
METHODS
We conducted a retrospective cohort study (2014–2022) on consecutive VA-ECMO patients in the medical and infectious disease intensive care unit of Bichat-Claude Bernard University Hospital, Paris, France. UGIB was defined as (i) an overt bleeding (haematemesis, melena, haematochezia) or (ii) acute anaemia associated with a lesion diagnosed on upper gastrointestinal endoscopy. VA-ECMO-associated UGIB was defined as an UGIB occurring during VA-ECMO, or up to 10 days after decannulation in patients weaned off extracorporeal membrane oxygenation (ECMO). Cause-specific models were used to identify factors associated with UGIB and death, respectively.
RESULTS
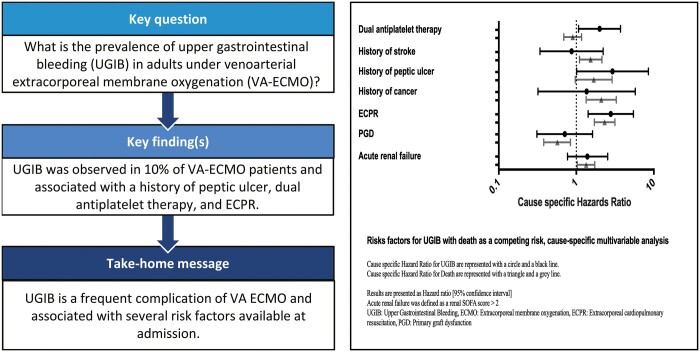
Among the 455 patients included, 48 (10%) were diagnosed with UGIB after a median of 12 [7; 23] days following ECMO cannulation. Mortality occurred in 36 (75%) patients with UGIB and 243 (60%) patients without. UGIB patients had longer intensive care unit stays (32 [19; 60] vs 18 [7; 37] days; P < 0.01), longer ECMO (14 [9; 18] vs 7 [4; 11] days; P < 0.01) and mechanical ventilation durations (21 [16; 36] vs 10 [5; 20] days; P < 0.01), as compared to non-UGIB patients. Ninety upper gastrointestinal endoscopies were performed, and the most frequent lesions detected were gastro-duodenal ulcers (n = 23, 26%), leading to 11/90 therapeutic procedures. By multivariable analysis, a history of peptic ulcer [cause-specific hazard ratio (CSHR) 2.93, 95% confidence interval (CI) [1.01; 8.51]], a dual antiplatelet therapy (CSHR 2.0, 95% CI [1.07; 3.72]) and extracorporeal cardiopulmonary resuscitation (CSHR 2.78, 95% CI [1.42; 5.45]) were independently associated with an increased risk of UGIB.
CONCLUSIONS
In adult patients under VA-ECMO, a history of gastric ulcer, dual antiplatelet therapy and extracorporeal cardiopulmonary resuscitation were independently associated with an increased risk of UGIB. This study highlights the potential role of acute ischaemia–reperfusion injury in the pathophysiology of VA-ECMO-associated UGIB.
Keywords: Extracorporeal membrane oxygenation, Intensive care units, Gastrointestinal haemorrhage, Endoscopy, gastrointestinal, Shock, cardiogenic
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is an emergency technique used in adult patients to treat refractory cardiogenic shock or refractory cardiac arrest [1].
INTRODUCTION
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is an emergency technique used in adult patients to treat refractory cardiogenic shock or refractory cardiac arrest [1]. There has been a massive increase in its use over the last decade [2, 3].
Upper gastrointestinal bleeding (UGIB) is a frequent complication of critical illness, occurring in 0.6–7% of cases [4–6]. It is associated with adverse outcomes, including increased intensive care unit (ICU) length of stay and increased mortality [7].
In a multicentre study, in 1994, on 2252 critically ill patients, Cook et al.identified 2 independent risk factors for gastrointestinal bleeding, namely respiratory failure and coagulopathy [8]. In the recent SUP-ICU trial conducted on 1034 patients in 2016, variables independently associated with clinically important ‘stress ulcers’ were 3 or more coexisting diseases, coexisting coagulopathy, coexisting liver disease, use of renal replacement therapy (RRT), acute coagulopathy and a high organ failure score [6].
The main pathophysiological mechanism of UGIB in critically ill patients is splanchnic hypoperfusion, resulting from both hypotension and release of proinflammatory cytokines at the acute phase. This can notably be increased by an ischaemia–reperfusion phenomenon, leading paradoxically to an increase in mucosal ischaemia [9]. The clinical presentation of UGIB is non-specific in the absence of overt bleeding and is usually suspected in the presence of unexplained acute anaemia, haemodynamic instability and/or hyperuricaemia. A definitive diagnosis is made with upper gastrointestinal endoscopy (UGE), which can sometimes lead to a therapeutic action (haemostatic injection, vasoconstrictor injection, clipping, etc.).
Haemorrhagic complications, including UGIB, are frequent during extracorporeal membrane oxygenation (ECMO) support and are associated with a poor outcome [10]. Haemorrhagic complications may be observed in VA-ECMO patients for multiple reasons: first, anticoagulation is often used to avoid thrombosis of the oxygenation membrane. Second, ECMO circuits induce a short-term loss of high molecular weight von Willebrand factor multimer, which is necessary for primary haemostasis [11]. Last, thrombopenia and low fibrinogen concentrations are frequent under ECMO [12–14].
Pathophysiological mechanisms involved in the development of UGIB under ECMO may include patient-related factors (i.e. previous ulcer, chronic use of antiplatelet therapy, etc.), illness-related factors (i.e. mechanical ventilation, illness severity anticoagulation, thrombopenia, etc.) [9] and potential ECMO-related factors (i.e. decreased gastric mucosal perfusion [15, 16], ischaemia–reperfusion injury [17], etc.).
Mazzeffi et al. [18] described a prevalence of 13.6% of gastrointestinal bleeding in a mixed cohort of 132 patients placed on ECMO, including 64 patients on veno-venous ECMO. To date, there are no specific studies regarding UGIB in patients on VA-ECMO.
In the present study, we aimed to identify risk factors, prevalence and outcomes associated with UGIB in adult patients supported with VA-ECMO.
METHODS
Design
We performed a single-centre retrospective cohort study in the 26-bed medical ICU of the Bichat-Claude Bernard University Hospital, APHP, Paris, France.
Ethics
This study was approved by the ethical committee of the French Society of Intensive Care (SRLF). The database was declared to the French National Commission of data processing (CNIL).
Patients
We included consecutive adult patients who received VA-ECMO support for refractory cardiogenic shock or refractory cardiac arrest, between 1 January 2014 and 31 December 2021. Exclusion criteria were (i) age <18 years and (ii) missing data on UGIB, UGE and/or outcome in the ICU.
Data collection
Clinical parameters were extracted from the medical charts. These included baseline characteristics at admission (weight, height, comorbidities: chronic use of antiplatelet therapy), history of stroke (ischaemic or haemorrhagic cerebral stroke reported in the medical report), history of cancer (cured or ongoing cancer reported in the medical report), peptic ulcer, Simplified Acute Physiology Score [19], Charlson score [20], Sepsis-related Organ Failure Assessment (SOFA) score [21], characteristics at the time of cannulation (i.e. survival after veno-arterial ECMO score [22]), use of antiplatelet therapy during ICU stay, site of implantation, place of implantation, aetiology of cardiogenic shock or refractory cardiac arrest, occurrence of pre-ECMO cardiopulmonary resuscitation (CPR), occurrence of extracorporeal cardiopulmonary resuscitation (ECPR) corresponding to the implantation of VA-ECMO during CPR and outcome characteristics (duration of mechanical ventilation, ICU length of stay, use of RRT, duration and outcome of VA-ECMO, date and cause of death in non-survivors).
Biological parameters were collected at the time of cannulation (haemoglobin, platelets, prothrombin time, creatinine, and lactate) and 24 h before bleeding events (haemoglobin, platelets, prothrombin time, activated partial thromboplastin time) in patients who developed bleeding events.
Transfusion data were extracted retrospectively from the blood bank database of our hospital Etablissement Français du Sang. For each patient, all red blood cell (RBC) units, fresh frozen plasma and platelet units transfused were identified and the date of transfusion was noted.
Upper gastrointestinal bleeding definitions and upper gastrointestinal endoscopy procedures
UGIB was described as an overt bleeding (haematemesis, melena, haematochezia), which was considered when either blood was visible or detected with oxygenated water in the faeces or in emesis or in gastric aspiration, or acute anaemia associated with a lesion found on the UGE. VA-ECMO-associated UGIB was defined as a UGIB occurring during VA-ECMO, or up to 10 days after decannulation in patients weaned off ECMO. UGE was prescribed by the physician in charge in cases of overt bleeding or acute anaemia with a suspicion of GI bleeding. UGE was performed by endoscopy specialists. The results of UGE procedures were classified in a pre-defined list of aetiologies. If 2 or more lesions were found on the same UGE, only the most clinically significant one was described in our study. Endoscopic therapeutic procedures were performed according to the lesions found, and the choice of the procedure was left at the discretion of the endoscopy specialist.
Stress ulcer prophylaxis, thromboprophylaxis, feeding and transfusion policies
All patients placed on VA-ECMO received a stress ulcer prophylaxis with a proton pump inhibitor (intravenous omeprazole, 40 mg per day) from the day of ICU admission, until ICU discharge. To prevent thrombotic events and membrane clotting during VA-ECMO support, every patient received a continuous infusion of unfractionated heparin, with an anti-Xa target of 0.2–0.3 UI/ml that was continued for at least 1 month after ECMO explantation. The decision to stop heparin because of thrombopenia or haemorrhagic events was left to the discretion of the treating physician. Enteral feeding via an oral gastric tube was started within the first 24 h of admission, if feasible, to achieve appropriate energy needs (25–35 kcal/kg/day) within the first 7 days. The haemoglobin threshold for transfusion was 8.0 g/dl and the platelet level was 50 000/mm3 in the absence of bleeding. In the case of bleeding events, transfusions were determined by the physician in charge to maintain a platelet level of >80 000/mm3, a prothrombin time of >70% and a fibrinogen plasma concentration of >1.5 g/l. There was no specific protocol regarding anticoagulation management in case of isolated and uncomplicated suspected bleeding. In cases of suspected or confirmed massive bleeding (haematemesis/melena associated with shock) and/or severe coagulopathy (thrombocytopaenia <50 000/mm3 and/or severe diffuse intravascular coagulation), anticoagulation was stopped and ECMO could be run without heparin.
Statistical analysis
Data are described as numbers (%) for categorical variables and medians (interquartile range) for continuous variables. Comparisons relied on Fisher’s exact test for categorical data and the Wilcoxon test for continuous data. Using cause-specific models, we determined risk factors for the occurrence of UGIB in adult patients on VA-ECMO during their ICU stay, considering death as a competing risk. For this purpose, risk factors were explored by univariate analyses. Then, variables yielding a P-value of <0.1 were entered into a multivariable model using a forward selection procedure. Results were expressed as cause-specific hazard ratios (CSHR) with their 95% confidence intervals (CIs). Missing data were imputed to the median or to the highest frequency. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc, Cary, NC). A P-value of <0.05 was considered statistically significant, and P-values may not be interpreted as confirmatory but rather descriptive. There was no prespecified plan to adjust for multiple comparisons.
RESULTS
Study population
Between January 2014 and December 2022, 459 patients received VA-ECMO support in our ICU. Four patients were excluded because of missing files and 455 patients were finally studied. A study flow chart is provided in Supplementary Material, File S1.
Patient characteristics at ICU admission are shown in Table 1. The median age was 59 [48; 67] years, patients were mainly men (n = 340, 75%), the median Charlson score was 3 [1; 5], the median Simplified Acute Physiology Score was 61 [43; 80] and the median SOFA was 11 [7; 13]. Compared to non-UGIB patients, patients with UGIB had more frequent chronic renal disease (29% vs 16%; P = 0.03). Other characteristics were comparable between UGIB and non-UGIB patients.
Table 1:
Baseline characteristics
| Characteristic | Total (n = 455) | Without UGIB (n = 407) | With UGIB (n = 48) | P-Value |
|---|---|---|---|---|
| Age (years) | 59 [48; 67] | 59 [47; 66] | 61 [51; 68] | 0.28 |
| Males | 340 (75) | 301 (74) | 39 (81) | 0.27 |
| BMI (kg/m2) | 27 [23; 30] | 27 [23; 30] | 27 [24; 31] | 0.29 |
| Comorbidities | ||||
| Charlson score | 3 [1; 5] | 3 [1; 5] | 4 [2; 6] | 0.22 |
| Myocardial infarction | 123 (27) | 110 (27) | 13 (27) | 0.99 |
| Congestive heart failure | 193 (42) | 174 (43) | 19 (40) | 0.67 |
| Peripheral vascular disease | 46 (10) | 40 (10) | 6 (12) | 0.56 |
| Peptic ulcer | 24 (5) | 19 (5) | 5 (10) | 0.09 |
| Chronic liver disease | 11 (2) | 8 (2) | 2 (4) | 0.40 |
| Diabetes | 137 (30) | 117 (29) | 20 (42) | 0.06 |
| Chronic renal disease | 80 (18) | 66 (16) | 14 (29) | 0.03 |
| Hypertension | 177 (39) | 158 (39) | 19 (40) | 0.92 |
| Smoking | 90 (20) | 82 (20) | 8 (17) | 0.57 |
| Dyslipidaemia | 125 (27) | 111 (27) | 14 (29) | 0.78 |
| Alcoholism | 32 (7) | 29 (7) | 3 (6) | 0.82 |
| Antiplatelet therapy | 153 (34) | 129 (34) | 16 (33) | 0.96 |
| Severity on admission | ||||
| SAPS II | 61 [43; 80] | 61 [43; 82] | 58 [46; 72] | 0.77 |
| SOFA | 11 [7; 13] | 10 [7; 13] | 11 [7; 13] | 0.85 |
Results are expressed as n (%) or median [interquartile range].
BMI: body mass index; SAPS: Simplified Acute Physiology Score; SOFA: Sepsis-related Organ Failure Assessment; UGIB: upper gastrointestinal bleeding.
Patient characteristics at the time of cannulation are presented in Table 2. Medical indication was the main reason for VA-ECMO implantation (n = 248, 54%): myocardial infarction was the main diagnosis (n = 115, 25%) followed by dilated cardiomyopathy (n = 67, 15%). Two hundred and seven patients (46%) received VA-ECMO support because of cardiac surgery, of whom 87 patients (19%) had primary graft dysfunction after heart transplant. Overall, 158 (35%) patients received pre-ECMO CPR, including 88 (19%) ECPR patients. Patients were mainly cannulated in the operating room (n = 301, 66%). Principal sites of implantation were femoral–axillary (n = 213, 47%) and femoral–femoral (n = 230, 50%). The median SOFA at the time of cannulation was 11 [8; 14] and the median survival after veno-arterial ECMO score was −6 [−10; −2].
Table 2:
Characteristics at time of cannulation
| Characteristic | Total (n = 455) | Without UGIB (n = 407) | With UGIB (n = 48) | P-Value |
|---|---|---|---|---|
| Reason for ECMO implantation | ||||
| Cardiac surgery | 207 (45) | 188 (46) | 19 (40) | 0.59 |
| Bypass or valve surgery | 120 (26) | 108 (26) | 12 (25) | 0.82 |
| PGD | 87 (19) | 80 (20) | 7 (15) | 0.40 |
| Myocardial infarction | 115 (25) | 98 (24) | 17 (35) | 0.09 |
| Dilated cardiomyopathy | 67 (15) | 63 (16) | 4 (8) | 0.19 |
| Myocarditis | 33 (7) | 30 (7) | 3 (6) | 0.78 |
| Pulmonary embolism | 20 (4) | 16 (4) | 4 (8) | 0.16 |
| Endocarditis | 34 (7) | 31 (8) | 3 (6) | 0.73 |
| Septic shock | 17 (4) | 15 (4) | 2 (4) | 0.87 |
| Chronic graft rejection | 5 (1) | 5 (1) | 0 (0) | 0.44 |
| Others | 15 (3) | 14 (3) | 1 (2) | 0.62 |
| Pre-ECMO CPR | 158 (35) | 141 (35) | 17 (35) | 0.92 |
| ECPR | 88 (19) | 75 (18) | 13 (27) | 0.15 |
| Place of implantation | ||||
| ICU | 154 (34) | 135 (33) | 19 (40) | 0.37 |
| Operating room | 301 (66) | 272 (67) | 29 (60) | – |
| Site of implantation | ||||
| Centralized | 12 (3) | 11 (3) | 1 (2) | 0.95 |
| Femoral–axillary | 213 (47) | 191 (47) | 22 (46) | – |
| Femoral–femoral | 230 (50) | 205 (50) | 25 (52) | – |
| Severity | ||||
| SOFA | 11 [8; 14] | 11 [8; 14] | 11 [8; 13] | 0.47 |
| Platelets (g/l) | 152 [102; 214] | 153 [103; 216] | 146 [100; 198] | 0.61 |
| Haemoglobin (g/dl) | 10 [8.5; 12] | 10 [8.6; 12.1] | 9.4 [8.4; 11] | 0.13 |
| Prothrombin time (%) | 57 [41; 72] | 57 [41; 71] | 60 [48; 80] | 0.08 |
| Creatinine (µmol/l) | 140 [99; 209] | 140 [100; 202] | 143 [101; 250] | 0.34 |
| Lactate (mmol/l) | 5.6 [2.8; 9.8] | 5.6 [2.9; 9.6] | 5.5 [2.7; 10.1] | 0.76 |
| SAVE score | −6 [−10; −2] | −6 [−10; −2] | −7 [−11; −2] | 0.50 |
Results are expressed as n (%) or median [interquartile range].
CPR: cardio pulmonary resuscitation; ECMO: extracorporeal membrane oxygenation; ECPR: extracorporeal cardio pulmonary resuscitation; ICU: intensive care unit; PGD: primary graft dysfunction; SAVE: survival after veno-arterial ECMO; SOFA: Sepsis-related Organ Failure Assessment; UGIB: upper gastrointestinal bleeding.
Outcomes are presented in Table 3. Two hundred and seventy-nine patients (61%) died in the ICU. Mechanical ventilation duration and ICU length of stay were 12 [5; 21] and 19 [9; 38] days, respectively. The median numbers of units transfused were 12 [5; 23] for RBCs, 4 [0; 10] for platelets and 4 [0; 12] for fresh frozen plasma. One hundred and thirty-nine (30%) patients received intermittent RRT and 123 (27%) patients received continuous RRT during their ICU stay. ECMO duration was 8 [4; 13] days. Overall, 176 (39%) patients were successfully weaned, 221 (49%) patients were never weaned, 42 cases (9%) were bridged to a heart transplant and 16 cases (3%) were bridged to a left ventricular assist device. The main causes of death were refractory multiple organ failure (n = 189, 64%) (death after ECPR with early termination was classified as refractory multiple organ failure), withdrawal of life sustaining therapies (n = 50, 17%) and brain death (n = 28, 9%). Compared to non-UGIB patients, UGIB patients died more frequently (75% vs 60%; P = 0.04), had longer mechanical ventilation duration (21 [16; 36] vs 10 [5; 20] days; P < 0.01), longer ICU length of stay (32 [19; 60] vs 18 [7; 37] days; P < 0.01) and longer ECMO duration (14 [9; 18] vs 7 [4; 11] days; P < 0.01) and received more RBC units (20 [11; 40] vs 11 [4; 21]; P < 0.01) and more platelet units (7 [2; 15] vs 4 [0; 9]; P < 0.01).
Table 3:
Outcome characteristics and comparison between patients with and without upper gastrointestinal bleeding
| Characteristic | Total (n = 455) | Without UGIB (n = 407) | With UGIB (n = 48) | P-Value |
|---|---|---|---|---|
| ICU outcomes | ||||
| ICU death | 279 (61) | 243 (60) | 36 (75) | 0.04 |
| MV duration (days) | 12 [5; 21] | 10 [5; 20] | 21 [16; 36] | <0.01 |
| ICU LOS (days) | 19 [9; 38] | 18 [7; 37] | 32 [19; 60] | <0.01 |
| No RRT | 193 (42) | 179 (44) | 14 (29) | 0.13 |
| Intermittent RRT | 139 (30) | 115 (30) | 24 (36) | – |
| Continuous RRT | 123 (27) | 108 (26) | 15 (31) | – |
| RBC transfusion | 12 [5; 23] | 11 [4; 21] | 20 [11; 40] | <0.01 |
| Platelet transfusion | 4 [0; 10] | 4 [0; 9] | 7 [2; 15] | <0.01 |
| FFP transfusion | 4 [0; 12] | 4 [0; 12] | 6 [1; 10] | 0.50 |
| ECMO outcomes | ||||
| Successfully weaned | 176 (39) | 156 (38) | 20 (42) | 0.03 |
| Never weaned | 221 (49) | 200 (49) | 21 (44) | – |
| Bridge to transplant | 42 (9) | 40 (10) | 2 (4) | – |
| Bridge to LVAD | 16 (3) | 11 (3) | 5 (10) | – |
| ECMO duration (days) | 8 [4; 13] | 7 [4; 11] | 14 [9; 18] | <0.01 |
| Re-cannulation | 52 (12) | 44 (11) | 8 (16) | 0.23 |
| Cause-specific hospital mortalitya | 0.19 | |||
| Refractory MOF | 189 (64) | 168 (65) | 21 (57) | – |
| WLST | 50 (17) | 42 (16) | 8 (22) | – |
| Septic shock | 24 (8) | 21 (8) | 3 (8) | – |
| Brain death | 28 (9) | 24 (9) | 4 (11) | – |
| Cardiac arrest | 5 (2) | 4 (1.5) | 1 (3) | – |
| Haemorrhagic shock | 1 (0.3) | 1 (0.4) | 0 (0) | – |
Results are expressed as n (%) or median [interquartile range].
One hundred and sixty-five patients (64%) died during their hospital stay.
ECMO: extracorporeal membrane oxygenation; FFP: fresh frozen plasma; ICU: intensive care unit; LOS: length of stay; LVAD: left ventricular assist device; MOF: multiple organ failure; MV: mechanical ventilation; RBC: red blood cell; RRT: renal replacement therapy; UGIB: upper gastrointestinal bleeding; WLST: withdrawal of life support treatment.
Variables associated with upper gastrointestinal bleeding
Central image presents the multivariable analysis (univariate analysis is presented in Supplementary Material, File S2) for UGIB in a cause-specific manner with death as a competitive risk. Characteristics associated with UGIB were: history of peptic ulcer (CSHR 2.93 [1.01; 8.51]; P = 0.05), a dual antiplatelet therapy (CSHR 2.00 [1.07; 3.72]; P = 0.03) and an ECPR (CSHR 2.78 [1.42; 5.45]; P < 0.01). Characteristics associated with ICU mortality were: a history of stroke (CSHR 1.53 [1.10; 2.14]; P = 0.01), a history of cancer (CSHR 2.10 [1.34; 3.28]; P < 0.01), ECPR (CSHR 2.32 [1.71; 3.15]; P < 0.01) and an acute renal failure (CSHR 1.34 [1.03; 1.73]; P = 0.03). In contrast, a primary graft dysfunction (CSHR 0.57 [0.38; 0.84]; P < 0.01) was independently associated with a reduced risk of death.
Upper gastrointestinal endoscopy characteristics
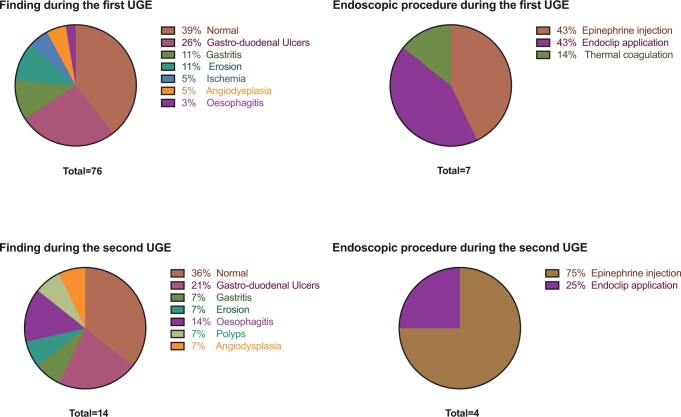
UGE findings are presented in Fig. 1. Seventy-six (17%) patients underwent at least 1 UGE, 12 [7; 23] days after cannulation. Thirty-two (42%) were performed for acute anaemia, 18 (24%) for haematemesis, 21 (28%) for melena and 5 (7%) for haematochezia. Among UGEs, 31/76 (41%) were reported as normal, 20/76 (26%) revealed at least 1 gastro-duodenal ulcer, and 7/76 (9%) revealed gastritis. Seven out of 76 (9%) patients were submitted to an endoscopic therapeutic procedure during the first UGE (n = 3 epinephrine injection therapy; n = 3 endoclip application and n = 1 thermal coagulation). Among the 76 patients who underwent UGE, 14/76 (18%) patients were submitted to a second endoscopy. Among these patients, 3/14 (21%) were found to have gastro-duodenal ulcers, 5/14 (36%) were reported as normal and 2/14 (14%) had esophagitis. Four out of 14 (28%) patients underwent an endoscopic procedure during the second UGE (n = 1 endoclip application, n = 3 epinephrine injection therapy).
Figure 1:
Characteristics of upper gastrointestinal endoscopy (UGE). Risk factors for upper gastrointestinal bleeding, cause-specific multivariable analysis. Results are presented as hazard ratio [95% confidence interval]. Acute renal failure was defined as a renal Sepsis-related Organ Failure Assessment score >2.
Transfusion characteristics on the day of the UGE procedure are presented in Supplementary Material, File S2.
Measures of diagnostic accuracy of main clinical and biological characteristics present in the last 24 h before UGE are presented in Supplementary Material, File S2. Platelet count 24 h before UGE between patient described as normal or pathological was not significantly different (120 [80; 169] vs 95 [54; 203]; P = 0.45). The existence of haematemesis and haematochezia had the highest specificities (93% and 91%, respectively). However, these parameters had low sensitivities (32% and 5%, respectively).
DISCUSSION
In this single-centre retrospective study that evaluated incidence, risk factors and outcomes associated with UGIB in adult VA-ECMO patients, we found that UGIB occurred in 10% of patients, after a median of 12 [7; 23] days following ECMO cannulation. The main findings during UGE were gastro-duodenal ulcers, and a few UGEs led to endoscopic therapeutic procedures. A history of peptic ulcer, dual antiplatelet therapy and ECPR were the main independent risk factors. UGIB patients had longer ICU stays and longer ECMO and mechanical ventilation durations, as compared to non-UGIB patients. Of note, no UGIB was responsible for death in this cohort or reported as a life-threatening situation.
In large studies conducted in the general ICU population, UGIB occurred in 2–5% of cases [6, 8, 23]. The high proportion of UGIB in VA-ECMO patients observed in our cohort could be simply explained by the severe condition of patients on VA-ECMO. These patients often present several risk factors, including respiratory failure, use of RRT and coagulopathy [6, 8]. These variables were not associated with UGIB in our study, as almost every patient received mechanical ventilation, and more than half of the patients received RRT.
It is noteworthy that UGIB was rarely diagnosed within the first days following ICU admission. It is thus possible that UGIB was undetected in the most severe patients who died early and in whom diagnostic endoscopic procedures were not performed.
In our study, we used a competing risk regression model with death as a competing risk to identify risk factors for UGIB. This model allowed to evaluate the relationship of covariates to cause-specific events [24]. Our results suggest that UGIB may be more frequent in patients with a history of gastric ulcer, in patients receiving dual antiplatelet therapy during ICU stay and in those cannulated under chest compressions (ECPR patients). It is likely that gastric ulcer prevention measures with proton pump inhibitor and early enteral feeding might be of benefit in patients presenting at least one of these characteristics. However, the potential benefit of a high-dose proton pump inhibitor therapeutic strategy in this population remains to be investigated. The different risk factors identified in our study could reflect the importance of ischaemia–reperfusion injury in splanchnic territories [25]. The digestive tissue might be very sensitive to ischaemia–reperfusion-related damage: intestinal villosities contain the highest concentration in the body of xanthine dehydrogenase which is responsible for the production of superoxide anions, radical oxygen species, in the case of ischaemia–reperfusion [26]. Mechanisms involving radical oxygen species that increase the damage caused by ischaemia–reperfusion are well known, including endothelial dysfunction and platelet and leucocyte activation [27–29].
In our study, among the 90 UGEs performed, the main findings were lesions of the digestive mucosa and gastro-duodenal ulcers. In a previous study, stress gastritis was the most frequent diagnosis among ECMO patients but UGE was only performed in 7 patients [18]. In another study carried out in a medico-surgical ICU in non-ECMO patients, 84 UGEs were performed: erosion (21%) was the most frequent diagnosis, followed by gastritis (17%) and peptic ulcer (15%), and 25 endoscopies (30%) were described as normal [30]. This cohort seems to present the lesions found in our population in approximately in the same proportion as in our group of severe VA-ECMO patients. This suggests that the lesions found in our population may not be specific to the presence of VA-ECMO. We assume that the relatively high rate of UGIB in our study can be explained by several factors, including patients’ characteristics (comorbidities, severity at time of cannulation), a low threshold for performing endoscopy and local gastric ulcer and anticoagulation protocols. However, this remains speculative and larger multicentre studies are needed to confirm our findings. In our cohort, 35 UGEs (46%) were described as normal. This high proportion of normal endoscopy may reflect the difficulty in diagnosing acute anaemia in patients with VA-ECMO. Overt bleeding appeared to be more frequently associated with lesions. Information about increases in norepinephrine and urea–creatinine dissociation was missing and could perhaps be of interest to determine a priori patients with lesions. Although some of these lesions may not result in therapeutic changes, they remain a reflection of splanchnic hypoperfusion and may be responsible for progressive anaemia. Eleven UGEs led to haemostatic endoscopic procedures, a prevalence that, although low, remains high compared to other studies on ICU patients [30]. UGIB definition is not standardized in the literature and often refers only to clinical findings [6, 10], which is an important drawback. That is why we chose a harsh definition that included both clinical and UGE findings, making our UGIB definition more relevant for clinical practice. Moreover, because VA-ECMO-associated UGIB has not been previously defined in the literature, we arbitrarily chose a cut-off duration of 10 days after decannulation in patients weaned off ECMO, since UGIB events occurring after 10 days could be linked to non-ECMO-related factors.
Limitations
Our study has several limitations: (i) this is a single-centre study, and our results may not be extrapolatable to other ECMO centres: it should be noted that we had a relatively high proportion of both post-cardiac surgery VA-ECMO patients and patients with primary graft dysfunction following heart transplantation. However, cardiac infarction and dilated cardiopathy remained the main reasons for ECMO implantation; (ii) our study is based on a retrospective analysis of data. However, all UGE reports were computer based and we can therefore safely assume that no important information about endoscopic procedures was missing; (iii) because the decision to perform UGE was based on clinical suspicion, some asymptomatic gastrointestinal lesions may have been missed; (iv) some lesions may have been missed or inaccessible to endoscopists and therefore be falsely described as normal; (v) we lacked quantitative information on enteral feeding, which is associated with a decrease in the prevalence of UGIB; we also lacked information concerning anticoagulant therapy and antiplatelet agent management (the occurrence of UGIB could lead to the discontinuation of the anticoagulants); (vi) we lacked information about vasopressor dosages, intra-abdominal pressure and pre-ECMO mesenteric arterial status, which can influence splanchnic perfusion; and (vii) lower intestinal endoscopy or video capsule endoscopy was not performed systematically in case of normal UGE and patients could be falsely described without intestinal bleeding. To note lower intestinal endoscopy was performed for 51 patients during this period, among them only 11 found either erosions or ischaemia, on these 11 patients 8 had a UGE and among them 5 were described as normal. In conclusion with these findings, lower intestinal endoscopy could increase the number of patients with intestinal bleeding by 5 patients.
CONCLUSION
UGIB is a frequent complication among adult patients under VA-ECMO. A history of peptic ulcer, dual antiplatelet therapy and ECPR are independently associated with UGIB in this population. This study highlights the potential role of acute ischaemia–reperfusion injury in the pathophysiology of VA-ECMO-associated UGIB.
Supplementary Material
Glossary
ABBREVIATIONS
- CI
Confidence interval
- CPR
Cardiopulmonary resuscitation
- CSHR
Cause-specific hazard ratio
- ECMO
Extracorporeal membrane oxygenation
- ECPR
Extracorporeal cardiopulmonary resuscitation
- ICU
Intensive care unit
- RBC
Red blood cell
- RRT
Renal replacement therapy
- SOFA
Sepsis-related Organ Failure Assessment
- UGE
Upper gastrointestinal endoscopy
- UGIB
Upper gastrointestinal bleeding
- VA-ECMO
Veno-arterial extracorporeal membrane oxygenation
Contributor Information
Jules Stern, APHP.Nord, Department of Intensive Care Medicine, Hôpital Bichat - Claude Bernard, Paris, France.
Claire Dupuis, APHP.Nord, Department of Intensive Care Medicine, Hôpital Bichat - Claude Bernard, Paris, France; Medical Intensive Care Unit, Gabriel Montpied University Hospital, Clermont Ferrand, France.
Hervé Kpeglo, APHP.Nord, Department of Intensive Care Medicine, Hôpital Bichat - Claude Bernard, Paris, France.
Jean Reuter, APHP.Nord, Department of Intensive Care Medicine, Hôpital Bichat - Claude Bernard, Paris, France.
Camille Vinclair, APHP.Nord, Department of Intensive Care Medicine, Hôpital Bichat - Claude Bernard, Paris, France.
Marylou Para, APHP.Nord, Department of Cardiac Surgery, Hôpital Bichat - Claude Bernard, Paris, France; Université Paris Cité, INSERM UMR 1148, LVTS, Paris, France.
Patrick Nataf, APHP.Nord, Department of Cardiac Surgery, Hôpital Bichat - Claude Bernard, Paris, France.
Anne-Laure Pelletier, APHP.Nord, Department of Hepato-Gastroenterology, Hôpital Bichat - Claude Bernard, Paris, France.
Etienne de Montmollin, APHP.Nord, Department of Intensive Care Medicine, Hôpital Bichat - Claude Bernard, Paris, France.
Lila Bouadma, APHP.Nord, Department of Intensive Care Medicine, Hôpital Bichat - Claude Bernard, Paris, France; Université Paris Cité, INSERM U1137, F-75018 Paris, France.
Jean-François Timsit, APHP.Nord, Department of Intensive Care Medicine, Hôpital Bichat - Claude Bernard, Paris, France; Université Paris Cité, INSERM U1137, F-75018 Paris, France.
Romain Sonneville, APHP.Nord, Department of Intensive Care Medicine, Hôpital Bichat - Claude Bernard, Paris, France; Université Paris Cité, INSERM U1137, F-75018 Paris, France.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Conflict of interest: none declared.
DATA AVAILABILITY
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
Jules Stern: Conceptualization; Investigation; Writing—original draft; Writing—review & editing. Claire Dupuis: Data curation; Formal analysis; Methodology. Hervé Kpeglo: Data curation. Jean Reuter: Data curation; Methodology. Camille Vinclair: Data curation; Methodology. Marylou Para: Writing—review & editing. Patrick Nataf: Writing—review & editing. Anne-Laure Pelletier: Writing—review & editing. Etienne de Montmollin: Writing—review & editing. Lila Bouadma: Data curation; Writing—review & editing. Jean-François Timsit: Supervision; Validation; Writing—review & editing. Romain Sonneville: Conceptualization; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Samuel Heuts and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Makdisi G, Wang IW.. Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015;7:E166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T. et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med 2016;42:889–96. [DOI] [PubMed] [Google Scholar]
- 3. Mao J, Paul S, Sedrakyan A.. The evolving use of ECMO: the impact of the CESAR trial. Int J Surg 2016;35:95–9. [DOI] [PubMed] [Google Scholar]
- 4. Liolios A, Oropello JM, Benjamin E.. Gastrointestinal complications in the intensive care unit. Clin Chest Med 1999;20:329–45, viii. [DOI] [PubMed] [Google Scholar]
- 5. Plummer MP, Blaser AR, Deane AM.. Stress ulceration: prevalence, pathology and association with adverse outcomes. Crit Care 2014;18:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krag M, Perner A, Wetterslev J, Wise MP, Borthwick M, Bendel S. et al. ; the SUP-ICU co-authors. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med 2015;41:833–45. [DOI] [PubMed] [Google Scholar]
- 7. Cook DJ, Griffith LE, Walter SD, Guyatt GH, Meade MO, Heyland DK. et al. ; Canadian Critical Care Trials Group. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care 2001;5:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R. et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994;330:377–81. [DOI] [PubMed] [Google Scholar]
- 9. Stollman N, Metz DC.. Pathophysiology and prophylaxis of stress ulcer in intensive care unit patients. J Crit Care 2005;20:35–45. [DOI] [PubMed] [Google Scholar]
- 10. Mazzeffi M, Greenwood J, Tanaka K, Menaker J, Rector R, Herr D. et al. Bleeding, transfusion, and mortality on extracorporeal life support: ECLS Working Group on Thrombosis and Hemostasis. Ann Thorac Surg 2016;101:682–9. [DOI] [PubMed] [Google Scholar]
- 11. Tauber H, Ott H, Streif W, Weigel G, Loacker L, Fritz J. et al. Extracorporeal membrane oxygenation induces short-term loss of high-molecular-weight von willebrand factor multimers. Anesth Analg 2015;120:730–6. [DOI] [PubMed] [Google Scholar]
- 12. Dornia C, Philipp A, Bauer S, Stroszczynski C, Schreyer AG, Müller T. et al. D-dimers are a predictor of clot volume inside membrane oxygenators during extracorporeal membrane oxygenation. Artif Organs 2015;39:782–7. [DOI] [PubMed] [Google Scholar]
- 13. Arachchillage DRJ, Laffan M, Khanna S, Vandenbriele C, Kamani F, Passariello M. et al. Frequency of thrombocytopaenia and heparin-induced thrombocytopaenia in patients receiving extracorporeal membrane oxygenation compared with cardiopulmonary bypass and the limited sensitivity of pretest probability score. Crit Care Med 2020;48:e371–9. [DOI] [PubMed] [Google Scholar]
- 14. Jiritano F, Serraino GF, ten Cate H, Fina D, Matteucci M, Mastroroberto P. et al. Platelets and extra-corporeal membrane oxygenation in adult patients: a systematic review and meta-analysis. Intensive Care Med 2020;46:1154–69. 10.1007/s00134-020-06031-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaer JA, Shaw AD, Wild R, Swift RI, Munsch CM, Smith PL. et al. Effect of cardiopulmonary bypass on gastrointestinal perfusion and function. Ann Thorac Surg 1994;57:371–5. [DOI] [PubMed] [Google Scholar]
- 16. Crow S, John R, Boyle A, Shumway S, Liao K, Colvin-Adams M. et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg 2009;137:208–15. [DOI] [PubMed] [Google Scholar]
- 17. McILwain RB, Timpa JG, Kurundkar AR, Holt DW, Kelly DR, Hartman YE. et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest 2010;90:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazzeffi M, Kiefer J, Greenwood J, Tanaka K, Menaker J, Kon Z. et al. Epidemiology of gastrointestinal bleeding in adult patients on extracorporeal life support. Intensive Care Med 2015;41:2015. [DOI] [PubMed] [Google Scholar]
- 19. Le Gall JR, Lemeshow S, Saulnier F.. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957–63. [DOI] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 21. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H. et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT. et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015;36:2246–56. [DOI] [PubMed] [Google Scholar]
- 23. Krag M, Perner A, Møller MH.. Stress ulcer prophylaxis in the intensive care unit. Curr Opin Crit Care 2016;22:186–190. [DOI] [PubMed] [Google Scholar]
- 24. Dignam JJ, Zhang Q, Kocherginsky MN.. The use and interpretation of competing risks regression models. Clin Cancer Res 2012;18:2301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dorweiler B, Pruefer D, Andrasi TB, Maksan SM, Schmiedt W, Neufang A. et al. Ischemia-reperfusion injury : pathophysiology and clinical implications. Eur J Trauma Emerg Surg 2007;33:600–12. [DOI] [PubMed] [Google Scholar]
- 26. Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol 1988;255:H1269–1275. [DOI] [PubMed] [Google Scholar]
- 27. McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 1985;312:159–63. [DOI] [PubMed] [Google Scholar]
- 28. Cai H, Harrison DG.. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000;87:840–4. [DOI] [PubMed] [Google Scholar]
- 29. Leo R, Praticò D, Iuliano L, Pulcinelli FM, Ghiselli A, Pignatelli P. et al. Platelet activation by superoxide anion and hydroxyl radicals intrinsically generated by platelets that had undergone anoxia and then reoxygenated. Circulation 1997;95:885–91. [DOI] [PubMed] [Google Scholar]
- 30. Jean-Baptiste S, Messika J, Hajage D, Gaudry S, Barbieri J, Duboc H. et al. Clinical impact of upper gastrointestinal endoscopy in critically ill patients with suspected bleeding. Ann Intensive Care 2018;8:75. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6031555/. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
Jules Stern: Conceptualization; Investigation; Writing—original draft; Writing—review & editing. Claire Dupuis: Data curation; Formal analysis; Methodology. Hervé Kpeglo: Data curation. Jean Reuter: Data curation; Methodology. Camille Vinclair: Data curation; Methodology. Marylou Para: Writing—review & editing. Patrick Nataf: Writing—review & editing. Anne-Laure Pelletier: Writing—review & editing. Etienne de Montmollin: Writing—review & editing. Lila Bouadma: Data curation; Writing—review & editing. Jean-François Timsit: Supervision; Validation; Writing—review & editing. Romain Sonneville: Conceptualization; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Samuel Heuts and the other anonymous reviewer(s) for their contribution to the peer review process of this article.