Abstract
The COVID-19 disease continues to cause devastation for almost 3 years of its identification. India is one of the leading countries to set clinical trials, production, and administration of COVID-19 vaccination. Recent COVID-19 vaccine tracker record suggests that 12 vaccines are approved in India, including protein subunit, RNA/DNA, non-replicating viral vector, and inactivated vaccine. Along with that 16 more vaccines are undergoing clinical trials to counter COVID-19. The availability of different vaccines gives alternate and broad perspectives to fight against viral immune resistance and, thus, viruses escaping the immune system by mutations. Using the recently published literature on the Indian vaccine and clinical trial sites, we have reviewed the development, clinical evaluation, and registration of vaccines trial used in India against COVID-19. Moreover, we have also summarized the status of all approved vaccines in India, their associated registered clinical trials, manufacturing, efficacy, and their related safety and immunogenicity profile.
Keywords: SARS-CoV-2, Covaxin, Covishield, Vaccination, Sputnik
Introduction
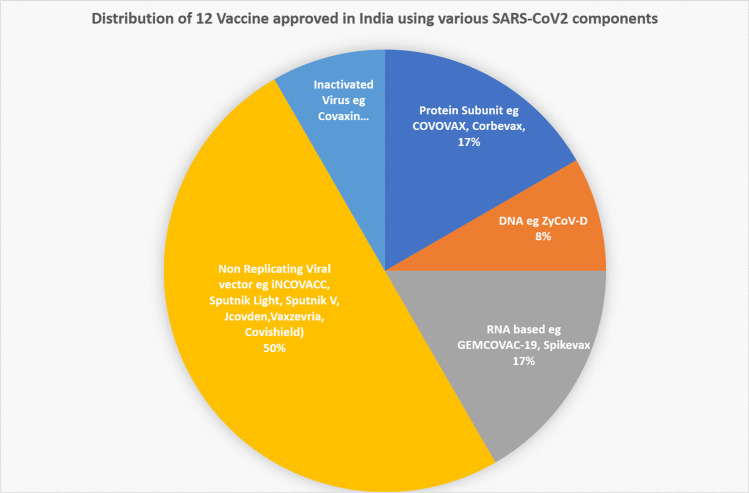
The sudden emergence of SARS-CoV-2 and its newly emerging variant has swept the world. Scientists worldwide have been working hard to understand and fight this disease. The only preventive measures are wearing masks, maintaining social distancing, self-isolation, and, most importantly, vaccination. Mass vaccination is the most effective and decisive option to prevent COVID-19. After sequencing the genome in January 2020, scientists set themselves at the compulsory production of vaccines in a short time. However, with the emergence of new mutant strains of the SARS-CoV2 variants, there was an urgency for a concrete and effective plan. World Health Organization (WHO) declared a severe respiratory disorder syndrome that originated in Wuhan city, China, as a global public health emergency on January 30, 2020, and named the disease COVID-19 on February 11, 2020 [1–3]. On March 11, 2020, WHO declared it a global pandemic [4, 5]. India reported the first confirmed case in Kerala on January 27, 2020 [6]. The spread of COVID-19 was relentless spreading in almost all over the countries, leading to severe public health and social and economic upheaval. A detailed study on novel coronavirus led to a mutual understanding that a COVID-19 vaccine is likely the most effective approach to sustainably controlling the COVID-19 pandemic globally [7]. Operation Warp Speed (OWS) USA was initiated, which cooperates with the National Institute of Health (NIH), Centers for Disease Control and Prevention (CDC), and other organizations to develop strategies for vaccine development, production, and distribution of large quantities of vaccines (https://www.cdc.gov/washington/testimony/2020/t20200702.htm). With a massive surge in COVID-19 disease infections and deaths during the second wave of infection, India needed to take the urgent implementation of steps to develop an indigenous vaccine followed by mass vaccination. Similar to the USA, in India, the CARES (Coronavirus Aid, Relief, and Economic Security) Fund Trust decided to allocate 100 million Indian rupees (INR) to support efforts to develop a vaccine against the coronavirus (https://www.kff.org/coronavirus-covid-19/issue-brief/the-coronavirus-aid-relief-and-economic-security-act-summary-of-key-health-provisions/). The Department of Biotechnology (DBT), India, is an Indian government institution, made the central coordinating agency for determining the vaccine development pathway. Moreover, the Indian government and private companies stepped up their efforts to develop a vaccine to curb the spread of COVID-19. In India, as of November 2022, more than 530 K deaths and more than 44.7 million cases have been reported by the world meter tracking site (https://www.worldometers.info/coronavirus/country/india/). Since the coronavirus outbreak, many efforts have been directed toward the development of a vaccine against COVID-19 all over the world. Since early 2020, to immunize against COVID-19 infection, global vaccine development has been accelerated through unprecedented collaboration in the global pharmaceutical industry and between different countries, governments, international health organizations, and university research groups. Finally, the vaccine drive in India has started to come to fruition with the approval of many indigenous COVID-19 vaccines developed by local pharmaceutical companies and collaborative agreements with many foreign pharma companies. India implemented the world’s largest vaccine program on January 13, 2021, with 2 vaccines, including Covishield and Covaxin [8]. Among the various COVID vaccines used today, COVAXIN, ZycovD, GEMCOVAC-19, and Corbevax are indigenously produced in India; the rest are being developed in other countries but are manufactured in India. In India, three vaccines are initially primarily used, i.e., Covaxin, Covishield, and Sputnik. Two vaccines received approval for emergency use in India at the onset of the program at the beginning of the pandemic; Covishield—a brand of the Oxford—AstraZeneca vaccine manufactured by the Serum Institute of India (SII), and Covaxin, which was developed by Bharat Biotech [8]. A list of vaccines approved in India is detailed in Table 1, and ongoing clinical trials of Indian COVID-19 vaccines are shown in Table 2. In this review, we have discussed the different vaccines against COVID-19, either approved in India or under clinical trial. We have also discussed the strategy and method used to develop those vaccines. Currently, in November 2022, 16 vaccines are being evaluated in clinical trials in India, out of which 12 vaccines have been approved for use in India (https://covid19.trackvaccines.org/country/india/). At the time of submission, there were 12 different products approved, including 2 protein subunits, 1 DNA, 2 RNA, 6 non-replicating vectors, and one inactivated virus, as shown in Fig. 1. Recent data suggests that so far, 2.1 billion total COVID-19 doses have been administered in India (https://pib.gov.in/newsite/pmreleases.aspx?mincode=31).
Table 1.
List of approved vaccines in India
| Vaccine | Sponsor | Vaccine type | Some of the trials listed | Approval |
|---|---|---|---|---|
| Covovax (Novavaz formulation) | Serum Institute of India | Protein subunit |
• CTRI/2022/04/042017 • CTRI/2021/02/031554 |
Bangladesh, India, Indonesia, Philippines, Thailand |
| Corbevax (BECOV2A) | Biological E limited | Protein subunit |
• CTRI/2021/08/036074 • CTRI/2021/10/037066 • CTRI/2021/06/034014 • CTRI/2020/11/029032 |
Botswana, India |
| ZyCoV-D | Zydus Cadila | DNA |
• CTRI/2021/01/030416 • CTRI/2022/06/043365 • CTRI/2020/07/026352 • CTRI/2021/03/032051 |
India |
| GEMCOVAC-19 | Gennova Biopharmaceuticals | RNA | • CTRI/2022/04/041880 | India |
| Spikevax | Moderna | RNA |
• NCT05230953 etc |
88 countries including India |
| iNCOVACC | Bharat Biotech | Non replicating viral vector |
• CTRI/2022/02/040065 |
India |
| Sputnik Light | Gamaleya | Non replicating viral vector |
• CTRI/2022/04/041792 |
26 countries including India |
| Sputnik V | Gamaleya | Non replicating viral vector | 74 countries including India | |
| Jcovden | Johnson & Johnson | Non replicating viral vector | 113 countries including India | |
| Vaxzevria | Oxford/Astrazeneca | Non replicating viral vector |
• jRCT2031210679 |
149 countries including India |
| Covishield | Serum Institute of India | Non replicating viral vector |
• CTRI/2022/04/042017 • CTRI/2022/04/041792 • CTRI/2020/08/027170 |
49 countries including India |
| Covaxin | Bharat biotech | Inactivated virus |
• CTRI/2022/04/042017 • CTRI/2022/04/041792 |
14 countries including India |
Table 2.
List of vaccines under clinical trials in India
| Formulation | Vaccine name | Company | Status |
|---|---|---|---|
| Protein subunit | Nuvaxovid | Novavax |
Approved in 40 countries, 22 trials in 14 countries |
| Corbevax | Biological E limited |
Approved in 2 countries, 7 trials in 1 country |
|
| Covovax (Novavax formulation) | Serum Institute of India |
Approved in 6 countries, 7 trials in 3 countries |
|
| AKS- 452 | University Medical center Groningen | 4 trials in 2 countries | |
| BECOV2B, BECOV2C, BECOV2D | Biological E limited | 2 trials in 1 country | |
| DNA | ZyCov-D | Zydus Cadila |
Approved in 1 country, 6 trials in 1 country |
| RNA | GEMCOVAC-19 | Gennova Biopharmaceutical Limited |
Approved in 1 country, 2 trials in 1 country |
| HGCO19 | Gennova Biopharmaceutical Limited | 2 trials in 1 country | |
| Inactivated virus | Covaxin | Bharat Biotech |
Approved in 14 countries, 16 trials in 2 countries |
| Non-replicating viral vector | INCOVACC | Bharat Biotech |
Approved in 1 country, 4 trials in 1 country |
| Sputnik Light | Gamaleya |
Approved in 26 countries, 7 trials in 3 countries |
|
| Sputnik V | Gamaleya |
Approved in 74 countries, 25 trials in 8 countries |
|
| Vaxzevria | Oxford/AstraZeneca |
Approved in 149 countries, 77 trials in 33 countries |
|
| Covishield (Oxford/AstraZeneca formulation) | Serum Institute of India |
Approved in 149 countries, 6 trials in 1 country |
Fig. 1.
Various platforms of approved Indian COVID-19 vaccine in India
Covaxin
Bharat Biotech, an Indian manufacturer, collaborated with the National Institute of Virology (NIV) and the Indian Council for Medical Research (ICMR) to develop Covaxin (code name BBV152) based on SARS-CoV-2 vaccine strain NIV-2020–770 (spike variant Asp614Gly), an inactivated coronavirus vaccine [9, 10]. After the successful preclinical studies, Bharat Biotech commenced the clinical trials of Covaxin in the country in July [11]. Covaxin is based on the SARS-CoV2 Spike protein. Neutralizing antibodies are produced against Spike protein and their components such as RBD and NTD upon vaccination [12]. The SARS-CoV2 virus was cultured and propagated at NIV. Beta-propiolactone was used for the inactivation of these viral cultures, which inactivates the whole virion while the spike protein remains intact to the virus [13–15]. The inactivated virus was then combined with a small quantity of an adjuvant toll-like receptor 7/8 agonist with an aluminum-based chemical alum (Algel-IMDG) that stimulates the immune system to boost its response to vaccination [16, 17] (Fig. 2). Phase 3 clinical trial (NCT04641481) in India (CTRI/2020/11/028976) showed BBV152 vaccine efficacy was approximately 77.8% 2 weeks post-second dose without any major side effects and anaphylaxis [18]. This efficacy was higher in severe COVID cases at 93.4%, whereas in asymptomatic cases, efficacy was 63.6% [9]. WHO Strategic Advisory Group of Experts on Immunization (SAGE) recommended an interim policy for COVAXIN usage. Per their recommendation, this vaccine was safe for adults above 18 years old, including pregnant women, breastfeeding and non-breast-feeding women, old age people, health care workers, and immunocompromised persons. This vaccine should not administer to children and persons who are suffering from anaphylaxis, fever, or acute COVID infection. Despite distributing the vaccine in 23 other countries, it is mostly used in India. It is given in two doses (0.5 mL each) via the intramuscular route in 4 weeks intervals. A booster dose can be taken after 4- to 6-month intervals, especially in older and immunocompromised people. SAGE also suggested taking a second dose of either mRNA vaccine (Pfizer or Moderna) or adenovirus vector-based vaccines such as Covishield or Vaxzevria followed by the first dose of COVAXIN. This combination either provides equivalent or better immunogenic responses (https://www.who.int/news-room/feature-stories/detail/the-bharat-biotech-bbv152-covaxin-vaccine-against-covid-19-what-you-need-to-know).
Fig. 2.
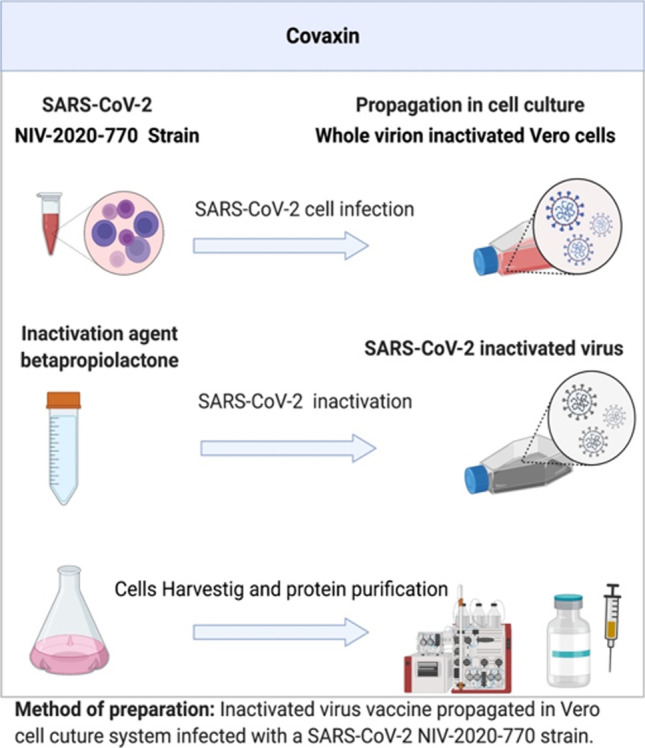
Methodology used for Covaxin preparation. This figure was prepared using BioRender software. It involved 4 major steps: transfection, virus chemical inactivation, cell harvesting, and protein purification
Administration of Covaxin results in the activation of both B cells and T cells which results in.antibody production and T cell cytotoxic activity. B and T cells also form memory B cells, which respond upon natural SARS-CoV2 infection, thus subsequent rechallenge [8, 19–21]. Antibodies prevent the virus from entering lung cells by targeting the spike protein. The durability of BBV152 vaccine-mediated antibody response declines after 6 months of their initial two doses regimen [22, 23]. Phase 2 trial (NCT04471519) showed improvement in neutralization titers as measured by PRNT50 (plaque reduction neutralization test) and seroconversion rate after BBV152 booster after 6 months of the first series of vaccination. Booster-dose-vaccinated individuals also showed better protection against various circulating variants of concern, such as Alpha, Beta, Delta, and Delta plus [24, 25]. This shows antibody cross-reactivity to spike variants. They also found an enhanced B and T cell memory population without any severe side effects [26]. COVAXIN has also been shown to be well tolerated and immunogenic in children from 2 to 18 years of age. Phase 2/3 trials conducted in India (CTRI/2021/05/033752) evaluating the safety and immunogenicity of COVAXIN in children from age 2–18 receiving two doses of 0.5 mL each of COVAXIN demonstrated a higher humoral immune response compared to adults [27]. The major vaccine response was found to be Th1 (T helper cell 1) with IgG1/IgG4 ratios above 1, without any serious adverse side effects or deaths [28].
Covishield
The University of Oxford partnered with AstraZeneca to generate a coronavirus vaccine called ChAdOx1 nCoV-19 or AZD1222 [29]. In February 2020, SII began testing vaccine candidates on animals. The institute also announced the application for the conduction of clinical trials in April 2020 from the Drug Controller General of India (DCGI) [30]. SARS-CoV2 spike protein is cloned into Adenovirus viral vector backbone in this vaccine. For vaccine preparation, ChAdOx1, a modified form of chimpanzee adenovirus, is used [31]. The Adenovirus activates the immune system by releasing DAMPs (damage-associated molecular patterns) and activating the anti-viral state in neighboring immune cells [32, 33] (Fig. 3). The Oxford-AstraZeneca vaccine causes the immune system to react more forcefully to the spike proteins by raising this alert. The antibodies get themselves attached to coronavirus spikes, killing the virus and further helping to prevent infection as it blocks the spikes from attaching to other healthy cells, as reported in a phase III clinical trial study (ISRCTN89951424). Pooled analysis of clinical trials for Covishield (ISRCTN89951424, NCT04324606, NCT04400838, and NCT04444674) confirmed the vaccine efficacy of 76% after a single dose of vaccination. Efficacy was found to be 81.3% in participants receiving two standard doses with a longer prime-boost interval than those with a short interval (efficacy 55.1%) [34, 35]. SAGE also recommended an interim policy for recombinant ChAdOx1 vector-based vaccine trade names such as Vaxzevria and COVISHIELD [36]. The vaccine efficacy of these vaccines is 72% (95% CI: 63–79%) for asymptomatic infected patients and 85% (95% CI: 58–94%) for old age people. Vaccines are given in two doses (0.5 mL) in a 4- to 12-week interval via the intramuscular route. Longer intervals gave improved immune responses [37]. Regarding mix and match, vaccine combination showed that ChAdOx1 vector-based vaccine followed by mRNA vaccines (Pfizer or Moderna) generate enhanced neutralizing antibody and T cell responses [38]. As per WHO recommendations, if people suffer from anaphylaxis reaction, allergic reaction, acute fever, and thrombosis with thrombocytopenia syndrome (TTS) after the first dose of this vaccine, they should be prohibited from subsequent doses. People suffering from underlying conditions or comorbidities such as respiratory illness, HIV, diabetes, etc., and immunocompromised persons at risk of getting COVID-19 should get the vaccination, which has been shown to be safe and effective. The vaccine is also effective in pregnant and breastfeeding women [36].
Fig. 3.
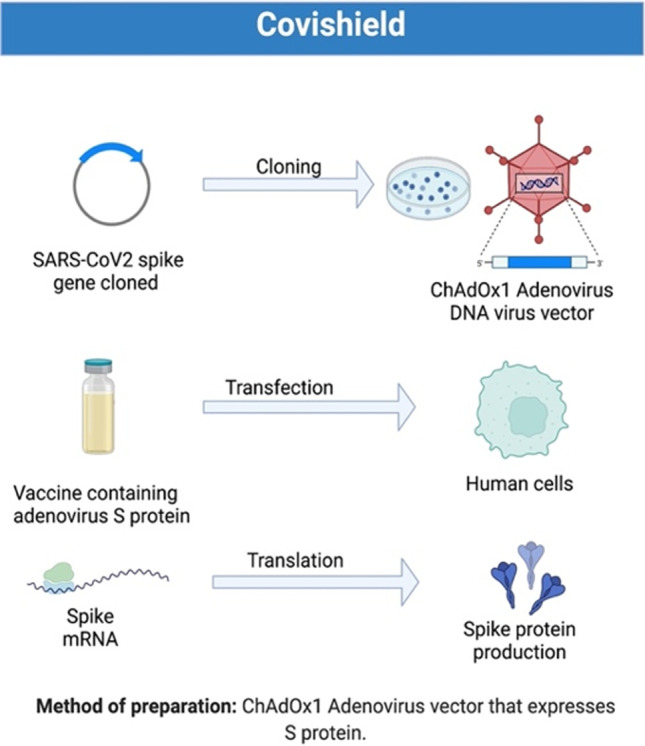
Methodology used for Covishield preparation. This figure was prepared using BioRender software. The steps in preparing Covishield are as follows: 1. Spike cloning into adenovirus vector backbone, 2. transfection, and 3. translation and protein purification
Sputnik V
Ministry of Health, Russia, undertaking body “Gamaleya Research Institute” developed a vaccine for coronavirus and named it Sputnik V or Gam Covid Vac which collaborated with Dr. Reddy’s Laboratories, Hyderabad, India [39, 40]. Spike protein gene is cloned in two kinds of adenovirus Ad26 and Ad5 backbone. Adenovirus vectors serve as a carrier to deliver. The use of two serotypes intends to eliminate any previous Sputnik demonstrated 97.6% efficacy without any strong adverse effects in vaccinated persons in Russia [41]. Indian pharmaceutical giant Dr. Reddy made a deal with phase 2/3 human trials in India of Russia’s Sputnik V vaccine and produced 100 million doses [42]. The study suggests that both Sputnik V and Sputnik Light are effective against SARS-CoV2 delta variant [43, 44].
ZyCoV-D
ZyCoV-D was developed by Zydus Cadila and supported by DBT-BIRAC. It received emergency use authorization in August 2021 for children and adults 12 years and above [45]. It is a plasmid DNA-based COVID vaccine that is administered intradermally through a PharmaJet needle free applicator. Phase 1/2 study showed that ZycoV-D was able to elicit a good immune response with no safety concerns [46]. Interim results from the phase 3 trial ZycoV-D showed an efficacy of 66.6% with adverse effects similar to placebo upon administration of three doses of intradermal needle-free vaccination [47].
Covovax
On September 15, 2020, Novavax, Inc. (NVAX), a late-stage biotechnology agency developing next-generation vaccines for severe infectious diseases, announced an amendment to its agreement made on August 2020 with Serum Institute of India Private Limited (SIIPL). With this agreement, SIIPL started manufacturing the antigen component of NVX CoV2373; Novavax’s COVID-19 vaccine candidate with which Novavax production capability of NVX-CoV2373 increased to over a billion doses annually. NVX CoV2373 is a stable, prefusion protein made using Novavax’s recombinant protein nanoparticle technology and consists of Novavax’s proprietary Matrix M™ adjuvant. The agreement with SIIPL augments an international supply chain to supply over billion doses of NVX-CoV2373 annually as of 2021. Protein or peptide vaccines are easy to produce and have better stability in outside environments [48, 49] (https://www.novavax.com/science-technology/vaccine-pipeline/covid-19-investigational-vaccine).
Corbevax (BECOV2A)
Corbevax manufactured by Biological E limited is based on the RBD subunit which interacts with human host cell ACE2 (angiotensin converting enzyme) receptor. Safety, immunogenicity, and reactogenicity of Corbevax were assessed in phase 1/2 clinical study as CTRI/2021/06/034014 and CTRI/2020/11/029032. Low or no incidence of adverse events was observed after receiving two doses scheduled in 28-day intervals. CpG1018 and aluminum hydroxide were used as adjuvants in this vaccine formulation which showed higher anti RBD-IgG binding and neutralization titers and improved cellular response [50, 51]. Corbevax has been shown to be immunologically superior and safer compared to Covishield in the larger and relatively aged populations [52].
GEMCOVAC.™-19
It is an indigenously developed mRNA vaccine in India developed primarily by Genova Biopharmaceutical limited which is part of Emcure Biopharmaceutical Limited, headquarters situated in Pune, India. It received emergency use authorization for adults on June 22, 2022, from Drugs Controller General. Phase 2 and 3 studies (CTRI/2022/04/041880) found that the vaccine showed less or no incidence of adverse side effects (www.ctri.nic.in).
Spikevax (MOD)
Spikevax, previously known as Moderna COVID-19 vaccine, is an mRNA-based vaccine developed by Moderna. The active substance of the vaccine is CX-024414, which is an mRNA encoding SARS-CoV2 spike protein. It was approved by FDA on January 31, 2022, for individuals 18 years of age and over (https://spikevax.com/). NCT04470427 phase III clinical trial suggests that its vaccine efficacy was more than 90% and it is safe to use. It is a lipid nanoparticle-encapsulated mRNA vaccine [53]. The vaccine is administered as a series of two doses, 1 month apart.
Sputnik Light
This vaccine is a recombinant adenovirus type 26 (rAd26)-based vector containing the SARS-CoV2 spike gene. It is administered as a single dose. Phase 1 clinical trial results showed that there were mild or moderate symptoms post-injection with no severe adverse events observed in all participants. Both seropositive and seronegative participants showed strong humoral (enhanced neutralizing antibody response) and cellular immune response (increased antigen-specific T cell proliferation, production of IFNγ-producing cells, and their cytokine secretion) upon vaccination [54]. Sputnik Light is also proven to be effective against the Delta variant of coronavirus [43, 44].
iNCOVACC
An intranasal COVID-19 vaccine also known as BBV154 is manufactured by Bharat Biotech. Clinical trial (NCT04751682) shows the safe and immunogenic potential of this vaccine. This vaccine provided not only neutralizing antibody and T cell proliferation but also mucosal IgA. Thus, it is effective at the site of both infection as well as transmission and protects the upper and lower respiratory tract. It is targeted at the nasal mucosa site. It is the world’s first intranasal vaccine that got the first restricted use authorization approval in India for adults. It is based on an adenovirus-based vector and stable at 2–8 °C temperature [55, 56]. On September 22, 2020, Codagenix, Inc. announced the production of CDX-005, an intranasal, live-attenuated vaccine by the Serum Institute of India. It is funded by investors such as Adjuvant Capital and Topspin partners. The preclinical studies yielded positive safety and efficacy results for the vaccine [57]. Nasal vaccines are given as booster shots.
Jcovden
Previously known as COVID-19 vaccine Janssen. This vaccine is based on an adenovirus vector (recombinant Ad26.COV2-S). The clinical trials showed its vaccine efficacy was 67%. This vaccine is primarily manufactured by Janssen Biotech, Inc., a Janssen Pharmaceutical company, Johnson & Johnson. It is administered as a single dose [58].
Vaxzevria
Vaxzevria (ChAdOx1 nCov19) is a chimpanzee adenovirus (ChAd)-based COVID-19 vaccine developed by AstraZeneca and is quite effective against Delta variant. The vaccine is approved by EMA for people aged 18 years or older. It is given in two doses with the second dose 4 to 12 weeks after the first dose [59]. The results from a 90-patient study demonstrated that antibody levels were elevated in those with a longer interval between second and the third dose [60].
Mixed inoculation of vaccine in India
Heterologous prime-boost vaccine delivery is based on the administration of SARS-CoV2 spike antigen. This is an important aspect as it leads not only to improved protective efficacy in terms of durable and stronger immune response but also to optimize the usage of available vaccines at that time in developing countries like India compared to homologous vaccine regimens. Usage of adenovirus-based vaccines such as Sputnik or Covishield followed by mRNA-based (Pfizer or Moderna) or protein-based (Novavax) reduces the chance of adverse immune response against the adenovirus-based vector [61]. The example includes the primary vaccine from Covishield, and then a booster with Corbevax showed better B cell and T cell immune response [62]. Another recent report tested Covishield and Covaxin in a homologous and heterologous booster in all possible combinations and showed that Covishield boost followed by Covaxin primary series of combinations shows an improved immune response compared to all other possible combinations [63].
Indian government drive in the development of COVID-19 vaccine
The Indian government announced a $120 million grant for COVID-19 vaccine research on November 29, 2020 for Mission COVID Suraksha (Safety) to vaccinate the larger population of India and to accelerate the preclinical and clinical development of different vaccine candidates. The government should have also included a very fundamental objective of producing raw materials for the vaccine candidates. The fund should have encouraged the production of raw materials for the vaccines within the country itself, thus standing completely on the slogan of “Atamanirbhar Bharat” (self-reliant India). So far, a total of 12 vaccine candidates have been supported by the Department of Biotechnology (DBT), India [42, 64, 65].
After Pfizer’s COVID-19 vaccine approval, BioNTech received emergency clearance for the mass distribution of the vaccine in the UK and Bahrain on December 2, 2020, via Emergency Use Authorization (EUA) [66, 67]. However, this vaccine needs to be stored at a temperature of – 70 °C, which is not sustainable in India and poses the biggest challenge for India. Serum Institute of India (SII) applied for the EUA of the Covishield vaccine on December 7, 2020, during its phase-3 trials, conducted not just across India but also in the UK and Brazil. One of the significant advantages of this vaccine candidate is that it can be easily stored at temperatures between 2 and 8 °C. It got approval from the UK on December 30, 2020 [68]. On January 2, 2021, the Drugs Controller General of India (DCGI) granted the approval to AZD1222, COVID-19 vaccine developed by the Oxford-AstraZeneca, previously called ChAdOx1, a chimpanzee adenovirus vaccine and currently manufactured by Serum Institute of India as COVISHIELD and indigenously developed BBV152 or COVAXIN by Bharat Biotech for restricted emergency use in the country [69–71]. January 16, 2021 marked the beginning of a nationwide vaccination drive in India, aimed to immunize 3 billion people, including 0.3 billion healthcare and frontline workers, along with 2.7 billion people above 50 years of age and the under-50 population groups with co-morbidities [42]. India launched a campaign called “Vaccine Maitri,”(Vaccine Friendly), a humanitarian and commercial initiative undertaken by the Indian government to provide COVID-19 vaccines to countries across the globe, with our neighbors Maldives and Bhutan becoming the first recipients [72, 73]. The second dose of COVID-19 vaccination started on February 13, 2021 for those recipients who have completed 28 days after getting their 1st dose. Pre-registration facility was provided on Cowin 2.0, and the Arogya Setu app, also on spot registration facility was provided for the beneficiaries [74] (https://www.aarogyasetu.gov.in/). As per Co-Win (Winning over COVID-19) website data, in India so far, among all vaccines available; the most widely used vaccine is Covishield followed by Covaxin and then Corbevax, with very few dose administration of another available vaccine such as Sputnik V and Covovax (https://dashboard.cowin.gov.in/). To accelerate the process, under Ayushman Bharat PMJAY, 10,000 private hospitals and 600 private hospitals under CGHS (central government health scheme) were used for this phase, 529 government hospitals and 761 private hospitals announced to offer the vaccine which the government hospitals provided free of cost, 250 was the MRP in the private hospitals [75, 76]. Thus, Indian government actively participates in preventing COVID-19 disease from India.
Strategies for allocation of COVID-19 vaccines in India
The Indian government announced free COVID-19 vaccination to all citizens on January 16, 2021. However, at the beginning of the epidemic, COVID-19 vaccines were preferentially administered to 30 million healthcare workers, initially having limited resources of manpower and cold chain facilities. Per the National Expert Group on Vaccine Administration for COVID-19 (NEGVAC), committee recommendations gave the priority list of COVID-19 vaccines. It included healthcare workers or frontline workers (~ 3 million) as the first and foremost priority. The second priority in line was given to elderly persons and people with underlying comorbidities, pregnant women, and a large number of medical and paramedical staff.
[42, 77]. Vaccinating healthcare workers is important as they are at high risk of exposure while treating SARS-CoV-2-infected patients. And further, if they get infected, all other healthcare services will be reduced. An initial report from Wuhan hospital (where the initial outbreak happened) showed that among the first 138 patients infected with SARS-CoV2, 40 (29%) were healthcare workers. [78]. This illustrated the significance of vaccinating healthcare workers as a first preference to control epidemics.
Indian COVID-19 vaccines compared to the rest of the world
In India, Covishield and Covaxin constitute a large portion of vaccines administered. India has contributed significantly to the overall COVID-19 vaccine drives globally. India approved their first nasal iNCOVACC vaccine, similar to China [79]. Comparing efficacy, storage conditions, doses, and cost per dose play an essential role in the distribution and administration of vaccines. [80]. Table 3 shows the comparison of the Indian vaccine to other approved and most-used vaccines globally. It showed that mRNA vaccines (both Pfizer and Moderna) require ultra-low temperature storage (− 15 to – 80 °C), so expensive per dose compared to adenovirus-based such as Covishield, or inactivated virus-based such as Covaxin, which require regular 4 °C refrigeration which can be feasible in developing countries [81, 82]. In the USA, bivalent mRNA vaccines were also approved for a booster to deal with the Omicron variant, but no such vaccines are available in India so far [83]. Similarly, mRNA vaccines were also approved for children 6 months and above in the USA, but there was limited success in pediatric vaccine availability in India. Covaxin (BB152) was approved for adolescents (12–17 years old) under the EUA category. For younger kids aged 2–18 years, it is still under phase 2–3 clinical trial. Similarly, Corbevax for 5–17 years and ZyCoV-D (Zydus Cadila) for 12–17 years old are also under phase 3 clinical trial [84].
Table 3.
Comparison of Indian vaccines vs. other vaccines used globally in terms of storage feasibility and cost per dose
| Vaccine type | Manufacturer | Doses administer | Cold storage conditions | Cost per dose |
|---|---|---|---|---|
| BNT16b2 | Pfizer/BioNtech |
30 μg 2 doses 21 days interval |
− 80 °C to − 60 °C for long-term storage or at – 25 °C to – 15 °C for up to 2 weeks Vials prior to use may be stored at 4 °C refrigerator for up to 31 days or 2 h for room temperature |
EU and USA: $19.50 African Union: $6.75 Brazil: $10 Colombia: $12 |
| mRNA-1273 | Moderna |
100 μg 2 doses 28 days interval |
Long-term storage in frozen suspension stored between − 50 and – 15 °C. For short-term unopened vial storage at 4 °C refrigerator for up to 30 days. Room temperature for up to 24 h |
EU: $25.5 USA: $15 Argentina: $21.5 Botswana: $28.8 |
| AZD1222 ChAdOx1 nCoV-19 vaccine | AstraZeneca/University of Oxford |
5 × 1010 viral particles (standard dose) 2 doses 4- to 12-week interval |
Do not freeze. The unopened vial can be stored for up to 6 months. Short-term storage at 4 °C refrigerator after opening in a refrigerator for no more than 48 h. Storage at room temperature up to 6 h |
$2.15 in the EU $4–6 elsewhere |
| Ad26.COV2.S | Johnson & Johnson |
5 × 1010 viral particles A single dose |
Should be protected from light. Supplied as a liquid suspension Unopened vial can be stored at in 4 °C refrigerator until the expiration date or at room temperature for up to 12 h. After the first dose has been withdrawn, the vial is held between 4 °C refrigerator for up to 6 h or at room temperature for up to 2 h | < $10 |
| Gam-COVID-Vax Sputnik V | Gamaleya Research Institute |
1011 viral particles per dose for each recombinant adenovirus 0.5 mL/dose 2 doses 21 days interval |
Transport: two forms: lyophilized or frozen storage in 4 °C refrigerator | < $10 |
| NVX-CoV2373 | Novavax |
5 μg protein and 50 ug matrix-M adjuvant 2 doses 21 days interval |
Shipped in a ready-to-use liquid formulation storage in 4 °C refrigerator | $20.9 for Denmark COVAX: $3 |
| Convidecia™ Ad5-nCoV | CanSino |
1010 viral particles per 0.5 mL in a vial Single dose |
Do not freeze. Supplied as a vial of 0.5 mL. Storage in 4 °C refrigerator | Pakistan private market: $27.2 |
| CoronaVac | Sinovac Biotech |
3 μg 0.5 mL per dose 2 doses 28 days interval |
Supplied as a vial or syringe of 0.5 mL. Do not freeze. Protect from light Storage and transport in 4 °C refrigerator. Shake well before use Shelf-life: 12 months | China: $29.75 Ukraine: $18 Philippines: $14.5 Brazil: $10.3 Cambodia: $10 |
| BBIBP-COrV | Sinopharm/Beijing Institute of Biological Products |
4 μg 0.5 mL per dose Intramuscularly 2 doses 21- to 28-day interval |
Supplied as a pre-filled syringe or vial. Cannot be frozen. Protect from light. Store and transport in 4 °C refrigerator | Argentina, Mongolia: $15 Senegal: $18.6 China: $30 Hungary: $36 |
| Covaxin | Bharat Biotech |
6 μg single dose: 0.5 mL 10-dose or 20-dose vial Intramuscularly 2 doses 28 days interval |
Supplied as a single dose or multidose vial. Do not freeze. Stored in 4 °C refrigerator |
India: $3–5 Brazil: $15 Botswana: $16 |
Conclusion
Most studies are currently available delineating vaccine status in the USA and Europe, with limited data available on developing countries like India. Limited scientific literature is available describing Indian contribution to vaccine availability, clinical trials, and vaccine during the COVID-19 pandemic duration. In order to find recent available literature on Indian vaccine research and current vaccine availability, we did a PubMed search until November 2022 based on the query “(Indian vaccine) OR (Vaccine trials in Indian vaccine) OR (Contribution of Indian vaccine) AND (Indian vaccine Immune response).” We did not keep a filter for our search by either language or any type of publication. We found many studies primarily illustrating Covishield, Covaxin, Sputnik, and nasal vaccine immune response and their clinical trial information. None of the studies have evaluated updated vaccine trials with all available 12 vaccines and 16 ongoing clinical trials. As a result of all efforts, in November 2022, India became the second country after China to cover 2.1 billion COVID-19 vaccination doses, strengthening our global fight against COVID-19. To our knowledge, this is the first review that describes vaccination in India in detail, including strategy, methods used, production, and distribution in India. While most vaccine studies have been reported for mRNA vaccines such as Pfizer-BioNTech and Moderna and mostly USA or Europe based, it is important to understand vaccine resource availability in developing countries like India. This review also emphasizes the importance of diverse vaccine availability in the current SARS-CoV2 newly emerging variants and developing mutations worldwide. Figure 1 in this review highlights the proportion of usage of various types of available COVID-19 vaccines. While Figs. 2 and 3 depict laboratory method and steps involved in developing Covaxin and Covishield, respectively. Tables in this review summarize 12 vaccine availability and 16 ongoing clinical trials. The positive side of demonstrating current and future scientific ongoing contributions in this review is to develop mental peace and trust in medical research to deal with vaccine hesitancy. This is more relevant in a country like India, which is home to diverse socio-economic and cultural variations. More and more data available in the public domain related to vaccine efficacy, immunogenicity, and safety data will increase reliability in mass-scale vaccination and herd immunity.
Current challenges in vaccine success
COVID-19 is an ongoing pandemic that has resulted in global health concerns, economic burdens, and social life disturbance. The vaccination campaign is the first and foremost successful method to counteract the COVID-19 pandemic. Moreover, sufficient vaccination coverage is conditioned by the people’s acceptance of these vaccines. All vaccines reviewed here are based on ancestral SARS-CoV2 (Wuhan strain) spike. However, the newly emerging SARS-CoV2 variant with increased transmissibility and escaping from their neutralization poses a serious concern for achieving herd immunity. Thus, despite vaccine availability, constant efforts are needed to get boosters and development of more effective strategies to design the COVID-19 vaccine.
Author contribution
Conceptualization, S.L.G, S.G., R.K.J, and P.S.; writing original draft preparation, S.L.G., S.G., R.K.J, and P.S; writing review and editing, S.L.G, S.G., R.K.J, P.S., A.A, N.N., and P.K; supervision, R.K.J. and P.S. All authors have read and agreed to the published version of the manuscript.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any authors.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sneh Lata Gupta and Surbhi Goswami shared first author.
Contributor Information
Priyanka Sharma, Email: priyankajnu89@gmail.com.
Rishi K. Jaiswal, Email: rishijai24@gmail.com
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta bio-medica : Atenei Parmensis. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohrabi C, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg (London, England) 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu B, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carneiro DC, Sousa JD, Monteiro-Cunha JP. The COVID-19 vaccine development: a pandemic paradigm. Virus Res. 2021;301:198454. doi: 10.1016/j.virusres.2021.198454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews MA, et al. First confirmed case of COVID-19 infection in India: a case report. Indian J Med Res. 2020;151(5):490–492. doi: 10.4103/ijmr.IJMR_2131_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu F, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das S, et al. Immunogenic and reactogenic efficacy of Covaxin and Covishield: a comparative review. Immunol Res. 2022;70(3):289–315. doi: 10.1007/s12026-022-09265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ella R, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet (London, England) 2021;398(10317):2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ella R, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royal A, et al. An altmetric analysis of online news on India's first indigenous COVID-19 vaccine. J Ed Health Promotion. 2021;10:348–348. doi: 10.4103/jehp.jehp_1603_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Flores D, et al. SARS-CoV-2 vaccines based on the spike glycoprotein and implications of new viral variants. Front Immunol. 2021;12:701501. [DOI] [PMC free article] [PubMed]
- 13.Liu C, et al. The architecture of inactivated SARS-CoV-2 with postfusion spikes revealed by Cryo-EM and Cryo-ET. Structure (London, England : 1993), 2020;28(11):1218–1224.e4. [DOI] [PMC free article] [PubMed]
- 14.de Castro Barbosa E, et al. Influence of SARS-CoV-2 inactivation by different chemical reagents on the humoral response evaluated in a murine model. Mol Immunol. 2022;147:199–208. doi: 10.1016/j.molimm.2022.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke Z, et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020;588(7838):498–502. doi: 10.1038/s41586-020-2665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang N, et al. A perspective on the roles of adjuvants in developing highly potent COVID-19 vaccines. Viruses. 2022;14(2):387. doi: 10.3390/v14020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Z, et al. Adjuvants for coronavirus vaccines. Front Immunol. 2020;11:589833. [DOI] [PMC free article] [PubMed]
- 18.Ella R, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398(10317):2173–84. [DOI] [PMC free article] [PubMed]
- 19.Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldblatt D, et al. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol Rev. 2022;310(1):6–26. doi: 10.1111/imr.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta SL, Jaiswal RK. Relevant of neutralizing antibody during SARS-CoV-2 infection and their therapeutic usage. Mol Biol Rep. 2022;49(10):10137–10140. doi: 10.1007/s11033-022-07493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeyanathan M, et al. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta SL, Jaiswal RK. Neutralizing antibody: a savior in the Covid-19 disease. Mol Biol Rep. 2022;49(3):2465–2474. doi: 10.1007/s11033-021-07020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dotiwala F, Upadhyay AK. A comprehensive review of BBV152 vaccine development, effectiveness, safety, challenges, and prospects. Front Immunol. 2022;13:940715. [DOI] [PMC free article] [PubMed]
- 25.Gupta SL, et al. Loss of Pfizer (BNT162b2) Vaccine-induced antibody responses against the SARS-CoV-2 Omicron variant in adolescents and adults. J Virol. 2022;96(17):e00582–e622. doi: 10.1128/jvi.00582-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vadrevu KM, et al. Persistence of immunity and impact of a third (booster) dose of an inactivated SARS-CoV-2 vaccine, BBV152; a phase 2, double-blind, randomised controlled trial. medRxiv. 2022;2022.01.05.22268777.
- 27.Vadrevu KM, et al. Immunogenicity and reactogenicity of an inactivated SARS-CoV-2 vaccine (BBV152) in children aged 2–18 years: interim data from an open-label, non-randomised, age de-escalation phase 2/3 study. Lancet Infect Dis. 2022;22(9):1303–1312. doi: 10.1016/S1473-3099(22)00307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vadrevu KM, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine (BBV152) in children from 2 to 18 years of age: an open-label, age-de-escalation phase 2/3 study. medRxiv. 2021;2021.12.28.21268468.
- 29.Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet (London, England) 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doerfler W. Adenoviral vector DNA- and SARS-CoV-2 mRNA-based Covid-19 vaccines: possible integration into the human genome - are adenoviral genes expressed in vector-based vaccines? Virus Res. 2021;302:198466–198466. doi: 10.1016/j.virusres.2021.198466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atasheva S, Shayakhmetov DM. Adenovirus sensing by the immune system. Curr Opin Virol. 2016;21:109–113. doi: 10.1016/j.coviro.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greber UF, Flatt JW. Adenovirus entry: from infection to immunity. Annual Review of Virology. 2019;6(1):177–197. doi: 10.1146/annurev-virology-092818-015550. [DOI] [PubMed] [Google Scholar]
- 34.Voysey M, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. The Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet (London, England) 2020;396(10262):1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health, O, Interim recommendations for use of the ChAdOx1-S [recombinant] vaccine against COVID-19 (AstraZeneca COVID-19 vaccine AZD1222 Vaxzevria™, SII COVISHIELD™): interim guidance, first issued 10 February 2021, updated 21 April 2021, last updated 30 July 2021;2021, World Health Organization: Geneva.
- 37.Emary KRW, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. The Lancet. 2021;397(10282):1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hillus D, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9(11):1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Callaway E. Russia's fast-track coronavirus vaccine draws outrage over safety. Nature. 2020;584(7821):334–335. doi: 10.1038/d41586-020-02386-2. [DOI] [PubMed] [Google Scholar]
- 40.Logunov DY, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. The Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cazzola M, et al. Controversy surrounding the Sputnik V vaccine. Respir Med. 2021;187:106569–106569. doi: 10.1016/j.rmed.2021.106569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar VM, et al. Strategy for COVID-19 vaccination in India: the country with thesecond highest population and number of cases. npj Vaccines. 2021;6(1):60. [DOI] [PMC free article] [PubMed]
- 43.Dolzhikova IV, et al. One-shot immunization with Sputnik Light (the first component of Sputnik V vaccine) is effective against SARS-CoV-2 Delta variant: efficacy data on the use of the vaccine in civil circulation in Moscow. medRxiv. 2021;202110.08.21264715.
- 44.Sukhikh GT, et al. Sputnik Light and Sputnik V vaccination is effective at protecting medical personnel from COVID-19 during the period of Delta variant dominance. Vaccines. 2022;10(11):1804. doi: 10.3390/vaccines10111804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, et al. Immune evasive effects of SARS-CoV-2 variants to COVID-19 emergency used vaccines. Front Immunol. 2021;12:771242–771242. doi: 10.3389/fimmu.2021.771242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Momin T, et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine. 2021;38:101020. doi: 10.1016/j.eclinm.2021.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khobragade A, et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet. 2022;399(10332):1313–1321. doi: 10.1016/S0140-6736(22)00151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kudlay D, Svistunov A. COVID-19 vaccines: an overview of different platforms. Bioengineering (Basel, Switzerland) 2022;9(2):72. doi: 10.3390/bioengineering9020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heath PT, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thuluva S, et al. Evaluation of safety and immunogenicity of receptor-binding domain-based COVID-19 vaccine (Corbevax) to select the optimum formulation in open-label, multicentre, and randomised phase-1/2 and phase-2 clinical trials. EBioMedicine. 2022;83:104217. [DOI] [PMC free article] [PubMed]
- 51.Marchese AM, Beyhaghi H, Orenstein WA. With established safe and effective use, protein vaccines offer another choice against COVID-19. Vaccine. 2022;40(46):6567–6569. doi: 10.1016/j.vaccine.2022.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thuluva S, et al. Immunogenic superiority and safety of Biological E’s CORBEVAX™ vaccine compared to COVISHIELD™ (ChAdOx1 nCoV-19) vaccine studied in a phase III, single blind, multicenter, randomized clinical trial. medRxiv. 2022;2022.03.20.22271891.
- 53.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tukhvatulin AI, et al. An open, non-randomised, phase 1/2 trial on the safety, tolerability, and immunogenicity of single-dose vaccine “Sputnik Light” for prevention of coronavirus infection in healthy adults. Lancet Regional Health – Eur. 2021;11:100241. [DOI] [PMC free article] [PubMed]
- 55.Chavda VP, et al. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discovery Today. 2021;26(11):2619–2636. doi: 10.1016/j.drudis.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alu A, et al. Intranasal COVID-19 vaccines: from bench to bed. EBioMedicine. 2022;76:103841–103841. doi: 10.1016/j.ebiom.2022.103841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc Natl Acad Sci USA. 2021;118(29):e2102775118. doi: 10.1073/pnas.2102775118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliver SE, et al. Use of the Janssen (Johnson & Johnson) COVID-19 vaccine: updated interim recommendations from the Advisory Committee on Immunization Practices - United States, December 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):90–95. doi: 10.15585/mmwr.mm7103a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hammerschmidt SI, et al. Robust induction of neutralizing antibodies against the SARS-CoV-2 Delta variant after homologous Spikevax or heterologous Vaxzevria-Spikevax vaccination. Eur J Immunol. 2022;52(2):356–359. doi: 10.1002/eji.202149645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flaxman A, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) The Lancet. 2021;398(10304):981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kunal S, et al. Mix and match COVID-19 vaccines: potential benefit and perspective from India. Postgrad Med J. 2022;98(e2):e99–e101. doi: 10.1136/postgradmedj-2021-140648. [DOI] [PubMed] [Google Scholar]
- 62.Jaggaiahgari S, et al. Heterologous booster dose with CORBEVAX following primary vaccination with COVISHIELD enhances protection against SARS-CoV-2. Vaccines (Basel). 2022;10(12):2146. [DOI] [PMC free article] [PubMed]
- 63.Rose W, et al. Immunogenicity and safety of homologous and heterologous booster vaccination of ChAdOx1 nCoV-19 (COVISHIELD™) and BBV152 (COVAXIN®): a non-inferiority phase 4, participant and observer-blinded, randomised study. Lancet Regional Health - Southeast Asia. [DOI] [PMC free article] [PubMed]
- 64.Goel I, Sharma S, Kashiramka S. Effects of the COVID-19 pandemic in India: an analysis of policy and technological interventions. Health Policy Technol. 2021;10(1):151–164. doi: 10.1016/j.hlpt.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghosh A, Nundy S, Mallick TK. How India is dealing with COVID-19 pandemic. Sensors Int. 2020;1:100021–100021. doi: 10.1016/j.sintl.2020.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fortner A, Schumacher D. First COVID-19 vaccines receiving the US FDA and EMA emergency use authorization. Discoveries (Craiova, Romania) 2021;9(1):e122–e122. doi: 10.15190/d.2021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kashte S, et al. COVID-19 vaccines: rapid development, implications, challenges and future prospects. Hum Cell. 2021;34(3):711–733. doi: 10.1007/s13577-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joshi G, et al. Exploring the COVID-19 vaccine candidates against SARS-CoV-2 and its variants: where do we stand and where do we go? Hum Vaccin Immunother. 2021;17(12):4714–4740. doi: 10.1080/21645515.2021.1995283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar VM, et al. Strategy for COVID-19 vaccination in India: the country with the second highest population and number of cases. NPJ vaccines. 2021;6(1):60–60. doi: 10.1038/s41541-021-00327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chavda VP, et al. The vaccine world of COVID-19: India’s contribution. Vaccines. 2022;10(11):1943. doi: 10.3390/vaccines10111943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharun K, and Dhama K. India’s role in COVID-19 vaccine diplomacy. J Travel Med. 2021;28(7). [DOI] [PMC free article] [PubMed]
- 72.Singh B, et al. India’s neighbourhood vaccine diplomacy during COVID-19 pandemic: humanitarian and geopolitical perspectives. J Asian African Stud. 0(0): 00219096221079310.
- 73.Ariyawardana N. India’s vaccine diplomacy and changing geopolitics in the global south. J Soc Sci Human Rev. 2022;7.
- 74.Singh K, Verma A, Lakshminarayan M. India’s efforts to achieve 1.5 billion COVID-19 vaccinations: a narrative review. Osong Public Pealth Res Perspect. 2022;13(5):316–327. doi: 10.24171/j.phrp.2022.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joseph J, H. Sankar D, and D. Nambiar, Empanelment of health care facilities under Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (AB PM-JAY) in India. PloS one. 2021;16(5):0251814–0251814. doi: 10.1371/journal.pone.0251814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sriee GV, V.P. and G.R. Maiya. Coverage, utilization, and impact of Ayushman Bharat scheme among the rural field practice area of Saveetha Medical College and Hospital, Chennai. J Family Med Primary Care. 2021;10(3):1171–1176. doi: 10.4103/jfmpc.jfmpc_1789_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Surendranath M, et al. A modern perspective on vaccinating healthcare service providers in India: a narrative review. Infect Dis Ther. 2022;11(1):81–99. doi: 10.1007/s40121-021-00558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koh D. Occupational risks for COVID-19 infection. Occup Med. 2020;70(1):3–5. doi: 10.1093/occmed/kqaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waltz E. China and India approve nasal COVID vaccines - are they a game changer? Nature. 2022;609(7927):450. doi: 10.1038/d41586-022-02851-0. [DOI] [PubMed] [Google Scholar]
- 80.Fiolet T, et al. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202–221. doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uddin MN, Roni MA. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines. 2021;9(9):1033. doi: 10.3390/vaccines9091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crommelin DJA, et al. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. 2021;110(3):997–1001. doi: 10.1016/j.xphs.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta SL, Jaiswal RK. An assessment of the bivalent vaccine as a second booster for COVID-19. Vaccines. 2023;11(1):79. doi: 10.3390/vaccines11010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta SL, et al. Children’s SARS-CoV-2 infection and their vaccination. Vaccines. 2023;11(2):418. doi: 10.3390/vaccines11020418. [DOI] [PMC free article] [PubMed] [Google Scholar]