Keywords: Cystic echinococcosis, donkeys, eastern Africa, Echinococcus equinus, Echinococcus granulosus sensu lato
Abstract
Cystic echinococcosis (CE) is endemic in humans and domestic animals in eastern Africa. All the species of the Echinococcus granulosus sensu lato complex have been reported in this region except for E. equinus, possibly due to the small number of studies involving equids. This study reports the frequency of different Echinococcus species in donkeys from eastern Africa. A total of 5961 donkeys were examined during meat inspection in 3 slaughterhouses in Kenya. Identification of Echinococcus spp. was achieved through polymerase chain reaction-restriction fragment-length polymorphism and sequencing of the mitochondrial nicotinamide adenine dinucleotide (NADH) dehydrogenase subunit 1 gene. The prevalence of CE was 5.7% (337/5961). The 263 genotyped cysts belonged to E. equinus (n = 163), E. granulosus sensu stricto (n = 70), E. canadensis (G6/7) (n = 26) and E. ortleppi (n = 4). One donkey harboured a metacestode of Spirometra theileri. All E. equinus cases, except 2, originated from southern Ethiopia, whereas the other species were more evenly distributed across the study area. Most of the cysts belonging to E. equinus were fertile (111/163), while those of the other species were non-fertile. This is the first report of Echinococcus spp. in donkeys from sub-Saharan Africa and the first confirmation of E. equinus in East Africa. The frequent fertility of E. equinus cysts in donkeys affirms their suitability as intermediate hosts of this species, while low frequency and cyst fertility suggest a marginal role of donkeys in the transmission of E. granulosus s. s., E. canadensis (G6/7) and E. ortleppi.
Introduction
Cystic echinococcosis (CE) is a zoonotic disease caused by metacestodes of the cestode Echinococcus granulosus sensu lato (s. l.). The World Health Organization (WHO) has listed echinococcosis among 20 neglected diseases targeted for control or elimination by 2050 (WHO, 2012). Although CE has a worldwide distribution, it has major public health and economic impact in areas of extensive livestock keeping (Deplazes et al., 2017). The lifecycles of the various species within E. granulosus s. l. involve mainly canids as definitive hosts, and a range of herbivorous or omnivorous intermediate hosts in which the metacestode develops (Thompson, 2017).
The E. granulosus s. l. complex includes at least 5 cryptic species and some distinct genotypes (G), namely E. granulosus sensu stricto (s. s.) (G1, G3, GOmo), E. equinus (G4), E. ortleppi (G5), E. canadensis (G6–8, G10) and E. felidis (Nakao et al., 2013a, 2013b; Wassermann et al., 2016; Vuitton et al., 2020). Epidemiological studies in eastern Africa have reported all these taxa except for E. canadensis G8 and G10 (which are wildlife parasites from the northern Holarctic) and E. equinus. The latter mainly infects intermediate hosts of the horse family (Equidae), is rarely zoonotic and occurs around the globe mainly in domestic lifecycles involving dogs and horses or donkeys (Romig et al., 2017).
In Africa, E. equinus is known to infect donkeys north of the Sahara at considerable frequency (Azlaf and Dakkak, 2006; Haridy et al., 2008; Taha, 2012; Aboelhadid et al., 2013; Boufana et al., 2014; Lahmar et al., 2014; Mahdy et al., 2014a, 2014b; Barghash et al., 2017; Desouky et al., 2017), and in southern Africa, it has been reported from horses, zebras and rhinos (Kumaratilake et al., 1986; Wassermann et al., 2015; Romig et al., 2017; Zaffarano et al., 2021). However, there are no records from the west, central and east of the continent. At least for eastern Africa, the absence of E. equinus can be explained by sampling bias. While numerous studies in the last decade contributed to our understanding of CE epidemiology, mainly in Kenya, by screening livestock, wildlife and humans (Kagendo et al., 2014; Mbaya et al., 2014; Addy et al., 2017a; Romig et al., 2017; Mulinge et al., 2018; Odongo et al., 2018; Kere et al., 2019; Nungari et al., 2019; Omondi et al., 2020), none of these surveys involved donkeys, horses or zebras. This was mainly due to the absence of slaughter facilities for donkeys, as they were not slaughtered for human consumption even though the government of Kenya had gazetted donkeys as food animals in 1999 (Legal Act Notice No146, 1999). Only recently, several export slaughterhouses for donkeys were licensed to operate in Kenya, which provided a research opportunity to close the gap of knowledge on the presence, frequency and causative species of CE in donkeys and to determine their role in the transmission cycles.
Materials and methods
Study sites
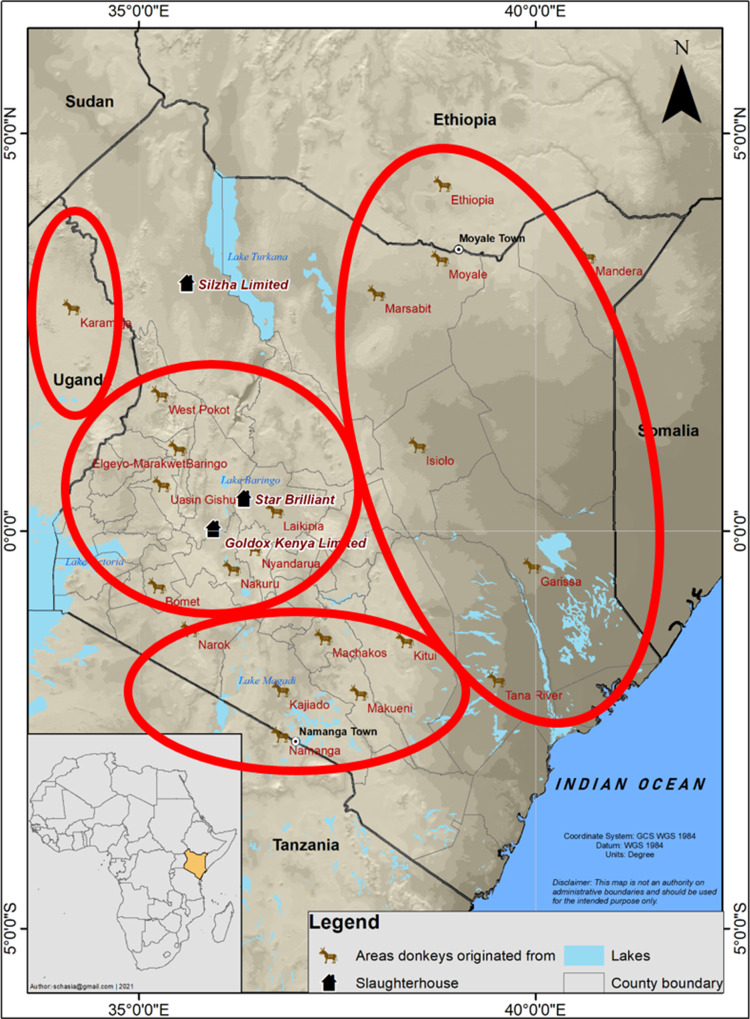
The study was done in 3 private-owned slaughterhouses, 1 in Turkana County (Lodwar: Silzha Ltd.) and 2 in central Kenya (Naivasha: Star Brilliant Ltd. and Mogotio: Goldox Kenya Ltd.) (Fig. 1). The origin of the donkeys was identified to county level in Kenya; donkeys from Uganda were from the Karamoja area, donkeys from Tanzania were from the Maasai area in northern Tanzania, and donkeys from Ethiopia entering Kenya through the border town of Moyale originated from the adjacent Borena zone of southern Ethiopia (Fig. 1).
Fig. 1.
A map of eastern Africa showing the location of the 3 slaughterhouse, the origin of the donkeys in Kenya and the neighbouring countries, and the 4 sub-regions listed in Table 2.
Collection of CE cysts and microscopic examination
The study was done at 2 intervals; the first between January and September 2017 which involved all the 3 slaughterhouses (3197 donkeys). The second phase was carried out only in Naivasha slaughterhouse between February and August 2019 (2764 donkeys). Carcasses were examined for cysts by palpation and subsequent incision of the major organs. The cysts/lesions were excised in toto, cleaned and stored individually in 70% ethanol. The cysts were examined microscopically for the presence of protoscoleces, and with sterile cysts (not containing protoscoleces), the integrity of the germinal layer was visually assessed to discriminate between viable and degenerated cysts.
DNA extraction
Cyst material or a single protoscolex were lysed in 0.02 M NaOH at 99°C for 10 min (Nakao et al., 2003). The lysate was used as template immediately following polymerase chain reaction (PCR). Cyst samples that failed to yield a PCR product following the lysis procedure were subjected to DNA extraction using DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
Polymerase chain reaction-restriction fragment-length polymorphism, PCR purification and sequencing
Two nested PCR assays based on NADH dehydrogenase subunit 1 gene (nad1) were used for genotyping of cysts depending on their conditions. The first PCR targeted the entire nad1 gene (894 bp) and was carried out according to Hüttner et al. (2008). The second PCR amplified part of the nad1 gene (550–552 bp) as described by Mulinge et al. (2018) and was carried out on samples that failed to yield an amplicon with the first PCR. In both PCR assays, the reaction mixture contained 2 μL of the DNA, 1 × DreamTaq Green Buffer [20 mm Tris-HCl (pH 8.0), 1 mm Dithiothreitol (DTT), 0.1 mm Ethylenediamine tetraacetic acid (EDTA), 100 mm KCl, 0.5% (v/v) Nonidet P40, 0.5% (v/v) Tween 20] (Thermo Scientific, Waltham, MA, USA), 0.2 mm deoxynucleotide triphosphate (New England Biolabs, Ipswich, MA, USA), 0.25 μm of forward and reverse primers each, 2 mm MgCl2 and 0.625 units of DreamTaq Green DNA Polymerase (Thermo Scientific) in 25 μL final volume. The PCR cycling conditions were 5 min for initial denaturation at 94°C, 40 cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 60 s, and a final extension at 72°C for 5 min (Hüttner et al., 2008).
The nad1 PCR products were digested using the restriction enzyme, HphI (New England Biolabs), either the entire or partial nad1 gene (Hüttner et al., 2009; Mulinge et al., 2018). The total reaction mixture was 20 μL including 7.5 μL nuclease-free water, 2.0 μL of 10 × CutSmart buffer, 0.5 μL HphI (5 units) and 10 μL PCR product. The restriction digests were incubated overnight at 37°C and separated on 3% agarose gel alongside positive controls for E. granulosus s. s., E. equinus, E. ortleppi, E. canadensis (G6/7) and E. felidis (Hüttner et al., 2009). In addition, for confirmation purposes and in case of ambiguous banding patterns, PCR products were purified using QIAquick PCR purification kit (Qiagen) following the manufacturer's guidelines. The purified amplicons were sent to Macrogen Europe BV (Amsterdam, the Netherlands) for sequencing using the nested reverse primer. The sequences were identified by comparing with those available in the National Centre for Biotechnology Information database (NCBI) using the basic local alignment search tool (http://www.ncbi.nlm.nih.gov/BLAST/) (Altschul et al., 1997).
Results
Cyst condition and organ location of Echinococcus spp.
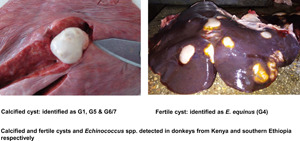
Out of 338 donkeys, 528 cystic structures were collected. Of these, 263 were characterized as Echinococcus spp. and 1 as a metacestode of Spirometra theileri. The remaining cysts either did not yield sequences or banding patterns of sufficient quality, or were excluded from molecular examination due to advanced degeneration with low probability of amplification success. Four E. granulosus s. l. species were detected: E. equinus (n = 163), E. granulosus s. s. (n = 70), E. canadensis (G6/7) (n = 26) and E. ortleppi (n = 4) (Tables 1 and 2). Details of representative sequences obtained in this study are available on GenBank under accession numbers OK489943–OK489955 (Table 3). Details of sequences from GenBank that were identical to those reported in this study are shown in Table 3. Fertility of E. equinus cysts was high (111/163), but low for the other species: 7/70 for E. granulosus s. s., 1/4 for E. ortleppi and 3/26 for E. canadensis (G6/7). The most frequently infected organs were the liver for E. equinus (115/163) and E. canadensis (G6/7) (15/26) and the lungs for E. granulosus s. s. (43/70), while 2/4 E. ortleppi cysts occurred in the lungs and the kidneys each (Table 1).
Table 1.
Cyst condition and organ location of Echinococcus spp. from donkeys in eastern Africa
| E. granulosus s. s. | E. ortleppi | E. canadensis (G6/7) | E. equinus | |
|---|---|---|---|---|
| Fertile | ||||
| Liver | 0 | 0 | 3 | 83 |
| Lungs | 7 | 1 | 0 | 28 |
| Total | 7 | 1 | 3 | 111 |
| Sterile (viable) | ||||
| Liver | 1 | 0 | 0 | 0 |
| Lungs | 3 | 0 | 0 | 1 |
| Kidney | 1 | 1 | 0 | 0 |
| Total | 5 | 1 | 0 | 1 |
| Non-viable (caseated/calcified) | ||||
| Liver | 24 | 0 | 12 | 31 |
| Lungs | 33 | 1 | 10 | 20 |
| Kidney | 1 | 1 | 1 | 0 |
| Total | 58 | 2 | 23 | 51 |
| All conditions | ||||
| Liver | 25 | 0 | 15 | 115 |
| Lungs | 43 | 2 | 10 | 48 |
| Kidney | 2 | 2 | 1 | 0 |
| Total | 70 | 4 | 26 | 163 |
Table 2.
Geographical origin and prevalence of Echinococcus spp. from donkeys in eastern Africa
| Origin | N examined | N infected (prevalence) | N cysts genotyped | Echinococcus spp. (N cysts) |
|---|---|---|---|---|
| 1. North/East Kenya, southern Ethiopia | ||||
| S-Ethiopia (Borena) | 2363 | 124 (5.2%) | 191 | EG (23), EC (8), EE (160) |
| Mandera | 75 | 0 (0.0%) | 0 | |
| Marsabit | 93 | 11 (11.8%)a | 3 | EC (3) |
| Isiolo | 42 | 2 (4.8%)a | 0 | |
| Garissa | 102 | 8 (7.8%) | 3 | EO (2), EE (1) |
| Tana River | 32 | 0 (0.0%)a | 0 | |
| Sub-total | 2707 | 145 (5.4%)a | 197 | EG (23), EC (11), EO (2), EE (161) |
| 2. Northeast Uganda | ||||
| Karamoja | 70 | 11 (15.7%)a | 2 | EC (2) |
| Sub-total | 70 | 11 (15.7%)a | 2 | EC (2) |
| 3. Central/West Kenya | ||||
| West Pokot | 30 | 0 (0.0%)a | 0 | |
| Elgeyo Marakwet | 102 | 0 (0.0%)a | 0 | |
| Baringo | 23 | 0 (0.0%)a | 0 | |
| Uasin Gishu | 14 | 0 (0.0%)a | 0 | |
| Laikipia | 58 | 4 (6.9%)a | 1 | EG (1) |
| Nyandarua | 24 | 0 (0.0%)a | 0 | |
| Nakuru | 93 | 6 (6.5%)a | 1 | EG (1) |
| Bomet | 419 | 1 (0.2%)a | 0 | |
| Sub-total | 763 | 11 (1.4%)a | 2 | EG (2) |
| 4. South Kenya/northern Tanzania | ||||
| Kitui | 95 | 2 (2.1%)a | 0 | |
| Machakos | 30 | 0 (0.0%)a | 0 | |
| Narok | 839 | 17 (2.0%)a | 6 | EG (5), EC (1) |
| Kajiado/Tanzania | 810 | 13 (1.6%) | 3 | EG (1), EO (1), EE (1) |
| Makueni | 33 | 0 (0.0%) | 0 | |
| Sub-total | 1807 | 32 (1.8%)a | 9 | EG (6), EC (1), EO (1), EE (1) |
| Grand totalb | 5961 | 337 (5.7%) | 263 | EG (70), EC (26), EO (4), EE (163) |
EG, E. granulosus s. s.; EO, E. ortleppi; EC, E. canadensis G6/7; EE, E. equinus.
Possible prevalence underestimate, as some positive animals were not included for which the information on the geographical origin was lost during carcass processing.
Figures include donkeys of unknown origin (within the study region) and positive animals whose data on origin were lost during carcass processing.
Table 3.
Echinococcus granulosus sensu lato representative nad1 sequences from donkeys in eastern Africa and details of identical sequences in the GenBank
| Isolate | Source | Species | Accession number | Identity sequence accession number | Percentage identity (%) | Length of nad1 gene sequence (bp) | Reference | Host | Country |
|---|---|---|---|---|---|---|---|---|---|
| NSD 001 | Donkey | Echinococcus granulosus sensu stricto | OK489943 | MN199128a | 100 | 483 | Ohiolei et al. (2019a) | Camel | Nigeria |
| NSD 024 | Donkey | Echinococcus granulosus sensu stricto | OK489945 | MN199128a | 99.79 | 483 | Ohiolei et al. (2019a) | Camel | Nigeria |
| DMT 077 | Donkey | Echinococcus granulosus sensu stricto | OK489946 | MG672196a | 100 | 483 | Kinkar et al. (2018) | Sheep | Turkey |
| NSD 006 | Donkey | Echinococcus granulosus sensu stricto | OK489944 | MG672196a | 99.79 | 483 | Kinkar et al. (2018) | Sheep | Turkey |
| NSD 228 | Donkey | Echinococcus granulosus sensu stricto | OK489947 | MN269987a | 99.79 | 894 | Ohiolei et al. (2019b) | Sheep | China |
| DMT 085 | Donkey | Echinococcus equinus | OK489948 | KP161212 | 100 | 483 | Wassermann et al. (2015) | Zebra | Namibia |
| NSD 023 | Donkey | Echinococcus ortleppi | OK489949 | KX010904a | 100 | 483 | Addy et al. (2017a) | Cattle | Kenya |
| DMT 086 | Donkey | Echinococcus ortleppi | OK489950 | KX010904a | 99.79 | 483 | Addy et al. (2017a) | Cattle | Kenya |
| NSD 195 | Donkey | Echinococcus ortleppi | OK489951 | KU842044 | 100 | 894 | Addy et al. (2017a) | Cattle | Ethiopia |
| NSD 053 | Donkey | Echinococcus canadensis (G6/7) | OK489952 | MT525967a | 100 | 483 | Omondi et al. (2020) | Camel | Kenya |
| NSD 256 | Donkey | Echinococcus canadensis (G6/7) | OK489954 | KX010875a | 100 | 894 | Addy et al. (2017b) | Camel | Kenya |
| NSD 033 | Donkey | Spirometra theileri | OK489955 | MN244299 | 100 | 332 | Eom et al. (2019) | Leopard | Tanzania |
Representative reference sequence with closest geographic origin, others with same percentage identity exist.
Prevalence and geographical distribution of Echinococcus spp.
In total, 5961 donkeys were examined, of which 337 (5.7%) were infected with CE and 1 with a metacestode of S. theileri. The geographical origin of the donkeys is presented in Table 2 at regional and county level. For 614 donkeys, the origin was unknown from the onset. In addition, from those with known origin, in case of 77 of the 337 positive animals, this information was lost during carcass processing. These positive records were omitted from Table 2 (except for the grand total), resulting for some counties in prevalence underestimates (see footnote ‘a’ in Table 2). Generally, CE prevalence estimates were highest for animals from Karamoja (Uganda), northeastern Kenya and southern Ethiopia, and low for animals from central, western and southern Kenya and northern Tanzania. Geographical structuring was obvious for E. equinus, where all except 2 infected animals originated from southern Ethiopia. The other Echinococcus spp. were widely spread across the study area; all of the (few) characterized cyst from central Kenya were E. granulosus s. s., all cysts from Karamoja were E. canadensis (G6/7) (Table 2). The donkey infected with S. theileri originated from Kajiado county, southern Kenya.
Discussion
This study reports for the first time the presence and prevalence of CE in donkeys, and any member of the horse family, in eastern Africa. Data on CE in donkeys are few worldwide, e.g. from Turkey, Italy and Israel (Abo-Shehada, 1988; Mukbel et al., 2000; Thompson and McManus, 2002; Oge et al., 2004; Varcasia et al., 2008; Simsek et al., 2015). In Africa, such reports are restricted to the North (Egypt, Tunisia and Morocco), while no data exist from sub-Saharan Africa despite the ubiquity of donkeys in many countries (Pandey, 1980; Azlaf and Dakkak, 2006; Haridy et al., 2008; Taha, 2012; Aboelhadid et al., 2013; Boufana et al., 2014; Lahmar et al., 2014; Mahdy et al., 2014a, 2014b; Barghash et al., 2017; Desouky et al., 2017). The prevalence of donkey CE found in this study (5.7%) was close to that reported from northern Africa with 4.2% in Morocco (Pandey, 1980), 8.5% in Tunisia (Lahmar et al., 2014) and 6.9–14.2% in Egypt (Haridy et al., 2008; Aboelhadid et al., 2013; Mahdy et al., 2014b; Barghash et al., 2017; Desouky et al., 2017). In addition to the scarcity of CE data from donkeys, few studies have identified the causative species of Echinococcus. Thus, E. equinus has been identified in Tunisia and Egypt (Aboelhadid et al., 2013; Boufana et al., 2014; Lahmar et al., 2014; Desouky et al., 2017; Mousa et al., 2020) and E. granulosus s. s. in Tunisia and Morocco (Azlaf, 2007; Boufana et al., 2014; Lahmar et al., 2014).
Echinococcus equinus is assumed to be the species most closely adapted to donkeys and other Equidae as intermediate hosts, reaching high levels of fertility. This was confirmed by 68% fertility among our samples of E. equinus. The distribution and host range of this species in sub-Saharan Africa are still rather enigmatic: in our study, almost all donkeys with E. equinus originated from southern Ethiopia, only 2 animals from Kenya. This is unexplained, but the scarcity of this species in Kenya is supported by its absence in recent large-scale faecal surveys of dogs (Mulinge et al., 2018) and wild carnivores (Kagendo et al., 2014) across the country. Older data from Chad (no case of CE in 163 horses and donkeys) suggest that this region of absence of scarcity of E. equinus may stretch from eastern to central Africa (Graber et al., 1969). No wild equids (zebras) have ever been examined for CE in eastern and central Africa. This is in clear contrast to the situation in southern Africa, where E. equinus is obviously common in plains zebras and wild carnivores in northern Namibia (Wassermann et al., 2015), was reported from a mountain zebra in ‘South West Africa’ (Kumaratilake et al., 1986), and where reports of 60% ‘incidence’ (sic) and fertility of cysts in plains zebra of Kruger National Park in South Africa also suggest the frequent presence of E. equinus there (Young, 1975a, 1975b). The latter is supported by a recent report of fertile cysts of E. equinus in a white rhino from Kruger National Park, RSA (Zaffarano et al., 2021). There are no reports of E. equinus in donkeys, horses or domestic dogs from southern Africa. Although our data close a gap of knowledge by reporting the presence of E. equinus in eastern Africa, data on the obviously patchy geographical spread of this species on the continent are still far from complete. In particular, further surveys are needed to investigate whether the endemic area in southern Ethiopia is an isolated focus of this parasite, or is linked to the northern African endemic region. Reasons for differences in local frequency of this parasite are unclear. The principal hosts, donkeys and domestic dogs, are abundant in all parts of our study area. Even though in most parts of eastern Africa, donkeys are rarely slaughtered for human consumption and dogs may not get infected via slaughter offal, it is unlikely that carcasses of donkeys that died due to age or accidents are disposed in a way that they are out of reach for (stray) dogs or wild scavengers. Ongoing research on Echinococcus transmission in southern Ethiopia may shed light on the risk factors in that focus. This is not irrelevant, as E. equinus – long assumed to be apathogenic for humans – has recently been reported as causative agent of human CE (Kim et al., 2020; Macin et al., 2021), and the rarity of reported human cases may at least partly be due to the general rarity of the parasite in large parts of Africa (and the world).
The numbers of donkeys infected with Echinococcus spp. other than E. equinus (E. granulosus s. s. n = 70; E. canadensis G6/7 n = 26; E. ortleppi n = 4) do approximately reflect the relative frequencies of these species in their typical intermediate hosts (sheep, goats, camels and cattle) in the study area. In Kenya, E. granulosus s. s. is the most abundant species in sheep, goats and cattle followed by E. canadensis (G6/7) and E. ortleppi, this frequency can also be seen in donkeys. However, the prevalence of these 3 species in donkeys was far lower than in their ruminant hosts in the same region (Dinkel et al., 2004; Maillard et al., 2007; Casulli et al., 2010; Addy et al., 2012; Hailemariam et al., 2012; Mutwiri et al., 2013; Mbaya et al., 2014; Chamai et al., 2016; Tigre et al., 2016; Odongo et al., 2018; Nungari et al., 2019; Terefe et al., 2019; Tamarozzi et al., 2022). Also, cyst fertility rates in donkeys [10% for E. granulosus s. s., 12% for E. canadensis (G6/7), 1/4 for E. ortleppi] were far lower compared to their typical hosts. In case of E. granulosus s. s., this is in accordance with studies from the Mediterranean area, where few or no cysts of this species were found fertile in donkeys and horses (Azlaf, 2007; Varcasia et al., 2008; Utuk and Simsek, 2013; Boufana et al., 2014; Lahmar et al., 2014), indicating a poor host adaptation. For E. canadensis (G6/7) and E. ortleppi, the presented results are even the first confirmation that these species can infect donkeys and reach fertility there; apart from our findings, an E. ortleppi cyst of unknown fertility status previously reported from an unspecified species of zebra in Namibia was the only record of E. ortleppi from any member of the Equidae (Obwaller et al., 2004). Yet, given the low prevalence and fertility rate, donkey infections with E. granulosus s. s., E. canadensis (G6/7) and E. ortleppi are likely the result of spillover from the typical lifecycles between dogs and domestic ruminants, and donkeys probably play a small role, if any, in the transmission of these parasites.
This study reports the unusual finding of S. theileri calcified cyst in a donkey that was located in the kidney and initially misidentified as a degenerated Echinococcus cyst. Domestic and wild carnivores serve as the definitive hosts of Spirometra spp., while the first intermediate hosts are copepods and the second intermediate hosts are amphibians, reptiles or herbivorous mammals (paratenic hosts). A sylvatic cycle for Spirometra spp. involving wild herbivores (zebra, warthog and antelope) as second intermediate hosts (paratenic) and carnivores (hyenas) was proposed in Maasai Mara (Nelson et al., 1965; Muller-Graf, 1995). A small number of human infections with plerocercoids of Spirometra spp. are known from Kenya (Schmid and Watschinger, 1972; MOH, 2016). Although the zoonotic potential of S. theileri is unknown, infections with adult worms of this species have been found in leopards and spotted hyenas in Tanzania and recently in domestic dogs in Maasai Mara (Eom et al., 2019; Mulinge et al., 2021). As the plerocercoid found in our donkey was calcified, the significance of this finding is unclear, and the lifecycle of S. theileri in the area is in need of investigation.
Acknowledgements
The authors sincerely thank the management of the 3 slaughterhouses in Naivasha, Mogotio and Lodwar for permission to access and collect of cysts from donkeys. The meat inspectors are appreciated for their corporation during the sampling period. This manuscript is published with permission from the Director General, KEMRI.
Data availability
Data supporting results are provided within the article and available on GenBank under accession numbers OK489943-OK489955.
Author contributions
E. M., E. Z., P. K., M. W., U. M. and T. R. conceived and designed the study. E. M., E. Z. and J. M. carried out fieldwork. E. M., C. M., L. G., T. K. and M. W. performed laboratory analysis. All authors wrote and revised the manuscript.
Financial support
This study was funded by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – KE282-9/1, -12/1, RO3753-3/1, -9/1 – and conducted in the context of the ‘Cystic Echinococcosis in sub-Saharan Africa Research initiative (CESSARi)’.
Conflict of interest
None.
Ethical standards
The KEMRI Scientific Ethics Review Unit (SERU) approved this study SSC. No. 1684 as well as the Department of Veterinary Services, Kenya.
References
- Abo-Shehada M (1988) Prevalence of hydatidosis in donkeys from central Jordan. Veterinary Parasitology 30, 125–130. [DOI] [PubMed] [Google Scholar]
- Aboelhadid S, El-Dakhly K, Yanai T, Fukushi H and Hassanin K (2013) Molecular characterization of Echinococcus granulosus in Egyptian donkeys. Veterinary Parasitology 193, 292–296. [DOI] [PubMed] [Google Scholar]
- Addy F, Alakonya A, Wamae N, Magambo J, Mbae C, Mulinge E, Zeyhle E, Wassermann M, Kern P and Romig T (2012) Prevalence and diversity of cystic echinococcosis in livestock in Maasailand, Kenya. Parasitology Research 111, 2289–2294. [DOI] [PubMed] [Google Scholar]
- Addy F, Wassermann M, Banda F, Mbaya H, Aschenborn J, Aschenborn O, Koskei P, Umhang G, De La Rue M, Elmahdi I, Mackenstedt U, Kern P and Romig T (2017a) Genetic polymorphism and population structure of Echinococcus ortleppi. Parasitology 144, 450–458. [DOI] [PubMed] [Google Scholar]
- Addy F, Wassermann M, Kagendo D, Ebi D, Zeyhle E, Elmahdi I, Umhang G, Casulli A, Harandi M, Aschenborn O, Kern P, Mackenstedt U and Romig T (2017b) Genetic differentiation of the G6/7 cluster of Echinococcus canadensis based on mitochondrial marker genes. International Journal for Parasitology 47, 923–931. [DOI] [PubMed] [Google Scholar]
- Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W and Lipman D (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azlaf R (2007) Echinococcosis/Hydatidosis in Morocco: Epidemiology, Modeling, E. granulosus Genotyping and Molecular Analysis. PhD thesis, Hassan II Institute of Agronomy and Veterinary Medicine, Rabat, Morocco. [Google Scholar]
- Azlaf R and Dakkak A (2006) Epidemiological study of the cystic echinococcosis in Morocco. Veterinary Parasitology 137, 83–93. [DOI] [PubMed] [Google Scholar]
- Barghash SM, El Sayed RA, El-Alfy N, Abou-Elnour B, El-Kattan A and Sadek A (2017) Prevalence and molecular identification of Echinococcus granulosus in humans and slaughtered animals in Egypt. European Journal of Biomedical and Pharmaceutical Sciences 4, 34–42. [Google Scholar]
- Boufana B, Lahmar S, Rebai W, Ben Safta Z, Jebabli L, Ammar A, Kachti M, Aouadi S and Craig PS (2014) Genetic variability and haplotypes of Echinococcus isolates from Tunisia. Transactions of the Royal Society of Tropical Medicine and Hygiene 108, 706–714. [DOI] [PubMed] [Google Scholar]
- Casulli A, Zeyhle E, Brunetti E, Pozio E, Meroni V, Genco F and Filice C (2010) Molecular evidence of the camel strain (G6 genotype) of Echinococcus granulosus in humans from Turkana, Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 104, 29–32. [DOI] [PubMed] [Google Scholar]
- Chamai M, Omadang L, Erume J, Ocaido M, Oba P, Othieno E, Bonaventure S and Kitibwa A (2016) Identification of Echinococcus granulosus strains using polymerase chain reaction-restriction fragment length polymorphism amongst livestock in Moroto district, Uganda. Onderstepoort Journal of Veterinary Research 83, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplazes P, Rinaldi L, Alvarez Rojas C, Torgerson P, Harandi M, Romig T, Antolova D, Schurer J, Lahmar S, Cringoli G, Magambo J, Thompson R and Jenkins E (2017) Global distribution of alveolar and cystic echinococcosis. Advances in Parasitology 95, 315–493. [DOI] [PubMed] [Google Scholar]
- Desouky A, Helmy N, Sorour S and Amer M (2017) Prevalence and molecular studies on Echinococcus equinus isolated from necropsied donkeys. Iraqi Journal of Veterinary Sciences 31, 101–106. [Google Scholar]
- Dinkel A, Njoroge E, Zimmermann A, Walz M, Zeyhle E, Elmahdi I, Mackenstedt U and Romig T (2004) A PCR system for detection of species and genotypes of the Echinococcus granulosus-complex, with reference to the epidemiological situation in eastern Africa. International Journal for Parasitology 34, 645–653. [DOI] [PubMed] [Google Scholar]
- Eom KS, Park H, Lee D, Choe S, Kang Y, Bia MM, Ndosi BA, Nath TC, Eamudomkarn C, Keyyu J, Fyumagwa R, Mduma S and Jeon HK (2019) Identity of Spirometra theileri from a leopard (Panthera pardus) and spotted hyena (Crocuta crocuta) in Tanzania. Korean Journal of Parasitology 57, 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber M, Troncy P, Tabo R, Service J and Oumatie O (1969) Echinococcosis-hydatidosis in Central Africa. I. Echinococcosis of domestic and wild animals. Revue d'Elevage et de Medecine Veterinaire des Pays Tropicaux 22, 55–67. [PubMed] [Google Scholar]
- Hailemariam Z, Nakao M, Menkir S, Lavikainen A, Yanagida T, Okamoto M and Ito A (2012) Molecular identification of unilocular hydatid cysts from domestic ungulates in Ethiopia: implications for human infections. Parasitology International 61, 375–377. [DOI] [PubMed] [Google Scholar]
- Haridy F, Gawad A, Ibrahim B, Hassan A, El-Sherbi G, El Shazly A and Morsy T (2008) Zoonotic hydatidosis in donkeys: post mortem examination in the Zoo, Giza, Egypt. Journal of the Egyptian Society of Parasitology 38, 305–312. [PubMed] [Google Scholar]
- Hüttner M, Nakao M, Wassermann T, Siefert L, Boomker J, Dinkel A, Sako Y, Mackenstedt U, Romig T and Ito A (2008) Genetic characterization and phylogenetic position of Echinococcus felidis (Cestoda: Taeniidae) from the African lion. International Journal for Parasitology 38, 861–868. [DOI] [PubMed] [Google Scholar]
- Hüttner M, Siefert L, Mackenstedt U and Romig T (2009) A survey of Echinococcus species in wild carnivores and livestock in East Africa. International Journal for Parasitology 39, 1269–1276. [DOI] [PubMed] [Google Scholar]
- Kagendo D, Magambo J, Agola E, Njenga S, Zeyhle E, Mulinge E, Gitonga P, Mbae C, Muchiri E, Wassermann M, Kern P and Romig T (2014) A survey for Echinococcus spp. of carnivores in six wildlife conservation areas in Kenya. Parasitology International 63, 604–611. [DOI] [PubMed] [Google Scholar]
- Kere OJ, Joseph E, Jessika B and Maina K (2019) Prevalence and monetary loss due to cystic echinococcosis in slaughter house livestock: a case study of Migori County, Kenya. Parasite Epidemiology and Control 5, e00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Yong TS, Shin MH, Lee KJ, Park GM, Suvonkulov U, Kovalenko D and Yu HS (2020) Phylogenetic characteristics of Echinococcus granulosus sensu lato in Uzbekistan. Korean Journal of Parasitology 58, 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkar L, Laurimäe T, Acosta-Jamett G, Andresiuk V, Balkaya I, Casulli A, Gasser RB, van der Giessen J, González LM, Haag KL, Zait H, Irshadullah M, Jabbar A, Jenkins DJ, Kia EB, Manfredi MT, Mirhendi H, M'Rad S, Rostami-Nejad M, Oudni-M'rad M, Pierangeli NB, Ponce-Gordo F, Rehbein S, Sharbatkhori M, Simsek S, Soriano SV, Sprong H, Šnábel V, Umhang G, Varcasia A and Saarma U (2018) Global phylogeography and genetic diversity of the zoonotic tapeworm Echinococcus granulosus sensu stricto genotype G1. International Journal for Parasitology 48, 729–742. [DOI] [PubMed] [Google Scholar]
- Kumaratilake LM, Thompson RC and Eckert J (1986) Echinococcus granulosus of equine origin from different countries possess uniform morphological characteristics. International Journal for Parasitology 16, 529–540. [DOI] [PubMed] [Google Scholar]
- Lahmar S, Boufana B, Jebabli L, Craig P, Ayari H, Basti T, Dhibi M and Torgerson P (2014) Modelling the transmission dynamics of cystic echinococcosis in donkeys of different ages from Tunisia. Veterinary Parasitology 205, 119–124. [DOI] [PubMed] [Google Scholar]
- Macin S, Orsten S, Samadzade R, Colak B, Cebeci H and Fındık D (2021) Human and animal cystic echinococcosis in Konya, Turkey: molecular identification and the first report of E. equinus from human host in Turkey. Parasitology Research 120, 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdy O, Abdel-Maogood S, Abdel-Wahab A and El-Bahy M (2014a) Molecular characterization of Echinococcus granulosus cysts isolated from some animals in Egypt. Global Veterinaria 12, 594–598. [Google Scholar]
- Mahdy O, Maogood A, Wahab A and El-Bahy M (2014b) Epidemiological and molecular characterization of antigens extracted from hydatid cysts of camel, cattle and donkeys in Egypt. International Journal of Basic and Applied Sciences 3, 93. [Google Scholar]
- Maillard S, Benchikh-Elfegoun M, Knapp J, Bart J, Koskei P, Gottstein B and Piarroux R (2007) Taxonomic position and geographical distribution of the common sheep G1 and camel G6 strains of Echinococcus granulosus in three African countries. Parasitology Research 100, 495–503. [DOI] [PubMed] [Google Scholar]
- Mbaya H, Magambo J, Njenga S, Zeyhle E, Mbae C, Mulinge E, Wassermann M, Kern P and Romig T (2014) Echinococcus spp. in central Kenya: a different story. Parasitology Research 113, 3789–3794. [DOI] [PubMed] [Google Scholar]
- MOH (2016) Report for the Medical Reconnaissance Visit to Narok County Following an Increase in Sparganosis Cases. Kenya: Ministry of Health, Nairobi, pp. 1–21. [Google Scholar]
- Mousa WM, Abdel-Wahab AM, El-Gameel Sohila M and Mahdy OA (2020) Genetic characterization of hydatid cysts of different intermediate hosts. Helminthologia 57, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukbel R, Torgerson P and Abo-Shehada M (2000) Prevalence of hydatidosis among donkeys in northern Jordan. Veterinary Parasitology 88, 35–42. [DOI] [PubMed] [Google Scholar]
- Mulinge E, Magambo J, Odongo D, Njenga S, Zeyhle E, Mbae C, Kagendo D, Addy F, Ebi D, Wassermann M, Kern P and Romig T (2018) Molecular characterization of Echinococcus species in dogs from four regions of Kenya. Veterinary Parasitology 255, 49–57. [DOI] [PubMed] [Google Scholar]
- Mulinge E, Zeyhle E, Mpario J, Mugo M, Nungari L, Ngugi B, Sankale B, Gathura P, Magambo J and Kachani M (2021) A survey of intestinal helminths in domestic dogs in a human-animal-environmental interface: the Oloisukut Conservancy, Narok County, Kenya. Journal of Helminthology 95, e59. [DOI] [PubMed] [Google Scholar]
- Muller-Graf C (1995) A coprological survey of intestinal parasites of wild lions (Panthera leo) in the Serengeti and the Ngorongoro Crater, Tanzania, east Africa. Journal of Parasitology 81, 812–814. [PubMed] [Google Scholar]
- Mutwiri T, Magambo J, Zeyhle E, Mkoji G, Wamae C, Mulinge E, Wassermann H, Kern P and Romig T (2013) Molecular characterisation of Echinococcus granulosus species/strains in human infections from Turkana, Kenya. East African Medical Journal 90, 235–240. [PubMed] [Google Scholar]
- Nakao M, Sako Y and Ito A (2003) Isolation of polymorphic microsatellite loci from the tapeworm Echinococcus multilocularis. Infection Genetics and Evolution 3, 159–163. [DOI] [PubMed] [Google Scholar]
- Nakao M, Lavikainen A, Yanagida T and Ito A (2013a) Phylogenetic systematics of the genus Echinococcus (Cestoda: Taeniidae). International Journal for Parasitology 43, 1017–1029. [DOI] [PubMed] [Google Scholar]
- Nakao M, Yanagida T, Konyaev S, Lavikainen A, Odnokurtsev V, Zaikov V and Ito A (2013b) Mitochondrial phylogeny of the genus Echinococcus (Cestoda: Taeniidae) with emphasis on relationships among Echinococcus canadensis genotypes. Parasitology 140, 1625–1636. [DOI] [PubMed] [Google Scholar]
- Nelson G, Pester F and Rickman R (1965) The significance of wild animals in the transmission of cestodes of medical importance in Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 59, 507–524. [DOI] [PubMed] [Google Scholar]
- Nungari L, Mbae C, Gikunju J, Mulinge E, Kaburu T, Zeyhle E and Magambo J (2019) Prevalence and genotyping of Echinococcus species from livestock in Kajiado County, Kenya. BioMed Research International 2019, 4798906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obwaller A, Schneider R, Walochnik J, Gollackner B, Deutz A, Janitschke K, Aspock H and Auer H (2004) Echinococcus granulosus strain differentiation based on sequence heterogeneity in mitochondrial genes of cytochrome c oxidase-1 and NADH dehydrogenase-1. Parasitology 128, 569–575. [DOI] [PubMed] [Google Scholar]
- Odongo D, Tiampati C, Mulinge E, Mbae C, Bishop R, Zeyhle E, Magambo J, Wasserman M, Kern P and Romig T (2018) Prevalence and genotyping of Echinococcus granulosus in sheep in Narok County, Kenya. Parasitology Research 117, 2065–2073. [DOI] [PubMed] [Google Scholar]
- Oge S, Kircali F, Yildirim A and Oge H (2004) Hydatidosis (hydatid cyst) in equines. Ankara Universitesi Veteriner Fakultesi Dergisi 51, 75–76. [Google Scholar]
- Ohiolei J, Yan H, Li L, Isaac C, Fu B and Jia W (2019a) First report of Echinococcus granulosus sensu stricto (G1) in Nigeria, West Africa. Journal of Helminthology 94, e109. [DOI] [PubMed] [Google Scholar]
- Ohiolei JA, Xia CY, Li L, Liu JZ, Tang WQ, Wu YT, Danqulamu Zhu GQ, Shi B, Fu BQ, Yin H, Yan HB and Jia WZ (2019b) Genetic variation of Echinococcus spp. in yaks and sheep in the Tibet Autonomous Region of China based on mitochondrial DNA. Parasite & Vectors 12, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omondi H, Gitau G, Gathura P, Mulinge E, Zeyhle E, Kimeli P and Bett B (2020) Prevalence and genotyping of Echinococcus granulosus sensu lato from livestock in north-eastern Kenya. Journal of Helminthology 94, e205. [DOI] [PubMed] [Google Scholar]
- Pandey V (1980) Hydatidosis in donkeys in Morocco. Annals of Tropical Medicine and Parasitology 74, 519–521. [DOI] [PubMed] [Google Scholar]
- Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig P, Wassermann M, Takahashi K and de la Rue M (2017) Ecology and life cycle patterns of Echinococcus species. Advances in Parasitology 95, 213–314. [DOI] [PubMed] [Google Scholar]
- Schmid H and Watschinger H (1972) Sparganosis in the Masailand. Acta Tropica 29, 218–230. [PubMed] [Google Scholar]
- Simsek S, Roinioti E and Eroksuz H (2015) First report of Echinococcus equinus in a donkey in Turkey. Korean Journal of Parasitology 53, 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha H (2012) Genetic variations among Echinococcus granulosus isolates in Egypt using RAPD-PCR. Parasitology Research 111, 1993–2000. [DOI] [PubMed] [Google Scholar]
- Tamarozzi F, Kibona T, de Glanville WA, Mappi T, Adonikamu E, Salewi A, Misso K, Maro V, Casulli A, Santoro A, Santolamazza F, Mmbaga BT and Cleaveland S (2022) Cystic echinococcosis in northern Tanzania: a pilot study in Maasai livestock-keeping communities. Parasite & Vectors 15, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terefe Y, Addy F, Alemu S, Mackenstedt U, Romig T and Wassermann M (2019) Genetic characterization of Echinococcus species in eastern Ethiopia. Veterinary Parasitology, Regional Studies and Reports 17, 100302. [DOI] [PubMed] [Google Scholar]
- Thompson R (2017) Biology and systematics of Echinococcus. Advances in Parasitology 95, 65–109. [DOI] [PubMed] [Google Scholar]
- Thompson R and McManus D (2002) Towards a taxonomic revision of the genus Echinococcus. Trends in Parasitology 18, 452–457. [DOI] [PubMed] [Google Scholar]
- Tigre W, Deresa B, Haile A, Gabriel S, Victor B, Pelt J, Devleesschauwer B, Vercruysse J and Dorny P (2016) Molecular characterization of Echinococcus granulosus s.l. cysts from cattle, camels, goats and pigs in Ethiopia. Veterinary Parasitology 215, 17–21. [DOI] [PubMed] [Google Scholar]
- Utuk A and Simsek S (2013) Molecular characterization of the horse isolate of Echinococcus granulosus in Turkey. Journal of Helminthology 87, 305–308. [DOI] [PubMed] [Google Scholar]
- Varcasia A, Garippa G, Pipia A, Scala A, Brianti E, Giannetto S, Battelli G, Poglayen G and Micagni G (2008) Cystic echinococcosis in equids in Italy. Parasitology Research 102, 815–818. [DOI] [PubMed] [Google Scholar]
- Vuitton D, McManus D, Rogan M, Romig T, Gottstein B, Naidich A, Tuxun T, Wen H and Menezes da Silva A (2020) International consensus on terminology to be used in the field of echinococcoses. Parasite 27, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann M, Aschenborn O, Aschenborn J, Mackenstedt U and Romig T (2015) A sylvatic lifecycle of Echinococcus equinus in the Etosha National Park, Namibia. International Journal for Parasitology. Parasites and Wildlife 4, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann M, Woldeyes D, Gerbi B, Ebi D, Zeyhle E, Mackenstedt U, Petros B, Tilahun G, Kern P and Romig T (2016) A novel zoonotic genotype related to Echinococcus granulosus sensu stricto from southern Ethiopia. International Journal for Parasitology 46, 663–668. [DOI] [PubMed] [Google Scholar]
- WHO (2012) Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for Implementation: Executive Summary. Geneva: World Health Organization. [Google Scholar]
- Young E (1975a) Echinococcosis (hydatodosis) in wild animals of the Kruger National Park. Journal of the South African Veterinary Association 46, 285–286. [PubMed] [Google Scholar]
- Young E (1975b) Some important parasitic and other diseases of lion, Panthera leo, in the Kruger National Park. Journal of the South African Veterinary Association 46, 181–183. [PubMed] [Google Scholar]
- Zaffarano GP, de Klerk-Lorist LM, Junker K, Mitchell E, Bhoora RV, Poglayen G and Govender D (2021) First report of cystic echinococcosis in rhinos: a fertile infection of Echinococcus equinus in a Southern white rhinoceros (Ceratotherium simum simum) of Kruger National Park, South Africa. International Journal for Parasitology. Parasites and Wildlife 14, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting results are provided within the article and available on GenBank under accession numbers OK489943-OK489955.