Abstract
Purpose
We examined cancer screening practices and related beliefs in cancer survivors and individuals with family or close friends with a cancer diagnosis compared to individuals without the above cancer history for 5 population-based (gastric, colorectal, lung, breast, cervical) and 1 opportunistic (prostate) cancer screenings using nationally representative cross-sectional survey in Japan.
Methods
We analyzed 3269 data from 3605 respondents (response rate, 37.1%) and compared the screening beliefs and practices of cancer survivors (n = 391), individuals with family members (n = 1674), and close friends with a cancer diagnosis (n = 685) to those without any cancer history (n = 519).
Results
Being a cancer survivor was associated with screening for gastric (OR, 1.75; 95% CI, 1.04–2.95), colorectal (OR, 1.56; 95% CI, 1.03–2.36), and lung cancer (OR, 1.71; 95% CI, 1.10–2.66) but not breast, cervical cancer or PSA test. Having a family cancer diagnosis was associated with colorectal and lung cancer screening. Having friends with a cancer diagnosis was associated with PSA test. Cancer survivors and family members perceived themselves as being more susceptible and worried about getting cancer than individuals without any cancer history. Cancer survivors strongly believed screening can detect cancer and were more likely to undergo screening. Subgroup analysis indicated an interrelation between gastric and colorectal cancer screening among survivors.
Conclusions
A cancer diagnosis in oneself or family or friend influences an individual’s health-related belief and risk perception, which can increase the likelihood of cancer screening.
Implications for Cancer Survivors
Targeted and tailored communication strategies can increase awareness of cancer screening.
Keywords: Cancer screening, Cancer survivor, Cancer diagnosis in family, Cancer diagnosis in friend, Health belief model, Screening behavior
Introduction
A cancer diagnosis has both negative and positive impacts on the patients and their loved ones, including family members and close friends [1–8]. Studies indicate that a cancer diagnosis can serve as a “teachable moment” or “cue to action” for positive behavior change in not only the cancer survivors but also their family [2, 9–11] and friends [2], who may come to realize the risk of cancer and be motivated to make positive lifestyle changes. Life partners of cancer survivors are sometimes referred to as “second survivors” [4, 12] or “co-survivors” [13–15], and their interaction with the survivor is an important contributing factor to the quality of survivorship among cancer survivors [13, 15–19]. Previous studies have reported that both cancer survivors and their family members are more likely to engage in regular physical activity [2, 9] and consume a healthy diet such as by increasing fruit and vegetable intake [10, 11]. One survey conducted at cancer fundraising events in Australia showed that cancer survivors, family members, and close friends of cancer survivors all made improvements to their diet and physical activity [2]. These studies suggest that a cancer diagnosis can motivate cancer survivors and their family and close friends to improve their health behaviors and that some, in fact, do make health behavior changes. In contrast, the impact of a cancer diagnosis on other preventive behavior such as screening practice is not well studied.
Cancer screening is an important cancer prevention measure as it can lead to timely diagnosis and early treatment [20–23]. Evidence-based cancer screening is recommended and provided as part of organized screening programs in many developed countries [20–23], and public health professionals have made efforts to increase the screening rate [20, 24–26]. In Japan, current screening recommendations are divided into population-based screening and opportunistic screening based on the best available research evidence and expert discussions that scrutinize the benefits and harms of different screening tests [27]. Population-based cancer screening in Japan is recommended for gastric (X-ray and endoscopic examination for adults aged ≥ 50 years), colorectal (fecal occult blood test for adults aged ≥ 40 years), breast (mammography for women aged ≥ 40 years), cervical (Papanicolaou test for women aged ≥ 20 years), and lung cancer (X-ray examination for adults aged ≥ 40 years). Unlike the USA and other western countries, where lung cancer screening is only recommended for high-risk populations, Japanese guidelines for lung cancer screening recommend chest X-rays as a population-based screen. Thus, screening programs for five types of cancer (gastric, lung, colorectal, cervical, and breast cancers) have become continuously available at both community and occupational sites [28]. Although the prostate-specific antigen (PSA) test is not included in the current national mass screening guideline, the Japanese Urological Association has been recommending to take a PSA test for men aged 50 years and older [29], and 80.4% of municipalities were providing a PSA test in 2016 [30]. The person found to have an elevated PSA level (the cutline is 4.0 ng/ml) will be introduced to a urologist for further examination, normally internal examination and, sometimes, biopsy, if necessary, to confirm the diagnoses.
Although major cancer screening tests are provided for free or at very low cost to the general public in Japan, screening rates are much lower than those in major OECD countries. For example, the screening rate for breast cancer in 2019 was only 44.6% in Japan compared to 76.5%, 74.5%, and 70.2% in the USA, Australia, and Korea, respectively [31]. The factors that influence cancer screening uptake are not well understood. Thus, investigations into whether cancer experience influences screening uptake among survivors, secondary survivors, and other populations are warranted and could be useful for public health decision makers to update their strategy to promote cancer screening.
The screening practices of cancer survivors are of interest to researchers [12, 32–34] since survivors are at higher risk of developing additional cancers than the general population [20, 35–40]. However, studies that have synthesized evidence comparing cancer screening practices between cancer survivors and non-cancer controls [12, 33, 34] have reported conflicting results. Two studies reported that cancer survivors were more likely to undergo colorectal, breast, and cervical cancer screening [12, 33]. Conversely, one systematic review concluded that cancer survivors were less likely to adopt secondary prevention practices than cancer-free controls [34]. In terms of the family members of cancer survivors, one cross-sectional study in Minnesota reported that female relatives of breast cancer survivors undergo regular mammograms compared to women without a family history of cancer [41]. Another cross-sectional study in Israel explored colonoscopy rates among first-degree relatives and reported that greater adherence was associated with sociodemographic variables (older age, having siblings, having a spouse, higher level of education, and income) and behavioral variables (healthier lifestyle, utilization of preventive health services) [42]. However, to our knowledge, no study has examined comprehensive screening practices among cancer survivors and secondary survivors such as the family members and close friends of someone diagnosed with cancer compared to a cancer-free population.
The health belief model (HBM) [43, 44] has been used in many studies as a conceptual framework to explore beliefs about cancer and screening in the general public and cancer survivors [32, 45–48]. This model posits that a person’s health behavior, in this case, engaging in cancer screening, depends on their perception of four areas: (1) their susceptibility to that illness; (2) the severity of a potential illness; (3) the benefits of taking a preventative action; and (4) perceived barriers to taking the preventative action. Additionally, the HBM suggests that an individual’s behavior is influenced if they believe they have the ability to perform an action to control the illness (self-efficacy) and there are cues to action that move them to change their behavior (events or people that act as triggers for the action). Thus, the perception of cancer and beliefs about screening may differ among cancer survivors, their family members, their close friends, and the cancer-free population.
The aim of the current study was to examine the impact of a cancer diagnosis on cancer screening practices and beliefs among individuals with a prior personal cancer diagnosis (CanSurvivors), individuals with a prior family cancer diagnosis (FamCancer), and individuals with close friends with a cancer diagnosis (FriendCancer), compared to individuals without any of the above cancer history (NoCancer). Our specific research question was as follows: What are the screening practices and related beliefs of cancer survivors, their family members, and close friends compared to a group without any history of cancer? We used the HBM as a guiding framework [32, 49] to understand individuals’ cancer-related beliefs, concerns, and perceptions of risk related to screening as the model fit the study aims and available data.
Materials and methods
Study design and procedures
This cross-sectional, correlational study used data from INFORM Study 2020, a nationally representative cross-sectional survey on health information access for consumers in Japan. The INFORM study’s methodology is reported in detail elsewhere [50]. Briefly, the survey used two-stage stratified random sampling, the same sampling strategy as that used for the National Census by the Japanese government. From 35 strata identified by crossing nine regions and four municipality groups by population size, we randomly selected 500 census areas with probability proportional to the size of the stratum. From the 500 areas, we sampled 10,000 individuals aged 20 years or older.
Core items of the questionnaires were initially selected from the Health Information National Trends Survey (HINTS) in the USA [51]. We additionally included items considered important for the Japanese population [50]. While the questionnaire consists of 95 questions in 11 sections, only questions relevant to the research question in this study were used in the analysis.
Data collection for INFORM Study 2020 began on August 1, 2020 and ended in September 30, 2020. The invitation letter and questionnaire were mailed to subjects, who were asked to complete the questionnaire and mail it back. We considered participants who selected the item “agree to participate” in the introductory statement of the questionnaire as consenting to participate in the study. The ethical and scientific validity of this study was confirmed by the institutional review board of the National Cancer Center Japan (research project number: 290) and Keio University SFC (research project number: 342), and those of all participating institutions.
Measures
Demographic characteristics, chronic disease history, cancer screening practices, self-reported health status, health beliefs and engagement related to cancer and screening, and experience with cancer information seeking and communication were collected. Demographic characteristics included age, sex, marital status, education, and household income. We also asked about the respondents’ history of chronic disease (i.e., hypertension, heart failure, cerebrovascular diseases) besides cancer. Self-reported health status was rated on a 5-item scale (1, excellent; 2, very good; 3, good; 4, fair; or 5, poor). Participants were also asked if they had ever sought out information about cancer and if they had friends or family members they had talked to about their health.
Cancer history
In the present study, participants were classified into four groups based on their cancer history: (1) CanSurvivors, if they reported having ever been diagnosed with any kind of cancer; (2) FamCancer, if they reported never having been diagnosed with cancer themselves but that their family members had experienced cancer; (3) FriendCancer, if they reported that neither themselves nor family members had been diagnosed but close friends had experienced cancer; and (4) NoCancer, if they reported none of the above experiences with cancer. All participants were categorized in any of these four distinct groups. Those categorized as CanSurvivors we asked to provide their cancer sites and the time since diagnosis in years. CanSurvivors were classified by cancer site for subgroup analysis.
Cancer screening
Participants were asked if they had ever undergone screening for gastric, colorectal, lung, breast, cervical, and prostate cancer. According to the National Guidance[28, 52], both male and female participants ≥ 50 years of age were included for analysis of gastric cancer screening, and those ≥ 40 years of age were included for analysis of colorectal and lung cancer screening. Female participants ≥ 40 of age were included for analysis of mammography, and those ≥ 20 of age were included for analysis of the Papanicolaou test. In addition, men ≥ 50 years of age were included for analysis of the PSA test.
Cancer survivors diagnosed with a cancer that formed the object of a screening test of interest were excluded from calculation of screening rates for that site (i.e., breast cancer survivors were excluded from calculations for mammography).
Beliefs about cancer and screening
By using HBM as a guide, we selected relevant questions regarding beliefs about cancer and cancer screening in the questionnaire to examine the four areas of HBM. The selected statements included both correct and incorrect knowledge about cancer and cancer screening. The respondents’ “perceived susceptibility” was examined using two questions: “How likely are you to get cancer in your lifetime?” and “How worried are you about getting cancer?” We asked participants to answer these questions on 5-item scales (1, very unlikely; 2, unlikely; 3, neither unlikely nor likely; 4, likely; and 5, very likely; and 1, not at all; 2, slightly; 3, somewhat; 4, moderately; 5, extremely, respectively). Respondents’ “perceived severity” was examined using two items: “Every cancer detected by cancer screening grows to a life-threatening extent” and “When I think about cancer, I automatically think about death,” which were rated on a 5-point Likert-type scale (1, strongly disagree; 2, disagree; 3, unsure; 4, agree; 5, strongly agree). For “perceived benefits” of screening, we used two statements: “Getting checked regularly for cancer helps find cancer when it’s easy to treat” (4-item scale from “strongly agree” to “strongly disagree”) and “These tests can definitely tell that a person has cancer” (3 items: 1, true; 2, false; 3, don’t know). For “perceived barriers,” the statement “Cancer screening can cause further physical burden depending on additional testing” (3 items: 1, true; 2, false; 3, don’t know) was used. The responses were used to indicate the respondents’ beliefs and trust in screening regardless of whether the statement represented correct or incorrect knowledge.
The likelihood of engaging in cancer screening was determined using the statement “I will keep on undergoing cancer screening as long as I live no matter how old I get,” assessed on a 4-item scale (1, strongly think so; 2, think so; 3, don’t think so; 4, don’t think so at all). We also asked participants whether they have ever sought out information about cancer from any source (yes/no) and whether they have friends or family members they talk to about their health (yes/no).
Statistical analysis
Descriptive statistics were calculated for questions related to demographics, beliefs about cancer and screening, and screening practices. Group comparisons were performed using analysis of variance (ANOVA) for continuous variables and the chi-squared test for categorical variables. Results from CanSurvivors, FamCancer, and FriendCancer were compared to the NoCancer group as the reference group and also reported by cancer site. Tests for linear trends across groups were conducted using logistic regression for beliefs about cancer and screening. Logistic regression analyses were performed with screening (yes/no) as the dependent variable and experience with cancer and cancer site as independent variables for each screening test to estimate the adjusted odds ratio (OR) and 95% confidence interval (CI). All models included age in years as a covariate.
The sampling weight for each participant was calculated as the reciprocal of the probability of selecting the participant for the survey in the stratum. The probabilities of nonresponse were estimated based on strata, sex, and age information. The final weight was calculated by multiplying the sampling weight by the reciprocal of the probability of nonresponse. We conducted a weighted analysis to account for the complex sampling design and missing responses to calculate accurate population parameter estimates and CIs for the Japanese general population using the Taylor series linearization method [53]. All analyses were performed using SURVEY PROCEDURES in SAS version 9.4 (SAS Institute, Inc, Cary, NC, USA) with 500 census areas as a variable for selecting the primary sampling units and the 35 sampling strata as a variable for selecting strata. All P-values reported in this study were two sided, and P < 0.05 was considered statistically significant.
Results
Characteristics of the study sample
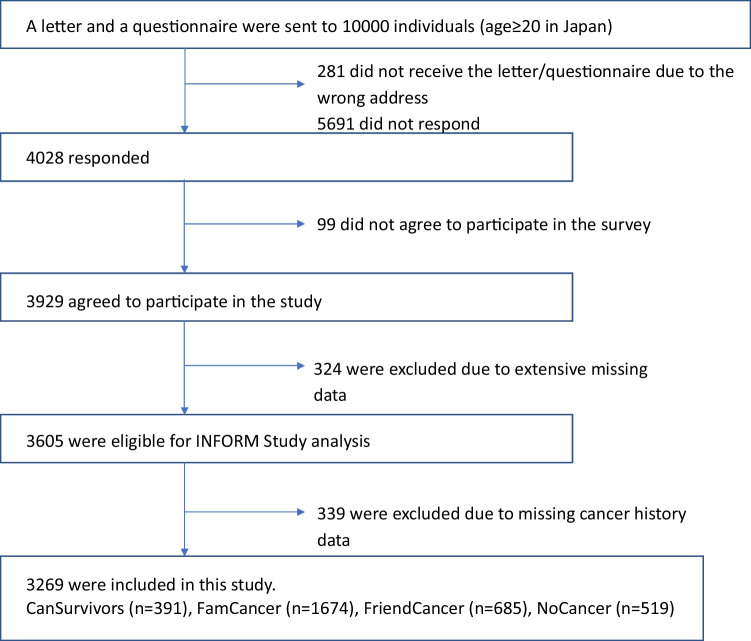
A total of 3605 participants completed the survey (response rate, 37.1%). After excluding participants with missing data for items related to cancer history, 3269 participants were included in the analysis (Fig. 1). Within the present study sample, 11.5% (n = 391) identified themselves as cancer survivors when asked if they had ever been diagnosed with cancer. Meanwhile, 50.5% (n = 1674) and 20.7% (n = 685) of the sample were allocated to the FamCancer (they themselves had never received a diagnosis but family members had been diagnosed with cancer) and FriendCancer (they and family members had never received a diagnosis but friends had been diagnosed with cancer) groups, respectively, and 17.3% (n = 519) to the NoCancer (they, family, and friends had never experienced cancer) group. Age, gender, education, marital status, employment status, income, self-reported general health status, and prevalence of chronic diseases were significantly different (p < 0.001) among the four groups (CanSurvivors, FamCancer, FriendCancer, and NoCancer) (Table 1). Overall, the CanSurvivors group was older, less educated, more likely to be retired, and reported poorer health than the other three groups. In contrast, The NoCancer group was younger, more educated, more likely to be employed, and reported better health than the other three groups.
Fig. 1.
Participant flowchart
Table 1.
Demographics of all respondents
| CanSurvivors n = 391 (11.5%) |
FamCancer n = 1674 (50.5%) |
FriendCancer n = 685 (20.7%) |
NoCancer n = 519 (17.3%) |
P-valuea | |
|---|---|---|---|---|---|
| Age, mean (min, max) | 69.1 (23, 95) | 54.3 (20, 94) | 56.2 (20, 92) | 43.3 (20, 86) | < 0.0001 |
| Gender, N (%) | < 0.0001 | ||||
| Male | 188 (49.0) | 699 (45.4) | 347 (53.5) | 257 (55.3) | |
| Female | 203 (51.0) | 975 (54.6) | 338 (46.5) | 262 (44.7) | |
| Education, N (%) | < 0.0001 | ||||
| ≤ Junior high school | 54 (15.5) | 117 (7.9) | 57 (9.1) | 30 (6.2) | |
| High school | 179 (45.5) | 640 (38.1) | 268 (39.3) | 179 (33.9) | |
| Junior college | 63 (14.6) | 415 (23.2) | 153 (21.0) | 128 (23.8) | |
| ≥ College | 94 (24.5) | 497 (30.8) | 203 (30.6) | 182 (36.2) | |
| Marital status, N (%) | < 0.0001 | ||||
| Married | 298 (73.7) | 1140 (65.7) | 530 (75.3) | 334 (60.3) | |
| Divorced | 28 (6.8) | 98 (5.4) | 35 (4.7) | 23 (4.2) | |
| Widowed | 38 (12.4) | 140 (9.6) | 31 (5.4) | 12 (3.0) | |
| Never married | 27 (7.1) | 289 (19.3) | 85 (14.7) | 150 (32.5) | |
| Employment status, N (%) | N/Ac | ||||
| Employed full-time/Self-employed | 118 (29.7) | 833 (51.4) | 340 (50.8) | 321 (63.9) | |
| Part-time | 44 (10.0) | 256 (13.5) | 108 (14.2) | 68 (11.5) | |
| Unemployed | 90 (25.5) | 201 (13.2) | 84 (13.4) | 42 (8.7) | |
| Retired | 66 (17.6) | 105 (5.9) | 58 (8.1) | 18 (3.1) | |
| Homemaker | 73 (17.2) | 253 (14.1) | 84 (11.7) | 47 (7.8) | |
| Student | 0 (0.0) | 23 (1.8) | 9 (1.8) | 22 (5.1) | |
| Income, N (%) | < 0.0001 | ||||
| < ¥4mil | 197 (52.3) | 596 (36.9) | 230 (34.6) | 155 (30.1) | |
| ¥4mil to < 8mil | 125 (31.3) | 641 (38.8) | 265 (39.1) | 226 (44.8) | |
| > ¥8mil | 60 (16.3) | 391 (24.2) | 170 (26.3) | 126 (25.1) | |
| Smoking status, N (%) | 0.0005 | ||||
| Never | 295 (78.8) | 1207 (73.7) | 470 (70.2) | 370 (69.8) | |
| Former | 40 (10.5) | 172 (10.8) | 94 (13.9) | 47 (9.3) | |
| Current | 41 (10.7) | 248 (15.5) | 106 (16.0) | 100 (20.9) | |
| History of other diseases, N (%) | < 0.0001 | ||||
| Yes | 228 (60.2) | 722 (43.5) | 316 (46.3) | 148 (27.9) | |
| No | 158 (39.8) | 930 (56.5) | 362 (53.7) | 367 (72.1) | |
| Self-reported health status, N (%) | < 0.0001 | ||||
| Excellent/very good/good | 249 (64.2) | 1298 (77.8) | 511 (74.3) | 437 (83.7) | |
| Fair/poor | 137 (35.8) | 367 ( 22.2) | 172 (25.7) | 82 (16.3) | |
| Healthy lifestyle (i.e., exercise or diet), N (%) | |||||
| “Already started (longer than 6 months)” | 74 (19.2) | 255 (14.8) | 91 (13.7) | 49 (9.5) | 0.0008 |
All percentages were adjusted for nonresponse bias by weighting
aCategorical data were compared using a chi-squared test. Age differences were compared using ANOVA
bHistory of other diseases includes diagnosis of one or more of diabetes, hypertension, heart diseases, stroke, chronic lung disease, arthritis or rheumatism, and depression
cN/A: the p-value could not be obtained due to the 0 cell
Of the 391 CanSurvivors, 218 (58%) reported that it had been more than 5 years since their diagnoses, and 46 (12%) reported that they had experienced more than one cancer. Within the CanSurvivors group, cervical cancer survivors were younger (mean age, 56.7: min, 28; max, 85) compared to those with other cancers (mean age over 65 years) (Table 2). Time since diagnosis varied among survivors of different cancers: It was shorter among prostate cancer and liver cancer survivors and longer among gastric/esophageal, breast, and cervical cancer survivors (Table 2).
Table 2.
Characteristics of cancer survivors
| All cancer survivors (n = 391) | Gastric/esophageal (n = 55) | Colorectal (n = 48) | Lung (n = 16) | Breast (n = 70) | Cervical (n = 29) | Prostate (n = 30) | Liver (n = 7) | Other (n = 88) | > One cancer (n = 46) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Current age, mean (min, max) | 69.1 (23, 95) | 73.2 (25, 93) | 70.8 (38, 95) | 73.6 (61, 85) | 66.9 (40, 85) | 56.7 (28, 85) | 76.7 (58, 93) | 76.2 (61, 85) | 65.2 (23, 91) | 73.5 (46, 94) |
| Age at diagnosis, mean (min, max) | 59.1 (17, 90) | 63.3 (25, 85) | 61.7 (37, 79) | 66.9 (51, 83) | 56.9 (36, 83) | 43.9 (20, 70) | 69.7 (48, 87) | 73.1 (58,82) | 54.1 (17, 90) | 61.6 (20, 81) |
| Time since diagnosis, N (%) | ||||||||||
| ≤ 5 years | 162 (42.3) | 19 (35.6) | 24 (48.8) | 9 (53.1) | 26 (36.8) | 12 (43.8) | 17 (60.1) | 6 (83.6) | 31 (37.2) | 17 (39.1) |
| 6–10 years | 82 (21.3) | 17 (30.0) | 9 (20.1) | 3 (20.6) | 18 (26.8) | 2 (6.5) | 4 (14.2) | 1 (16.4) | 17 (18.5) | 10 (20.7) |
| 11–15 years | 55 (15.3) | 5 (11.8) | 5 (9.6) | 3 (18.5) | 9 (13.4) | 6 (24.6) | 5 (18.1) | 0 (0.0) | 15 (17.3) | 7 (19.8) |
| ≥ 16 years | 81 (21.2) | 13 (22.6) | 8 (21.5) | 1 (7.7) | 16 (23.0) | 8 (25.2) | 2 (7.6) | 0 (0.0) | 24 (27.1) | 9 (20.3) |
| Health status, N (%) | ||||||||||
| Excellent/very good/good | 249 (64.2) | 31 (57.6) | 28 (56.2) | 11 (67.5) | 51 (73.1) | 19 (59.6) | 19 (61.9) | 5 (67.2) | 57 (70.6) | 26 (57.2) |
| Fair/poor | 137 (35.8) | 23 (42.4) | 19 (43.8) | 5 (32.5) | 19 (26.9) | 10 (40.4) | 11 (38.1) | 2 (32.8) | 28 (29.4) | 20 (42.8 |
All percentages were adjusted for nonresponse bias by weighting
Two cancer survivors did not indicate their cancer sites
Screening practices
We compared the percentage of individuals who underwent cancer screening among the four groups and the prevalence ORs of having experienced cancer according to screens for different types of cancer (Table 3). Overall, the screening rate was highest for gastric cancer screening (86.6% of CanSurvivors, 82.9% of FamCancer, 81.7% of FriendCancer, and 78.6% of NoCancer), following by lung cancer screening (86.2% of CanSurvivors, 82.9% of FamCancer, 78.3% of FriendCancer, and 76.3% of NoCancer). In contrast, the screening rate was lowest for PSA (38.3% of CanSurvivors, 27.9% of FamCancer, 26.9% of FriendCancer, and 17.7% of NoCancer). In logistic regression adjusted for age, the CanSurvivors group showed significantly higher screening rates for gastric cancer screening (OR, 1.75; 95% CI, 1.04–2.95), colorectal cancer screening (OR, 1.56; 95% CI, 1.03–2.36), and lung cancer screening (OR, 1.71; 95% CI, 1.10–2.66). However, being a cancer survivor was not significantly associated with screening rates for mammography (OR, 1.28; 95% CI, 0.68–2.39) or the Papanicolaou test (OR, 1.39; 95% CI, 0.82–2.34).
Table 3.
Cancer screening practices and beliefs about cancer and screening among the four groups
| CanSurvivors n = 391 (11.46%) |
FamCancer n = 1674 (50.51%) |
FriendCancer n = 685 (20.74%) |
NoCancer n = 519 (17.29%) |
|||
|---|---|---|---|---|---|---|
| Screening practices | ||||||
| Gastric cancer screening (men and women, ≥ 50 years) | ||||||
| N (screened/eligible for screening) (%) | 216/248 (86.6) | 869/1042 (82.9) | 359/442 (81.7) | 129/166 (78.6) | ||
| OR (95% CI) for screening | 1.75 (1.04–2.95) | 1.33 (0.86–2.03) | 1.21 (0.76–1.93) | 1 | ||
| Colorectal cancer screening (men and women, ≥ 40 years) | ||||||
| N (screened/eligible for screening) (%) | 221/281 (77.5) | 1009/1340 (75.0) | 406/574 (70.7) | 188/281 (67.1) | ||
| OR (95% CI) for screening | 1.56 (1.03–2.36) | 1.43 (1.08–1.91) | 1.14 (0.84–1.56) | 1 | ||
| Lung cancer screening (men and women, ≥ 40 years) | ||||||
| N (screened/eligible for screening) (%) | 270/314 (86.2) | 1113/1332 (82.9) | 455/572 (79.3) | 219/288 (76.3) | ||
| OR (95% CI) for screening | 1.71 (1.10–2.66) | 1.43 (1.05–1.95) | 1.12 (0.81–1.55) | 1 | ||
| Breast cancer screening (mammography) (women, ≥ 40 years) | ||||||
| N (screened/eligible for screening) (%) | 76/108 (69.5) | 585/785 (71.5) | 212/286 (71.4) | 95/132 (72.1) | ||
| OR (95% CI) for screening | 1.28(0.68–2.39) | 1.14 (0.71–1.85) | 1.13 (0.66–1.93) | 1 | ||
| Cervical cancer screening (pap test) (women, ≥ 20 years) | ||||||
| N (screened/eligible for screening) (%) | 111/153 (72.0) | 739/970 (74.2) | 242/334 (68.7) | 189/260 (71.4) | ||
| VOR (95% CI) for screening | 1.39 (0.82–2.34) | 1.34 (0.96–1.87) | 1.03 (0.69–1.56) | 1 | ||
| Prostate-specific antigen test (men, ≥ 40 years) | ||||||
| N (screened/eligible for screening) (%) | 43/109 (38.3) | 154/516 (27.9) | 101/263 (36.9) | 28/136 (17.7) | ||
| OR (95% CI) for screening | 1.11 (0.56–2.22) | 1.46 (0.90–2.37) | 1.98 (1.19–3.30) | 1 | ||
| Beliefs about cancer and screening | P value | P for trend | ||||
| Perceived susceptibility, N (%) | ||||||
| “Very likely” or “Likely” to get cancer in my lifetime | 244 (61.3) | 711 (43.8) | 164 (25.5) | 121 (23.1) | < 0.0001 | < 0.0001 |
| Being “Extremely” or “Moderately” worried about getting cancer | 171 (42.3) | 494 (30.2) | 159 (23.4) | 110 (21.8) | < 0.0001 | < 0.0001 |
| Perceived severity, N (%) | ||||||
| “Every cancer detected by cancer screening grows to a life-threatening extent.” | 41 (11.5) | 130 (8.2) | 44 (7.1) | 37 (8.1) | 0.0096 | 0.093 |
| “When I think about cancer, I automatically think about death.” | 259 (68.5) | 1248 (75.8) | 492 (72.6) | 391 (76.6) | 0.0223 | 0.0392 |
| Perceived benefits of screening, N (%) | ||||||
| “Getting checked regularly for cancer helps find cancer when it’s easy to treat.” | 374 (96.2) | 1622 (97.4) | 662 (97.3) | 506 (98.4) | 0.3666 | 0.0944 |
| “These tests can definitely tell that a person has cancer.” | 184 (48.3) | 480 (28.9) | 230 (33.9) | 142 (27.1) | < 0.0001 | < 0.0001 |
| Perceived barriers of screening, N (%) | ||||||
| “Cancer screening can cause further physical burden depending on additional testing.” | 129 (32.4) | 586 (35.4) | 216 (31.3) | 169 (33.5) | 0.2026 | 0.9438 |
| Likelihood of engaging in screening, N (%) | ||||||
| “I will keep on undergoing cancer screening as long as I live no matter how old I get.” | 288 (73.64) | 1131 (68.52) | 442 (65.9) | 363 (71.8) | 0.046 | 0.3939 |
| Information seeking and communication, N (%) | ||||||
| “I have looked for information about cancer from some source.” | 330 (82.9) | 1088 (63.4) | 356 (50.9) | 194 (35.7) | < 0.0001 | < 0.0001 |
| “I have friends or family members that I talk to about my health.” | 376 (95.3) | 1581 (94.0) | 635 (92.4) | 469 (90.0) | 0.0062 | 0.0058 |
Screening criteria (e.g., age) were based on Ministry of Health, Labor, and Welfare recommendations for the general population for all screening approaches except PSA
For all values, weighted percentages are shown
For screening practices, multiple logistic regression analysis was performed with adjustment for age as a continuous variable
The FamCancer group had higher screening rates than the NoCancer group for colorectal cancer screening (OR, 1.43; 95% CI, 1.08–1.91) and lung cancer screening (OR, 1.43; 95% CI, 1.05–1.95). The FriendCancer group had higher screening rates than the NoCancer group for PSA (OR, 1.98; 95% CI, 1.19–3.30) only.
Beliefs about cancer and screening
As shown in Table 3, we also compared the items of the HBM (perceived susceptibility, severity, benefits, and barriers of screening), likelihood of engaging in cancer screening, and experience with information seeking and communication among the four groups.
CanSurvivors, followed by the FamCancer group, perceived themselves to be more susceptible and worried about getting cancer than the NoCancer group. Further, 42.3% of CanSurvivors, 30.2% of FamCancer, 23.4% of FriendCancer, and 21.8% of NoCancer participants indicated they were “extremely worried” or “moderately worried” about getting cancer (Table 3).
Analysis of the two items used to assess perceived severity revealed different results. When presented with the statement, “Every cancer detected by cancer screening grows to a life-threatening extent,” 11.5% of CanSurvivors, 8.2% of FamCancer, 7.1% of FriendCancer, and 8.1% of NoCancer participants indicated agreement. However, when presented with the statement, “When I think about cancer, I automatically think about death,” 68.5% of CanSurvivors agreed, while 75.8%, 72.6%, and 76.6% of FamCancer, FriendCancer, and NoCancer participants indicated agreement, respectively (Table 3).
Among the two items used to assess the perceived benefits of screening, over 96% of individuals in all four groups agreed with the statement, “Getting checked regularly for cancer helps find cancer when it’s easy to treat,” and no statistically significant difference was observed. In contrast, significant differences were noted in the remaining item, “These tests can definitely tell that a person has cancer.” While 48.3% of CanSurvivors agreed with the statement, just 28.9% of FamCancer, 33.9% of FriendCancer, and 27.1% of NoCancer agreed (p < 0.0001). Meanwhile, there was no statistically significant difference among the four groups regarding the item used to assess perceived barriers, “Cancer screening can cause further physical burden depending on additional testing.”
Regarding the likelihood of engaging in cancer screening, 73.6% of cancer survivors agreed with the statement, “I will keep on undergoing cancer screening as long as I live no matter how old I get,” compared to 68.5%, 65.9%, 71.8% of FamCancer, FriendCancer, and NoCancer participants, respectively (p < 0.05). Among the four groups, the highest proportion of individuals in CanSurvivors had sought out cancer information, followed by the FamCancer, FriendCancer, and NoCancer groups. Similarly, a greater proportion of individuals in CanSurvivors, followed by the FamCancer, FriendCancer, and NoCancer, indicated that they had friends or family members they talk to about their health.
Screening practices of cancer survivors for new primary cancers
We compared the screening practices of cancer survivors for other cancers and determined the adjusted OR in reference to the NoCancer group (Table 4). Overall, no significant differences were observed in ORs in each cancer group compared to the NoCancer group. Gastric cancer screening was performed by 93.6% of colorectal cancer survivors, 74.3% of lung cancer survivors, 84.4% of breast cancer survivors, 89.0% of cervical cancer survivors, 88.8% of prostate cancer survivors, and 100% of liver cancer survivors. The OR of colorectal cancer survivors performing gastric cancer screening compared to the NoCancer group was 3.19 (95% CI, 0.90–11.37).
Table 4.
Screening for new primary cancers in cancer survivors compared to the NoCancer group
| Reported ever having | NoCancer (n = 519) | Gastric cancer survivor (n = 55) | Colorectal cancer survivor (n = 48) | Lung cancer survivor (n = 16) | Breast cancer survivor (n = 70) | Cervical cancer survivor (n = 29) | Prostate cancer survivor (n = 30) | Liver cancer survivor (n = 7) | Multiple cancer survivor (n = 46) |
|---|---|---|---|---|---|---|---|---|---|
| Gastric cancer screening (men and women, ≥ 50 years) | |||||||||
| Weighted % | 78.6 | – | 93.6 | 74.3 | 84.4 | 86.0 | 88.8 | 100 | 89.6 |
| N (screened/eligible for screening) | 129/166 | – | 41/44 | 12/16 | 53/62 | 16/19 | 26/29 | 6/6 | 38/42 |
| OR (95% CI) for screening | 1 | – |
3.19 (0.90–11.37) |
0.58 (0.17–2.04) |
1.38 (0.60–3.16) |
1.80 (0.52–6.16) |
1.54 (0.41–5.84) |
N/A |
1.67 (0.51–5.47) |
| Colorectal cancer screening (men and women, ≥ 40 years) | |||||||||
| Weighted % | 67.1 | 84.7 | – | 70.7 | 76.9 | 82.6 | 75.0 | 100 | 83.1 |
| N (screened/eligible for screening) | 188/281 | 45/54 | – | 11/16 | 55/68 | 21/26 | 23/30 | 6/6 | 40/46 |
| OR (95% CI) for screening | 1 |
2.02 (0.86–4.73) |
– |
0.85 (0.26–2.80) |
1.44 (0.70–2.95) |
2.19 (0.80–5.96) |
1.10 (0.39–3.06) |
N/A |
1.93 (0.72–5.18) |
| Lung cancer screening (men and women, ≥ 40 years) | |||||||||
| Weighted % | 76.3 | 83.5 | 84.0 | – | 83.8 | 89.0 | 85.0 | 100 | 89.1 |
| N (screened/eligible for screening) | 219/288 | 45/54 | 38/46 | – | 59/70 | 22/25 | 26/30 | 6/6 | 42/46 |
| OR (95% CI) for screening | 1 |
1.24 (0.51–3.03) |
1.30 (0.48–3.55) |
– |
1.39 (0.65–2.96) |
2.37 (0.67–8.31) |
1.42 (0.46–4.45) |
N/A |
2.14 (0.68–6.72) |
| Breast cancer screening (mammography) (women, ≥ 40 years) | |||||||||
| Weighted % | 72.1 | 82.7 | 62.5 | 64.8 | – | 80.5 | – | * | 70.6 |
| N (screened/eligible for screening) | 95/132 | 11/14 | 12/19 | 2/3 | – | 22/26 | – | * | 14/18 |
| OR (95% CI) for screening | 1 |
2.55 (0.52–12.56) |
0.92 (0.35–2.40) |
1.06 (0.08–14.13) |
– |
1.67 (0.48–5.80) |
– | * |
1.27 (0.39–4.11) |
| Cervical cancer screening (pap test) (women, ≥ 20 years) | |||||||||
| Weighted % | 71.4 | 59.6 | 54.3 | 70.4 | 81.8 | * | 54.7 | ||
| N (screened/eligible for screening) | 189/260 | 8/14 | 9/19 | 2/3 | 58/70 | – | – | * | 12/18 |
| OR (95% CI) for screening | 1 |
0.59 (0.14–2.41) |
0.46 (0.15–1.44) |
0.94 (0.07–11.89) |
1.92 (0.88–4.19) |
– | – | * |
0.54 (0.19–1.54) |
| Prostate-specific antigen test (men, ≥ 40 years) | |||||||||
| Weighted % | 17.7 | 31.9 | 42.7 | 42.6 | – | – | – | 76.1 | 65.3 |
| N (screened/eligible for screening) | 28/136 | 12/37 | 12/26 | 6/13 | – | – | – | 3/4 | 17/26 |
| OR (95% CI) for screening | 1 |
0.78 (0.28–2.20) |
1.50 (0.53–4.27) |
1.23 (0.29–5.27) |
– | – | – |
5.45 (0.68–43.39) |
2.26 (0.79–6.46) |
Screening criteria (e.g., age) were based on Ministry of Health, Labor, and Welfare recommendations for the general population for all screening approaches except PSA. For the screening rate, weighted percentages are shown
Values indicate odd ratios and 95% confidence intervals obtained using multiple logistic regression analysis adjusted for age as a continuous variable
Data for the “other cancer” category (n = 88) are not shown
N/A: OR and 95% CI could not be obtained due to the 0 cell
* Five liver cancer survivors were men. One woman did not respond to the question about mammography and Papanicolaou test
The colorectal cancer screening rate exceeded 80% among gastric cancer survivors (84.7%), cervical cancer survivors (82.6%), and liver cancer survivors (100%). ORs of gastric cancer survivors and cervical cancer survivors performing colorectal cancer screening were 2.02 (95% CI, 0.86–4.73) and 2.19 (95% CI, 0.80–5.96), respectively, compared to the NoCancer group.
The lung cancer screening rate exceeded 80% among all cancer survivors. Further, the ORs of all cancer survivor groups exceeded one, although no statistically significant differences were observed.
Interestingly, although the PSA test is only recommended as an opportunistic screening method, 42.7% of colorectal cancer survivors, 42.6% of lung cancer survivors, and 76.1% of liver cancer survivors had taken the PSA test.
Discussion
Previous studies have suggested that a cancer diagnosis could be a “cue to action” to motivate positive health behavior changes among cancer survivors [2, 54, 55] and their family and friends [2, 10]. The aim of this study was to further explore the effect of a cancer diagnosis on the screening practices of survivors and their family and close friends. To our knowledge, this is the first study to compare the cancer screening practices and related beliefs of four populations (cancer survivors, individuals with a family member who experienced cancer, individuals with a close friend who experienced cancer, and individuals without any history of cancer). We also conducted subgroup analysis to identify the practices of cancer survivors related to non-primary cancer screening according to cancer type.
In our study, the adjusted screening rates for gastric, colorectal, and lung cancer were highest among CanSurvivors followed by FamCancer, FriendCancer, and NoCancer groups. These results indicate that a cancer history in oneself, a family member, or close friend is positively related to screening practice. However, the trend was not equally observed among all cancer screens.
Compared to the NoCancer group, CanSurvivors were more likely to perform gastric cancer screening (OR, 1.75; 95% CI, 1.04–2.95) and colorectal screening (OR = 1.56, CI: 1.03, 2.36). The positive finding for colorectal screening is comparable to the results of a meta-analysis by Corkum et al., who reported an OR for colorectal cancer screening of 1.19 (95% CI, 1.10–1.30) [12]. The present study also adds the new finding that a family history of cancer is positively associated with colorectal screening (OR, 1.43; 95% CI, 1.08–1.91). In addition, our subgroup analysis of survivors suggested an interrelation between gastric cancer screening and colorectal cancer screening, with 95.6% of colorectal cancer survivors indicating they had performed gastric cancer screening and 84.7% of gastric cancer survivors indicating they had performed colorectal cancer screening. Given the historically high incidence and mortality of gastric cancer in Japan, the nation has a well-established gastric cancer screening system that facilitates widespread screening at both community and occupational sites, similar to the colorectal cancer screening program. Since stomach- and colon-related cancers are both treated in the gastroenterology department, patients with cancer at one site may have been provided information about and recommended by their physicians to undergo screening for the other site at follow-up.
To date, very few studies have examined participation in lung cancer screening as it is not recommended to the general population as part of organized mass screening programs in many countries. In contrast, Japan recommends mass screening for lung cancer using chest X-ray examination for non-high-risk groups in its screening guideline [56]. The present study showed that CanSurvivors and FamCancer groups had significantly higher screening rates for lung cancer than the NoCancer group. Subgroup analysis showed that only the lung cancer screening rate exceeded 80% in all cancer survivors. The mortality of lung cancer is highest for men and second highest for women among all cancers in Japan [57]. The lung is also the most common site for cancer metastases [58]. Thus, both cancer survivors and their family members may perceive greater susceptibility to lung cancer, leading them to undergo screening for early detection.
The screening rate for PSA, which is not a recommended population-based screening approach in Japan [27, 59], was much lower (18–38%) than that for other cancer screens (69–87%). In our study, undergoing PSA was significantly associated with a friend’s cancer history (OR, 1.98; 95% CI, 1.19–3.30) but not with any other cancer history. This finding is consistent with other evidence that a cancer diagnosis in a friend can prompt an increase in prostate cancer screening behavior [60, 61]. The decision to undergo opportunistic screening approaches such as the PSA test is left to an individual. For men in higher-risk age groups, the impact of a friend’s diagnosis may be great enough to prompt them to undergo screening even at their own financial expense. Further studies are warranted to examine the reason for the different trends in PSA compared to other cancer screening approaches.
In our study, analysis using logistic regression adjusted for age did not show a statistically significant association of cervical and breast cancers with cancer history. In a prior meta-analysis, Uhlig et al. reported that cancer survivors were 27% and 38% more likely to undergo breast cancer screening and cervical cancer screening than cancer-free controls, respectively [33]. Similarly, another meta-analysis by Corkum et al. reported that cancer survivors were 19% and 22% more likely to undergo breast cancer screening and cervical cancer screening, respectively [12]. However, most of the studies included in the articles by Uhlig et al. and Corkum et al. were conducted in North America, mainly in the USA. Even within studies conducted in the USA, heterogeneity among study populations has led to disparate results. Analysis of data from the nationwide HINTS survey in the USA by Mayer et al. showed that being a cancer survivor significantly influenced colorectal cancer screening practice but had no effect on screening practices for cervical, breast, prostate cancers [32]. Our results show the same trend. One survey-based study in South Korea showed a weaker association than our study for breast cancer screening (OR, 1.12; 95% CI, 0.87–1.45) and cervical cancer screening (OR, 1.16; 95% CI, 0.92–1.47) [62]. One possible explanation for the discrepant results may be related to the national campaigns and efforts to increase screening rates for breast and cervical cancers in different countries. In Japan, movements like the “pink ribbon” and “teal and white ribbon” campaigns have brought attention to breast and cervical cancers, respectively, and television advertisements highlight the importance of screening for these diseases. Health insurers and municipalities have also made efforts to improve invitation leaflets and brochures to increase breast and cervical cancer screening rates by tailoring messages to their target audience [63, 64], that the NoCancer group was also likely exposed to such campaigns and written material may explain why we did not observe differences among groups. However, further study is needed to explore explanations for the observed effect.
Overall, the four groups had different beliefs and perceptions about cancer and screening. CanSurvivors and FamCancer were more likely to perceive susceptibility and worry about getting cancer than the other two groups. However, among these two groups, although CanSurvivors strongly believed that screening can detect cancer, the FamCancer group did not have such a belief and showed lower likelihood of engaging in screening than CanSuvivors. This result suggests that, for family members of cancer survivors, messages that increase their self-efficacy for screening to prevent cancer may be more effective than messages based on fear appeals. As previous studies have suggested [63, 65], for those who are already aware of their susceptibility and the severity of the disease, using gain-frame messages such as “detecting cancer early can lead to a higher chance of cure” to increase self-efficacy can motivate them to undergo screening. In the present study, no statistical difference was observed in the four items of HBM between the FriendCancer group and NoCancer group, even though a greater proportion of the former group sought cancer information than the latter group. Given that we could not determine how close the respondents were to the friend that had received a cancer diagnosis, further studies are needed to clarify the associations.
This study has several limitations. First, we performed analysis of data from INFORM Study 2020, which was not designed to evaluate survivorship-related outcomes, nor was the sampling designed to be representative of the population of Japanese cancer survivors. Thus, the actual response rate for each group may have been different. It is possible that those who were not interested in cancer did not complete the survey; in which case, we may have underestimated the impact of cancer experience on screening. Second, we did not have an adequate sample size to explore the screening practices of some subsets of cancer survivors (e.g., there were only seven liver cancer survivors). Third, self-report of cancer history and screening practices are subject to recall bias, and no verification of the self-report was conducted. Fourth, because of the cross-sectional nature of the data, we were unable to examine the temporality of screening practices. We were also unable to confirm whether survivors were encouraged to undergo screening as a result of their diagnosis. Fifth, the HBM has not been evaluated for its predictive ability. However, it has been used to guide the identification and inclusion of relevant variables for understanding screening behavior [49]. Despite knowledge that oncologists or primary care physicians can drive behaviors related to screening among cancer survivors, we were unable to determine whether they or other public health resources influenced the survivors’ decision to undergo screening. Finally, data collection was conducted in the midst of the COVID-19 pandemic. It is unclear how this may have affected participants’ responses to the survey.
Nevertheless, this population-based study of Japanese stratified into four relatively robust groups (survivors, individuals with a family history, individuals with history among close friends, and individuals with no history of cancer) uniquely explored how their cancer experience affects their screening practices and related perceptions and beliefs. Our findings suggest the importance of targeting and tailoring communication strategies to raise awareness of screening. For people with no experience with cancer, such messages as “Cancer is not someone else’s problem. It can happen to you and your loved ones at any time” to evoke familiarity of cancer may be effective to encourage them to undergo screening. For cancer survivors, physicians should recommend screening for other cancers during follow-up care. The period during which patients are transitioning from a hospital-based specialist to follow-up care with a community-based general practitioner may be a good opportunity to provide inclusive information about cancer screening.
Acknowledgements
This work was supported by the National Cancer Center Research and Development Fund (grant numbers 30-A-18, 2021-A-19). The items of the questionnaire used in this survey were selected and developed by members of the INFORM Study group: Maiko Fujimori, Masayo Hayakawa, Satoyo Hosono, Manami Inoue, Yuki Kaji, Kota Katanoda, Aya Kuchiba, Tomohiro Matsuda, Yutaka J. Matsuoka, Miyuki Odawara, Aki Otsuki, Junko Saito, Hirokazu Takahashi, Miyako Takahashi, Yosuke Uchitomi, Jun Umezawa, Otome Watanabe, Akiko Yaguchi-Saito, Itsuro Yoshimi, and Taichi Shimazu (National Cancer Center); Gary L. Kreps (George Mason University); Naomi Sakurai (Cancer Solutions Co., Ltd.); Takumi Momosaka (Hitotsubashi University); Miki Akiyama (Keio University); Rina Miyawaki (Meiji University); Ryo Okubo (National Center of Neurology and Psychiatry); Rie Akamatsu, Yui Kawasaki (Ochanomizu University); Kahori Fujisaki (Sagami Women’s University); Hirono Ishikawa, Runa Ogawa, and Yoshiharu Fukuda (Teikyo University); Haruhiko Imamura (Toho University); Kumi Nakaya and Naoki Nakaya (Tohoku University); and Seigo Mitsutake and Hiroyuki Sasai (Tokyo Metropolitan Institute of Gerontology). Miki Akiyama and Taichi Shimazu equally contributed to the manuscript.
Data Availability
The datasets used in this study are not publicly available. However, anonymized datasets may be available after approval by the INFORM study group and the institutional review board. Proposals for use of the data (Research question, Aim, Background, Design, and analytical plan) should be submitted to the corresponding author.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heinsch M, Cootes H, Wells H, Tickner C, Wilson J, Sultani G, et al. Supporting friends and family of adults with a primary brain tumour: a systematic review. Health Soc Care Community. 2022;30:869–887. doi: 10.1111/hsc.13586. [DOI] [PubMed] [Google Scholar]
- 2.Humpel N, Magee C. Jones SC The impact of a cancer diagnosis on the health behaviors of cancer survivors and their family and friends. Support Care Cancer. 2007;15:621–630. doi: 10.1007/s00520-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y. Given BA Quality of life of family caregivers of cancer survivors. Cancer. 2008;112:2556–2568. doi: 10.1002/cncr.23449. [DOI] [PubMed] [Google Scholar]
- 4.Clark PG, Brethwaite DS. Gnesdiloff S Providing support at time of death from cancer: results of a 5-year post-bereavement group study. J Soc Work End Life Palliat Care. 2011;7:195–215. doi: 10.1080/15524256.2011.593156. [DOI] [PubMed] [Google Scholar]
- 5.Kent EE, Rowland JH, Northouse L, Litzelman K, Chou WSS, Shelburne N, et al. Caring for caregivers and patients: research and clinical priorities for informal cancer caregiving. Cancer. 2016;122:1987. doi: 10.1002/cncr.29939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong A, Shin DW, Kim SY, Yang HK, Shin JY, Park K, et al. The effects on caregivers of cancer patients’ needs and family hardiness. Psycho-oncology (Chichester, England) 2016;25:84–90. doi: 10.1002/pon.3878. [DOI] [PubMed] [Google Scholar]
- 7.Northouse LL, Katapodi MC, Schafenacker AM. Weiss D The impact of caregiving on the psychological well-being of family caregivers and cancer patients. Semin Oncol Nurs. 2012;28:236. doi: 10.1016/j.soncn.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Ramasamy T, Veeraiah S. Balakrishnan K Psychosocial issues among primary caregivers of patients with advanced head and neck cancer - a mixed-method study. Indian J Palliat Care. 2021;27:503–512. doi: 10.25259/IJPC_76_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Audrain J, Schwartz M, Herrera J, Goldman P. BUSH A Physical activity in first-degree relatives of breast cancer patients. J Behav Med. 2001;24:587–603. doi: 10.1023/A:1012943411367. [DOI] [PubMed] [Google Scholar]
- 10.Lemon SC, Zapka JG. Clemow L Health behavior change among women with recent familial diagnosis of breast cancer. Prev Med. 2004;39:253–262. doi: 10.1016/j.ypmed.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Rabin C. Pinto B Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psycho-oncology (Chichester, England) 2006;15:701–712. doi: 10.1002/pon.1000. [DOI] [PubMed] [Google Scholar]
- 12.Corkum M, Hayden JA, Kephart G, Urquhart R, Schlievert C. Porter G Screening for new primary cancers in cancer survivors compared to non-cancer controls: a systematic review and meta-analysis. J Cancer Surviv. 2013;7:455–463. doi: 10.1007/s11764-013-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGillivray HMK, Piccolo EEL. Wassersug RJ “Partner”, “caregiver”, or “co-survivor”—might the label we give the partners of cancer patients affect the health outcome of the patients and their partners? Curr Oncol. 2021;29:122–129. doi: 10.3390/curroncol29010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall CA, Curran MA, Koerner SS, Weihs KL, Hickman AC, García FAR. Information and support for co-survivors during or after cancer treatment: consideration of Un Abrazo Para la Familia as a model for family-focused intervention in cancer rehabilitation. Work. 2013;46(4):395–405. [DOI] [PMC free article] [PubMed]
- 15.Bottorff JL, Oliffe JL, Halpin M, Phillips M, McLean G. Mroz L Women and prostate cancer support groups: the gender connect? Soc Sci Med. 2008;66:1217–1227. doi: 10.1016/j.socscimed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Ghamary L, Foodani N, Sadeghi N. Azarbarzin M Relationship between perception of family and friends’ support and psychosocial adaptation in adolescents suffering from cancer. J Nurs Midwifery Sci. 2022;9:124–131. doi: 10.4103/jnms.jnms_61_21. [DOI] [Google Scholar]
- 17.Aizer AA, Chen M, Mccarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–76. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Yang K, Zhang Y, Wu C, Wen D, Lv J, et al. Assessment of modifiable factors for the association of marital status with cancer-specific survival. JAMA Netw Open. 2021;4(5):2111813. doi: 10.1001/jamanetworkopen.2021.11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Wang X, Chen M, Shi Y, Hu Y. Family interaction among young Chinese breast cancer survivors. BMC Fam Pract. 2021;22(1):122. doi: 10.1186/s12875-021-01476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Regional Office for Europe A short guide to cancer screening increase effectiveness, maximize benefits and minimize harm. 2022.
- 21.International Agency for Reserach on Cancer IARC Handbooks of cancer prevention, Volume 15: Breast Cancer Screening . IARC Handbooks 2016.
- 22.Internatinal Agency for Research on Cancer IARC handbooks of cancer prevention, volume 18: cervical cancer screening. IARC Handbook. 2022; 18.
- 23.Internatinal Agency for Research on Cancer IARC Handbooks of cancer prevention, volume 17: colorectal cancer screening. 2019.
- 24.Center for Cancer Control and Information Services. National Cancer Center [Internet] Cancer Screening Rate.
- 25.Center for Disease Control and Prevention Screen Out Cancer, Advancing Cancer Screening Nationwide. Available from: https://www.cdc.gov/screenoutcancer/. Accessed 7 Jan 2023.
- 26.Institute of Medicine, National Research Council, National Cancer Policy Board, Susan J. Curry, Tim Byers, Maria Hewitt. Fulfilling the potential of cancer prevention and early detection. 2003. [PubMed]
- 27.Hamashima C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn J Clin Oncol. 2018;48:278–286. doi: 10.1093/jjco/hyx190. [DOI] [PubMed] [Google Scholar]
- 28.Ministry of Health Labour and Welfare Cancer Screening (in Japanese). Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000059490.html. Accessed 7 Jan 2023.
- 29.Japanese Urological Association. Screening gudeline for prostate cancer 2018. https://www.urol.or.jp/lib/files/other/guideline/32_prostate_cancer_screening_2018.pdf. Accecced 10 Mar 2023.
- 30.Center for Cancer Control and Information Services, National Cancer Center. Screening Performance Measures-2016 Data Book. 2017. https://ganjoho.jp/public/qa_links/report/pdf/Cancer_Screening_Performance_Measures_2016.pdf. Accecced 10 Mar 2023.
- 31.OECD. Health Care Utilisation: Screening, Breast cancer screening, survey data. OECD.Stat. https://stats.oecd.org/index.aspx?queryid=30159. Accessed 1 Nov 2022.
- 32.Mayer DK, Terrin NC, Menon U, Kreps GL, McCance K, Parsons SK, et al. Screening practices in cancer survivors. J Cancer Surviv. 2007;1:17–26. doi: 10.1007/s11764-007-0007-0. [DOI] [PubMed] [Google Scholar]
- 33.Uhlig A, Mei J, Baik I, Meyer C. Uhlig J Screening utilization among cancer survivors: a meta-analysis. J Public Health (Oxf) 2018;40:129–137. doi: 10.1093/pubmed/fdx030. [DOI] [PubMed] [Google Scholar]
- 34.Wilkins KL. Woodgate RL Preventing second cancers in cancer survivors. Oncol Nurs Forum. 2008;35:E12–E22. doi: 10.1188/08.ONF.E12-E22. [DOI] [PubMed] [Google Scholar]
- 35.Zhu G, Chen Y, Zhu Z, Lu L, Bi X, Deng Q, et al. Risk of second primary cancer after treatment for esophageal cancer: a pooled analysis of nine cancer registries. Dis Esophagus. 2012;25:505–511. doi: 10.1111/j.1442-2050.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- 36.Travis LB. The Epidemiology of Second Primary Cancers. Cancer Epidemiol Biomark Prev. 2006;15:2020–2026. doi: 10.1158/1055-9965.EPI-06-0414. [DOI] [PubMed] [Google Scholar]
- 37.Preyer O, Concin N, Obermair A, Concin H, Ulmer H, Oberaigner W. The relative risk of second primary cancers in Austria’s western states: a retrospective cohort study. BMC Cancer. 2017;17(1):699. doi: 10.1186/s12885-017-3683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youlden DR, Baade PD. The relative risk of second primary cancers in Queensland, Australia: a retrospective cohort study. BMC Cancer. 2011;11:83. doi: 10.1186/1471-2407-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feller A, Matthes KL, Bordoni A, Bouchardy C, Bulliard J, Herrmann C, et al. The relative risk of second primary cancers in Switzerland: a population-based retrospective cohort study. BMC Cancer. 2020;20(1):51. 10.1186/s12885-019-6452-0. Erratum in: BMC Cancer. 2020;20(1):87. [DOI] [PMC free article] [PubMed]
- 40.Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P. Travis LB Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30:3734–3745. doi: 10.1200/JCO.2012.41.8681. [DOI] [PubMed] [Google Scholar]
- 41.Madlensky L, Vierkant RA, Vachon CM, Pankratz VS, Cerhan JR, Vadaparampil ST, et al. Preventive health behaviors and familial breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2340–2345. doi: 10.1158/1055-9965.EPI-05-0254. [DOI] [PubMed] [Google Scholar]
- 42.Bronner K, Mesters I, Weiss-Meilik A, Geva R, Rozner G, Strul H, et al. Determinants of adherence to screening by colonoscopy in individuals with a family history of colorectal cancer. Patient Educ Couns. 2013;93:272–281. doi: 10.1016/j.pec.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 43.Becker MH. The health belief model and sick role behavior. Health Educ Behav. 1974;2:409–419. [Google Scholar]
- 44.Janz NK. Health belief the health belief model: a decade later. Patient Educ Couns. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs L. Health beliefs of first-degree relatives of individuals with colorectal cancer and participation in health maintenance visits: a population-based survey. Cancer Nurs. 2002;25:251–265. doi: 10.1097/00002820-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Koc S. Esin M Screening behaviors, health beliefs, and related factors of first-degree relatives of colorectal cancer patients with ongoing treatment in Turkey. Cancer Nurs. 2014;37:E51–E60. doi: 10.1097/NCC.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 47.Bai Y, Wong CL, Peng X, So WKW. Colonoscopy screening behaviour and associated factors amongst first-degree relatives of people with colorectal cancer in China: testing the health belief model using a cross-sectional design. Int J Environ Res Public Health. 2020;17(14):4927. doi: 10.3390/ijerph17144927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manne S, Markowitz A, Winawer S, Guillem J, Meropol NJ, Haller D, et al. Understanding intention to undergo colonoscopy among intermediate-risk siblings of colorectal cancer patients: a test of a mediational model. Prev Med. 2003;36:71–84. doi: 10.1006/pmed.2002.1122. [DOI] [PubMed] [Google Scholar]
- 49.Yarbrough SS, Braden CJ, Yarbrough S. Utility of health belief model as a guide for explaining or predicting breast cancer screening behaviours. J Adv Nurs. 2001;33(5):677–88. doi: 10.1046/j.1365-2648.2001.01699.x. [DOI] [PubMed] [Google Scholar]
- 50.Otsuki A, Saito J, Yaguchi-Saito A, Odawara M, Fujimori M, Hayakawa M, et al. A nationally representative cross-sectional survey on health information access for consumers in Japan: a protocol for the INFORM study. World Med Health Policy. 2022;14:220–270. doi: 10.1002/wmh3.506. [DOI] [Google Scholar]
- 51.Finney Rutten LJ, Blake KD, Skolnick VG, Davis T, Moser RP. Hesse BW Data resource profile: the National Cancer Institute’s Health Information National Trends Survey (HINTS) Int J Epidemiol. 2019;49:17. doi: 10.1093/ije/dyz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamashima C. Update version of the Japanese Guidelines for gastric cancer screening. Jpn J Clin Oncol. 2018;48:673–683. doi: 10.1093/jjco/hyy077. [DOI] [PubMed] [Google Scholar]
- 53.Walter K. Introduction to variance estimation. 2nd edition, New York: Springer-Verlag.
- 54.Hoedjes M, Nijman I, Hinnen C. Psychosocial determinants of lifestyle change after a cancer diagnosis: a systematic review of the literature. Cancers (Basel). 2022;14(8):2026. doi: 10.3390/cancers14082026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tollosa DN, Tavener M, Hure A. James EL Adherence to multiple health behaviours in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2019;13:327–343. doi: 10.1007/s11764-019-00754-0. [DOI] [PubMed] [Google Scholar]
- 56.The Japan Lung Cancer Society Lung Cancer Screening Guideline 2022. In: Anonymous , 2022.
- 57.Center for Cancer Control and Information Services, National Cancer Center Cancer Statistics in Japan. Available from: https://ganjoho.jp/reg_stat/statistics/stat/summary.html. Accessed 7 Jan 2023.
- 58.Gerull WD, Puri V. Kozower BD The epidemiology and biology of pulmonary metastases. J Thorac Dis. 2021;13:2585–2589. doi: 10.21037/jtd.2020.04.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamashima C, Saito H, Nakayama T, Nakayama T. Sobue T The standardized development method of the Japanese guidelines for cancer screening. Jpn J Clin Oncol. 2008;38:288–295. doi: 10.1093/jjco/hyn016. [DOI] [PubMed] [Google Scholar]
- 60.Weinrich S, Holdford D, Boyd M, Creanga D, Cover K, Johnson A, et al. Prostate cancer education in African American churches. Publ Health Nurs (Boston, Mass.) 1998;15:188–195. doi: 10.1111/j.1525-1446.1998.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 61.McKee JM. Cues to action in prostate cancer screening. Oncol Nurs Forum. 1994;21(7):1171–6. [PubMed] [Google Scholar]
- 62.Oh MG, Han MA, Park J, Ryu SY, Park C. Choi SW Health behaviors of cancer survivors: the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV, 2007–09) Jpn J Clin Oncol. 2013;43:981–987. doi: 10.1093/jjco/hyt118. [DOI] [PubMed] [Google Scholar]
- 63.Ishikawa Y, Hirai K, Saito H, Fukuyoshi J, Yonekura A, Harada K, et al. Cost-effectiveness of a tailored intervention designed to increase breast cancer screening among a non-adherent population: a randomized controlled trial. BMC Public Health. 2012;12:760. doi: 10.1186/1471-2458-12-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okuhara T, Ishikawa H, Goto E, Okada M, Kato M. Kiuchi T Processing fluency effect of a leaflet for breast and cervical cancer screening: a randomized controlled study in Japan. Psychol Health Med. 2018;23:1250–1260. doi: 10.1080/13548506.2018.1492732. [DOI] [PubMed] [Google Scholar]
- 65.Tannenbaum MB, Hepler J, Zimmerman RS, Saul L, Jacobs S, Wilson K, et al. Appealing to fear: a meta-analysis of fear appeal effectiveness and theories. Psychol Bull. 2015;141:1178–1204. doi: 10.1037/a0039729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this study are not publicly available. However, anonymized datasets may be available after approval by the INFORM study group and the institutional review board. Proposals for use of the data (Research question, Aim, Background, Design, and analytical plan) should be submitted to the corresponding author.