Abstract
AIM
To determine the clinical characteristics, pathological types, tumor markers, treatments, and outcomes of Chinese patients with primary lacrimal sac lymphoma.
METHODS
This case-based retrospective study analyzed 15 Chinese patients with primary lacrimal sac lymphoma. The clinical data collected included gender, age at diagnosis, symptoms, imaging examination results, pathologic diagnosis, pathogen identification, tumor markers, treatments, follow-up, and prognosis. Descriptive statistics were used to characterize the patients. Progression-free survival (PFS) was defined as the time from surgery to the last follow-up, first record of tumor recurrence, or death.
RESULTS
There were 7 males and 8 females with unilateral primary lacrimal sac lymphoma in the left eye (n=6) or right eye (n=9). The initial symptom in 13 patients was epiphora, and 2 patients had redness and swelling in the lacrimal sac area. All patients ultimately developed epiphora, and 12 had masses in the lacrimal sac area. Analysis of preoperative plasma tumor markers indicated 14 patients had elevated homocysteine, 9 had elevated β2-microglobulin, and 2 had elevated lactate dehydrogenase (LDH); 2 patients had elevations of all three markers, and 1 patient had no elevation of any marker. All patients underwent surgical resection and 12 patients received postoperative chemotherapy. The pathological types were DLBCL (n=8), MALT lymphoma (n=5), and NK/T-cell lymphoma, nasal type (n=2). The mean follow-up time was 25.8mo (range: 4-41) and 2 patients died. Seven patients who underwent mass excision combined with dacryocystorhinostomy (DCR) had no postoperative epiphora. Eight patients who only underwent mass excision had varying degrees of postoperative epiphora. Preoperative LDH elevation and NK/T-cell lymphoma, nasal type were associated with poor prognoses.
CONCLUSION
Early diagnosis and treatment can lead to a good prognosis for most patients with primary lacrimal sac lymphoma. Mass resection combined with DCR can reduce the occurrence of post-surgical epiphora. The pathology type and tumor marker status are associated with prognosis.
Keywords: primary lacrimal sac lymphoma, pathological type, tumor markers, treatment and prognosis
INTRODUCTION
Primary lymphoma of the lacrimal sac is very rare, accounting for only 2% to 6% of lacrimal sac malignancies[1]–[6]. This tumor is a non-epithelial malignancy[2]–[5], and is considered an extranodal non-Hodgkin's lymphoma (NHL)[5],[7]. Patients often present with symptoms of secondary nasolacrimal duct obstruction with epiphora and dacryocystitis. Clinicians classify this malignancy as a lymphoma of the ocular appendage, a group that accounts for approximately 2% of all lymphomas and 5% of all non-Hodgkin's lymphomas[2]–[3],[5],[7]. The proportion of lacrimal sac lymphomas among ocular appendage lymphomas is uncertain.
Lymphomas of the lacrimal sac may be primary or secondary. Although lymphoma patients typically have systemic symptoms, lacrimal sac lymphoma manifests as an isolated ocular appendage lesion[5]. Primary lacrimal sac lymphoma refers to a lesion that originates from the lacrimal sac without systemic lesions, or the early stage of disease when there is no systemic disease[5].
Previous studies have characterized populations with lacrimal sac lymphoma in Europe[5], the United States[8], Japan[7], and China[9]. No literature has been found to assess tumor markers and prognostic factors associated with primary lacrimal sac lymphoma in PubMed. The objectives of the present case-based retrospective study were to describe the clinical characteristics, pathological types, tumor markers, treatments, and outcomes of a series of patients from China who had primary lacrimal sac lymphomas.
SUBJECTS AND METHODS
Ethical Approval
The retrospective review was approved by the Ethics Committee of the Third Medical Center of PLA General Hospital (No.KY2021-009), and all procedures were performed in accordance with the Declaration of Helsinki. Fifteen eligible patients were finally included. The surviving patients and the families of deceased patients were consulted at the beginning of this study and signed informed consent documents.
Inclusion and Exclusion Criteria
This was a retrospective study of the clinical data of patients who were diagnosed with primary lacrimal sac lymphoma at the Senior Department of Ophthalmology, the Third Medical Center of Chinese PLA General Hospital (Beijing, China). Lymphoma was based on pathological diagnosis[10], and the diagnosis of lacrimal sac lymphoma was based on the presence of the main tumor body of the lymphoma in the lacrimal sac region. According to the lymphoma staging system of the American Joint Committee on Cancer (AJCC), all patients had primary stage T2d nasolacrimal drainage system involvement[11], and none of them had systemic lymphoma. Patients with secondary lacrimal sac lymphoma, binocular lymphoma, or incomplete medical records were excluded. All patients received prolonged follow-up.
Data Collection and Statistical Analysis
The clinical data collected included gender, age at diagnosis, symptoms, imaging examination results, pathologic diagnosis, pathogen identification, tumor markers, treatments, follow-up, and prognosis. Descriptive statistics were used to characterize the patients. Progression-free survival (PFS) was defined as the time from surgery to the last follow-up, first record of tumor recurrence, or death. The Kaplan-Meier method was used to analyze PFS and overall survival (OS). Statistical analysis was performed using GraphPad version 8.0.
RESULTS
We examined the records of 15 patients who had primary lacrimal sac lymphoma (Table 1). There were 7 males and 8 females, the mean age was 56.5 years old (range: 29–88), and each patient had lymphoma in one eye (9 in the right eye and 6 in the left eye). One patient had hepatitis B and a pathological diagnosis of diffuse large B-cell lymphoma (DLBCL; Figure 1), and two patients were positive for the Epstein-Barr virus (EBV) and had natural killer/T-cell (NK/T-cell) lymphoma, nasal type (Figure 2). Thirteen patients had epiphora as an initial presenting symptom and two had redness and swelling in the medial canthal at that time. All patients had epiphora of varying severity at the first visitand 12 patients had masses in the medial canthal. Two patients had histories of fever. The pathological types were DLBCL (n=8), mucosa-associated lymphoid tissue lymphoma (MALT lymphoma; n=5), and NK/T-cell lymphoma, nasal type (n=2).
Table 1. Clinical details of patients with primary lacrimal sac lymphoma.
| Patient | Age/sex | Eye | Anatomy | Pathologic diagnosis | Symptoms at first visit | Pathogen | Tumor marker positivity | History | Surgery | Chemotherapy | Follow-up |
| 1 | 37/F | R | Lacrimal sac, nasolacrimal duct | DLBCL | Epiphora, mass of lacrimal sac | HBsAg | Hcy | Hepatitis B | Mass resection and DCR | 4 cycle R-CHOP | Survival: 41 mo, no epiphora |
| 2 | 88/M | R | Canaliculus, larimal sac, nasolarimal duct | DLBCL | Epiphora, mass of medial canthal | Hcy B2M | Mass resection and DCR | none (refusal) | Survival: 39 mo, no epiphora | ||
| 3 | 49/M | L | Canaliculus, larimal sac, nasolarimal duct | DLBCL | Epiphora, mass | Not determined | Hcy | Mass resection and DCR | 6 cycle R-CHOP | Survival: 37 mo, no epiphora | |
| 4 | 47/F | R | Larimal sac, nasolarimal duct | DLBCL | Epiphora, mass | Not determined | Hcy | Mass resection and DCR | 6 cycle R-CHOP | Survival: 34 mo, no epiphora | |
| 5 | 43/F | R | Larimal sac | MALT lymphoma | Epiphora, mass of medial canthal, mucopurulent discharge | Hcy B2M | Intubationa | Mass resection | 4 cycle R-CHOP | Survival: 33 mo, epiphora | |
| 6 | 57/M | R | Larimal sac, nasolarimal duct | DLBCL | Epiphora, mass | Hcy B2M | Mass resection | 6 cycle R-CHOP | Survival: 32 mo, epiphora | ||
| 7 | 55/M | R | Canaliculus, larimal sac | MALT lymphoma | Epiphora, mass of medial canthal | ND | Hcy B2M | Mass resection and DCR | 4 cycle R-CHOP | Survival: 30 mo, no epiphora | |
| 8 | 29/F | L | Canaliculus, larimal sac, nasolarimal duct | NK/T-cell lymphoma, nasal type | Epiphora, red and swelling of medial canthal | Epstein-Barr virus | Hcy B2M LDH | No initial fever; fever developed | Mass resection | 2 cycle R-CHOP | Death: 4 mo, epiphora |
| 9 | 58/M | R | Larimal sac | MALT lymphoma | Epiphora, mass | Not determined | Hcy | Mass resection | None | Survival: 26 mo, epiphora | |
| 10 | 69/F | R | Larimal sac, nasolarimal duct | DLBCL | Epiphora, mass | Not determined | Hcy B2M | Mass resection | 6 cycle R-CHOP | Survival: 25 mo, epiphora | |
| 11 | 71/M | L | Canaliculus, larimal sac | MALT lymphoma | Epiphora, mass | Not determined | Hcy B2M | Mass resection | None | Survival: 21 mo, epiphora | |
| 12 | 60/F | R | Canaliculus, larimal sac, nasolarimal duct | DLBCL | Epiphora, mass of medial canthal, mucopurulent discharge | Mass resection and DCR | 4 cycle R-CHOP | Survival: 21 mo, no epiphora | |||
| 13 | 62/F | L | Canaliculus, larimal sac | MALT lymphoma | Epiphora | Not determined | Hcy | Mass resection | 4 cycle R-CHOP | Survival: 19 mo, epiphora | |
| 14 | 73/F | L | Larimal sac, nasolarimal duct | DLBCL | Epiphora, mass | Not determined | Hcy B2M | Mass resection and DCR | 4 cycle R-CHOP | Survival: 19 mo, no epiphora | |
| 15 | 50/M | L | Canaliculus, larimal sac, nasolarimal duct | NK/T-cell lymphoma, nasal type | Epiphora, discharge, redness and swelling of medial canthal | Epstein-Barr virus | Hcy B2M LDH | No initial fever; fever developed | Mass resection | 3 cycle R-CHOP | Death: 6 mo, epiphora |
B2M: β2 microglobulin; DCR: dacryocystorhinostomy; DLBCL: Diffuse large B-cell lymphoma; F: Female; Hcy: Homocysteine; L: Left; LDH: Lactate dehydrogenase; M: Male; MALT lymphoma: Mucosa-associated lymphoid tissue lymphoma; NK/T-cell lymphoma, nasal type: Natural killer/T-cell lymphoma, nasal type; R: Right; R-CHOP: Rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone. aPatient-5 received intubation for epiphora, which did not improve after the surgery, followed by the growth of a medial canthus mass.
n=15
Figure 1. Patient No.1, a 37-year-old female with primary lacrimal sac lymphoma (DLBCL) of the right eye.
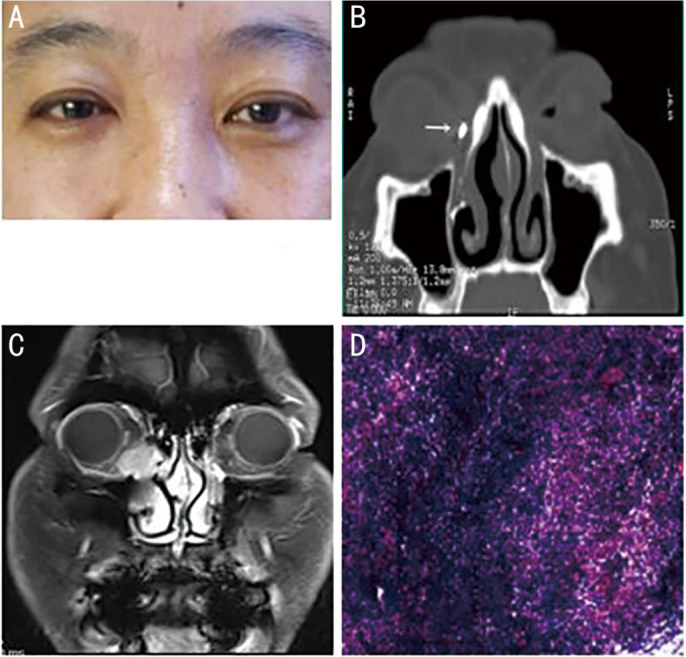
A: Clinical photograph. B: Computed tomography dacryocystography (CT-DCG), showing right post-saccal stenosis, a medial canthus soft tissue mass, and no bone destruction. The arrow indicates the contrast agent (nasolacrimal duct, coronal view). C: Magnetic resonance imaging (MRI), showing the mass had T1 hyperintensity. D: Postoperative pathological examination.
Figure 2. Patient No.8, a 29-year-old female with primary lacrimal sac lymphoma of the left eye (NK/T-cell lymphoma, nasal type) of the left eye.
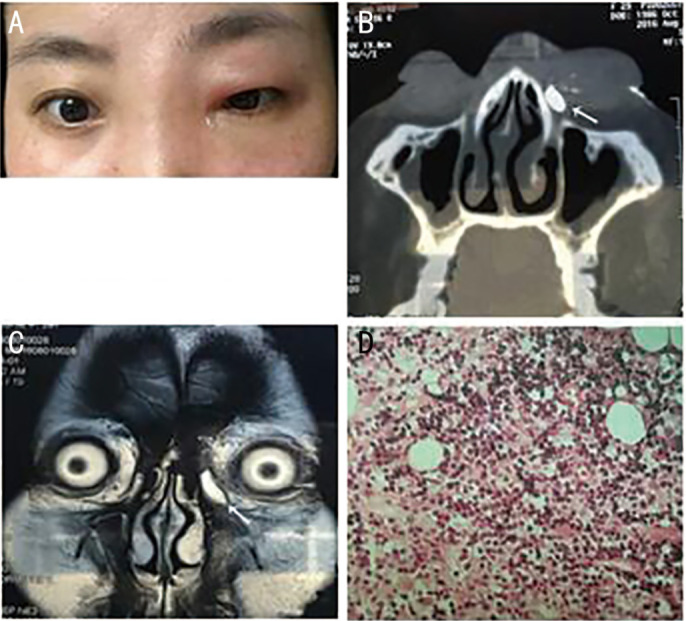
A: Clinical photograph during early-stage disease, when her symptoms her symptoms suggested acute dacryocystitis. B: Computed tomography dacryocystography (CT-DCG), showing a left post-saccal obstruction, a medial canthus soft tissue mass, and no bone destruction. The arrow indicates the contrast agent (nasolacrimal duct, coronal view). C: Magnetic resonance imaging (MRI), showing the mass had T2 hyperintensity. D: Postoperative pathological examination.
We checked all patients preoperatively for three plasma tumor markers, homocysteine [Hcy, upper-limit of normal (ULN): 15 µmol/L], β2 microglobulin (B2M, ULN: 3.0 mg/L), and lactate dehydrogenase (LDH, ULN: 240 IU/L; Table 2). One patient who had DLBCL had normal levels of all three markers. The Hcy level was elevated in 14 patients (7 with DLBCL, 5 with MALT lymphoma, and 2 with NK/T-cell lymphoma, nasal type); the B2M level was elevated in 9 patients (5 with DLBCL, 2 with MALT lymphoma, and 2 with NK/T-cell lymphoma, nasal type); and the LDH level was elevated in 2 patients (each with NK/T-cell lymphoma, nasal type and also with elevated levels of both other markers).
Table 2. Clinical characteristics of patients with primary lacrimal sac lymphoma.
| Characteristics | Value |
| Age, mean (range) | 56.5 (29–88)y |
| Gender, n | |
| Male | 7 |
| Female | 8 |
| Affected eye, n | |
| Right | 9 |
| Left | 6 |
| Follow-up time, mean (range) | 25.8 (4–41)mo |
| Initial presenting symptoms, n | |
| Epiphora | 13 |
| Redness and swelling of medial canthal | 2 |
| Symptoms at subsequent visits, n | |
| Epiphora | 15 |
| Mass | 12 |
| Redness and swelling of medial canthal | 2 |
| Late systemic symptoms, n | |
| Fever | 2 |
| Pathologic type, n | |
| DLBCL | 8 |
| MALT lymphoma | 5 |
| NK/T-cell lymphoma, nasal type | 2 |
| CT-DCG, n | |
| No bone destruction | 15 |
| Serum tumor marker positivity, n | |
| Hcy | 14 |
| B2M | 9 |
| LDH | 2 |
| All three high | 2 |
| All three normal | 1 |
| Symptoms during follow-up, n | |
| Epiphora | 8 |
n=15
All patients underwent complete tumor resection or combined with DCR, and 12 patients received postoperative chemotherapy. Patients with high malignant lymphoma, or with rapid progression of the disease, or with stage II or above need chemotherapy. The patients with DLBCL which was highly malignant received chemotherapy. Patients with pathological types which were between diffuse large B-cell lymphoma and MALT lymphoma, and have a clear tendency to transform to diffuse large B-cell lymphoma, received chemotherapy. Patients with simple MALT lymphoma which is low-grade malignant did not receive chemotherapy. In this study, 2 patients with NK/T-cell lymphoma, nasal type which was highly malignant received chemotherapy. The mean follow-up time after surgery was 25.8mo (range: 4-41mo). All 7 patients who underwent mass resection with postoperative chemotherapy achieved disease control and had no postoperative epiphora. The eight patients who only underwent mass resection had postoperative epiphora.
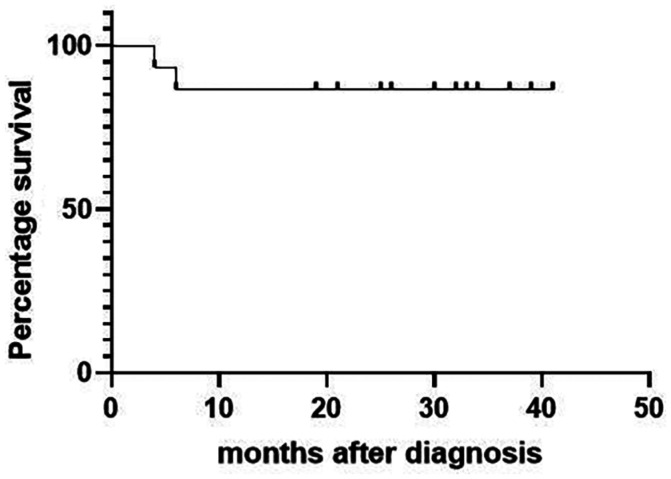
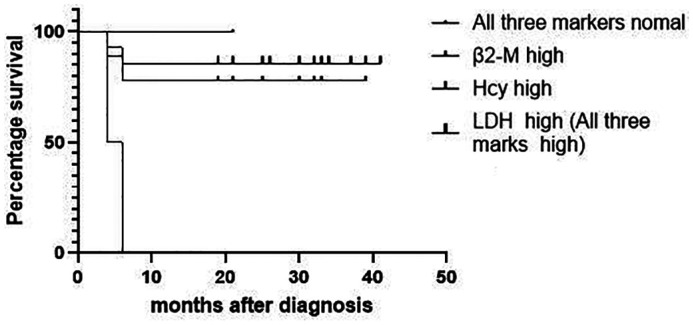
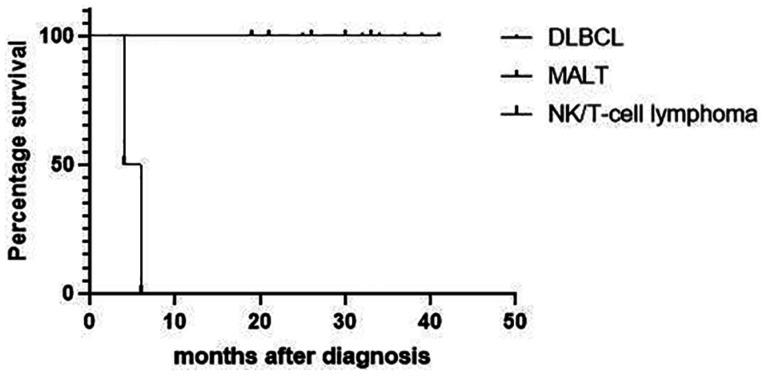
As of the last follow-up (mean: 25.8mo, range: 4-41mo), 13 patients were alive (Figure 3). Analysis of the three tumour markers indicated that patients with an elevated preoperative level of LDH level had the worst prognosis (Figure 4). Analysis of the three pathological types of lymphomas indicated patients with NK/T-cell lymphoma, nasal type had the worst prognosis (Figure 5). Two patients died, and both had NK/T-cell lymphoma, nasal type and elevated preoperative levels of LDH. One died of lymphoma-related hemophagocytic syndrome and the other died of systemic metastasis. The 13 other patients had no recurrence of the mass as of the last follow-up.
Figure 3. Kaplan-Meier analysis of overall survival in patients with primary lacrimal sac lymphoma.
Figure 4. Kaplan-Meier analysis of progression-free survival in patients with primary lacrimal sac lymphoma who tested positive for different tumor markers.
Figure 5. Kaplan-Meier analysis of overall survival in patients who had three different pathological types of primary lacrimal sac lymphoma.
DISCUSSION
Primary lacrimal sac lymphoma is a non-epithelial lacrimal sac malignancy that accounts for fewer than 10% of lacrimal sac malignancies[1]–[6]. Most studies of this malignancy are case reports and there are few clinical studies. The most common symptom is epiphora and swelling[4]–[5],[12]–[14]; some patients also present with dacryocystitis[4]–[5],[15] due to obstruction of the nasolacrimal duct, or rigidity of lacrimal sac or nasolacrimal duct tissue, resulting in drainage dysfunction. Thus, many patients with early-stage cancer are misdiagnosed as having common lacrimal duct stenosis or obstruction, and clinicians may consider lymphoma only after development of a mass in the medial canthus. Some patients experience bloody tears or discharge[4]. Because lacrimal sac lymphoma occurs in the submucosal lymphoid tissue of the lacrimal sac—not in the epithelial tissue — bloody tears are rare. Bloody tears may suggest a malignancy, whereas patients with lacrimal sac lymphoma often present only with epiphora and swelling, leading to misdiagnosis. Many patients receive an accurate diagnosis after a pathological examination that includes DCR[4],[16]. Some of these patients may only receive intubation, thus resulting in missed or delayed diagnosis, leading to a lost opportunity for treatment or to spread of the lesion. There is evidence that patients with lacrimal sac lymphoma have a greater risk for acute dacryocystitis[4], possibly because of immune deficiency and nasolacrimal duct obstruction from the lymphoma[3]. However, none of our patients developed acute dacryocystitis, although some patients presented with pseudo-acute dacryocystitis.
Lymphoma of the lacrimal sac is classified as a lymphoma of the ocular appendage, and ocular adnexal lymphoma is generally considered more common in older adults[11]. Although our patients had diverse ages, 10 of them (67%) were over 50 years old and there was a slight female predominance (53%). Primary lacrimal sac lymphoma is mostly unilateral, but there are reports of bilateral involvement[15]. Bilateral lacrimal sac lymphomas appear to occur more often as secondary than primary lymphomas[4], and bilateral involvement may be a manifestation of occult systemic disease, rather than restrictive disease.
Several studies of ocular adnexal lymphoma found that most of these lymphomas were low-grade B-cell lymphomas, and MALT lymphoma was the most common type[17]–[18] , with up to 80% MALT lymphoma in one study[19]. High-grade B-cell lymphomas are rarer, as are T-cell lymphomas, NK-cell lymphomas, and other types[18].
Although lacrimal sac lymphoma is an ocular adnexal lymphoma, we found that highly aggressive DLBCL was the most common pathological type, followed by inert MALT lymphoma, and T-cell lymphoma, different from the findings regarding ocular appendage lymphomas. Our results are consistent with several other studies of lacrimal sac lymphoma[4]–[5],[7]. In particular, Sjö et al[5] performed a European Organization for Research and Treatment of Cancer study of primary lacrimal sac lymphoma in a predominantly white population and reported DLBCL in 33% of cases, MALT lymphoma in 33%, transitional MALT lymphoma in 20%, and unclassified B-cell lymphoma in 13%. Sabundayo et al[7] studied primary lacrimal sac lymphoma in a Japanese population and found that DLBCL was more common than MALT lymphoma, and that NK/T-cell lymphoma was rare, although they only examined 5 patients.
Singh and Ali[4] conducted a PubMed search of English language literature on lacrimal sac lymphoma and found that primary lymphomas involving the lacrimal sac were more prevalent (63%). Furthermore, among primary lacrimal sac lymphomas, DLBCL (43%) was the most common, followed by MALT lymphoma (24%), unclassified B-cell lymphoma (21%), lymphoid hyperplasia (5%), small lymphocytic lymphoma (SLL, 3%) and NK/T-cell lymphoma (3%). But some other results have been reported. Meng et al[9] retrospectively analyzed 8 Chinese patients with primary dacryocystoid lymphomas, including five MALT lymphoma, one DLBCL, one NK/T-cell lymphoma, and one mantle cell lymphoma. Although Singh and Ali[4] found more reports of primary than secondary lacrimal sac lymphoma, there could have been a possible publication bias. Neerukonda et al[8] recently reported more cases of secondary than primary lacrimal sac lymphoma.
Extranodal NK/T-cell lymphoma, nasal type is a rare and highly aggressive non-Hodgkin's lymphoma (NHL)[20] that is more common in Asia and Central America than in the United States and Europe[21] and is highly associated with EBV infection[6],[21]–[22]. This malignancy mostly involves the nasal cavity and paranasal sinuses, and more rarely the lacrimal sac and nasolacrimal duct, and the prognosis is worse for patients with lymphoma in the lacrimal sac than in the nose[22]. NK/T-cell lymphoma, nasal type involving the lacrimal sac may resemble acute dacryocystitis, because patients typically present with redness, swelling, masses of the lacrimal sac, and epiphora[23]. Two of our 15 patients (13%) had NK/T-cell lymphoma, nasal type, and both presented early with symptoms that were similar to acute dacryocystitis.
The Chinese Southwest Oncology Group and Asia Lymphoma Study Group ENKTL (CA) staging system classifies NK/T-cell lymphoma involving the lacrimal sac as stage-I/II NK/T-cell[21]. Most patients with stage-I/II NK/T-cell are classified as low-risk in the International Prognostic Index (IPI), but some patients have poor prognoses[24]–[25]. Both of our patients with NK/T-cell lymphoma died within 6mo.
A high plasma Hcy level is associated with a variety of cancers, including lymphoma[26]–[28] and is indicative of cancer cell proliferation. However, Hcy has high sensitivity but only average specificity for diangosis of cancer[26]. Hcy is not elevated during the early stages of cancer, but is significantly elevated in the later stages[26]. Only 1 of our patients had a normal Hcy level, presumably due to early-stage lymphoma in this patient; however, Hcy was elevated in the two patients who died, suggesting it may be useful as an indicator of prognosis. A high plasma C level is a major marker of NHL, and the level corresponds with tumor load[29] and overall survival in lymphoma patients[30] Our two patients with NK/T-cell lymphoma had B2M levels within the normal range during the early stage of disease, but their levels gradually increased over time, and there were significant elevations upon definitive diagnosis. LDH is the only biological parameter incorporated into the IPI score for DLBCL, NK/T-cell lymphoma, and follicular lymphoma, and may be related to the proliferative activity of this cancer[21],[29]. However, serum LDH elevation is less common in patients with NHL (10.2%-37%)[31]. Only 2 of our patients had elevated LDH; both of them also had elevated Hcy and B2M levels, NK/T-cell lymphoma, nasal type, and died within 6mo. Overall, patients with elevations of all three tumor markers had the worst prognosis, and patients with no elevation of any marker had smaller tumors and fewer invasive sites.
Surgical excision for patients with primary lacrimal sac lymphoma is necessary to remove the primary lesion and to obtain tissue for pathological examination. DCR is the gold standard treatment for nasolacrimal duct obstruction. When the tumor leads to nasolacrimal duct obstruction, combined DCR was required. When most of the lacrimal sac was excised at the same time as the tumor, DCR was also performed to avoid postoperative epiphora. For patients with rapid disease progression, or with poor general condition, only the tumor was removed. When the tumor was relatively limited and the scope of sac resection was limited, only the tumor was removed and the residual sac was repaired. Whether combined with DCR or not, it is important to remove the tumor as completely as possible and provide adequate specimens for pathological examination. Some researchers suggested that the combined use of DCR with excision might increase the risk of tumor spread and metastasis[2]. However, we found that DCR combined with surgical resection did not affect prognosis, and it appeared to reduce the incidence of postoperative epiphora. The classical chemotherapy regimens that combine rituximab with other agents also have good efficacy in patients with B-cell lymphoma.
In conclusion, primary lacrimal sac lymphoma is associated with more limited lesions than systemic lymphoma, and early definitive diagnosis and treatment can provide better patient outcome. Our results suggest that resection of the mass combined with DCR reduced the incidence of postoperative epiphora, and that tumor markers and pathological type were related to prognosis. Preoperative symptoms correlated with the type of lesion, preoperative plasma tumor markers were suggestive of prognosis, and postoperative pathological type appeared directly related to prognosis.
This study was limited by the small sample size, short observation period, and examination of patients at a single center. Examination of more patients at multiple centers, and long-term follow-up studies are needed to confirm our results.
Acknowledgments
Conflicts of Interest: Bai F, None; Tao H, None; Zhou XB, None; Wang F, None; Wang P, None; Wang LH, None; Zhang HY, None.
REFERENCES
- 1.Zhao Y, Hui JW, Yang LL, Zhao H, Lin JY, Pan Y. Clinical and pathological analysis of 64 patients with primary neoplasms of the lacrimal drainage system. Zhonghua Yan Ke Za Zhi. 2020;56(5):364–369. doi: 10.3760/cma.j.cn112142-20190414-00216. [DOI] [PubMed] [Google Scholar]
- 2.Krishna Y, Coupland SE. Lacrimal sac tumors—a review. Asia Pac J Ophthalmol (Phila) 2017;6(2):173–178. doi: 10.22608/APO.201713. [DOI] [PubMed] [Google Scholar]
- 3.Kadir SMU, Rashid R, Sultana S, Nuruddin M, Nessa MS, Mitra MR, Haider G. Lacrimal sac tumors: a case series. Ocul Oncol Pathol. 2022;8(1):42–51. doi: 10.1159/000520086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh S, Ali MJ. Lymphoproliferative tumors involving the lacrimal drainage system: a major review. Orbit. 2020;39(4):276–284. doi: 10.1080/01676830.2019.1634104. [DOI] [PubMed] [Google Scholar]
- 5.Sjö LD, Ralfkiaer E, Juhl BR, Prause JU, Kivelä T, Auw-Haedrich C, Bacin F, Carrera M, Coupland SE, Delbosc B, Ducrey N, Kantelip B, Kemeny JL, Meyer P, Sjö NC, Heegaard S, European Organization for Research and Treatment of Cancer Primary lymphoma of the lacrimal sac: an EORTC ophthalmic oncology task force study. Br J Ophthalmol. 2006;90(8):1004–1009. doi: 10.1136/bjo.2006.090589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramberg I, Toft PB, Heegaard S. Carcinomas of the lacrimal drainage system. Surv Ophthalmol. 2020;65(6):691–707. doi: 10.1016/j.survophthal.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Sabundayo MS, Takahashi Y, Kakizaki H. Lacrimal sac lymphoma: a series of Japanese patients. Eur J Ophthalmol. 2019;29(6):678–684. doi: 10.1177/1120672118803510. [DOI] [PubMed] [Google Scholar]
- 8.Neerukonda VK, Stagner AM, Wolkow N. Lymphoma of the lacrimal sac: the Massachusetts eye and ear experience with a comparison to the previously reported literature. Ophthalmic Plast Reconstr Surg. 2022;38(1):79–86. doi: 10.1097/IOP.0000000000001997. [DOI] [PubMed] [Google Scholar]
- 9.Meng FX, Yue H, Yuan YQ, Zhang R, Yuan YF, Bi YW, Qian J. Lacrimal sac lymphoma: a case series and literature review. Int J Ophthalmol. 2022;15(10):1586–1590. doi: 10.18240/ijo.2022.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Leval L, Jaffe ES. Lymphoma classification. Cancer J. 2020;26(3):176–185. doi: 10.1097/PPO.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 11.Graue GF, Finger PT, Maher E, Della Rocca D, Della Rocca R, Lelli GJ, Jr, Milman T. Ocular adnexal lymphoma staging and treatment: American Joint Committee on Cancer versus Ann Arbor. Eur J Ophthalmol. 2013;23(3):344–355. doi: 10.5301/ejo.5000224. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kim J, Baek S. Diffuse large B-cell lymphoma arising in the lacrimal sac. J Craniofacial Surg. 2021;33(1):e19–e21. doi: 10.1097/SCS.0000000000007861. [DOI] [PubMed] [Google Scholar]
- 13.Kakutani S, Takahashi Y, Valencia M, Kakizaki H. Diffuse large B-cell lymphoma of the lacrimal sac in a Japanese patient. Case Rep Ophthalmol. 2019;9(3):516–519. doi: 10.1159/000495745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueathaweephol S, Wongwattana P, Chanlalit W, Trongwongsa T, Sutthinont S. Lacrimal sac lymphoma: a case report. Arch Craniofac Surg. 2022;23(1):43–47. doi: 10.7181/acfs.2022.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz AAV, Gomes DM, Chahud F. Bilateral mantle cell lymphoma of the lacrimal sac and nasolacrimal duct. JAMA Ophthalmol. 2020;138(12):e202394. doi: 10.1001/jamaophthalmol.2020.2394. [DOI] [PubMed] [Google Scholar]
- 16.Mihailovic N, Steenken J, Elges S, Merté RL, Grenzebach U, Eter N, Alnawaiseh M. Masquerade lacrimal duct stenosis: prevalence of lacrimal sac tumors. Ophthalmologe. 2020;117(5):439–444. doi: 10.1007/s00347-019-00971-z. [DOI] [PubMed] [Google Scholar]
- 17.Ponzoni M, Govi S, Licata G, et al. A reappraisal of the diagnostic and therapeutic management of uncommon histologies of primary ocular adnexal lymphoma. Oncologist. 2013;18(7):876–884. doi: 10.1634/theoncologist.2012-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferry JA, Fung CY, Zukerberg L, Lucarelli MJ, Hasserjian RP, Preffer FI, Harris NL. Lymphoma of the ocular adnexa: a study of 353 cases. Am J Surg Pathol. 2007;31(2):170–184. doi: 10.1097/01.pas.0000213350.49767.46. [DOI] [PubMed] [Google Scholar]
- 19.Rootman DB, Mavrikakis I, Connors JM, Rootman J. Primary, unilateral ocular adnexal lymphoma: disease progression and long-term survival. Ophthalmic Plast Reconstr Surg. 2011;27(6):405–409. doi: 10.1097/IOP.0b013e31821cc4bb. [DOI] [PubMed] [Google Scholar]
- 20.Mei M, Wang YJ, Zhang MZ. Causes of mortality in cases with extra nodal natural killer/T-cell lymphoma, nasal type: a cohort study. PLoS One. 2019;14(4):e0214860. doi: 10.1371/journal.pone.0214860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Fu BB, Gale RP, Liang Y. NK-/T-cell lymphomas. Leukemia. 2021;35(9):2460–2468. doi: 10.1038/s41375-021-01313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki R. Pathogenesis and treatment of extranodal natural killer/T-cell lymphoma. Semin Hematol. 2014;51(1):42–51. doi: 10.1053/j.seminhematol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Eze AN, Kalejaiye AO. An unusual case of extranodal natural killer/T-cell lymphoma, nasal type masquerading as dacryocystitis and sinusitis. Ear Nose Throat J. 2023;102(3):NP114–NP118. doi: 10.1177/0145561320987643. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Xia ZJ, Huang HQ, Lu Y, Zhang YJ. Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in the treatment of stage IE/IIE extranodal natural killer/T cell lymphoma, nasal type: 13-year follow-up in 135 patients. Int J Hematol. 2012;96(5):617–623. doi: 10.1007/s12185-012-1174-y. [DOI] [PubMed] [Google Scholar]
- 25.Almeida de Arruda JA, de Castro Abrantes T, Cunha JLS, et al. Mature T/NK-cell lymphomas of the oral and maxillofacial region: a multi-institutional collaborative study. J Oral Pathol Med. 2021;50(6):548–557. doi: 10.1111/jop.13205. [DOI] [PubMed] [Google Scholar]
- 26.Hasan T, Arora R, Bansal AK, Bhattacharya R, Sharma GS, Singh LR. Disturbed homocysteine metabolism is associated with cancer. Exp Mol Med. 2019;51(2):1–13. doi: 10.1038/s12276-019-0216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang DH, Wen XM, Wu W, Guo Y, Cui W. Elevated homocysteine level and folate deficiency associated with increased overall risk of carcinogenesis: meta-analysis of 83 case-control studies involving 35, 758 individuals. PLoS One. 2015;10(5):e0123423. doi: 10.1371/journal.pone.0123423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y, Wei XF, Feng YF, et al. The influence of serum homocysteine level to the prognosis of newly diagnosed multiple myeloma patients and analysis of related factors. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29(2):530–534. doi: 10.19746/j.cnki.issn.1009-2137.2021.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Gui W, Wang T, Wang J, Wang L, He J, Yang B, Zhao Z, Zhang H, Zhang Q. An improved prognostic parameter for non-Hodgkin's lymphoma based on the combination of three serum tumor markers. Int J Biol Markers. 2008;23(4):207–213. doi: 10.1177/172460080802300402. [DOI] [PubMed] [Google Scholar]
- 30.Wang XL, Wang XL, He S, Zhai HL. Association of β2-microglobulin with the prognosis of non-Hodgkin's lymphoma: a meta analysis. Int J Clin Exp Med. 2015;8(3):3992–3999. [PMC free article] [PubMed] [Google Scholar]
- 31.Economopoulos T, Papageorgiou S, Dimopoulos MA, et al. Non-Hodgkin's lymphomas in Greece according to the WHO classification of lymphoid neoplasms. A retrospective analysis of 810 cases. Acta Haematol. 2005;113(2):97–103. doi: 10.1159/000083446. [DOI] [PubMed] [Google Scholar]