The Significance of Deprescribing in Older Adults Living with AD/ADRD
People living with Alzheimer’s disease and related dementias (AD/ADRD) are particularly burdened by the potential hazards of taking medications with diminished value as they progress to severe stage disease and near end of life. More than half of NH residents with severe AD/ADRD receive at least one medication with questionable benefit1 with nearly 40% of these occurring in the last year of life.2 Given the high rates of dysphagia,3 polypharmacy,4,5 increased vulnerability to adverse effects,6 and potential inability to comply with directions for appropriate use, it is likely that overly complex medication regimens that favor prevention over comfort will cause more harm than benefit.7,8
As AD/ADRD progresses and goals of care change near the end of life, the potential benefits of medications begin to be outweighed by the risk for adverse effects, burdens of administration, and prioritized health outcomes. Common examples include cholinesterase inhibitors in individuals with severe AD/ADRD, or use of statins, aspirin, or bisphosphonates for long-term prevention in adults with advanced age or life expectancy <1 year.9 In addition to assessing the clinical benefits and risks of medications, attention should also be given to the behavioral and functional symptoms of AD/ADRD that make it difficult and potentially unsafe to administer medications. Deprescribing is a patient-centered approach to reduce or stop medications that are no longer appropriate when considering time until benefit (i.e., the time from initiating treatment to when it begins to provide meaningful benefit), life expectancy, and goals of care.10 Optimizing medications through deprescribing is a pillar of goal-concordant care, defined as the alignment of treatment decisions with patients’ known goals and values, for people with serious or advanced illness, including AD/ADRD.
Imagine an 88-year-old patient with multiple chronic conditions with early-stage dementia who presents to clinic for a Medicare annual wellness visit. They have very few functional limitations, and after a discussion with the patient and their adult child, you agree on a set of treatment goals focused on preserving life expectancy. Over the next year, this patient has progressed to symptoms of moderate cognitive impairment, including difficulty remembering to take their medications, but continues to maintain their functional independence. At this stage, thinking in the long-term is less a priority and attention may be better spent focusing on preserving functional capacity so that they can continue to live as independently as possible. A year later, the patient’s cognition has significantly worsened to the point where they are no longer able to live alone, but still maintain much of their mobility. However, their adult child is very distressed by frequent outbursts, challenging behaviors, and difficulty swallowing medications. At what point in this patient’s trajectory is a lengthy medication list no longer tenable and at what point is deprescribing warranted?
To date, there has been little integration of medication preferences and deprescribing into research focused on evaluating goal concordant care for older adults living with AD/ADRD. Here, we highlight the need to address deprescribing as an integral component of care for older adults living with AD/ADRD as they progress to severe-stage disease and near end of life. We also highlight the need for future research to develop and validate measures of goal-concordant deprescribing to be used in intervention studies that aim to improve medication management for this population.
Deprescribing is Essential for Achieving Goal-Concordant Care in AD/ADRD
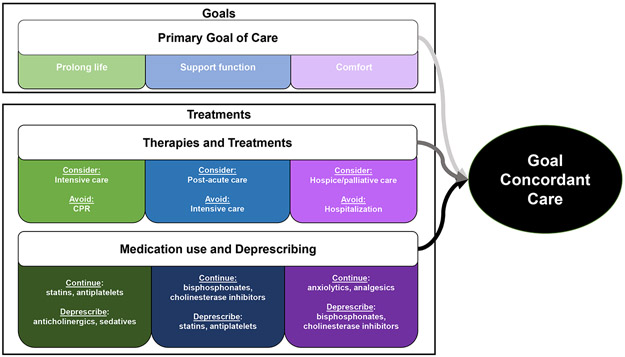
Goal-concordant care is an important outcome for high-quality AD/ADRD care and serious illness care research, allowing patients to receive care that supports their goals(e.g., prolong life, support function, improve comfort) and avoid treatments that do not (Figure 1).11 For example, comfort-oriented orders (e.g., do-not-hospitalize, hospice referral) aim to reduce treatment use that would not improve comfort for people with serious illness.12 Deprescribing provides an additional approach for improving comfort-focused care that has been under-studied.
Figure 1.
Goal-Concordant Dementia Care Continuum and Treatments
Frameworks operationalize goal-concordant carevia patient-specific and population-specific measures, including12 assessment from previously completed advance care planning and family reports of patient goals matched against healthcare utilization (e.g., cardiopulmonary resuscitation (CPR), intubation, hospitalization, hospice) and location of death.12 Deprescribing of medications whose risk-benefit profile is not consistent with goals of care at a particular stage of AD/ADRD is a logical additional component of utilization-based assessment of goal-concordant care. Of note, the progression of AD/ADRD from early to late-stage disease often coincides with a shift away from life-prolonging medications (e.g., statins) and those that help to maintain function (e.g., bisphosphonates) to those that focus on comfort only (e.g., analgesics and anxiolytics). Figure 1 illustrates this continuum and how evolving goals and priorities coincide with such shifts.
Challenges to Integrating Deprescribing into Goal-Concordant Care for AD/ADRD
Clinicians may face difficulty when reconciling medication use with goals of care due to a lack of clear and consistent documentation of goals-of-care. As part of these discussions, preferences for specific medications are typically absent, so goals-of-care must be extrapolated to specific treatments. Although there are sometimes clear indications for deprescribing due to risks and lack of continued effectiveness, goals of care are particularly relevant when there are clear pros and cons of continuing treatment, when patient and caregiver preferences should play a prominent role. For example, studies demonstrate high rates of use of cholinesterase inhibitors in nursing home residents with severe AD/ADRD13 and overly stringent diabetes management among nursing home residents at end of life,14 populations that tend to prioritize comfort.8,11 In the absence of standardized data elements to contextualize patients’ and caregivers’ specific preferences for medication use and potential reasons for discontinuation or continuation, many studies are limited in their ability to evaluate whether prescribing (and deprescribing) is appropriate and consistent with a patient or caregiver goals and preferences. Although there has been an increased interest in deprescribing research, there is currently no validated outcome measure for evaluating goal-concordant deprescribing, which limits researchers’ ability to contextualize findings from studies of medication discontinuation.
Although research could inform how and when to deprescribe, evidence on the benefits and potential harms of deprescribing is lacking. Randomized studies evaluating the benefits of medications seldom include older adults with AD/ADRD that impairs their ability to consent. Thus, there is a lack of empiric data to inform clinical decision-making regarding continued medication use in this population. Challenges in participant recruitment combined with ethical issues of consent in the presence of late-stage AD/ADRD and evaluation of medication withdrawal are barriers to generating evidence. Additionally, AD/ADRD care suffers from fragmentation across multiple specialists (i.e., neurology, cardiology) and lack of specialists (e.g., geriatricians, palliative care) to assist with deprescribing.15,16
Emerging Opportunities to Integrate Deprescribing into Goal-Concordant Care for AD/ADRD
To address the above challenges, studies utilizing routinely collected clinical data (e.g., from electronic health records [EHRs], Minimum Data Set [MDS] for nursing homes) are uniquely poised to inform strategies to align prescribing with patient goals and preferences. However, a necessary first step is to evaluate the ability of these data sources to characterize goals of care and determine whether they can be aligned with specific drugs. Accurate measures that distinguish intentional deprescribing from nonadherence or discontinuation due to other reasons in detailed clinical data have yet to be developed. Similarly, limited research has explored characterization of medication preferences in routinely collected clinical data. One of the few standardized data elements being integrated into research datasets is the recent addition of the patient attitudes toward deprescribing (PATD) questionnaire in the 2016 wave of the NHATS data. However, the validity and feasibility of even this type of assessment for people living with AD/ADRD or their family caregivers requires further exploration.17
Challenges notwithstanding, opportunities remain to measure deprescribing in the context of goal-concordant care. Data technologies are improving to evaluate deprescribing patterns across providers, health systems, and nursing homes. The IMPACT Collaboratory, funded by the National Institute of Aging, is developing a data repository to consolidate nursing home EHR data for research purposes.18 Other common data models, such as PCORNET, may have similar utility for exploring such research questions.19 Natural language processing technologies are increasingly being applied to free-text EHR notes, including to discern goals of care.20 Leveraging the strengths of these advances, future research should seek to address gaps in knowledge regarding characterizing deprescribing as a measure of goal-concordant care, including: 1) exploring the utility of data elements already available in routinely collected data sources; 2) exploring the utility of natural language processing techniques to characterize patient and caregiver goals and preferences; and 3) evaluating the feasibility of incorporating standardized assessments such as the PATD into routinely collected data.
An enhanced ability to compare deprescribing practices with documented goals of care would enable researchers and clinicians to evaluate the quality of medication use with greater certainty. This is particularly salient in situations where the use of seemingly low-value or inappropriate medications may be warranted based on patient or caregiver preferences, for example, the use of antipsychotic medications to manage refractory behavioral symptoms of AD/ADRD. The development of a measure for goal-concordant medication use would be instrumental for identifying deprescribing for interventional studies. Finally, measures for goal-concordant deprescribing that rely on existing data elements could be feasibly incorporated as benchmarks or quality indicators to evaluate the impact of system-wide interventions or policies that seek to improve outcomes through deprescribing.
Conclusion
Deprescribing is a logical component of quality hospice and palliative AD/ADRD care, yet it is infrequently addressed in literature evaluating goal-concordant care. Research to improve the measurement of medication goals and preferences through advancements in data linkage and natural language processing presents a tremendous opportunity to advance the fields of research for deprescribing and hospice and palliative AD/ADRD care.
ACKNOWLEDGEMENTS
DISCLOSURES:
Drs. Niznik, Ernecoff, Hanson, and Mitchell were supported by the National Institute on Aging (NIA) of the National Institutes of Health under Award Number U54AG063546, which funds NIA Imbedded Pragmatic Alzheimer’s Disease and AD-Related Dementias Clinical Trials Collaboratory (NIA IMPACT Collaboratory). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Niznik is supported by a career development award from the National Institutes on Aging (1K08AG071794).
Footnotes
Conflicts of Interest: All authors confirm they have no conflicts of interest to disclose.
References
- 1.Tjia J, Briesacher BA, Peterson D, Liu Q, Andrade SE, Mitchell SL. Use of Medications of Questionable Benefit in Advanced Dementia. JAMA Intern Med. 2014;174(11):1763–1771. doi: 10.1001/JAMAINTERNMED.2014.4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morin L, Vetrano DL, Grande G, Fratiglioni L, Fastbom J, Johnell K. Use of Medications of Questionable Benefit During the Last Year of Life of Older Adults With Dementia. J Am Med Dir Assoc. 2017;18(6):551.e1–551.e7. doi: 10.1016/J.JAMDA.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 3.Streicher M, Wirth R, Schindler K, Sieber CC, Hiesmayr M, Volkert D. Dysphagia in Nursing Homes-Results From the NutritionDay Project. J Am Med Dir Assoc. 2018;19(2):141–147.e2. doi: 10.1016/J.JAMDA.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 4.McNeil MJ, Kamal AH, Kutner JS, Ritchie CS, Abernethy AP. The Burden of Polypharmacy in Patients Near the End of Life. J Pain Symptom Manage. 2016;51(2):178. doi: 10.1016/J.JPAINSYMMAN.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jokanovic N, Tan ECK, Dooley MJ, Kirkpatrick CM, Bell JS. Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Dir Assoc. 2015;16(6):535.e1–535.e12. doi: 10.1016/J.JAMDA.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 6.Shi S, Klotz U. Age-related changes in pharmacokinetics. Curr Drug Metab. 2011;12(7):601–610. doi: 10.2174/138920011796504527 [DOI] [PubMed] [Google Scholar]
- 7.Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. American Journal of Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136 [DOI] [PubMed] [Google Scholar]
- 8.Ernecoff NC, Lin FC, Wessell KL, Hanson LC. Quality of Life with Late-Stage Dementia: Exploring Opportunities to Intervene. J Am Geriatr Soc. 2019;67(6). doi: 10.1111/jgs.15794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes HM, Sachs GA, Shega JW, Hougham GW, Cox Hayley D, Dale W. Integrating palliative medicine into the care of persons with advanced dementia: identifying appropriate medication use. J Am Geriatr Soc. 2008;56(7):1306–1311. doi: 10.1111/J.1532-5415.2008.01741.X [DOI] [PubMed] [Google Scholar]
- 10.Bruyère Research Institute. What Is Deprescribing?; 2019. [Google Scholar]
- 11.Ernecoff NC, Zimmerman S, Mitchell SL, et al. Concordance between Goals of Care and Treatment Decisions for Persons with Dementia. J Palliat Med. 2018;21(10):1442–1447. doi: 10.1089/jpm.2018.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernecoff NC, Wessell KL, Bennett A v., Hanson LC. Measuring Goal-Concordant Care in Palliative Care Research. J Pain Symptom Manage. 2021;62(3):e305–e314. doi: 10.1016/J.JPAINSYMMAN.2021.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niznik JD, Zhao X, He M, et al. Factors Associated With Deprescribing Acetylcholinesterase Inhibitors in Older Nursing Home Residents With Severe Dementia. J Am Geriatr Soc. 2019;67(9):1871–1879. doi: 10.1111/JGS.15985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niznik JD, Hunnicutt JN, Zhao X, et al. Deintensification of Diabetes Medications among Veterans at the End of Life in VA Nursing Homes. J Am Geriatr Soc. 2020;68(4):736–745. doi: 10.1111/JGS.16360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy LM, Farrell B, BScPhm PH, Quast T. Supporting deprescribing in long-term care: An approach using stakeholder engagement, behavioural science and implementation planning. Exploratory Research in Clinical and Social Pharmacy. Published online August 13, 2022:100168. doi: 10.1016/J.RCSOP.2022.100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeve E, Thompson W, Farrell B. Deprescribing: A narrative review of the evidence and practical recommendations for recognizing opportunities and taking action. Eur J Intern Med. 2017;38:3–11. doi: 10.1016/J.EJIM.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 17.Reeve E, Anthony AC, Kouladjian O’Donnell L, et al. Development and pilot testing of the revised Patients’ Attitudes Towards Deprescribing questionnaire for people with cognitive impairment. Australas J Ageing. 2018;37(4):E150–E154. doi: 10.1111/AJAG.12576 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell SL, Mor V, Harrison J, McCarthy EP. Embedded Pragmatic Trials in Dementia Care: Realizing the Vision of the NIA IMPACT Collaboratory. J Am Geriatr Soc. 2020;68(S2):S1–S7. doi: 10.1111/JGS.16621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleurence RL, Curtis LH, Califf RM, Platt R, Selby J v., Brown JS. Launching PCORnet, a national patient-centered clinical research network. Journal of the American Medical Informatics Association. 2014;21(4):578–582. doi: 10.1136/AMIAJNL-2014-002747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee RY, Brumback LC, Lober WB, et al. Identifying Goals of Care Conversations in the Electronic Health Record Using Natural Language Processing and Machine Learning. J Pain Symptom Manage. 2021;61(1):136–142.e2. doi: 10.1016/J.JPAINSYMMAN.2020.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]