Abstract
Due to the photocatalytic property of titanium dioxide (TiO2), its application may be dependent on the growing light environment. In this study, radish plants were cultivated under four light intensities (75, 150, 300, and 600 μmol m−2 s−1 photosynthetic photon flux density, PPFD), and were weekly sprayed (three times in total) with TiO2 nanoparticles at different concentrations (0, 50, and 100 μmol L−1). Based on the obtained results, plants used two contrasting strategies depending on the growing PPFD. In the first strategy, as a result of exposure to high PPFD, plants limited their leaf area and send the biomass towards the underground parts to limit light-absorbing surface area, which was confirmed by thicker leaves (lower specific leaf area). TiO2 further improved the allocation of biomass to the underground parts when plants were exposed to higher PPFDs. In the second strategy, plants dissipated the absorbed light energy into the heat (NPQ) to protect the photosynthetic apparatus from high energy input due to carbohydrate and carotenoid accumulation as a result of exposure to higher PPFDs or TiO2 concentrations. TiO2 nanoparticle application up-regulated photosynthetic functionality under low, while down-regulated it under high PPFD. The best light use efficiency was noted at 300 m−2 s−1 PPFD, while TiO2 nanoparticle spray stimulated light use efficiency at 75 m−2 s−1 PPFD. In conclusion, TiO2 nanoparticle spray promotes plant growth and productivity, and this response is magnified as cultivation light intensity becomes limited.
Subject terms: Light responses, Photosynthesis
Introduction
Global food demand is steadily rising as a result of a growing population1. To ensure global food security, further improvements in crop yields are necessary1,2. Intensifying agricultural production by increasing the associated inputs is commonly employed as an effective strategy to stimulate plant growth and performance3–6. Yet, improving input use efficiency stands out as a more attractive alternative6, since the intensification-associated environmental pressure is evaded7.
Noteworthy, the application of nanoparticles has proven to be a powerful tool in advancing yield8. For instance, the application of titanium dioxide (TiO2) nanoparticles has been associated with stimulation of carbohydrate production, chlorophyll formation, as well as improvements in photosynthetic rate and eventually yield9,10. These promotive effects are concentration dependent, and vary excessively among species9,11. In addition, the positive effect of TiO2 nanoparticles on plant growth and productivity depends on the environmental conditions during cultivation10,11. In this regard, their application may be exercised when the remaining conditions allow optimal impact on plants. For instance, plant growth and productivity also strongly depends on light availability12–15. Light is the main energy source-driver of photosynthesis. Light intensity is the pivotal determinant of plant growth and performance16–18. Production of crops in greenhouses and controlled environments has nowadays attracted a lot of attention19. There is a need to optimize the photosynthetic photon flux density (PPFD) needed for plant growth in an approach with the highest resource use efficiency6,18. TiO2 nanoparticles are used to improvemeyield during winter cultivation of plants20. However, the effect of either TiO2 nanoparticles application or light level has been limitedly addressed. It, therefore, remains unknown whether or not these two factors interact to determine plant yield.
Chlorophyll fluorescence is often employed for non-invasive assessments of the electron transport system properties21–24. Polyphasic chlorophyll fluorescence induction curves may be additionally utilized to study the fate of absorbed light energy and provide further insights into the structure and function of the photosynthetic system25–27.
TiO2 nanoparticles have special properties such as high specific area, easy operation, high absorption capacity, and photocatalytic activity. They have emerged as antimicrobial agents and growth promoters. Chahardoli et al.28, showed that promoting effects of TiO2 application on the growth of plants are concentration-dependent. Indeed, in low concentrations (50 and 100 mg L−1) TiO2 nanoparticles had a stimulating effect; while increasing the concentration to 2500 mg L−1 impose an inhibitory effect on plant growth24. It has been reported that TiO2 nanoparticles increase the rate of photosynthesis and plant immunity, leading to a 30% increase in yield11. In the study of Hossein et al.29, foliar application of 2.5 mg L−1 Ti, chlorophyll pigments (chlorophyll a, b, carotenoid), plant biomass, photochemical efficiency of photosystem II (Fv/Fm) and electron transfer rate (ETR) of soybean improve under natural light conditions. Choi et al.30 reported that TiO2 foliar application affected CO2 absorption and chlorophyll fluorescence of cherry tomato plants. Foliar spraying of TiO2 during cloudy days with low light intensity increased the electron transfer rate from QA to QB in the reaction center of photosystem II (ET0/RC) and carbon dioxide (CO2) stabilization. The use of TiO2 nanoparticles for strawberry cultivation in the winter, which is characterized by low light intensity, increased the chlorophyll content, fruit yield, and firmness of strawberry fruit20.
Controlled environment agriculture (CEA) has emerged as a new sustainable approach to increase the productivity of crops irrespective of natural environmental variations27. However, increasing the efficacy of artificial light in CEA is of vital importance to make it energy-cost effective31. There is scarce information on the interaction of light intensity and TiO2 nanoparticles in the CEA. Considering that TiO2 nanoparticles have photocatalytic activity, they can be capable of increasing the efficiency of light use32. Therefore, detailed information on their practical impacts on photosynthesis and crop growth is needed.
The aim of this study was to investigate for the first time the joint inputs of cultivation light level and TiO2 nanoparticles application on determining photosynthetic efficiency, biomass allocation and yield in controlled-environment agriculture. Radish (Raphanus raphanistrum L.) was employed as a model species, owing to the high harvest index (i.e., edible leaves and tuber), and short growth cycle33. The obtained data serve as a conceptual basis for understanding the approaches that plants utilized under different scenarios of light energy inputs, as well as for the stimulation of photosynthetic capacity with the aim of maximizing plant growth and productivity, especially in indoor environments where light use efficiency is of obvious concern.
Results
Characterization of TiO2
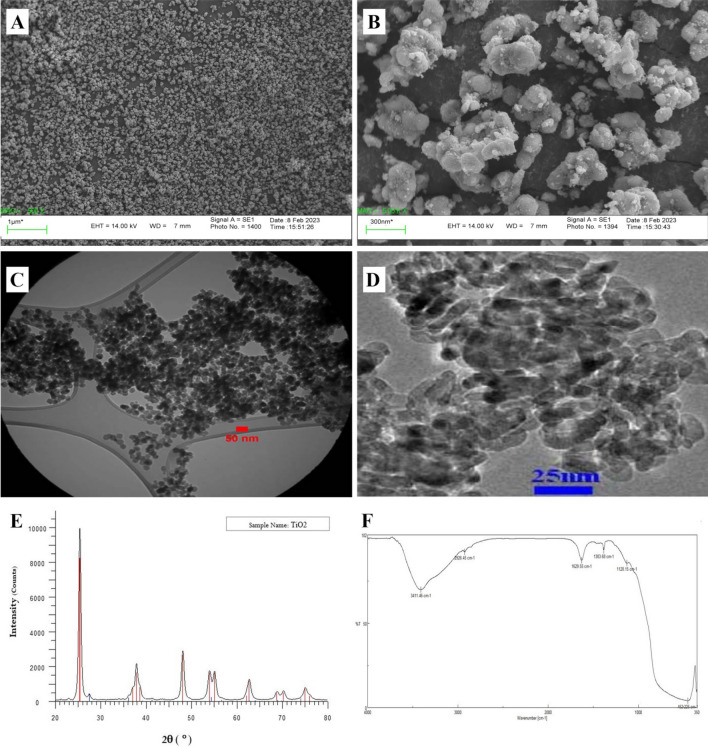
The surface morphology of TiO2 nanoparticles was determined by scanning electron microscope (SEM) and transmission electron microscopy (TEM) (Fig. 1A–D). Briefly, TiO2 nanoparticles were spherical or rod-shaped with -32 mV zeta potential and good dispersion. The size of most nanoparticles was in the range of 10–25 (average 18 nm) nm. Using BET method, SSA, pore volume, and average pore diameter were evaluated as 69.679 m2g−1, 0.2588 cm3g−1, and 14.857 nm, respectively.
Figure 1.
Representative scanning electron microscope (SEM), transmission electron microscopy (TEM) images (A–D), X-ray diffraction (XRD) pattern (E), and fourier-transform infrared spectroscopy (F) of titanium dioxide (TiO2) nanoparticles employed in the present study.
X-ray diffraction showed all diffraction peaks of the anatase phase well (Fig. 1E). The characteristic peak of the anatase phase was 2Ɵ = 25.334. Several sharp peaks are observed in the 2Ɵ range at 20–80°. The sharp peaks in the 2Ɵ range were 25.12°, 38.25°, 48.10°, 54°, 55.1°, 63°, 69.0°, 70.50°, and 75.10°.
The FTIR spectrum of TiO2 nanoparticles is shown in Fig. 1F. The used spectrometer has measured vibrations in the wavelength range of 350–4000 cm−1. The broad peak at 3411.46 cm−1 was related to the intermolecular interaction of the hydroxyl group (‒OH) attached to Ti. The 1629.55 peak corresponds to the -OH bending vibration. The broad peak at 452.225 cm−1 is assigned to the bending vibration bonds (Ti–O–Ti) in the TiO2 lattice34. The observation of the mentioned peaks in the FTIR spectrum indicates the presence of Ti in the nanoparticle structure and the formation of TiO2 nanoparticles.
In present study, TiO2 foliar application affected chlorophyll fluorescence parameters of radish plants under different light intensities. Our results showed that foliar spraying of TiO2 nanoparticles reduced the negative influence of low light intensity on radish. TiO2 treatment led to an increase in EI0/RC under low light conditions, which indicates that TiO2 improves the rate of electron transfer flux in photosystem II reaction centers. In addition, in the reaction center of TiO2-treated plants, the dissipated energy flux DI0/RC decreased, indicating that TiO2 contributed to the photosynthetic light response.
Increasing cultivation light intensity improved plant growth, and triggered biomass allocation towards the tuber in a TiO2 concentration-dependent manner
Plants were cultivated under four light intensities (75, 150, 300 and 600 μmol m−2 s−1 PPFD), and weekly sprayed with three TiO2 nanoparticle levels (0, 50, and 100 μmol L−1).
An increase in cultivation light intensity from 75 to 150 μmol m−2 s−1 PPFD resulted in a two-fold plant leaf area (Table 1; see also Fig. 2). This increase was mostly due to larger individual leaf area, and secondarily due to an increase in leaf number. A two-step increase in cultivation light intensity from 150 (though 300) to 600 μmol m−2 s−1 PPFD led to a smaller increase in plant leaf area. This increase was exclusively related to an increase in leaf number.
Table 1.
Effect of cultivation light intensity and spray treatment (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles on growth and morphology of radish cv.
| Light intensity (µmol m−2 s−1) | TiO2 (µmol L−1) | Leaf | Petiole length (leaf −1) | Dry weight (g) | Tuber volume (mL) | SLA (cm2 g−1) | LMR (g g−1) | TMR (g g−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number (plant−1) | Area (cm2 leaf−1) | Area (cm2 plant−1) | Shoot | Tuber | Plant | |||||||
| 75 | 0 | 4.0d | 11.0d | 43.9f | 7.11a | 0.09e | 0.07f | 0.15f | 1.50d | 520a | 0.56ab | 0.44de |
| 50 | 4.0d | 17.9abc | 71.5e | 5.47bcd | 0.16d | 0.11f | 0.27ef | 1.00d | 447ab | 0.60a | 0.40e | |
| 100 | 4.0d | 16.8c | 67.3e | 5.88bc | 0.17d | 0.13f | 0.30ef | 1.00d | 403b | 0.56ab | 0.44de | |
| 150 | 0 | 4.7bcd | 18.3abc | 85.2cd | 6.37ab | 0.22cd | 0.21ef | 0.43e | 2.00d | 415b | 0.50bc | 0.50cd |
| 50 | 5.3ab | 20.8abc | 109.8a | 4.93cde | 0.42b | 0.37de | 0.79d | 9.50c | 262c | 0.53ab | 0.47de | |
| 100 | 4.3cd | 21.9ab | 93.4bc | 7.79a | 0.25c | 0.57d | 0.81d | 2.50d | 383b | 0.30d | 0.70b | |
| 300 | 0 | 5.7a | 16.7c | 94.0bc | 4.91cde | 0.43b | 0.56d | 0.99d | 2.00d | 223cde | 0.43c | 0.57c |
| 50 | 5.0abc | 21.9ab | 110a | 3.92ef | 0.43b | 0.89c | 1.31c | 9.50c | 258cd | 0.33d | 0.67b | |
| 100 | 5.0abc | 22.6a | 110a | 3.77ef | 0.41b | 1.11b | 1.52c | 8.50c | 271c | 0.27de | 0.73ab | |
| 600 | 0 | 5.7a | 17.8bc | 100ab | 4.39def | 0.55a | 1.32b | 1.86b | 19.0b | 186de | 0.30d | 0.70b |
| 50 | 5.3ab | 19.4abc | 103ab | 3.29gf | 0.57a | 1.58a | 2.14a | 25.0a | 182e | 0.27de | 0.73ab | |
| 100 | 4.3cd | 19.0abc | 76.9de | 2.17g | 0.44b | 1.74a | 2.18a | 19.0b | 177e | 0.20e | 0.80a | |
Cherry belle plants. Six replicate plants were assessed per treatment. In traits, where the interaction of the two factors (light intensity, TiO2 nanoparticle concentration) was significant, different letters indicate significant differences.
LMR leaf mass ratio, SLA specific leaf area, TMR tuber mass ratio.
Figure 2.
The growth environment (top panels), and representative images of radish cv. Cherry belle plants cultivated under different light intensities and sprayed (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles at different levels.
At 75, 150, and 300 μmol m−2 s−1 PPFD, TiO2 nanoparticle spray stimulated plant leaf area (Table 1; see also Fig. 2). This increase was exclusively related to an increase in individual leaf area. At 150 μmol m−2 s−1 PPFD, the low TiO2 nanoparticle level (50 μmol L−1) was optimal for plant leaf area, while at 75 and 300 μmol m−2 s−1 PPFD no significant difference was noted among the two TiO2 nanoparticle levels. At 600 μmol m−2 s−1 PPFD, the low TiO2 nanoparticle level (50 μmol L−1) did not affect plant leaf area, while the high TiO2 nanoparticle level (100 μmol L−1) decreased it. This decrease was exclusively related to a decrease in leaf number.
Increasing cultivation light intensity resulted in shorter leaf petioles (Table 1). Within each light intensity, TiO2 nanoparticle spray decreased leaf petiole in a concentration-dependent manner in all cases, besides one (150 μmol m−2 s−1 PPFD, 100 μmol L−1).
Increasing cultivation light intensity strongly stimulated plant dry weight (Table 1; see also Fig. 2). For instance, plant dry weight was 0.15 g at 75 μmol m−2 s−1 PPFD, while it was 1.86 g at 600 μmol m−2 s−1 PPFD. This increase in plant dry weight was mediated via respective increases in both shoot and tuber dry weights.
At each cultivation light intensity, TiO2 nanoparticle spray stimulated plant dry weight (Table 1; see also Fig. 2). No significant difference was noted between the two TiO2 nanoparticle levels.
Increasing cultivation light intensity generally led to thinner leaves (i.e., lower SLA; Table 1). At 75 μmol m−2 s−1 PPFD, TiO2 nanoparticle spray was also associated with thinner leaves (Table 1). At 300 μmol m−2 s−1 PPFD, the opposite trend was noted (Table 1).
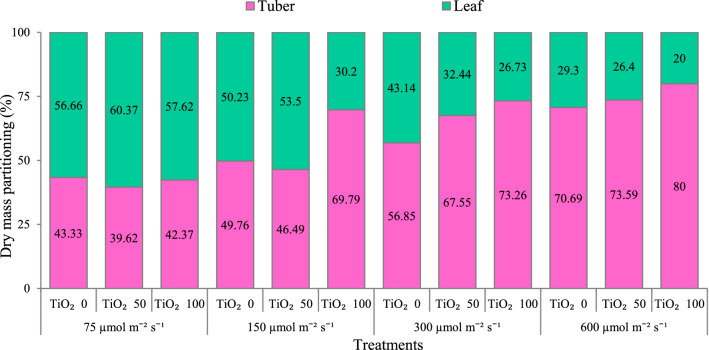
Increasing cultivation light intensity generally decreased dry mass allocation to leaves, and increased dry mass allocation to the tuber (Table 1; Fig. 3). At 300 and 600 μmol m−2 s−1 PPFD, this trend was magnified by TiO2 nanoparticle spray in a TiO2 concentration-dependent manner (Table 1; Fig. 3).
Figure 3.
Effect of cultivation light intensity (75, 150, 300 and 600 µmol m−2 s−1) and spray treatment (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles (0, 50 and 100 µmol L−1) on biomass partitioning of radish cv. Cherry belle plants. Six replicate plants were assessed per treatment.
Increasing cultivation light intensity generally improved leaf chlorophyll and carotenoid contents in a TiO2 concentration-dependent manner
Increasing cultivation light intensity up to 300 μmol m−2 s−1 PPFD led to an increase in leaf chlorophyll and carotenoid contents, while a further increase to 600 μmol m−2 s−1 PPFD resulted in a respective decrease (Fig. 4).
Figure 4.
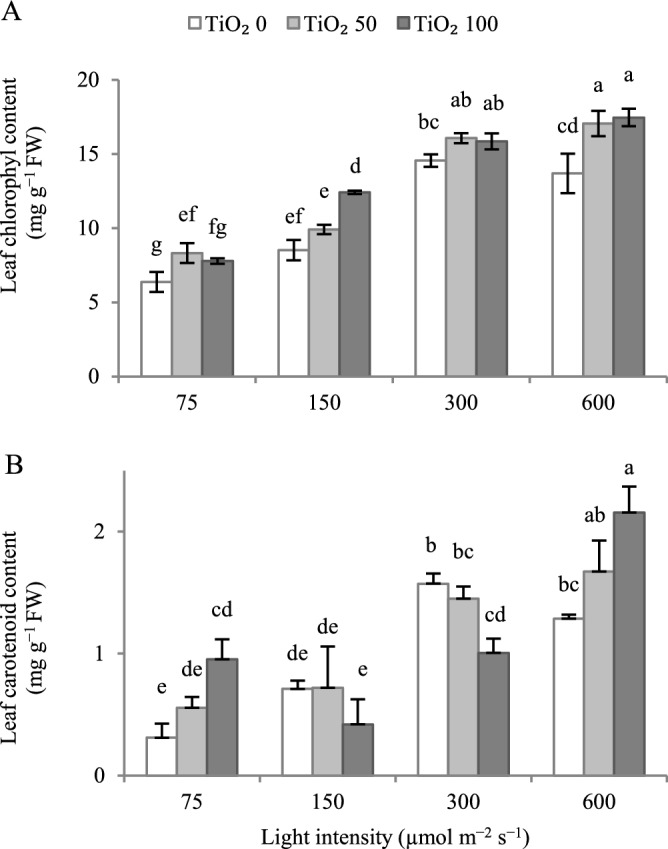
Effect of cultivation light intensity (75, 150, 300 and 600 µmol m−2 s−1) and spray treatment (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles (open, grey, and dark grey for 0, 50 and 100 µmol L−1, respectively) on leaf chlorophyll, and carotenoid contents of radish cv. Cherry belle plants. Six replicate plants were assessed per treatment. Bars represent SEM. In traits, where the interaction of the two factors (light intensity, TiO2 nanoparticle level) was significant, different letters indicate significant differences. FW, fresh weight.
TiO2 nanoparticle spray generally stimulated leaf chlorophyll content, and counteracted the noted decrease at 600 μmol m−2 s−1 PPFD (Fig. 4A).
At 75, and 600 µmol m−2 s−1 PPFD, TiO2 nanoparticle spray stimulated leaf carotenoid content, and counteracted the noted decrease at 600 μmol m−2 s−1 PPFD (Fig. 4B). At 150, and 300 µmol m−2 s−1 PPFD, the high TiO2 nanoparticle level (100 μmol L−1) tended to decrease leaf carotenoid content, with this effect being only significant at 300 µmol m−2 s−1 PPFD.
Increasing cultivation light intensity and applying TiO2 nanoparticles generally induce accumulation of soluble carbohydrates in both leaves and tuber
Increasing cultivation light intensity above 150 μmol m−2 s−1 PPFD tended to increase leaf total soluble carbohydrates content, with this effect being only significant at 600 µmol m−2 s−1 PPFD (Fig. 5A). At 300, and 600 µmol m−2 s−1 PPFD, the high TiO2 nanoparticle level (100 μmol L−1) stimulated leaf total soluble carbohydrates content.
Figure 5.
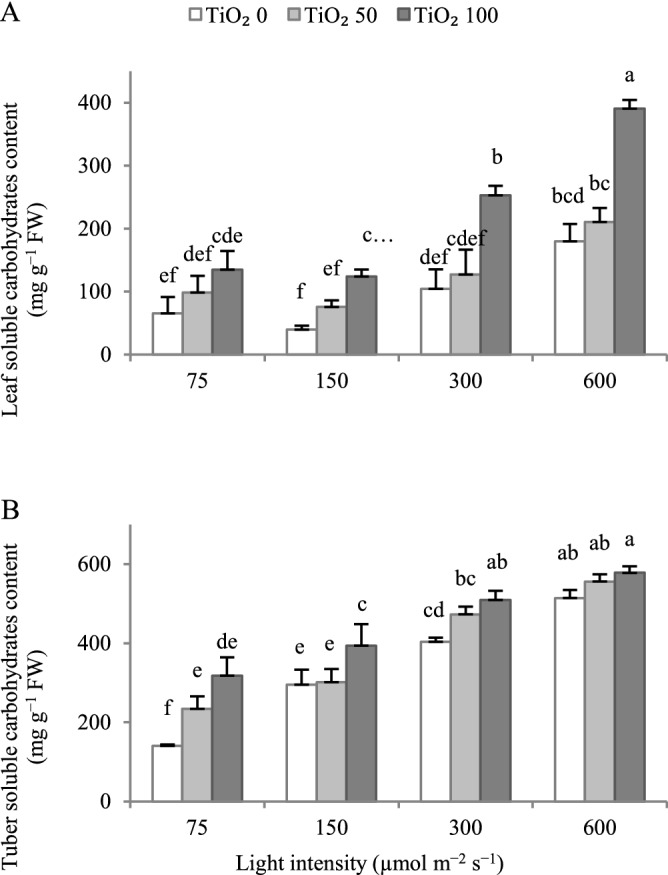
Effect of cultivation light intensity (75, 150, 300 and 600 µmol m−2 s−1) and spray treatment (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles (open, grey, and dark grey for 0, 50 and 100 µmol L−1, respectively) on total carbohydrates content in leaves and tuber (A,B, respectively) of radish cv. Cherry belle plants. Six replicate plants were assessed per treatment. Bars represent SEM. In traits, where the interaction of the two factors (light intensity, TiO2 nanoparticle level) was significant, different letters indicate significant differences. The difference in the y-axis scale among panels ought to be noted. FW, fresh weight.
Increasing cultivation light intensity enhanced tuber total soluble carbohydrates content (Fig. 5B). At 75, 150, and 300 µmol m−2 s−1 PPFD, TiO2 nanoparticle spray generally stimulated tuber total soluble carbohydrates content.
TiO2 nanoparticle application up-regulated photosynthetic functionality under low cultivation light intensity, while down-regulated photosynthetic functionality under high cultivation light intensity
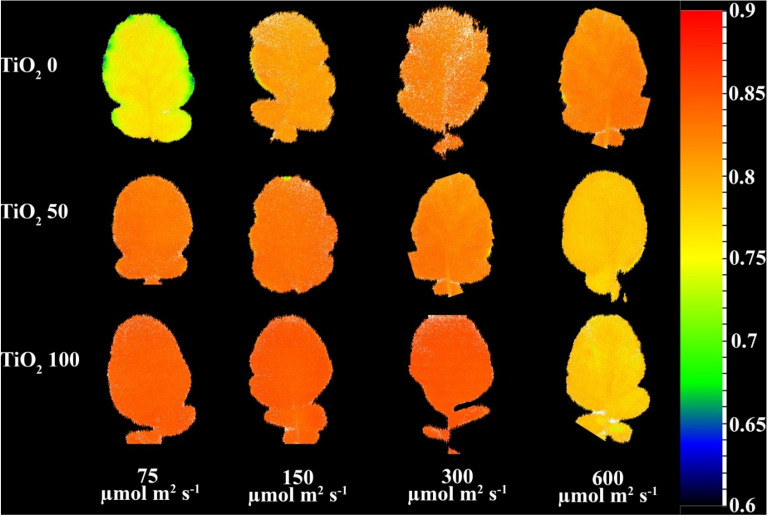
At the lowest cultivation intensity (75 µmol m−2 s−1 PPFD), the lowest Fv/Fm was noted (Fig. 6). At the highest cultivation intensity (600 µmol m−2 s−1 PPFD), intermediate Fv/Fm values were apparent, with the highest ones being noted at 150, and 300 µmol m−2 s−1 PPFD.
Figure 6.
Representative image of the maximal quantum yield of PSII photochemistry (Fv/Fm) (equation and explanation in Table 3) of radish cv. Cherry belle plants cultivated under different light intensities (75, 150, 300 and 600 µmol m−2 s−1) and sprayed (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles at different concentrations (0, 50 and 100 µmol L−1).
At 75, and 150 µmol m−2 s−1 PPFD, TiO2 nanoparticle spray stimulated Fv/Fm (Fig. 6). At 300 µmol m−2 s−1 PPFD, the high TiO2 nanoparticle level (100 μmol L−1) promoted Fv/Fm. At 600 µmol m−2 s−1 PPFD, TiO2 nanoparticle spray decreased Fv/Fm.
Increasing the cultivation light intensity generally decreased the minimum fluorescence when all PSII reaction centers are open (F0; Fig. 7A). At 75 µmol m−2 s−1 PPFD, TiO2 nanoparticle spray decreased F0.
Figure 7.
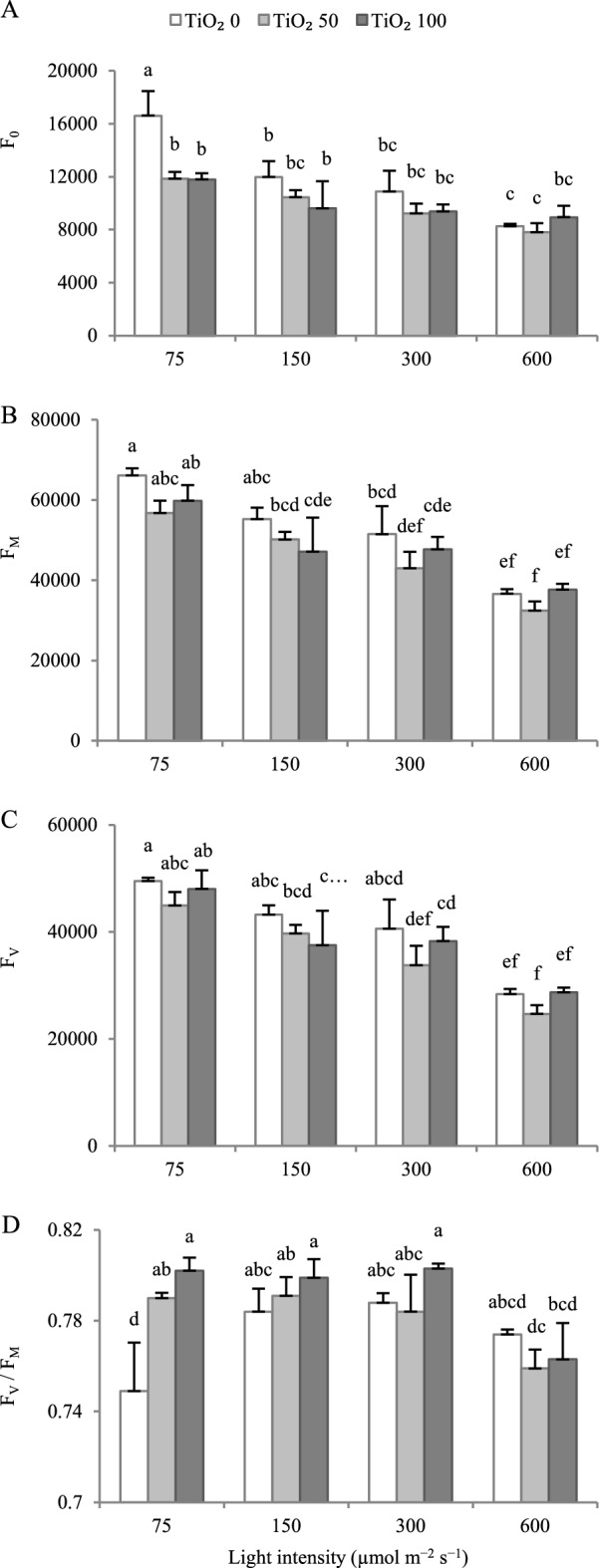
Effect of cultivation light intensity (75, 150, 300 and 600 µmol m−2 s−1) and spray treatment (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles (open, grey, and dark grey for 0, 50 and 100 µmol L−1, respectively) on minimum fluorescence when all PSII reaction centers are open (F0; A), maximum fluorescence when all PSII reaction centers are closed (Fm; B), variable fluorescence of the dark-adapted sample (Fv; C) and maximal quantum yield of PSII photochemistry (Fv/Fm; D) (equations and explanations in Table 3) of radish cv. Cherry belle plants. Nine replicate plants were assessed per treatment. Bars represent SEM. In traits, where the interaction of the two factors (light intensity, TiO2 nanoparticle level) was significant, different letters indicate significant differences.
Increasing the cultivation light intensity generally decreased the maximum fluorescence when all PSII reaction centers are closed (Fm; Fig. 7B) and the variable fluorescence of the dark-adapted sample (Fv; Fig. 7C). Within each light intensity regime, TiO2 nanoparticle spray did not have any effect on Fm and Fv.
Fv/Fm was higher at 150 and 300 µmol m−2 s−1 PPFD, as compared to 75 µmol m−2 s−1 PPFD (Fig. 7D). TiO2 nanoparticle spray increased Fv/Fm at 75 µmol m−2 s−1 PPFD.
Cultivation light intensity did not affect the quantum yield of electron transport (φE0; Fig. 8A) and the performance index in light absorption basis (PIABS; Fig. 8D). TiO2 nanoparticle spray increased φE0 and PIABS at 75 µmol m−2 s−1 PPFD.
Figure 8.
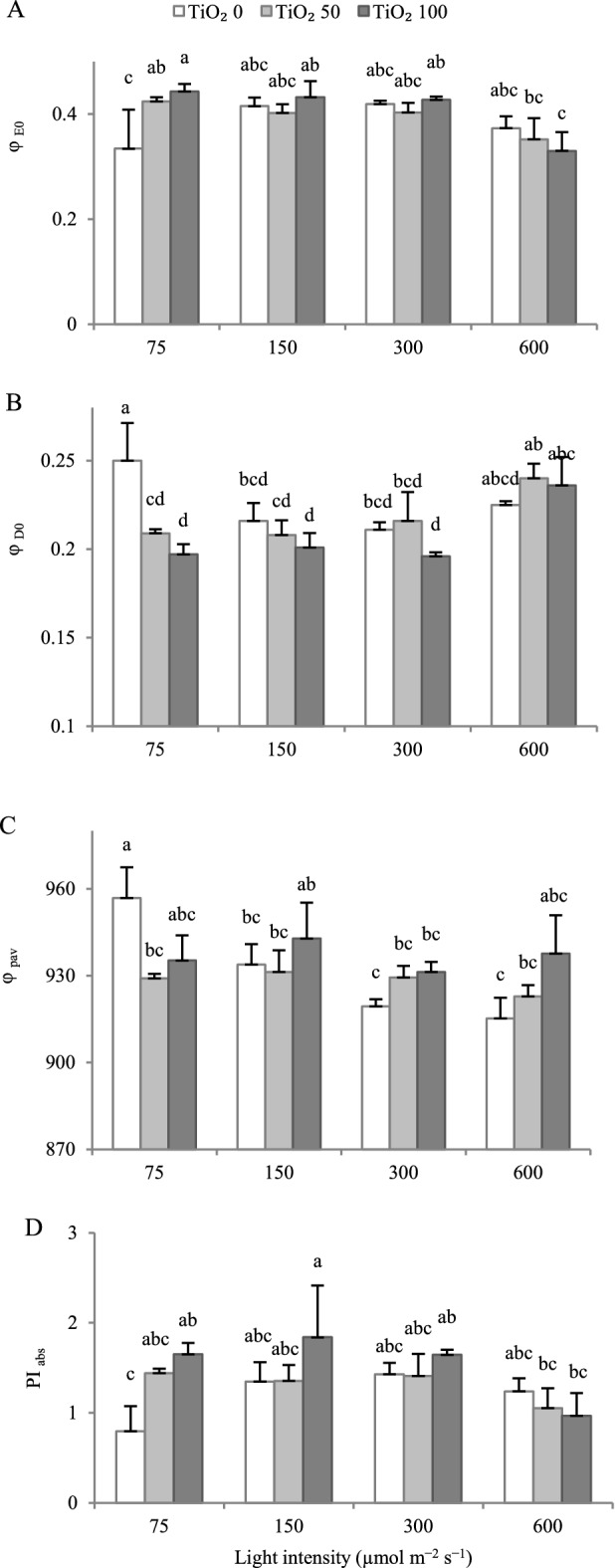
Effect of cultivation light intensity (75, 150, 300 and 600 µmol m−2 s−1) and spray treatment (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles (open, grey, and dark grey for 0, 50 and 100 µmol L−1, respectively) on quantum yield of electron transport (φE0; A), quantum yield of energy dissipation (φD0; B), quantum yield for primary photochemistry (φPAV; C), performance index in light absorption basis (PIABS; D) (equations and explanations in Table 3) of radish cv. Cherry belle plants. Nine replicate plants were assessed per treatment. Bars represent SEM. In traits, where the interaction of the two factors (light intensity, TiO2 nanoparticle level) was significant, different letters indicate significant differences.
The quantum yield of energy dissipation (φD0) was higher at 75 µmol m−2 s−1 PPFD as compared to 150 and 300 µmol m−2 s−1 PPFD (Fig. 8B). TiO2 nanoparticle spray decreased φD0 at 75 µmol m−2 s−1 PPFD.
Increasing the cultivation intensity generally decreased the quantum yield for primary photochemistry (φPAV; Fig. 8C). TiO2 nanoparticle spray decreased φPAV at 75 µmol m−2 s−1 PPFD.
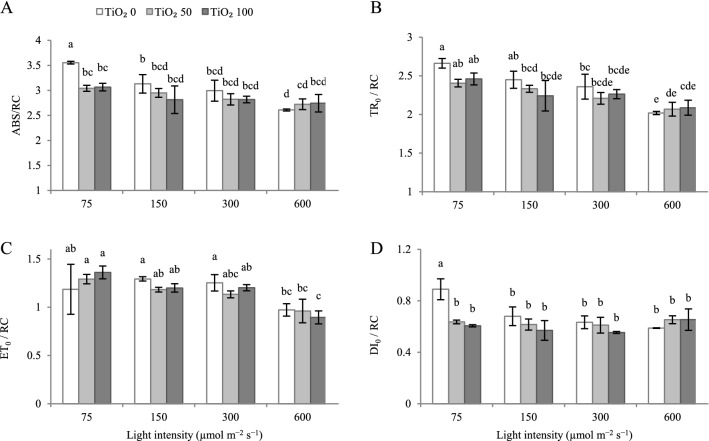
Increasing cultivation light intensity generally decreased the specific energy fluxes per reaction center for energy absorption (ABS/RC; Fig. 9A), and the trapped energy flux per reaction center (TR0/RC; Fig. 9B). TiO2 nanoparticle spray decreased ABS/RC at 75 µmol m−2 s−1 PPFD (Fig. 9A).
Figure 9.
Effect of cultivation light intensity (75, 150, 300 and 600 µmol m−2 s−1) and spray treatment (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles (open, grey, and dark grey for 0, 50 and 100 µmol L−1, respectively) on specific energy fluxes per reaction center for energy absorption (ABS/RC; A), trapped energy flux per reaction center (TR0/RC; B), electron transport flux per reaction center (ET0/RC; C), dissipated energy flux (DI0/RC; D) (equations and explanations in Table 3) of radish cv. Cherry belle plants. Nine replicate plants were assessed per treatment. Bars represent SEM. In traits, where the interaction of the two factors (light intensity, TiO2 nanoparticle level) was significant, different letters indicate significant differences.
The electron transport flux per reaction center (ET0/RC) was lower at 600 µmol m−2 s−1 PPFD as compared to lower intensities (Fig. 9C). TiO2 nanoparticle spray did not affect ET0/RC.
The dissipated energy flux (DI0/RC) was higher at 75 µmol m−2 s−1 PPFD as compared to higher light intensities (Fig. 9D). TiO2 nanoparticle spray decreased DI0/RC at 75 µmol m−2 s−1 PPFD (Fig. 9D). At 0 µmol m−2 s−1 PPDF measuring light intensity, ΦPSII was not affected by cultivation light intensity (Fig. 10A). As measuring light intensity increased, ΦPSII decreased. Leaves cultivated under higher light intensities generally sustained higher ΦPSII values across measurement light levels (100−1000 μmol m−2 s−1 PPFD). At 75 µmol m−2 s−1 PPFD during cultivation, the high TiO2 nanoparticle level (100 μmol L−1) tended to sustain higher ΦPSII values. As measurement light intensity increased, rETR also increased (Fig. 10B). Differences among cultivation light intensities were magnified, as measurement light intensity increased. The lower rETR was noted in leaves cultivated under 75 µmol m−2 s−1 PPFD.
Figure 10.
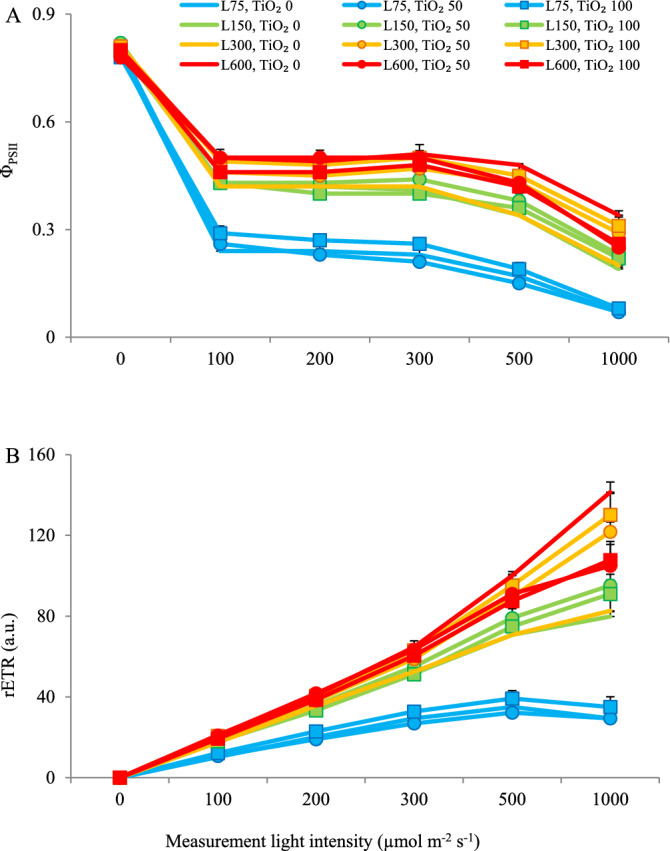
Rapid light curve of effective quantum yield of PSII (ΦPSII, A) and electron transport rate (rETR, B) (equations and explanations in Table 3) as a function of measurement light intensity in radish cv. Cherry belle plants cultivated under different light intensities (75, 150, 300 and 600 µmol m−2 s−1) and sprayed (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles at different concentrations (0, 50 and 100 µmol L−1). Nine replicate plants were assessed per treatment. Bars represent SEM.
Increasing cultivation light intensity and applying TiO2 nanoparticles generally stimulate non-photochemical quenching
The non-photochemical quenching (NPQ) was higher at 600 µmol m−2 s−1 PPFD as compared to lower light intensities (Fig. 11). TiO2 nanoparticle spray generally increased NPQ across cultivation light intensities. This effect was comparable at different cultivation light intensities.
Figure 11.
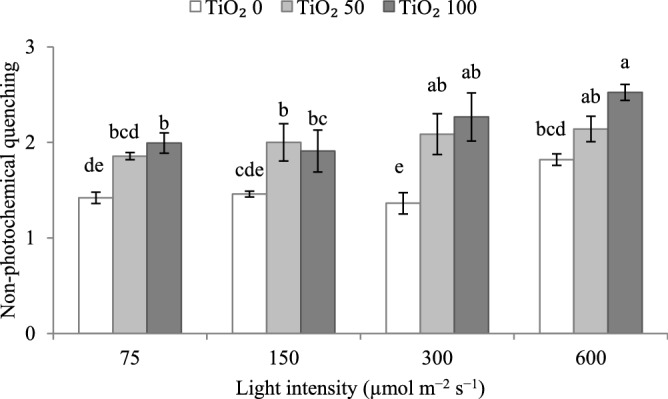
Effect of cultivation light intensity (75, 150, 300 and 600 µmol m−2 s−1) and spray treatment (once a week, and three times in total) with titanium dioxide (TiO2) nanoparticles (open, grey, and dark grey for 0, 50 and 100 µmol L−1, respectively) on non-photochemical quenching (equation and explanation in Table 3) of radish cv. Cherry belle plants. Nine replicate plants were assessed per treatment. Bars represent SEM. The interaction of the two factors (light intensity, TiO2 nanoparticle level) was significant, and different letters indicate significant differences.
Discussion
In this study, the optimal concentration (50, and 100 μmol L−1) of TiO2 nanoparticles foliar application for stimulating photosynthetic efficiency, and eventually yield was evaluated in radish. There are some previous indications highlighting more effectiveness of the foliar application of TiO2 nanoparticles on crop growth, photosynthesis, and yield. For instance, the application of TiO2 nanoparticles (average diameter 25 nm, in the form of anatase, zeta potentials ˗22.5 mV) in aerosol format was more effective compared to TiO2 nanoparticles as a soil amendment for increasing photosynthesis and lycopene content35. The dependence of the respective promotive effect on cultivation light intensity (75, 150, 300 and 600 μmol m−2 s−1 PPFD) was also investigated in the present study.
Increasing cultivation light intensity resulted in a larger plant leaf area (Table 1; see also Fig. 2), and in this way improved light capture36. The plant leaf area increase was most prominent when light intensity increased from 75 to 150 μmol m−2 s−1 PPFD, while a further increase in light intensity led to a more moderate (plant leaf area) increase. In the former case (from 75 to 150 μmol m−2 s−1 PPFD), the increase in plant leaf area was mostly due to larger individual leaf area, whereas in the latter case (≥ 300 μmol m−2 s−1 PPFD), it was exclusively due to the larger number of leaves (thus enhanced leaf initiation). Therefore, the initial light level (rather than the percentage of increase) strongly determines both the promoting effect of increasing light intensity on plant leaf area, and the underlying process by which this increase is realized (i.e., individual leaf area and/ or the number of leaves).
When cultivation light intensity did not exceed 300 μmol m−2 s−1 PPFD, TiO2 nanoparticle spray also stimulated plant leaf area (Table 1; see also Fig. 2). The TiO2 nanoparticle spray-induced increase in plant leaf area was due to increased individual leaf area. The TiO2 nanoparticle spray-induced increase in plant leaf area was more prominent as cultivation light intensity was lower. Therefore, applying TiO2 nanoparticles provides an additional advantage in plants cultivated at low light levels.
Increasing cultivation light intensity resulted in larger plant biomass (Table 1; see also Fig. 2). The applied light levels (75–600 m−2 s−1 PPFD) were thus below the light saturation point of the crop12. The light intensity-induced increase in plant biomass was more prominent as the initial light intensity was lower. Across cultivation light intensities, TiO2 nanoparticle spray stimulated plant biomass (Table 1; see also Fig. 2). The TiO2 nanoparticle application-induced increase in plant biomass was also more prominent as the cultivation light intensity was lower. Both TiO2 nanoparticle levels (50, and 100 μmol L−1) were equally effective in stimulating plant biomass.
More plant mass was allocated to the leaves (i.e., higher LMR), as cultivation light intensity was lower (Table 1; Fig. 3). Thus, plants favored biomass partitioning to the light-intercepting organ (leaves) to amplify carbon gain under low light level conditions, whereas this partitioning gradually shifted to the tuber, which is evidently affiliated with the supply of other resources (i.e., water and nutrients), under higher light level circumstances12. Fine-tuning of leaf thickness (SLA) by regulating the light capture surface was another important aspect of adaptation to the light level in radish. Leaves were consistently thinner (i.e., higher SLA) as the light level decreased (Table 1). Increasing SLA stimulates the amount of light which is intercepted, and in this way maximizes carbon assimilation. This adaptation in leaf morphology is common in low-light situations, and has been reported in several taxa12.
Throughout the distribution chain of edible greens, the intensity of leaf greenness is an index of quality5. At inadequate leaf chlorophyll level, photosynthetic efficiency is also impeded23. Carotenoids are the main antioxidant metabolites, contributing to plant defense, and exhibiting health-promoting properties when consumed5. Increasing cultivation light intensity up to 300 μmol m−2 s−1 PPFD improved both leaf chlorophyll and carotenoid contents, while a further increase (600 μmol m−2 s−1 PPFD) exerted an adverse effect (Fig. 4). At 75, 150, and 600 μmol m−2 s−1 PPFD, TiO2 nanoparticle spray promoted leaf chlorophyll content (Fig. 4A). At 75, and 600 μmol m−2 s−1 PPFD, TiO2 nanoparticle spray also enhanced leaf carotenoid content (Fig. 4B). In either case, the optimum TiO2 nanoparticle concentration depended on light level. Therefore, applying TiO2 nanoparticles improves both leaf chlorophyll and carotenoid contents, especially under extreme light environments (i.e., low or very high).
Increasing cultivation light intensity generally stimulated the content of total soluble carbohydrates (primary energy reserves) in both leaves and tuber, with this effect being more prominent in the tuber (Fig. 5). TiO2 nanoparticle spray further improved both leaf and tuber total soluble carbohydrates contents, with this effect being more pronounced in the leaf (Fig. 5). The level of primary energy reserves has been earlier associated with prolonged postharvest longevity22,37. For instance, an increased content of soluble carbohydrates may support maintenance needs (by sustaining ATP synthesis), and protect membrane integrity (by scavenging reactive oxygen species)22,37.
Growth environment determines the light energy absorbance by the PSII apparatus, and this is readily reflected by chlorophyll fluorescence analysis38. As growth PPFD increased, a decreasing trend was noted in the F0, Fm, and Fv (Fig. 7A–C). These parameters (F0, Fm, and Fv) were generally not affected by TiO2 nanoparticle spray, except for a decrease in F0 at 75 µmol m−2 s−1 PPFD. The decrease in F0 may be associated with a reduction in plastoquinone electron receptors and incomplete oxidation due to retardation, which results in electron transfer chain postponement in PSII. Alternatively, F0 may be decreased by the separation of light-harvesting Chl a/b protein complexes in PSII39. The reduction in Fm is probably related to the deactivation of proteins in chlorophyll structure.
Higher values of Fv/Fm [(Fm – F0)/Fm; Table 3] reflect a better light use efficiency39. Under stress, both the rising trend of F0 and the declining trend of Fm typically underlie the decrease in Fv/Fm15,25,40. This decline in Fv/Fm denotes a stress-induced damage in the structure or function of PSII. As growth PPFD increased up to 300 µmol m−2 s−1 PPFD, Fv/Fm also increased (Fig. 7D; see also Fig. 6). A further increase in growth PPFD to 600 µmol m−2 s−1 led to a decrease in Fv/Fm. Therefore, the optimum growth light intensity for light use efficiency was clearly the 300 µmol m−2 s−1 PPFD. In lettuce, the optimum growth light intensity was 600 µmol m−2 s−1 PPFD16,17,41–44, while for Aloe vera L. it was 50% of sunlight as compared to 75 or 100%44. Fv/Fm was generally not affected by TiO2 nanoparticle spray, except for an increase noted at 75 µmol m−2 s−1 PPFD. Therefore, TiO2 nanoparticle spray improved light use efficiency at the lowest growth light intensity.
Table 3.
Abbreviations, definitions and formulas of the chlorophyll fluorescence parameters assessed in the current study.
| Category | Abbreviation | Definition | Equation |
|---|---|---|---|
| Basic parameters | F0 | Minimum fluorescence, when all PSII reaction centers are open (O-step of OJIP transient) | F50µs |
| FJ | Fluorescence intensity at the J-step (2 ms) of OJIP | F2ms | |
| FI | Fluorescence intensity at the I-step (30 ms) of OJIP | F30ms | |
| Fluorescence parameters | Fm | Maximum fluorescence, when all PSII reaction centers are closed (P-step of OJIP transient) | F1s = Fp |
| Fv | Variable fluorescence of the dark-adapted sample | Fm – F0 | |
| VJ | Relative variable fluorescence at time 2 ms (J-step) after start of actinic light pulse | (FJ – F0)/(Fm – F0) | |
| VI | Relative variable fluorescence at time 30 ms (I-step) after start of actinic light pulse | (F30ms – F0)/(Fm – F0) | |
| Fv/Fm | Maximal quantum yield of PSII photochemistry | 1 – (F0/Fm) = (Fm – F0)/Fm = φP0 = TR0/ABS | |
| Quantum yields and efficiencies/probabilities | φE0 | The quantum yield of electron transport | [1– ( F0/ Fm)](1 – VJ) = ET0/ABS |
| φD0 | Quantum yield of energy dissipation | F0/Fm | |
| φPAV | Average (from time 0 to tFM) quantum yield for primary photochemistry | φP0 (1 − VJ) = φP0 (SM/tFM) | |
| Specific energy fluxes (per QA reducing PSII reaction center) | ABS/RC | The specific energy fluxes per reaction center for energy absorption | M0 (1/VJ)(1/φP0) |
| TR0/RC | Trapped energy flux (leading to QA reduction) per reaction center | M0 (1/VJ) | |
| ET0/RC | Electron transport flux (further than QA−) per reaction center | M0 (1/VJ)(1 − VJ) | |
| DI0/RC | Dissipated energy flux | (ABS/RC) − (TR0/RC) | |
| Performance indices (products of terms expressing partial potentials at steps of energy bifurcations) | PIABS | Performance index for the photochemical activity | [(γRC/1 − γRC)( φP0 /1 − φP0)(ψE0 /1 − ψE0)] |
| NPQ | Non-photochemical quenching | (Fm/Fm´) – 1 | |
| Rapid light curve as a function of PPFD | ΦPSII | Effective quantum yield of PSII | ∆F/Fm´ = (Fm´ – F)/ Fm´ |
| rETR | Relative electron transport rate | ΦPSII × PPFD × 0.5 × 0.84 |
PIABS is an index of the quantum yield of absorbed photons, and is highly sensitive to changes in the concentration of reaction centers, initial photochemistry, and electron transport14,45. Exposure to non-optimal PPFD leads to alterations in absorption and energy trapping, thereby adversely influencing PIABS17. Similarly to Fv/Fm, PIABS increased as growth PPFD increased up to 300 µmol m−2 s−1 PPFD, and then (600 µmol m−2 s−1 PPFD) slightly decreased (Fig. 8D). Likewise to Fv/Fm, TiO2 nanoparticle spray increased PIABS at 75 µmol m−2 s−1 PPFD. A decrease in PIABS may be due to the higher light energy absorption, dissipated energy flux, and lower electron transport per reaction center (expressed by ABS/RC, DI0/RC, and ET0/RC, respectively)46.
ABS/RC flux is divided into TR0/RC, ET0/RC, and DI0/RC, while the share of ABS is converted to DI and TR. As growth PPFD increased, ABS/RC tended to decrease (Fig. 9A). ABS/RC was generally not affected by TiO2 nanoparticle spray, except for a decrease apparent at 75 µmol m−2 s−1 PPFD. The decreasing trend in ABS/RC indicates the more efficient function of the electron transport system, which decreases the excitation force on each reaction center and directs that energy mostly toward photosystem I.
At 600 µmol m−2 s−1 PPFD, ET0/RC was lower (Fig. 9C), indicating an adverse effect in the rate of electron transport flux on PSII reaction centers. Increasing cultivation light intensity generally decreased TR0/RC (Fig. 9B). At 75 µmol m−2 s−1 PPFD, DI0/RC was higher (Fig. 9D). These parameters (ET0/RC, TR0/RC, and DI0/RC) were generally not affected by TiO2 nanoparticle spray, except a decrease in DI0/RC at 75 µmol m−2 s−1 PPFD. A high DI0/RC is generally associated with decreased Fv/Fm and an increased incidence of photoinhibition17,47. Since DI0/RC is related to the partial deactivation of PSII reaction centers, it usually increases when PSII reaction centers cannot transfer energy upstream of PSII as a consequence of damage to the thylakoid membrane14,48. Shabbir et al.49 reported that the photochemical efficiency of PSII increased by applying 90 mg L−1 TiO2 to vetiver plants. In the study by Gao et al.50 treatment of spinach with TiO2 resulted in the induction of a complex of Rubisco and Rubisco activase to accelerate the carboxylation of Rubisco and ultimately improve photosynthetic efficiency. In the study of Azmat et al.51, the treatment of spinach with TiO2 nanoparticles increased the starch content due to its photocatalytic properties.
The absorbed light energy is quenched via photochemistry, fluorescence, or heat dissipation. In a healthy photosystem, a large portion is used to perform photosynthesis (the so-called photochemical quenching), and a trivial one is released via fluorescence. The third fate is NPQ, in which the excess excitation energy of the chlorophyll in the PSII complex is harmlessly dissipated into thermal energy52. Therefore, NPQ signifies an effective way for dissipating excessive irradiation into heat by the photosynthetic apparatus53. Studying the behavior of NPQ provides information regarding the xanthophyll cycle activity and other energy-quenching pathways which are induced by the proton concentration inside thylakoids54–56. NPQ was higher at 600 µmol m−2 s−1 PPFD (Fig. 11). Across cultivation light intensities, TiO2 nanoparticle spray generally increased NPQ. Thus, it can be concluded that plants grown under the highest PPFD and/ or TiO2 nanoparticle spray directed more efficiently the excess excitation energy through non-radiative dissipative processes. This high energy input can be due to feedback inhibition of carbohydrate accumulation on the electron transport chain. An increase in the level of carotenoids as a result of exposure to higher PPFDs or TiO2 concentrations is a helpful strategy to direct the energy input toward the NPQ. In this way, the photosynthetic apparatus remains adequately protected under conditions of excessively high light energy input.
The rapid light curves illustrate the short-term photosynthetic response to rising light levels. Studying them is a proper way to gain knowledge on overall photosynthetic performance as well as the saturation characteristics of electron transport49. In the present study, rapid light curves of both ΦPSII and rETR were assessed. As measuring light intensity increased, ΦPSII decreased whereas rETR increased (Fig. 10). The decline in ΦPSII denotes a limited capacity for photochemical energy usage. In rETR, both the lower increase and the development of a plateau depict the light intensity, where the photosynthetic pathway became limited. Across measurement light levels (100 − 1000 μmol m−2 s−1 PPFD), leaves cultivated under higher light intensities generally sustained higher ΦPSII and rETR values (Fig. 10). At 75 µmol m−2 s−1 PPFD during cultivation, TiO2 nanoparticle spray (100 μmol L−1) tended to sustain higher ΦPSII and rETR values. In accordance, it has been shown that foliar spraying of 100 mg L−1 of TiO2 nanoparticles under low light intensity increased the rate of photosynthesis and yield in cherry tomato plants30.
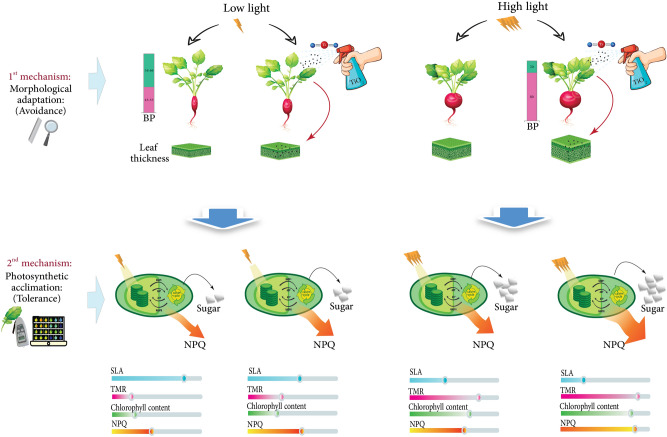
In conclusion, radish plants employed two mechanisms under contrasting lighting conditions. In the first mechanism (morphological adaptation), under low light, plants partitioned their biomass toward upper-ground parts which resulted in smaller tubers. Furthermore, plants allocated their biomass toward leaf expansion rather than leaf thickness which was indicated by the high SLA of plants under low light intensity (shade avoidance response). Conversely, plants under high light partitioned most of biomass toward underground parts which resulted in production of bigger tubers. They also produced thicker leaves with limited area to avoid capturing excessive light photons. In the second mechanism (photosynthetic acclimation), plants decreased NPQ and accumulated less carbohydrates under low light condition, while increased NPQ and produced more carbohydrates under high light condition. TiO2 nanoparticle application mitigate the shade avoidance response under low light intensities, while increased NPQ and reduced electron transport under high light due to higher energy perception (Fig. 12).
Figure 12.
A schematic illustration on the effect of low and high cultivation light intensity and titanium dioxide (TiO2) nanoparticles application on radish plants. Under low light, plants partitioned less biomass towards the tubers and allocated their biomass toward leaf expansion rather than leaf thickness. Low light-exposed plants decreased NPQ and accumulated less carbohydrates. Conversely, under high light, plants partitioned most of their biomass into the tubers and produced thick leaves with limited area. They increased NPQ and produced more carbohydrates under high light condition. TiO2 nanoparticle application directed more biomass into the tubers under low light intensities, while increased NPQ under high light condition.
Materials and methods
Plant material and growth conditions
Seeds of radish (R. raphanistrum) cv. Cherry Belle were sterilized (3 min) in sodium hypochlorite solution (5%; v/v), and then rinsed (3 min) with distilled water. Seeds were then manually planted in a seedling tray (30 × 18 × 5 cm) filled with a mixture of peat and perlite (1:1, v/v). The sowing depth was kept constant, and seeds were covered with substrate immediately after sowing. At the two-leaf stage, plants were transferred to 2 L pots [diameter (top) × diameter (bottom) × height = 15 × 13 × 15 cm] containing the same mixture. To facilitate drainage, 5 ± 1 g of pebbles (particle size of 16–30 mm) had been earlier placed at the bottom of each pot. Next, 120 pots were equally distributed to four environmentally-controlled growth chambers (l × w × h = 1.5 × 1.0 × 1.3 m) for realizing treatments. A density of 20 plants per m2 was employed.
Twelve factors (4 light intensities × 3 TiO2 nanoparticle spray treatments) were applied as a factorial experiment based on a completely randomized design. Each growth chamber included a single light intensity level, which was set at 75, 150, 300 and 600 μmol m−2 s−1 PPFD for 16 h d−1 (06:00–22:00), respectively. In all chambers, light was provided by red (660 nm peak wavelength) and blue (450 nm peak wavelength) LED modules (Parcham Co, Tehran, Iran) with a (red to blue) ratio of 3 to 1. Light intensity was determined by a handy fluorometer (PAR-FluorPen FP 100-MAX, Photon Systems Instruments, Drásov, Czech Republic), while the spectrum was defined by a handy spectrometer (Seconic C7000, Japan).
The remaining environmental variables were identical among the four growth chambers. Day/ night air temperature was set to 26/ 16 °C, and relative air humidity was adjusted to 65–70%. Two ventilation fans (12 V, 0.90A) were installed in each growth chamber to ensure uniform air circulation. Plants were cultivated in a customized liquid culture hydroponic system, by using a modified Hoagland nutrient solution (pH ≈ 5.8; EC ≈ 1.8 dS m−1; detailed composition in Table 2). Growth media moisture was maintained at or near maximum water-holding capacity. To prevent salt accumulation, pots were also irrigated with distilled water twice a week (> 20% drainage).
Table 2.
Stock solutions' ingredients employed in the present study. From each stock solution, 10 mL was added to prepare 1 L of nutrient solution.
| Ingredient | Quantity for 1 L of stock solution (g) |
|---|---|
| Calcium nitrate | 97 |
| Potassium nitrate | 61 |
| Magnesium sulfate (16% MgO) | 25 |
| Mono-potassium phosphate | 19 |
| Potassium sulfate | 17 |
| Iron EDDHA (6%) | 3.723 |
| Mono-ammonium phosphate | 1 |
| Borax (11.3% B) | 0.383 |
| Zinc sulfate | 0.35 |
| Manganese sulfate | 0.3 |
| Copper sulfate | 0.04 |
| Sodium molybdate (39.6%) | 0.024 |
Plant- and leaf-level measurements were conducted. For leaf-level measurements, sampled leaves had grown under direct light, and were fully-expanded. Replicate leaves were selected from separate plants. To minimize border effects, experimental plants were surrounded by border plants (adjacent to chamber walls) that were not sampled. Samples were collected at the onset of the light period (06:00–07:00 h). Different treatments were always assessed simultaneously. In growth and biomass allocation measurements, the time between sampling and the start of the evaluation did not exceed 15 min. In the remaining evaluations, samples were placed in vials, flash frozen in liquid nitrogen, and transferred to a freezer (− 80 °C) for storage. In all cases, sampling was conducted at the end of the cultivation period, besides the chlorophyll fluorescence evaluation, which was performed 1 d earlier. In all determinations, six replicates were assessed per treatment, besides chlorophyll fluorescence assessments, where nine replicates were evaluated.
Preparation of TiO2 suspension
The methods used for the synthesis of TiO2 nanoparticles are Sol–gel route, fame hydrolysis, co-precipitation, impregnation and chemical vapor deposition. Three crystalline phases of titanium dioxide, are anatase (tetragonal), rutile (tetragonal), and brookite (orthorhombic), that brookite has no commercial value11. TiO2 nanoparticles in the anatase form (˃ 99% purity, APS: 10–25 nm, Color: white, Bulk density: 0.24 g/cm3, True density: 3.9 g/cm3, PH: 6–6.5) were obtained from a commercial supplier (Iranian Nanomaterial Pioneers Company, Mashhad, Iran). The shape, size and crystal structure of TiO2 nanoparticles were evaluated using SEM, TEM, XRD and FTIR. The Specific surface area (SSA) and pores were analyzed by BET (Brunauer–Emmett–Teller) instrument.
In each light intensity regime, following adaptation (3 d), TiO2 nanoparticles at different concentrations (0, 50, and 100 μmol L−1) were weekly applied (three times in total) via foliar spray. The suitable concentration range was selected based on both a comprehensive literature survey20,28,57, and a pre-experiment. Each time before application, the TiO2 nanoparticles solution was ultrasonically homogenized using a sonicator (UP100H, Hielscher Ultrasonics, Germany)58–60. At the time of the first application, plants were still at the two-leaf stage.
Plant growth, morphology, and biomass allocation
The petiole (stalk) length (from the base to the leaf joint), the number of leaves, individual leaf area (one-sided surface area), and plant leaf area were first determined. For leaf area assessment, leaves were scanned (HP Scanjet G4010, Irvine, CA, USA) and then evaluated by using the Digimizer software (version 5.3.5, MedCalc Software, Ostend, Belgium)21,61.
Following removal of the substrate from the tuber via gentle washing, tuber volume was measured by employing a volume-displacement technique62,63. Tubers were suspended in a cylinder filled with water, and tuber volume was then determined by measuring the volume of displaced water.
Leaf and tuber (fresh and dry) masses were also recorded (± 0.01 g; MXX-412; Denver Instruments, Bohemia, NY, USA). For measuring dry weight, samples were placed in a forced-air drying oven for 72 h at 80 °C. By using dry mass, specific leaf area (SLA; leaf area/leaf mass), leaf mass ratio (LMR; leaf mass/plant mass), and tuber mass ratio (TMR; tuber mass/plant mass) were calculated.
Prior to the destructive measurements, plant images were obtained by using a digital camera (Canon EOS M2; Canon Inc., Tokyo, Japan). The plants were manually moved to the image capture station. The imaging station included top and side lighting units (fluorescent tubes; Pars Shahab Lamp Co., Tehran, Iran). The camera-to-plant distance was maintained constant. One image (RGB) was obtained per plant.
Leaf chlorophyll and carotenoid content
Samples (0.1 g) were homogenized with the addition of 10 mL of 100% acetone. The extract was then centrifuged (14,000 g for 15 min), and the supernatant was collected. Since chlorophyll is light sensitive, the extraction took place in a dark room58,59. The obtained extract was subjected to reading on a spectrophotometer (Optizen pop, Mecasys Co. Ltd. Daejeon, Korea). Total chlorophyll and carotenoid contents were calculated according to Lichtenthaler and Wellburn64.
Leaf total soluble carbohydrates content
Colorimetric quantification of leaf total soluble carbohydrates (i.e., reducing and non-reducing sugars) content was performed65. Shortly before analysis (< 30 min), the anthrone reagent was prepared under darkness by dissolving 0.1 g of anthrone (0.2%) in 100 mL of concentrated sulfuric acid (98%). Sample (0.1 g) and anthrone reagent (1 mL) were loaded in tubes, which were placed in a water bath (90 °C for 15 min), cooled (0 °C for 5 min), and vortexed (1 min). Before reading, a heating step (20 min) to room temperature (25 °C) was performed. The absorbance was measured at 620 nm with a spectrophotometer (Optizen pop, Mecasys Co. Ltd. Daejeon, Korea). A standard curve based on a series of known glucose concentrations was prepared.
Maximum quantum yield of photosystem II (PSII)
As a sensitive indicator of photosynthetic performance, dark-adapted values of the maximum quantum yield of PSII (Fv/Fm; equation and explanation in Table 3) were non-invasively recorded in leaves of each treatment22,37,63. Measurements were performed by using a portable imaging fluorometer (Handy FluorCam FC 1000-H, Photon Systems Instruments, Drásov, Czech Republic). Before taking measurements, samples were dark-adapted (≥ 20 min). Then, Fv/Fm was evaluated by a custom-made protocol21,23, where leaves were exposed to short flashes followed by long saturated light pulses (3900 µmol m−2 s−1 PPFD) to cause a reduction in the primary quinone acceptor of PSII.
Polyphasic chlorophyll fluorescence transient (O–J–I–P) evaluation
A polyphasic chlorophyll fluorescence induction curve (O–J–I–P-transient) was obtained in the attached leaves of each treatment. By employing this test, the shape changes of the O–J–I–P transient are quantitatively translated to a set of parameters (see Table 3), which relate to the in vivo adaptive behavior of the photosynthetic apparatus (especially PSII) to the growth environment25,26. Measurements were conducted by using a PAR- FluorPen FP 100-MAX (Photon Systems Instruments, Drásov, Czech Republic) following dark adaptation (≥ 20 min). The employed light intensity (3900 μmol m−2 s−1 PPFD) was sufficient to generate maximal fluorescence in all treatments.
Following dark adaptation, leaves exhibit a polyphasic chlorophyll fluorescence rise during the first second of illumination. The fluorescence transient, plotted on a logarithmic time scale, typically includes the following phases: O to J, J to I, and I to P. F0 was measured at 50 μs, fluorescence intensity at J-step was assessed at 2 ms, and fluorescence intensity at I-step was evaluated at 30 ms39,66.
Non-photochemical quenching
Another set of dark-adapted attached leaves were used to determine non-photochemical quenching (NPQ; equation and description in Table 3) by using a PAR-FluorPen FP 100-MAX (Photon Systems Instruments, Drásov, Czech Republic). Calculations were performed by using the FluorPen software (v. 7; Photon Systems Instruments, Drásov, Czech Republic).
Rapid light curve of effective quantum yield of PSII and electron transport rate
The rapid light curve of effective quantum yield of PSII (ΦPSII; equation and description in Table 3) was determined with a handy fluorometer (PAR-FluorPen FP 100-MAX, Photon Systems Instruments, Drásov, Czech Republic). The dark-adapted leaves were exposed to a gradual increase of actinic blue light illumination in six steps (0, 100, 200, 300, 500 and 1000 μmol m−2 s−1 PPFD). Each illumination step was separated by 10 s intervals. Illuminating the saturating flash required 0.8 s. Data were analyzed by using the FluorPen software (v. 7; Photon Systems Instruments, Drásov, Czech Republic). Relative electron transport rate (rETR; equation and description in Table 3) was also calculated54.
Statistical analysis
Data were subjected to analysis of variance using SAS software (v. 9.4, SAS Institute Inc., Cary, NC, USA). Data were firstly tested for normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test). A split-plot design was employed, with cultivation light intensity (75, 150, 300 and 600 μmol m−2 s−1 PPFD) as the main factor, and TiO2 nanoparticles concentration (0, 50, and 100 μmol L−1) as the split factor. Treatment effects were tested at a 5% probability level and mean separation was carried out using the least significant differences based on Duncan's multiple range test (P ≤ 0.05).
Acknowledgements
The authors gratefully acknowledge the laboratory staff for their contributions, continued diligence, and dedication to their craft.
Abbreviations
- ABS/RC
The specific energy fluxes per reaction center for energy absorption
- DI0/RC
The dissipated energy flux
- ET0/RC
The electron transport flux per reaction center
- Fm
The maximum fluorescence when all PSII reaction centers are closed
- Fv
The variable fluorescence of the dark-adapted sample
- Fv/Fm
Ratio of variable to maximum fluorescence
- FW
Fresh weight
- F0
The minimum fluorescence when all PSII reaction centers are open
- LMR
Leaf mass ratio
- NPQ
Non-photochemical quenching
- PIABS
The performance index in light absorption basis
- PPFD
Photosynthetic photon flux density
- PSII
Photosystem II
- rETR
Relative electron transport rate
- SLA
Specific leaf area
- TEM
Transmission electron microscopy
- TiO2
Titanium dioxide
- TMR
Tuber mass ratio
- TR0/RC
The trapped energy flux per reaction center
- XRD
X-ray diffraction
- ∆F/Fm′
Effective quantum yield of PSII
- φD0
The quantum yield of energy dissipation
- ΦPSII
Effective quantum yield of PSII
- φE0
The quantum yield of electron transport
- φPAV
The quantum yield for primary photochemistry
Author contributions
Conceptualization, A.V., M.M-N, S.Al., and D.F.; data curation, A.V., S.Ab., M.M-N., and S.R.; formal analysis, A. V., M.M-N.; investigation, A. V., S.Ab., Z.M., M.M-N.; project administration, S.Al.; supervision, S.Al., S.R. and D.F.; funding, A. V, S. Al., and S.R.; visualization, M.M-N; writing—original draft, D.F.; writing—review and editing, S.Al., M.M-N., G.T., and D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by a research grant from the Ministry of Science, Research and Technology of Iran as a post-doctoral project to Akram Vatankhah (No: 5547981).
Data availability
The main data are contained within the article. Data are available upon request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meyfroidt P. Trade-offs between environment and livelihoods: Bridging the global land use and food security discussions. Glob. Food Sec. 2018;16:9–16. doi: 10.1016/j.gfs.2017.08.001. [DOI] [Google Scholar]
- 2.Simkin AJ, López-Calcagno PE, Raines CA. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019;70:1119–1140. doi: 10.1093/jxb/ery445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godfray HCJ, et al. Food security: The challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- 4.Xie H, Huang Y, Chen Q, Zhang Y, Wu Q. Prospects for agricultural sustainable intensification: A review of research. Land. 2019;8:157. doi: 10.3390/land8110157. [DOI] [Google Scholar]
- 5.Paschalidis K, et al. Pilot cultivation of the vulnerable cretan endemic verbascum arcturus l. (scrophulariaceae): Effect of fertilization on growth and quality features. Sustain. 2021;13:14030. doi: 10.3390/su132414030. [DOI] [Google Scholar]
- 6.Moosavi-Nezhad M, Salehi R, Aliniaeifard S, Winans KS, Nabavi-Pelesaraei A. An analysis of energy use and economic and environmental impacts in conventional tunnel and LED-equipped vertical systems in healing and acclimatization of grafted watermelon seedlings. J. Clean. Prod. 2022;361:132069. doi: 10.1016/j.jclepro.2022.132069. [DOI] [Google Scholar]
- 7.Sarkar S, et al. Management of crop residues for improving input use efficiency and agricultural sustainability. Sustain. 2020;12:1–24. [Google Scholar]
- 8.Shang Y, et al. Applications of nanotechnology in plant growth and crop protection: A review. Molecules. 2019;24:2558. doi: 10.3390/molecules24142558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013;10:1–33. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irshad MA, et al. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021;212:111978. doi: 10.1016/j.ecoenv.2021.111978. [DOI] [PubMed] [Google Scholar]
- 11.Waghmode MS, Gunjal AB, Mulla JA, Patil NN, Nawani NN. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019;1:310. doi: 10.1007/s42452-019-0337-3. [DOI] [Google Scholar]
- 12.Fanourakis D, et al. Stomatal anatomy and closing ability is affected by supplementary light intensity in rose (Rosa hybrida L.) Hortic. Sci. 2019;46:81–89. doi: 10.17221/144/2017-HORTSCI. [DOI] [Google Scholar]
- 13.Sørensen HK, et al. Using artificial lighting based on electricity price without a negative impact on growth, visual quality or stomatal closing response in Passiflora. Sci. Hortic. 2020;267:109354. doi: 10.1016/j.scienta.2020.109354. [DOI] [Google Scholar]
- 14.Moradi S, et al. Blue light improves photosynthetic performance and biomass partitioning toward harvestable organs in saffron (Crocus sativus L.) Cells. 2021;10:1994. doi: 10.3390/cells10081994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashrostaghi T, et al. Light intensity: The role player in cucumber response to cold stress. Agronomy. 2022;12:201. doi: 10.3390/agronomy12010201. [DOI] [Google Scholar]
- 16.Esmaeili S, et al. Elevated light intensity compensates for nitrogen deficiency during chrysanthemum growth by improving water and nitrogen use efficiency. Sci. Rep. 2022;12:10002. doi: 10.1038/s41598-022-14163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghorbanzadeh P, et al. Dependency of growth, water use efficiency, chlorophyll fluorescence, and stomatal characteristics of lettuce plants to light intensity. J. Plant Growth Regul. 2021;40:2191–2207. doi: 10.1007/s00344-020-10269-z. [DOI] [Google Scholar]
- 18.Collado, C. E. & Hernández, R. Effects of light intensity, spectral composition, and paclobutrazol on the morphology, physiology, and growth of petunia, geranium, pansy, and dianthus ornamental transplants. J. Plant Growth Regul. 1–18 (2022).
- 19.Gómez C, et al. Controlled environment food production for urban agriculture. HortScience. 2019;54:1448–1458. doi: 10.21273/HORTSCI14073-19. [DOI] [Google Scholar]
- 20.Choi HG, et al. Effects of foliar fertilization containing titanium dioxide on growth, yield and quality of strawberries during cultivation. Hortic. Environ. Biotechnol. 2015;56:575–581. doi: 10.1007/s13580-015-0023-3. [DOI] [Google Scholar]
- 21.Moosavi-Nezhad M, et al. Blue light improves photosynthetic performance during healing and acclimatization of grafted watermelon seedlings. Int. J. Mol. Sci. 2021;22:8043. doi: 10.3390/ijms22158043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, et al. Contrary to red, blue monochromatic light improves the bioactive compound content in broccoli sprouts. Agronomy. 2021;11:2139. doi: 10.3390/agronomy11112139. [DOI] [Google Scholar]
- 23.Seifikalhor M, et al. The regulatory role of γ-aminobutyric acid in chickpea plants depends on drought tolerance and water scarcity level. Sci. Rep. 2022;12:7034. doi: 10.1038/s41598-022-10571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moosavi-Nezhad M, Alibeigi B, Estaji A, Gruda NS, Aliniaeifard S. Growth, biomass partitioning, and photosynthetic performance of chrysanthemum cuttings in response to different light spectra. Plants. 2022;11:3337. doi: 10.3390/plants11233337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalaji HM, et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017;132:13–66. doi: 10.1007/s11120-016-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seif M, et al. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021 doi: 10.1071/FP20280. [DOI] [PubMed] [Google Scholar]
- 27.Moradi, S. et al. Monochromatic blue light enhances crocin and picrocrocin content by upregulating the expression of underlying biosynthetic pathway genes in saffron (Crocus sativus L.). Front. Hortic.1, (2022).
- 28.Chahardoli A, Sharifan H, Karimi N, Kakavand SN. Uptake, translocation, phytotoxicity, and hormetic effects of titanium dioxide nanoparticles (TiO2NPs) in Nigella arvensis L. Sci. Total Environ. 2022;806:151222. doi: 10.1016/j.scitotenv.2021.151222. [DOI] [PubMed] [Google Scholar]
- 29.Hussain S, et al. Changes in morphology, chlorophyll fluorescence performance and Rubisco activity of soybean in response to foliar application of ionic titanium under normal light and shade environment. Sci. Total Environ. 2019;658:626–637. doi: 10.1016/j.scitotenv.2018.12.182. [DOI] [PubMed] [Google Scholar]
- 30.Choi HG. Effect of TiO2 nanoparticles on the yield and photophysiological responses of cherry tomatoes during the rainy season. Horticulturae. 2021;7:563. doi: 10.3390/horticulturae7120563. [DOI] [Google Scholar]
- 31.Lastochkina O, et al. Novel approaches for sustainable horticultural crop production: Advances and prospects. Horticulturae. 2022;8:910. doi: 10.3390/horticulturae8100910. [DOI] [Google Scholar]
- 32.Zhao X, et al. Exploring engineering reduced graphene oxide-titanium dioxide (RGO-TiO2) nanoparticles treatment to effectively enhance lutein biosynthesis with Chlorella sorokiniana F31 under different light intensity. Bioresour. Technol. 2022;348:126816. doi: 10.1016/j.biortech.2022.126816. [DOI] [PubMed] [Google Scholar]
- 33.Levine LH, et al. Quality characteristics of the radish grown under reduced atmospheric pressure. Adv. Sp. Res. 2008;41:754–762. doi: 10.1016/j.asr.2007.03.082. [DOI] [Google Scholar]
- 34.Viana MM, Soares VF, Mohallem NDS. Synthesis and characterization of TiO2 nanoparticles. Ceram. Int. 2010;36:2047–2053. doi: 10.1016/j.ceramint.2010.04.006. [DOI] [Google Scholar]
- 35.Raliya R, Nair R, Chavalmane S, Wang W-N, Biswas P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics. 2015;7:1584–1594. doi: 10.1039/C5MT00168D. [DOI] [PubMed] [Google Scholar]
- 36.Zou J, et al. Lettuce growth, morphology and critical leaf trait responses to far-red light during cultivation are low fluence and obey the reciprocity law. Sci. Hortic. 2021;289:104555. doi: 10.1016/j.scienta.2021.110455. [DOI] [Google Scholar]
- 37.Chen Y, et al. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021;172:111376. doi: 10.1016/j.postharvbio.2020.111376. [DOI] [Google Scholar]
- 38.Lichtenthaler HK, Ač A, Marek MV, Kalina J, Urban O. Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol. Biochem. 2007;45:577–588. doi: 10.1016/j.plaphy.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Baker NR. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- 40.Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 41.Esmaili M, et al. Assessment of adaptive neuro-fuzzy inference system (ANFIS) to predict production and water productivity of lettuce in response to different light intensities and CO2 concentrations. Agric. Water Manag. 2021;258:107201. doi: 10.1016/j.agwat.2021.107201. [DOI] [Google Scholar]
- 42.Hosseinzadeh M, Aliniaeifard S, Shomali A, Didaran F. Interaction of light intensity and CO2 concentration alters biomass partitioning in chrysanthemum. J. Hortic. Res. 2021;29:45–56. doi: 10.2478/johr-2021-0015. [DOI] [Google Scholar]
- 43.Esmaili M, et al. CO2 enrichment and increasing light intensity till a threshold level, enhance growth and water use efficiency of lettuce plants in controlled environment. Not. Bot. Horti Agrobot. Cluj-Napoca. 2020;48:2244–2262. doi: 10.15835/nbha48411835. [DOI] [Google Scholar]
- 44.Hazrati S, Tahmasebi-Sarvestani Z, Modarres-Sanavy SAM, Mokhtassi-Bidgoli A, Nicola S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol. Biochem. 2016;106:141–148. doi: 10.1016/j.plaphy.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 45.Kalaji HM, et al. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016;38:102. doi: 10.1007/s11738-016-2113-y. [DOI] [Google Scholar]
- 46.Oukarroum A, Madidi SE, Schansker G, Strasser RJ. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007;60:438–446. doi: 10.1016/j.envexpbot.2007.01.002. [DOI] [Google Scholar]
- 47.Martins JPR, Schimildt ER, Alexandre RS, Falqueto AR, Otoni WC. Chlorophyll a fluorescence and growth of Neoregelia concentrica (Bromeliaceae) during acclimatization in response to light levels. Vitr. Cell. Dev. Biol. - Plant. 2015;51:471–481. doi: 10.1007/s11627-015-9711-z. [DOI] [Google Scholar]
- 48.Rapacz M, Sasal M, Kalaji HM, Kościelniak J. Is the OJIP test a reliable indicator of winter hardiness and freezing tolerance of common wheat and triticale under variable winter environments? PLoS ONE. 2015;10:e0134820. doi: 10.1371/journal.pone.0134820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shabbir A, et al. Efficacy of TiO2 nanoparticles in enhancing the photosynthesis, essential oil and khusimol biosynthesis in Vetiveria zizanioides L. Nash. Photosynthetica. 2019;57:599–606. doi: 10.32615/ps.2019.071. [DOI] [Google Scholar]
- 50.Gao F, et al. Mechanism of nano-anatase TiO 2 on promoting photosynthetic carbon reaction of spinach: Inducing complex of rubisco-rubisco activase. Biol. Trace Elem. Res. 2006;111:239–253. doi: 10.1385/BTER:111:1:239. [DOI] [PubMed] [Google Scholar]
- 51.Azmat R, Altaf I, Moin S. The reflection of the photocatalytic properties of TiO 2 nanoparticles on photosynthetic activity of Spinacia oleracea plants. Pak. J. Bot. 2020;52:1229–1234. doi: 10.30848/PJB2020-4(2). [DOI] [Google Scholar]
- 52.Fu W, Li P, Wu Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. (Amsterdam) 2012;135:45–51. doi: 10.1016/j.scienta.2011.12.004. [DOI] [Google Scholar]
- 53.Pinnola A, et al. Zeaxanthin binds to light-harvesting complex stress-related protein to enhance nonphotochemical quenching in Physcomitrella patens. Plant Cell. 2013;25:3519–3534. doi: 10.1105/tpc.113.114538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ralph PJ, Gademann R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005;82:222–237. doi: 10.1016/j.aquabot.2005.02.006. [DOI] [Google Scholar]
- 55.Jahns P, Holzwarth AR. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta - Bioenerg. 2012;1817:182–193. doi: 10.1016/j.bbabio.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Lacour T, Babin M, Lavaud J. Diversity in Xanthophyll Cycle Pigments Content and Related Nonphotochemical Quenching (NPQ) Among Microalgae: Implications for Growth Strategy and Ecology. J. Phycol. 2020;56:245–263. doi: 10.1111/jpy.12944. [DOI] [PubMed] [Google Scholar]
- 57.Lian J, et al. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.) Chemosphere. 2020;239:124794. doi: 10.1016/j.chemosphere.2019.124794. [DOI] [PubMed] [Google Scholar]
- 58.Ahmadi-Majd M, Mousavi-Fard S, Rezaei Nejad A, Fanourakis D. Carbon nanotubes in the holding solution stimulate flower opening and prolong vase life in carnation. Chem. Biol. Technol. Agric. 2022;9:15. doi: 10.1186/s40538-021-00264-1. [DOI] [Google Scholar]
- 59.Ahmadi-Majd M, Rezaei Nejad A, Mousavi-Fard S, Fanourakis D. Postharvest application of single, multi-walled carbon nanotubes and nanographene oxide improves rose keeping quality. J. Hortic. Sci. Biotechnol. 2022;97:346–360. doi: 10.1080/14620316.2021.1993755. [DOI] [Google Scholar]
- 60.Vatankhah A, Reezi S, Izadi Z, Ghasemi-Varnamkhasti M, Motamedi A. Development of an ultrasensitive electrochemical biosensor for detection of Agrobacterium tumefaciens in Rosa hybrida L. Measurement. 2022;187:110320. doi: 10.1016/j.measurement.2021.110320. [DOI] [Google Scholar]
- 61.Yousefzadeh K, et al. Joint effects of developmental stage and water deficit on essential oil traits (content, yield, composition) and related gene expression: A case study in two thymus species. Agronomy. 2022;12:1008. doi: 10.3390/agronomy12051008. [DOI] [Google Scholar]
- 62.Javadi Asayesh E, et al. Supplementary light with increased blue fraction accelerates emergence and improves development of the inflorescence in aechmea, guzmania and vriesea. Horticulturae. 2021;7:485. doi: 10.3390/horticulturae7110485. [DOI] [Google Scholar]
- 63.Estaji A, Kalaji HM, Karimi HR, Roosta HR, Moosavi-Nezhad SM. How glycine betaine induces tolerance of cucumber plants to salinity stress? Photosynthetica. 2019;57:753–761. doi: 10.32615/ps.2019.053. [DOI] [Google Scholar]
- 64.Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983;11:591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- 65.Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zushi K, Kajiwara S, Matsuzoe N. Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit. Sci. Hortic. 2012;148:39–46. doi: 10.1016/j.scienta.2012.09.022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The main data are contained within the article. Data are available upon request from the corresponding author.