Abstract
Background
Unhealthy alcohol use is an unaddressed barrier to achieving and maintaining control of the human immunodeficiency virus (HIV) epidemic. Integrated screening, treatment of common behavioral and mental health comorbidities, and telemedicine can improve alcohol treatment and HIV clinical and quality of life outcomes for rural and underserved populations.
Objective
In a randomized controlled clinical trial, we will evaluate the effectiveness and implementation of telephone-delivered Common Elements Treatment Approach (T-CETA), a transdiagnostic cognitive behavioral therapy protocol, on unhealthy alcohol use, HIV, other substance use and mental health outcomes among predominantly rural adults with HIV receiving care at community clinics in Alabama.
Methods
Adults with HIV receiving care at four selected community clinics in Alabama will receive a telephone-delivered alcohol brief intervention (BI), and then be assigned at random (stratified by clinic and sex) to no further intervention or T-CETA. Participants will be recruited after screening positively for unhealthy alcohol use or when referred by a provider. The target sample size is 308. The primary outcome will be change in the Alcohol Use Disorder Identification Test (AUDIT) at six- and 12-months post-enrollment. Additional outcomes include HIV (retention in care and viral suppression), patient-reported mental health (anxiety, depression, posttraumatic stress), and quality of life. A range of implementation measures be evaluated including T-CETA provider and client acceptability, feasibility, cost and cost-effectiveness.
Conclusions
This trial will inform alcohol treatment within HIV care programs, including the need to consider comorbidities, and the potential impact of alcohol interventions on HIV and quality of life outcomes.
Keywords: Common elements treatment approach, Unhealthy alcohol use, Mental health, HIV, Telemedicine
Abbreviations
- PLWH
people living with HIV
- AUDs
alcohol use disorders
- PrEP
pre-exposure prophylaxis
- ART
anti-retro viral
- BI
brief intervention
- HIV
human immunodeficiency virus
- US
United States
- CETA
Common Elements Treatment Approach
- TALC
Telemedicine for Unhealthy Alcohol Use in Persons Living with HIV using Common Elements Treatment Approach
- T-CETA
CETA, delivered via teletherapy
- RWHP
Ryan White HIV/AIDs Program
- CPSS-SBIRT
CETA Psychosocial Services – Screening, Brief Intervention, and Referral to Treatment
- AUDIT
Alcohol Use Disorder Identification Test
- PEth
Phosphatidylethanol
- RCT
randomized controlled clinical
- ePROs
electronic Patient Reported Outcomes
- UAB
University of Alabama at Birmingham
- IRBs
Institutional Review Boards
- M&E
monitoring and evaluation
- DSMB
Data Safety and Monitoring Board
- DBS
dried blood spot
- D4C
Data for Care
- PRISM
Practical, Robust Implementation and Sustainability Model
- LMIC
low- and middle-income countries
- QALY
quality-adjusted life year
Funding
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism (PIs: Cropsey and Vinikoor, 5P01AA029540-02). The funders did not play any role in the study design, data collection and analysis, preparation of the manuscript, nor the decision to publish. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Introduction
Globally, 30% of people living with human immunodeficiency virus (HIV; PLWH) have ‘unhealthy alcohol use’ [1], which broadly comprises heavy and hazardous use, heavy episodic drinking, and alcohol use disorders (AUDs) [2]. In PLWH, the prevalence of unhealthy alcohol use is 2–4 times higher than the general population [[3], [4], [5]]. Alcohol use not only increases risk for initial HIV acquisition but can also undermine each step in the HIV care continuum [3]. Interruptions to care can occur from uptake and continuation on pre-exposure prophylaxis (PrEP), testing and diagnosis, to linkage to an HIV treatment program, initiation and adherence to antiretroviral treatment, and continuous engagement in care leading to sustained HIV viral load suppression [3]. Unhealthy alcohol use challenges both HIV treatment and prevention goals, since antiretroviral therapy (ART) induced viral suppression is a critically important mechanism to lower HIV incidence (i.e., Treatment = Prevention, Undetectable = Untransmissible) [6].
People with unhealthy alcohol and substance use often have comorbid mental health problems and these two components tend to have a bidirectional link, potentially reinforcing each other over time [7,8]. Behavioral treatments focused on substance use reduction, such as motivational interviewing, brief intervention (BI), and cognitive behavioral therapy, are efficacious in reducing unhealthy alcohol use in the general population; however, their efficacy in PLWH has been inconclusive [[9], [10], [11], [12], [13], [14], [15], [16], [17],17,18,18,19]. To date, BI interventions in HIV treatment have yielded isolated effects on quantity and frequency of alcohol use, with no changes in HIV-related outcomes (viral load, ART adherence, and sexual behavior) [20]. These limitations may be, in part, be due to inconsistent measurement in assessing study participants’ abstinence from alcohol, such as limited utilization of biomarkers [17,18,20]. However, it is also possible that these suboptimal results are influenced by untreated mental health comorbidities (e.g., depression), which impact treatment efficacy for AUD, regardless of HIV status [19]. Historically, addiction and mental health programs have been siloed and treatment guidelines recommend evidence-based single diagnosis protocols [21,22]. This is problematic in the real world, as mental health and behavioral health comorbidity is the norm, with many patients presenting with complex and/or overlapping issues [23], including many PLWH and unhealthy alcohol use [24]. Combining effective treatment elements shows promise for treating comorbid mood and anxiety symptoms among people with AUD [25]. Thus, transdiagnostic approaches that address both alcohol use and mental health comorbidities may help reduce the impact of these conditions on HIV outcomes.
To address siloed care in the health system and improve management of complex and overlapping clinical presentations, transdiagnostic cognitive behavioral treatments (CBTs) were created. Transdiagnostic CBTs allow a provider to use a single protocol to treat comorbid mental and behavioral health issues [26,27]. In the United States (US) however, such approaches have not been widely scaled up for several reasons, including challenges in adapting well-engrained systems of siloed care. Recognizing limitations of existing CBT models in the US, the Common Elements Treatment Approach (CETA) was developed. CETA was originally designed specifically for low and middle income countries where the professional mental/behavioral health workforce is extremely limited. Therefore, CETA was designed for delivery by both experts and lay health care workers [28]. In controlled clinical trials mostly conducted in low and middle-income countries, CETA demonstrated the ability to reduce unhealthy alcohol use, depression, anxiety, interpersonal violence, and posttraumatic stress symptoms [[29], [30], [31], [32]].
In this protocol, we describe a randomized controlled trial that will evaluate CETA among PLWH in the US Deep South. The Deep South is a logical place to promote domestic use of CETA because of its substance use and mental health syndemics. PLWH in the Deep South often face high levels of poverty, reduced social support, pervasive stigma, and disparities in access to evidence-based treatment of substance use and mental health services [33,34]. PLWH in under-served and rural parts of the Deep South have limited access to care due to substantial transportation and other psychosocial barriers [35,36]. Therefore, CETA in this trial will be delivered using telemedicine approaches. Telemedicine can address geographic disparities by increasing access to care for PLWH in rural settings [37]. In management of mental health and substance use, teletherapy is both feasible and acceptable [38,39]. The CETA manual was adapted for telephone delivery (T-CETA), following a review of evidence-based telehealth strategies, ethical guidelines, clinical recommendations from telehealth providers, and two pilot studies in Zambia [40,41].
Telemedicine for Unhealthy Alcohol Use in Persons Living with HIV using Common Elements Treatment Approach (TALC) is a randomized controlled clinical trial (RCT) to evaluate CETA, delivered via teletherapy, in PLWH who are receiving care at community clinics across the state of Alabama. Participants with HIV and unhealthy alcohol use will be randomly allocated to receive CETA plus an alcohol BI, or BI alone. Both clinical and implementation outcomes will be measured. The overall aims of this study are to.
-
1)
Evaluate the effectiveness of T-CETA on unhealthy alcohol use among PLWH. We hypothesize that those randomized to T-CETA will have larger reductions in alcohol use.
-
2)
Evaluate the effectiveness of T-CETA on HIV outcomes. We hypothesize that those randomized to T-CETA will have better HIV outcomes.
-
3)
Evaluate the effectiveness of T-CETA on substance use disorder and mental health comorbidities. We hypothesize that those randomized to T-CETA will have larger reductions in other substance use and symptoms of mental health.
-
4)
Evaluate implementation factors related to brief alcohol intervention and T-CETA provision to people with HIV and unhealthy alcohol use in care at community clinics in Alabama including provider and client acceptability, feasibility, cost and cost-effectiveness.
2. Methods
2.1. Study setting
This study will be implemented with patients at four community health centers funded by the Ryan White HIV/AIDs Program (RWHP). The RWHP provides funding for clinics to serve low-income, uninsured, and/or under-insured PLWH. Alabama has 12 RWHP-supported sites, whose patients have a median age of 47 and are 76% male, 65% non-Hispanic black, 10% with unstable housing/homelessness. Based on these sociodemographic data and prior research, we estimate 24% of our sample will be female and 65% will be Non-Hispanic, Black PLWH. Services provided by the four study clinics vary by site, but include HIV medical care, routine blood work, pharmacotherapy, and social work services.
We selected four that serve predominantly rural and under-resourced patients and have some degree of burden from behavioral health issues (Fig. 1). Participating sites all employ electronic Patient-Reported Outcomes (ePROs) to accurately capture sensitive behavioral and psychological information while patients are in the waiting or exam room [42,43]. Leveraging existing patient-reported outcomes infrastructure and procedures, this study will recruit a sample of PWLH from selected RWHP clinics in Alabama who report unhealthy alcohol use as defined by self-reported AUDIT-C assessment, or clinician referral.
Fig. 1.
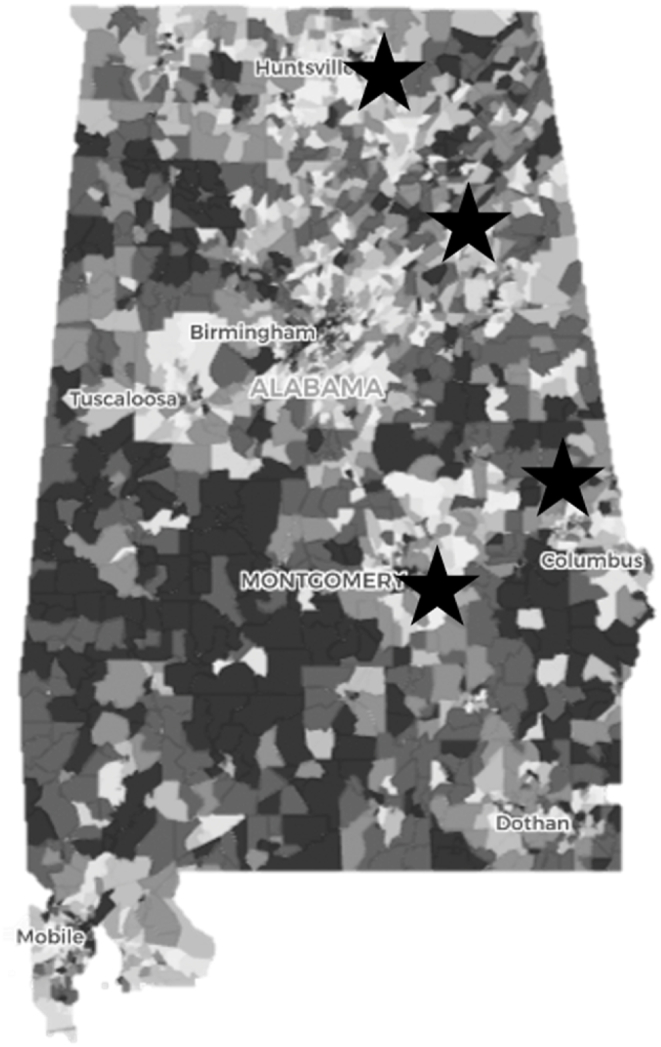
Distribution of 4 ryan white HIV/AIDs program clinics collecting patient reported outcomes by area deprivation index.
*Stars are Ryan White HIV/AIDs Program clinics; Dark grey represents the most disadvantaged regions; White are least disadvantaged in US (Kind et al., 2018).
2.2. Intervention
The components, content, and targets of CETA are listed in Table 1. The CETA intervention was designed by combining elements from evidence-based cognitive behavioral therapy treatments for trauma, behavioral problems, anxiety, and depression [34]. In addition, CETA providers are trained in multi-problem clinical decision-making, allowing a counselor to decide on which element(s), order, and dose are most appropriate for each client based on presentation. The CETA providers will be early-stage clinical psychology doctoral students at the University of Alabama at Birmingham. The doctoral students will receive a 40-h, in-person training to become “CETA providers”, followed by several weeks of practice sessions with CETA supervisors prior to recruitment beginning. Once participants are randomized to the BI + CETA arm and assigned to a CETA provider, the providers will be supervised and authorized by a licensed clinical psychologist that will oversee counseling sessions. CETA sessions will last approximately 45 min and participants will have six to 12 sessions, depending on the clinical needs of the participant. For example, participants who report suicidal ideation or polysubstance use may need more sessions than others only presenting with mild depression and/or alcohol use. The frequency of sessions will be decided by the CETA provider, their supervisor, and the participant.
Table 1.
Components of alcohol brief intervention and common elements treatment approach.
| Component | Content | Target | |
|---|---|---|---|
| Alcohol BI | Assess/screen for alcohol use | Two-week alcohol timeline follow-back measure | Establish baseline frequency and quantity of alcohol use |
| Understand the impacts of alcohol use | Review core ways alcohol use can negatively impact an individual, family, and the community | Increase client motivation to reduce use by highlighting negative effects; help client understand that positive effects of alcohol use are short-term, the negatives are long-term | |
| Explore possibilities for change | Explore potential ways the client would consider changing or reducing their alcohol use | Brainstorm measurable changes the client could make to reduce use | |
| Set goals | Set a goal for one way the client could reduce their alcohol use in the next few weeks | Set a measurable target for the client to work toward | |
| Identify reasons for alcohol use | Understand client motivations for alcohol use | Use the client's motivations for alcohol use to determine the best strategies for reducing it | |
| Build skills | Teach one coping skill to help the client combat one main reason for alcohol use | Build skills that address reasons for alcohol use | |
| CETA | Psychoeducation/introduction | Program information, normalize symptoms and problems | Psychoeducation; reduce stigma |
| Substance use reduction | CBT and MI merged to set goals and reduce substance use; identification and strategies for ‘drivers’ of substance use | Reduce substance use, increase social support | |
| Behavioral activation | Identify and engage in pleasurable activities | Reduce depression symptoms; activate action to engage in helpful programs (i.e., HIV care) | |
| Cognitive coping/restructuring | Identify and correct thoughts, feelings, and behaviors; replace unhealthy thoughts with helpful ones in order to feel better and behave in a more healthy, productive way | Reduce depression, anxiety, and trauma-related symptoms; reduce self-blame and stigma; reduce negative thoughts on HIV care; reduce aggressive/violent behavior, reduce risk taking, improve retention and adherence | |
| Relaxation | Breathing exercises, imagery, etc. | Reduce anxiety and stress-related symptoms | |
| Exposure | Talk about trauma memories or confront fears using gradual desensitization | Reduce trauma and anxiety symptoms | |
| Problem solving | Teach a process of steps to solve problems and make healthy decisions | Promote health decision making; skills training for problem solving; improve relationships and communication |
2.3. Study design
TALC is a RCT (Clinical trial ID: NCT04955795) to test the efficacy and implementation of BI + CETA, compared to BI alone, on unhealthy alcohol use in PLWH. Additionally, we will evaluate the impact of T-CETA on mental health and HIV outcomes including substance use and depressive symptoms. At 6-months (primary endpoint) and 12 months, the effect of T-CETA on unhealthy alcohol use will be measured by both the Alcohol Use Disorder Identification Test (AUDIT) and a Phosphatidylethanol (PEth) test, an alcohol biomarker. We will also explore the impact of T-CETA on HIV outcomes such as retention in HIV care and viral suppression.
3. Recruitment
3.1. Eligibility criteria
The criteria for enrollment in this RCT are displayed in Table 2. Participants who demonstrate need for intensive alcohol treatment services (i.e., acute withdrawal, etc.) will be referred to inpatient services.
Table 2.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • 18 years of age or older • Living with HIV infection • Receiving HIV care at a participating Ryan White HIV/AIDS Program-funded community clinic in Alabama • Unhealthy alcohol use documented on the AUDIT survey, defined as ≥ 4 points for women and ≥8 points for men |
• Inability to use a phone due to cognitive or physical impairments • Unable to speak sufficient English to provide informed consent and receive cognitive behavioral therapy • Active suicidality or psychosis • Risk for acute alcohol withdrawal or seizures. |
3.2. Recruitment procedures
Participants will be recruited through ePROs which are implemented at each clinic as part of standard of care assessment, in which the AUDIT-C [44] screening tool is utilized for hazardous alcohol use. Potentially eligible patients who are interested in study participation will then be referred to the study team by clinic staff; regular meetings between study staff and partner sites will allow identification and referral of eligible participants.
3.3. Screening, consent, and baseline procedure
Upon identifying potentially eligible patients, a study coordinator will contact the patient via telephone, contingent on them giving verbal permission to clinic staff for the study staff to contact. During this call, staff will screen and obtain informed consent if the patient is eligible. After informed consent is obtained, the study staff will transition straight into conducting the baseline session, which includes several questionnaires and the BI. The BI component of this study is the CETA Psychosocial Services – Screening, Brief Intervention, and Referral to Treatment (CPSS-SBIRT) intervention for substance use. CPSS-SBIRT are single-sessions that last 20–30 min and include psychoeducation, a substance use intervention, and screening for mental health problems and safety risks, with referral to elevated care (CETA) if indicated.
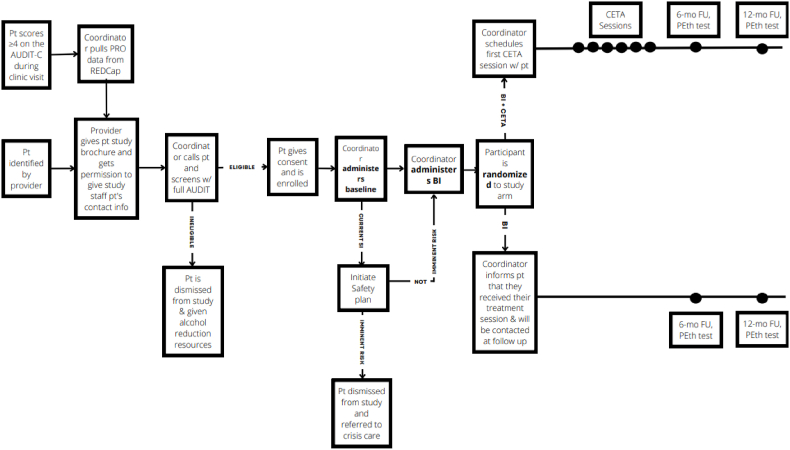
After receiving BI, the participant will be assigned at random to either BI alone or BI + T-CETA (Fig. 2). Randomization will be done via REDCap and stratified by clinic and sex. Research staff will not know the randomization until after the baseline and BI is complete. To improve retention in the study, participants will be asked to provide information for two personal contacts in the event the participant falls out of care.
Fig. 2.
Flow diagram of TALC.
3.4. Ethics and consent
This study was approved by the University of Alabama at Birmingham (UAB) and Johns Hopkins Institutional Review Boards (IRBs). All subjects enrolled in this study will complete an informed consent process prior to enrollment. The consent form will cover (1) study procedures, (2) benefits and risks; (3) participant demands and time commitment; (4) compensation plan; (5) alternatives to participation; and (6) voluntary nature of the study. Contact information for the study team and the UAB IRB will be provided.
3.5. Data collection and management
Study staff will collect clinical data including records of ePROs from the participants’ HIV care appointments and study data will be obtained via self-report surveys at study visits and CETA sessions. We will obtain data on HIV viral load and clinic retention from participating clinics. In addition to self-reported measures, PEth will also be measured. We will control consistency and quality of data collection through.
-
1.
The use of electronic data collection (precluding data entry errors);
-
2.
The use of standardized data collection tools (quantitative and qualitative) in both sites that have previously been used and/or validated;
-
3.
Weekly data monitoring and evaluation (M&E) conference calls with Zambia and US investigators in charge;
-
4.
Weekly M&E data reports;
-
5.
Weekly quality control checks on all data types with reports sent back to the sites and instruction for follow-ups on irregularities, if needed.
To maintain integrity, validity, and confidentiality of the data, data will be stored in UAB's HIPAA-compliant data platform, and only accessed by study staff through assigned privileges. A Data Safety and Monitoring Board (DSMB) will be created as an independent body of investigators not otherwise involved in the project charged with ensuring the safety of study subjects and that the scientific goals of the study are being met.
4. Measures
4.1. Alcohol use
Self-reported alcohol use by use of the AUDIT and the testing of PEth are valid, complementary measures to improve identification of AUD [45]. To measure PEth at baseline, 6 and 12 months, participants will self-collect a dried blood spot (DBS) using a fingerstick analogous to a diabetic checking their glucose. Participants will receive a kit with all materials needed for a DBS collection from the clinic staff at the time they express interest in the study. With this, participants will also receive a picture guide demonstrating the DBS procedure and given the option to be guided virtually by staff. Once collected and dried, the DBS will be packaged in a pre-addressed and stamped envelope to send via mail to study staff. Such an approach has proven feasible in previous HIV and sexually transmitted infection screening studies [46,47]. If necessary, a family member or friend may help the participant with the DBS collection. Also, the participant may bring the supplies to their next routine HIV care clinic visit for assistance.
4.2. HIV outcomes
We will collect outcomes in HIV care retention, viral suppression, and sustained viral suppression (HIV RNA <200 at 12 months) from the electronic HIV medical records at study clinics. HIV care retention will be measured by evaluating the number of visits scheduled, cancelled, or not attended during the study period. Patients will be considered “not retained in care” if they do not schedule or attend follow up visits for their HIV care. All data entry will be done at the point of collection using desktop and laptop computers. All RWHAP Clinics in Alabama participate in Data for Care (D4C) initiative whereby data on HIV viral load and clinic engagement are stored in a central database [48]. This database is part of the routine standard of care; hence, all participants will have data stored in this database. We will extract all HIV laboratory results and clinical visit data at baseline and after exiting the trial [49]. These will allow us to determine CD4+ T cell count, HIV viral load, and clinic engagement over the course of the study.
4.3. Patient-reported outcomes
We will collect patient-reported outcomes on alcohol use, depression, anxiety, posttraumatic stress disorder, substance use, and health-related quality of life using standard measures (Table 3). These outcomes will be collected and entered into our system as the patient responds.
Table 3.
Patient-reported outcomes and measurement tools.
| Outcome | Measure | Description | Collection Timepoint |
|---|---|---|---|
| Alcohol use | Alcohol Use Disorders Identification Test (AUDIT) [50,51] | AUDIT is a 10-item measure of hazardous alcohol use. A total score is calculated across the items with a possible range of 0–40 and higher scores indicating more severe alcohol use problems. The AUDIT was previously validated for use in the US [50]. Unhealthy use defined as: ≥4 among women or ≥8 among men; eligibility for more severe problem/higher risk of AUD: ≥12 among women or ≥16 among men [25,52]. | Baseline, 6-mo, 12-mo |
| Depression symptoms | Patient Health Questionnaire-9 (PHQ-9) [53] | PHQ-9 is a standard, widely used depression screener with nine items evaluating depression severity with a possible total score of 0–27. Scores ≥10 suggest moderate depression; ≥20 suggests severe depression [53]. | Baseline, 6-mo, 12-mo |
| Anxiety symptoms | General Anxiety Disorder Questiionnaire-7 (GAD-7) [54] | GAD-7 is a standard, widely used general anxiety disorder screener with seven items evaluating anxiety and a possible total score of 0–21. Scores ≥10 suggest moderate anxiety and ≥15 suggest severe anxiety [54]. | Baseline, 6-mo, 12-mo |
| Posttraumatic stress disorder symptoms | PTSD Checklist for DSM-5 (PCL-5) [55] | The PCL-5 is a 20-item measure of trauma symptoms with a possible total score of 0–80. Scores ≥31 suggest moderate PTSD symptoms [55]. | Baseline, 6-mo, 12-mo |
| Substance use | Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) [56,57] | ASSIST is a 7-item measure that evaluates frequency of use, abuse, and dependence symptoms for a range of substance types, including tobacco, alcohol, marijuana, inhalants, cocaine, sedatives, hallucinogens, methamphetamines, and opioids. A specific substance involvement (SSI) score is calculated for each substance type that a participant reports ever having used in their lifetime. An SSI score can range from 0 to 39. The ASSIST was previously validated in the US An SSI score on the ASSIST of ≥27 is considered high risk substance use [56,57]. | Baseline, 6-mo, 12-mo |
| Health-related quality of life | Mobility, Self-care, Usual activities, Pain/discomfort, Anxiety/depression (EQ- 5D)58,59 | EQ-5D is a brief quality of life measure encompassing five dimensions of current health and allows quality-adjusted life-years to be calculated. This measure has been used widely for people living HIV and been shown to be an appropriate tool in the sub-Saharan Africa setting. Those waiting for ART (CD4<200) have a score of 0.69 while those on treatment are better off having a score of 0.80. A score of 1 indicates full health, 0 for death [58,59]. | Baseline, 6-mo, 12-mo |
4.4. Sample size estimation
A power analysis was conducted to estimate the sample size needed to achieve sufficient power. The primary endpoint will be the difference in change in AUDIT score from baseline to 6-month follow-up between the BI alone and BI + T-CETA. We believe that an effect size of BI + T-CETA ≥0.5 would be clinically significant and is in line with the effect size found in a previous trial in Zambia [60]. Further assuming α = 0.05 and β = 80%, we will require a sample size of 128 (n = 64 per arm). To have additional statistical power to detect potentially important moderators of treatment effectiveness, including gender and mental health comorbidities which are critical to interpreting trial results, we will increase the sample size by 50%. Finally, to conservatively account for possible loss-to-follow-up/drop-out of 20% (i.e., 20% will not complete the 6-month primary outcome assessment), the final sample size will be N = 308.
4.5. Statistical analysis of alcohol use
We will conduct intention to treat analyses at study end. The primary outcome of change in AUDIT score, along with other continuous secondary outcomes, will be modelled with linear mixed models. Fixed effects will include treatment arm (0 = BI; 1 = BI + CETA), time (0 = Baseline; 1 = 6-months post-baseline; 2 = 12-months post-baseline), and interaction terms between treatment arm and time. This will allow estimation of the mean difference in change in score between arms and 95% confidence intervals. We will calculate Cohen's d to estimate effect size. Covariates will be included if they differ meaningfully at baseline or predict significant change in the outcome. For non-normally distributed continuous outcomes, generalized linear mixed effects models will be estimated. For dichotomous HIV outcomes, we will calculate and compare risk differences with 95% confidence intervals between treatment arms. Missing data will be addressed through multiple imputation with chained equations. In secondary analysis, we will incorporate PEth levels. Those reporting abstinence at the outcome but have detectable PEth will be excluded from analysis. PEth of ≥ 50 ng/ml is considered “hazardous use.” We will consider the % with abstinence (PEth <8) or moderate use (<50) as an alternative trial arm.
4.5.1. Mediator/moderator analysis
We will assess the role of potential mediating variables (e.g., mental health) in the impact of treatment on alcohol use to differentiate direct and indirect effects of CETA. To allow for the possibility of exposure-mediator interactions, we will use a counterfactual approach. Mediator analyses will be used in combination with the qualitative data to explore mechanisms of change for CETA. In addition to main treatment effects, we will evaluate the impact of potential moderators (e.g., age, gender) on alcohol, HIV, and mental health outcomes. For these analyses, a 3-way interaction between the moderator, treatment arm, and time will be included in the models.
5. Evaluation of the implementation of TALC
5.1. Cost effectiveness analysis of T-CETA
Total cost will include expenditures for personnel, recurring supplies, furniture, and utilities (including electricity, water, internet, phone calls, building space and other utilities – which are often covered by overhead costs). Personnel costs may include the costs of provision of alcohol BI and CETA sessions, scheduling patient follow-ups, counselors’ time on regular meeting with supervisors to review or plan for CETA sessions, and the costs of provider supervision. Program management costs such as costs of coordination and fiscal management, and regular meetings will also be estimated. Research (e.g., personnel costs for ePRO assessment) and routine clinical costs (e.g., for HIV care, sexually transmitted infections and Hepatitis C testing) will be excluded.
We will estimate the cost-effectiveness of BI + T-CETA compared to BI alone for the TALC study following standard guidelines [61,62]. We will calculate incremental cost-effectiveness ratios (ICER) comparing CETA versus BI. The numerator of the ratio is the difference in costs; the denominator is the difference in effectiveness. Costs of medical care (including HIV treatments, HIV comorbidity management) from existing literature will be used in addition to our collected data [63,64]. Our effectiveness measure will be quality-adjusted life year (QALY) developed from the collected EQ-5D data. We will build a state-transition decision analytic model using TreeAge Pro 2022. R1 (TreeAge Software, Williamtown, MA) to simulate the cost and effectiveness outcomes of the participants at their time of entering the trial, at quarterly intervals, plus an additional post-intervention year. In the model, we will project the possible alcohol use and HIV outcomes at the end of this additional post-intervention year using our collected data (at 6 and 12 month follow-up), calibrate the results using relevant literature, and explore the cost-effectiveness of the interventions against uncertainties of this projection [65]. Both costs and QALY will be discounted at a 3% rate in Alabama [61]. The ICER will be referenced to the $100,000/QALY threshold to determine the cost-effectiveness. We will assess the cost-effectiveness results against uncertainties in the patient characteristics (e.g., age, gender), intervention efficacy, clinical practice factors (e.g., uptake and completion of unhealthy alcohol use treatment, retention in HIV care, comorbidities) and varying costs. This assessment will be conducted in one-way, multiple-way, and probabilistic sensitivity analyses to determine the factors or the combinations of factors that most affect the cost-effectiveness of the experimental treatments for unhealthy alcohol use.
5.2. Provider knowledge, competency, and intervention fidelity of T-CETA
Provider competency, defined as the extent to which a therapist has the knowledge and skill required to deliver a treatment to the standard needed for it to achieve its expected effects, is critical to dissemination and implementation of psychological therapies [66]. All T-CETA providers in training will be evaluated on their comprehension of CETA elements and appropriate delivery of T-CETA. Tests will be administered: i) after the training and before pilot T-CETA/BI cases, ii) after the completion of 1–2 pilot cases, and iii) mid-way through the study, according to our approaches in other CETA studies. We will conduct descriptive analyses on knowledge test results in both sites and compare to previous trials of T-CETA.
We will evaluate providers’ application of knowledge in clinical practice in two ways: by audio recording a sample of therapy sessions. We will (with client permission and consent) audio record approximately 5 sessions per counselor over the course of the study (assuming 6 counselors in TALC this would equate to 30 recorded sessions). We will purposively choose sessions that represent a range of CETA elements and client symptom severities. Expert CETA trainers who are not involved in direct supervision of the CETA counselors (and therefore are masked to the identity of the counselors) will independently code the competency of the counselors using a standardized form developed by our team. Descriptive statistics on the competency form will be generated and compared to previous CETA studies. If there is significant variation in CETA knowledge or competency across counselors, we may explore these factors as moderators.
In addition to information on competency, we will also analyze fidelity to the CETA treatment model. CETA clinical supervisors will use standardized checklists in each supervision session with counselors to evaluate the degree to which the counselors have implemented and sequenced CETA elements according to the CETA model decision rules. We will describe overall fidelity to the CETA model and explore if there is variation in fidelity across counselors and CETA elements.
5.3. Process evaluation
We will also conduct a mixed methods process evaluation with multiple key informants to understand how contextual factors impact treatment completion. Based on the Practical, Robust Implementation and Sustainability Model (PRISM) [67], we will consider both participant, organizational, and policy perspectives. We will include a subset of around 50 T-CETA clients (i.e., study participants with HIV and unhealthy alcohol use) in a mixed methods survey of implementation outcomes. Implementation measures will focus on the acceptability, appropriateness, cost, and feasibility of T-CETA and the BI. We will deliberately include trial participants who were randomized to, but did not complete T-CETA.
Focus group discussions with T-CETA providers and HIV clinic staff in Alabama will be used to further explore the acceptability, appropriateness, reach, feasibility, and attitudes, thoughts, feelings, and barriers and facilitators related to implementation [68]. Finally we will conduct key informant interviews with approximately 10–15 policymakers and clinical administrators at participating clinics to gain insight on intervention sustainability, scale-up, and collaborator buy-in. Qualitative data will be digitally recorded and transcribed. Data will be analyzed using framework analysis based on hybrid inductive-deductive reasoning and following best practice guidelines to integrate quantitative and qualitative data [69]. Descriptive analyses will be used to investigate the quantitative data on implementation constructs.
6. Discussion
CETA is a unique, evidence-based transdiagnostic CBT-based protocol originally designed for use in low- and middle-income countries (LMIC) settings, but holds promise in the US where evidence-based mental health and substance use care are siloed and access to care is fragmented [70]. Prior clinical trial evidence on CETA was generated in LMIC and has been implemented a limited number of times in the US [71]. TALC represents among the first randomized evaluations of CETA in the US and among PLWH. Further, this protocol will be among the first to evaluate T-CETA in the US. To date, T-CETA implementation has been limited in LMIC settings, with technology limitations and cultural challenges in phone/internet based clinical care. Thus, TALC will generate new data on CETA in multiple ways, which can support its future use, particularly for disadvantaged populations in the US.
While TALC focuses on an evaluation of CETA in adults with HIV and unhealthy alcohol use, the results may have broader implications. TALC will test the hypothesis that treating behavioral health problems in PLWH can improve clinical outcomes measured by programs, which could catalyze increased investment in screening and treatment of unhealthy alcohol use within the RWHPs and other HIV care funders. Our primary outcome of alcohol use will be assessed by traditional self-reported methods and an alcohol biomarker, which can help to reduce social desirability bias that has called into question the results of most prior alcohol treatment studies. By assessing a range of comorbidities, including posttraumatic stress, TALC will also be able to describe behavioral health syndemics in PLWH. Implementation data will inform policymakers and program coordinators on whether and how to adopt elements of TALC and what it will cost to introduce and sustain. Our ultimate vision is for HIV treatment and prevention programs worldwide to have tools in place for the measurement and treatment of unhealthy alcohol use and common substance use and mental health comorbidities.
6.1. Potential limitations
This trial is recruiting study populations that are at high risk of poor HIV outcomes, including viral suppression and health-related quality of life. However, we acknowledge that by recruiting clinic-based populations we are not including those in the community who are not receiving continuous HIV care and/or are undiagnosed; groups that may be at the highest risk for poor HIV outcomes. We believe that these studies are a critical step in testing and establishing the efficacy of CETA and BI in reducing unhealthy alcohol use and improving HIV outcomes. Subsequent follow-up studies will investigate implementation strategies and reach community-based samples. In our health economic analyses, if BI alone and BI + T-CETA are found to have equivalent effectiveness, we will conduct a cost-minimization analysis, where no incremental cost-effectiveness ratios will be calculated. Only the cost of intervention implementation per participant will be estimated and reported. This alternative approach would remain informative to policymakers.
7. Conclusions
TALC will make substantive contributions in several areas. Given evidence that a common elements approach such as T-CETA is feasible, efficacious, and scalable, it is likely this will be an effective intervention for Alabamians with HIV, especially when delivered via telemedicine (a more accessible platform). The knowledge to be gained in understanding the preliminary efficacy of this approach and its ability to be integrated within HIV care is likely to have a major impact on HIV services and policy in the US Deep South.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Duko B., Ayalew M., Ayano G. The prevalence of alcohol use disorders among people living with HIV/AIDS: a systematic review and meta-analysis. Subst. Abuse Treat. Prev. Pol. 2019;14(1):1–9. doi: 10.1186/s13011-019-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guha M. Diagnostic and statistical manual of mental disorders: DSM-5. Ref. Rev. 2014;28(3):36–37. doi: 10.1108/RR-10-2013-0256. [DOI] [Google Scholar]
- 3.Shuper P.A., Neuman M., Kanteres F., Baliunas D., Joharchi N., Rehm J. Causal considerations on alcohol and HIV/AIDS—a systematic review. Alcohol Alcohol. 2010;45(2):159–166. doi: 10.1093/alcalc/agp091. [DOI] [PubMed] [Google Scholar]
- 4.Puryear S.B., Balzer L.B., Ayieko J., et al. Associations between alcohol use and HIV care cascade outcomes among adults undergoing population-based HIV testing in East Africa. AIDS (London, England) 2020;34(3):405. doi: 10.1097/QAD.0000000000002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nouaman M.N., Vinikoor M., Seydi M., et al. High prevalence of binge drinking among people living with HIV in four African countries. J. Int. AIDS Soc. 2018;21(12) doi: 10.1002/jia2.25202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen M.S., Chen Y.Q., McCauley M., et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo-Carniglia A., Keyes K.M., Hasin D.S., Cerdá M. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatr. 2019;6(12):1068–1080. doi: 10.1016/S2215-0366(19)30222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrakis I.L., Gonzalez G., Rosenheck R., Krystal J.H. Comorbidity of alcoholism and psychiatric disorders: an overview. Alcohol Res. Health. 2002;26(2):81. [Google Scholar]
- 9.Knox J., Hasin D.S., Larson F.R., Kranzler H.R. Prevention, screening, and treatment for heavy drinking and alcohol use disorder. Lancet Psychiatr. 2019;6(12):1054–1067. doi: 10.1016/S2215-0366(19)30213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke B.L., Arkowitz H., Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J. Consult. Clin. Psychol. 2003;71(5):843. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- 11.Ray L.A., Bujarski S., Grodin E., et al. State-of-the-art behavioral and pharmacological treatments for alcohol use disorder. Am. J. Drug Alcohol Abuse. 2019;45(2):124–140. doi: 10.1080/00952990.2018.1528265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCaul M.E., Hutton H.E., Cropsey K.L., et al. Decreased alcohol consumption in an implementation study of computerized brief intervention among HIV patients in clinical care. AIDS Behav. Dec 2021;25(12):4074–4084. doi: 10.1007/s10461-021-03295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huis in ‘t Veld D E-MC. Pengpid S., Peltzer K., Colebunders R. The efficacy of a brief intervention to reduce alcohol use in persons with HIV in South Africa, a randomized clinical trial. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0220799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wandera B., Tumwesigye N.M., Nankabirwa J.I., et al. Efficacy of a single, brief alcohol reduction intervention among men and women living with HIV/AIDS and using alcohol in Kampala, Uganda: a randomized trial. J. Int. Assoc. Phys. AIDS Care. 2017;16(3):276–285. doi: 10.1177/2325957416649669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huis in ‘t Veld D. Ensoy-Musoro C., Pengpid S., Peltzer K., Colebunders R. The efficacy of a brief intervention to reduce alcohol use in persons with HIV in South Africa, a randomized clinical trial. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0220799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott-Sheldon L.A., Carey K.B., Johnson B.T., Carey M.P. Behavioral interventions targeting alcohol use among people living with HIV/AIDS: a systematic review and meta-analysis. AIDS Behav. 2017;21(2):126–143. doi: 10.1007/s10461-017-1886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samet J.H., Walley A.Y. Interventions targeting HIV-infected risky drinkers: drops in the bottle. Alcohol Res. Health. 2010;33(3):267. [PMC free article] [PubMed] [Google Scholar]
- 18.Brown J.L., DeMartini K.S., Sales J.M., Swartzendruber A.L., DiClemente R.J. Interventions to reduce alcohol use among HIV-infected individuals: a review and critique of the literature. Curr. HIV AIDS Rep. 2013;10(4):356–370. doi: 10.1007/s11904-013-0174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madhombiro M., Musekiwa A., January J., Chingono A., Abas M., Seedat S. Psychological interventions for alcohol use disorders in people living with HIV/AIDS: a systematic review. Syst. Rev. 2019;8(1):1–16. doi: 10.1186/s13643-019-1176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madhombiro M., Musekiwa A., January J., Chingono A., Abas M., Seedat S. Psychological interventions for alcohol use disorders in people living with HIV/AIDS: a systematic review. Syst. Rev. 2019;8:1–16. doi: 10.1186/s13643-019-1176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arlington V. Association, AP diagnostic and statistical manual of mental disorders. Am Psychiatr Assoc. 2013;5:612–613. [Google Scholar]
- 22.Kelly T.M., Daley D.C., Douaihy A.B. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict. Behav. 2012;37(1):11–24. doi: 10.1016/j.addbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morojele N.K., Saban A., Seedat S. Clinical presentations and diagnostic issues in dual diagnosis disorders. Curr. Opin. Psychiatr. 2012;25(3):181–186. doi: 10.1097/YCO.0b013e328351a429. [DOI] [PubMed] [Google Scholar]
- 24.Common Comorbidities with Substance Use Disorders Research Report. National Institutes on Drug Abuse (US); 2020. [PubMed] [Google Scholar]
- 25.Kane J.C., Sharma A., Murray L.K., et al. Common Elements Treatment Approach (CETA) for unhealthy alcohol use among persons with HIV in Zambia: study protocol of the ZCAP randomized controlled trial. Addictive behaviors reports. 2020;12 doi: 10.1016/j.abrep.2020.100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barlow D.H., Farchione T.J., Bullis J.R., et al. The unified protocol for transdiagnostic treatment of emotional disorders compared with diagnosis-specific protocols for anxiety disorders: a randomized clinical trial. JAMA Psychiatr. 2017;74(9):875–884. doi: 10.1001/jamapsychiatry.2017.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chorpita B.F., Weisz J.R. MATCH-ADTC; 2009. Modular Approach to Therapy for Children with Anxiety, Depression, Trauma, or Conduct Problems. [Google Scholar]
- 28.Murray L.K., Dorsey S., Haroz E., et al. A common elements treatment approach for adult mental health problems in low-and middle-income countries. Cognit. Behav. Pract. 2014;21(2):111–123. doi: 10.1016/j.cbpra.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray L.K., Kane J.C., Glass N., et al. Effectiveness of the Common Elements Treatment Approach (CETA) in reducing intimate partner violence and hazardous alcohol use in Zambia (VATU): a randomized controlled trial. PLoS Med. 2020;17(4) doi: 10.1371/journal.pmed.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolton P., Lee C., Haroz E.E., et al. A transdiagnostic community-based mental health treatment for comorbid disorders: development and outcomes of a randomized controlled trial among Burmese refugees in Thailand. PLoS Med. 2014;11(11) doi: 10.1371/journal.pmed.1001757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss W.M., Murray L.K., Zangana G.A.S., et al. Community-based mental health treatments for survivors of torture and militant attacks in Southern Iraq: a randomized control trial. BMC Psychiatr. 2015;15(1):1–16. doi: 10.1186/s12888-015-0622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonilla-Escobar F.J., Fandiño-Losada A., Martínez-Buitrago D.M., et al. A randomized controlled trial of a transdiagnostic cognitive-behavioral intervention for Afro-descendants’ survivors of systemic violence in Colombia. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0208483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAuliffe W.E., Dunn R. Substance abuse treatment needs and access in the USA: interstate variations. Addiction. 2004;99(8):999–1014. doi: 10.1111/j.1360-0443.2004.00783.x. [DOI] [PubMed] [Google Scholar]
- 34.McAuliffe W.E., LaBrie R., Woodworth R., Zhang C., Dunn R.P. State substance abuse treatment gaps. Am. J. Addict. 2003;12(2):101–121. [PubMed] [Google Scholar]
- 35.Bensley K.M., McGinnis K.A., Fortney J., et al. Patterns of alcohol use among patients living with HIV in urban, large rural, and small rural areas. J. Rural Health. 2019;35(3):330–340. doi: 10.1111/jrh.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellowski J.A. Barriers to care for rural people living with HIV: a review of domestic research and health care models. J. Assoc. Nurses AIDS Care. 2013;24(5):422–437. doi: 10.1016/j.jana.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith E., Badowski M.E. Telemedicine for HIV care: current status and future prospects. HIV/AIDS (Auckland, NZ) 2021;13:651. doi: 10.2147/HIV.S277893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalmida S.G., Payne-Foster P., Kirkpatrick B., Mugoya G. 2017. Telemedicine Perceptions of Rural Patients with HIV and Mental Health Issues. [Google Scholar]
- 39.Mehrotra A., Huskamp H.A., Souza J., et al. Rapid growth in mental health telemedicine use among rural Medicare beneficiaries, wide variation across states. Health Aff. 2017;36(5):909–917. doi: 10.1377/hlthaff.2016.1461. [DOI] [PubMed] [Google Scholar]
- 40.Kane J.C., Sharma A., Murray L.K., et al. Efficacy of the Common Elements Treatment Approach (CETA) for unhealthy alcohol use among adults with HIV in Zambia: results from a pilot randomized controlled trial. AIDS Behav. 2022:1–14. doi: 10.1007/s10461-021-03408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson R., Darnell D., Berliner L., Dorsey S., Murray L., Monroe-DeVita M. Implementing transdiagnostic cognitive behavioral psychotherapy in adult public behavioral health: a pilot evaluation of the feasibility of the common elements treatment approach (CETA) J. Behav. Health Serv. Res. 2019;46:249–266. doi: 10.1007/s11414-018-9631-x. [DOI] [PubMed] [Google Scholar]
- 42.Kozak M.S., Mugavero M.J., Ye J., et al. Patient reported outcomes in routine care: advancing data capture for HIV cohort research. Clin. Infect. Dis. 2012;54(1):141–147. doi: 10.1093/cid/cir727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitahata M.M., Rodriguez B., Haubrich R., et al. Cohort profile: the centers for AIDS research network of integrated clinical systems. Int. J. Epidemiol. 2008;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. Sep 14 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 45.Eyawo O., Deng Y., Dziura J., et al. Validating self‐reported unhealthy alcohol use with phosphatidylethanol (PEth) among patients with HIV. Alcohol Clin. Exp. Res. 2020;44(10):2053–2063. doi: 10.1111/acer.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saberi P., Ming K., Shrestha I., Scott H., Thorson B., Liu A. Feasibility and acceptability of home-collected samples for human immunodeficiency virus preexposure prophylaxis and severe acute respiratory syndrome coronavirus 2 laboratory tests in san francisco primary care clinics. Open Forum Infect. Dis. Feb 2022;9(2):ofab657. doi: 10.1093/ofid/ofab657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan S.P., Sullivan P.S., Stephenson R. Acceptability and feasibility of a telehealth intervention for STI testing among male couples. AIDS Behav. Dec 2021;25(12):4029–4043. doi: 10.1007/s10461-021-03173-4. [DOI] [PubMed] [Google Scholar]
- 48.Sohail M., Rastegar J., Long D., et al. Data for care (D4C) Alabama: clinic-wide risk stratification with enhanced personal contacts for retention in HIV care via the Alabama quality management group. J. Acquir. Immune Defic. Syndr. Dec 2019;82(Suppl 3):S192–S198. doi: 10.1097/QAI.0000000000002205. [DOI] [PubMed] [Google Scholar]
- 49.Sohail M., Rastegar J., Long D., et al. Data for care (D4C) Alabama: clinic-wide risk stratification with enhanced personal contacts for retention in HIV care via the Alabama quality management group. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2019;82:S192–S198. doi: 10.1097/qai.0000000000002205. [DOI] [PubMed] [Google Scholar]
- 50.Babor T.F., Higgins-Biddle J.C., Saunders J.B., Monteiro M.G. World Health Organization; Geneva: 2001. The Alcohol Use Disorders Identification Test. [Google Scholar]
- 51.Saunders J.B., Aasland O.G., Babor T.F., De La Fuente J.R., Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 52.Helping Patients Who Drink Too Much: A Clinician's Guide. 2005. https://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf [Google Scholar]
- 53.Kroenke K., Spitzer R.L., Williams J.B. The PHQ‐9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spitzer R., Kroenke K., Williams J. Generalized anxiety disorder 7-item (GAD-7) scale. Arch. Intern. Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 55.Blevins C., Weathers F.W., Davis M.T., Witte T.K., Domino J. Prevalence and psychological correlates of complicated. J. Trauma Stress. 2015;28:489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- 56.Humeniuk R., Ali R., Babor T.F., et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST) Addiction. 2008;103(6):1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 57.Humeniuk R., Henry-Edwards S., Ali R., Poznyak V., Monteiro M.G., Organization W.H. 2010. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Manual for Use in Primary Care. [Google Scholar]
- 58.Wu A.W., Hanson K.A., Harding G., et al. Responsiveness of the MOS-HIV and EQ-5D in HIV-infected adults receiving antiretroviral therapies. Health Qual. Life Outcome. 2013;11(1):1–8. doi: 10.1186/1477-7525-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robberstad B., Olsen J.A. The health related quality of life of people living with HIV/AIDS in sub-Saharan Africa-a literature review and focus group study. Cost Eff. Resour. Allocation. 2010;8(1):1–11. doi: 10.1186/1478-7547-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kane J.C., Sharma A., Murray L.K., et al. Efficacy of the common elements treatment approach (CETA) for unhealthy alcohol use among adults with HIV in Zambia: results from a pilot randomized controlled trial. AIDS Behav. Feb 2022;26(2):523–536. doi: 10.1007/s10461-021-03408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanders G., Neumann P., Basu A. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine (vol 316, pg 1093, 2016) JAMA, J. Am. Med. Assoc. 2016;316(18) doi: 10.1001/jama.2016.12195. 1924-1924. [DOI] [PubMed] [Google Scholar]
- 62.Edejer T.T.-T., Baltussen R., Tan-Torres T., et al. Vol. 1. World Health Organization; 2003. (Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis). [Google Scholar]
- 63.Girouard M.P., Sax P.E., Parker R.A., et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin. Infect. Dis. 2016;62(6):784–791. doi: 10.1093/cid/civ981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kimaro G.D., Guinness L., Shiri T., et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced human immunodeficiency virus infection starting antiretroviral therapy in Tanzania and Zambia: a cost-effectiveness analysis. Clin. Infect. Dis. 2020;70(8):1652–1657. doi: 10.1093/cid/ciz453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wutzke S.E., Shiell A., Gomel M.K., Conigrave K.M. Cost effectiveness of brief interventions for reducing alcohol consumption. Soc. Sci. Med. 2001;52(6):863–870. doi: 10.1016/s0277-9536(00)00189-1. [DOI] [PubMed] [Google Scholar]
- 66.Fairburn C.G., Cooper Z. Therapist competence, therapy quality, and therapist training. Behav. Res. Ther. 2011;49(6–7):373–378. doi: 10.1016/j.brat.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCreight M.S., Rabin B.A., Glasgow R.E., et al. Using the Practical, Robust Implementation and Sustainability Model (PRISM) to qualitatively assess multilevel contextual factors to help plan, implement, evaluate, and disseminate health services programs. Translational Behavioral Medicine. 2019;9(6):1002–1011. doi: 10.1093/tbm/ibz085. [DOI] [PubMed] [Google Scholar]
- 68.Peters D.H., Tran N.T., Adam T. World Health Organization; 2013. Implementation Research in Health: a Practical Guide. [Google Scholar]
- 69.Creswell J.W., Klassen A.C., Plano Clark V.L., Smith K.C. Vol. 2013. National Institutes of Health; Bethesda (Maryland): 2011. pp. 541–545. (Best Practices for Mixed Methods Research in the Health Sciences). [Google Scholar]
- 70.Meidlinger P.C., Hope D.A. The new transdiagnostic cognitive behavioral treatments: commentary for clinicians and clinical researchers. J. Anxiety Disord. 2017;46:101–109. doi: 10.1016/j.janxdis.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Peterson R., Darnell D., Berliner L., Dorsey S., Murray L., Monroe-DeVita M. Implementing transdiagnostic cognitive behavioral psychotherapy in adult public behavioral health: a pilot evaluation of the feasibility of the common elements treatment approach (CETA) J. Behav. Health Serv. Res. Apr 2019;46(2):249–266. doi: 10.1007/s11414-018-9631-x. [DOI] [PubMed] [Google Scholar]