Abstract
In this narrative review, we describe the epidemiology (prevalence, incidence, temporal trends, and projections) of type 2 diabetes among children and adolescents (<20 years), focusing on data from the U.S. and reporting global estimates where available. Secondarily, we discuss the clinical course of youth-onset type 2 diabetes, from prediabetes to complications and comorbidities, drawing comparisons with youth type 1 diabetes to highlight the aggressive course of this condition, which, only recently, has become recognized as a pediatric disease by health care providers. Finally, we end with an overview of emerging topics in type 2 diabetes research that have potential to inform strategies for effective preventive action at the community and individual levels.
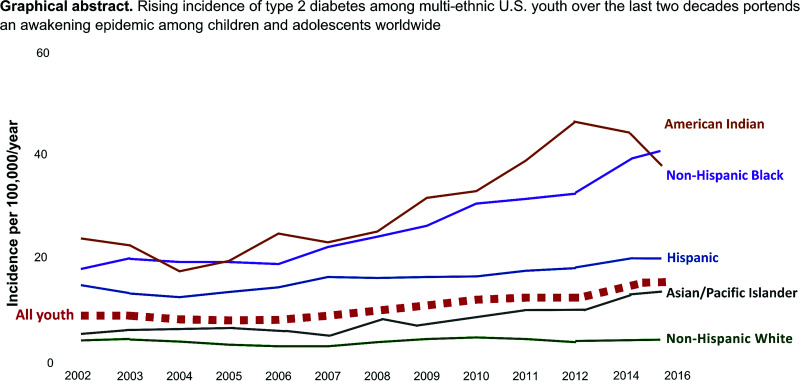
Graphical Abstract
Introduction
Four decades ago, “juvenile diabetes” referred to type 1 diabetes and “adult-onset diabetes” was synonymous with type 2 diabetes. Three decades ago, pediatric type 2 diabetes cases began to emerge from clinic-based investigations (1) and studies monitoring youth-onset type 2 diabetes trends in American Indian communities (2–4). Today, incidence of type 2 diabetes in adolescents is twice that of type 1 diabetes in several non-White racial and ethnic groups (5,6) and has, only recently, become recognized as a pediatric disease in clinical settings (7). While youth-onset type 2 diabetes is still relatively rare, any occurrence of this condition in children and adolescents is concerning given its aggressive clinical course (8), association with risk of debilitating complications by young adulthood (9), and high all-cause mortality (10).
In this narrative review, we describe the epidemiology of type 2 diabetes and its clinical course among people <20 years of age, focusing on data from the U.S. and reporting global estimates where available. Secondarily, we discuss key drivers of current trends and put forth emerging research areas with potential to inform strategies for type 2 diabetes prevention in youth.
Burden
Prevalence
Most of our current knowledge about the epidemiology of youth-onset diabetes in the U.S. comes from the SEARCH For Diabetes in Youth study, a population-based study that surveilled physician-diagnosed diabetes in Washington, South Carolina, Ohio, Colorado, and California, plus select American Indian reservations, from 2000 to 2020 (11). Based on the most recent SEARCH data, type 2 diabetes prevalence among U.S. youth in 2017 was 0.67 (95% CI 0.63, 0.70) case subjects per 1,000, corresponding to 1,230 cases among 1,848,899 youth aged 10–19 years (12).
Racial and ethnic minority populations carry the largest burden of youth-onset type 2 diabetes. In SEARCH, the highest prevalence of type 2 diabetes per 1,000 youth in 2017 was observed among Black or African American youth at 1.80, followed by 1.63 in American Indian youth, 1.03 among youth of Hispanic origin, 0.59 among Asian/Pacific Islander youth, and 0.20 among non-Hispanic White youth (12). While type 1 diabetes remains the predominant form of diabetes in children and adolescents, prevalence of type 2 diabetes is now higher than that of type 1 diabetes among American Indian youth (1.63 cases per 1,000 vs. 0.56 cases per 1,000 in 2017). Moreover, the annual percent change (APC) in prevalence between 2009 and 2017 was steeper for type 2 than type 1 diabetes among Asian/Pacific Islander youth (7.3% vs. 2.9%), Black youth (7.1% vs. 2.4%), and Hispanic youth (3.2% vs. 2.4%).
Across all racial and ethnic groups, the prevalence of type 2 diabetes increased with age: 0.29 per 1,000 for 10- to 14-year-old individuals and 1.04 per 1,000 for 15- to 19-year-old individuals (12). Additionally, females consistently have higher prevalence than males (0.82 [95% CI 0.77, 0.88] vs. 0.51 [95% CI 0.47, 0.56] per 1,000) (12).
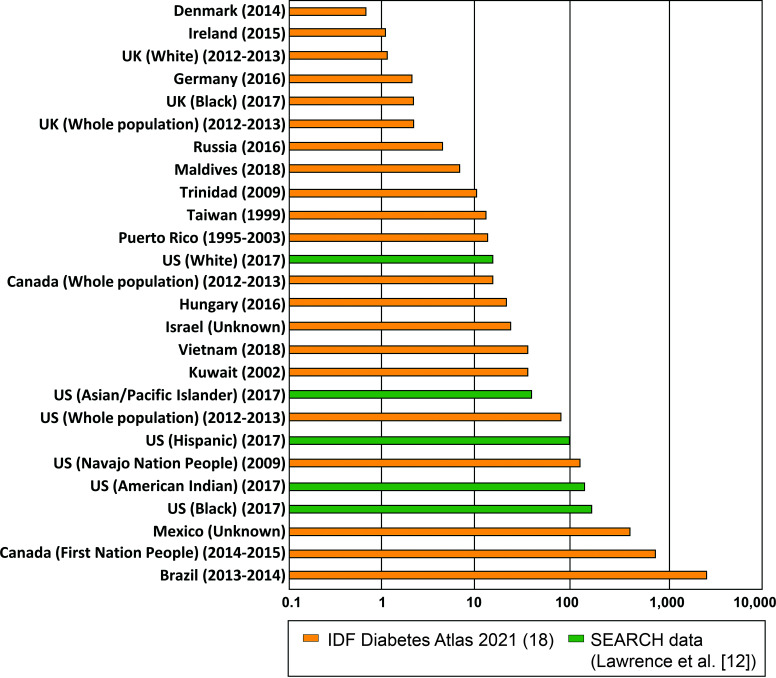
Globally, type 2 diabetes prevalence is highest among adolescents in Brazil (33 per 1,000) (13), the Ontario First Nations People (5.7 per 1,000) (14), and youth in Mexico (4 per 1,000) (15), followed by Black youth (1.8 per 1,000) and American Indian youth (1.63 per 1,000) in the U.S. (12). Lowest prevalences are observed in Denmark (0.6 per 100,000) (16) and England and Wales (2.9 per 100,000) (17). Figure 1 shows worldwide prevalence estimates ranked by region and ethnicity, comprising data from the 2021 IDF Diabetes Atlas (18) and SEARCH (12). Note that direct comparisons of country-specific statistics should be made with caution given the lack of universal diagnostic criteria for youth-onset type 2 diabetes.
Figure 1.
Global prevalence of type 2 diabetes among children and adolescents (age <20 years), per 100,000.
Incidence
Incidence of type 2 diabetes among children and adolescents in the U.S. is 13.8 per 100,000 youth/year according to SEARCH 2014–2015 data (5). Incidence increases with age (per 100,000/year: 12.4 vs. 15.2 for age 10–14 vs. 15–19 years), female sex (per 100,000/year: 16.7 vs. 11.1 for females vs. males), and youth from racial and ethnic minority populations (per 100,000/year: 37.8 for Black youth, 32.8 for American Indian youth, 20.9 for Hispanic youth, 11.9 for Asian/Pacific Islander youth, and 4.5 for non-Hispanic White youth) (5).
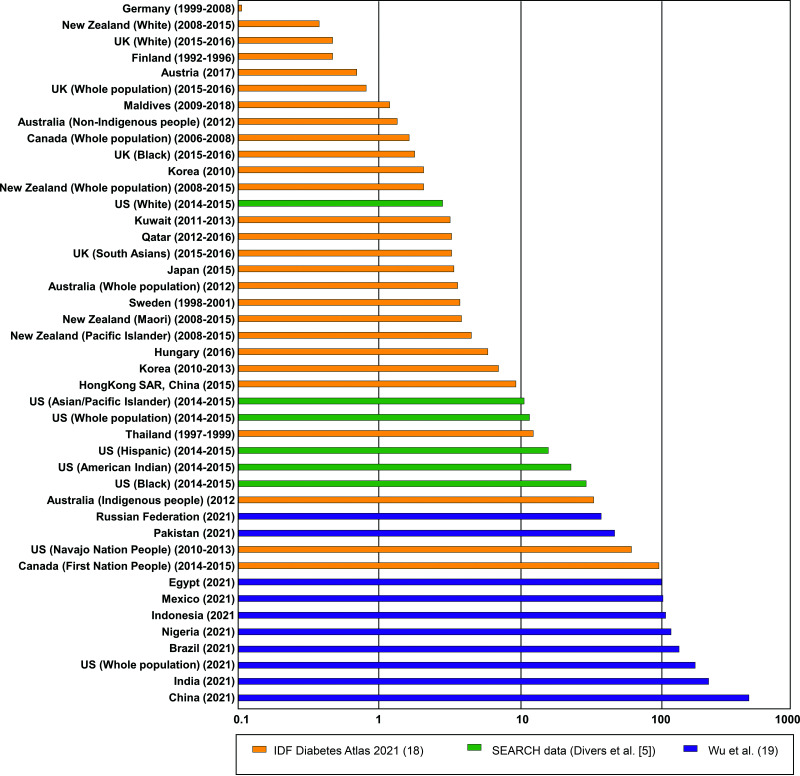
In a recent systematic review of literature on type 2 diabetes incidence among children and adolescents from 25 countries/territories, Wu et al. (19) estimated ∼41,600 new cases of youth-onset type 2 diabetes globally in 2021. One-third of incident cases were in China (734 per 100,000), India (397 per 100,000), and the U.S. (285 per 100,000). Other countries within the top 10 highest incidence rates per 100,000 in 2021 include Brazil (n = 154), Nigeria (n = 143), Indonesia (n = 133), Mexico (n = 119), Egypt (n = 116), Pakistan (n = 88), and the Russian Federation (n = 65) (19). On the other hand, non-Hispanic White youth in the U.K., Germany, and the U.S. had the lowest incidence (0.1 to 0.8 per 100,000/year) (5,20,21). Figure 2 shows global incidence estimates by region and ethnicity based on data from the 2021 IDF Diabetes Atlas (18), SEARCH (5), and Wu et al. (19). Generally, global patterns for prevalence and incidence of youth-onset type 2 diabetes align with those observed in the U.S., with a disproportionate burden among non-White youth.
Figure 2.
Global incidence of type 2 diabetes among children and adolescents (age <20 years), per 100,000.
Temporal Trends and Projections
In SEARCH (12), type 2 diabetes prevalence per 1,000 youth was 0.34 in 2001, 0.46 in 2009, and 0.67 in 2017, with a steeper APC in prevalence during the latter half of the follow-up period (4.8% vs. 3.7% increase). During this time, the APC significantly increased among 15- to 19-year-old individuals (2.9% in 2001–2009 to 5.5% in 2009–2017; P = 0.04) but not among 10- to 14-year-old individuals (4.9% in 2001–2009 vs. 3.1% in 2009–2017; P = 0.46). Both sexes experienced increased type 2 diabetes prevalence during the abovementioned study periods (12). Disaggregation by race and ethnicity revealed the largest increase in prevalence among Black youth, for whom the APC increased from 1.2% (−1.3%, 3.8%) in 2001–2009 to 7.1% (4.8%, 9.4%) in 2009–2017 (P = 0.007). Across these two periods, marginally significant increases in APC were observed for American Indian youth (APC −1.0% in 2009–2017 vs. 4.8% in 2009–2017; P = 0.11) and Asian/Pacific Islander youth (APC −0.7% in 2001–2009 vs. 7.3% in 2009–2017; P = 0.07). In contrast, the APC in prevalence decreased among Hispanic youth (7.3% in 2001–2009 and 3.2% in 2009–2017; P = 0.03) and remained stable in non-Hispanic White youth (2.5% in 2001–2009 and 1.4 in 2009–2017; P = 0.64) (12).
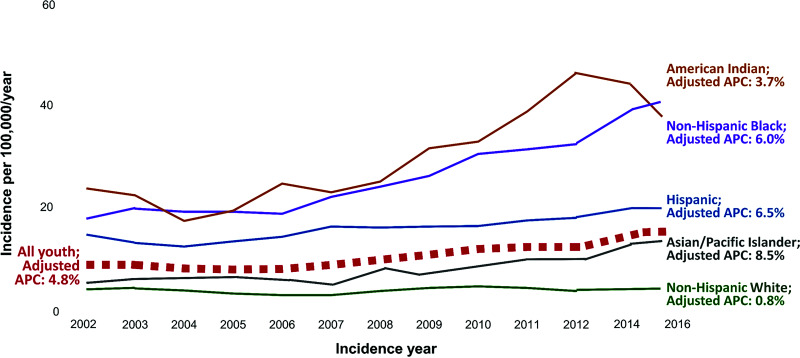
SEARCH data also indicate a steady rise in type 2 diabetes incidence. In 2002–2003, the incidence of youth-onset type 2 diabetes was 9 new cases per 100,000 individuals/year. This estimate increased to 12.2 cases in 2009–2010, 12.5 in 2011–2012, and 13.8 in 2014–2016 (5), corresponding to an age- and sex-adjusted APC in incidence of 4.8% from 2002 to 2015 (5). This statistic was higher for females than males (5.1% vs. 4.4%) and for older than younger adolescents (5.0% for 15- to 19-year-old individuals vs. 4.6% for 10- to 14-year-old individuals). As shown in Fig. 3, disaggregation by race and ethnicity reveals the highest APC in incidence among Asian/Pacific Islander youth at 7.7% (3.4%, 12.2%), followed by 6.5% (4.4%, 8.5%) in Hispanic youth, 6.0% (4.1%, 7.9%) in Black youth, 3.7% (0.1%, 7.4%) in American Indian youth, and 0.8% (−1.4%, 2.9%) among non-Hispanic White youth.
Figure 3.
Temporal trends and APC in incidence of type 2 diabetes among multiethnic U.S. youth in the SEARCH study from 2002 to 2015. APC estimates for all youth are adjusted for age, sex, race, and ethnicity; estimates within racial and ethnic strata are adjusted for age and sex. Created from data reported in Mayer-Davis et al. (15) and Divers et al. (6).
Based on these estimates and those of other studies (6), researchers project a fourfold increase in prevalence of youth-onset type 2 diabetes in the U.S. by 2050 after accounting for anticipated changes in demography, with the most substantial increases occurring among racial and ethnic minority youth, particularly those of Black or Indigenous populations (22). As these groups also have the highest prevalence of overweight/obesity, current temporal trends in Black and Indigenous youth may portend future trends in other racial and ethnic groups as the obesity epidemic continues to evolve. We note, however, that population-level patterns of change in obesity prevalence may have differential effects on type 2 diabetes trends across racial and ethnic groups as some populations, e.g., those in East Asia, have high incidence of youth-onset type 2 diabetes despite a lower burden of childhood obesity than White youth in the U.S. and Europe (23,24). Genetic predisposition, heterogeneous pathophysiology of type 2 diabetes, disparities in socioeconomic status, access to health care, and cultural practices likely contribute to such differences in incidence.
Clinical Course
Prediabetes
In adults, the progression from normal glucose tolerance to type 2 diabetes involves the intermediate stage of prediabetes, comprising impaired fasting glucose (IFG) (fasting plasma glucose 100–126 mg/dL) and/or impaired glucose tolerance (IGT) (plasma glucose 140–199 mg/dL 2 h after oral glucose tolerance test). For both adults and children, the American Diabetes Association (ADA) defines prediabetes as having IFG, IGT, or hemoglobin A1c (HbA1c) level between 5.7% and 6.4%. Here, we describe the epidemiology of prediabetes in youth according to the current ADA definition, acknowledging that there is need for research investigating the natural history of prediabetes in children and adolescents and the clinical relevance of current thresholds for predicting youth-onset type 2 diabetes (25).
A recent analysis of 2005–2016 National Health and Nutrition Examination Survey (NHANES) data estimated 18.0% prevalence of prediabetes among adolescents 12–18 years of age based on HbA1c values (26). Within the categorization of prediabetes, 9.2% of adolescents had IFG, 2.8% had IGT, and 0.7% had both. After accounting for age, race, ethnicity, and BMI, the prevalence of prediabetes was higher among males than females during adolescence (22.5% [19.5%, 25.4%] vs. 13.4% [10.8%, 16.5%]) (26). In a subsequent study that analyzed 10 NHANES cycles spanning 1999–2018, investigators observed an increase in prediabetes among 12- to 19-year-old individuals from 11.5% in 1999–2002 to 28.2% in 2015–2018 (27). This increase was steeper in males (15.8% to 36.4%) than females (7.1% to 19.6%) (27). We note that while relative differences in these statistics across subgroups and trends over time are likely valid, estimates at each time point may be lower than in actuality, given evidence of markedly lower prediabetes prevalence when using HbA1c (4.4%) versus fasting glucose (15.0%) thresholds (28). Additionally, national-level U.S. data indicate that Black youth consistently exhibit ∼0.2% higher HbA1c values than their White peers both in the presence and absence of diabetes (29). Mexican American youth have values that are between those of Black and White youth (29). These racial and ethnic differences are not explained by glycemia, sociodemographic characteristics, clinical factors, or access to or quality of health care, pointing toward the need for additional research to assess the need for race- or ethnicity-specific HbA1c norms.
Given that prediabetes is a precursor to type 2 diabetes in adults, it is tempting to surmise that trends in youth prediabetes portend a continued rise in prevalence and incidence of youth-onset type 2 diabetes. However, it is important to note that a substantial proportion (∼70%) of youth categorized as having prediabetes revert to normoglycemia after puberty (30). Moreover, the paradoxical sex differences for prediabetes versus type 2 diabetes suggest that male youth with prediabetes are more likely to revert to normoglycemia after puberty, whereas female youth with prediabetes are more likely to progress to type 2 diabetes. Although reasons for this discrepancy remain unclear, hypotheses include a more “harmful” effect of puberty on metabolic health in female individuals, especially in the context of obesity (31), and/or higher levels of physical activity among male individuals (32), a pattern that has been observed as early as age 4–5 years (33). More research is required to determine whether extrapolation of adult prediabetes definitions is appropriate for children and adolescents; to assess clinical relevance of current definitions for predicting youth-onset type 2 diabetes; and to identify determinants of progression to type 2 diabetes versus reversion to normoglycemia (25).
Complications and Comorbidities
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study, a U.S. clinical trial assessing treatment efficacy among youth with recently diagnosed type 2 diabetes (9), exemplifies the high burden of type 2 diabetes complications following a relatively short disease duration. Additionally, the SEARCH study, which was purposefully designed to provide a direct comparison of burden of diabetes-related complications and comorbidities among youth with type 1 and type 2 diabetes of similar disease duration, glycemic control, and age, provides compelling evidence that youth-onset type 2 diabetes is an aggressive disease associated with higher rates of complications than type 1 diabetes. Here, we summarize evidence from TODAY and SEARCH, among others, to describe the epidemiology of complications and comorbidities among youth with type 2 diabetes, drawing comparisons to type 1 diabetes where possible.
Microvascular Complications
In TODAY, cumulative incidence of any microvascular complication was 50% by 9 years and 80% by 15 years of type 2 diabetes duration, corresponding to a 60% prevalence of having any microvascular complication approximately 13 years after diagnosis (34). Diabetic retinopathy, or damage to light-sensitive blood vessels in the retina, is the most common complication of type 2 diabetes (9). Diabetic retinopathy was present in 50% of TODAY participants by age 25 years, after ∼12 years of disease duration (35). Equally alarming is the 35% cumulative incidence peripheral neuropathy, or damage to nerves outside the brain, 12–15 years after diagnosis (36). As diabetes is the leading cause of kidney disease (37), it is not surprising that up to 50% of people with diabetes develop diabetic kidney disease (DKD) (38), for which changes in kidney structure indicative of nephropathy are detectable as soon as 1.5 years after disease onset (39). Although the baseline prevalence of DKD at the time of enrollment into the TODAY study was only 8%, investigators reported a cumulative incidence of 55% after 15 years of follow-up at age 26 years (9), an estimate comparable to the 43.5% DKD prevalence among adults 65 years or older with type 2 diabetes (40). A comparison of these statistics to the 25% of young people with type 1 diabetes who suffer from any microvascular complication within 10 years of diagnosis underscores the gravity of these data.
In comparisons of youth with type 2 vs. type 1 diabetes, the prevalence of all microvascular complications is consistently higher among cases of type 2 diabetes despite similar disease duration, glycemic control, and age. In SEARCH, participants with type 2 diabetes had 3.5% higher prevalence of diabetic retinopathy (9.1% vs. 5.6%), 9.2% higher prevalence of peripheral neuropathy (17.7% vs. 8.5%), and 14.0% higher prevalence of DKD (19.9% vs. 5.8%) than those with type 1 diabetes after an average disease duration of 8 years (41). SEARCH also showed that DKD was present in 20% of participants with type 2 diabetes 8 years after diagnosis (average age 22 years), an estimate that is threefold higher than that for participants with type 1 diabetes (41). Similarly, in an Australian study of patients with diabetes diagnosed before age 18 years, the prevalence of peripheral and autonomic neuropathy was 21% and 57%, respectively, after a median of just 1.3 years’ disease duration, comparable to the prevalence of neuropathy among youth with type 1 diabetes in the same clinic but 20 years after diagnosis (42).
The discrepancies in prevalence of microvascular complications in TODAY versus SEARCH is due, in part, to differing definitions. Specifically, the lower prevalence of diabetic retinopathy in SEARCH compared with TODAY reflects the fact that SEARCH included more advanced cases of diabetic retinopathy comprising proliferative retinal changes (41), whereas TODAY included all cases of diabetes retinopathy, including nonproliferative cases (35).
Beyond comparisons of diabetes complications among youth-onset type 2 and type 1 diabetes, indirect comparisons have been made to older adults in the Diabetes Prevention Program Outcomes Study (DPPOS) (9), for which participants were ∼51 years of age at baseline. In this setting, prevalence of composite microvascular complications is relatively low (11.3–12.4%) (43), but the comparison is somewhat misleading, as TODAY participants were youth with clinically diagnosed type 2 diabetes while DPPOS participants represent a high-risk population screened for type 2 diabetes with periodic oral glucose tolerance tests.
Macrovascular Complications
Macrovascular complications of type 2 diabetes include coronary heart disease, cardiomyopathy, arrhythmias, cerebrovascular disease, and peripheral artery disease. In SEARCH, youth with type 2 versus type 1 diabetes had much higher age-adjusted prevalence of arterial stiffness (47.4% vs. 11.1%) and hypertension (21.6% vs. 10.1%) after an average of 8 years’ disease duration, although these discrepancies were attenuated after accounting for obesity status, pointing toward a key role for excess adiposity in the pathophysiology of macrovascular complications (41). Similarly, in a study of 824 Australian participants in Sydney diagnosed with diabetes between 15 and 30 years of age, ischemic heart disease and/or cerebrovascular disease was observed in 14% of participants with type 2 diabetes vs. 6% of those with type 1 diabetes after 12 and 15 years of disease duration, respectively (10). In another Australian study that took place in New South Wales, hypertension was more frequent among youth with type 2 than type 1 diabetes (36% vs. 16% prevalence) at age 15 years (42). Together, these findings highlight the markedly higher burden of macrovascular complications and comorbidities among youth with type 2 than type 1 diabetes.
SEARCH data reveal that the higher prevalence of diabetes complications among youth with type 2 than type 1 diabetes is driven by youth from minority populations (41). For example, the higher prevalence of retinopathy for type 2 (9.1%) versus type 1 diabetes (4.6%) is driven by non-White minority youth, among whom type 2 diabetes cases have 5.5% (1.0%, 11.0%) higher prevalence of retinopathy than type 1 diabetes. On the other hand, no significant difference was detected with respect to diabetes type among non-Hispanic White adolescents. This pattern holds true for all other diabetes complications, except for cardiovascular autonomic neuropathy, for which no significant difference in prevalence was observed by diabetes type. Importantly, these racial and ethnic disparities are not explained by differences in glycemic control, blood pressure levels, or obesity, suggesting other potential explanations, such as social determinants of health (see social determinants of health, below), and the need for additional research in this area.
Mortality
Among SEARCH participants diagnosed with diabetes between 2002 and 2015, short-term mortality among youth and young adults with type 2 diabetes was 1.5 times higher than in those with type 1 diabetes (standardized mortality ratio 2.3 [95% CI 1.7, 3.0] vs. 1.5 [95% CI 1.2, 1.8] during 8.5 years of follow-up among 19,717 participants) (44). The excess mortality among young people with type 2 diabetes was higher among racial and ethnic minorities and those younger than 25 years of age at time of death (44). Among participants with type 2 diabetes, only 9.1% of deaths were attributable to diabetes, while 34.5% were due to external causes such as motor vehicle accidents, accidental injuries, and assault. The proportion of deaths attributable to external causes is consistent with the general U.S. population, for which the leading cause of death (31–40%) for people 1–44 years of age is unintentional injuries (45). However, there remains an excess in mortality among youth and young adults with type 2 diabetes beyond what is experienced by the general population, potentially due to socioeconomic disadvantage, as evidenced by lower household income and parental education and reliance on Medicaid (41).
Among global deaths due to youth-onset diabetes, the majority (73.7% [68.3%, 77.4%]) are classified as being due to type 1 diabetes (46). Age-standardized death rates due to diabetes among people <25 years declined between 1990 and 2019, with a steeper decline for type 1 than type 2 diabetes (APC −21.0% [−33.9%, −5.9%] vs. −2.5% [−17.7%, 15.8%]). The highest age-standardized death rates due to any type of diabetes among young people worldwide are reported for low- to middle-income countries, ranging from 0.23 (0.19, 0.28) in Kyrgyzstan to 1.93 (1.30, 2.68) in Myanmar (46). In comparison, the lowest and highest age-standardized death rates reported for high-income countries are 0.03 (0.02, 0.04) in Cyprus and 0.45 (0.34, 0.62) in Kuwait (46). These global trends, albeit for combined type 1 and 2 diabetes, emphasize the wide-ranging impact of social factors not only on diabetes prevalence and incidence but also on short- and long-term complications and comorbidities.
Contributors to Trends in Youth-Onset Type 2 Diabetes
The Obesity Epidemic
Parallel trends in childhood obesity and youth-onset type 2 diabetes, albeit with an average 10-year latency between the two conditions (47), are not surprising given that obesity is a leading risk factor for development of insulin resistance and eventual failure of the β-cells to maintain adequate insulin secretion. The effect of excess adiposity on type 2 diabetes risk takes root very early in life. With increasing rates of obesity and/or diabetes among reproductive-aged women (48), exposure to developmental overnutrition in utero through excess maternal adiposity (e.g., high prepregnancy BMI and excessive gestational weight gain [49]), hyperglycemia (50), and poor prenatal diet quality (51) are recognized as risk factors for early-onset metabolic disease (52). Additionally, postnatal and early-life exposures and experiences that occur at the family and individual levels, e.g., shorter breastfeeding duration (53), exposure to environmental pollutants (e.g., ambient air pollution and endocrine-disrupting hormones) (54,55), and a home environment that promotes unfavorable eating behaviors and low physical activity levels (56), can further compound the effects of exposure to in utero overnutrition. Finally, upstream of family and individual-level risk factors are social determinants of health or structurally embedded contexts that trickle down to affect our physical and social environment, health behaviors, and ultimately individual-level physiology, a topic discussed in the next section.
Social Determinants of Health
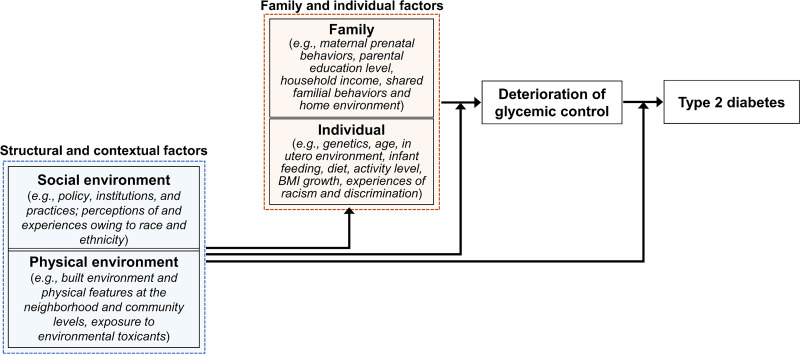
Research on type 2 diabetes prevention has historically focused on individual-level biological traits (e.g., genetic predisposition, in utero exposures, and biochemical markers of disease progression) and behavioral risk factors (e.g., diet and physical activity). In recent years, social determinants of health, i.e., the conditions and environment into which we are born, live, play, and work, have risen to the forefront as upstream drivers of chronic metabolic conditions. Social factors such as income, education, housing/neighborhood context, and access to health care and nutritious food are central to the development and progression of type 2 diabetes (57,58). Moreover, the prevalence and incidence of childhood obesity and type 2 diabetes, as well as related complications and comorbidities, are socially graded with a disparate burden among children from families of lower socioeconomic status and historically marginalized populations (57,59). As shown in Fig. 4, these disparities point toward a need to address the social and environmental contexts surrounding youth-onset type 2 diabetes. These upstream, contextual risk factors include experiences of poverty and structural racism, which occur at and, thus, require action at multiple levels (i.e., individual [60], county [61], and state [62]) and dimensions (e.g., segregation, educational inequities, employment, income, and home ownership [63]) in order to identify avenues by which to mount broad-ranging prevention and intervention.
Figure 4.
A conceptual diagram of multilevel risk factors for youth-onset type 2 diabetes.
The multilevel nature of type 2 diabetes risk factors suggests that individual- and family-level lifestyle interventions may not be sufficient, although we note that such approaches have had moderate success among certain high-risk subsets of youth. One example is Tribal Turning Point, a randomized trial that modified and culturally tailored the Diabetes Prevention Program lifestyle intervention for American Indian children (64). While such intensive and tailored approaches are crucial for mitigating type 2 diabetes risk among the highest-risk segments of the population, a complementary approach is to take the onus off individuals and families and instead improve community resources that forge healthy lifestyles. Examples include increasing neighborhood green space and walkability to promote physical activity and facilitating access to healthy foods and health care. Importantly, community engagement and involvement in implementation of interventions are key to effective and sustained impact, especially in low-resource settings (65). We include suggestions for extending both tailored/targeted strategies as well as broader approaches to diabetes prevention in a two-pronged approach to youth-onset type 2 diabetes prevention, below.
Future Directions
Characterizing the Spectrum of Youth-Onset Type 2 Diabetes
Compared with the breadth of literature on the epidemiology of type 2 diabetes in adults, such data on children and adolescents are limited, especially at the global level. This scarcity is due to the relative rarity of youth-onset type 2 diabetes, which contributes to incomplete knowledge about the etiology and pathophysiology of this relatively new disease that may be distinct from adult-onset type 2 diabetes.
At present, a diagnosis of type 2 diabetes is typically considered among pubertal youth with hyperglycemia who also have obesity, a family history of type 2 diabetes, and/or features of metabolic syndrome (abdominal adiposity; high blood pressure, plasma glucose, and serum triglycerides; and low serum high-density lipoprotein) in the absence of islet autoantibodies (66). However, overlap in symptoms (i.e., presence of polydipsia and polyuria, presence of obesity, and presentation with ketoacidosis) among youth with newly diagnosed diabetes, despite distinct etiologies (e.g., autoimmune diabetes vs. monogenic forms of diabetes vs. type 2 diabetes), may lead to misclassification, diagnostic challenges, and inappropriate treatment. In addition, youth-onset type 2 diabetes may have a heterogeneous pathophysiology. Among young and middle-aged adults in Sweden (67) and India (68), researchers identified distinct subgroups of individuals based on characteristics at diagnosis, including age, BMI, HbA1c, and indices of insulin resistance and β-cell function. The subgroups had different sociodemographic characteristics and differential risk of complications. Additionally, insulin deficiency was a key pathophysiological driver of type 2 diabetes among young Indians, whereas insulin resistance with obesity was the predominant feature among young adults of European origin (68). Use of such clustering approaches to identify distinct subgroups of type 2 diabetes phenotypes in youth, while considering inclusion of data on genetics, environmental factors, and social determinants of health in addition to characteristics at diagnosis, is a promising direction to improve understanding of youth-onset type 2 diabetes pathophysiology, which in turn will improve prevention, diagnosis, and treatment.
The Intergenerational Cycle of Obesity and Type 2 Diabetes
The longitudinal study of Pima Indians in the Gila River Indian Community (69) was one of the first to explore associations of in utero exposure to maternal diabetes with risk of type 2 diabetes in offspring. In this high-risk population, offspring of women with diabetes had higher birth weight and 10-fold greater risk of type 2 diabetes by adolescence and young adulthood (3). Further, maternal diabetes was the single strongest risk factor for youth-onset type 2 diabetes, accounting for most of the increase in youth-onset type 2 diabetes in this population over the last three decades (70). In the SEARCH Case-Control Study, participants whose mothers had diabetes during pregnancy were at sevenfold greater risk of type 2 diabetes than their unexposed counterparts (71). A discordant sibling pair study of Pima Indians demonstrated threefold greater odds of youth-onset type 2 diabetes among the siblings born after versus before the diagnosis of maternal diabetes (72), supporting a specific in utero programming effect of maternal diabetes on offspring type 2 diabetes risk. Such findings highlight the need to consider exposures and experiences, starting in gestation, if not before conception, for prevention of type 2 diabetes.
A Two-Pronged Approach to Youth-Onset Type 2 Diabetes Prevention
Understanding the Pathophysiology of Youth-Onset Type 2 Diabetes to Identify High-Risk Youth
Prevention of youth-onset type 2 diabetes is crucial, as this form of disease is more aggressive (73,74) and less responsive to treatment than adult-onset type 2 diabetes (75). However, our limited knowledge of type 2 diabetes pathophysiology prevents us from identifying high-risk youth for intervention. To date, most literature on the pathophysiology of youth-onset type 2 diabetes comprises cross-sectional studies (76–80) and small longitudinal protocols that did not span all of puberty (81–84). Much remains unknown about type 2 diabetes cases that occur with increasing frequency in adolescence (85) and young adulthood (86). Longitudinal studies following initially normoglycemic youth to onset of dysglycemia and full-blown type 2 diabetes during puberty are needed to understand subclinical disease progression, characterize the spectrum of glycemic transitions, and identify determinants of progression versus reversion. Such efforts will inform targeted approaches for diabetes prevention early in disease progression and complement findings from clinical trials like TODAY (9) and the Restoring Insulin Secretion (RISE) (87), which focus on the pathophysiology of youth-onset type 2 diabetes after diagnosis to identify effective treatment options.
Concerted Prevention Efforts Across Government, Public Health, and Clinical Care
While targeted approaches for type 2 diabetes prevention are crucial for mitigating disease risk as well as future complications/comorbidities among individuals at the upper end of the risk distribution, an overarching goal of chronic disease prevention is to deploy such efforts at the population level. Within this context, successful prevention requires concerted efforts across three societal sectors (88). First, governments, in concert with the private sector, need to promote healthy nutritional and agricultural policies, implement modifications in the built environment that encourage physical activity, and make prevention affordable for the most high-risk and vulnerable individuals. Second, public health is crucial for translating evidence-based research findings for use in clinical settings, developing accessible and cost-effective programs that can be deployed at the community level, and monitoring the progress of prevention initiatives. Finally, the clinical sector bears the charge of screening and identifying youth at high risk for type 2 diabetes based on risk factors and referring these individuals to intervention programs. At present, such intervention programs are not widely available. Coordination among public health researchers and clinicians will be instrumental to identifying evidence-based strategies for diabetes prevention and subsequent investment in the resources and programs to accomplish this end. Further, a concerted effort across government, public health, and clinical care sectors is required not only to develop and test cost-effective community-level interventions that meet individual needs but also to ensure that access to medical treatment and other support are sustainable in the long term and accessible to the most vulnerable and impoverished segments of the population.
Conclusions
The rising prevalence and incidence of youth-onset type 2 diabetes in the U.S. and globally is a public health concern. The relative newness and rarity of this condition, paired with our limited knowledge of its pathophysiology, hamper effective prevention and treatment. Research priorities include the following:
Perform systematic surveillance of the entire course of youth-onset type 2 diabetes, from normoglycemia to dysglycemia to full-blown type 2 diabetes and its complications/comorbidities;
Improve understanding of youth-onset type 2 diabetes pathophysiology, an end point that will provide insight on the spectrum of type 2 diabetes in children and adolescents and aid in identifying high-risk individuals for preventive action;
Apply a holistic approach for identifying lifetime exposures and experiences, including multilevel analyses of upstream contextual risk factors (e.g., social determinants of health) as well as downstream family- and individual-level characteristics that independently and interactively promote risk of type 2 diabetes;
Develop multilevel strategies for type 2 diabetes prevention that will be beneficial to multiple other cardiometabolic conditions on the rise among young people worldwide.
Article Information
Funding. W.P. is supported by the Colorado Clinical and Translational Sciences Institute (KL2-TR002534) and the ADA (ADA-7-22-ICTSPM-08).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. W.P. wrote the initial draft of the manuscript, and R.C. contributed to this draft. R.C., E.M.D., and D.D. reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Footnotes
This article is featured in podcasts available at diabetesjournals.org/care/pages/diabetes_care_on_air and diabetesjournals.org/journals/pages/diabetes-core-update-podcasts.
References
- 1. Cowie CC, Casagrande SS, Menke A, et al., Eds. Diabetes in America. Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [PubMed] [Google Scholar]
- 2. Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 1996;128:608–615 [DOI] [PubMed] [Google Scholar]
- 3. Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ. Increasing prevalence of type II diabetes in American Indian children. Diabetologia 1998;41:904–910 [DOI] [PubMed] [Google Scholar]
- 4. Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American adolescents: an epidemiologic health perspective. J Pediatr 2000;136:664–672 [DOI] [PubMed] [Google Scholar]
- 5. Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths–selected counties and Indian reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep 2020;69:161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes 2013;4:270–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. RISE Consortium . Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bjornstad P, Drews KL, Caprio S, et al.; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 2013;36:3863–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamman RF, Bell RA, Dabelea D, et al.; SEARCH for Diabetes in Youth Study Group . The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 2014;37:3336–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawrence JM, Divers J, Isom S, et al.; SEARCH for Diabetes in Youth Study Group . Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA 2021;326:717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Telo GH, Cureau FV, Szklo M, Bloch KV, Schaan BD. Prevalence of type 2 diabetes among adolescents in Brazil: findings from Study of Cardiovascular Risk in Adolescents (ERICA). Pediatr Diabetes 2019;20:389–396 [DOI] [PubMed] [Google Scholar]
- 14. Shulman R, Slater M, Khan S, et al. Prevalence, incidence and outcomes of diabetes in Ontario First Nations children: a longitudinal population-based cohort study. CMAJ Open 2020;8:E48–E55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simental-Mendía LE, Gamboa-Gómez CI, Aradillas-García C, Rodríguez-Morán M, Guerrero-Romero F. The triglyceride and glucose index is a useful biomarker to recognize glucose disorders in apparently healthy children and adolescents. Eur J Pediatr 2020;179:953–958 [DOI] [PubMed] [Google Scholar]
- 16. Oester IM, Kloppenborg JT, Olsen BS, Johannesen J. Type 2 diabetes mellitus in Danish children and adolescents in 2014. Pediatr Diabetes 2016;17:368–373 [DOI] [PubMed] [Google Scholar]
- 17. Khanolkar AR, Amin R, Taylor-Robinson D, Viner R, Warner J, Stephenson T. Ethnic minorities are at greater risk for childhood-onset type 2 diabetes and poorer glycemic control in England and Wales. J Adolesc Health 2016;59:354–361 [DOI] [PubMed] [Google Scholar]
- 18. International Diabetes Federation . IDF Diabetes Atlas. 10th ed. Brussels, Belgium, International Diabetes Foundation, 2021 [Google Scholar]
- 19. Wu H, Patterson CC, Zhang X, et al. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res Clin Pract 2022;185:109785. [DOI] [PubMed] [Google Scholar]
- 20. Galler A, Stange T, Müller G, et al.; Childhood Diabetes Registry in Saxony, Germany . Incidence of childhood diabetes in children aged less than 15 years and its clinical and metabolic characteristics at the time of diagnosis: data from the Childhood Diabetes Registry of Saxony, Germany. Horm Res Paediatr 2010;74:285–291 [DOI] [PubMed] [Google Scholar]
- 21. Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH. Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabet Med 2018;35:737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imperatore G, Boyle JP, Thompson TJ, et al.; SEARCH for Diabetes in Youth Study Group . Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012;35:2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afshin A, Forouzanfar MH, Reitsma MB, et al.; GBD 2015 Obesity Collaborators . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei JN, Sung FC, Lin CC, Lin RS, Chiang CC, Chuang LM. National surveillance for type 2 diabetes mellitus in Taiwanese children. JAMA 2003;290:1345–1350 [DOI] [PubMed] [Google Scholar]
- 25. Mangione CM, Barry MJ, Nicholson WK, et al.; US Preventive Services Task Force . Screening for prediabetes and type 2 diabetes in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA 2022;328:963–967 [DOI] [PubMed] [Google Scholar]
- 26. Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-2016. JAMA Pediatr 2020;174:e194498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Li Y, Zhang D, Yi SS, Liu J. Trends in prediabetes among youths in the US from 1999 through 2018. JAMA Pediatr 2022;176:608–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee AM, Fermin CR, Filipp SL, Gurka MJ, DeBoer MD. Examining trends in prediabetes and its relationship with the metabolic syndrome in US adolescents, 1999-2014. Acta Diabetol 2017;54:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saaddine JB, Fagot-Campagna A, Rolka D, et al. Distribution of HbA(1c) levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Survey. Diabetes Care 2002;25:1326–1330 [DOI] [PubMed] [Google Scholar]
- 30. Mehreen TS, Kamalesh R, Pandiyan D, et al. Incidence and predictors of dysglycemia and regression to normoglycemia in Indian adolescents and young adults: 10-year follow-up of the ORANGE study. Diabetes Technol Ther 2020;22:875–882 [DOI] [PubMed] [Google Scholar]
- 31. Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction 2010;140:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishii K, Shibata A, Adachi M, Nonoue K, Oka K. Gender and grade differences in objectively measured physical activity and sedentary behavior patterns among Japanese children and adolescents: a cross-sectional study. BMC Public Health 2015;15:1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perng W, Francis EC, Schuldt C, Barbosa G, Dabelea D, Sauder KA. Pre- and perinatal correlates of ideal cardiovascular health during early childhood: a prospective analysis in the Healthy Start Study. J Pediatr 2021;234:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bjornstad P, Drews KL, Caprio S, et al.; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. TODAY Study Group . Development and progression of diabetic retinopathy in adolescents and young adults with type 2 diabetes: results from the TODAY study. Diabetes Care 2021;45:1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. TODAY Study Group . Risk factors for diabetic peripheral neuropathy in adolescents and young adults with type 2 diabetes: results from the TODAY study. Diabetes Care 2021;45:1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saran R, Robinson B, Abbott KC, et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019;75(Suppl. 1):A6–A7 [DOI] [PubMed] [Google Scholar]
- 38. KDOQI . KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007;49:S12–S154 [DOI] [PubMed] [Google Scholar]
- 39. Dalla Vestra M, Saller A, Bortoloso E, Mauer M, Fioretto P. Structural involvement in type 1 and type 2 diabetic nephropathy. Diabetes Metab 2000;26(Suppl. 4):8–14 [PubMed] [Google Scholar]
- 40. Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes 2014;7:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 2006;29:1300–1306 [DOI] [PubMed] [Google Scholar]
- 43. Diabetes Prevention Program Research Group . Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lawrence JM, Reynolds K, Saydah SH, et al.; SEARCH for Diabetes in Youth Study Group . Demographic correlates of short-term mortality among youth and young adults with youth-onset diabetes diagnosed from 2002 to 2015: the SEARCH for Diabetes in Youth study. Diabetes Care 2021;44:2691–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep 2019;68:1–77 [PubMed] [Google Scholar]
- 46. GBD 2019 Diabetes Mortality Collaborators . Diabetes mortality and trends before 25 years of age: an analysis of the Global Burden of Disease Study 2019. Lancet Diabetes Endocrinol 2022;10:177–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Everhart JE, Pettitt DJ, Bennett PH, Knowler WC. Duration of obesity increases the incidence of NIDDM. Diabetes 1992;41:235–240 [DOI] [PubMed] [Google Scholar]
- 48. Rezai S, LoBue S, Henderson CE. Diabetes prevention: reproductive age women affected by insulin resistance. Womens Health (Lond) 2016;12:427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol 2014;24:793–800.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Francis EC, Dabelea D, Ringham BM, Sauder KA, Perng W. Maternal blood glucose level and offspring glucose-insulin homeostasis: what is the role of offspring adiposity? Diabetologia 2021;64:83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Francis EC, Dabelea D, Shankar K, Perng W. Maternal diet quality during pregnancy is associated with biomarkers of metabolic risk among male offspring. Diabetologia 2021;64:2478–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perng W, Oken E, Dabelea D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia 2019;62:1779–1788 [DOI] [PubMed] [Google Scholar]
- 53. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr 2015;104:30–37 [DOI] [PubMed] [Google Scholar]
- 54. Perng W, Cantoral A, Soria-Contreras DC, et al. Exposure to obesogenic endocrine disrupting chemicals and obesity among youth of Latino or Hispanic origin in the United States and Latin America: a lifecourse perspective. Obes Rev 2021;22(Suppl. 3):e13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parasin N, Amnuaylojaroen T, Saokaew S. Effect of air pollution on obesity in children: a systematic review and meta-analysis. Children (Basel) 2021;8:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Appelhans BM, Fitzpatrick SL, Li H, et al. The home environment and childhood obesity in low-income households: indirect effects via sleep duration and screen time. BMC Public Health 2014;14:1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care 2016;39:1635–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hill J, Nielsen M, Fox MH. Understanding the social factors that contribute to diabetes: a means to informing health care and social policies for the chronically ill. Perm J 2013;17:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ogden CL, Carroll MD, Fakhouri TH, et al. Prevalence of obesity among youths by household income and education level of head of household—United States 2011–2014. MMWR Morb Mortal Wkly Rep 2018;67:186–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Powell CA, Rifas-Shiman SL, Oken E, et al. Maternal experiences of racial discrimination and offspring sleep in the first 2 years of life: Project Viva cohort, Massachusetts, USA (1999–2002). Sleep Health 2020;6:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dougherty GB, Golden SH, Gross AL, Colantuoni E, Dean LT. Measuring structural racism and its association with BMI. Am J Prev Med 2020;59:530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lukachko A, Hatzenbuehler ML, Keyes KM. Structural racism and myocardial infarction in the United States. Soc Sci Med 2014;103:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chantarat T, Van Riper DC, Hardeman RR. The intricacy of structural racism measurement: a pilot development of a latent-class multidimensional measure. EClinicalMedicine 2021;40:101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sauder KA, Dabelea D, Bailey-Callahan R, et al. Targeting risk factors for type 2 diabetes in American Indian youth: the Tribal Turning Point pilot study. Pediatr Obes 2018;13:321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shaibi GQ, Konopken Y, Hoppin E, Keller CS, Ortega R, Castro FG. Effects of a culturally grounded community-based diabetes prevention program for obese Latino adolescents. Diabetes Educ 2012;38:504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Copeland KC, Zeitler P, Geffner M, et al.; TODAY Study Group . Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–369 [DOI] [PubMed] [Google Scholar]
- 68. Prasad RB, Asplund O, Shukla SR, et al. Subgroups of patients with young-onset type 2 diabetes in India reveal insulin deficiency as a major driver. Diabetologia 2022;65:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bennett PH, Rushforth NB, Miller M, LeCompte PM. Epidemiologic studies of diabetes in the Pima Indians. Recent Prog Horm Res 1976;32:333–376 [DOI] [PubMed] [Google Scholar]
- 70. Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med 2000;9:83–88 [DOI] [PubMed] [Google Scholar]
- 71. Dabelea D, Mayer-Davis EJ, Lamichhane AP, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 2008;31:1422–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–2211 [DOI] [PubMed] [Google Scholar]
- 73. RISE Consortium . Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zeitler P, Hirst K, Pyle L, et al.; Today Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. RISE Consortium . Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Roemmich JN, Clark PA, Lusk M, et al. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord 2002;26:701–709 [DOI] [PubMed] [Google Scholar]
- 77. Guzzaloni G, Grugni G, Mazzilli G, Moro D, Morabito F. Comparison between beta-cell function and insulin resistance indexes in prepubertal and pubertal obese children. Metabolism 2002;51:1011–1016 [DOI] [PubMed] [Google Scholar]
- 78. Bloch CA, Clemons P, Sperling MA. Puberty decreases insulin sensitivity. J Pediatr 1987;110:481–487 [DOI] [PubMed] [Google Scholar]
- 79. Moran A, Jacobs DR Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 1999;48:2039–2044 [DOI] [PubMed] [Google Scholar]
- 80. Caprio S, Plewe G, Diamond MP, et al. Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. J Pediatr 1989;114:963–967 [DOI] [PubMed] [Google Scholar]
- 81. Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 2006;60:759–763 [DOI] [PubMed] [Google Scholar]
- 82. Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes 2001;50:2444–2450 [DOI] [PubMed] [Google Scholar]
- 83. Ball GD, Huang TT, Gower BA, et al. Longitudinal changes in insulin sensitivity, insulin secretion, and beta-cell function during puberty. J Pediatr 2006;148:16–22 [DOI] [PubMed] [Google Scholar]
- 84. Hoffman RP, Vicini P, Sivitz WI, Cobelli C. Pubertal adolescent male-female differences in insulin sensitivity and glucose effectiveness determined by the one compartment minimal model. Pediatr Res 2000;48:384–388 [DOI] [PubMed] [Google Scholar]
- 85. Khawandanah J. Double or hybrid diabetes: a systematic review on disease prevalence, characteristics and risk factors. Nutr Diabetes 2019;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee JM. Why young adults hold the key to assessing the obesity epidemic in children. Arch Pediatr Adolesc Med 2008;162:682–687 [DOI] [PubMed] [Google Scholar]
- 87. RISE Consortium . Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bergman M, Buysschaert M, Schwarz PE, Albright A, Narayan KV, Yach D. Diabetes prevention: global health policy and perspectives from the ground. Diabetes Manag (Lond) 2012;2:309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]