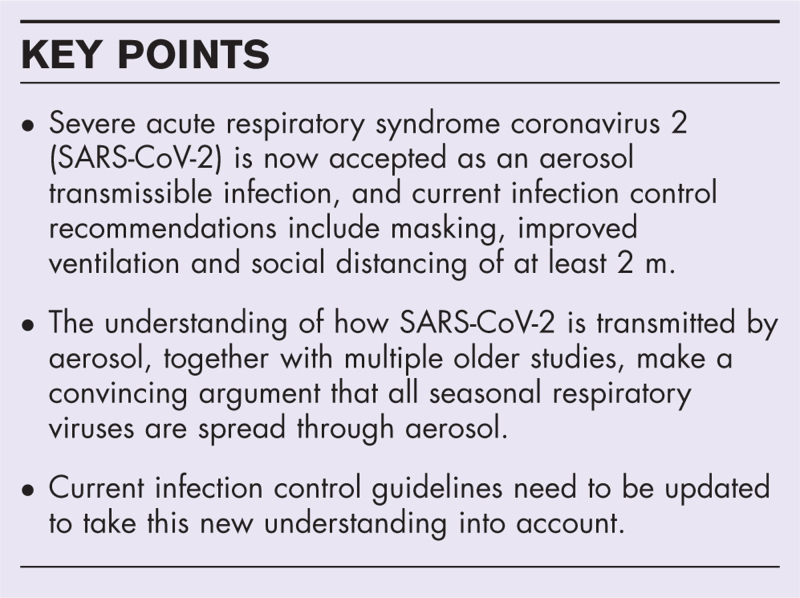
Purpose of review
The coronavirus disease 2019 pandemic has had a wide-ranging and profound impact on how we think about the transmission of respiratory viruses This review outlines the basis on which we should consider all respiratory viruses as aerosol-transmissible infections, in order to improve our control of these pathogens in both healthcare and community settings.
Recent findings
We present recent studies to support the aerosol transmission of severe acute respiratory syndrome coronavirus 2, and some older studies to demonstrate the aerosol transmissibility of other, more familiar seasonal respiratory viruses.
Summary
Current knowledge on how these respiratory viruses are transmitted, and the way we control their spread, is changing. We need to embrace these changes to improve the care of patients in hospitals and care homes including others who are vulnerable to severe disease in community settings.
Keywords: aerosol, coronavirus disease 2019, respiratory viruses, severe acute respiratory syndrome coronavirus 2, transmission
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has highlighted many flaws in the way we think about the transmission of respiratory viruses (e.g. Middle East respiratory syndrome-coronavirus – MERS-COV, influenza, respiratory syncytial virus – RSV, rhinoviruses and enteroviruses, and adenoviruses). It has forced us to re-examine the fundamental mechanisms underlying the transmission process, as well as the validity of the evidence that has been used to support these beliefs.
The pandemic has also revealed the difficulties in changing the mindset of some infection control specialists who have resisted modern techniques for interpreting such evidence and how this may be applied to improve not just the prevention and control of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but other respiratory viruses as well.
Here, we discuss the evidence for the aerosol transmission of respiratory viruses from different angles, with the aim of educating a new generation of infection control specialists to improve infection prevention and control and therefore reduce the seasonal healthcare burden of these pathogens.
Box 1.
no caption available
NATURE OF THE EVIDENCE
For each respiratory virus examined, studies on transmission fall into one of several over-lapping categories, such as: environmental (air-sampling studies with PCR detection, with or without viral culture); epidemiological (based on outbreak investigations); experimental infections; and interventional (where an intervention - deliberate or unintentional - suggests the most likely type of transmission) [1▪].
For the purposes of this article, the component of the traditional ‘droplet’ transmission, where exhaled particles containing virus land on the mucous membranes (e.g. eyes, nasal vaults and lips) of others, is not considered - as this applies only to droplets (typically >100 μm in diameter) that are too large to be inhaled [2▪]. The traditional cut-off of 5 μm between droplets and aerosols, in terms of how they move through the air, has been shown to be erroneous [3▪▪]. The only component of the traditional ‘droplet’ transmission’ that is considered is that which can be inhaled - when, by definition, the ‘droplet’ then behaves as an aerosol regardless of the distance it has travelled [4▪].
Note that in this context, droplets under the influence of gravity will not travel ‘upwards’ to the nose or eyes of others (unless they are lying horizontal) since they are then behaving like an aerosol. The fact that a respiratory virus can be transmitted as an aerosol clearly does not preclude more direct modes of transmission such as kissing.
Although viruses are often classified as transmitting mainly by one route or another, these routes are not mutually exclusive, particularly when people are in close proximity. It is possible for a virus to be transmitted by more than one route, through aerosols in one situation and fomites in another. Also of note, there is no robust evidence for traditional fomite (e.g. a virus-contaminated inanimate object) or droplet transmission between individuals. Such evidence would require confirmation by viral sequencing of the source and recipient, along with tracking of the transmission pathway by a video record to the exclusion of another potential third party sources of the infection (including exposure via aerosol). Not only is there no substantive evidence supporting transmission via the more traditional direct contact, fomite, or droplet routes but these routes are not mutually exclusive; for example, viruses that are resuspended from fomites can be inhaled as aerosols, as occurs in the transmission of hantaviruses from desiccated urine and faecal deposits on the ground during sweeping [5].
ENVIRONMENTAL EVIDENCE
There have been many air-sampling studies of various respiratory viruses, with viral RNA or DNA detected by PCR testing, sometimes further sequenced to confirm epidemiological linkage with the source and occasionally including culture to demonstrate virus viability.
MERS-CoV [6], SARS-CoV [7], SARS-CoV-2 [8,9▪▪,10▪▪], influenza virus [11,12], and RSV [13] have been detected in air-sampling studies from a variety of sources, including healthcare and community settings, and naturally-infected volunteers in laboratories.
Kim and colleagues collected air-samples from hospital areas during the 2015 MERS-CoV outbreaks in South Korea and detected MERS-CoV RNA by PCR. This study also managed to culture live virus from some of the air samples collected into virus culture media [6].
Air-samplers draw airborne viruses into collection media using high velocity airflows. It is now realized that the high shear stresses created during this process can damage the physical integrity of viruses such as coronavirus, influenza, and RSV, leaving them unable to bind to permissive host cells in viral culture [14]. So the apparent lack of live virus in many air-sampling studies does not necessarily indicate the correct proportion of virus viability in naturally exhaled or inhaled airborne viruses.
Recent advances in air sampling methods that better preserve the virus have led to new studies demonstrating the presence of infectious virus in air. For example, Lednicky et al.[8] found viable SARS-CoV-2 in air samples collected in a hospital several meters away from patients, and Vass et al.[10▪▪] detected viable SARS-CoV-2 in the air of a residence occupied by an infected person.
Milton and colleagues detected viable influenza virus [11,12] and SARS-CoV-2 [9▪▪] in exhaled air samples collected directly from naturally-infected human volunteers using their custom-designed and built Gesundheit II (GII) machine. The results for both viruses were further partitioned into ‘fine’ (<5 μm diameter) and ‘coarse’ (>5 μm diameter) particles. There was a greater concentration of virus in the fine particles collected over a 30-min sampling time, during which volunteers performed various breathing, talking, shouting and singing activities.
Kulkarni et al.[13] screened air samples in general and intensive care paediatric wards (using a modified Andersen slit sampler) for RSV by virus culture and quantified them using plaque-forming assays. They showed that in a busy paediatrics bronchiolitis ward, 1000–1 000 000 viable RSV were detected over 30 min.
Adenoviruses (Ads), especially Ad types 4 and 7 (Ad4, Ad7) have long been known to cause explosive outbreaks of respiratory illnesses, in crowded indoor areas such as military barracks [15,16], consistent with, and suggestive of, aerosol transmission. Adenoviruses have been recovered by isolation in cell culture from aerosol samples (diameters < 15 μm) in rooms with infected subjects [17] and by PCR from air filters of ventilation systems in military barracks [16].
Detractors of such evidence for the aerosol transmission of these viruses have argued that insufficient viable virus was detected, despite the fact that a tangible infectious dose-range for any individual remains unknown. One review suggests that 100–1000 virions of SARS-CoV-2 might be sufficient in an otherwise immunologically naïve individual [18▪], which is similar to other aerosol-transmitted viruses, such as influenza [19] and smallpox [20].
Furthermore, when considering this environmental ‘virus cloud inhalation’ mechanism, we cannot know exactly how much virus is inhaled by different individuals. For example, some might be exhaling as they walk through a viral cloud; others, at rest, would also inhale but not so deeply. This individual variability - including the threshold infectious dose - may explain why some people fail to become infected despite a clear opportunity for exposure.
EPIDEMIOLOGICAL EVIDENCE
Epidemiological evidence comes from detailed outbreak investigations, which are often retrospective because ongoing outbreaks may not attract attention until significant numbers of cases present themselves. So, there are weaknesses in this type of evidence due to recall bias and paucity of initial screening, etc. Another limitation is that such investigations often focus on ‘close contacts’ and so miss those who might have been infected by inhalation of the virus at a distance. Ideally, the availability of surveillance videos at the time of exposure would enable exploration of the relative contribution of close contact and distant exposure.
Nevertheless, with PCR detection and viral sequencing to confirm epidemiological linkage, this type of evidence has been used for decades to demonstrate the links and transmissibility of other viruses such as measles and chickenpox.
The main weakness of this approach for detecting respiratory viruses lies partially in the nonspecific clinical presentation of respiratory illness compared with the distinctive febrile rash presentation of measles and chickenpox. Also, respiratory virus infections can be asymptomatic or mildly symptomatic, such that some of those infected may not realize that they are sick, unless they are enrolled within a study examining a potential exposure-infection linkage.
In this context, several outbreaks of COVID-19 have been investigated, where secondary cases with confirmed epidemiological linkage indicated by viral sequencing were mostly likely to have occurred by aerosol transmission.
In Australia, at a time when very few community COVID-19 cases were present, a singing chorister on an elevated balcony infected multiple members of the congregation sitting below at horizontal distances of 3–15 m from the singer. No close, or direct contact, could have occurred [21▪▪].
In New Zealand, in a quarantine hotel manned by fully vaccinated staff, one infected traveller transmitted the virus to other travellers on the opposite of the corridor when both room doors were open as meals were passed in [22▪▪].
In both situations above, viral sequencing and phylogenetic analysis confirmed that the contacts were infected with a virus identical to that of the source (index case).
Detractors of this evidence have suggested that there may have been a common exposure (of the index case and contacts) to an unknown source. This seems unlikely, however, as the New Zealand travellers came from different flights, and the chorister and infected members of the congregation did not socialize together prior to the service.
Another outbreak in a Hong Kong gym similarly used traditional epidemiological data with viral sequencing to confirm transmission, though in such a close contact situation, other direct contact and fomite routes of transmission could not be excluded [23]. This example shows that even if other nonaerosol routes cannot be ruled out, neither can the aerosol route. This counterfactual reasoning is often omitted by traditionalist infection control arguments against aerosol transmission.
EVIDENCE FROM EXPERIMENTAL HUMAN VOLUNTEER INFECTIONS
Experimental infections in the laboratory permit clear separation of aerosols and large droplets, as preparations of homogeneous aerosols free of large droplets is readily achieved. For influenza A, studies by Alford involved exposing volunteers to carefully titrated, aerosolized influenza virus [24]. They established that the ID50 (i.e. the infectious dose required to infected 50% of the volunteers) by aerosols was about 0.6–3.0 TCID50 and that experimental infections could replicate the whole spectrum of disease. In contrast, inoculation by nasal instillation required a larger dose and was associated with milder illness [25]. In addition, several experiments using different animal models show that influenza A can be transmitted by aerosols [25,26].
Experimental infection in human volunteers with adenovirus 4 (Ad4) suggests that intranasal inoculation seldom results in illness [27]. In addition, it was found that intestinal inoculation with enteric-coated capsules resulted in asymptomatic infection [15]. In contrast, aerosol inoculation of human volunteers with Ad4 resulted in successful infections in 16 of 21 subjects, 15 of whom became severely ill with fever, cough, prostration, and in one case, a left lower lobe pneumonia [27]. Significantly, the ID50 required by aerosol was about 70 times less than the ID50 required by the intranasal route.
Coxsackie A21, an enterovirus well known to cause respiratory infections and easily recoverable in cell culture, has been studied in similar ways. Experimental infections on human volunteers with Coxsackie A21 can be achieved by both intranasal and aerosol inoculations. However, illness resulting from intranasal inoculation was invariably restricted to the upper respiratory tract whereas aerosol inoculation also caused disease involving the lower respiratory tract [28]. Interestingly, and in contrast to Ad4, the ID50 by either route was approximately the same (although the authors pointed out that the aerosol inoculum was only a fraction of the administered dose that would be inhaled and retained, whereas most of the aerosol dose would be exhaled and therefore discharged) [28]. It is worth noting that for rhinovirus A15 the ID50 by the intranasal route is in fact considerably lower (approximately 20-fold) than by the aerosol route [29].
INTERVENTIONAL EVIDENCE
Interventional evidence explores whether an intervention impedes transmission if this occurs only by the traditional direct contact, fomite or ‘droplet landing onto mucous membranes’ routes (inhaled droplets are classified as ‘aerosol transmission’). Such studies may be deliberate or unintentional.
In 1957 during the A(H2N2) pandemic, patients in the tuberculosis (TB) wing of a veteran's hospital were protected from contracting TB by a ceiling ultraviolet germicidal irradiation (UVGI) system. Patients in this wing were also found to be protected from influenza infection - unlike patients in other neighbouring wings where ceiling UVGI was absent [30,31]. The ceiling UVGI could only be effective if, like the TB bacillus, the virus was airborne and was inactivated following UV exposure. Seroconversion in some of the attending nursing staff and in a small number of patients established that the virus was introduced but did not spread.
As a counterpart to this experiment, zanamivir administered by intranasal drops is highly protective against experimental infection by nasal instillation [32]. This replicates infection by large droplets and/or contact with mucosa. However, intranasal zanamivir is not protective in natural infection settings [33], which imply that aerosol infections must have occurred. Similarly, Cowling et al.[34], when re-analysing the data from randomized controlled trial of hand hygiene and surgical face masks to prevent household transmission of influenza, found that these measures did not prevent all cases and that approximately half of all transmissions must have occurred through aerosol transmission.
Another older study performed at the University of Wisconsin demonstrated that rhinovirus had to be airborne to cause infection. Volunteers sat around a card table playing with rhinovirus contaminated cards, with some wearing contraptions designed to prevent them from touching their face with their hands. After a 12-h exposure period, a high proportion (10/18, 56%) of the players became infected, despite being unable to touch facial sites. This was not statistically different from players in the control group who were unrestrained, and who could have become infected via aerosol, direct contact or indirect fomite routes (12/18, 67%). This indicated that the virus must have been able to transmit successfully via aerosol to cause infection [35].
In another study, 10 experimentally infected subjects were housed in one half of a barrack separated by a 54 inches double wired barrier from the other half harbouring uninfected volunteers [36]. All of the 19 volunteers across the barrier were infected over the course of the 26-day study, with 5 and 10 volunteers, respectively, infected on days 6 and 12, within a few days of detection of Coxsackie A21 in collected air samples. This gave an estimated mean incubation period of 2.5 days.
A more recent study using the GII machine compared the ability of a surgical mask to contain influenza, coronavirus and rhinovirus in naturally-infected volunteers. If the traditional transmission routes for rhinovirus were correct (via direct contact, fomites or droplets landing on mucous membranes) then surgical masks should effectively contain any leakage of these respiratory viruses for both fine and coarse particle populations. It was found that the surgical mask contained both fine and coarse particles containing coronavirus; partially contained influenza was mostly at the coarse particle size level, but the mask failed to prevent rhinovirus escaping in either particle size, indicating that rhinovirus was airborne [37]. This confirmed earlier results from the University of Wisconsin [35].
Various national and international surveillance studies have shown that rhinoviruses have persisted throughout the pandemic, despite the various pandemic restrictions, for example, lockdowns and nonpharmaceutical interventions such as the wearing of face masks, social distancing, curfews, etc. Based on the epidemiological and interventional evidence, it seems that rhinoviruses are even more likely to be airborne than SARS-CoV-2 [38▪], as could be inferred from the earlier study by Leung et al.[37].
An earlier study using the GII device also showed that whilst a surgical mask could reduce the leakage of influenza from an infected person, it did not stop the leakage of influenza completely [11], which again confirms the findings of Leung et al.[37].
Detractors of this type of evidence argue that the airborne viral infections/detections could have originated from other sources from elsewhere via the more traditional ‘fomite’ and ‘droplet’ transmission routes, but again, these do not exclude the possibility of aerosol transmission, and furthermore there is no substantial evidence to support these more traditional transmission routes.
CONCLUSION
Whilst seasonal respiratory viruses cause only mild clinical illness for most people, the recognition that transmission can occur via the aerosol route allows updated guidance to take this into account. Such guidance should recommend face masks and social distancing, in the knowledge that these measures can reduce the aerosol transmission risk component of these viruses, especially for those individuals who are more vulnerable to severe disease. This paradigm shift in thinking about how such respiratory viruses can transmit via aerosols was recently reiterated in a Lancet commissioned report on COVID-19 and other respiratory viruses [39▪▪].
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Tang JW, Tellier R, Li Y. Hypothesis: all respiratory viruses (including SARS-CoV-2) are aerosol-transmitted. Indoor Air 2022; 32:e12937. [DOI] [PubMed] [Google Scholar]; One of several articles that essentially make the case for aerosol transmission as the most common way that respiratory viruses transmit.
- 2▪.Marr LC, Tang JW. A paradigm shift to align transmission routes with mechanisms. Clin Infect Dis 2021; 73:1747–1749. [DOI] [PubMed] [Google Scholar]; One of several articles that essentially make the case for aerosol transmission as the most common way that respiratory viruses transmit.
- 3▪▪.Randall K, Ewing ET, Marr LC, et al. How did we get here: what are droplets and aerosols and how far do they go? A historical perspective on the transmission of respiratory infectious diseases. J R Soc Interface 2021; 11:20210049. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent overview of how the history of the misunderstandings around aerosol transmission led to the modern day resistance in accepting this mode of transmission for respiratory viruses.
- 4▪.Tang JW, Marr LC, Milton DK. Aerosols should not be defined by distance travelled. J Hosp Infect 2021; 115:131–132. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of several articles that essentially make the case for aerosol transmission as the most common way that respiratory viruses transmit.
- 5. US Centers for Disease Control and Prevention. Hantavirus Pulmonary Syndrome. Transmission. 29 August 2012. Available at: https://www.cdc.gov/hantavirus/hps/transmission.html [Accessed 13 September 2022]. [Google Scholar]
- 6.Kim SH, Chang SY, Sung M, et al. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis 2016; 63:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth TF, Kournikakis B, Bastien N, et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis 2005; 191:1472–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lednicky JA, Lauzard M, Fan ZH, et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis 2020; 100:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪▪.Adenaiye OO, Lai J, Bueno de Mesquita PJ, et al. Infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in exhaled aerosols and efficacy of masks during early mild infection. Clin Infect Dis 2022; 75:e241–e248. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first studies to demonstrate the viability of exhaled SARS-CoV-2 from naturally-infected human volunteers.
- 10▪▪.Vass WB, Lednicky JA, Shankar SN, et al. Viable SARS-CoV-2 Delta variant detected in aerosols in a residential setting with a self-isolating college student with COVID-19. J Aerosol Sci 2022; 165:106038. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of several community-based studies demonstrating the aerosol transmission of SARS-CoV-2.
- 11.Milton DK, Fabian MP, Cowling BJ, et al. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog 2013; 9:e1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan J, Grantham M, Pantelic J, et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci USA 2018; 115:1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkarni H, Smith CM, Lee Ddo H, et al. Evidence of respiratory syncytial virus spread by aerosol. Time to revisit infection control strategies? Am J Respir Crit Care Med 2016; 194:308–316. [DOI] [PubMed] [Google Scholar]
- 14.Verreault D, Moineau S, Duchaine C. Methods for sampling of airborne viruses. Microbiol Mol Biol Rev 2008; 72:413–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaydos CA, Gaydos JC. Adenovirus vaccines in the U.S. military. Mil Med 1995; 160:300–304. [PubMed] [Google Scholar]
- 16.Echavarria M, Kolavic SA, Cersovsky S, et al. Detection of adenoviruses (AdV) in culture-negative environmental samples by PCR during an AdV-associated respiratory disease outbreak. J Clin Microbiol 2000; 38:2982–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artenstein MS, Miller WS, Lamson TH, Brandt BL. Large-volume air sampling for meningococci and adenoviruses. Am J Epidemiol 1968; 87:567–577. [DOI] [PubMed] [Google Scholar]
- 18▪.Karimzadeh S, Bhopal R, Nguyen Tien H. Review of infective dose, routes of transmission and outcome of COVID-19 caused by the SARS-COV-2: comparison with other respiratory viruses. Epidemiol Infect 2021; 149:e96. [DOI] [PMC free article] [PubMed] [Google Scholar]; Interesting paper trying to estimate and compare the infective dose of SARS-CoV-2 with other respiratory viruses
- 19.Nikitin N, Petrova E, Trifonova E, Karpova O. Influenza virus aerosols in the air and their infectiousness. Adv Virol 2014; 2014:859090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franz DR, Jahrling PB, Friedlander AM, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA 1997; 278:399–411. [DOI] [PubMed] [Google Scholar]
- 21▪▪.Katelaris AL, Wells J, Clark P, et al. Epidemiologic evidence for airborne transmission of SARS-CoV-2 during church singing, Australia, 2020. Emerg Infect Dis 2021; 27:1677–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of several community-based studies demonstrating the aerosol transmission of SARS-CoV-2.
- 22▪▪.Fox-Lewis A, Williamson F, Harrower J, et al. Airborne transmission of SARS-CoV-2 delta variant within tightly monitored isolation facility, New Zealand (Aotearoa). Emerg Infect Dis 2022; 28:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of several community-based studies demonstrating the aerosol transmission of SARS-CoV-2.
- 23.Chu DKW, Gu H, Chang LDJ, et al. SARS-CoV-2 superspread in fitness center, Hong Kong, China, March 2021. Emerg Infect Dis 2021; 27:2230–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alford RH, Kasel JA, Gerone PJ, Knight V. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med 1966; 122:800–804. [DOI] [PubMed] [Google Scholar]
- 25.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis 2006; 12:1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface 2009; 6:S783–S790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couch RB, Knight V, Douglas RG, Jr, et al. The minimal infectious dose of adenovirus type 4; the case for natural transmission by viral aerosol. Trans Am Clin Climatol Assoc 1969; 80:205–211. [PMC free article] [PubMed] [Google Scholar]
- 28.Couch RB, Cate TR, Douglas RG, Jr, et al. Effect of route of inoculation on experimental respiratory viral disease in volunteers and evidence for airborne transmission. Bacteriol Rev 1966; 30:517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight V, Couch RB, Landahl HD. Effect of lack of gravity on airborne infection during space flight. JAMA 1970; 214:513–518. [PubMed] [Google Scholar]
- 30.McLean RL. The mechanism of spread of Asian influenza: general discussion. Am Rev Respir Dis 1961; 83:36–38. [DOI] [PubMed] [Google Scholar]
- 31.Reed NG. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep 2010; 125:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calfee DP, Peng AW, Hussey EK, et al. Safety and efficacy of once daily intranasal zanamivir in preventing experimental human influenza A infection. Antivir Ther 1999; 4:143–149. [PubMed] [Google Scholar]
- 33.Kaiser L, Henry D, Flack NP, et al. Short-term treatment with zanamivir to prevent influenza: results of a placebo-controlled study. Clin Infect Dis 2000; 30:587–589. [DOI] [PubMed] [Google Scholar]
- 34.Cowling BJ, Ip DK, Fang VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Nat Commun 2013; 4:1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dick EC, Jennings LC, Mink KA, et al. Aerosol transmission of rhinovirus colds. J Infect Dis 1987; 156:442–448. [DOI] [PubMed] [Google Scholar]
- 36.Couch RB, Douglas RG, Jr, Lindgren KM, et al. Airborne transmission of respiratory infection with coxsackievirus A type 21. Am J Epidemiol 1970; 91:78–86. [DOI] [PubMed] [Google Scholar]
- 37.Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020; 26:676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪.Tang JW, Bialasiewicz S, Dwyer DE, et al. Where have all the viruses gone? Disappearance of seasonal respiratory viruses during the COVID-19 pandemic. J Med Virol 2021; 93:4099–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Not all respiratory viruses were controlled by the COVID-19 pandemic restrictions. Here we look at why rhinoviruses may be potentially even more aerosol-transmissible than either SARS-CoV-2 or influenza.
- 39▪▪.Sachs JD, Karim SSA, Aknin L, et al. The Lancet Commission on lessons for the future from the COVID-19 pandemic. Lancet 2022; 400:1224–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wide-ranging and critical overview of what went wrong and what could have been done better during the COVID-19 pandemic. The delayed recognition of SARS-CoV-2 aerosol transmission and the delayed instigation of appropriate infection control policies likely led to many unnecessary infections and deaths.