Background
Intra-articular injections are widely used for conservative treatment of knee osteoarthritis (OA). However, rigorous data are lacking regarding the comparative therapeutic effectiveness of these injections.
Purpose
The aim of this study was to compare clinical outcomes after intra-articular injections of glucocorticoid, hyaluronic acid, platelet-rich plasma (PRP), or placebo in patients with mild or moderate OA of the knee.
Materials and Methods
In a double-blinded, placebo-controlled, single-center trial, we randomly assigned knees with early- to middle-stage knee OA (Kellgren-Lawrence grade 1–3) to an intra-articular injection with one of these substances: glucocorticoid, hyaluronic acid, PRP, or placebo. Primary outcome was pain reduction within 6 months after the injection, assessed with the numeric rating scale (NRS; range, 0–100). Secondary outcome parameters included WOMAC scores, Tegner Activity Scale, knee mobility, and adverse events. Finally, a linear mixed-effects model was calculated and corrected for possible patient and covariate effects.
Results
One hundred twenty knees (30 knees per treatment group) in 95 patients (41 female) were included in the final analysis. The median age of patients was 60 years (interquartile range, 54.0–68.0). There was no evidence that the drug effects of primary and secondary outcome parameters differed over time. The median pain at baseline was 32.5 (interquartile range, 15.00–50.00) on NRS. The changes in pain level during the first 6 months compared with baseline were small (within ±5 points on NRS), whereas the intrapatient variability was large between −20 and +20 points. Secondary outcome parameters did not differ significantly among the groups. Kellgren-Lawrence grade did not have a statistically significant effect on pain reduction (P = 0.61).
Conclusions
There is no evidence that knee injections with glucocorticoid, PRP, or hyaluronic acid have superior short- or long-term effects in patients with low pain level at baseline and early- to middle-stage knee OA when compared with placebo.
Key Words: knee, osteoarthritis, intra-articular, injection, glucocorticoid, steroid, hyaluronic acid, PRP, platelet-rich plasma, placebo
Osteoarthritis (OA) of the knee is a chronic and progressive disease, which can severely affect patient mobility and quality of life.1–3 The causes remain incompletely understood but are primarily attributed to aging, anatomical factors, obesity, biomechanical factors, and previous trauma.4–7 Nonsurgical treatment of OA is useful for patients with early stages of OA (Kellgren-Lawrence [KL] grade 1–3), whereas in an advanced stage of OA (KL grade 4), surgical treatment is often needed as definitive treatment.8 Current nonsurgical treatments focus on symptomatic pain relieve and include physical therapy, oral anti-inflammatory medication, and intra-articular injections9,10 with glucocorticoids, hyaluronic acid, or platelet-rich plasma (PRP).9
Intra-articular injections with glucocorticoids have been used for over 50 years and have shown to achieve pain relief and functional improvement in patients with OA for at least 26 weeks.2,11 The use of hyaluronic acid injections tends to return less favorable clinical results than glucocorticoids,12 but also reduce pain in mild OA up to 24 weeks.13 Recent studies on PRP injections indicate promising results for relieving pain as an alternative to hyaluronic acid for patients with mild knee OA.1 However, it remains unclear which drug should be used or when other treatments should be preferred. Furthermore, most published studies were either not double-blinded, had no control group, or were performed on severe cases with high pain levels at baseline.
In this randomized controlled trial, we compared clinical outcomes after intra-articular injections of glucocorticoids, hyaluronic acid, PRP, or placebo in patients with mild or moderate OA of the knee.
METHODS
Study Design and Oversight
This was a prospective, single-center, placebo-controlled, multiarm parallel trial with a 1:1:1:1 randomization conducted in Switzerland. The study's aim was to compare the effects of intra-articular knee injections with glucocorticoid, hyaluronic acid, or PRP, with placebo. Ethics approval for the trial was granted by the Cantonal Ethics Commission Zurich, Switzerland (KEK-Nr. 2015-0376) and Swissmedic (Swiss Agency for Therapeutic Products; W-560-UKB), Switzerland. The study was carried out in accordance with the Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use E6 guideline, and EN ISO 14155, as well as national obligations. The trial was registered with the Swiss National Clinical Trials Portal and ClinicalTrials.gov (trial number: NCT02776514). All patients provided written informed consent and were informed that they may be given a placebo. Information specifically included potential effects of not receiving medication and that patient's condition may worsen while treated with the placebo injection. The authors vouch for the accuracy and completeness of the data and for the confidentiality of the trial to the protocol. The trial was initiated by the investigators and was funded by 2 private, noncommercial foundations on an equal share: The “Foundation for Research in Rheumatology” (German title: “Stiftung für Rheumaforschung”), Zurich, Switzerland and the “Marie-Lou Ringgenberg Foundation,” Bern, Switzerland. Funders did not participate in the conduct of the study, data collection, data analysis, or the writing of the manuscript.
Enrollment
Patients were enrolled at a tertiary referral center (Balgrist University Hospital in Zurich, Switzerland) between February 2016 and November 2018. We included patients with mild to moderate knee OA of grades 1, 2, and 3 according to the KL classification14 along with pain or swelling for at least 3 months. All patients had a clinical indication for a knee injection due to symptomatic knee pain. The detailed inclusion and exclusion criteria can be found in Table 1.
TABLE 1.
Summary of Inclusion and Exclusion Criteria
| Inclusion criteria |
| • Radiographically confirmed knee osteoarthritis (Kellgren-Lawrence grades 1–3) |
| • Knee pain and/or knee swelling for at least 3 mo |
| • German speaking |
| • Informed consent to study trial |
| Exclusion criteria |
| • Younger than 18 y |
| • End-stage knee osteroarthritis (Kellgren-Lawrence grade 4) |
| • Anticoagulant therapy and/or coagulation disorders |
| • Inflammatory disease: rheumatoid arthritis, spondyloarthritis, collagenosis, crystal deposit disease |
| • Severe heart disease (NYHA Grade IV) |
| • Infection |
| • Immunosuppressive therapy of immunocompromising disease |
| • Contraindication for magnetic resonance imaging |
| • Pregnancy |
| • Nonsteroidal anti-inflammatory drugs during the last 3 d before injection |
| • Injections in the same joint during the last 6 mo |
| • Allergic reactions to the applied medications (hyaluronic acid, iodinated contrast media, steroids) |
| • Symptomatic chronic fibromyalgia |
Randomization and Blinding
Standard radiographs were used to confirm the OA and KL grade14 and were each rated by an experienced rheumatologist or fellowship-trained musculoskeletal radiologist (MT or ABR). We obtained demographic information and baseline characteristics. Participants received financial compensation for their travel expenses.
All patients were clinically examined (routine knee examination, for details see Table 2) by a rheumatologist (MT or FB) or an orthopedic surgeon (SFF) before the start of the trial and at a 3-month follow-up appointment.
TABLE 2.
Baseline Characteristics
| Variable | Overall | Missing | Placebo | Glucocorticoid | Hyaluronic Acid | PRP | P† |
|---|---|---|---|---|---|---|---|
| n | 120 | 30 | 30 | 30 | 30 | ||
| Age (median [IQR]) | 60.00 [54.00, 68.00] | 0.0 | 58.00 [54.00, 61.00] | 59.00 [49.00, 65.00] | 64.00 [54.75, 72.00] | 62.00 [56.00, 68.00] | 0.27 |
| Gender = female (%) | 43.2 | 0.0 | 40.0 | 52.0 | 37.5 | 42.9 | 0.77 |
| BMI (median [IQR]) | 25.70 [23.10, 29.80] | 2.1 | 25.10 [23.00, 28.75] | 27.00 [24.35, 30.75] | 25.50 [22.65, 30.25] | 26.00 [21.30, 28.70] | 0.64 |
| Oral analgesic medication = yes (%) | 29 (24.6) | 1.7 | 6 (20.0) | 9 (31.0) | 7 (23.3) | 7 (24.1) | 0.82 |
| Other medication = yes (%) | 72 (61.0) | 1.7 | 15 (50.0) | 15 (51.7) | 20 (66.7) | 22 (75.9) | 0.13 |
| Mechanical leg axis (%) | 3.3 | 0.94 | |||||
| Valgus | 25 (21.6) | 6 (21.4) | 6 (20.7) | 8 (26.7) | 5 (17.2) | ||
| Varus | 50 (43.1) | 14 (50.0) | 12 (41.4) | 12 (40.0) | 12 (41.4) | ||
| Neutral | 41 (35.3) | 8 (28.6) | 11 (37.9) | 10 (33.3) | 12 (41.4) | ||
| Kellgren-Lawrence grade (%) | 0.0 | 0.36 | |||||
| 1 | 30 (31.6) | 8 (32.0) | 4 (16.0) | 9 (37.5) | 9 (42.9) | ||
| 2 | 23 (24.2) | 8 (32.0) | 6 (24.0) | 4 (16.7) | 5 (23.8) | ||
| 3 | 42 (44.2) | 9 (36.0) | 15 (60.0) | 11 (45.8) | 7 (33.3) | ||
| Side = right (%) | 65 (55.1) | 1.7 | 19 (65.5) | 17 (56.7) | 19 (63.3) | 10 (34.5) | 0.07 |
| Joint effusion (%) | 0.0 | 0.41 | |||||
| No | 93 (77.5) | 23 (76.7) | 21 (70.0) | 24 (80.0) | 25 (83.3) | ||
| + | 22 (18.3) | 7 (23.3) | 6 (20.0) | 4 (13.3) | 5 (16.7) | ||
| ++ | 5 (4.2) | 0 (0.0) | 3 (10.0) | 2 (6.7) | 0 (0.0) | ||
| +++ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Active_rom_extension (median [IQR]) | 0.00 [−5.00, 0.00] | 0.0 | 0.00 [−5.00, 0.00] | 0.00 [−3.75, 0.00] | 0.00 [0.00, 0.00] | 0.00 [−5.00, 0.00] | 0.93 |
| Active_rom_flexion (median [IQR]) | 130.00 [120.00, 135.00] | 0.0 | 130.00 [125.00, 140.00] | 130.00 [120.00, 133.75] | 130.00 [120.00, 135.00] | 130.00 [121.25, 140.00] | 0.44 |
| Passive_rom_flexion (median [IQR]) | 135.00 [130.00, 140.00] | 0.0 | 135.00 [130.00, 140.00] | 130.00 [130.00, 135.00] | 135.00 [126.25, 140.00] | 135.00 [130.00, 140.00] | 0.44 |
| Passive_rom_extension (median [IQR]) | 0.00 [−5.00, 0.00] | 0.0 | 0.00 [−5.00, 0.00] | 0.00 [−3.75, 0.00] | 0.00 [0.00, 3.75] | 0.00 [−5.00, 3.75] | 0.65 |
| Thigh circumference (cm) left (median [IQR]) | 47.00 [44.00, 52.00] | 0.0 | 48.25 [45.00, 51.00] | 49.25 [44.25, 53.38] | 46.00 [44.62, 49.00] | 46.25 [43.00, 51.00] | 0.47 |
| Thigh circumference (cm) right (median [IQR]) | 47.00 [44.00, 52.00] | 0.0 | 48.00 [44.25, 51.38] | 49.50 [44.25, 53.88] | 46.50 [44.12, 50.00] | 47.00 [43.62, 51.88] | 0.55 |
| Pain NRS* (median [IQR]) | 32.50 [15.00, 50.00] | 0.0 | 32.00 [20.00, 50.00] | 39.50 [17.75, 47.75] | 42.50 [21.50, 50.75] | 20.00 [10.00, 38.50] | 0.10 |
| WOMAC pain: total score* (median [IQR]) | 2.00 [1.00, 3.50] | 0.0 | 2.00 [1.50, 3.50] | 2.25 [1.00, 3.50] | 2.25 [1.50, 3.75] | 2.00 [0.50, 3.50] | 0.57 |
| WOMAC stiffness: total score* (median [IQR]) | 2.60 [1.30, 5.00] | 0.8 | 2.60 [1.30, 3.80] | 3.20 [1.30, 5.00] | 2.60 [1.30, 6.30] | 2.60 [1.60, 4.70] | 0.91 |
| WOMAC physical function: total score* (median [IQR]) | 1.70 [0.75, 3.00] | 0.8 | 1.40 [0.70, 2.30] | 2.05 [0.90, 3.72] | 1.85 [0.80, 3.45] | 1.40 [0.72, 2.48] | 0.53 |
| Tegner Activity Scale (median [IQR]) | 4.00 [3.00, 4.00] | 5.8 | 4.00 [3.00, 4.00] | 4.00 [3.00, 4.50] | 3.00 [3.00, 4.00] | 4.00 [3.00, 4.75] | 0.42 |
*NRS scores for knee pain range from 0 to 100, with 0 indicating no pain and 100 indicating “pain as bad as you can imagine.”
†P values are based on Fisher exact test and Kruskal-Wallis rank sum test; for detailed WOMAC score results, see Supplementary Table S1, http://links.lww.com/RLI/A779.
IQR, interquartile range; BMI, body mass index: weight in kilograms divided by the square of the height in meters; NRS, numerical rating scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis; ROM, range of motion.
In addition, we collected 15 mL of venous blood in all patients for PRP preparation; blood samples were discarded for those not assigned to the PRP group.
Patients were randomly assigned to receive 1 of the 3 drugs or placebo (for details, see Supplemental Material, http://links.lww.com/RLI/A779). Randomization was performed using a permuted block randomization with a block size of 60 patients. Every randomization block consisted of 60 opaque envelops, each containing a card with a drug name (15 with “PRP,” 15 with “glucocorticoids,” 15 with “hyaluronic acid,” and 15 with “placebo”) on it. The envelopes were stored in a locked safe. Before each injection, one of the envelops was drawn out of the safe by the injecting radiologist determining patient's drug group. After 60 patients, the next block randomization was performed in the same way.
Patients with both knees included were randomized for each knee independently with a minimum of 3 months between the injections. Except for the radiologists performing the injection, all patients and investigators were blinded. A blanket was placed between the injecting radiologist and the patient's head in order to avoid that the patients can recognize the color of the injected fluid. Patients were enrolled by one of the study nurses, who checked all inclusion and exclusion criteria with the patient. In cases of unclear criteria, the principal investigator (MT) or sponsor-investigator (ABR) was consulted.
For the PRP injection, the “ACP-System” (Arthrex, Munich, Germany) was used; patients' blood was centrifuged at 1500 rpm for 5 minutes (Angel Centrifuge; Arthrex, Naples, FL), which separated the platelets from the red blood cell, resulting in leukocyte-poor plasma with a platelet concentration approximately 500 × 103 μL.15
Intervention
On day 1 of the trial, all patients received a single-dose fluoroscopy-guided intraarticular injection under sterile conditions from 1 of 3 investigators (ABR, CWAP, and RS): 1 mL of contrast agent (Iopamidol, “Iopamiro”; Bracco Imaging SPA, Milano, Italy) followed by 1 mL of glucocorticoid (triamcinolon, “Triamcort Depot”; Zentiva, Prague, Czech Republic), or 6 mL of hyaluronic acid (sodium hyaluronate solution, “Suplasyn 1-shot”; Viatris, Canonsburg, PA), or 3 mL of the patient's own PRP. The placebo group received 1 mL of contrast agent only.
Outcomes
We sent questionnaires immediately after the injection, as well as at 1 week and every 3 months up to 24 months postintervention. Primary outcomes for pain assessment within 6 months included the numeric rating scale (NRS; range, 0–100). Secondary outcomes included the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) component for pain (range, 0–20), WOMAC components for stiffness (range, 0–8) and physical function (range, 0–68), and the Tegner Activity Scale (range, 0–10). Furthermore, a clinical examination at baseline and after 3 months (knee mobility, thigh circumference) was done. All adverse events were recorded.
Study Dropout
Patients were replaced in the study if dropout occurred within the first 3 months. Dropout reasons were noncompliance to the study protocol (missed appointments, etc), withdrawal of the informed consent, and necessary intervention (additional injection in the same knee or knee surgery) due to increasing knee pain.
The detailed study flowchart is shown in Figure 1.
FIGURE 1.
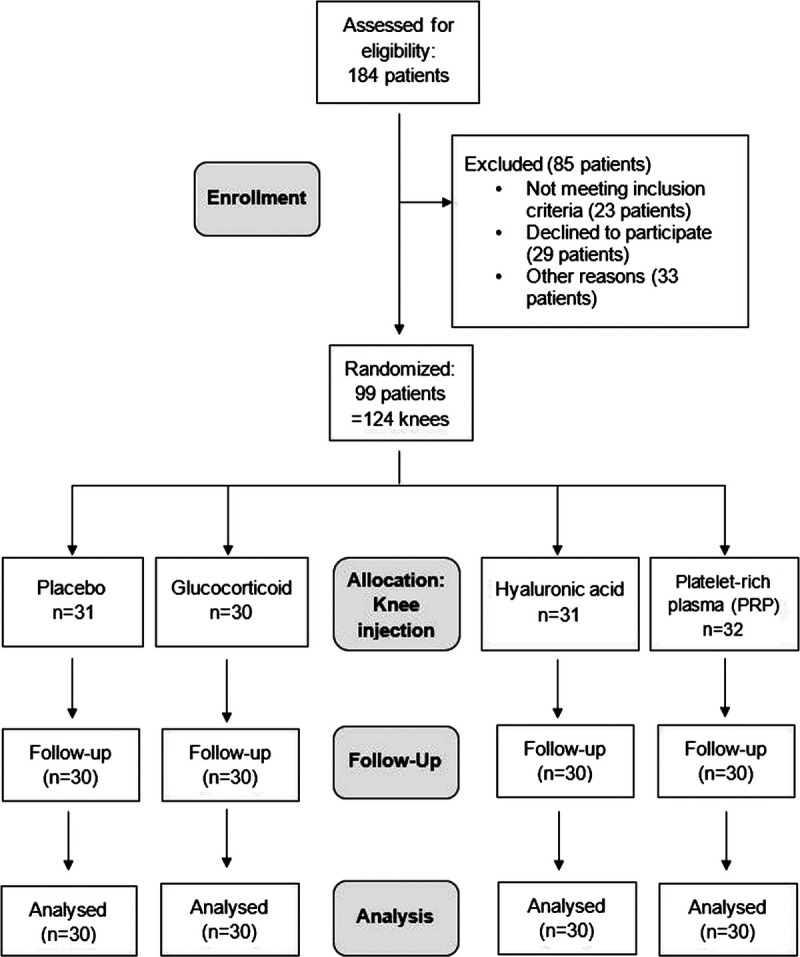
Trial enrollment, randomization, and follow-up numbers. Note: n = number of knees; 4 knees of 4 different patients had to be replaced due to dropout within the first 3 months after injection. Follow-up numbers are not shown for all follow-up time points in order to avoid an unwieldy figure. Most common reasons for failed inclusion criteria were Kellgren-Lawrence grade IV, anticoagulant therapy, and immunosuppressive therapy.
Statistical Analysis
The statistical analysis was done by 3 professional statisticians (MT, TG, and NG) and performed with the R statistical package version 4.1.0 (The R Foundation for Statistical Computing, Vienna, Austria).16 Study data were stored and managed with REDCap versions 6.12.1 to 6.14.1 (REDCap, Vanderbilt University, Nashville, TN).
For the descriptive analysis, mean and standard deviation or median and interquartile range (IQR), respectively, were used for continuous variables, and number and percentage were used for categorical variables. To assess differences in baseline data, Fisher exact test and Kruskal-Wallis rank sum test were used. A P value of <0.05 was considered statistically significant.
For sample size estimation, we assumed that 6 months after the injection the placebo group would show no improvement, an improvement of 20 pain NRS units for glucocorticoid, an improvement of 40 units for PRP, and an improvement of 50 units for the hyaluronic acid. The within-group standard deviation was assumed to be 30. These assumptions were based on a recently published meta-analysis.2 We also accounted for a dropout rate of 5%. Missing values were not imputed.
The improvement of pain NRS with respect to baseline was the primary end point.
Pain NRS was measured at baseline and at 9 successive time points (Fig. 2A). Positive values of this response are thus associated with more pain compared with baseline. On the contrary, negative values are associated with less pain than at baseline and therefore are an improvement for the patient. To account for the structure of the data, a linear mixed-effects model (LMM) was used. In particular, “knee” and “patient” were taken as random effects. Knee was included as a random effect as they have been measured repeatedly over time. The patient random effect was necessary to account for the nonindependence of the observations of those patients that had 2 treated knees. Treatment and time (a categorical variable) and their interaction were taken as fixed effects. In addition, the model also contained the following control variables: sex, age, body mass index, KL grade, pain (NRS) at baseline (as a quadratic effect), knee side (left or right), amount of oral analgesic medication with respect to baseline, and another drug used. The LMM was implemented with the lme4 package version 1.1-26.17 The statistical inference on the predictors contained in the LMM was performed via likelihood ratio test, but also via semiparametric bootstrap as the residuals happened to slightly, but clearly deviate from the normal distribution.
FIGURE 2.
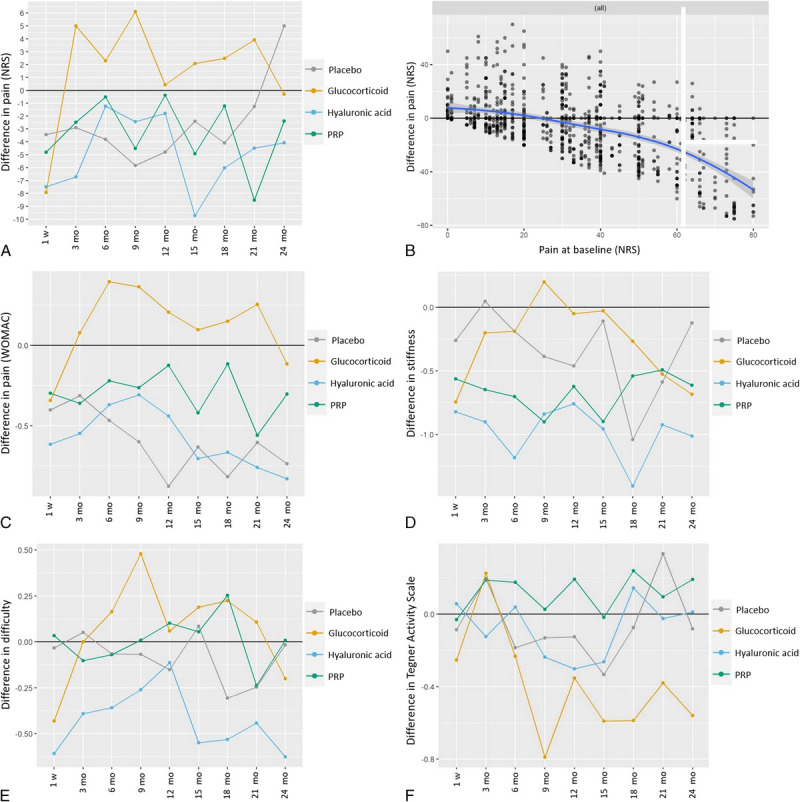
Primary outcome (A and B) and secondary outcomes (C–F). Panel A shows the difference in pain on NRS compared with baseline based on the linear mixed-effects model (LMM). Negative difference indicates pain reduction. Predicted values are created by setting all predictors other than drug and time point to a given value. The graph shows the expected effects for “an average patient” when we control for all other predictors in the model. Note: Confidence intervals for the predicted values are not reported as currently there is no satisfactory way to compute them for LMMs.17 Nevertheless, Supplementary Table S2, http://links.lww.com/RLI/A779, does report the estimated post hoc contrasts along with standard errors and P values. Panel B demonstrates the difference in pain versus pain at baseline in all knees. The blue line represents the “smoother line.” The effect of baseline value is very clear and strong. Those having low baseline values (eg, 0) won't show any improvement (see left-hand side of this graph). Note that the effect here does not seem to be linear. Therefore, this effect was implemented into the prediction model as a quadratic effect. Panel C shows the difference in pain (WOMAC pain: total) compared with baseline based on the LMM. Negative difference indicates pain reduction. Panel D demonstrates the difference in stiffness (WOMAC stiffness: total) compared with baseline based on the LMM. Negative difference indicates improvement of stiffness; positive difference indicates worsening of stiffness. E, The difference in physical function (WOMAC physical function: total) compared with baseline based on the LMM is shown is panel E. Negative difference indicates improvement of physical function. Panel F shows the difference in Tegner Activity Scale compared with baseline based on the LMM. Positive difference indicates increase in level of activity.
Post hoc tests were performed using the multcomp package version 1.4-15.18 P values of post hoc tests were adjusted according to the “single-step method.”18
Clinical relevance of the effects was set at ≥30% reduction of baseline pain value.19 The coefficients of the LMM model (and their linear combinations) were assumed to follow a normal distribution (confirmed by simulations).
The analyses for all questionnaire variables followed the same scheme described here for “pain NRS.”
RESULTS
Patients
We assessed 184 patients for eligibility (Fig. 1). Of these, we excluded 23 patients for not meeting the inclusion criteria, 29 for declining to participate, and 33 for other reasons. Overall, 124 knees in 99 patients met the inclusion criteria and were assigned to 1 of the 4 treatment groups (Fig. 1). Four of these knees/patients represent replacements for study dropouts within the first 3 months after injection.
One hundred twenty knees in 95 patients were available for final data assessment: 41 females, 54 males, and 25 patients with bilateral knee inclusion. All baseline characteristics can be found in Table 2 and Supplementary Table S1, http://links.lww.com/RLI/A779. Some baseline P values were close to significance or slightly significant, which is expected due to the number of tests performed. Nevertheless, our statistical models included and controlled for several covariate including baseline characteristics.
Primary Outcome
Pain (Numeric Rating Scale)
As expected, starting conditions played a relevant role. In particular, “pain NRS” at baseline had a strong effect on the variable pain improvement (Table 3). Patients with low pain starting values (<20 pain NRS units) showed no to little improvement over time (ie, flooring effect).
TABLE 3.
Predictors in the Generalized Linear Mixed-Effects Model and Their P Values for Pain Improvement in NRS Score
| Baseline Parameter | P |
|---|---|
| Body mass index | 0.32 |
| Side | 0.28 |
| Kellgren-Lawrence grade | 0.61 |
| Age | 0.19 |
| Gender | 0.74 |
| Pain (polynomial second degree) | <0.001 |
| Oral analgesic medication | <0.001 |
| Other medication | 0.17 |
| Treatment: time | 0.25 |
The P values were computed via likelihood ratio tests. The last term in this table (ie, treatment: time) represents the interaction between time and drug.
To compare patients and treatments on a fair basis, baseline pain and other variables were taken into consideration using a model-based approach. All results refer to the estimated effects where we controlled for baseline pain and other covariates.
The P values associated with all predictors are summarized in Table 3. Interestingly, the only 2 predictors that seem to play a role for improvement in NRS scores were pain at baseline (P < 0.0001) and amount of oral analgesic medication (P < 0.0001). Patients taking “more” oral analgesic medication for 6 months compared with baseline had an NRS pain score of 18 points higher than those taking less oral analgesic medication than at baseline. There was no evidence that the drug effects differed over time (ie, the P value for the interaction between time and treatment is P = 0.25). The predicted values graph can be seen in Figure 2A. This figure shows the estimated treatment effects over time, when the effect of all other explanatory variables present in the model is corrected for. In other words, Figure 2A shows the model-corrected treatment effects over time. The majority of the estimated difference in pain values were found between −5 and +5 on the NRS. The magnitude of the treatment effects is approximately 4 times smaller compared with the among patients variation (95% of them lay within −20/+20 on the NRS). Furthermore, a quite large intrapatient variability was found when compared with the treatment effect. Within a patient, the amount of unexplained variability is quite large. In particular, the standard deviation of the residuals was 14, which implies that within a given patient the unexplained jumps over time are in most cases within the range ±28. In other words, the treatment effects are approximately 4 times smaller than the natural differences observed among patients or 6 times smaller than the natural fluctuations observed within patients over time.
The predicted graph shows that the greatest reduction in pain occurs in the hyaluronic acid and glucocorticoid group after 1 week, but the glucocorticoid effect vanishes completely after 3 months, the placebo effect after >21 months. Interestingly, patients with low NRS pain values at baseline showed no or only little improvement (Fig. 2B). The post hoc contrasts for the effect model showed no significant differences between the effects of placebo or glucocorticoid and the other drugs within the first 6 months after injection (see Supplementary Table S2, http://links.lww.com/RLI/A779).
Secondary Outcomes
All results in this section refer to the model-based (LMM) approach.
WOMAC (Pain)
The calculated difference to baseline pain values can be seen in Figure 2C. There was no evidence that drug effects differed over time (P = 0.75). Post hoc contrasts showed no significant differences between the effects of placebo or glucocorticoid and other drugs (P = 0.21–0.98).
WOMAC (Stiffness and Physical Function) and Tegner Activity Scale
Difference to baseline values can be seen for WOMAC stiffness in Figure 2D, for WOMAC physical function in Figure 2E, and for Tegner Activity Scale in Figure 2F. The post hoc contrasts for the effect model showed no significant differences, and there was no evidence that the drug effects differed over time (P = 0.31–1.00).
Clinical Parameters
Knee mobility did not differ significantly between baseline and at the follow-up examination (3 months after the injection): mean range of motion difference was −0.7 degrees (standard deviation: ±7.2 degrees) for active knee extension, 0.5 degrees (±9.9 degrees) for active knee flexion, 0.2 degrees (±9.0 degrees) for passive knee flexion, and −0.3 degrees (±6.8 degrees) for passive knee extension. The mean difference in thigh circumference was −0.1 cm (±2.8) on the right side and 0 cm (±2.3) on the left side.
Raw Data
The calculated differences to baseline based on the raw data can be found in Supplementary Figure S1–5, http://links.lww.com/RLI/A779. Seventy-eight knees completed the study regularly after 24 months (18 in the placebo, 20 in the glucocorticoid, 22 in the hyaluronic acid, and 18 in the PRP group). There is no evidence that some drugs had higher dropout rates. Reasons for dropout included joint injection (8 knees: 6 in same knee joint, 2 in other joints), knee surgery (3 knees), death (2 knees), lost-to-follow-up (1 knee), accident (1 knee), active withdraw from trial (27 knees).
Adverse Events
We encountered 5 adverse events in 2 patients (2 knees). One patient experienced facial redness and palpitations immediately after glucocorticoid injection and presented with knee joint swelling with effusion at 3 months, but no signs of infection. The other patient experienced nausea and vomiting immediately after receiving the glucocorticoid. Two nonrelated severe adverse events occurred: one patient died due to heart failure, another one died due to cancer during the study period.
DISCUSSION
This single-center randomized placebo-controlled trial compared intra-articular injection treatments in patients with mild to moderate OA of the knee. We found no significant difference in pain relief comparing glucocorticoid, hyaluronic acid, PRP, and placebo during the first 6 months after injection. Secondary outcomes including stiffness, Tegner Activity Scale, and clinical parameters of knee mobility were small and comparable among treatment groups. To take into account the structure of the data (patients measured over time) and to control for covariates, all results discussed are based on the LMM.
In the glucocorticoid group, pain reduction assessed with “pain NRS” score and WOMAC questionnaire was pronounced 1 week after the injection and vanished after 3 months. This is similar to a systematic review2 of 27 trials by the Cochrane group in 2015, which found a moderate reduction in knee pain in the first 1 to 2 weeks and no more pain reduction after 26 weeks. Although data in this review showed a difference in improved pain scores of 1.0 cm on a 10-cm NRS between corticosteroids and sham injections, our data found no difference in pain outcome between patients in the placebo and glucocorticoid group. Furthermore, the pain reduction in all our groups stayed mostly below a clinically relevant threshold, which is 30% of the baseline pain. For functional improvements, our observed effects were similar to the Cochrane analysis. In our study, the Tegner Activity Scale showed only small changes, with minimal improvement 3 months after the glucocorticoid injection. Glucocorticoid injections are frequently used in patients with knee OA, but whether the benefit exceeds the potential harms remains controversial.20 Recent studies indicated potential negative side such as loss of cartilage after repeated injections,21 transient meniscal thickness reduction,22 and increased risk of knee OA progression.23 Structural changes after the injection in our patients will be assessed in a separate article covering using magnetic resonance imaging data.
A meta-analysis in 2015 showed that intra-articular glucocorticoids reduce pain more effectively than hyaluronic acid in short term (up to 1 month), whereas hyaluronic acid is more effective in long term (up to 6 months).24 Similarly, our study shows a longer-lasting effect of hyaluronic acid, although the changes were small. Pain values (NRS and WOMAC) in the hyaluronic acid group stayed below baseline during follow-up with a peak of pain reduction after 15 months (NRS). It is thought that patients with mild-to-moderate OA are more likely to respond positively to hyaluronic injections than higher grades,25 but KL grade had neither statistically significant nor clinically relevant effect here. Similar to the study by Tammachote et al,26 injection of corticosteroids provided a similar improvement in knee pain, function, and range of motion compared with 1-shot hyaluronic acid at the 6 months follow-up. Contrary to their data, our hyaluronic acid group showed no better pain relief in the first week after injection.
A meta-analysis published in 202027 concluded that patients with PRP injection had a better functional recovery than hyaluronic acid injection at the 3 months, 6 months, and 12 months follow-up, as evaluated by WOMAC function scores. The pooled results demonstrated that PRP injection reduced pain more effectively than hyaluronic acid injection at 6 months and 12 months follow-up evaluated by WOMAC pain scores and visual analog scale (VAS) scores. Our study results do not support those data. It has to be stated that there is no clear standard concentration for PRP production.28 Depending on the used system and patient factors (baseline platelet concentration and function), platelet concentration is variable. Furthermore, the published studies are very variable regarding the injection frequency (single-injection or multiple injections). This makes comparison of PRP studies difficult and might explain the variation in outcome data. Another meta-analysis29 stated that a combination of hyaluronic acid and PRP shows the best results improving stiffness, physical function, and total scores, whereas PRP alone seems best for pain reduction. Various studies reported that OA knee patients treated with PRP had better results within the first 6 months after injection compared with placebo, especially with mild to moderate knee OA.30–32 In our study, patients with PRP treatment showed a slightly lower reduction in pain than hyaluronic acid and glucocorticoids 1 week after injection. After 3 months, pain levels were worst in the glucocorticoid group, followed by PRP and placebo (both at similar level). After 12 months, pain levels did not differ significantly from the placebo group, which is consistent with a recently published study comparing PRP and saline injections in patients with KL grades 2 and 3.33
Overall, the expected effect of pain reduction in our LMM prediction model was small (±5 points on an NRS from 0 to 100 points) for all drug groups, which is similar to some other studies.21,25 From a statistical point of view, the drug effects were practically not relevant compared with the high natural variability (±28 NRS units) within patients (±20 NRS units). Other studies observed a much larger pain reduction with changes between 15 and 30 points on the NRS.26,33 Baseline pain was quite low in our study patients compared with other studies.21,25,32,34 Because we found that the baseline pain was a significant predictor for pain reduction in our prediction model, the low improvement rate might be explained by a study cohort with low pain levels at the beginning. Nevertheless, these results question the practical effectiveness of these treatments for patients with low to moderate pain.
The strengths of our study included its single-center study setup and the single-dose intra-articular injection. The image-guided injection with contrast media assured correct intra-articular application, which is a crucial factor.35 This study is, to our knowledge, the first randomized trial comparing the use of glucocorticoids, hyaluronic acid, PRP, and placebo intra-articular injections.
Limitations
Up to now, there is no clear recommendation how often PRP should be injected. Because of the blinded study design, we could not offer multiple PRP injections to our patients.36
Our study included patients with 1 or 2 knees treated. We took this into account in our statistical modeling and implemented a model with 2 random effects (for the patient and patient's knee) and an additional variable predictor that accounts for the treatment applied to the contralateral knee. Because systemic effects of glucocorticoid injections have only been reported up to 1 month after the initial injection, we injected the second knee at least 3 months after the injection in the contralateral knee.37
Lastly, intra-articular injections are known for strong placebo effect.38 Self-reported parameters such as pain and stiffness are susceptible to this phenomenon.12 Future studies with larger study cohorts are warranted to confirm our findings.
In summary, there is no evidence that knee injections with glucocorticoid, PRP, or hyaluronic acid have superior short- or long-term effects in patients with mild or moderate OA when compared with placebo.
Based on these results, treating physicians might avoid these injection therapies for the management of knee OA.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the "Stiftung für Rheumaforschung" (Zurich, Switzerland) and the "Stiftung Marie-Lou Ringgenberg" (Bern, Switzerland) foundations for the financial support of this trial. The funding foundations had no involvement in the study design, data collection, data analysis or interpretation of data, the manuscript writing, or the decision to submit the article for publication. The authors also thank Professors Ulrike Held, Klaus Steigmiller, and Nicole Graf (NG) for their statistical support to this work.
Footnotes
Conflicts of interest and sources of funding: None of the authors has anything to disclose. Data generated or analyzed during the study and the study protocol are available from the corresponding author by request.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.investigativeradiology.com).
Contributor Information
Marcel Tschopp, Email: marcel.tschopp@olympian.org.
Christian W.A. Pfirrmann, Email: christian.pfirrmann@pfirrmann.ch.
Sandro F. Fucentese, Email: sandro.fucentese@balgrist.ch.
Florian Brunner, Email: florian.brunner@balgrist.ch.
Sabrina Catanzaro, Email: sabrina.catanzaro@balgrist.ch.
Nathalie Kühne, Email: nathalie.kuehne@balgrist.ch.
Iwan Zwyssig, Email: iwan.zwyssig@gmail.com.
Reto Sutter, Email: reto.sutter@balgrist.ch.
Tobias Götschi, Email: tobias.goetschi@balgrist.ch.
Matteo Tanadini, Email: matteo.tanadini@zurich-data-scientists.ch.
REFERENCES
- 1.Di Y Han C Zhao L, et al. Is local platelet-rich plasma injection clinically superior to hyaluronic acid for treatment of knee osteoarthritis? A systematic review of randomized controlled trials. Arthritis Res Ther. 2018;20:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jüni P Hari R Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;2015:CD005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384:51–59. [DOI] [PubMed] [Google Scholar]
- 4.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. [DOI] [PubMed] [Google Scholar]
- 5.Safiri S Kolahi AA Cross M, et al. Prevalence, incidence, and years lived with disability due to gout and its attributable risk factors for 195 countries and territories 1990–2017: a systematic analysis of the global burden of disease study 2017. Arthritis Rheumatol. 2020;72:1916–1927. [DOI] [PubMed] [Google Scholar]
- 6.Alshami AM. Knee osteoarthritis related pain: a narrative review of diagnosis and treatment. Int J Health Sci (Qassim). 2014;8:85–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325:568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaishya R Pariyo GB Agarwal AK, et al. Non-operative management of osteoarthritis of the knee joint. J Clin Orthop Trauma. 2016;7:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole BJ, Karas V, Fortier LA. Hyaluronic acid versus platelet-rich plasma: response. Am J Sports Med. 2017;45:NP21–NP22. [DOI] [PubMed] [Google Scholar]
- 10.Martin CL, Browne JA. Intra-articular corticosteroid injections for symptomatic knee osteoarthritis: what the orthopaedic provider needs to know. J Am Acad Orthop Surg. 2019;27:e758–e766. [DOI] [PubMed] [Google Scholar]
- 11.Deyle GD Allen CS Allison SC, et al. Physical therapy versus glucocorticoid injection for osteoarthritis of the knee. N Engl J Med. 2020;382:1420–1429. [DOI] [PubMed] [Google Scholar]
- 12.Jones IA Togashi R Wilson ML, et al. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannuru RR Natov NS Dasi UR, et al. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis—meta-analysis. Osteoarthritis Cartilage. 2011;19:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oudelaar BW Peerbooms JC Huis In 't Veld R, et al. Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med. 2019;47:479–487. [DOI] [PubMed] [Google Scholar]
- 16.Team RC . R: A language and environment for statistical computing. Vienna, Austria: RFoundation for Statistical Computing; 2021. [Google Scholar]
- 17.Bates DMM, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 18.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. [DOI] [PubMed] [Google Scholar]
- 19.Farrar JT Young JP LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. [DOI] [PubMed] [Google Scholar]
- 20.Kompel AJ Roemer FW Murakami AM, et al. Intra-articular corticosteroid injections in the hip and knee: perhaps not as safe as we thought? Radiology. 2019;293:656–663. [DOI] [PubMed] [Google Scholar]
- 21.McAlindon TE LaValley MP Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelletier JP Raynauld JP Abram F, et al. Intra-articular corticosteroid knee injection induces a reduction in meniscal thickness with no treatment effect on cartilage volume: a case-control study. Sci Rep. 2020;10:13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng C Lane NE Hunter DJ, et al. Intra-articular corticosteroids and the risk of knee osteoarthritis progression: results from the osteoarthritis initiative. Osteoarthr Cartil. 2019;27:855–862. [DOI] [PubMed] [Google Scholar]
- 24.He WW Kuang MJ Zhao J, et al. Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: a meta-analysis. Int J Surg. 2017;39:95–103. [DOI] [PubMed] [Google Scholar]
- 25.Bowman EN Hallock JD Throckmorton TW, et al. Hyaluronic acid injections for osteoarthritis of the knee: predictors of successful treatment. Int Orthop. 2018;42:733–740. [DOI] [PubMed] [Google Scholar]
- 26.Tammachote N Kanitnate S Yakumpor T, et al. Intra-articular, single-shot Hylan G-F 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis: a double-blind, randomized controlled trial. J Bone Joint Surg Am. 2016;98:885–892. [DOI] [PubMed] [Google Scholar]
- 27.Tang JZ Nie MJ Zhao JZ, et al. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. J Orthop Surg Res. 2020;15:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal H Leon J Pont JL, et al. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Sci Rep. 2021;11:3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Zhang Y, Bi L. Comparative efficacy of treatments for patients with knee osteoarthritis: a network meta-analysis. Eur J Med Res. 2020;25:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel S Dhillon MS Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356–364. [DOI] [PubMed] [Google Scholar]
- 31.Lin KY Yang CC Hsu CJ, et al. Intra-articular injection of platelet-rich plasma is superior to hyaluronic acid or saline solution in the treatment of mild to moderate knee osteoarthritis: a randomized, double-blind, triple-parallel, placebo-controlled clinical trial. Arthroscopy. 2019;35:106–117. [DOI] [PubMed] [Google Scholar]
- 32.Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884–891. [DOI] [PubMed] [Google Scholar]
- 33.Bennell KL Paterson KL Metcalf BR, et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. 2021;326:2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang PH Wang CJ Chou WY, et al. Short-term clinical results of intra-articular PRP injections for early osteoarthritis of the knee. Int J Surg. 2017;42:117–122. [DOI] [PubMed] [Google Scholar]
- 35.Jones A Regan M Ledingham J, et al. Importance of placement of intra-articular steroid injections. BMJ. 1993;307:1329–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bahrami MH Raeissadat SA Cheraghi M, et al. Efficacy of single high-molecular-weight versus triple low-molecular-weight hyaluronic acid intra-articular injection among knee osteoarthritis patients. BMC Musculoskelet Disord. 2020;21:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habib GS. Systemic effects of intra-articular corticosteroids. Clin Rheumatol. 2009;28:749–756. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W. The powerful placebo effect in osteoarthritis. Clin Exp Rheumatol. 2019;37 Suppl 120:118–123. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


