Purpose of review
COVID-19 pandemic has caused more than 6.6 million deaths globally. Tremendous efforts have been committed for the development of new and repurposed drugs for the treatment of COVID-19. Although different international and national guidelines share consensus in the management of COVID-19 disease with different levels of severity, new challenges have emerged, steering the need for ongoing research in advancing the clinical management of COVID-19.
Recent findings
This review focuses on recent data from randomized trials and postmarketing real-world evidence for the treatment of mild to moderate disease in the outpatient setting and patients hospitalized for COVID-19 with varying level of severity. Relevant data for treatment of the latest omicron sub-variants in people who received vaccination are presented. Challenges in special populations, including immunocompromised hosts, patients with renal failure and pregnant women, are also discussed.
Summary
Treatment of COVID-19 should be personalized according to host characteristics, degree of severity and available treatment options.
Keywords: antiviral, disease severity, monoclonal antibody, SARS-CoV-2
INTRODUCTION
Since the beginning of the COVID-19 pandemic in early 2020, more than 6.6 million people around the world died of the disease, but the real health burden of the pandemic is still far to be evaluated [1]. Nevertheless, in an incredibly short period of time, scientific research advances on prevention and treatment of SARS-CoV-2 infection were developed. The widespread rollout of SARS-CoV-2 vaccines and improvement in the standard of care in treatment has dramatically reduced morbidity and mortality of COVID-19 [2,3].
This review focuses on recent data on drug treatment of adults with COVID-19 disease published from January 2022 to November 2022. Where applicable, data from randomized controlled trials are presented. Real-world evidence is also reviewed, as large real-life observational were often more representative of populations infected with SARS-CoV-2 in real-life setting. This review also focuses on studies relevant to the omicron variant and the currently circulating the sub-variants viruses.
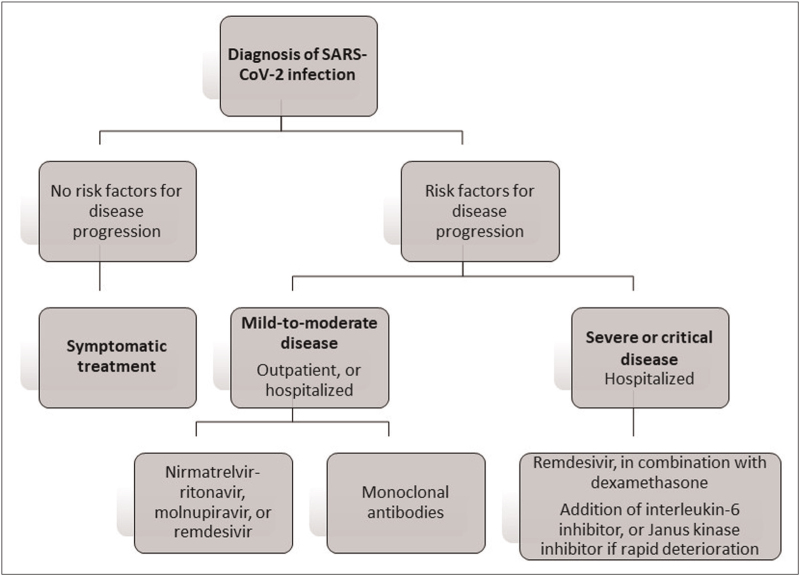
In this review, treatment of COVID-19 disease of the whole spectrum of severity in both outpatient and hospitalized settings is presented (Fig. 1). Then, particular issues related to treatment of special populations are discussed.
FIGURE 1.
Treatment of COVID-19 in outpatients and hospitalized patients.
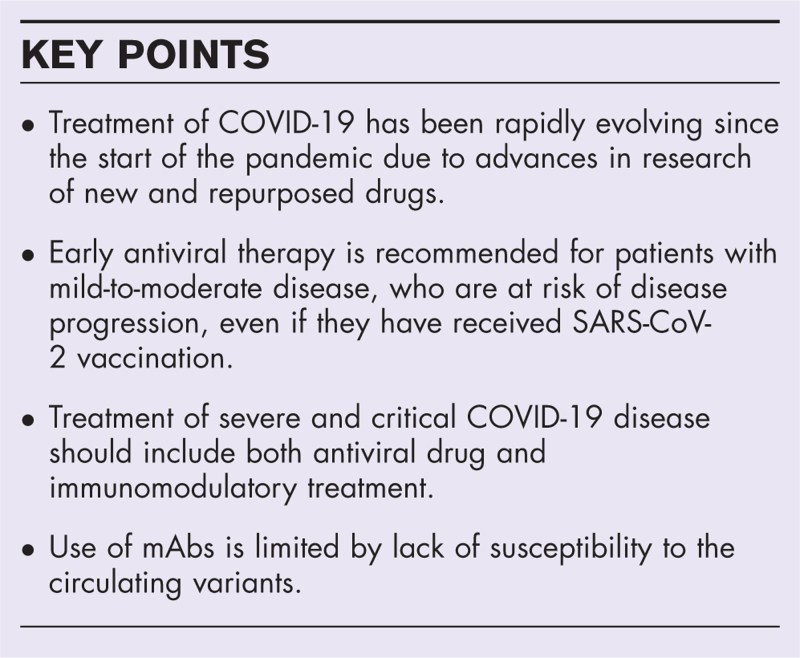
Box 1.
no caption available
TREATMENT OF MILD-TO-MODERATE COVID-19 DISEASE IN THE OUTPATIENT SETTING
Patients with mild COVID-19 disease commonly present with fever, cough, sore-throat and myalgia, while patients with moderate disease have clinical or radiographic evidence of lower respiratory tract involvement, while maintaining oxygen saturation at least 94% [4]. Most of these patients can be safely managed in outpatient settings, including telemedicine consultation, in-person clinics and emergency departments [5,6].
Antiviral treatment
Two landmark phase 3 randomized trials, the MOVe-OUT and EPIC-HR trials, evaluated the efficacy of two oral anti-SARS-CoV-2 antiviral drugs, molnupiravir and nirmatrelvir-ritonavir, prescribed to outpatients. When given to unvaccinated individuals with mild-to-moderate COVID-19 disease within 5 days of symptom onset, who were at risk of disease progression, molnupiravir and nirmatrelvir-ritonavir reduced hospitalization or death by 30 and 89%, respectively [7,8]. The pharmacological characteristics, mechanism of action and clinical use of these drugs have been reviewed previously [9▪].
These randomized trials were performed when delta and earlier variants were circulating, and involved unvaccinated individuals and relatively small proportions of older adults [10]. It was thus uncertain whether similar efficacy would be observed in vaccinated (with and without boosters) individuals who are older and had a wider spectrum of comorbidities, or affected by the omicron variant, which had a 60–70% lower risk of hospitalization and death than the delta variant [11].
Recent observational studies provided evidence for clinical effectiveness of these antiviral treatments given to outpatients in real-world settings (Table 1). These studies were performed when the omicron variant was circulating, and involved predominantly patients older than 60 years, with a wide range of comorbidities and immunocompromising conditions. Previous immunity secondary to vaccination or natural infection was greater than 70% for most studies.
Table 1.
Real-world evidence for use of oral antivirals in the treatment of mild to moderate COVID-19 disease in the outpatient setting
| Study design and setting | Sample size | Characteristics of antiviral users | Major findings |
| Molnupiravir | |||
| Najjar-Debbiny et al.[14] Retrospective cohort Two healthcare organization databases in Israel Jan to Feb 2022 |
2661 molnupiravir users 2661 PS-matched nonusers |
Age 73.1 ± 11.7 Male 50.4% ≥2 doses of vaccine with last dose within 180 days: 77.3% Hypertension 73.6% Cardiovascular disease 47.7% Diabetes 44.0% |
Severe COVID-19a or mortality HR 0.83 (95% CI 0.57–1.21) Subgroup analysis Age>75 years: HR 0.54 (0.34–0.86) Females: HR 0.41 (0.22–0.77) Inadequately vaccinated: HR 0.45 (0.25–0.82) |
| Wai et al.[12] Retrospective outpatient cohort Hong Kong Feb to Mar 2022 |
5345 molnupiravir users 23430 nonusers |
Age >60 years: 97.8% Male 46.7% Diabetes 9.6% Stroke 4.9% |
Hospital admission within 28 days OR 0.72 (95% CI 0.52–0.98) |
| Wong et al.[13] Retrospective cohort study Hong Kong Feb to Jun 2022 |
4983 molnupiravir users 49 234 matched nonusers |
Age >60 years: 88.7% Male 47.5% ≥2 doses of mRNA or ≥3 doses of inactivated vaccine 16% Charlson comorbidity index score 0–4: 89.8% |
All-cause mortality 17.9 vs. 22.1 per 100 000 person-days, HR 0.76 (95% CI 0.61–0.95) Hospital admission due to COVID-19 107.6 vs. 104.0 per 100 000 person-days, HR 0.98 (95% CI 0.89–1.06) >60 years: HR 0.89, 95% CI 0.81–0.97 |
| Yip et al.[68] Retrospective cohort study Electronic healthcare database in Hong Kong Feb to Mar 2022 |
4798 molnupiravir users 4758 nonusers with PS weighting |
Age 71.1 ± 11.7 Male 46.8% ≥2 doses of mRNA or ≥3 doses of inactivated vaccine 43% Diabetes 28% |
Hospital admission by 30 days 7.5 vs. 1.6%, weighted HR 1.17 (95% CI 0.99–1.39) Death, ICU or mechanical ventilation 0.9 vs. 0.2%, weighted HR 1.12 (95% CI 0.68–1.82) |
| Nirmatrelvir-ritonavir | |||
| Arbel et al.[17] Retrospective cohort Electronic health record from large healthcare organization in Israel Jan to Mar 2022 |
3902 nirmatrelvir-ritonavir users 105352 nonusers |
Age 40–64: 36%, ≥65: 64% Male 40% Previous immunity induced by vaccination and/or infection 90% Hypertension 49% Obesity 42% Diabetes 40% Immunosuppression 23% |
40–64 years Adjusted HR for hospitalization: 0.74 (95% CI 0.35–1.58) Adjusted HR for death: 1.32 (95% CI 0.16–10.75) ≥65 years Adjusted HR for hospitalization: 0.27 (95% CI 0.15–0.49) (without previous immunity: 0.15, 95% CI 0.04–0.60; with previous immunity 0.32, 95% CI 0.17–0.63) Adjusted HR for death: 0.21 (95% CI 0.05–0.82) |
| Dryden-Peterson et al.[18] Retrospective analysis of an electronic healthcare system database in US Jan to July 2022 |
11797 nirmatrelvir-ritonavir users 32248 nonusers |
Age ≥65 years: 46% Male 41% Vaccinated 23%, boosted 68% Last vaccine dose >20 weeks: 74% Immunocompromised 36% Solid tumour 23% Diabetes 19% |
Hospitalization with 14 days or death within 28 days 0.55 vs. 0.97%, adjusted HR 0.56 (95% CI 0.42–0.75) Subgroup analysis Age 50–64 years: relative risk 0.55 (95% CI 0.30–1.03) Age ≥65 years: 0.55 (0.40–0.77) Not fully vaccinated 0.19 (0.08–0.49) Vaccinated 0.69 (0.50–0.94) Last vaccine <20 weeks 0.87 (0.51–1.50) Last vaccine >20 weeks 0.45 (0.32–0.64) |
| Ganatra et al.[69] Retrospective analysis of electronic health records of >120 healthcare organizations in US Dec 2021 to Apr 2022 |
1131 nirmatrelvir-ritonavir users 1130 PS-matched nonusers |
Mean age 57.5 ± 16.3 Male 37% Vaccinated 100% Hypertension 52.2% Malignancy 45.3% Diabetes 22.1% |
Emergency room visits, hospitalization or death at 30 days 7.87 vs. 14.4%, OR 0.51 (95% CI 0.39–0.67) Hospitalization at 30 days 0.8 vs. 2.0%, OR 0.43 (95% CI 0.20–0.91) 30-day mortality 0 vs. 0.8% |
| Najjar-Debbiny et al.[19] Retrospective cohort Two healthcare organization databases in Israel Jan to Feb 2022 |
4737 nirmatrelvir-ritonavir users 175614 nonusers |
Age 68.5 ± 12.5 Male 42.1% ≥2 doses of vaccine with last dose within 180 days 77.8% Hypertension 51.7% Obesity 40.9% Diabetes 38.5% |
Severe COVID-193 or mortality adjusted HR 0.54 (95% CI 0.39–0.75) Subgroup analysis Adequate vaccination: adjusted HR 0.62 (95% CI 0.39–0.98) No adequate vaccination: adjusted HR 0.52 (95% CI 0.32–0.82) Age <60 years: adjusted HR 1.06 (0.36–3.15) Age ≥60 years: adjusted HR 0.52 (0.36–0.73) |
| Shah et al.[5] Retrospective analysis of electronic health record data set in USA Apr to Aug 2022 |
198 927 nirmatrelvir-ritonavir users 500 921 nonusers |
Age ≥65 years 37.9% Male 38.2% Previous infection 15.0% ≥2 doses of vaccine 68.8% ≥2 underlying health conditions 66.8% Immunocompromised 9.9% |
Overnight COVID-19-associated hospitalization by day 30 0.47 vs. 0.86%, adjusted HR 0.49 (95% CI 0.46–0.53) Subgroup analysis Similar reduction in persons with 2 and ≥3 doses of vaccines, and in all age groups (18–49, 50–64 and ≥65 years) No significant reduction in age group 18–49 with ≥3 mRNA doses, or only one underlying health condition |
| Wai et al.[12] Retrospective outpatient cohort Hong Kong Feb to Mar 2022 |
4442 nirmatrelvir-ritonavir users 23430 nonusers |
Age >60 years: 98.3% Male 45.4% Diabetes 6.6% Cancer 2.7% |
Hospital admission within 28 days OR 0.37 (95% CI 0.23–0.60) |
| Wong et al.[13] Retrospective cohort study Hong Kong Feb to Jun 2022 |
5542 nirmatrelvir-ritonavir users 54672 matched nonusers |
Age >60 years: 85.9% Male 46.3% ≥2 doses of mRNA or ≥3 doses of inactivated vaccine 33.4% Charlson comorbidity index score 0–4: 95.5% |
All-cause mortality 4.2 vs. 11.6 per 100 000 person-days, HR 0.34 (95% CI 0.22–0.52) Hospital admission due to COVID-19 48.5 vs. 61.0 per 100 000 person-days, HR 0.76 (95% CI 0.67–0.86) |
| Yip et al.[68] Retrospective cohort study Electronic healthcare database in Hong Kong Feb to Mar 2022 |
4921 nirmatrelvir-ritonavir users 4758 nonusers with PS weighting |
Age 70.8 ± 12.1 Male 45.7% ≥2 doses of mRNA or ≥3 doses of inactivated vaccine 43% Diabetes 27% |
Hospital admission by 30 days 3.5 vs. 1.6%, weighted HR 0.79 (95% CI 0.65–0.95) Death, ICU or mechanical ventilation 0.4 vs. 0.2%, weight HR 0.81 (95% CI 0.47–1.39) |
CI, confidence interval; HR, hazard ratio; OR, odds ratio; PS, propensity score.
Oxygen saturation <94%, arterial partial pressure of oxygen to fraction of inspired oxygen <300 mmHg or respiratory rate >30 breaths.
Studies evaluating effectiveness of molnupiravir yielded inconclusive results, with one study from Hong Kong demonstrating 28% reduction in hospital admission [12] and another showing 24% reduction in mortality [13]. In two studies, protection against disease progression was only observed among older adult and inadequately vaccinated subgroups [13,14].
In the PANORAMIC trial performed in the UK, molnupiravir given in outpatient setting failed to demonstrate a reduction of hospitalization or death, given the very low rate of these outcomes (0.8%) in the usual care arm. However, molnupiravir was able to reduce time to recovery by 4.2 days [15]. Secondary analyses of the MOVe-OUT trial data also showed additional benefits of molnupiravir in reducing the need of respiratory support and subsequent acute care visit [16].
Real-world studies evaluating effectiveness of nirmatrelvir-ritonavir demonstrated benefits in reducing hospital admission by 21–73% and mortality by 66–79% (Table 1). In subgroup analyses, clinical benefit was not observed in adults aged less than 60–65 years [17–19], or in younger individuals who received three doses of vaccine [5]. On the contrary, the greatest benefit was observed in people more than 65 years of age [17], older adults with a higher number of comorbidities [5] and underlying immunosuppression [19], and individuals not adequately vaccinated or who received last vaccine dose more than 20 weeks before infection [17–19].
Cost-effectiveness analyses from Hong Kong and USA analysing data from large electronic health databases showed that the use of SARS-CoV-2 oral antivirals, vs. symptomatic care, in outpatient setting reduced healthcare costs and increased quality-adjusted life years, by reducing subsequent hospital admissions, readmissions and long-term sequelae [12,20].
In the PINETREE randomized controlled trial, 3 days of intravenous remdesivir administered in the outpatient setting also reduced COVID-19-related hospitalization or death by 87% when administered within 7 days of symptom onset in at-risk unvaccinated populations [21].
At present, there are no randomized head-to-head comparative trials for the above-mentioned oral and intravenous antiviral treatments. In a prospective observational cohort of 562 outpatients with COVID-19 treated with either these three antivirals in Italy, the type of antiviral treatment was not associated with hospitalization or death, after adjusting for age, comorbidities, vaccination status and symptoms [22]. In this study, adverse events were more common with the oral antivirals, particularly gastrointestinal symptoms, and dysgeusia with nirmatrelvir-ritonavir, while remdesivir had a higher risk of bradycardia [22]. The use of nirmatrelvir-ritonavir is also challenged by drug–drug interactions. Some studies figured out that 12–20% of at-risk populations had potential severe drug–drug interactions or required to change chronic treatments when treated with nirmatrelvir-ritonavir [6,23].
Cases of viral rebound after cessation of nirmatrelvir-ritonavir, which was associated with symptoms and viral transmission, were reported shortly after rollout of antiviral treatment [24]. Recent data from EPIC-HR trial and two retrospective cohort studies each involving 13 000 patients showed that viral rebound was observed in 1.0–5.4% of patients treated with nirmatrelvir-ritonavir, and the incidence was similar to patients not treated with antiviral (0.6–1.7%) or with molnupiravir (0.8–8.6%) [25–27]. Antiviral resistance was not identified. Robust B and T cell immune responses were detected, suggesting a low risk of progression to severe disease during viral rebound [28].
Live-virus neutralization assays confirmed that molnupiravir, nirmatrelvir-ritonavir and remdesivir remain effective against omicron subvariants, including BQ.1.1 and XBB [29].
mAbs
In randomized trials, mAbs, such as sotrovimab and tixagevimab-cilgavimab, reduced progression to severe disease, hospitalization or death by approximately 50–80%, when given to unvaccinated nonhospitalized patients at risk for disease progression within 5–7 days of symptom onset [30,31]. A real-world study showed that in a population with 40% vaccination rate, mAbs reduced hospitalization and mortality by 52 and 89%, respectively, at the time when alpha and delta variants were circulating [32]. Bebtelovimab was also shown to have a trend in reducing risk of hospitalization or death in high-risk populations in a retrospective cohort study of patients treated in the outpatient care setting [33].
Comparative observational studies have evaluated clinical effectiveness of mAbs and antiviral treatments in outpatients. Bebtelovimab and nirmatrelvir-ritonavir performed similarly in preventing progression to severe disease [34], while sotrovimab had a lower risk of progression, hospital admission or death than molnupiravir [35].
These data supported the role of early administration of mAbs in the prevention of progression to severe disease. Unfortunately, none of the commercially available mAbs, including tixagevimab-cilgavimab and bebtelovimab, were susceptible in vitro against BQ.1.1 and XBB omicron sub-variants, which are the predominantly circulating variants globally at the last quarter of 2022 [29].
In summary, with regards to the treatment of mild-to-moderate COVID-19 disease in the outpatient setting, early use of antiviral treatment in individuals with risk factors for disease progression is highly recommended, and supported by randomized trials, real-world evidence and cost-effectiveness analyses. In settings wherein resources are limited, prioritization should be given to older adults with multiple comorbidities or immunosuppression, and those who are inadequately or not recently vaccinated. Nirmatrelvir-ritonavir is the recommended treatment in the outpatient setting. Where nirmatrelvir-ritonavir is contraindicated due to drug interactions, or severe renal or hepatic impairment [6], oral molnupiravir or intravenous remdesivir, if feasible, should be prescribed. The use of mAbs should only be considered if the circulating variant is susceptible to the available treatment.
TREATMENT OF PATIENTS HOSPITALIZED WITH MILD-TO-MODERATE DISEASE
Patients with mild-to-moderate disease may also be hospitalized due to symptomatic COVID-19 disease, or non-COVID-related indications. These patients are at a higher risk of severe clinical presentation and disease progression than those managed as outpatients.
Molnupiravir and nirmatrelvir-ritonavir
A randomized phase 2 trial including 304 patients hospitalized for COVID-19 to three different doses of molnupiravir or placebo failed to show clinical benefit [36]. To the best of our knowledge, no randomized trials were performed to evaluate efficacy of nirmatrelvir-ritonavir in hospitalized patients.
Three real-world studies performed in Hong Kong and Japan evaluated clinical effectiveness of nirmatrelvir-ritonavir and molnupiravir in people hospitalized with mild-to-moderate COVID-19 disease (Table 2). The large majority of included patients were more than 60 years old, and had adequate vaccination rates ranging from 10 to 80% [12,37,38]. Compared with matched controls, molnupiravir and nirmatrelvir-ritonavir were consistently associated with 40–55% lower risk of clinical deterioration. Moreover, molnupiravir was associated with 52–69%, and nirmatrelvir-ritonavir 66–90% lower risk of death respectively [12,37,38].
Table 2.
Real-world evidence for use of oral antivirals in the treatment of mild to moderate COVID-19 disease in hospitalized patients
| Study design and setting | Sample size | Characteristics of antiviral users | Major findings |
| Molnupiravir | |||
| Suzuki et al.[37] Retrospective cohort 23 hospitals in Fukushima, Japan Jan to Apr 2022 |
230 molnupiravir users 690 nonusers matched by PS |
Age 64.1 ± 20.0 Male 53.0% ≥2 doses of vaccine 82.2% Hypertension 55.3% Diabetes 26.1% Cardiac disease 18.3% |
Clinical deteriorationa 3.9 vs. 8.4% (P = 0.034), adjusted OR 0.45 (95% CI 0.21–0.97) |
| Wai et al.[12] Retrospective inpatient cohort Hong Kong Feb to Mar 2022 |
799 molnupiravir users 20057 nonusers |
Age >60 years: 98.2% Male 50.6% Diabetes 15.2% Stroke 8.1% |
All-cause mortality HR 0.31 (95% CI 0.24–0.40) 28-day hospital readmission OR 0.71 (95% CI 0.52–0.97) |
| Wong et al.[38] Retrospective cohort study Hospitalized adults in Hong Kong Feb to May 2022 |
1856 molnupiravir users 1856 nonusers matched by PS |
Age 80.3 ± 13.0 Male 49.2% ≥2 doses of mRNA or ≥3 doses of inactivated vaccine 6.2% Charlson's comorbidity index 5.8 ± 1.9 |
All-cause mortality 8.1 vs. 15.9%, HR 0.48 (95% CI 0.40–0.59) Composite disease progressionb 16.5 vs. 25.9%, HR 0.60 (95% CI 0.52–0.69) |
| Nirmatrelvir-ritonavir | |||
| Wai et al.[12] Retrospective inpatient cohort Hong Kong Feb to Mar 2022 |
282 Nirmatrelvir-ritonavir users 20057 nonusers |
Age >60 years: 99.3% Male 52.1% Stroke 9.2% Diabetes 7.4% |
All-cause mortality HR 0.10 (95% CI 0.05–0.21) 28-day hospital readmission OR 0.47 (95% CI 0.24–0.93) |
| Wong et al.[38] Retrospective cohort study in Hong Kong Hospitalized adults Feb to May 2022 |
890 nirmatrelvir-ritonavir users 890 nonusers matched by PS |
Age 77.2 ± 14.1 Male 50.0% ≥2 doses of mRNA or ≥3 doses of inactivated vaccine 10.5% Charlson's comorbidity index 5.1 ± 1.7 |
All-cause mortality 3.6 vs. 10.3%, HR 0.34 (95% CI 0.23–0.50) Composite disease progressionb 11.3 vs. 19.4%, HR 0.57 (95% CI 0.45–0.72) |
CI, confidence interval; HR, hazard ratio; OR, odds ratio; PS, propensity score.
Worsened respiratory condition requiring escalation of treatment or respiratory support.
All-cause mortality, invasive mechanical ventilation, intensive care admission or oxygen therapy.
A cost-effectiveness analysis using real-world data showed that in the inpatient setting, both oral antivirals saved costs due to reduction in hospital stay and readmissions, as compared with standard care. Although molnupiravir cost an Incremental cost-effectiveness ratio (ICER) of USD2629 per death averted, nirmatrelvir-ritonavir was cost-saving with an ICER of -USD5503 per death averted. This was due to shorter length of stay and higher survival rate for nirmatrelvir-ritonavir users [12].
Remdesivir
Randomized trials failed to demonstrate benefit in clinical recovery and survival in hospitalized patients with mild-to-moderate COVID-19 disease not requiring oxygen therapy; a possible reason was the limitation of small sample size in this subgroup [39,40]. Subsequent retrospective studies involving 200–250 patients not requiring oxygen therapy also showed similar results [41,42]. However, in two recent retrospective cohort studies from large hospital-based databases in the USA, including, respectively, 8000 and 16 000 hospitalized patients not requiring oxygen therapy who received remdesivir, the risk of mortality was reduced by 12–20% when compared with propensity score matched controls [43,44].
In summary, with regards to the treatment of patients hospitalized with mild-to-moderate disease, in the lack of randomized trial data, real-world data show the benefit from early use of antivirals, with improved survival. The option of antiviral should be personalized depending on clinical effectiveness, route of administration convenience and contraindications for individual treatment.
TREATMENT OF PATIENTS HOSPITALIZED WITH SEVERE AND CRITICAL DISEASE
Patients with severe disease present with hypoxemia (oxygen saturation <94%) and tachypnoea, with lung infiltrates detected on imaging in more than 50% of cases [4]. Patients with critical disease progress to respiratory failure requiring noninvasive or mechanical ventilation, with potential multiorgan failure [4].
Antiviral treatment
Early randomized trials and real-world data showed that remdesivir reduced mortality and progression to ventilation in hospitalized patients requiring low-flow supplemental oxygen, but clinical benefits were not observed in people requiring high-flow oxygen, or noninvasive or mechanical ventilation [39–42]. The latter findings were limited by inadequate sample size in detecting differences in outcomes. Recently, two retrospective cohort studies of large hospital-based databases in the USA, involving, respectively, 3500 and 7000 patients undergoing high-flow oxygen, or noninvasive or mechanical ventilation, showed that remdesivir was associated with 19–30% lower mortality risk [43,44].
In a retrospective study in Poland including 590 patients, a significantly lower mortality rate was observed in adults aged more than 80 years and hypoxia receiving molnupiravir within 5 days of symptom onset (14.6 vs. 35.2%, P = 0.016) [45]. No randomized clinical trial evaluated efficacy of oral antiviral treatment in hospitalized patients with severe COVID-19 disease so far.
Immunomodulatory therapy
The use of immunomodulatory agents in the treatment of severe and critical COVID-19 disease has been extensively reviewed [46▪]. Current guidelines recommend corticosteroid, interleukin-6 inhibitor, for example tocilizumab, and Janus kinase inhibitor, for example baricitinib, in severe COVID-19 disease [47,48].
Corticosteroids were the first drugs, which demonstrated survival benefit in the treatment of COVID-19 [49]. Recent studies compared effectiveness between standard dose of corticosteroid (dexamethasone 6 mg daily) and higher doses (dexamethasone 10–20 mg daily) in patients with severe or critical disease, but did not observe significant difference in mortality and other clinical outcomes [50–52].
In patients with rapid disease progression and evidence of systemic inflammation, for example elevated C reaction protein, interleukin-6 or Janus kinase inhibitors are recommended to be added to corticosteroid [53].
Anti-C5a antibody was recently tested in a large randomized trial, in which 368 patients receiving invasive mechanical ventilation were randomized to vilobelimab or placebo, in addition to standard of care, which included corticosteroid, anticoagulants and immunomodulators [54]. Vilobelimab significantly reduced all-cause mortality at 28 days by 33%, without increasing treatment-emergent serious adverse events.
In summary, with regards to the treatment of patients hospitalized with severe and critical disease, current evidence supported the use of remdesivir for the whole spectrum of respiratory support. Oral antivirals warrant further evaluation for patients on low-flow supplemental oxygen. Corticosteroid and other immunomodulatory therapy constitute an essential component in their clinical management.
SPECIAL POPULATIONS
Immunocompromised populations
Most patients infected with SARS-CoV-2 achieve clearance of infectious viruses by day 10 [55]. However, in immunocompromised individuals, persistent infection with persistent viral replication has been observed, particularly in those with impaired humoral immunity [56]. This condition causes long duration of symptoms, risk of emergence of new variants with potential antiviral resistance and delays immunosuppressant treatment for the underlying conditions [56,57].
There is currently no consensus for the treatment of this group of difficult-to-treat patients. In a survey of a cohort of 31 patients with COVID-19 and B cell immunodeficiency in the UK, those who received a combination of remdesivir and antibody therapy, including monoclonal antibody and convalescent plasma, had significantly higher rate of sustained viral clearance than those receiving remdesivir monotherapy or no antiviral treatment [58]. Repeated and prolonged courses of remdesivir up to 30 days [59], in combination with antibody treatment, or in combination with nirmatrelvir-ritonavir [57] have been reported to successfully achieve viral clearance.
Renal disease
People with moderate to severe renal impairment were excluded from randomized trials of antiviral treatments for COVID-19 [7,8,21]. A few observational studies reported use of remdesivir in people with impaired renal function. In people with estimated glomerular filtration rate less than 30 ml/min, use of remedesivir was not associated with increased adverse events, including acute kidney injury and abnormal liver function [60–62].
Remdesivir was also used, at half of the standard dose, in patients receiving haemodialysis in South Korea, and it was independently associated with a lower risk of composite outcome of mortality, high-flow nasal oxygen and intensive care admission [63]. Nirmatrelvir-ritonavir is currently being evaluated in various clinical trials in patients with varying degrees of renal impairment (trials registered in ClinicalTrials.gov: NCT05366192, NCT05487040, NCT05624840, NCT05386433).
Pregnancy
Antiviral treatments were seldom evaluated in pregnant women in randomized trials. Molnupiravir is contraindicated in pregnancy due to potential embryo-foetal toxicity [9▪]. Remdesivir use in hospitalized pregnant women was well tolerated, without an increase in adverse maternal or neonatal outcomes [64]. Early administration of remdesivir within 7 days of symptom onset was associated with lower intensive care admission, progression to severe disease and shorter length of hospital stay [65].
Nirmatrelvir-ritonavir was prescribed to 47 pregnant women in the USA [66]. Women were 57% in the third trimester and 34% in the second trimester. Two patients were subsequently hospitalized for non-COVID-related reasons. The treatment was well tolerated, with two women discontinuing medication due to adverse effects, and no serious adverse effects reported for women or new-borns.
A recent retrospective study evaluated the use of high-dose inhaled nitric oxide for pregnant women with severe COVID-19 pneumonia [67]. Inhaled nitric oxide was associated with more oxygen supplementation-free days, and shorter length of stay in intensive care and in hospital, and did not increase adverse maternal and neonatal outcomes.
CONCLUSION
The landscape of scientific research in the treatment of COVID-19 disease has evolved rapidly since the start of the pandemic. Effective antiviral treatment is currently available for early administration to people with mild-to-moderate disease at risk of progression even in people who received SARS-CoV-2 vaccination. The use of mAbs is limited by the rapid emergence of variants resistant to available products. Combination of antiviral treatment and immunomodulatory therapy is essential for the treatment of severe and critical disease. Advances are expected in the future for difficult-to-treat groups who are immunocompromised or have existing comorbidities limiting the use of currently available options, who may require personalised treatment regarding choice, combination and duration of antiviral treatments.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
Lui G. and Guaraldi G. have received research grants and consultancy fees from Gilead Sciences, MSD, GSK and Janssen outside of this work.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/on. [Accessed 12 December 2022]. [Google Scholar]
- 2.Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis 2022; 22:1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potter GE, Bonnett T, Rubenstein K, et al. Temporal improvements in COVID-19 outcomes for hospitalized adults: a post hoc observational study of remdesivir group participants in the adaptive COVID-19 treatment trial. Ann Intern Med 2022; 175:1716–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med 2020; 383:1757–1766. [DOI] [PubMed] [Google Scholar]
- 5.Shah MM, Joyce B, Plumb ID, et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19 - United States, April-September 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim S, Tignanelli CJ, Hoertel N, et al. Prevalence of medical contraindications to nirmatrelvir/ritonavir in a cohort of hospitalized and nonhospitalized patients with COVID-19. Open Forum Infect Dis 2022; 9:ofac389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022; 386:1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Rahmah L, Abarikwu SO, Arero AG, et al. Oral antiviral treatments for COVID-19: opportunities and challenges. Pharmacol Rep 2022; 74:1255–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reviews the pharmacologic characteristics, mechanism of action, evidence supporting clinical use and practical issues concerning prescription in clinical practice.
- 10.Zarębska-Michaluk D, Flisiak R. Early oral antiviral use in patients hospitalised with COVID-19. Lancet Infect Dis 2022; 22:1650–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet (London, England) 2022; 399:1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wai AK, Chan CY, Cheung AW, et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health West Pac 2023; 30:100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong CKH, Au ICH, Lau KTK, et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet (London, England) 2022; 400:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of molnupiravir in high risk patients: a propensity score matched analysis. Clin Infect Dis 2023; 76:453–460. [DOI] [PubMed] [Google Scholar]
- 15. Butler C, Hobbs FDR, Gbinigie O, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): preliminary analysis from the United Kingdom randomised, controlled open-label, platform adaptive trial (4 October 2022). https://ssrn.com/abstract=4237902. [Accessed 15 December 2022] [Google Scholar]
- 16.Johnson MG, Puenpatom A, Moncada PA, et al. Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: a randomized, placebo-controlled trial. Ann Intern Med 2022; 175:1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbel R, Wolff Sagy Y, Hoshen M, et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge. N Engl J Med 2022; 387:790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. health system: a population-based cohort study. Ann Intern Med 2023; 176:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of Paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis 2023; 76:e342–e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goswami H, Alsumali A, Jiang Y, et al. Cost-effectiveness analysis of molnupiravir versus best supportive care for the treatment of outpatient COVID-19 in adults in the US. PharmacoEconomics 2022; 40:699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2022; 386:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiseo G, Barbieri C, Galfo V, et al. Efficacy and safety of nirmatrelvir/ritonavir, molnupiravir, and remdesivir in a real-world cohort of outpatients with COVID-19 at high risk of progression: the PISA Outpatient Clinic Experience. Infect Dis Ther 2022; 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen CS. Assessing the proportion of the Danish population at risk of clinically significant drug-drug interactions with new oral antivirals for early treatment of COVID-19. Int J Infect Dis 2022; 122:599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charness ME, Gupta K, Stack G, et al. Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment. N Engl J Med 2022; 387:1045–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Berger NA, Davis PB, et al. COVID-19 rebound after paxlovid and molnupiravir during January-June 2022. medRxiv 2022; 2022.06.21.22276724. [Google Scholar]
- 26.Wong GL, Yip TC, Lai MS, et al. Incidence of viral rebound after treatment with nirmatrelvir-ritonavir and molnupiravir. JAMA Netw Open 2022; 5:e2245086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson AS, Caubel P, Rusnak JM. Nirmatrelvir-ritonavir and viral load rebound in Covid-19. N Engl J Med 2022; 387:1047–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epling BP, Rocco JM, Boswell KL, et al. Clinical, virologic, and immunologic evaluation of symptomatic Coronavirus disease 2019 rebound following nirmatrelvir/ritonavir treatment. Clin Infect Dis 2023; 76:573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai M, Ito M, Kiso M, et al. Efficacy of antiviral agents against Omicron subvariants BQ.1.1 and XBB. N Engl J Med 2023; 388:89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2022; 327:1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2022; 10:985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wynia MK, Beaty LE, Bennett TD, et al. Real-world evidence of neutralizing monoclonal antibodies for preventing hospitalization and mortality in COVID-19 outpatients. Chest 2022; S0012-3692(22)04033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dryden-Peterson S, Kim A, Joyce MR, et al. Bebtelovimab for high-risk outpatients with early COVID-19 in a large US Health System. Open Forum Infect Dis 2022; 9:ofac565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Razonable RR, O’Horo JC, Hanson SN, et al. Outcomes of bebtelovimab treatment is comparable to ritonavir-boosted nirmatrelvir among high-risk patients with Coronavirus disease-2019 during SARS-CoV-2 BA.2 Omicron Epoch. J Infect Dis 2022; 226:1683–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng B, Green ACA, Tazare J, et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform. BMJ (Clinical research ed) 2022; 379:e071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arribas JR, Bhagani S, Lobo SM, et al. Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19. NEJM Evid 2022; 1:EVIDoa2100044. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y, Shibata Y, Minemura H, et al. Real-world clinical outcomes of treatment with molnupiravir for patients with mild-to-moderate coronavirus disease 2019 during the Omicron variant pandemic. Clin Exp Med 2022; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong CKH, Au ICH, Lau KTK, et al. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis 2022; 22:1681–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19: final report. N Engl J Med 2020; 383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet (London, England) 2022; 399:1941–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diaz GA, Christensen AB, Pusch T, et al. Remdesivir and mortality in patients with Coronavirus disease 2019. Clin Infect Dis 2022; 74:1812–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olender SA, Walunas TL, Martinez E, et al. Remdesivir versus standard-of-care for severe Coronavirus Disease 2019 infection: an analysis of 28-day mortality. Open Forum Infect Dis 2021; 8:ofab27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chokkalingam AP, Hayden J, Goldman JD, et al. Association of remdesivir treatment with mortality among hospitalized adults with COVID-19 in the United States. JAMA Netw Open 2022; 5:e2244505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalized patients with Coronavirus Disease 2019 (COVID-19): a comparative analysis of in-hospital all-cause mortality in a large multicenter observational cohort. Clin Infect Dis 2022; 75:e450–e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flisiak R, Zarębska-Michaluk D, Rogalska M, et al. Real-world experience with molnupiravir during the period of SARS-CoV-2 Omicron variant dominance. Pharmacol Rep 2022; 74:1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪.van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. A guide to immunotherapy for COVID-19. Nat Med 2022; 28:39–50. [DOI] [PubMed] [Google Scholar]; This review describes in detail the supporting evidence and options of immunotherapy for treatment of severe and critical COVID-19 disease.
- 47.Bartoletti M, Azap O, Barac A, et al. ESCMID COVID-19 living guidelines: drug treatment and clinical management. Clin Microbiol Infect 2022; 28:222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Infectious Diseases Society of America 2022; Version 10.1.1. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. [Accessed 12 December 2022]. [Google Scholar]
- 49.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouadma L, Mekontso-Dessap A, Burdet C, et al. High-dose dexamethasone and oxygen support strategies in intensive care unit patients with severe COVID-19 acute hypoxemic respiratory failure: the COVIDICUS Randomized Clinical Trial. JAMA Intern Med 2022; 182:906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Granholm A, Munch MW, Myatra SN, et al. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a preplanned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med 2022; 48:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langer-Gould A, Xu S, Myers LC, et al. High-dose corticosteroids in patients hospitalized for COVID-19 pneumonia: an observational study of comparative effectiveness. Int J Infect Dis 2022; 125:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. [Accessed 12 December 2022]. [Google Scholar]
- 54.Vlaar APJ, Witzenrath M, van Paassen P, et al. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med 2022; 10:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puhach O, Meyer B, Eckerle I. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol 2023; 21:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dioverti V, Salto-Alejandre S, Haidar G. Immunocompromised patients with protracted COVID-19: a review of “Long Persisters”. Curr Transplant Rep 2022; 9:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trottier CA, Wong B, Kohli R, et al. Dual antiviral therapy for persistent COVID-19 and associated organizing pneumonia in an immunocompromised host. Clin Infect Dis 2023; 76:923–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown LK, Moran E, Goodman A, et al. Treatment of chronic or relapsing COVID-19 in immunodeficiency. J Allergy Clin Immunol 2022; 149:557–561. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez MA, Chen TY, Choi H, et al. Extended remdesivir infusion for persistent Coronavirus disease 2019 infection. Open Forum Infect Dis 2022; 9:ofac382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Umemura T, Mutoh Y, Mizuno T, et al. Safety evaluation of remdesivir for COVID-19 patients with eGFR < 30 mL/min without renal replacement therapy in a Japanese single-center study. Healthcare (Basel, Switzerland) 2022; 10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ackley TW, McManus D, Topal JE, et al. A valid warning or clinical lore: an evaluation of safety outcomes of remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother 2021; 65: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seethapathy R, Zhao S, Long JD, et al. A propensity score-matched observational study of remdesivir in patients with COVID-19 and severe kidney disease. Kidney360 2022; 3:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim JH, Park SD, Jeon Y, et al. Clinical effectiveness and safety of remdesivir in hemodialysis patients with COVID-19. Kidney Int Rep 2022; 7:2522–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gutierrez R, Mendez-Figueroa H, Biebighauser JG, et al. Remdesivir use in pregnancy during the SARS-CoV-2 pandemic. J Matern Fetal Neonatal Med 2022; 35:9445–9451. [DOI] [PubMed] [Google Scholar]
- 65.Eid J, Abdelwahab M, Colburn N, et al. Early administration of remdesivir and intensive care unit admission in hospitalized pregnant individuals with Coronavirus disease 2019 (COVID-19). Obstet Gynecol 2022; 139:619–621. [DOI] [PubMed] [Google Scholar]
- 66.Garneau WM, Jones-Beatty K, Ufua MO, et al. Analysis of clinical outcomes of pregnant patients treated with nirmatrelvir and ritonavir for acute SARS-CoV-2 infection. JAMA Netw Open 2022; 5:e2244141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valsecchi C, Winterton D, Safaee Fakhr B, et al. High-dose inhaled nitric oxide for the treatment of spontaneously breathing pregnant patients with severe Coronavirus disease 2019 (COVID-19) pneumonia. Obstet Gynecol 2022; 140:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yip TCF, Lui GCY, Lai MSM, et al. Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients. Clin Infect Dis 2023; 76:e26–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ganatra S, Dani SS, Ahmad J, et al. Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with Covid-19. Clin Infect Dis 2023; 76:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]