OBJECTIVES:
We performed a systemic review and meta‐analysis to evaluate the diagnostic accuracy of monocyte distribution width (MDW) and to compare with procalcitonin and C‐reactive protein (CRP), in adult patients with sepsis.
DATA SOURCES:
A systematic literature search was performed in PubMed, Embase, and the Cochrane Library to identify all relevant diagnostic accuracy studies published before October 1, 2022.
STUDY SELECTION:
Original articles reporting the diagnostic accuracy of MDW for sepsis detection with the Sepsis-2 or Sepsis-3 criteria were included.
DATA EXTRACTION:
Study data were abstracted by two independent reviewers using a standardized data extraction form.
DATA SYNTHESIS:
Eighteen studies were included in the meta‐analysis. The pooled sensitivity and specificity of MDW were 84% (95% CI [79–88%]) and 68% (95% CI [60–75%]). The estimated diagnostic odds ratio and the area under the summary receiver operating characteristic curve (SROC) were 11.11 (95% CI [7.36–16.77]) and 0.85 (95% CI [0.81–0.89]). Significant heterogeneity was observed among the included studies. Eight studies compared the diagnostic accuracies of MDW and procalcitonin, and five studies compared the diagnostic accuracies of MDW and CRP. For MDW versus procalcitonin, the area under the SROC was similar (0.88, CI = 0.84–0.93 vs 0.82, CI = 0.76–0.88). For MDW versus CRP, the area under the SROC was similar (0.88, CI = 0.83–0.93 vs 0.86, CI = 0.78–0.95).
CONCLUSIONS:
The results of the meta-analysis indicate that MDW is a reliable diagnostic biomarker for sepsis as procalcitonin and CRP. Further studies investigating the combination of MDW and other biomarkers are advisable to increase the accuracy in sepsis detection.
Keywords: biomarker, meta-analysis, monocyte distribution width, sepsis
KEY POINTS.
Question: To evaluate the diagnostic accuracy of monocyte distribution width (MDW) and compare with procalcitonin and C‐reactive protein (CRP) in adult patients with sepsis.
Findings: In this meta-analysis, the pooled sensitivity of MDW was significantly higher than that of procalcitonin, whereas the pooled specificity of MDW was significantly lower than that of procalcitonin. The diagnostic accuracy of MDW was comparable with that of procalcitonin and CRP.
Meaning: The results of the meta-analysis indicate that MDW is a reliable diagnostic biomarker for sepsis as procalcitonin and CRP. Further studies investigating the combination of MDW and other biomarkers are advisable to increase the accuracy in sepsis detection.
Sepsis is defined as a dysregulated host response to infection leading to organ dysfunction (1). It is associated with a high risk of mortality and is a major global public health problem with millions of septic patients treated annually. With a mortality rate ranging from 20% to 50%, epidemiologic studies showed that over 20% of the septic patients required mechanical ventilation, which imposes high costs on the healthcare systems. Despite the development in the clinical guidelines for sepsis treatment, the early identification of patients at high risk for sepsis remains a significant challenge. The early diagnosis of sepsis is complicated by its overlapping symptoms with other diseases and the lack of reliable and readily available ancillary tests for sepsis determination (2). Therefore, the need for new and accurate biomarkers for the timely diagnosis and management of sepsis is urgent.
Monocyte distribution width (MDW), a novel biomarker for sepsis detection, is a measure of the dispersion around the mean of monocyte population volume, which is estimated with the volume, conductivity, and scatter technology (3). In the early phase of sepsis, MDW manifests with changes in its functional and morphologic properties—resulting in a highly heterogeneous population—in response to proinflammatory signals from pathogen-associated molecular patterns (4). Recent research has shown good diagnostic performance of MDW in sepsis identification (5). Most of the studies that compared the performance of biomarkers in detecting sepsis found higher accuracy in MDW than others. Therefore, we herein systematically examined the diagnostic accuracy of MDW and compared it with those of procalcitonin and C-reactive protein (CRP).
MATERIALS AND METHODS
The aim of the present study was to compile the summary estimates of the diagnostic performance (i.e., sensitivity and specificity) of MDW in detecting sepsis among existing studies and compared them with those of procalcitonin and CRP if relevant data were provided. Following the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines, the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, and the current guidelines for diagnostic accuracy reviews (6–8), two investigators (C.C.-Y., Y.-H.H.) systematically and independently reviewed the relevant published literatures to select the eligible studies, extract the data, and judge the methodological quality from the included studies. The discrepancies between the two investigators were resolved by consulting with another senior investigator (S.C.-S.). The study protocol was registered with the International prospective register of systematic reviews (CRD42017075964).
Data Sources and Searches
A systematic literature search was performed in PubMed, Embase, and the Cochrane Library to identify all relevant diagnostic accuracy studies published before October 1, 2022. We used the following keywords: (monocyte distribution width OR MDW) AND (sepsis OR “bacterial infection” OR “systemic inflammatory response syndrome” OR SIRS) in Pubmed and the Cochrane Library, and (“monocyte distribution width”/exp OR “monocyte distribution width” OR [“monocyte”/exp OR monocyte] AND [“distribution”/exp OR distribution] AND [“width”/exp OR width] OR mdw) AND (“sepsis”/exp OR sepsis OR “bacterial infection”/exp OR “bacterial infection” OR “systemic inflammatory response syndrome” OR sirs) in Embase. The list of references of each primary study was also examined to identify additional potential studies.
Study Selection
The studies included in the current research were selected with the following process. First, all abstracts and full-text articles of retrospective and prospective studies from the search output were identified. Based on the title and abstract, we excluded case reports, case series, animal studies, pediatric studies, and studies with repeated human subjects—for the studies that examined the same database, the one with the largest sample size was selected. Afterwards, full-text reviews were conducted, and studies that 1) involved adult patients with suspected infection; 2) were conducted in the emergency department (ED), hospital wards, or the ICU; and 3) used MDW for the detection of sepsis with the Sepsis-2 and Sepsis-3 criteria were further selected. All eligible studies contained sufficient information for the construction of the 2 × 2 table of true-positive, false-positive, true-negative, and false-negative counts—either extracted from the original article or calculated with the reported sensitivity and specificity. Therefore, we did not contact the corresponding authors for relevant information. Two investigators (C.-C.Y., Y.-H.H.) completed the search and the screening of all the studies independently. Interinvestigator discrepancies were resolved by reviewing the inclusion criteria and consulting a third investigator (S.-C.S.).
Data Extraction and Quality Assessment
The research data extraction was independently performed by two investigators (C.-C.Y., Y.-H.H.). The following information from the included studies were collected: the first author, year of publication, study design, eligibility criteria, setting (ED, general ward, or ICU), sample size, mean or median age, cutoffs of MDW, procalcitonin and CRP, type of anticoagulant, and diagnostic criteria (Sepsis-2 or Sepsis-3). Each investigator also recorded the values of true-positive, false-positive, true-negative, and false-negative and the sensitivity and specificity values of MDW, along with procalcitonin and CRP when available. The risk of bias for each of the included studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool (9), which has been developed for the quality evaluation of diagnostic test accuracy studies using four domains of bias and applicability—patient selection, index test, reference standard, and flow and timing. Discrepancies were resolved by reviewing the rating criteria and consulting a third investigator (S.-C.S.).
Data Synthesis and Analysis
For each study, a 2 × 2 contingency table was constructed to calculate the sensitivity and specificity and the corresponding 95% CIs. The sensitivity and specificity estimates of individual studies were displayed on forest plots and the receiver operating characteristic (ROC) space for the visual examination of heterogeneity. To pool the results, a hierarchical summary ROC (HSROC) model was used to calculate the summary points of various accuracy variables (sensitivity, specificity, positive and negative likelihood ratios, diagnostic odds ratio, and their associated confidence bounds) (10). The estimates of test accuracy were plotted on the ROC space, and the summary points were presented on the summary ROC curve (SROC) with confidence regions (11, 12). Threshold effect is considered a major contributor to heterogeneity in diagnostic test accuracy reviews (13). To assess the threshold effect, Spearman’ s correlation coefficient (r, of which ≥ 0.6 indicates a threshold effect) was calculated as the included studies used different cutoff values of MDW for the diagnosis of sepsis. The nonthreshold-related heterogeneity was calculated with the chi-square test, Cochran’s Q test, and the I2. Heterogeneity among the studies was considered to be present if Cochran’s Q p value is less than 0.1 or when I2 is greater than 50%. We further performed predefined subgroup analyses to examine the heterogeneity among studies stratified by the setting (ICU or ED patient population), number of patients (≥ 650 or < 650), diagnostic criteria (Sepsis-2 or Sepsis-3 criteria), study design (retrospective or prospective), region (Asia or non-Asia), prevalence (≥ 15% or < 15%), anticoagulant (K2-EDTA or K3-EDTA), and cutoff value (≥ 20.3 or < 20.3 U). Publication bias was assessed with the Deek’s effective sample size funnel plot and the log diagnostic odds ratio, and a regression test of asymmetry was performed. The statistical and meta-analyses were conducted using R Version 4.1.2 (R Foundation of Statistical Computing, Vienna, Austria).
RESULTS
Search Results
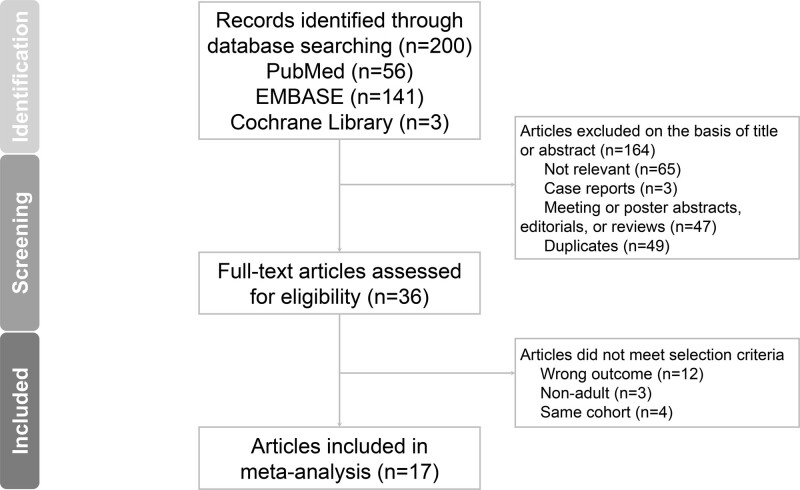
Our database search returned 200 articles. After the initial screening, 164 articles were excluded. Thirty-six articles were subjected to further full-text reviews, and 19 were excluded, leaving 17 for inclusion (Fig. 1) (4, 14–29). Because a study by Crouser et al (28) contained one derivation cohort and one validation cohort, we analyzed these cohorts separately and, therefore, included 18 studies in our analysis. The search in the reference lists of the identified articles did not return any more eligible study.
Figure 1.
Flow chart of study identification, screening, inclusion, and exclusion for meta‑analysis.
Study Characteristics
eTable 1 (http://links.lww.com/CCM/H301) presents the characteristics of the included studies. All eligible studies were published between 2017 and 2022, the median sample size is 626 (interquartile range: 332–1,440), and the final analysis included a total of 22,459 patients. For the geographic area, nine (50%) were conducted in Europe (4, 15, 16, 23–26), five (27.8%) were conducted in Asia (14, 18, 19, 22, 27), and four (22.2%) were conducted in the United States (17, 28, 29). For the study design, 12 studies (66.7%) were prospective cohort studies (15, 17–22, 25, 27–29), five (27.8%) were retrospective cohort studies (4, 14, 22, 24, 26), and one did not report the study design (16). No case-control studies or randomized controlled trials were included. Among all 22,459 patients, 2,124 patients (9.5%) were in the sepsis group (including Sepsis-2 and Sepsis-3 definition), and 20,335 (90.5%) were in the control group. These patients comprised of 21,482 ED patients (95.6%), 717 ICU patients (3.2%), and 260 ward patients (1.2%). The percentage of patients with sepsis ranged from 2.0% to 57.4% among the studies. The MDW cutoff value for detecting sepsis ranged from 19.19 to 24.63 U, with a median value of 20.3 U.
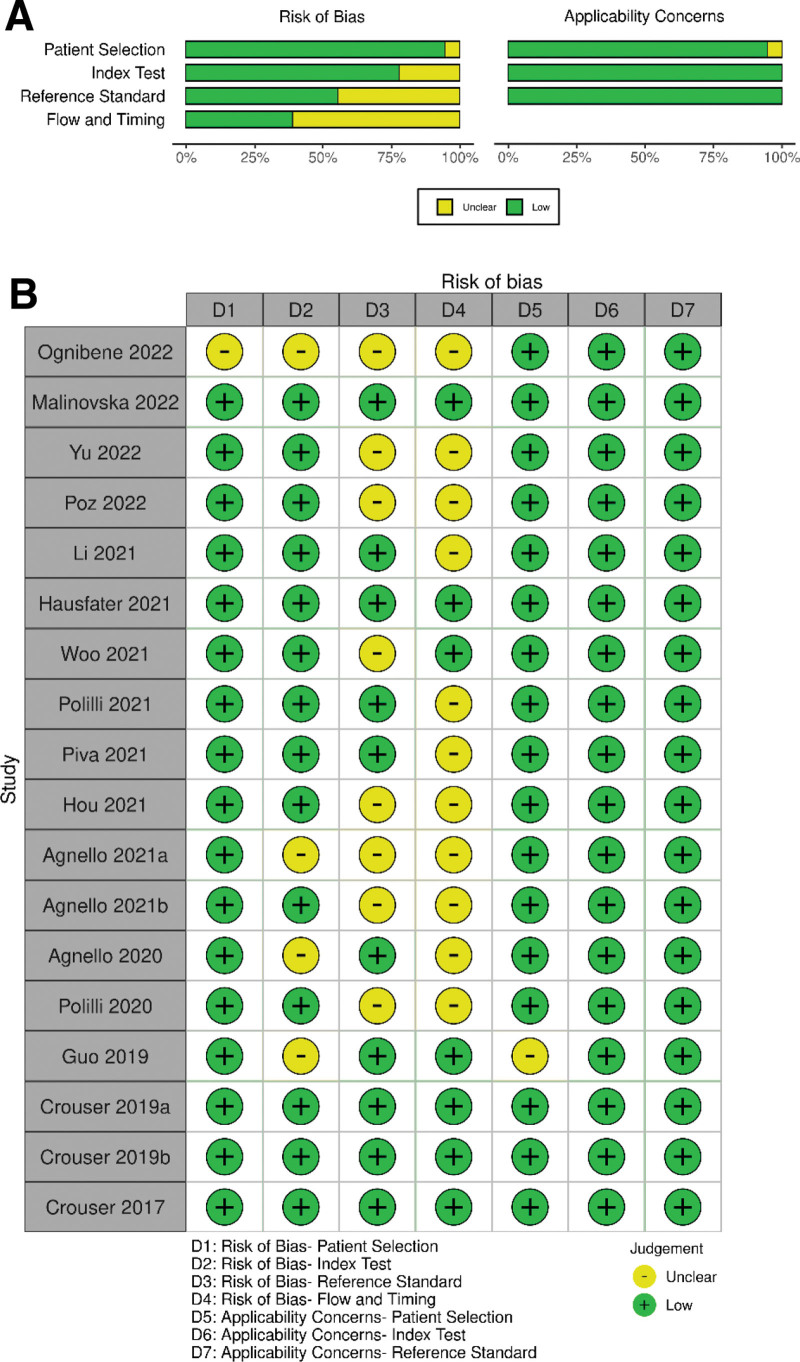
Quality Assessment
Quality assessments using the QUADAS-2 criteria are summarized in Figure 2. In the patient selection domain, one study (5.6%) had an unclear risk of bias due to its insufficient description of enrollment design (16). In the index test domain, four studies (22.2%) had an unclear risk of bias because they calculated sensitivity and specificity using the optimal cutoff value other than the predefined value (4, 16, 24, 27). In the reference standard domain, eight studies (44.4%) had an unclear risk of bias because whether their reference standard results were interpreted with the results of the index test was not explicitly stated (4, 14–16, 19, 22, 24, 25). For the flow and timing domain, the risk of bias was unclear in 11 studies (61.1%) since the interval between the index test and the reference standard was not clearly defined (4, 14–16, 18, 20–22, 24–26). As for the applicability, one study had an unclear applicability in the patient selection domain as it enrolled only patients with liver cirrhosis (27), whereas all other studies were at a low risk.
Figure 2.
Quality assessment for 18 studies (Quality Assessment of Diagnostic Accuracy Studies [QUADAS]–2).
Primary Analysis of Overall Accuracy
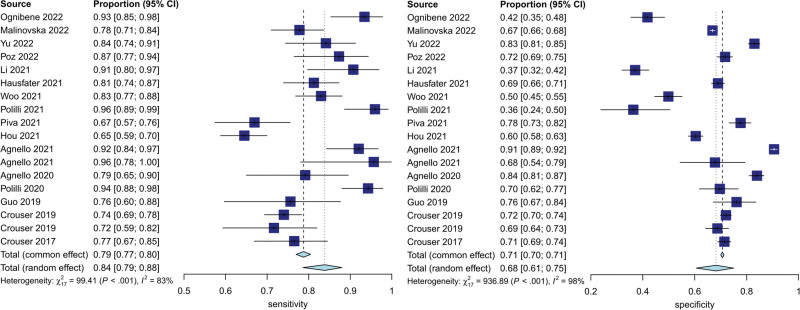
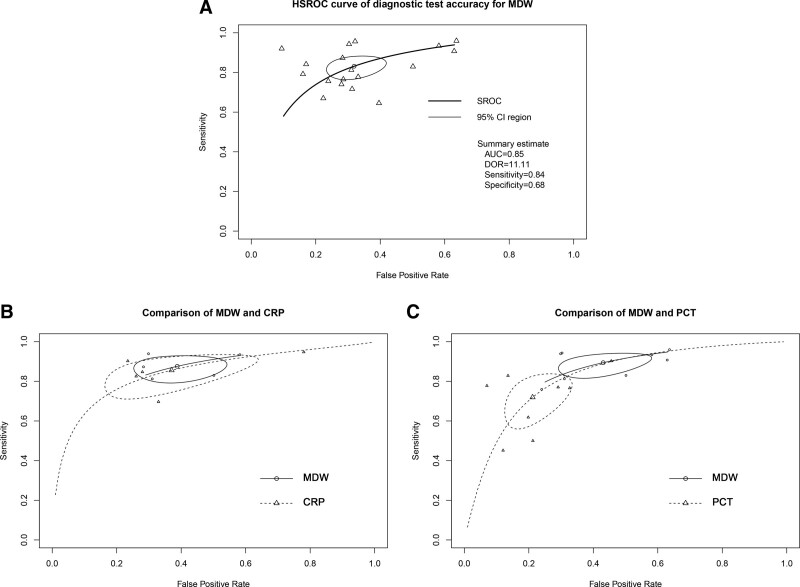
Figure 3 shows the forest plots for the sensitivity and specificity of MDW reported in the 18 included studies. The pooled sensitivity across all studies was 84% (95% CI [79–88%]), and the pooled specificity was 68% (95% CI [60–75%]). The estimated diagnostic odds ratio was 11.11 (95% CI [7.36–16.77]). The pooled estimates of positive and negative likelihood ratios were 2.63 (95% CI [2.11–3.28]) and 0.24 (CI [0.18–0.31]), respectively. The HSROC curves, together with the bivariate summary points of specificity and sensitivity and their 95% confidence regions, are shown in Figure 4. The area under the SROC curve was 0.85 (95% CI [0.81–0.89]).
Figure 3.
Forest plots of the sensitivity and specificity for monocyte distribution width across all included studies.
Figure 4.
A, Hierarchical summary receiver operating characteristic plot of monocyte distribution width (MDW) across all included studies. B, Comparisons of MDW and procalcitonin (PCT) with summary receiver operating characteristic (ROC) curves. C, Comparisons of MDW and C‑reactive protein (CRP) with summary ROC (SROC) curves. AUC = area under the curve, DOR = diagnostic odds ratio, HSROC = hierarchical summary ROC.
Heterogeneity Analyses
We assessed the nonthreshold effect by evaluating the heterogeneity in the studies’ sensitivity (Cochran’s Q test = 127.55; p < 0.01 and the I2 = 82.9%) and specificity (Cochran’s Q test = 1,130.84; p < 0.01 and the I2 = 98.2%). The results indicated the existence of significant heterogeneity among studies. The Spearman’s correlation coefficient (r) was 0.33 (95% CI [–0.16 to 0.69]), indicating that the threshold effect did not contribute to the heterogeneity among the studies.
Subgroup and Sensitivity Analysis
We performed subgroup analyses to analyze sources of heterogeneity (eTable 2, http://links.lww.com/CCM/H301). Studies with more than 650 patients had a significantly higher pooled specificity (76%, CI = 69–82% vs 59%, CI = 48–70%; p = 0.01) than those with fewer than 650 patients. Studies with the diagnostic criteria of Sepsis-2 had a significantly higher pooled specificity (76%, CI = 67%–83% vs 63%, CI = 53–72%; p = 0.046) than those with the Sepsis-3 criteria. Studies with K3-EDTA had a significantly higher pooled sensitivity (91%, CI = 84–95% vs 81%, CI = 75–86%; p = 0.02) than those with K2-EDTA. Studies with MDW cutoff value greater than or equal to 20.3 U had a significantly higher pooled specificity (76%, CI = 69–82% vs 59%, CI = 48–69%; p < 0.01) than those with cutoff value less than 20.3 U. The pooled sensitivity and specificity were not significantly different among the setting (ICU or ED patient population), study design (retrospective or perspective), region (Asia or non-Asia), and prevalence (≥ 15% or < 15%). A substantial degree of heterogeneity existed among each subgroup. The sensitivity analysis of MDW showed that the area under the SROC curve did not significantly differ with the removal of any study.
Comparison of the Performances of Procalcitonin and CRP
Eight of the 18 studies directly compared the diagnostic accuracies of MDW and procalcitonin (eTable 3, http://links.lww.com/CCM/H301). The pooled sensitivity and specificity values for MDW were 90% (95% CI [84–94%]) and 57% (95% CI [46–68%]) and those for procalcitonin were 72% (95% CI [60–82%]) and 79% (95% CI [70–86%]). The pooled sensitivity of MDW was significantly higher than that of procalcitonin (p < 0.01), whereas the pooled specificity of MDW was significantly lower than that of procalcitonin (p < 0.01). The diagnostic accuracy of MDW was similar to that of procalcitonin as measured by the area under the SROC curve (0.88, CI = 0.84–0.93 vs 0.82, CI = 0.76–0.88). Five of the 16 studies directly compared the diagnostic accuracies of MDW and CRP. The pooled sensitivity and specificity values for MDW were 88% (95% CI [82–92%]) and 61% (95% CI [50–71%]) and those for CRP were 86% (95% CI [76–92%]) and 63% (95% CI [44–79%]). The pooled sensitivity and specificity of MDW and CRP were not significantly different (p = 0.61; p = 0.66). The diagnostic accuracy of MDW was similar to that of CRP as measured by the area under the SROC curve (0.88, CI = 0.83–0.93 vs 0.86, CI = 0.78–0.95). The comparisons of the HSROC curves of MDW versus procalcitonin and CRP are presented in Figure 4.
Publication Bias
For MDW, the 18 studies showed no significant publication bias (p = 0.22) (eFig. 1, http://links.lww.com/CCM/H301). The studies associated with the comparison between MDW versus procalcitonin (p = 0.58) and MDW versus CRP (p = 0.28) also showed no significant publication bias.
DISCUSSION
Despite the widely recognized importance of the early identification and treatment of sepsis, the diagnosis of sepsis remains challenging to physicians. The systemic inflammatory response syndrome (SIRS) criteria were first introduced in 1991 and have been widely adopted in clinical practice since (30). Nevertheless, the low specificity of the SIRS criteria undermines their reliability since several noninfectious conditions, such as acute coronary syndrome, pulmonary embolism, acute pancreatitis, and even exercise, could be accompanied with SIRS (31). In 2016, the Sequential Organ Failure Assessment (SOFA) score was included as a component of the Sepsis-3 definitions. However, multiple laboratory blood tests are required to calculate the score and increase the complexity in clinical practice (32). The quick SOFA (qSOFA) score, a triage tool for suspected sepsis, was introduced because of its simplicity and high specificity for early sepsis detection (32). Nevertheless, the qSOFA score exhibits poor sensitivity in identifying sepsis and predicting in-hospital mortality, which impedes its effectiveness in guiding early sepsis treatment (33). The Sepsis-3 task force recognized that qSOFA excels in specificity rather than sensitivity, and it should not be used as a “rule-out” screening tool (1). In the present meta-analysis, MDW, with a pooled sensitivity of 84%, pooled specificity of 68%, and an area under the SROC curve of 0.85, was found to have a high diagnostic value for sepsis in adult patients. Due to its high sensitivity, MDW could serve as a screening tool to exclude the possibility of sepsis in suspected patients. One existing meta-analysis suggested that MDW was a reliable biomarker for sepsis screening and exhibited heterogeneity secondary to the nonthreshold effect (34); however, the study did not further examine the source of heterogeneity or perform subgroup analyses due to its small sample size of 10 studies. The strength of our meta-analysis is that in comparison with the previous review, we included eight additional studies from the United States, Italy, China, Korea, and Taiwan, which endow our findings with greater generalizability across different geographic regions. Furthermore, our meta-analysis is the first to compare the diagnostic accuracy of MDW with procalcitonin and CRP, and our results inform the determination of the appropriate biomarker for sepsis. Finally, our findings are relatively more reliable as we assessed the risk of bias by using the QUADAS-2 tools and examined potential heterogeneity among the included studies by performing several subgroup and sensitivity analyses.
Among a variety of inflammation markers, the diagnostic accuracies of procalcitonin and CRP in bacterial infection and sepsis have been most frequently studied (35). For the diagnostic accuracy in sepsis, a meta-analysis from Tan et al (36) found that the overall area under the SROC curve and the pooled specificity of procalcitonin were higher than those of CRP (0.85 vs 0.73; 0.77 vs 0.61). Our analyses indicated that the overall area under the SROC curve of MDW is similar to that of procalcitonin (0.88 vs 0.82) and CRP (0.88 vs 0.86). These results suggest that the overall diagnostic performance of MDW is comparable with that of procalcitonin and CRP. Although the pooled sensitivity of MDW is significantly higher than that of procalcitonin, the pooled specificity is significantly lower than that of procalcitonin. The possible reason is that the specificity of a biomarker can be influenced by the pretest probability of a positive result. In contrast to procalcitonin, which is ordered for patients who are highly suspected of sepsis, MDW is available for all patients. Therefore, the pretest probability of sepsis is likely lower for MDW compared with procalcitonin, which could possibly explain the lower specificity.
To identify the sources of the heterogeneity among studies, we conducted subgroup analyses and found the diagnostic criteria, anticoagulant, cutoff value, and sample size to be the contributors of the observed heterogeneity. MDW in the Sepsis-3 group has a significantly lower specificity and a nonsignificant but higher sensitivity than in the Sepsis-2 group. A validation study conducted by Freund et al (37) indicated that patients identified with the Sepsis-3 criteria had a higher in-hospital mortality rate, along with a higher specificity but a lower sensitivity, than those identified with the Sepsis-2 criteria. MDW is not only a diagnostic indicator of early sepsis but also a good predictor for disease progression and sepsis prognosis (20). Given the higher proportion of critically ill patients in the Sepsis-3 group, MDW has a higher sensitivity with a lower associated specificity. We also found that the sensitivity of MDW in blood samples in the K2 EDTA tubes is significantly lower than that of samples in the K3 EDTA tubes. Furthermore, the specificity of MDW is significantly higher in the cutoff value greater than or equal to 20.3 U. As the manufacturer described, the values in K2 EDTA anticoagulant would be lower than those in K3 EDTA anticoagulant. If inappropriate cutoffs of MDW are used for the anticoagulant, the numbers of false-positive and false-negative may increase. The heterogeneous study characteristics and different anticoagulant samples limit the ability for our study to identify a universal cutoff. Further research is required to determine the optimal cutoffs for different anticoagulants.
In clinical practice, a biomarker with a high sensitivity could be efficiently used for disease exclusion. MDW has a high sensitivity and a moderate specificity for sepsis detection (34), rendering it reliable for the early identification of sepsis. For frontline physicians, MDW can be measured routinely in blood cell counts with differential through a hematologic analyzer during the initial patient encounter, and it is relatively fast, low-cost, and easily available (38). The potential disadvantage of using MDW as a biomarker lies in its specificity. Studies have shown that the combination of MDW and other biomarkers could improve the diagnostic performance in specificity under a similar sensitivity (16, 17, 22, 23, 28, 29). More evidence is required to determine the optimal combination of biomarkers for sepsis diagnosis.
Our systematic review and meta-analysis should be considered in the context of several limitations. First, although we performed subgroup and sensitivity analyses to identify the sources of heterogeneity, some sources remained unidentified. Second, the suggested cutoffs of MDW by the manufacturer are different in the K2 EDTA tubes and the K3 EDTA tubes. However, several studies did not use the predefined cutoff values to evaluate the diagnostic accuracy of MDW for sepsis. Further investigation is required to determine the optimal cutoff values for different anticoagulants. Third, the number of studies comparing MDW with procalcitonin or CRP is small. More studies are necessary to further increase the credibility of our findings. Finally, the studies used different criteria for the determination of “patients with suspected infection,” and resultantly, the findings of our study may not be applicable in all clinical settings.
CONCLUSIONS
In summary, our systematic review and meta-analysis demonstrated that MDW is an effective and convenient diagnostic biomarker for sepsis that is comparable with procalcitonin and CRP. Given the high sensitivity of MDW, it can serve as a promising tool for sepsis screening. Nevertheless, these results should be interpreted cautiously due to the considerable between-study heterogeneity. Furthermore, to improve the precision of sepsis detection, future studies examining the combination of MDW and other biomarkers are advisable.
Supplementary Material
Footnotes
Drs. Huang and Chen contributed equally to this work.
Drs. Huang, Chen, and Yen contributed to conceptualization. Drs. Li and Hsiao contributed to data curation. Drs. Yen and Niu contributed to formal analysis. Drs. Huang, Shao, and Yen contributed to quality assessment. Drs. Yen contributed to supervision. Drs. Huang, Jung, and Yen contributed to writing the original draft. All authors have read and agreed to the published version of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. Yen disclosed work for hire. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C-C, Lan H-M, Han S-T, et al. : Comparison of diagnostic accuracy in sepsis between presepsin, procalcitonin, and C-reactive protein: A systematic review and meta-analysis. Ann Intensive Care 2017; 7:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Iribarren A, Tejedor X, Sala Sanjaume A, et al. : Performance evaluation of the new hematology analyzer UniCel DxH 900. Int J Lab Hematol 2021; 43:623–631 [DOI] [PubMed] [Google Scholar]
- 4.Agnello L, Iacona A, Maestri S, et al. : Independent validation of sepsis index for sepsis screening in the emergency department. Diagnostics (Basel) 2021; 11:1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J, Li L, Luo J: Diagnostic and prognostic value of monocyte distribution width in sepsis. Journal of Inflammation Research 2022, 15:4107–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D, Shamseer L, Clarke M, et al. : Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macaskill P, Gatsonis C, Deeks J, et al. : Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 1.0 Edition. London, The Cochrane Collaboration, 2010 [Google Scholar]
- 8.Leeflang MM, Deeks JJ, Gatsonis C, et al. : Systematic reviews of diagnostic test accuracy. Ann Intern Med 2008; 149:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiting PF, Rutjes AW, Westwood ME, et al. : QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–536 [DOI] [PubMed] [Google Scholar]
- 10.Rutter CM, Gatsonis CA: A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 2001; 20:2865–2884 [DOI] [PubMed] [Google Scholar]
- 11.Reitsma JB, Glas AS, Rutjes AW, et al. : Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005; 58:982–990 [DOI] [PubMed] [Google Scholar]
- 12.Moses LE, Shapiro D, Littenberg B: Combining independent studies of a diagnostic test into a summary ROC curve: Data-analytic approaches and some additional considerations. Stat Med 1993; 12:1293–1316 [DOI] [PubMed] [Google Scholar]
- 13.Dinnes J, Deeks J, Kirby J, et al. : A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol Assess 2005; 9:1–113 [DOI] [PubMed] [Google Scholar]
- 14.Yu S, Song SA, Jun KR, et al. : Clinical performance of monocyte distribution width for early detection of sepsis in emergency department patients: A prospective study. Ann Lab Med 2022; 42:286–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poz D, Crobu D, Sukhacheva E, et al. : Monocyte distribution width (MDW): A useful biomarker to improve sepsis management in emergency department. Clin Chem Lab Med 2022; 60:433–440 [DOI] [PubMed] [Google Scholar]
- 16.Ognibene A, Lorubbio M, Montemerani S, et al. : Monocyte distribution width and the fighting action to neutralize sepsis (FANS) score for sepsis prediction in emergency department. Clin Chim Acta 2022; 534:65–70 [DOI] [PubMed] [Google Scholar]
- 17.Malinovska A, Hinson JS, Badaki-Makun O, et al. : Monocyte distribution width as part of a broad pragmatic sepsis screen in the emergency department. J Am Coll Emerg Physicians Open 2022; 3:e12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li CH, Seak CJ, Chaou CH, et al. : Comparison of the diagnostic accuracy of monocyte distribution width and procalcitonin in sepsis cases in the emergency department: A prospective cohort study. BMC Infect Dis 2022; 22:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo A, Oh DK, Park CJ, et al. : Monocyte distribution width compared with C-reactive protein and procalcitonin for early sepsis detection in the emergency department. PLoS One 2021; 16:e0250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polilli E, Frattari A, Esposito JE, et al. : Monocyte distribution width (MDW) as a new tool for the prediction of sepsis in critically ill patients: a preliminary investigation in an intensive care unit. BMC Emerg Med 2021; 21:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piva E, Zuin J, Pelloso M, et al. : Monocyte distribution width (MDW) parameter as a sepsis indicator in intensive care units. Clin Chem Lab Med 2021; 59:1307–1314 [DOI] [PubMed] [Google Scholar]
- 22.Hou SK, Lin HA, Chen SC, et al. : Monocyte distribution width, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio improves early prediction for sepsis at the emergency. J Pers Med 2021; 11:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hausfater P, Robert Boter N, Morales Indiano C, et al. : Monocyte distribution width (MDW) performance as an early sepsis indicator in the emergency department: comparison with CRP and procalcitonin in a multicenter international European prospective study. Crit Care 2021; 25:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agnello L, Lo Sasso B, Vidali M, et al. : Validation of monocyte distribution width decisional cutoff for sepsis detection in the acute setting. Int J Lab Hematol 2021; 43:O183–o185 [DOI] [PubMed] [Google Scholar]
- 25.Polilli E, Sozio F, Frattari A, et al. : Comparison of monocyte distribution width (MDW) and procalcitonin for early recognition of sepsis. PLoS One 2020; 15:e0227300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agnello L, Bivona G, Vidali M, et al. : Monocyte distribution width (MDW) as a screening tool for sepsis in the emergency department. Clin Chem Lab Med 2020; 58:1951–1957 [DOI] [PubMed] [Google Scholar]
- 27.Guo F, Feng YC, Zhao G, et al. : The leukocyte VCS parameters compared with procalcitonin, interleukin-6, and soluble hemoglobin scavenger receptor sCD163 for prediction of sepsis in patients with cirrhosis. Dis Markers 2019; 2019:1369798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crouser ED, Parrillo JE, Seymour CW, et al. : Monocyte distribution width: a novel indicator of sepsis-2 and sepsis-3 in high-risk emergency department patients. Crit Care Med 2019; 47:1018–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crouser ED, Parrillo JE, Seymour C, et al. : Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. Chest 2017; 152:518–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bone RC, Balk RA, Cerra FB, et al. : Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 31.Churpek MM, Zadravecz FJ, Winslow C, et al. : Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med 2015; 192:958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhodes A, Evans LE, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43:304–377 [DOI] [PubMed] [Google Scholar]
- 33.Fernando SM, Tran A, Taljaard M, et al. : Prognostic accuracy of the quick sequential organ failure assessment for mortality in patients with suspected infection: A systematic review and meta-analysis. Ann Intern Med 2018; 168:266–275 [DOI] [PubMed] [Google Scholar]
- 34.Agnello L, Vidali M, Lo Sasso B, et al. : Monocyte distribution width (MDW) as a screening tool for early detecting sepsis: A systematic review and meta-analysis. Clin Chem Lab Med 2022; 60:786–792 [DOI] [PubMed] [Google Scholar]
- 35.Kibe S, Adams K, Barlow G: Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother 2011; 66:ii33–ii40 [DOI] [PubMed] [Google Scholar]
- 36.Tan M, Lu Y, Jiang H, et al. : The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J Cell Biochem 2019; 120:5852–5859 [DOI] [PubMed] [Google Scholar]
- 37.Freund Y, Lemachatti N, Krastinova E, et al. : Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA 2017; 317:301. [DOI] [PubMed] [Google Scholar]
- 38.Laínez Martínez S, González Del Castillo J: Usefulness of monocyte distribution width (MDW) as a sepsis biomarker. Revista Española de Quimioterapia 2022; 35 Suppl 1(Suppl 1):2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.