ABSTRACT
Introduction
High-fructose diet (HFr) causes metabolic syndrome, and HFr-induced hypertension and renal damage are exaggerated in Dahl salt-sensitive (DS) rats. Exercise training (Ex) has antihypertensive and renal protective effects in rats fed HFr; however, there has been little discussion about the DS rats, which exhibit metabolic disturbances. This study thus examined the effects of Ex on DS rats fed HFr.
Methods
Male DS rats were divided into three groups. The control group was fed a control diet, and both the HFr group and the HFr–Ex group were fed an HFr (60% fructose). The HFr–Ex group also underwent treadmill running (20 m·min−1, 60 min·d−1, 5 d·wk−1). After 12 wk, renal function, histology, and renin–angiotensin system were examined.
Results
HFr increased blood pressure, urinary albumin, and creatinine clearance, and Ex inhibited these increases. HFr induced glomerular sclerosis, podocyte injury, afferent arteriole thickening, and renal interstitial fibrosis, and Ex ameliorated them. HFr reduced plasma renin activity, and Ex further reduced the activity. HFr also increased the expression of angiotensinogen, renin, angiotensin-converting enzyme (ACE), and angiotensin II type 1 receptor, and Ex restored the ACE expression to the control levels. HFr decreased the expression of ACE2, angiotensin II type 2 receptor, and Mas receptor, and Ex restored the ACE2 and Mas receptor expressions to the control levels and further decreased the angiotensin II type 2 receptor expression. HFr increased the ACE activity and decreased the ACE2 activity, and Ex restored these activities to the control levels.
Conclusions
Ex prevents HFr-induced hypertension and renal damages in DS rats. The changes in renal renin–angiotensin system may be involved in the mechanism of the antihypertensive and renal protective effects of Ex.
Key Words: HIGH-FRUCTOSE DIET, EXERCISE TRAINING, DAHL SALT-SENSITIVE RATS, RENAL DAMAGE, RENIN–ANGIOTENSIN SYSTEM
Dietary fructose consumption has increased significantly over the past decades and has led to an epidemic of metabolic syndrome, which increases the risk of hypertension and chronic kidney disease (CKD) (1–3). High-fructose diet (HFr) induces insulin resistance, hyperinsulinemia, hypertriglyceridemia, and hyperuricemia and elevated blood pressure in human and rodents (4–8). Most symptoms can be attenuated in Sprague-Dawley rats if exercise training is performed (9,10). However, the precise mechanisms of the hypertensive effects of fructose and the antihypertensive effects of exercises remain unknown. Previous research has shown a pathogenic role of the renin–angiotensin system (RAS) in the HFr-induced hypertension. It was observed that HFr increased plasma renin activity, urinary angiotensin II levels, and the renal expression of renin, angiotensin II, and angiotensin II type 1 receptor (AT1R) in Sprague-Dawley rats (7,11). RAS blockade by angiotensin-converting enzyme (ACE) inhibitors or AT1R blockers attenuate the HFr-induced elevation in blood pressure (12–15).
HFr causes renal hypertrophy, afferent arteriolar thickening, cortical vasoconstriction, glomerular hypertension, and tubulointerstitial injury in Sprague-Dawley rats (16,17). Moreover, using a genetic model of hypertension and kidney disease, Dahl salt-sensitive (DS) rats exhibited further exaggeration in the HFr-induced hypertension and renal damage than Sprague-Dawley rats. We recently reported that HFr elevates blood pressure in DS rats, but not in Dahl salt-resistant (DR) rats, and HFr-induced urinary albumin excretion, glomerular injury, and renal interstitial fibrosis are exaggerated in DS rats (18). HFr also increases the renal expression of AGT, renin, ACE, and AT1R. RAS inhibitors attenuate the HFr-induced hypertension and renal damage in DS rats (18).
Exercise has renal protection effects in rats with kidney disease (10,19–22). Exercise with treadmill running attenuates the progression of hypertension, renal dysfunction, glomerular sclerosis, and renal interstitial fibrosis in rats with 5/6 nephrectomy (21). Progression of glomerular sclerosis and renal interstitial fibrosis has no impact on blood pressure in rats with diabetic nephropathy (19). Exercise training also inhibits the progression of hypertension, albuminuria, glomerular sclerosis, and renal interstitial fibrosis in Sprague-Dawley rats fed HFr (10). However, exercise does not affect blood pressure in high salt-fed DS rats (20,22), whereas it slows the progress of renal dysfunction and glomerular sclerosis (20,22). We hypothesized that exercise training could inhibit the progression of HFr-induced hypertension and renal damage in DS rats. To test this hypothesis, the present study investigated the effects of exercise on HFr-induced hypertension, renal damage, and renal RAS in DS rats.
METHODS
Animals and diets
All animal experiments were approved by Tohoku University Committee for Animal Experiments and were performed in accordance with the Guidelines for Animal Experiments and Related Activities of Tohoku University, and the guiding principles of the Physiological Society of Japan and the US National Institutes of Health.
Five-week-old, male DS rats (Japan SLC Inc, Shizuoka, Japan) were housed in an animal care facility at Tohoku University Graduate School of Medicine at a controlled temperature, under a 12-h light/12-h dark cycle. All rats had free access to standard rat chow and tap water. After 1 wk of acclimation, their diet was changed to a control diet (Con) (TD.05075; Envigo Teklad Diets, Madison, WI) or a 60% HFr (TD.89247; Envigo Teklad Diets), which induced metabolic syndromes as previously reported (10,23). Previous study (24) showed that HFr diet (60% fructose) did not induced hypertension or hyperinsulinemia in female rats; thus, we only investigate male DS rats in this study.
To examine the effects of exercise training (Ex) in HFr-fed DS rats, DS rats were randomized into three groups: Con group, HFr group, and HFr and Ex group (HFr–Ex) (n = 8 in each group). The rats of the three groups were maintained on the control or HFr diet, and the HFr–Ex group underwent treadmill running (KN-73 Tread-Mill; Natsume Industries Co., Tokyo, Japan) at an aerobic intensity for 12 wk as previously described (10,19,20,22,25,26). Initially, the animals were started at 10 m·min−1, 0°, 10 min·d−1, after which the treadmill speed was increased from 10 to 20 m·min−1 progressively and to 60 min·d−1 in a week. After acclimation, the rats could undergo treadmill running for the remaining 11 wk, which was maintained at an aerobic intensity (20 m·min−1, 0°, 60 min·d−1, 5 d·wk−1). The sample size calculations are provided by G Power 3.1. With an α = 0.05 and power = 0.80, the projected total sample size needed with this effect size is approximately N = 21. Thus, our proposed sample size of N = 24 will be more than adequate for the main objective of this study.
Blood pressure measurement and plasma and urinary parameter
Every 2 wk, the body weight and systolic blood pressure (SBP) of the rats were measured. SBP was measured using the tail cuff method (MK-2000A; Muromachi Kikai, Tokyo, Japan). After an 8-h fasting, the plasma from tail vein was obtained to measure the glucose and insulin concentration at the 11th week of the experiments. The insulin resistance status was measured using the homeostasis model assessment for insulin resistance (HOMA-IR) (10,19). On the last day of the experiments, all rats were housed in an individual metabolic cage, and urine samples were collected on ice for 24 h. Urinary albumin and creatinine were measured (Oriental Yeast Co., Ltd., Nagahama, Japan). The urinary liver type-fatty acid binding protein (L-FABP) (27), a biomarker of proximal tubular stress and tubulointerstitial disorder and has a correlation with urine albumin excretion in diabetic kidney disease and CKD patients, was also measured as previously described (18). It can help predict the progression of diabetic kidney disease on renal damage at very early stage.
At the end of the experiments, all rats were anesthetized with sodium pentobarbital (50 mg·kg−1, i.p.) and blood samples were collected by decapitation. Blood samples were then centrifuged for 15 min at 1500g, and the supernatant was collected and stored at −80°C. Triglyceride, total cholesterol, free fatty acid, glucose, urea nitrogen, creatinine, and renin activity were measured (SRL Inc., Tokyo, Japan). Plasma fructose levels were measured by an enzyme-based chromogenic assay (EFRU-100; Bioassay Systems, Hayward, CA). Uric acid was measured using a colorimetric kit (Wako c-test; FUJIFILM Wako Pure Chemical Co., Osaka, Japan). Total angiotensinogen (AGT) concentration in plasma and urine was determined using a rat AGT ELISA kit (Immuno-Biological Laboratories, Fujioka, Japan) as previously described (28,29). All the assays were run in duplicate.
Tissue sample preparation
After sacrifice, the kidneys of the rats were quickly dissected. The renal cortex was homogenized in a buffer containing 0.1 mol·L−1 KH2PO4, 0.4 mol·L−1 Na2HPO4, 10 mmol·L−1 EDTA, and 10 mmol·L−1 dl-dithiothreitol. In addition, 0.1 mmol·L−1 phenylmethylsulfonyl fluoride was added before the homogenization. The homogenate was snap-frozen in liquid nitrogen and stored at −80°C until further analysis. The protein concentration of the samples was measured using the Bradford method, with bovine γ-globulin as standard (No. 500–0005; Bio-Rad Laboratories, Hercules, CA).
Histological analysis
The kidney was quickly removed after decapitation, was fixed in 10% formalin for over 24 h, and were then embedded in paraffin. Paraffin sections (3 μm thick) were cut and stained with Periodic acid–Schiff (PAS) and Masson’s trichrome staining to determine the index of glomerular sclerosis (IGS) and relative interstitial volume (RIV), respectively. The IGS and RIV were assessed in accordance with a previously described method (25). For histological analysis, five to six rats per group were prepared and 50 glomeruli in each rat were assessed for IGS. The percentages of interstitial space of the renal cortex and outer medulla except the glomerulus, blood vessels, and tubules per unit area were calculated; the values of RIV from 10 areas in each rat were measured, and the mean values were calculated. Quantifications were performed in a blinded manner.
Immunohistochemistry
Immunohistochemical analyses of desmin and α-smooth muscle actin (α-SMA) were performed to determine the severity of podocyte injury and afferent arteriolar thickening, respectively, as described previously (30,31). The deparaffinized tissues were rehydrated in graded ethanol and rinsed in phosphate-buffered saline, then the sections were treated with 0.3% hydrogen peroxide in absolute ethanol for 5 min, followed by processing for immunostaining with antibodies targeting desmin (Abcam, Cambridge, United Kingdom) and α-SMA (Dako, Glostrup, Denmark). Thirty glomeruli in each rat were assessed for desmin immunostaining, and the ratio of the desmin-expressing area to that of the whole glomerulus was determined using ImageJ (version 2.0.0; NIH, Bethesda, MD). For each arteriole, the outline of the vessel and its internal lumen (excluding the endothelium) was traced using computer analysis to calculate the total medial area (outline–inline) in 5–10 arterioles per slice. The media-to-lumen ratio was calculated using the outline–inline relationship (31). Quantifications were performed in a blinded manner.
Immunoblot analysis
Protein expression was examined using immunoblot analysis, as described previously (19). Renal cortical homogenates (20–30 μg) were loaded onto a sodium dodecyl sulfate polyacrylamide gels and separated by electrophoresis. Then the proteins were transferred electrophoretically to nitrocellulose membranes at 100 V in transfer buffer for 1 h. The membrane was blocked by immersion into a Tween-20 (TBS-T) and then incubated with primary antibodies raised against AGT (Immuno-Biological Laboratories, Fujioka, Japan); renin, ACE, ACE2, AT1R, and angiotensin II type 2 receptor (AT2R; Santa Cruz Biotechnology, Dallas, TX); Mas receptor (MasR; Alomone Labs, Jerusalem, Israel); and β-actin (Sigma-Aldrich, St. Louis, MO). Membranes were rinsed with TBST-20 and incubated at room temperature for 1 h with a horseradish peroxidase–conjugated antirabbit or antimouse IgG secondary antibody (Santa Cruz Biotechnology). After washing in TBST-20 for several times, immunoblots were developed using an enhanced chemiluminescent substrate (Super Signal; Thermo Fisher Scientific, Waltham, MA). The relative intensity of band was quantified using ImageJ. The intensity of band was normalized against β-actin, which was used as an internal standard; the intensity of the band in the Con group was assigned a value of 1.
Measurement of ACE and ACE2 activities
ACE and ACE2 activities were measured using a fluorometric assay kit (Abcam) as previously described (18).
Statistical analysis
The data are presented as mean ± SE. The data were analyzed using two-way repeated-measures ANOVA or one-way ANOVA, followed by a Tukey test for multiple comparisons among the groups. P < 0.05 was considered to indicate statistical significance.
RESULTS
Body weight and blood pressure
Figure 1 shows the time course of body weight and SBP. HFr did not affect body weight, whereas Ex significantly lowered body weight after the fourth week (P < 0.01; Fig. 1A). HFr significantly increased the SBP after the fourth week (P < 0.01). Ex significantly decreased the HFr-increased SBP after the sixth week (P < 0.01), but the SBP was still higher in the HFr–Ex group than in the Con group (P < 0.01; Fig. 1B; Supplemental Tables S1 and S2, Supplemental Digital Content, Effects of exercise on SBP and on body weight, http://links.lww.com/MSS/C762.)
FIGURE 1.
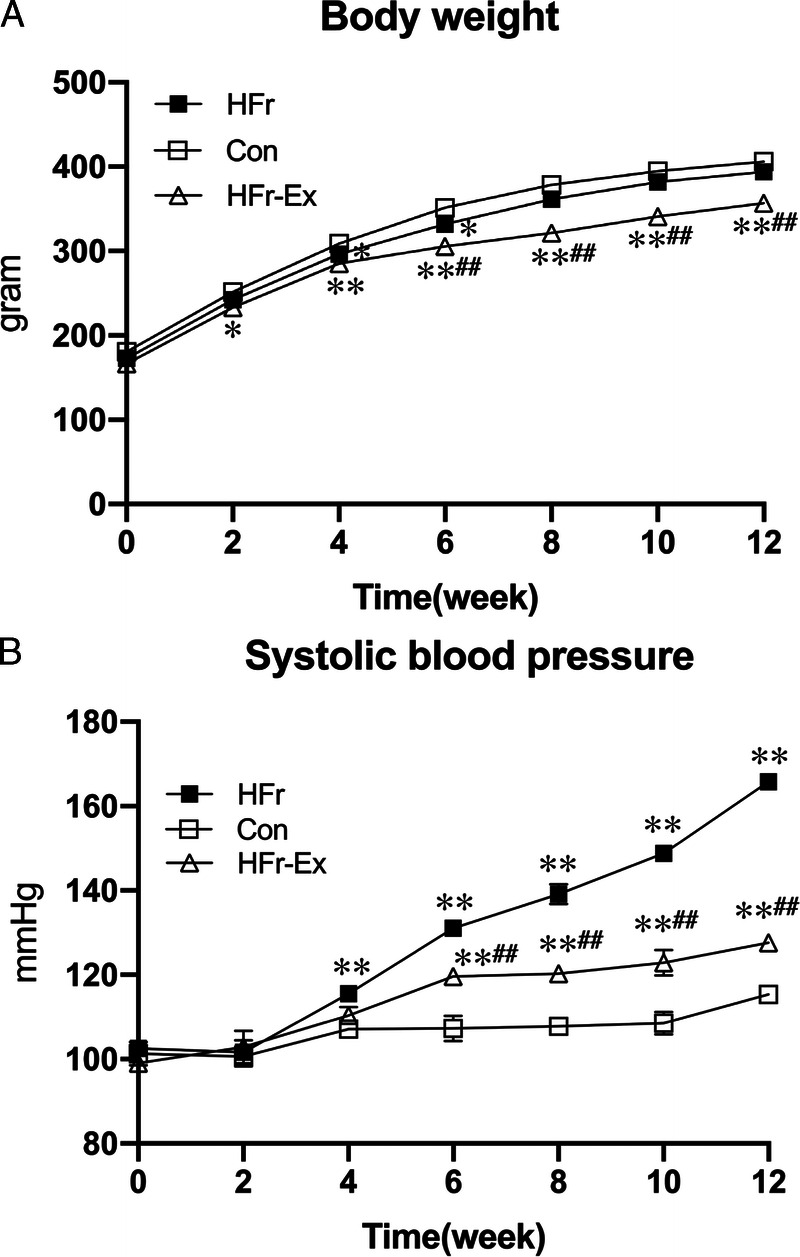
Blood pressure and body weight in DS rats. DS rats were divided into three groups and fed a control diet (Con) or a high-fructose diet (HFr; 60% fructose). A part of HFr-fed DS rats underwent exercise training (Ex) with treadmill running at an aerobic intensity for 12 wk. A, The time course of SBP in the Con (open squares), HFr (closed squares), and HFr and Ex (HFr–Ex; open triangles) groups. B, The time course of body weight in the Con (open squares), HFr (closed squares), and HFr–Ex (open triangles) groups. Data are presented as means ± SE for n = 8 rats per group. *P < 0.05, **P < 0.01 compared with the Con group; #P < 0.05, ##P < 0.01 compared with the HFr group.
Plasma parameters
Table 1 shows plasma parameters at the end of the experiment. HFr significantly increased HOMA-IR, triglyceride, total cholesterol, free fatty acid, glucose, fructose, and uric acid, and Ex significantly deceased the HFr-increased plasma parameters except triglyceride and fructose to the control levels. The levels of triglyceride were still higher in the HFr–Ex group than in the Con group, whereas the levels of fructose did not differ between the HFr group and the HFr–Ex group. HFr significantly decreased plasma renin activity, which was further suppressed by Ex.
TABLE 1.
Effects of exercise on plasma parameters in high fructose-fed DS rats.
| Con | HFr | HFr–Ex | P | |||
|---|---|---|---|---|---|---|
| Con vs HFr | HFr vs HFr–Ex | Con vs HFr–Ex | ||||
| HOMA-IR | 0.53 ± 0.07 | 0.67 ± 0.10 | 0.51 ± 0.04 | 0.02 | <0.01 | 0.51 |
| Triglyceride (mg·dL−1) | 263 ± 45 | 988 ± 155 | 606 ± 61 | <0.01 | <0.01 | <0.01 |
| Total cholesterol (mg·dL−1) | 75 ± 2 | 106 ± 7 | 80 ± 9 | <0.01 | <0.01 | 0.23 |
| Free fatty acid (mEq·L−1) | 189 ± 50 | 394 ± 98 | 203 ± 36 | <0.01 | <0.01 | 0.55 |
| Glucose (mg·dL−1) | 145 ± 18 | 194 ± 22 | 142 ± 5 | <0.01 | <0.01 | 0.68 |
| Fructose (mg·dL−1) | 3.68 ± 1.57 | 20.93 ± 5.03 | 19.38 ± 4.31 | <0.01 | 0.53 | <0.01 |
| Uric acid (mg·dL−1) | 1.67 ± 0.12 | 2.04 ± 0.13 | 1.68 ± 0.17 | <0.01 | <0.01 | 0.83 |
| Renin activity (ng·mL−1·h−1) | 6.1 ± 1.9 | 4.1 ± 0.8 | 2.2 ± 0.5 | 0.03 | <0.01 | <0.01 |
Data are presented as means ± SD.
P < 0.05 compared with the Con group.
P < 0.01 compared with the Con group.
P < 0.05 compared with the HFr group.
P < 0.01 compared with the HFr group.
Renal functions
HFr significantly decreased plasma creatinine (P < 0.01), and Ex significantly increased the HFr-decreased plasma creatinine to the control levels (P < 0.01; Fig. 2A). HFr significantly increased creatinine clearance (P < 0.05), and Ex significantly decreased the HFr-increased creatinine clearance to the control levels (P < 0.01; Fig. 2B). HFr significantly increased the urinary albumin excretion (P < 0.01), and Ex significantly decreased the HFr-increased excretion to the control levels (P < 0.01; Fig. 2C). HFr significantly increased the urinary L-FABP excretion (P < 0.01), and Ex significantly decreased the HFr-increased excretion to the control levels (P < 0.01; Fig. 2D).
FIGURE 2.
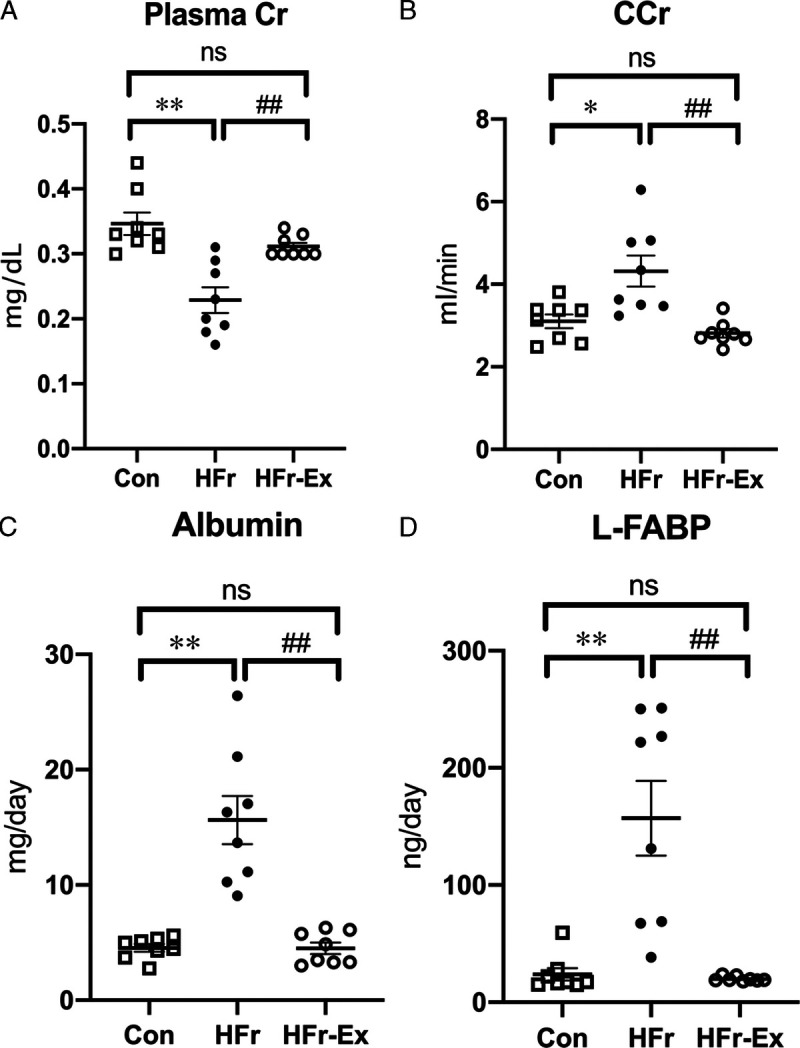
Renal functions in DS rats. Plasma creatinine (A), creatinine clearance (B), urinary albumin excretion (C), and urinary L-FABP excretion (D) were compared among the Con, HFr, and HFr–Ex groups. Data are presented as means ± SE for n = 8 rats per group. *P < 0.05, **P < 0.01 compared with the Con group; #P < 0.05, ##P < 0.01 compared with the HFr group.
Renal histology
Figure 3A shows representative photomicrographs of the glomeruli stained with PAS, against desmin, against α-SMA, and with Masson’s trichrome. HFr induced focal glomerular sclerosis, podocyte injury, afferent arteriole thickening, and renal interstitial fibrosis, which were ameliorated by Ex. HFr significantly increased IGS and desmin positive area (P < 0.01 and P < 0.01), whereas Ex significantly decreased them (P < 0.01 and P < 0.01; Figs. 3B, C). The IGS in the HFr–Ex group was still higher than that in the control levels (Fig. 3B), but Ex decreased the desmin positive area to the control levels (P < 0.05; Fig. 3C). HFr also significantly increased the media-to-lumen ratio of afferent arterioles and RIV (P < 0.01 and P < 0.01), and Ex significantly lowered them (P < 0.01 and P < 0.01; Figs. 3D, E). The media-to-lumen ratio in the HFr–Ex group was still higher than the control levels (P < 0.05; Fig. 3D), and Ex decreased the RIV to the control levels (Fig. 3E).
FIGURE 3.
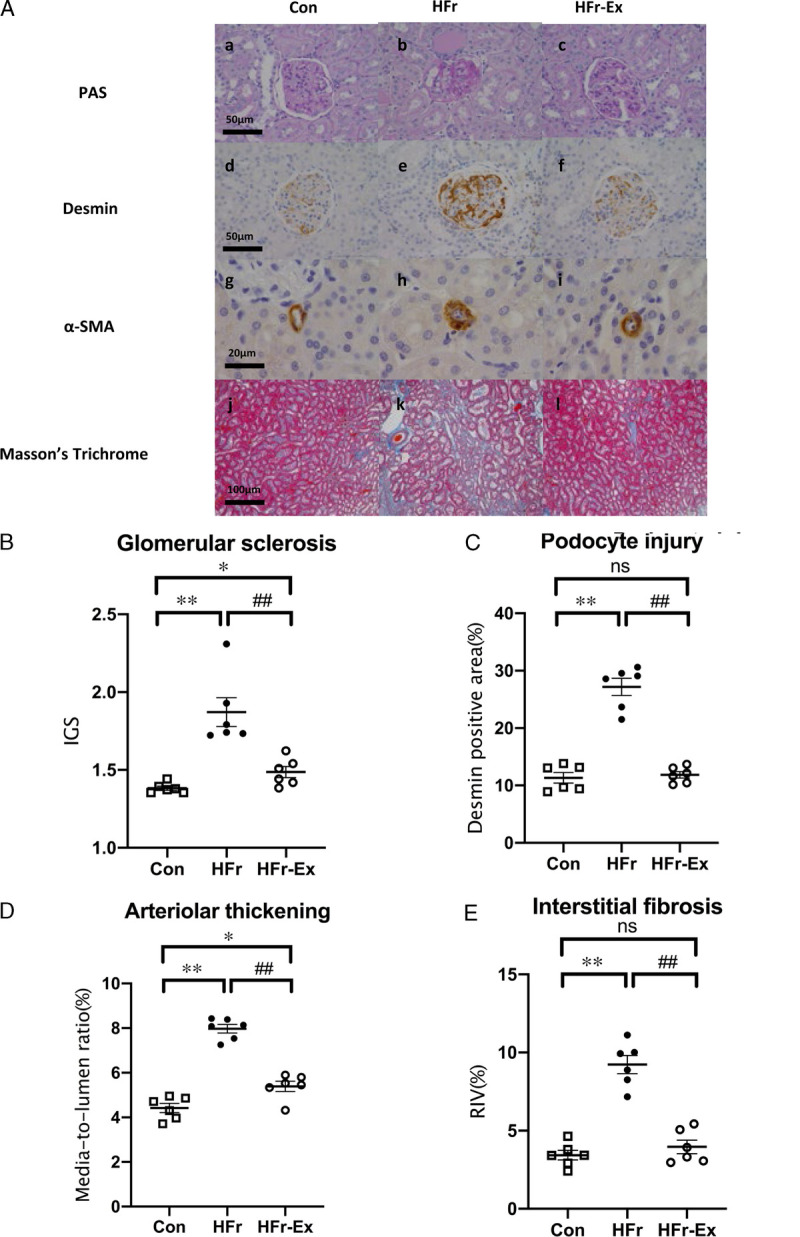
Renal histological characteristics in DS rats. A, Representative images of the glomerulus and the renal cortex stained with PAS (top panel), against desmin (second panel), against α-SMA (third panel), and with Masson trichrome (bottom panel) in the Con, HFr, and HFr–Ex groups. IGS (B), glomerular desmin-positive area (C), arteriolar media-to-lumen ratio (D), and RIV (E) were compared among the Con, HFr, and HFr–Ex groups. Data are presented as means ± SEM for n = 6 rats per group. *P < 0.05, **P < 0.01 compared with the Con group; #P < 0.05, ##P < 0.01 compared with the HFr group.
Renal expression of RAS components and activity of ACE and ACE2
HFr or Ex did not affect the plasma AGT concentration (Fig. 4A). However, HFr significantly increased the urinary AGT excretion (P < 0.01), and Ex significantly decreased the HFr-increased AGT excretion (P < 0.01) to levels lower than the control levels (P < 0.01; Fig. 4B). HFr significantly increased the renal AGT expression (P < 0.05), and Ex did not affect the HFr-increased AGT expression (Fig. 4C). HFr significantly increased the renal renin expression (P < 0.01), and Ex did not affect the HFr-increased renin expression (Fig. 4D).
FIGURE 4.
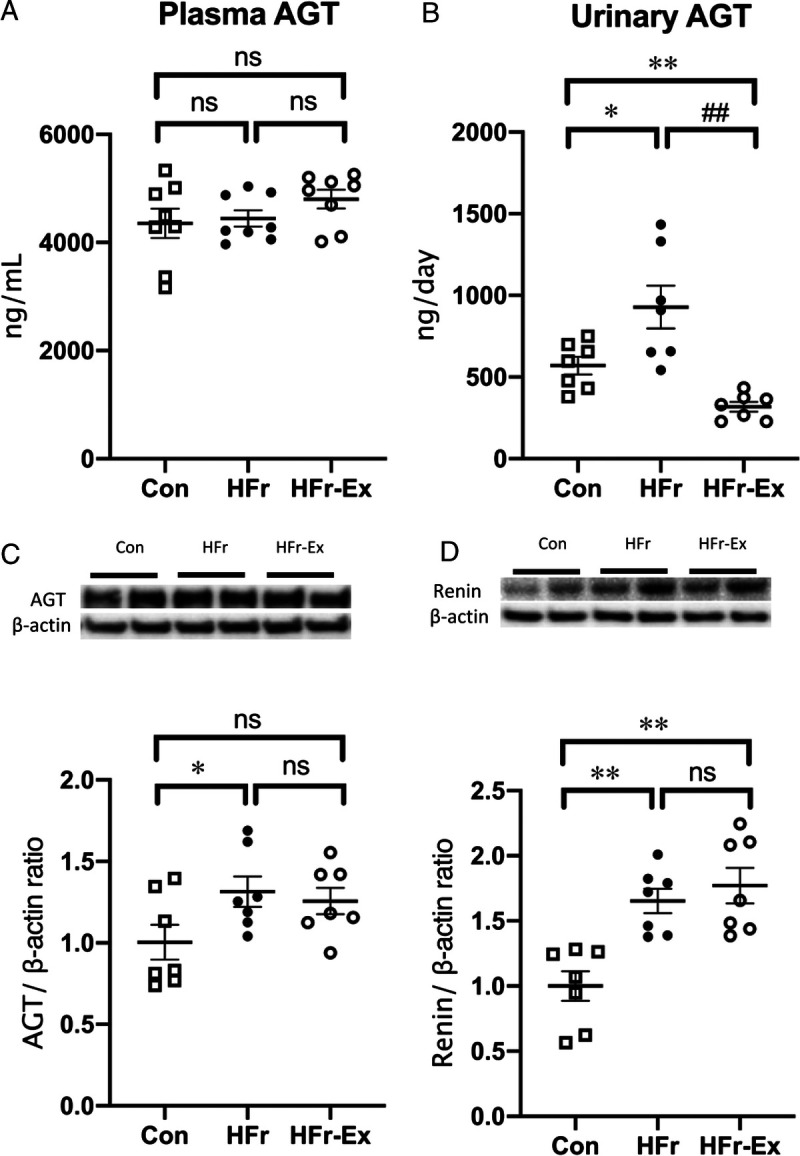
Levels of AGT in plasma and urine and expression of AGT and renin in the renal cortex of DS rats. The levels of plasma AGT (A) and urinary AGT (B) were compared among the Con, HFr, and HFr–Ex groups. The renal expression of AGT (C) and renin (D) were compared among the Con, HFr, and HFr–Ex groups. Top panels depict representative immunoblots from the different groups. The intensities of each specific protein band were normalized to that of β-actin, and the mean intensities of the values for the Con group. Data are presented as means ± SEM for n = 8 rats per group. *P < 0.05, **P < 0.01 compared with the Con group; #P < 0.05, ##P < 0.01 compared with the HFr group.
HFr significantly increased the renal ACE expression and activity (P < 0.01 and P < 0.01), and Ex significantly decreased the HFr-increased ACE expression and activity to the control levels (P < 0.01 and P < 0.01; Figs. 5A, C). HFr also significantly decreased the renal ACE2 expression and activity (P < 0.05 and P < 0.01), and Ex significantly increased the HFr-decreased ACE2 expression and activity to the control levels (P < 0.05 and P < 0.01; Figs. 5B, D).
FIGURE 5.
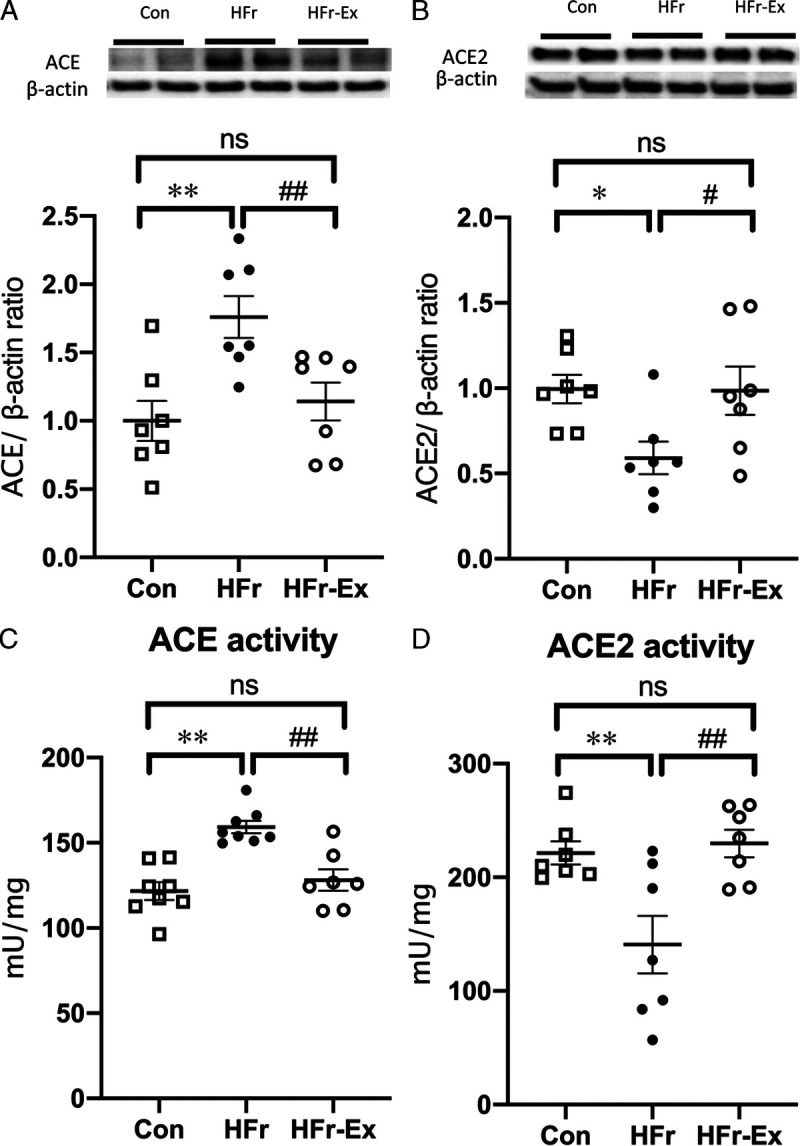
Expression and activity of ACE and ACE2 in the renal cortex of DS rats. The renal expression of ACE (A) and ACE2 (B) were compared among the Con, HFr, and HFr–Ex groups. Top panels depict representative immunoblots from the different groups. The intensities of each specific protein band were normalized to that of β-actin, and the mean intensities of the values for the Con group. The renal activity of ACE (C) and ACE2 (D)were compared among the Con, HFr, and HFr–Ex groups. Data are presented as means ± SEM for n = 8 rats per group. *P < 0.05, **P < 0.01 compared with the Con group; #P < 0.05, ##P < 0.01 compared with the HFr group.
HFr significantly increased the renal AT1R expression (P < 0.01), and Ex did not affect the HFr-increased AT1R expression (Fig. 6A). HFr significantly decreased the renal AT2R expression (P < 0.05), which was further decreased by Ex (P < 0.05). HFr significantly decreased the renal MasR expression (P < 0.05), and Ex significantly increased the HFr-decreased MasR expression to the control levels (P < 0.05; Figs. 6B, C).
FIGURE 6.
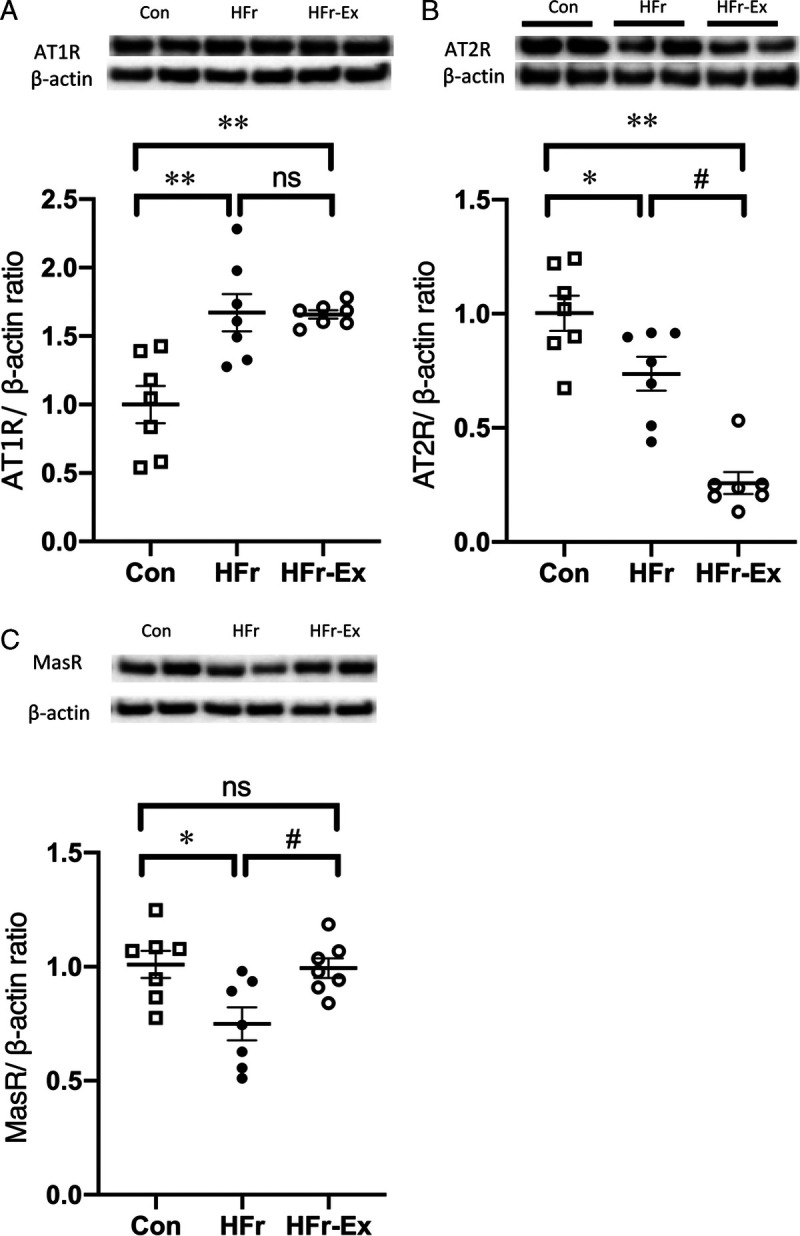
Expression of angiotensin receptors in the renal cortex of DS rats. The renal expression of AT1R (A), AT2R (B), and MasR (C) was compared among the Con, HFr, and HFr–Ex groups. Top panels depict representative immunoblots from the different groups. The intensities of each specific protein band were normalized to that of β-actin, and the mean intensities of the values for the Con group. Data are presented as means ± SEM for n = 8 rats per group. *P < 0.05, **P < 0.01 compared with the Con group; #P < 0.05, ##P < 0.01 compared with the HFr group.
DISCUSSION
The present study examined the effects of Ex on the HFr-induced hypertension, renal damage, and renal RAS components in DS rats. Ex restored degraded metabolic parameters of HFr plasma, other than triglyceride and fructose, to control levels. Ex also inhibited the progression of HFr-induced hypertension, albuminuria, and glomerular hyperfiltration. Moreover, Ex has strongly attenuated the HFr-induced renal damage, including glomerular sclerosis, podocyte injury, afferent arterial thickening, and renal interstitial fibrosis with changes in renal RAS components. To our knowledge, the present study is the first to report the great efficacy of Ex against HFr-induced hypertension and renal damage in DS rats.
Several studies have reported antihypertensive effects of Ex in HFr-fed rats. Voluntary wheel exercise for 2 wk attenuated HFr-induced blood pressure elevation and insulin resistance in Sprague-Dawley rats (9). Ex with treadmill for 12 wk attenuated the HFr-induced elevated blood pressure, insulin resistance, hypertriglyceridemia, and hyperuricemia in Sprague-Dawley rats (10). In this study, Ex strongly inhibited the progression of hypertension caused by HFr in DS rats. In contrast with the results obtained in HFr-fed DS rats, the antihypertensive effects of Ex are controversial in high salt fed DS rats. Voluntary wheel exercise was reported to have no effect on blood pressure in the high salt-fed DS rats (32). Ex with treadmill for 12 wk did not affect SBP in female DS rats fed high salt (33). However, Ex with treadmill for 8 wk reduced blood pressure in male DS rats who received high salt (34,35). In addition, Ex with treadmill for 8 wk attenuated the high salt-induced glomerular sclerosis in DS rats, although it did not affect blood pressure (20,22). An antihypertensive treatment with hydralazine, which lowered blood pressure to the control levels, did not ameliorate renal dysfunction and damage in the HFr-fed DS rats (18). These results suggest that the renal protective effects of Ex are not mediated through its antihypertensive effects in DS rats.
The ACE2–Ang-(1–7)–MasR axis exerts roles in antihypertrophic, antiproliferative, antifibrotic, vasoconstrictive, and renal protective effects, which are opposite to those elicited by the ACE–Ang II–AT1R axis (36). Previous studies reported that the renal expression of RAS components changed in CKD models (18,21,22). 5/6 nephrectomy was found to increase the AGT, ACE, and AT1R expressions and decreased the renin, ACE2, AT2R, and MasR expressions (21). In DS rats, high salt increased the AGT and ACE expressions in the renal cortex and the AGT and AT1R expressions in the outer medulla (22). high salt also decreased the renin, ACE2, and AT2R expressions in the renal cortex and the renin, ACE2, AT2R, and MasR expressions in the outer medulla (22). We also recently reported that the HFr-induced changes in renal expression of RAS components differ between DR rats and DS rats (18). In DR rats, HFr increased the ACE and AT2R expressions and decreased the renin and AT1R expressions. In DS rats, HFr increased the AGT, renin, ACE, and AT1R expressions and decreased the AT2R expression (18). The present study further revealed that HFr decreases the ACE2 and MasR expression in DS rats.
Ex restored the HFr-increased ACE expression and the HFr-decreased ACE2 and MasR expressions in the kidney of DS rats. Previous studies reported that Ex with treadmill for 8 wk restored the HFr-increased ACE expression and the HFr-decreased ACE2 and MasR expressions in the liver of Wistar rats (37), and that high-intensity interval training with treadmill for 12 wk restored the HFr-increased ACE and AT2R mRNA expressions and the HFr-decreased ACE2 and MasR mRNA expressions in the left ventricle of mice (38). However, there are few reports that examined the effects of Ex on the renal RAS. Ex with treadmill for 16 wk decreased the ACE and AT1R expressions and increased ACE2 and MasR expressions in the kidney of spontaneously hypertensive rats (39). Ex with treadmill for 8 wk restored the increased ACE expression and the decreased ACE2 and MasR expressions in the kidney of DS rats fed high salt (22). Ex with treadmill for 12 wk restored the increased AGT and ACE expression in rats with 5/6 nephrectomy (21). These results suggest that Ex can decrease the ACE expression and increase the ACE2 and MasR expressions in hypertensive and CKD model rats.
In contrast to the effects of Ex on renal RAS components, the ACE inhibitor, enalapril, and the AT1R blocker candesartan decreased the renal expression of all RAS components including renin, ACE, ACE2, AT1R, AT2R, and MasR in HFr-fed DS rats (18). A systematic review has also indicated that the treatment with RAS inhibitors does not change ACE2 levels in different animal models (40). These results suggest that RAS inhibitors suppress both the ACE–Ang II–AT1R axis and the ACE2–Ang-(1–7)–MasR axis, and that Ex not only suppresses the former axis but also activates the latter one. We previously reported that Ex ameliorates ACE2 and MasR in chronic renal failure rats with 5/6 nephrectomy and DS rats fed high salt (21,22). In this regard, Magalhaes et al. (41) reported that acute aerobic exercise increases plasma and urinary ACE2 as well as urinary levels of Ang-(1–7) in physically active young male volunteers. Taken together, these results suggest that exercise may ameliorate the renal RAS balance with a specific activation of the renal ACE2–Ang-(1–7)–MasR axis.
This study had several limitations. First, we studied only male DS rat because the female rats are protected against HFr-induced hypertension or hyperinsulinemia (24). Future study should be undertaken to explore the sex differences in impacts of HFr and Ex. Second, 60% fructose diet does not translate into diets for humans. We chose the 60% fructose diet, which is well established to induce metabolic syndrome and hypertension in rodents by numerous previous studies (1,17,23,42), and long-term impacts of fructose cannot be studied in rats unlike humans. Third, although Ex for 12 wk was shown to have antihypertensive and renal protective effects in the HFr-fed DS rats, it was not determined whether the current exercise protocol recommends a proper exercise frequency and intensity for antihypertensive and renal protective effects. In addition, plasma and intrarenal levels of Ang II or Ang-(1–7) were not examined. It is also unclear whether the Ex had a direct effect on renal RAS components. Lastly, to focus on the Ex effects on salt-sensitive induced hypertension, we did not include salt-resistant rat in this study. There is a need to clarify the mechanism behind the Ex-induced changes of renal RAS components in the future. There are several methods of measurement that could be improved. HOMA-IR for measuring insulin resistance is less precise. The telemetry would be better than tail cuff when measuring SBP; however, there is a possibility that the catheters in the femoral artery affect treadmill performance.
CONCLUSIONS
In conclusion, this study indicates that Ex strongly attenuated the HFr-induced hypertension and renal damage in DS rats. The renal protective and antihypertensive effects of Ex may be mediated by ameliorating the imbalance of renal RAS components. These results suggest that Ex may be highly efficacious against HFr-induced hypertension and renal damage in patients with salt sensitivity.
Supplementary Material
Acknowledgments
We would like to thank the Institute for Animal Experimentation of Tohoku University Graduate School of Medicine for animal care, and the Biomedical Research Unit of Tohoku University Hospital and the Biomedical Research Core of Tohoku University Graduate School of Medicine for technical assistance. We would like to thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this article.
This work was supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science grants 17H02119, 20H04054, and 22 K17656. The authors declare that they have no conflict of interests. The results of the study are presented clearly, honestly, and without fabrication or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
LUSI XU, Email: jyolx@yahoo.co.jp.
GAIZUN HU, Email: guinsooh@gmail.com.
JIAHE QIU, Email: jiaheeqiu@gmail.com.
TAKAHIRO MIURA, Email: takamiur@gmail.com.
SEIKO YAMAKOSHI, Email: seicouleur@gmail.com.
ASAKO NAMAI-TAKAHASHI, Email: hemp_nama@icloud.com.
MASAHIRO KOHZUKI, Email: kohzuki@med.tohoku.ac.jp.
REFERENCES
- 1.Gersch MS Mu W Cirillo P, et al. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Renal Physiol. 2007;293(4):F1256–61. [DOI] [PubMed] [Google Scholar]
- 2.Madero M, Perez-Pozo SE, Jalal D, Johnson RJ, Sanchez-Lozada LG. Dietary fructose and hypertension. Curr Hypertens Rep. 2011;13(1):29–35. [DOI] [PubMed] [Google Scholar]
- 3.Hannou SA, Haslam DE, McKeown NM, Herman MA. Fructose metabolism and metabolic disease. J Clin Invest. 2018;128(2):545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhingra R Sullivan L Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-age adults in the community. Circulation. 2007;116(5):480–8. [DOI] [PubMed] [Google Scholar]
- 5.Jalal DI, Smits G, Johnson RJ, Chonchol M. Increased fructose associates with elevated blood pressure. J Am Soc Nephrol. 2010;21(9):1543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R730–7. [DOI] [PubMed] [Google Scholar]
- 7.Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10(5):512–6. [DOI] [PubMed] [Google Scholar]
- 8.Klein AV, Kiat H. The mechanisms underlying fructose-induced hypertension: a review. J Hypertens. 2015;33(5):912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reaven GM, Ho H, Hoffman BB. Attenuation of fructose-induced hypertension in rats by exercise training. Hypertension. 1988;12(2):129–32. [DOI] [PubMed] [Google Scholar]
- 10.Hu G, Xu L, Ma Y, Kohzuki M, Ito O. Chronic exercise provides renal-protective effects with upregulation of fatty acid oxidation in the kidney of high fructose-fed rats. Am J Physiol Renal Physiol. 2020;318(3):F826–34. [DOI] [PubMed] [Google Scholar]
- 11.Xu C Lu A Lu X, et al. Activation of renal (pro)renin receptor contributes to high fructose-induced salt sensitivity. Hypertension. 2017;69(2):339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuhashi M Ura N Takizawa H, et al. Blockade of the renin–angiotensin system decreases adipocyte size with improvement in insulin sensitivity. J Hypertens. 2004;22(10):1977–82. [DOI] [PubMed] [Google Scholar]
- 13.Okada K, Hirano T, Ran J, Adachi M. Olmesartan medoxomil, an angiotensin II receptor blocker ameliorates insulin resistance and decreases triglyceride production in fructose-fed rats. Hypertens Res. 2004;27(4):293–9. [DOI] [PubMed] [Google Scholar]
- 14.Kamide K Rakugi H Higaki J, et al. The renin-angiotensin and adrenergic nervous system in cardiac hypertrophy in fructose-fed rats. Am J Hypertens. 2002;15(1 Pt 1):66–71. [DOI] [PubMed] [Google Scholar]
- 15.Nyby MD, Abedi K, Smutko V, Eslami P, Tuck ML. Vascular angiotensin type 1 receptor expression is associated with vascular dysfunction, oxidative stress and inflammation in fructose-fed rats. Hypertens Res. 2007;30(5):451–7. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama T Kosugi T Gersch M, et al. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Renal Physiol. 2010;298(3):F712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Lozada LG Tapia E Jimenez A, et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292(1):F423–9. [DOI] [PubMed] [Google Scholar]
- 18.Xu L Hu G Qiu J, et al. High fructose-induced hypertension and renal damage are exaggerated in Dahl salt-sensitive rats via renal renin–angiotensin system activation. J Am Heart Assoc. 2021;10(14):e016543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito D Cao P Kakihana T, et al. Chronic running exercise alleviates early progression of nephropathy with upregulation of nitric oxide synthases and suppression of glycation in Zucker diabetic rats. PLoS One. 2015;10(9):e0138037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa Y Takahashi J Sakuyama A, et al. Exercise training delays renal disorders with decreasing oxidative stress and increasing production of 20-hydroxyeicosatetraenoic acid in Dahl salt-sensitive rats. J Hypertens. 2020;38(7):1336–46. [DOI] [PubMed] [Google Scholar]
- 21.Yamakoshi S, Nakamura T, Mori N, Suda C, Kohzuki M, Ito O. Effects of exercise training on renal interstitial fibrosis and renin–angiotensin system in rats with chronic renal failure. J Hypertens. 2021;39(1):143–52. [DOI] [PubMed] [Google Scholar]
- 22.Sakuyama A Ogawa Y Xu L, et al. Effects of exercise training on the renin–angiotensin system in the kidneys of Dahl salt-sensitive rats. Med Sci Sports Exerc. 2022;54(7):1105–13. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T Hu H Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625–31. [DOI] [PubMed] [Google Scholar]
- 24.Galipeau D, Verma S, McNeill JH. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol. 2002;283(6):H2478–84. [DOI] [PubMed] [Google Scholar]
- 25.Kanazawa M Kawamura T Li L, et al. Combination of exercise and enalapril enhances renoprotective and peripheral effects in rats with renal ablation. Am J Hypertens. 2006;19(1):80–6. [DOI] [PubMed] [Google Scholar]
- 26.Tufescu A Kanazawa M Ishida A, et al. Combination of exercise and losartan enhances renoprotective and peripheral effects in spontaneously type 2 diabetes mellitus rats with nephropathy. J Hypertens. 2008;26(2):312–21. [DOI] [PubMed] [Google Scholar]
- 27.Kamijo-Ikemori A, Sugaya T, Kimura K. Urinary fatty acid binding protein in renal disease. Clin Chim Acta. 2006;374(1–2):1–7. [DOI] [PubMed] [Google Scholar]
- 28.Li L Guan Y Kobori H, et al. Effects of the novel nonsteroidal mineralocorticoid receptor blocker, esaxerenone (CS-3150), on blood pressure and urinary angiotensinogen in low-renin Dahl salt-sensitive hypertensive rats. Hypertens Res. 2019;42(6):769–78. [DOI] [PubMed] [Google Scholar]
- 29.Matsusaka T, Niimura F, Pastan I, Shintani A, Nishiyama A, Ichikawa I. Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int. 2014;85(5):1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou J Yaoita E Watanabe Y, et al. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch. 2006;448(4):485–92. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Lozada LG Tapia E Santamaria J, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67(1):237–47. [DOI] [PubMed] [Google Scholar]
- 32.Overton JM, VanNess JM, Takata HJ. Effects of chronic exercise on blood pressure in Dahl salt-sensitive rats. Am J Hypertens. 1998;11(1 Pt 1):73–80. [DOI] [PubMed] [Google Scholar]
- 33.Tipton CM, Overton JM, Pepin EB, Edwards JG, Wegner J, Youmans EM. Influence of exercise training on resting blood pressures of Dahl rats. J Appl Physiol (1985). 1987;63(1):342–6. [DOI] [PubMed] [Google Scholar]
- 34.Savage MV, Mackie GF, Bolter CP. Effect of exercise on the development of salt-induced hypertension in Dahl-S rats. J Hypertens. 1986;4(3):289–93. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd RE, Kuehne ML, Kenno KA, Durstine JL, Balon TW, Rapp JP. Attenuation of blood pressure increases in Dahl salt-sensitive rats by exercise. J Appl Physiol Respir Environ Exerc Physiol. 1982;52(6):1608–13. [DOI] [PubMed] [Google Scholar]
- 36.Iwai M, Horiuchi M. Devil and angel in the renin–angiotensin system: ACE–angiotensin II–AT1 receptor axis vs. ACE2–angiotensin-(1–7)–Mas receptor axis. Hypertens Res. 2009;32(7):533–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frantz EDC Medeiros RF Giori IG, et al. Exercise training modulates the hepatic renin–angiotensin system in fructose-fed rats. Exp Physiol. 2017;102(9):1208–20. [DOI] [PubMed] [Google Scholar]
- 38.de Oliveira Sa G, Dos Santos Neves V, de Oliveira Fraga SR, Souza-Mello V, Barbosa-da-Silva S. High-intensity interval training has beneficial effects on cardiac remodeling through local renin–angiotensin system modulation in mice fed high-fat or high-fructose diets. Life Sci. 2017;189:8–17. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal D, Elks CM, Reed SD, Mariappan N, Majid DS, Francis J. Chronic exercise preserves renal structure and hemodynamics in spontaneously hypertensive rats. Antioxid Redox Signal. 2012;16(2):139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kai H Kai M Niiyama H, et al. Overexpression of angiotensin-converting enzyme 2 by renin–angiotensin system inhibitors. Truth or myth? A systematic review of animal studies. Hypertens Res. 2021;44(8):955–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magalhaes DM Nunes-Silva A Rocha GC, et al. Two protocols of aerobic exercise modulate the counter-regulatory axis of the renin–angiotensin system. Heliyon. 2020;6(1):e03208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ares GR, Ortiz PA. Direct renal effects of a fructose-enriched diet: interaction with high salt intake. Am J Physiol Regul Integr Comp Physiol. 2015;309(9):R1078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


