ABSTRACT
Purpose
American-style football (ASF) players are at increased risk for head injuries and cardiovascular disease. n-3 polyunsaturated fatty acids are cardioprotective, and emerging evidence suggests benefits for protection against head injuries. However, fundamental knowledge of n-3 polyunsaturated fatty acid dosing in athletes such as ASF players remains poorly understood. Therefore, this study investigated the dose–response effect of docosahexaenoic acid (DHA) supplementation in red blood cells (RBC) and as the Omega-3 Index (O3I), in collegiate ASF players throughout a competitive season.
Methods
Sixty-nine ASF players were randomly assigned placebo (corn oil), or 2, 4, or 6 g·d−1 of DHA supplement. Blood samples were collected at eight time points (T1–T8) over 27 wk. RBC were extracted and analyzed by gas–liquid chromatography. Compliant players who had samples collected at all time points were analyzed. A repeated-measures ANOVA was conducted to assess the dose–response effect of DHA over time, and between-group differences at individual time points were assessed by one-way ANOVA followed by Tukey post hoc test.
Results
A significant dose and time interaction was found, and all supplement groups had significantly greater DHA in RBC compared with placebo from T2–T8 (P < 0.05). Athletes receiving 6 g·d−1 of DHA had the greatest O3I, relative to other groups, and the O3I reached steady state by 15 wk. The 6 g·d−1 group surpassed >8% on the O3I at approximately twice the rate of the 4 g·d−1 group (8 vs 15 wk).
Conclusions
Our findings provide important fundamental knowledge demonstrating a dose–response incorporation of DHA into RBC membranes up to 6 g·d−1. Furthermore, 6 g·d−1 of DHA can be used to rapidly achieve a desired O3I (>8%) in athletes in only 8 wk.
Key Words: OMEGA-3 FATTY ACID, RED BLOOD CELL, PLASMA, OMEGA-3 INDEX, CARDIOVASCULAR DISEASE, EXERCISE
American-style football (ASF) athletes have unique health concerns and nutritional requirements. ASF athletes have been found to consume an inadequate amount of omega-3 polyunsaturated fatty acids (n-3 PUFA) (1). However, there is currently a lack of studies assessing the dose–response supplementation of n-3 PUFA in ASF athletes. Increasing docosahexaenoic acid (DHA), a long-chain n-3 PUFA, consumption in nonathletes, up to 2 g·d−1, results in an increase of plasma DHA concentration in a dose-dependent manner, whereas quantities greater than 2 g·d−1 have shown minimal increases beyond that point (2). As previously shown, nonathletes with a greater body mass require greater intake of n-3 PUFA to raise tissue levels to similar values when compared with those with a lower body mass (3). Because of the greater size of ASF athletes compared with the general population (4) and potential for physical activity to modify lipid profiles (5,6), we posit that they will require larger quantities of n-3 PUFA to see a similar response to those with a lower body mass.
Worldwide consumption of n-3 PUFA, eicosapentaenoic acid (EPA; 20:5n-3), and DHA, is low with less than 20% of individuals consuming a recommended >250 mg·d−1 (7). However, humans have a limited capacity to synthesize EPA and DHA from the essential fatty acid alpha-linolenic acid (18:3n-3), as typically <1% DHA can be synthesized from alpha-linolenic acid (8). Thus, EPA and DHA must be obtained from the diet, which is predominantly found in marine oils and seafood. Blood plasma and red blood cells (RBC) are the two most common fractions used to assess fatty acid status (9). Both fractions are sensitive to fluctuations in fatty acid consumption and are known to reflect dietary intake (8,9). We have previously published work showing an increase of DHA (22:6n-3) in blood plasma of ASF players after supplementation with n-3 PUFA but not in RBC (10). The longer half-life of healthy RBC (~120 d) is thought to reflect long-term n-3 PUFA status, as it is less sensitive to acute consumption and fasting status, unlike the plasma fraction (8). The Omega-3 Index (O3I), which is the sum of EPA and DHA levels in RBC membranes expressed as a percentage of total RBC fatty acids, has been proposed as a clinical biomarker that provides information regarding cardiovascular disease (CVD) risk and overall n-3 PUFA status (11).
Understanding the specific nutritional needs of ASF athletes has important implications for brain health and CVD. Because of the physical nature of the sport, ASF athletes are consistently exposed to repetitive head impacts at a higher relative rate than other sports (12). Emerging evidence suggests that n-3 PUFA may differentially influence health outcomes and suggests that DHA can exhibit neuroprotective properties (12). A recent study conducted in ASF athletes corroborates the neuroprotective ability of DHA, when administered before insult (13–15), as measured by neurofilament light, a surrogate marker of head trauma (10). DHA along with EPA has also been shown to have important biological roles not only for brain health but also for cardiovascular health (2). A number of studies and surveys conducted in ASF athletes at both the collegiate and professional levels reported greater than 60% of ASF athletes to be prehypertensive or hypertensive, which was often attributed to high levels of low-density lipoprotein cholesterol (16–18). Furthermore, decreasing the n-6/n-3 PUFA ratio by increasing n-3 PUFA consumption further reduces CVD risk through anti-inflammatory mechanisms (19). The effects of n-3 PUFA on CVD risk are mixed but large human trials and meta-analyses show positive benefits of n-3 PUFA intake (20,21), particularly for those factors commonly known to affect ASF athletes—dyslipidemia and hypertension (16).
Given the lack of basic scientific studies regarding the kinetics and dosing status after n-3 PUFA supplementation in ASF athletes, the current study sought to examine the dose–response relationship of placebo, and 2, 4, and 6 g·d−1 of DHA supplementation in ASF athletes in RBC. By observing the fatty acid profile of RBC, we also sought to examine changes in the O3I over the course of an entire competitive season.
METHODS
Participants
This study is a secondary analysis of biobanked RBC samples collected in 2014. Further details of the methodology can be found in the primary study published by Oliver et al. (10). In brief, the current study was conducted according to the Declaration of Helsinki guidelines. All procedures involving human subjects were approved by the institutional review board (protocol no. 1404-68-1404). Participation was voluntary, procedures were explained, and written informed consent was obtained from all subjects. Participants were National Collegiate Athletic Association Division I ASF athletes medically cleared to participate in university athletics as determined by the team physician. Exclusion criteria included chronic daily anti-inflammatory (≥20 d) or antihypertensive medication use, medications for blood lipids, and fish oil or n-3 PUFA supplementation. Participants were advised as to which foods were high in DHA and asked to limit servings to no more than two per week. Athletes who sustained an injury and/or were unable to participate in regularly scheduled conditioning or competitions were excluded. Those athletes that missed two or more sample collections were also excluded from analysis. A consort diagram is provided in Figure 1 outlining specific exclusion criteria. A total of 69 athletes met the requirements for inclusion in this study.
FIGURE 1.

CONSORT diagram outlining reasoning for exclusion from data analysis.
Study design
A randomized, double-blind, placebo-controlled, parallel-design study was used to examine the dose–response and time course of supplemental DHA over the course of an ASF season (189 d). This time frame included off-season summer conditioning (57 d), preseason camp (23 d), and the competitive season (109 d). Before the start of summer conditioning, potential participants (n = 130) were randomized using a random number generator by player position (quarterback, offensive line, running back, tight end, wide receiver, defensive line, linebacker, cornerback, safety, specialist (i.e., kicker), incoming freshmen—no positions assigned) to one of four supplementation groups: 2 g·d−1 DHA (2G), 4 g·d−1 DHA (4G), or 6 g·d−1 DHA (6G), or a placebo (PLA) consisting of corn oil. Demographic information, including the breakdown of player positions per group, is described in Table 1.
TABLE 1.
Mean height and weight of ASF players across supplemental groups.
| 6G Group Total | N | 4G Group Total | N | 2G Group Total | N | PLA Group Total | N | Group | N |
|---|---|---|---|---|---|---|---|---|---|
| 6G | 18 | 4G | 15 | 2G | 18 | PLA | 18 | Total | 69 |
| QB | 2 | OL | 2 | QB | 1 | OL | 5 | QB | 3 |
| OL | 5 | CB | 1 | OL | 4 | CB | 4 | OL | 16 |
| CB | 1 | LB | 2 | LB | 2 | LB | 1 | CB | 6 |
| LB | 2 | PK | 1 | DL | 2 | TE | 2 | LB | 7 |
| PK | 3 | RB | 1 | S | 5 | S | 2 | PK | 4 |
| RB | 1 | DL | 3 | WR | 4 | WR | 4 | RB | 2 |
| TE | 2 | S | 1 | TE | 4 | ||||
| DL | 1 | WR | 4 | DL | 6 | ||||
| WR | 1 | S | 8 | ||||||
| WR | 13 | ||||||||
| Heighta | 74.8 ± 2.7 | 73.5 ± 3.3 | 73.7 ± 2.3 | 74.0 ± 2.6 | 74.0 ± 2.7 | ||||
| Weighta | 238.7 ± 39.9 | 228.8 ± 62.1 | 223.1 ± 42.0 | 229.9 ± 55.8 | 230.02 ± 49.5 | ||||
aOne-way ANOVA showed no statistical difference between height or weight among groups. Height and weight are reported in inches and pounds. Values are mean ± SD.
CB, cornerback; DL, defensive lineman; LB, linebacker; OL, offensive lineman; PK, place kicker; QB, quarterback; RB, running back; S, safety; TE, tight end; WR, wide receiver.
Blood was sampled at specific intervals to coincide with changes in training intensity. A baseline blood sample was taken when athletes returned from their off-season and reported for summer conditioning (baseline; T1). A second sample was obtained at the conclusion of summer training before preseason training camp (pre-camp; T2). A third sample was collected after preseason training camp (post-camp; T3). The remaining blood samples were taken throughout the competitive season on Mondays, 48 h after a Saturday game (T4–T8). All samplings throughout the season occurred within intervals of 14–28 d as permitted by scheduling and traveling. The timeline of blood sample collection is presented in Figure 2.
FIGURE 2.
Timeline of blood sample collection over the course of the season.
Supplementation
DHA was provided via algal oil (DHA-S oil; DSM Nutritional Products, Columbia, MD) derived from Schizochytrium sp. containing 35%–45% DHA by weight. Wesson® corn oil, containing <0.1% DHA, was used as the placebo and in the 2G and 4G DHA oil mixtures to ensure equal volume to the 6G mixture. The lower limit (35%) of DHA content by weight contained in the DHA oil was used for calculating the proper dose for each supplement group (10). All mixtures were flavored with artificial flavoring as a masking agent. The fatty acid composition for both oils is included in Oliver et al. (10).
Supplement mixtures were given on days participants reported to the training facility (5–7 d·wk−1), and adherence was monitored daily via visual supervision by the same research personnel (10). Supplement compliance was set at ≥80% for each individual over the course of the study. Days when players were not required to report to the team facility, the supplement was not given and considered for calculating compliance. From the 130 participants, 6 dropped out because of side effects (gastrointestinal distress, n = 4; poor palatability, n = 1; and smell, n = 1) (10).
Blood collection and fatty acid analysis
The night before each blood collection, participants were verbally reminded to ingest only water after 2200 h. On the day of blood sampling, participants reported to the athletic training facility after an overnight fast (≥8 h), and blood samples were collected via venipuncture from the antecubital fossa region using standard, sterile phlebotomy procedures while the athlete remained supine. Blood samples were collected in spray-coated K2 ethylenediaminetetraacetic acid vacutainer tubes (BD Diagnostics, Franklin Lakes, NJ). Vacutainer tubes were kept on ice before blood collection and immediately placed on ice after blood collection. All samples were centrifuged at 2000g for 30 min at 4°C (Allegra X-15R; Beckman Coulter, Brea, CA) within 30 min of collection. Aliquots of RBC, collected via sterile pipette from center of the RBC pack within the EDTA vacutainer tubes, were immediately transferred to prelabeled polypropylene vials.
Fatty acid measurement
Total lipid was extracted and methylated according to previously described procedures (3). Fatty acid methyl esters were analyzed with a Varian gas chromatograph (model CP-3800 fixed with a CP-8200 autosampler; Varian Inc., Walnut Creek, CA) equipped with a fused silica capillary column CP-Sil88 (100 m × 0.25 mm i.d.; Chrompack Inc., Middleburg, the Netherlands). Data are expressed as percent (%) total fatty acids. The O3I was calculated using the formula: %EPA + %DHA of total RBC fatty acids (22). Cut points that are used for classifying the risk of developing CVD are as follows: high risk, <4%; intermediate risk, 4%–8%; and low risk, >8%.
Statistical analyses
The study was originally powered to examine the effect of supplementation on blood biomarker associated with concussion injury (10). All available samples were used in the present study. The number of participants for each time point included the following: PLA, n = 14–18; 6G, n = 15–18; 4G, n = 11–15; and 2G, n = 14–18. The number of participants differs at each time point as any missing blood measures were not included in analysis. A mixed-model repeated-measures ANOVA was initially used to determine if there was a dose effect of DHA supplementation over time. Significant time–treatment (P < 0.05) interaction justified further analysis of specific time points by one-way ANOVA followed by a Tukey post hoc test (P < 0.05). All analyses were performed using SAS University Edition (SAS Institute Inc., Cary, NC).
RESULTS
Dose–response of DHA supplementation on the composition of DHA
A significant dose (P < 0.05), time (P < 0.05), and dose–time (P < 0.05) effect was observed for DHA (Fig. 3A). Post hoc analysis found that all supplement groups were greater (P > 0.05) than PLA at T2 through to T8. At the end of preseason training camp and 15 total wk of DHA supplementation (T4), %DHA in the supplemental groups began to plateau. From T3 to T8, 6G fluctuated between being significantly greater (T4, T7) and not different (T3, T5, T6, T8) to 4G. At no point were 4G and 2G statistically different. All values for Figure 3 can be found in Supplemental Table 1 (Supplemental Digital Content, http://links.lww.com/MSS/C787).
FIGURE 3.
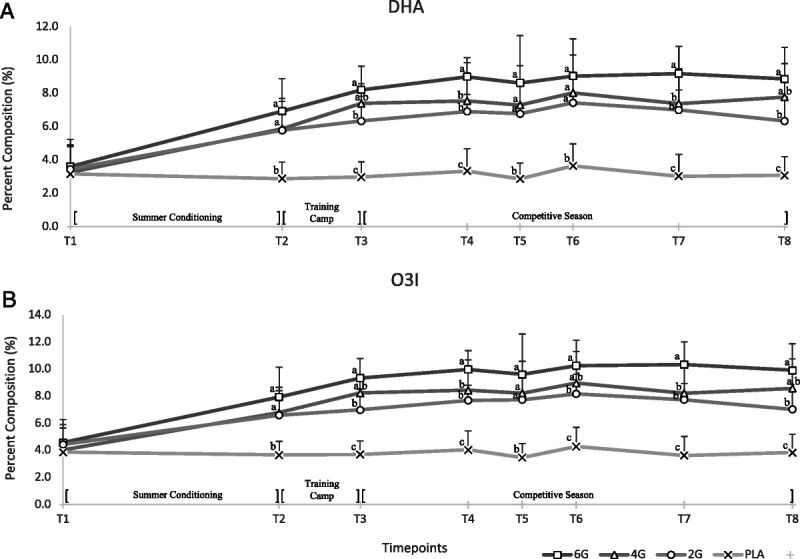
A, Dose–response curves of DHA after 27 wk of PLA (n = 14–18), or 6 g·d−1 (n = 15–18), 4 g·d−1 (n = 11–15), or 2 g·d−1 (n = 14–18) of DHA supplementation. B, Dose–response curve of the O3I. A mixed-model repeated-measures approach showed a significant (P < 0.05) effect of dose and time for DHA and O3I. Subsequently, a one-way ANOVA was conducted at each time point followed by Tukey post hoc to determine differences between means (P < 0.05). No significant difference was seen at baseline in either measure. Error bars represent SD of means. Letters correspond to which groups are considered significantly different at each time point (P < 0.05).
Changes in the O3I after DHA supplementation
Trends seen in the O3I after 27 wk of DHA supplementation follow a similar pattern to that of DHA values (Fig. 3B). A significant dose (P < 0.05), time (P < 0.05), and dose–time (P < 0.05) effect was observed. At baseline, each supplement group had an O3I slightly higher than 4%, and PLA fell below the 4% cutoff for high risk of CVD (4). After the first 8 wk (T2) of DHA supplementation, post hoc analysis found that the O3I significantly increased in 2G, 4G, and 6G compared with PLA (P < 0.05). At T2, 6G averaged an O3I value of 7.94, reaching the benchmark to be in the low-risk classification for CVD of >8% (22), which remained until T8. Players receiving 4G did not achieve this benchmark until T3, which was consistent until T8. Players receiving 2G reached this benchmark at T6 but fell below 8% at T7 and T8. Similar to the DHA results, players receiving 6G consistently maintained an O3I at least 1%–2% greater than that of 4G. Upon completion of the study, PLA was the only group to fall into the high-risk classification, and both 6G and 4G achieved a low-risk classification for CVD based upon the O3I (22).
Dose–response of DHA supplementation on the composition of EPA
Significant dose effect (P < 0.05) and dose–time effect (P > 0.05), but no time (P > 0.05) effect, were observed for EPA in Figure 4A. Post hoc analysis found that the 6G group had significantly higher EPA than all other groups at T3 and T5 (P < 0.05). 4G was statistically greater than PLA at T4, and at no point was different from 2G. By T8, no differences were noted between groups. All values for Figure 4 can be found in Supplemental Table 1 (Supplemental Digital Content, http://links.lww.com/MSS/C787).
FIGURE 4.
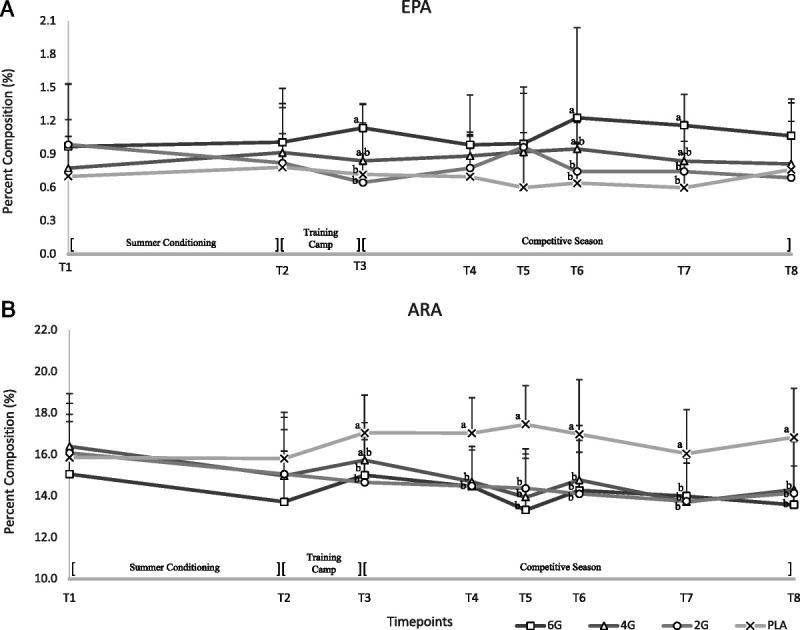
Dose–response of (A) EPA and (B) ARA after DHA supplementation between all treatment groups over the course of a competitive college football season. A mixed-model repeated-measures approach showed a significant (P < 0.05) effect of dose and time for EPA and ARA. Subsequently, a one-way ANOVA was conducted at each time point followed by Tukey post hoc to determine differences between means (P < 0.05). Letters indicate groups statistically significant from each other at that time point (P < 0.05); time points without letters indicate no statistical difference. Error bars represent SD.
Dose–response of DHA supplementation on the composition of arachidonic acid
The effect of supplemental DHA on RBC arachidonic acid (ARA) % composition is presented in Figure 4B. A significant dose (P < 0.05), time (P < 0.05), and dose–time (P < 0.05) effect was observed. Post hoc analysis showed that at T3, ARA in PLA was significantly (P < 0.05) greater than any of the supplement groups and continued to T8. The lowest RBC ARA was observed in those ingesting 6G, although it was not statistically different from 2G and 4G.
Percent composition of fatty acids in total RBCs
The percent composition of major fatty acids comprising >80% of total FA at each time point for seven FA including palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1n9), linoleic acid (18:2n6), ARA, EPA, and DHA are found in Supplemental Table 1 (Supplemental Digital Content, http://links.lww.com/MSS/C787). The n-6/n-3 ratio was calculated using the summation of n-6 and n-3 PUFA across all time points. Baseline values of the n-6/n-3 ratio were all quite similar between the supplementation and placebo groups. From T2 onward, ratios in 6G, 4G, and 2G saw large declines, whereas PLA ratios stayed consistent. The shift in the ratio of the supplement groups was driven largely by the increase of n-3 PUFA from DHA beginning at T2, as n-6 PUFA only exhibited modest declines over the course of the study.
DISCUSSION
The dose–response relationship of DHA supplementation in RBC fatty acid composition in ASF athletes was investigated over the course of a competitive season. We show a dose–response incorporation of DHA into RBC up to a dose of 6 g·d−1. In only 8 wk, 6 g·d−1 of DHA rapidly increased O3I, an objective marker of n-3 PUFA status, which is correlated with reduced CVD risk. As each dose resulted in a subsequently higher plateau, doses greater than 6 g·d−1 may still yield an even stronger dose–response and potential positive health benefits for ASF players.
After 27 wk of DHA supplementation, we found that the 6G group had the greatest %DHA and O3I increase compared with all other groups. All three supplementation groups reached different plateaus of %DHA and O3I, which can be seen in Figure 3. This is similar to previous work in which we found three distinct plateaus when fatty acid profiles were assessed in total plasma using the same quantities of DHA supplementation in the same population (10). In contrast, a review by Arterburn et al. (2) suggested that 2 g·d−1 of DHA supplementation results in near saturation of plasma in nonathletes, as there were only marginal increases when doses exceeded 2 g·d−1. However, upon examining the studies reviewed by Arterburn et al. (2), which used a treatment dose of >2 g·d−1 of DHA, only one published study was 8 wk in duration, whereas the remaining five studies were 6 wk or less in duration. Thus, the longer observation period of 27 wk in our study reveals a significantly higher level of saturation than previous short-term interventions, which has also been corroborated in one other long-term supplementation study over 12 months (23). Another potential reason for this discrepancy may be due to the focus on the plasma phospholipid fraction, which does not account for fatty acids in lipids pools that are unbound or being carried by lipoproteins or albumin, including triglycerides, free fatty acids, and cholesterol esters (24). Measures of fatty acid composition in RBC membranes are another relevant lipid fraction and distinct from plasma lipids, which are commonly used as a biomarker for long-term dietary fatty acid status (24). The %DHA in RBC membranes in the 4G and 6G supplementation groups also increased in a dose-dependent manner, which further supports our previous findings of increased saturation greater than 2 g·d−1 (10). Furthermore, as each supplemental group plateaued at different levels, it is possible that higher doses of sustained DHA supplementation may still lead to increased steady-state concentrations in elite athletes. This dose–response is not surprising even with the overall increased size of ASF players. We posited that ASF athletes would require larger doses of n-3 PUFA to elicit similar results compared with the general population based on their larger body mass (4) and increased physical activity levels, which can modify lipid profiles (5). Indeed, a small study of 115 men demonstrated that supplementation should be adjusted according to body weight to achieve a similar O3I response (3). Overall, few studies have examined the dose–response and health outcomes of n-3 PUFA supplementation and/or fish consumption at values >2 g·d−1. A recent meta-analysis demonstrated a dose–response reduction in CVD outcomes at a dose >2 g·d−1 (25). It remains to be determined how changes observed in blood fatty acid content translates to the tissue level and further biological changes relevant to human physiology and health. This warrants further investigation in functional studies.
Previously, the % composition of n-3 PUFA in total plasma lipids and RBC has previously been shown to be strongly correlated (7,9). Although there is a strong correlation, the time course of dietary n-3 PUFA incorporation into plasma has been reported to occur more rapidly compared with RBC (9). The %DHA in RBC in this study plateaued at week 15 (Fig. 3A/B). These results follow a similar time course seen in the general population, where % n-3 PUFA in RBC has been reported to plateau between 8 and 20 wk (2,26). As the dose increased, the duration of time it took to reach an O3I of 8%, a level associated with lower CVD risk (9,23), was also reduced (Fig. 3). The 6G group reached 8% in nearly half the time of the 4G group, 8 wk compared with 15 wk. This finding is beneficial for nutritional planning, which aligns with practice and game scheduling. However, future studies that evaluate specific health-related end points are needed to determine if doses of DHA up to 6 g·d−1 are necessary to elicit other beneficial effects in this cohort, as 4 g·d−1 also surpassed >8% on the O3I. A recent novel finding suggests that cycling DHA supplementation could potentially elicit similar tissue and blood concentrations as chronic DHA consumption over 12 wk using half the amount of DHA (27). This novel approach could allow athletes to maintain increased levels of supplementation on a more consistent and cost-effective basis; however, this effect has yet to be confirmed in human trials.
%EPA also increased significantly over the duration of the study in a dose-dependent manner because of DHA supplementation. The DHA supplement given to the ASF players in our study also contained a small quantity of EPA likely resulting in the increase seen in our results. On a biological basis, this effect was modest as the largest change of EPA status in any group from baseline to the end of the study was only ~0.5%. Metherel et al. (28) reported that there is limited to no retro conversion of EPA from DHA found in human plasma, even with high quantities of supplementation, and could be related instead to slowed EPA metabolism. In agreement, our results show that at increasingly high levels of DHA, ≥4 g·d−1, retro conversion of DHA to EPA is likely very minimal. Although players were counseled about their diet, this was not explicitly controlled; thus, increases of %EPA may have also been detected because of changes in consumption from dietary sources.
Surprisingly, DHA supplementation only resulted in a modest decrease of %ARA. Our results differ from previous work in nonathletes that reported DHA supplementation results in a decrease in ARA and its downstream metabolites (27). The difference in findings may be explained by the increased physical activity, and chronic injury in ASF compounded by the metabolic demands of games, practice, and training and may result in an increased inflammatory profile, which has been seen in athletes in various sports (29). A notable increase in %ARA can be seen in the PLA group (Fig. 4B), which corresponds to the beginning of training camp and players increasing physical contact and activity. This may be the initial response due to intense training, which then is maintained at a chronic level, independent of DHA supplementation.
In complement to the dose–response increase in O3I, a dose-dependent decrease in the n-6/n-3 ratio was also seen (Supplemental Table 1, Supplemental Digital Content, http://links.lww.com/MSS/C787). Decreasing the n-6/n-3 ratio has been strongly correlated to protect against CVD (19) and lowering the ratio to <4:1 has seen up to a 70% reduction in mortality rates (30). Although Division 1 ASF athletes are highly active, the risk of CVD in this cohort, particularly to offensive and defensive lineman, has been reported as high (1). Thus, decreasing the n-6/n-3 ratio is beneficial for this cohort, who is at greater risk of CVD.
Limitations
This study is not without limitations. Athlete diets were not restricted, and although information on what food sources to avoid was provided (i.e., primarily fish and n-3 PUFA supplements), there was no measure of compliance in this regard. Specific measures of exercise were not measured. Because of the defined number of players on the team, sample size was restricted. Outcome measures were limited to the fatty acid profile of RBCs; thus, tissue-specific effects remain to be determined in future studies. Lastly, n-3 PUFA supplements on the market are mainly a mixture of EPA + DHA; thus, the dose–response of such mixtures requires separate investigation.
CONCLUSIONS
This study was the first to assess the dose–response relationship of DHA supplementation on RBC fatty acid composition over the course of an entire season (27 wk). Supplementing with DHA in quantities greater than traditionally consumed by the general population, 2 g·d−1, may be considered in ASF to rapidly achieve an O3I associated with reduced CVD risk (≥8%) in only 8 wk. Results from this study will help to inform nutritionists working with athletes and teams considering n-3 PUFA supplementation. Future studies using doses greater than 6 g·d−1 and evaluating further health outcomes are warranted to determine a dose that yields the optimal benefits for this cohort.
Acknowledgments
Funding and supplements were provided by DSM Nutritional Products to J. M. O.
The authors declare they have no competing interests. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
CODY A. C. LUST, Email: clust@uoguelph.ca.
JESSIE L. BURNS, Email: jessica.burns@guelphhumber.ca.
MARGARET T. JONES, Email: mjones15@gmu.edu.
STEPHEN B. SMITH, Email: steve.smith@ag.tamu.edu.
SEONG HO CHOI, Email: seongho@cbnu.ac.kr.
MICHELE KRK, Email: michelle.kirk@tcu.edu.
DAVID A. GABLE, Email: d.gable@tcu.edu.
JONATHAN M. OLIVER, Email: jo2667@tc.columbia.edu.
REFERENCES
- 1.Anzalone A Carbuhn A Jones L, et al. The Omega-3 Index in National Collegiate Athletic Association Division I collegiate football athletes. J Athl Train. 2019;54(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(6 Suppl):1467S–6. [DOI] [PubMed] [Google Scholar]
- 3.Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose–response randomized controlled trial. J Am Heart Assoc. 2013;2(6):e000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto JB, Yamamoto BE, Yamamoto PP, Yamamoto LG. Epidemiology of college athlete sizes, 1950s to current. Res Sports Med. 2008;16(2):111–27. [DOI] [PubMed] [Google Scholar]
- 5.Nikolaidis MG, Mougios V. Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports Med. 2004;34(15):1051–76. [DOI] [PubMed] [Google Scholar]
- 6.Davinelli S Corbi G Righetti S, et al. Relationship between distance run per week, Omega-3 Index, and arachidonic acid (AA)/eicosapentaenoic acid (EPA) ratio: an observational retrospective study in non-elite runners. Front Physiol. 2019;10:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stark KD, Van Elswyk ME, Higgins MR, Weatherford CA, Salem N. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog Lipid Res. 2016;63:132–52. [DOI] [PubMed] [Google Scholar]
- 8.Harris WS. Assessing fatty acid biostatus: red blood cells or plasma? Lipid Technol. 2013;25(8):179–81. [Google Scholar]
- 9.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86(1):74–81. [DOI] [PubMed] [Google Scholar]
- 10.Oliver JM Jones MT Kirk KM, et al. Effect of docosahexaenoic acid on a biomarker of head trauma in American football. Med Sci Sports Exerc. 2016;48(6):974–82. [DOI] [PubMed] [Google Scholar]
- 11.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 12.Lust CAC, Mountjoy M, Robinson LE, Oliver JM, Ma DWL. Sports-related concussions & sub-concussive impacts in athletes: incidence, diagnosis, and the emerging role of EPA & DHA. Appl Physiol Nutr Metab. 2020;45(8):886–92. [DOI] [PubMed] [Google Scholar]
- 13.Oliver JM Jones MT Kirk KM, et al. Serum neurofilament light in American football athletes over the course of a season. J Neurotrauma. 2016;33(19):1784–9. [DOI] [PubMed] [Google Scholar]
- 14.Bailes JE, Mills JD. Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J Neurotrauma. 2010;27(9):1617–24. [DOI] [PubMed] [Google Scholar]
- 15.Oliver JM, Anzalone AJ, Turner SM. Protection before impact: the potential neuroprotective role of nutritional supplementation in sports-related head trauma. Sports Med. 2018;48(1 Suppl):39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JH Zafonte R Pascuale-Leon A, et al. American-style football and cardiovascular health. J Am Heart Assoc. 2018;7(8):e008620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang AY FitzGerald SJ Cannaday J, et al. Cardiovascular risk factors and coronary atherosclerosis in retired National Football League players. Am J Cardiol. 2009;104(6):805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borchers JR, Clem KL, Habash DL, Nagaraja HN, Stokley LM, Best TM. Metabolic syndrome and insulin resistance in division 1 collegiate football players. Med Sci Sports Exerc. 2009;41(12):2105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schunck WH, Konkel A, Fischer R, Weylandt KH. Therapeutic potential of omega-3 fatty acid–derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol Ther. 2018;183:177–204. [DOI] [PubMed] [Google Scholar]
- 20.Innes JK, Calder PC. The differential effects of eicosapentaenoic acid and docosahexaenoic acid on cardiometabolic risk factors: a systematic review. Int J Mol Sci. 2018;19(2):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernasconi AA, Wiest MM, Lavie CJ, Milani RV, Laukkanen JA. Effect of omega-3 dosage on cardiovascular outcomes: an updated meta-analysis and meta-regression of interventional trials. Mayo Clin Proc. 2021;96(2):304–13. [DOI] [PubMed] [Google Scholar]
- 22.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med (Baltim). 2004;39(1):212–20. [DOI] [PubMed] [Google Scholar]
- 23.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38(10):2012–22. [PubMed] [Google Scholar]
- 24.Furtado JD, Beqari J, Campos H. Comparison of the utility of total plasma fatty acids versus those in cholesteryl ester, phospholipid, and triglyceride as biomarkers of fatty acid intake. Nutrients. 2019;11(9):2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y, Hu FB, Manson JAE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 2019;8(19):e013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52(12):2265–72. [DOI] [PubMed] [Google Scholar]
- 27.Metherel AH, Irfan M, Chouinard-Watkins R, Trépanier MO, Stark KD, Bazinet RP. DHA cycling halves the DHA supplementation needed to maintain blood and tissue concentrations via higher synthesis from AlA in Long-Evans rats. J Nutr. 2019;149(4):586–95. [DOI] [PubMed] [Google Scholar]
- 28.Metherel AH, Irfan M, Klingel SL, Mutch DM, Bazinet RP. Compound-specific isotope analysis reveals no retroconversion of DHA to EPA but substantial conversion of EPA to DHA following supplementation: a randomized control trial. Am J Clin Nutr. 2019;110(4):823–31. [DOI] [PubMed] [Google Scholar]
- 29.Al-Khelaifi F Donati F Botrè F, et al. Metabolic profiling of elite athletes with different cardiovascular demand. Scand J Med Sci Sport. 2019;29(7):933–43. [DOI] [PubMed] [Google Scholar]
- 30.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233(6):674–88. [DOI] [PubMed] [Google Scholar]


