Abstract
Background
Idiopathic achalasia (AC) may be affected by anxiety and/or depression; however, reliable evidence is still lacking. The present retrospective cohort study aimed to explore the influence of psycho-mental factors on the severity of AC.
Methods
All patients in the AC database of the Tianjin Medical University General Hospital from 2012 to 2020 were divided into two subgroups, intervention (n = 202) and medication (n = 84), according to previous treatments. Healthy people (n = 300) who underwent gastrointestinal endoscopy comprised the control group. The severity of symptoms and the anxiety and depression score of AC patients and controls were monitored by telephone and compared before and during COVID-19. In addition, the factors of AC symptoms during the COVID-19 were discussed by multiple linear regression.
Results
During COVID-19, the anxiety and depression levels of AC patients and healthy individuals were deteriorated. For AC patients, before and after COVID-19, symptoms, anxiety, and depression scores in the medication group were more serious than those in the intervention group. Furthermore, previous therapy, depression, and gender were found to be significantly related to the severity of AC symptoms during COVID-19.
Conclusions
The outbreak of COVID-19 made AC patients and healthy people anxious and depressed. Depression rather than anxiety might worsen the AC symptoms. Interventional therapy might protect AC patients against psychological abnormalities during COVID-19.
Keywords: Achalasia, Anxiety, Depression
Introduction
Achalasia (AC) is a classic esophageal motility disorder of unknown etiology. The incidence of AC is approximately 1 case/100,000 [1, 2]. The main clinical manifestations, such as usually dysphagia, chest pain, fluid reflux, and weight loss, are based on the abnormal esophageal motility. Since the symptoms of abnormal esophageal motility are related to the underlying psychological abnormalities [3], esophageal hypervigilance and visceral anxiety are the strongest predictors of dysphagia severity [4]. It could be speculated that anxiety and/or depression may also influence the symptoms of AC. However, there is only little reliable evidence supporting this hypothesis [5].
Natural or social disasters can cause psychosocial abnormalities, such as anxiety and depression [6]. People's mental health is also deteriorated during the pandemics such as flu [7]. The outbreak of an epidemic has a broad psychological impact at the individual, community, and national levels. At the individual level, people are afraid of disease or death, helplessness, and shame [8]. COVID-19 is an emerging global health threat [9]. It was first detected in Wuhan, Hubei Province, China, in late December 2019 and had a great impact on people's psychology, with the pooled prevalence of depression (45%), anxiety (47%), and sleep disturbances (34%) [10, 11, 12]. Even outside Hubei, strict social control may cause anxiety and depression among the community population. Therefore, the outbreak of COVID-19 in China has provided us with an opportunity to observe the impact of anxiety and depression on the symptoms of AC patients in the community.
In this retrospective cohort study, we aimed to observe the impact of anxiety and depression on the severity of symptoms of AC patients in the community during COVID-19. Also, the factors affecting the symptoms of AC during COVID-19 were explored.
Methods
Setting and Participants
This retrospective cohort study was based on the database of AC patients (2012–2020). People undergoing gastrointestinal endoscopy at the physical examination center in Tianjin Medical University General Hospital from 2012 to 2020 were included as controls. AC is diagnosed by a physician based on esophageal barium meal examination, endoscopy, and high-resolution manometry and classified according to the Chicago Classification v3.0 [13].
The inclusion criteria for AC patients were as follows: (1) complete demographic data and at least one follow-up information (face-to-face or telephone interviews) for 1 year; (2) 18–65 years of age; (3) education level above primary school; (4) no history of serious digestive system diseases, such as peptic ulcer, liver disease, inflammatory bowel disease, or malignant tumor of the digestive system. The exclusion criteria for AC patients were as follows: (1) living abroad; (2) history of mental diseases such as obsessive-compulsive disorder; (3) history of severe organic diseases, such as hypertension, coronary heart disease, diabetes, and tumors; (4) history of neurological diseases affecting telephone follow-up, such as cerebral infarction or hearing impairment; (5) patients hospitalized after October 2019; (6) patients could not complete all the questionnaires at a time. The exclusion criteria for controls were a history of severe organic diseases, such as hypertension, coronary heart disease, diabetes, and tumors. The AC patients receiving per-oral endoscopic myotomy (POEM), Heller operation, or balloon dilatation were assigned to the intervention group, while patients receiving drug treatment were assigned to the medication group.
Procedure
The study was conducted from March 1, 2020, to May 25, 2020. Since COVID-19 emerged in China in January, December 2019 was deemed as the time prior to COVID-19 and February 2020 as during COVID-19. Due to the central and local ban on unnecessary face-to-face interaction, AC patients and healthy people were interviewed by telephone during the study. All the telephone interviews were completed by a trained gastroenterologist who received HAD scale training and was blinded to the patient's previous treatment. First, the investigators called the patients at night (19:00–22:00), according to the telephone number in the database, to ensure that the patients could be contacted and informed consent was obtained. If the subjects agreed to participate in the study, they were asked to stay in a quiet room for about 30 min and complete the questionnaire by telephone. Moreover, if participants heard the phone but did not have the time to conduct the survey, the investigators would arrange another appropriate time to complete the survey. If the subjects did not receive the first call, the investigators would make three consecutive calls the next day (morning [7:00–9:00], noon [11:00–14:00], and evening [19:00–22:00]). Subjects who did not receive at least four calls or refused to participate in the study were considered missing. After the completion of the survey, all the questionnaires were handed over to a statistician blinded to the study design to input and process the data. This present study was approved by the Institutional Review Board and Ethics Committee of the Tianjin Medical University General Hospital (IRB2020-WZ-096).
Survey Development
The questionnaire was prepared by three professional gastroenterologists. The structured questionnaire consisted of six parts: (1) demographic data, such as gender, age, and residence; (2) history, course of the disease, and medical history for healthy people; (3) the severity of AC symptoms before and during COVID-19; (4) anxiety and depression of AC patients and healthy people before and during COVID-19; (5) dietary habits of AC patients during COVID-19; (6) previous treatment for AC patients. If the patient could not recall the previous treatment, the medical record shall be considered.
Eckardt score [14] was used to assesses the AC patient's symptoms by combining the sum of symptom frequency scores for dysphagia, retrosternal pain, and regurgitation (the range for each symptom is 0–3; 0 indicates absent; 1, occasionally; 2, daily; 3, per meal) and the weight loss score (the range is 0–3; 0 indicates no weight loss; 1, <5.0 kg of weight loss; 2, 5.0–10.0 kg of weight loss; 3, >10.0 kg of weight loss). Hospital Anxiety and Depression Scale (HADS) [15] was used to measure the anxiety and depression of AC patients. Questions 1, 3, 5, 7, 9, 11, and 13 comprised the anxiety subscale. The total anxiety subscale score was divided into normal (0–7), mild anxiety (8–10), moderate anxiety (11–14), and severe anxiety (15–21). Questions 2, 4, 6, 8, 10, 12, and 14 formed the depression subscale. The total depression subscale score was categorized into normal (0–7), mild depression (8–10), moderate depression (11–14), and severe depression (15–21). In addition, the AC patient's compliance with doctor's dietary recommendations was assessed by 5-point Likert self-rating scale [16] (1 = worse than before; 2 = slightly worse than before; 3 = same as before; 4 = slightly better than before; 5 = better than before). All questionnaires and scales were surveyed during the same telephone follow-up.
Statistical Analyses
Normally distributed quantitative variables were recorded as mean ± standard deviation and assessed using analysis of paired sample t test or independent sample t test, as appropriate. Non-normally distributed variables were recorded as median (interquartile, range) and analyzed using the Kruskal-Wallis test or the Mann-Whitney posttest, as appropriate. The categorical variables were recorded as counts and percentages and evaluated by the χ2 or Fisher's exact test. Multiple linear regression was performed to identify the factors affecting the symptom severity of AC during COVID-19. In the regression analysis, we converted gender and previous treatment to dummy variables: male 0, female 1; interventional treatment 0, medication 1. Statistical analyses were conducted using SPSS 24.0 (IBM, Armonk, NY, USA). p value <0.05 was considered statistically significant.
Results
The number of AC patients who fulfilled all the inclusion criteria and did not meet the exclusion criteria was 350. The number of respondents was 286, with a response rate of 81.71%. The main cause of nonresponse was missing or erroneous phone numbers. All the respondents were included in the final analysis. AC patients included in the final data analysis were from northern China: Tianjin city (n = 200), Hebei province (n = 22), Shandong province (n = 18), Henan province (n = 16), Shanxi province (n = 12), Liaoning province (n = 11), and Heilongjiang province (n = 7). Apparently, according to the Chinese government's epidemic prevention policy, all the places listed above had implemented strict social control in February. Subsequently, 332 healthy subjects were followed up by telephone, of which 300 responded, showing a response rate of 90.36%.
Characteristics of the Subjects
According to the case information, all the subjects were divided into three subgroups: intervention group (n = 202), medication group (n = 84), and control group (n = 300). In the intervention group, 193 patients underwent POEM (14 received POEM as a remedial treatment after failed dilatation), 6 received Heller operation, and 3 received only balloon dilatation. On the other hand, patients in the medication group were treated with Nifedipine tablets (Shanghai Second Pharmaceutical Co., Ltd., 5 mg tid p.o. 5 min before meals). No significant difference was detected in the demographic data among the three groups except for age (Table 1).
Table 1.
Demographic characteristics of the subjects
| Intervention group (n = 202) | Medication group (n = 84) | Control group (n = 300) | Z (c2) | p value | |
|---|---|---|---|---|---|
| Age, years | 42.3±9.1 | 47.1±14.1 | 42.2±11.4 | 9.465 | 0.009 |
| Gender (male/female) | 101/101 | 44/40 | 160/140 | 0.542 | 0.763 |
| Course of disease, years | 5.6±4.2 | 5.2±3.1 | / | –0.421 | 0.674 |
| Type of AC (I/II/III) | 61/121/20 | 24/44/16 | / | 4.579 | 0.100 |
AC Symptoms Deteriorated during COVID-19
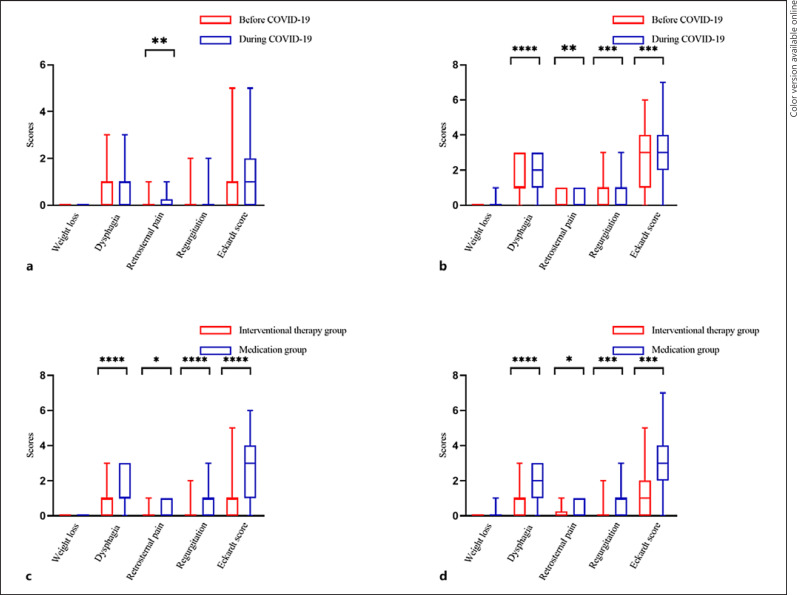
Regarding AC symptoms, only the symptoms of retrosternal pain aggravated during COVID-19 in the intervention group (p = 0.002) (Fig. 1a). However, except for weight loss, other symptoms (retrosternal pain [p < 0.01], dysphagia [p < 0.001], regurgitation [p = 0.011], and Eckardt score [p = 0.0004]) aggravated during COVID-19 in the medication group (Fig. 1b). Moreover, before and after the outbreak, almost all the symptoms (except for weight loss) in the medication group were severe than those in the intervention group (all p < 0.05) (Fig. 1c, d).
Fig. 1.
Comparison of symptoms before and during COVID-19. a Patients who had undergone POEM before and during COVID-19. b Patients who had not undergone POEM before and during COVID-19. c Patients who had or not undergone POEM before COVID-19. d Patients who had or not undergone POEM during COVID-19. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
AC Anxiety and Depression Level Deteriorated during COVID-19
Regarding psychological abnormality, all respondents were more anxious and depressed during the epidemic. Additionally, AC patients were more anxious and depressed than healthy people before and during COVID-19 (Table 2). In addition, the anxiety level of patients in the medication group was higher than that in the intervention group, both before and during COVID-19 (all p < 0.001) (Table 3).
Table 2.
Anxiety and depression before and during COVID-19
| Control group (n = 300) | AC patients (n = 286) | Z | p value | |
|---|---|---|---|---|
| Anxiety | ||||
| Before COVID-19 | 0 (0–1) | 1 (0–3) | 2.087 | <0.0001 **** |
| During COVID-19 | 0 (0–2) | 1 (0–4) | 2.326 | <0.0001 **** |
| Z | –4.477 | –6.195 | ||
| p | <0.0001**** | <0.0001 **** | ||
| Depression | ||||
| Before COVID-19 | 0 (0–2) | 1 (0–2) | 2.364 | <0.0001 **** |
| During COVID-19 | 0 (0–3) | 1 (0–4) | 2.394 | <0.0001 **** |
| Z | –7.723 | –7.210 | ||
| p | <0.0001 **** | <0.0001 **** |
*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Table 3.
Anxiety and depression of AC patients before and during COVID-19
| Intervention group (n = 202) | Medication group (n = 84) | Z | p value | |
|---|---|---|---|---|
| Anxiety | ||||
| Before COVID-19 | 0 (0–2.75) | 2 (1–3.75) | 4.881 | <0.0001 **** |
| During COVID-19 | 0 (0–2.75) | 2 (1–6) | 4.156 | <0.0001 **** |
| Z | –4.889 | –3.475 | ||
| p | <0.0001 **** | 0.001 ** | ||
| Depression | ||||
| Before COVID-19 | 2 (0–2) | 1 (0–3) | 2.896 | <0.0001 **** |
| During COVID-19 | 2 (0–4) | 1 (0–6) | 3.103 | <0.0001 **** |
| Z | –5.804 | –4.477 | ||
| p | <0.0001 **** | <0.0001 **** |
*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Diet Habits Improved in AC Patients, Especially in the Medication Group
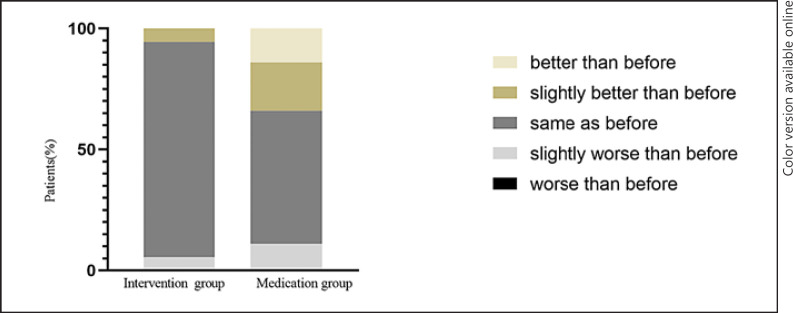
A total of 40 AC patients (11 from the interventional therapy and 29 from the medication group) reported that they paid more attention to their diet during COVID-19 than before (Fig. 2). Surprisingly, compared to those in the intervention group, patients in the medication group paid more attention to diet during COVID-19 and followed the doctor's diet guidance strictly (χ2 = 41.7, p < 0.05). However, this did not alter the fact that the symptoms of patients in the medication group were more severe during COVID-19 than before the epidemic.
Fig. 2.
Comparison of diet between groups.
Multiple Linear Regression of Affecting Factors of AC Symptoms during COVID-19
In order to avoid multicollinearity, we confirmed that no variable had a high correlation (r >±0.7) before conducting multiple regression analysis and calculated the tolerance of each independent variable. As a result, gender, age, course of the disease, previous treatment, mental state, subtypes of AC, and diet habits were independent variables, and Eckardt score was a dependent variable during COVID-19. Moreover, depression (B = 0.091, p < 0.05) and gender (female, B = 0.508, p < 0.05) were independently and positively correlated, while previous treatment (intention therapy, B = −1.880, p < 0.05) was negatively correlated with the severity of AC symptoms during COVID-19 (Table 4).
Table 4.
Multiple linear regression for factors affecting AC symptoms during COVID-19
| Variable | B | 95% CI |
t | p value | |
|---|---|---|---|---|---|
| lower | upper | ||||
| Gender | 0.508 | 0.180 | 0.836 | 3.053 | 0.002** |
| Age | 0.000 | –0.016 | 0.015 | –0.023 | 0.982 |
| Course of disease | –0.004 | –0.048 | 0.039 | –0.203 | 0.839 |
| Subtype of AC | 0.138 | –0.131 | 0.407 | 1.009 | 0.314 |
| Intention therapy | –1.880 | –1.498 | –2.262 | –9.686 | <0.0001 **** |
| Anxiety | 0.009 | –0.037 | 0.055 | 0.382 | 0.703 |
| Depression | 0.091 | 0.034 | 0.148 | 3.139 | 0.002** |
| Diet | 0.306 | –0.014 | 0.627 | 1.885 | 0.061 |
*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
Herein, we found that COVID-19 caused anxiety and depression in both AC patients and healthy individuals in China. This phenomenon was consistent with a previous study, which showed that during COVID-19, more than half of Chinese respondents rated psychological impact as moderate to severe [17], and health worries were positively associated with anxiety symptoms [5]. In addition, the psychological abnormalities during COVID-19 deteriorated the symptoms of AC. Idiopathic AC is characterized by abnormal esophageal motility and subsequent symptoms, such as dysphagia [18]. Due to persistent dysphasia, the patient's quality of life is affected severely [5]. Although psychological abnormalities have been suspected to influence the severity of AC symptoms, credible evidence is yet lacking [3, 5, 19]. To the best of our knowledge, some evidence suggested that mental disorders are associated with AC symptoms. For instance, in a retrospective investigation, 16 (34%) patients spontaneously reported that dysphagia was closely related to a psychic trauma in terms of time [5]. Compared to the previous cross-sectional designed study, the present study was a cohort study. The retrospective design was an obstacle for the present study to obtain reliable evidence. Inevitably, in a prospective intervention study, a moral hazard exists if psychological factors are used as intervention factors. However, during the outbreak of an epidemic, such as influenza, people may feel afraid of the disease or death, helplessness, and shame, and consequently be anxious and depressed [7, 17]. Therefore, by reviewing the changes in the symptoms in patients with AC during the epidemic, we elucidated the influence of psychological abnormalities on AC symptoms. Thus, this retrospective cohort study provides high-quality evidence with respect to the influence of psychological factors, such as anxiety and depression, on the AC symptoms.
Intriguingly, subgroup analysis revealed that the anxiety and depression of AC patients treated with interventional therapy were lower than those in the medication group during the epidemic outbreak. Similarly, the effect of interventional therapy is better than that of drug therapy [20, 21], and the intervention group presented a lower Eckardt score than that in the medication group before COVID-19 in the present study. We further speculated that the severity of symptoms affects patients' anxiety and depression in stressful situations.
Dietary habits affect the symptoms of the patients with AC; for instance, retrosternal pain and reflux can be aggravated by cold water or cold food and relieved by hot water [22]. Therefore, we require patients to eat warm diets, chew slowly, avoid irritating diets, and drink about 50 mL warm water during and after meals to rinse the esophagus. In this study, although AC patients in the medication group were likely to comply with the doctor's advice during COVID-19, the symptoms were aggravated and more serious than of those in the intervention group. Medication-treated patients tended to pay more attention to their diet but did not significantly change their anxiety and depression symptoms.
In addition, we explored the affecting factors of AC symptoms during COVID-19. As expected, interventional therapy was negatively correlated with the severity of AC symptoms, which was consistent with previous studies [20, 21, 23]. Gender is also related to AC, but the survey results were varied [24, 25, 26]. Conversely, in this study, women showed a higher Eckardt score during the epidemic. The difference between the results may be due to the differences in gender ratio, sample size, and research conditions of different studies. Moreover, age, course of the disease, diet habits, and subtypes were not related to AC symptoms, which could be attributed to the fact that this was a single-center study with a small sample number and short observation time.
Nevertheless, the present study has several deficiencies. First, due to the strict limitation of face-to-face communication, subjective evaluation such as a structured questionnaire rather than objective examination was used to evaluate the severity of AC. Second, the time span of observation was only about 1 month, which might have adverse effects on the study. However, with prolonged observation time, several mixed factors may be identified, and the psychological abnormalities of the population during the epidemic may change with time. Therefore, a long research duration may not always provide a reliable result.
In conclusion, the outbreak of COVID-19 has brought about anxiety and depression to AC patients and healthy people in China. In addition, depression rather than anxiety might worsen the AC symptoms. Thus, during COVID-19, interventional therapy may be a benefit factor for AC patients. However, multicenter, large sample size studies are needed to substantiate these findings in the future.
Statement of Ethics
The authors declare that they have no ethical conflicts to disclose. Written informed consent was obtained from participants. This present study was approved by the Institutional Review Board and Ethics Committee of the Tianjin Medical University General Hospital (IRB2020-WZ-096).
Conflict of Interest Statement
All authors declare no conflicts of interest in relation to this article.
Funding Statement
The authors would like to thank the Tianjin Science and Technology Commission (Grant No. 20JCQNJC00560, 20JCYBJC00450).
Author Contributions Statement
Wei Zhao, Lingyun Zhang, and Lilizhang: conception and design; Lingyun Zhang and Bangmao Wang: administrative support; Lingyun Zhang and Wei Zhao: provision of study materials or patients; Lingyun Zhang and Bangmao Wang: collection and assembly of data, data analysis, and interpretation; all authors: manuscript writing and final approval of manuscript.
Data Availability Statement
The data are not available. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Lingyun Zhang and Lili Zhang are co-first authors.
References
- 1.Pandolfino JE, Gawron AJ. Achalasia: a systematic review. JAMA. 2015;313((18)):1841–1852. doi: 10.1001/jama.2015.2996. [DOI] [PubMed] [Google Scholar]
- 2.Sadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil. 2010;22((9)):e256–61. doi: 10.1111/j.1365-2982.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- 3.Song CW, Lee SJ, Jeen YT, Chun HJ, Um SH, Kim CD, et al. Inconsistent association of esophageal symptoms, psychometric abnormalities and dysmotility. Am J Gastroenterol. 2001;96((8)):2312–2316. doi: 10.1111/j.1572-0241.2001.04035.x. [DOI] [PubMed] [Google Scholar]
- 4.Carlson DA, Gyawali CP, Roman S, Vela M, Taft TH, Crowell MD, et al. Esophageal hypervigilance and visceral anxiety are contributors to symptom severity among patients evaluated with high-resolution esophageal manometry. Am J Gastroenterol. 2020;115((3)):367–375. doi: 10.14309/ajg.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardi M, De Marchi C, Felli F, Paoletti PL, Paolini A. Cardial achalasia and emotional stress. Considerations on 47 clinical cases. Minerva Med. 1984;75((8)):405–408. [PubMed] [Google Scholar]
- 6.Maya-Mondragón J, Sánchez-Román FR, Palma-Zarco A, Aguilar-Soto M, Borja-Aburto VH. Prevalence of post-traumatic stress disorder and depression after the september 19(th), 2017 earthquake in Mexico. Arch Med Res. 2019;50((8)):502–508. doi: 10.1016/j.arcmed.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Rubin GJ, Potts HW, Michie S. The impact of communications about swine flu (influenza A H1N1v) on public responses to the outbreak: results from 36 national telephone surveys in the UK. Health Technol Assess. 2010;14((34)):183–266. doi: 10.3310/hta14340-03. [DOI] [PubMed] [Google Scholar]
- 8.Zhang WR, Wang K, Yin L, Zhao WF, Xue Q, Peng M, et al. Mental health and psychosocial problems of medical health workers during the COVID-19 epidemic in China. Psychother Psychosom. 2020;89((4)):242–250. doi: 10.1159/000507639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395((10223)):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Zheng D, Liu J, Gong Y, Guan Z, Lou D. Depression and anxiety among adolescents during COVID-19: a cross-sectional study. Brain Behav Immun. 2020;88:36–38. doi: 10.1016/j.bbi.2020.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu S, Tucker L, Wu C, Yang L. Beneficial effects of exercise on depression and anxiety during the covid-19 pandemic: a narrative review. Front Psychiatry. 2020;11:587557. doi: 10.3389/fpsyt.2020.587557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J, Zhou F, Hou W, Silver Z, Wong CY, Chang O. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. 2021;1486((1)):90–111. doi: 10.1111/nyas.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJPM, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27((2)):160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckardt AJ, Eckardt VF. Treatment and surveillance strategies in achalasia: an update. Nat Rev Gastroenterol Hepatol. 2011;8((6)):311–319. doi: 10.1038/nrgastro.2011.68. [DOI] [PubMed] [Google Scholar]
- 15.Julian LJ. Measures of anxiety: state-trait anxiety inventory (STAI), beck anxiety inventory (BAI), and hospital anxiety and depression scale-anxiety (HADS-A) Arthritis Care Res. 2011;63 Suppl 11((Suppl 110 11)):S467–72. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman G. Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theor Pract. 2010;15((5)):625–632. doi: 10.1007/s10459-010-9222-y. [DOI] [PubMed] [Google Scholar]
- 17.Achkar E. Achalasia. Gastroenterologist. 1995;3((4)):273–288. [PubMed] [Google Scholar]
- 18.Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17((5)):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue H, Sato H, Ikeda H, Onimaru M, Sato C, Minami H, et al. Per-oral endoscopic myotomy: a series of 500 patients. J Am Coll Surg. 2015;221((2)):256–264. doi: 10.1016/j.jamcollsurg.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 20.Werner YB, Costamagna G, Swanström LL, von Renteln D, Familiari P, Sharata AM, et al. Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years. Gut. 2016;65((6)):899–906. doi: 10.1136/gutjnl-2014-308649. [DOI] [PubMed] [Google Scholar]
- 21.Bergman YS, Cohen-Fridel S, Shrira A, Bodner E, Palgi Y. COVID-19 health worries and anxiety symptoms among older adults: the moderating role of ageism. Int Psychogeriatr. 2020;32((11)):1371–1375. doi: 10.1017/S1041610220001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Y, Ke M, Fang X, Zhu L, Sun X, Wang Z, et al. Response of esophagus to high and low temperatures in patients with achalasia. J Neurogastroenterol Motil. 2012;18((4)):391–398. doi: 10.5056/jnm.2012.18.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sediqi E, Tsoposidis A, Wallenius V, Axelsson H, Persson J, Johnsson E, et al. Laparoscopic Heller myotomy or pneumatic dilatation in achalasia: results of a prospective, randomized study with at least a decade of follow-up. Surg Endosc. 2021;35((4)):1618–1625. doi: 10.1007/s00464-020-07541-4. [DOI] [PubMed] [Google Scholar]
- 24.Hernández Mondragón OV, González Martinez MA, Blancas Valencia JM, Hernandez Reyes ML, Solórzano Pineda OM, Blanco Velasco G. Long-term quality of life after peroral endoscopic myotomy remains compromised in patients with achalasia type III. Endoscopy. 2017;49((12)):1209–1218. doi: 10.1055/s-0043-117401. [DOI] [PubMed] [Google Scholar]
- 25.Patel A, Patel A, Mirza FA, Soudagar S, Sayuk GS, Gyawali CP. Achalasia symptom response after Heller myotomy segregated by high-resolution manometry subtypes. J Gastroenterol. 2016;51((2)):112–118. doi: 10.1007/s00535-015-1088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Z, Sun Z, Sheng J, Li N, Lv H, Li S, et al. Discussion on the role of psychological factors in the pathogenesis of achalasia. J Chin Physician. 2010;12((008)):1145–1147. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not available. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.