Abstract
Pulmonary hypertension (PH) is a hemodynamic and pathophysiologic state present in many cardiovascular, respiratory, and systemic diseases. PH is considered to have a higher risk of cardiovascular events and mortality. The most common type of functional tricuspid regurgitation (FTR) is associated with PH. The aim of this study was to evaluate the association between FTR severity and mortality in PH in western China. This is a retrospective analysis in PH patients and all patients underwent right‐heart catheterization (RHC) for hemodynamic measurements. The FTR severity was determined according to the guidelines. Uni‐ and multivariate analyses were used to identify risk factors for mortality. From 2015 to 2021, 136 patients with PH with a median age of 50 years (interquartile range [IQR]: 35–64 years). During 26‐month median follow‐up (mean 27.7 ± 15.1 months), 40 (29.2%) patients died (mean after 21.7 ± 14.1 months). In the univariate Cox regression analysis, World Health Organization functional class (WHO FC) III/IV, elevated B‐type natriuretic peptide, pulmonary vascular resistance (≥16.2 Wood units), pulmonary artery oxygen saturation, severe FTR and right ventricular diameter/left ventricular diameter (≥0.62) were significantly associated with mortality. In the multivariate Cox regression analysis, severe FTR, WHO FC III/IV, and right ventricular end‐diastolic pressure (RVEDP) were risk factors for mortality. Severe FTR at baseline was strongly associated with mortality in both precapillary and postcapillary PH patients, independent of the other risk factors as RVEDP, HO FC III/IV, optimal pulmonary arterial hypertension targeted therapy.
Keywords: functional tricuspid regurgitation, mortality, pulmonary hypertension
INTRODUCTION
Pulmonary hypertension (PH), defined by increased pressure within the pulmonary vasculature, is a hemodynamic and pathophysiologic state present in a wide variety of cardiovascular, respiratory, and systemic diseases. PH is associated with progressive right ventricular (RV) remodeling with an increase in contractility, diastolic stiffening, eventual RV dilation, and clinical right heart failure. 1 PH is considered to have a higher risk of cardiovascular events and mortality. 2 , 3 , 4
Tricuspid regurgitation (TR) has been identified as a significant medical challenge in aging societies. TR, especially moderate/severe TR, is associated with increased mortality in patients with heart failure, atrial fibrillation, mitral valve surgery, and postoperative TR from left‐sided valve surgery. 5 Functional tricuspid regurgitation (FTR) develops due to structural alterations in the right heart myocardial geometry, leading to tricuspid annulus dilation and/or leaflet tethering, which are both associated with impaired leaflet coaptation. PH arising from causes other than left‐side heart disease, such as idiopathic pulmonary arterial hypertension (PAH), pulmonary embolism, and chronic pulmonary disease, can also cause TR due to increased RV afterload, RV dilation, and dysfunction. 6 The most common type of FTR is associated with PH.
The 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines recommend using the peak TR velocity as the key variable for assigning the echocardiographic probability of PH, additional variables related to RV morphology and function are used to define the echocardiographic probability of PH, this probability can be used to decide the need for further investigation, including cardiac catheterization in individual patients. Although echocardiography is widely used in screening and monitoring in PH patients follow‐up, few studies have evaluated the prognostic value of the FTR in all forms of PH patients. In this study, we sought to identify FTR in PH and correlate these characteristic findings with long‐term clinical outcomes, we also evaluated the association between FTR severity and mortality in PH in western China after adjustment for clinical, echocardiographic, and invasive hemodynamic parameters, and determined the association of changes in FTR severity with survival in PH patients. We hypothesized that a moderate or severe degree of FTR assessed noninvasively and conveniently by echocardiography has a predictive value for death in the long‐term follow‐up of PH.
METHOD
Study population
A retrospective analysis was performed entailing PH patients who were admitted to the Cardiology Department of The First Affiliated Hospital of Chongqing Medical University between October 1, 2015, and October 8, 2021. The analysis included 136 consecutive patients with complete echocardiographic and invasive hemodynamic data at the time of enrollment and complete follow‐up data. PH patients with Groups 1–4 were enrolled in the study. We excluded patients with severe comorbidities with limited life expectancy (end‐stage kidney or liver disease, metastatic cancer). Of these patients, 101 had serial transthoracic echocardiograms with a time interval of at least 3 months between echocardiograms. Age, sex, body mass index, major comorbidities, and laboratory tests were extracted from the electronic health records. The time to death was recorded for all patients. Clinical management was determined by PAH‐specialized physicians. PH is defined as an increase in mean pulmonary arterial pressure (mPAP) ≥25 mmHg at rest as assessed by right heart catheterization (RHC). Precapillary PH was defined by mPAP ≥25 mmHg, pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg, and pulmonary vascular resistance (PVR) > 3 Wood units (WU) at rest as assessed by RHC. Postcapillary PH is hemodynamically defined as mPAP ≥25 mmHg and PAWP > 15 mmHg. 7 The clinical classification of PH is intended to categorize multiple clinical conditions into five groups according to 2015 ESC/ERS Guidelines. The study was approved by the institutional review board.
Hemodynamic evaluation
All patients underwent RHC at rest for hemodynamic measurements. Mean right atrial pressure (RAP), systolic pulmonary artery pressure (sPAP), mPAP, and PAWP were measured. The cardiac output was measured in triplicate with the thermodilution technique (Edwards Lifesciences World Trade) in patients without congenital heart disease, and venous oxygen saturation was determined from the pulmonary arteries. PVR, cardiac index, and diastolic pressure gradient were calculated. Mixed‐venous blood was obtained during RHC.
Echocardiographic assessment
Transthoracic echocardiograms were performed at rest using commercially available equipment (Vivid7, GE Healthcare; iE33, Philips Medical Systems). The severity of TR was determined based on the vena contracta width and density of the continuous wave signal and was classified as mild, moderate, or severe according to the guidelines. The maximum measurement was taken. Severe TR was defined by a dense continuous wave signal as dense or denser than the forward flow with vena contracta ≥ 7 mm in width. Moderate TR was defined by a complete continuous wave signal that was moderate in density compared with the forward flow and a vena contracta width <7 mm. Mild TR as an exclusion was defined by an incomplete or low‐intensity continuous wave signal and a vena contracta width < 7 mm. 8 Right atrial size was obtained from the apical four‐chamber view at end‐systole. Right ventricle size was obtained from the parasternal left ventricular (LV) long axial view at end‐diastole. Tricuspid annular plane systolic excursion (TAPSE) was obtained with M‐mode recording of the distance of systolic excursion of the RV annular segment along its longitudinal plane in the apical four‐chamber view. RV S′ was evaluated by the peak systolic velocity of the tricuspid annulus by pulsed wave tissue Doppler imaging in a focused apical four‐chamber view. SPAP was calculated with the following formula: pressure gradient derived from the peak TR velocity plus the RAP according to the guidelines. RAP was estimated from the inferior vena cava diameter and inspiratory collapse. 9 LV end‐diastolic diameter was measured by M‐mode from the parasternal long‐axis view. LV ejection fraction (EF) was calculated by the Quinones method or Simpson's method in patients with segmental wall abnormalities. Left atrium, LV diameters, and LV mass were calculated from the two‐dimensional linear measurements obtained on the parasternal LV long‐axis view as recommended. 10
Clinical outcomes
All‐cause death was recorded during follow‐up. At study termination (October 8, 2021), the vital status of each patient was confirmed by a review of medical records and telephone interviews. Patients were excluded if they dropped out during follow‐up. All patients were followed up until the occurrence of clinical events or the end of the study period. Patients were evaluated at follow‐up as non‐responders (died) or as responders (alive).
Statistical analysis
Categorical variables are displayed as numbers (percentage), and continuous variables are expressed as the mean ± standard deviation or median (interquartile range [IQR]) with normal and nonnormal distributions, which were determined using the Kolmogorov–Smirnov test.
The patients were divided into groups according to their TR grade (none/minimal, mild, moderate, or severe). Continuous variables were compared between the groups using the t test or Mann–Whitney test, as appropriate. Categorical variables were compared using the χ 2 test or Fisher's exact test. The association of the clinical, functional, and echocardiographic parameters with the endpoint was identified by Cox proportional hazards models in the univariable analysis. A multivariable Cox proportional hazards regression analysis was performed to investigate the independent associations of mortality at the time‐point of significant TR in three models. Receiver operating characteristic curves were constructed to determine an optimal cutoff value for the variables retained in the Cox multivariate regression analysis. Overall survival curves were estimated with the Kaplan–Meier method and compared with the log‐rank test. A two‐tailed p < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS statistical software package (SPSS 26.0; IBM SPSS Statistics).
RESULTS
Baseline characteristics
The present study included 136 patients with PH with a median age of 50 years (IQR: 35–64 years). The baseline characteristics of the study population according to the grades of TR are illustrated in Table 1. Most of the patients were female (69.12%) and had precapillary PH (89.71%). Age, sex, heart rate, systolic blood pressure, World Health Organization functional class (WHO FC), hemoglobin, creatinine, urea, and targeted drugs were equally distributed. A history of congenital heart disease was more frequent in the patients with mild/moderate FTR, but a history of chronic obstructive pulmonary disease was more frequent in the severe FTR group (p < 0.05). Regarding the hemodynamic variables, the RAPs were significantly higher in the severe FTR group than in the mild/moderate FTR group (p < 0.05). Systolic and diastolic pulmonary arterial pressure, superior vena cava oxygen saturation, and pulmonary artery oxygen saturation were significantly lower in the severe FTR group than in the mild or moderate FTR group (p < 0.05). Echo assessment showed no differences in EF, sPAP, or TAPSE but a worsening right atrial diameter, RV diameter, and pericardial effusion in the severe FTR group (p < 0.05) (Tables 2 and 3).
Table 1.
Baseline demographics, clinical characteristics, and treatment regimens of the overall cohort and stratified by the FTR.
| All (n = 136) | Mild/moderate FTR (n = 83) | Severe FTR (n = 53) | p value | |
|---|---|---|---|---|
| Age (years) | 49.50 (35.00–63.75) | 47 (32.00–63.00) | 53 (36–65) | 0.121 |
| Sex, n (%) | 0.534 | |||
| Female | 94 (69.12%) | 59 (71.08%) | 35 (66.04%) | |
| Male | 42 (30.88%) | 24 (28.92%) | 18 (33.96%) | |
| Haemodynamic, n (%) | 0.163 | |||
| Precapillary | 122 (89.71%) | 77 (92.77%) | 45 (84.91%) | |
| Postcapillary | 5 (3.68%) | 1 (1.20%) | 4 (7.55%) | |
| Pre‐ and postcapillary | 9 (6.62%) | 5 (6.02%) | 4 (7.55%) | |
| Classification of pulmonary hypertension, n (%) | 0.286 | |||
| Group 1 | 79 (58.09%) | 54 (65.06%) | 25 (47.17%) | |
| Group 2 | 11 (8.09%) | 5 (6.02%) | 6 (11.32%) | |
| Group 3 | 14 (10.29%) | 8 (9.64%) | 6 (11.32%) | |
| Group 4 | 29 (21.32%) | 15 (18.07%) | 14 (26.42%) | |
| Group 5 | 3 (2.21%) | 1 (1.20%) | 2 (3.77%) | |
| Heart rate (bpm) | 86.06 ± 15.56 | 84.51 ± 13.72 | 88.49 ± 17.94 | 0.146 |
| Systolic blood pressure (mmHg) | 113.25 ± 18.92 | 114.98 ± 19.46 | 110.58 ± 17.91 | 0.189 |
| Body mass index (kg/m2) | 21.91 ± 4.14 | 21.85 ± 4.01 | 22.00 ± 4.37 | 0.836 |
| World Health Organization functional class, n (%) | 0.532 | |||
| I | 4 (2.94%) | 4 (4.82%) | 0 (0.00%) | |
| II | 49 (36.03%) | 30 (36.14%) | 19 (35.85%) | |
| III | 67 (49.26%) | 39 (46.99%) | 28 (52.83%) | |
| IV | 16 (11.76%) | 10 (12.05%) | 6 (11.32%) | |
| Chronic obstructive pulmonary disease, n (%) | 22 (16.18%) | 9 (10.84%) | 13 (24.53%) | 0.035 |
| History of congenital heart disease, n (%) | 25 (18.38%) | 20 (24.10%) | 5 (9.43%) | 0.041 |
| Surgery for congenital heart disease, n (%) | 8 (5.88%) | 4 (4.82%) | 4(7.55%) | 0.711 |
| Elevated B‐type natriuretic peptide | 87 (63.97%) | 50 (60.24%) | 37 (69.81%) | 0.101 |
| Hemoglobin (g/L) | 141.63 ± 27.68 | 144.91 ± 26.72 | 136.60 ± 28.61 | 0.089 |
| Urea (mmol/L) | 6.30 (4.90‐7.50) | 6.00 (4.90‐7.40) | 6.45 (5.30‐8.00) | 0.414 |
| Creatinine (umol/L) | 75.29 ± 38.99 | 71.85 ± 24.74 | 80.53 ± 53.78 | 0.214 |
| Targeted therapy, n (%) | 0.653 | |||
| Monotherapy | 49 (36.03%) | 31 (37.35%) | 18 (33.96%) | |
| Double combination | 49 (36.03%) | 32 (38.55%) | 17 (32.08%) | |
| Triple combination | 11 (8.09%) | 6 (7.23%) | 5 (9.43%) | |
| Endothelin receptor antagonist, n (%) | 55 (40.44%) | 39 (46.99%) | 16 (30.19%) | 0.052 |
| Phosphodiesterase type 5 inhibitor, n (%) | 64 (47.06%) | 39 (46.99%) | 25 (47.17%) | 0.983 |
| Guanylate cyclase stimulators, n (%) | 14 (10.29%) | 8 (9.64%) | 6 (11.32%) | 0.753 |
| Prostacyclinanalogues, n (%) | 39 (28.68%) | 25 (30.12%) | 14 (26.42%) | 0.641 |
| Prostacyclin receptor agonists, n (%) | 4 (2.94%) | 2 (2.41%) | 2 (3.77%) | 0.643 |
| Death, n (%) | 40 (29.41%) | 16 (19.28%) | 24 (45.28%) | 0.001 |
Abbreviation: FTR, functional tricuspid regurgitation.
Table 2.
Right heart catheterization characteristics of the overall cohort and stratified by the FTR at baseline.
| All (n = 136) | Mild/moderate FTR (n = 83) | Severe FTR (n = 53) | p Value | |
|---|---|---|---|---|
| Right atrial pressure (mmHg) | 11.34 ± 6.77 | 10.24 ± 6.45 | 13.08 ± 6.97 | 0.019 |
| Mean right atrial pressure (mmHg) | 8.17 ± 5.54 | 7.26 ± 5.04 | 9.62 ± 6.03 | 0.016 |
| Systolic pulmonary arterial pressure (mmHg) | 68.00 (54.25–87.00) | 72.00 (55.00–97.00) | 64.00 (53.50–79.00) | 0.048 |
| Mean pulmonary arterial pressure (mmHg) | 46.00 (38.25–60.00) | 39.00 (48.00–66.00) | 44.00 (35.00–52.00) | 0.028 |
| Diastolic pulmonary arterial pressure (mmHg) | 32.0 (25.00–44.00) | 35.00 (26.00–49.00) | 29.00 (22.50–35.50) | 0.028 |
| Cardiac output (L/min) | 3.83 (2.92–4.92) | 3.95 (4.02–4.79) | 3.40 (2.70–5.30) | 0.346 |
| Cardiac index (L/min/m2) | 2.18 (1.74–3.08) | 2.24 (1.76–2.89) | 1.97 (1.61–3.65) | 0.941 |
| Pulmonary vascular resistance (WU) | 9.13 (5.61–14.73) | 9.51 (6.30–14.81) | 8.00 (5.23–14.62) | 0.286 |
| Right ventricular end‐diastolic pressure (mmHg) | 10.00 (4.00–15.00) | 9.00 (4.00–16.25) | 10.00 (3.00–15.00) | 0.823 |
| Superior vena cava oxygen saturation (%) | 61.25 ± 11.02 | 62.98 ± 9.62 | 58.38 ± 12.62 | 0.022 |
| Pulmonary artery oxygen saturation (%) | 63.36 ± 11.41 | 65.04 ± 10.36 | 60.78 ± 12.53 | 0.038 |
| Mixed vena cava oxygen saturation (%) | 61.42 ± 10.54 | 62.67 ± 9.35 | 59.63 ± 11.93 | 0.122 |
| Mean pulmonary artery wedge pressure (mmHg) | 9.00 (6.00–13.00) | 9.00 (6.00–12.00) | 9.50 (5.00–13.50) |
Abbreviation: FTR, functional tricuspid regurgitation; WU, Wood units.
Table 3.
Echocardiography characteristics of the overall cohort and stratified by the FTR at baseline.
| All (n = 136) | Mild/moderate FTR (n = 83) | Severe FTR (n = 53) | p Value | |
|---|---|---|---|---|
| Right ventricular (mm) | 30.50 (25.00–35.00) | 26.00 (24.00–33.00) | 34.00 (30.00–39.00) | 0.000 |
| Right atrium (mm) | 47.00 (42.00–54.75) | 44.00 (38.00–48.00) | 54.00 (48.00–60.50) | 0.000 |
| Left atrium (mm) | 29.00 (26.00–33.00) | 29.00 (26.00–32.00) | 30.00 (27.00–35.50) | 0.060 |
| Left ventricular (mm) | 41.21 ± 7.38 | 41.72 ± 6.03 | 40.42 ± 9.13 | 0.316 |
| Left ventricular ejection fraction (%) | 65.00 (61.00–68.00) | 65.00 (62.00–68.00) | 66.00 (60.50–68.00) | 0.677 |
| Systolic pulmonary arterial pressure (mmHg) | 83.33 ± 25.83 | 82.68 ± 24.52 | 84.35 ± 27.97 | 0.716 |
| Tricuspid annular plane systolic excursion (mm) | 15.85 ± 4.02 | 16.25 ± 3.67 | 15.23 ± 4.46 | 0.148 |
| Right ventricular S′ (cm/s) | 10.08 ± 2.50 | 10.20 ± 2.42 | 9.90 ± 2.64 | 0.533 |
| Right ventricular diameter/left ventricular diameter | 0.85 ± 1.10 | 0.69 ± 0.20 | 1.11 ± 1.73 | 0.031 |
| Pericardial effusion | 18 (13.24%) | 7 (8.43%) | 11 (20.75%) | 0.039 |
Abbreviation: FTR, functional tricuspid regurgitation.
Treatment and outcome
A total of 109 (80.15%) patients received PAH‐targeted medications. Approximately 36.03% received monotherapy, 36.03% received doublet combination, and 8.09% received triple combination. Approximately 40.44% received endothelin receptor antagonists, 47.06% received phosphodiesterase type 5 inhibitors, and 28.68% received prostacyclin analogues. During a median follow‐up period of 2.2 years (mean 2.31 ± 1.27 months), 40 (29.41%) patients died (mean after 1.86 ± 1.22 years): 24 in patients with severe FTR (45.28%; 1‐, 3‐, and 5‐year survival 75.58%, 38.53%, and 19.27%, respectively) compared to 16 patients with mild or moderate FTR (19.28%; 1‐, 3‐, and 5‐year survival 84.78%, 73.27%, and 65.56%, respectively).
Echocardiographic changes in responders and nonresponders
Overall, 101 patients with serial echocardiograms were included in the analysis of echocardiographic changes and 30 patients were nonresponders. Patients of reducing in RV diameter and right atrium diameter were significantly more frequent in responders than in nonresponders (p < 0.05). Systolic pulmonary arterial pressure decreased, FTR regression and RV diameter/LV diameter (RV/LV) diminution were more frequent in responders but no statistical significance was found (Table 4).
Table 4.
Echocardiographic changes in responders and nonresponders.
| Responders (n = 71) | Nonreponders (n = 30) | p Value | |
|---|---|---|---|
| Right ventricular diameter diminution | 42 (59.2%) | 11 (36.7%) | 0.039 |
| Right atrium diameter diminution | 42 (59.2%) | 11 (36.7%) | 0.039 |
| Systolic pulmonary arterial pressure decreased | 41 (57.7%) | 14 (46.7%) | 0.307 |
| FTR regression | 19 (26%) | 3 (10%) | 0.062 |
| Right ventricular diameter/left ventricular diameter diminution | 42 (59.2%) | 14 (46.7%) | 0.249 |
Abbreviation: FTR, functional tricuspid regurgitation.
Predictors for the composite endpoint
The results of the univariate Cox regression analysis are shown in Table 5. WHO FC class III/IV (hazard ratio [HR] 3.263, 95% confidence interval [CI] 1.501–7.094, p = 0.003], elevated B‐type natriuretic peptide (HR 7.045, 95% CI 1.008–54.377, p = 0.049), PVR ( ≥ 16.2 WU) (HR 2.356, 95% CI 1.194–4.648, p = 0.013), and pulmonary artery oxygen saturation (HR 0.965, 95% CI 0.937–0.995, p = 0.020) were significantly associated with mortality. For the echocardiographic parameters, severe FTR (HR 2.534, 95% CI 1.343–4.784, p = 0.004) and RV/LV (≥0.62) (HR 3.884, 95% CI 1.517–9.942, p = 0.005) were identified as predictors of adverse outcomes.
Table 5.
Univariate Cox proportional hazards model for all‐cause mortality.
| Hazard ratio (95% CI) | p Value | |
|---|---|---|
| Age (years) | 1.013 (0.994–1.033) | 0.192 |
| Sex (male) | 0.946 (0.472–1.896) | 0.875 |
| Heart rate (bpm) | 1.007 (0.987–1.028) | 0.478 |
| Systolic blood pressure (mmHg) | 0.991 (0.971–1.010) | 0.344 |
| Body mass index (kg/m2) | 1.016 (0.938–1.101) | 0.956 |
| World Health Organization functional class (III/IV) | 3.263 (1.501–7.094) | 0.003 |
| Balloon pulmonary angioplasty | 0.315 (0.075–1.317) | 0.113 |
| Targeted therapy | 1.707 (0.667–4.366) | 0.265 |
| Elevated B‐type natriuretic peptide | 7.045 (1.008–54.377) | 0.049 |
| Right heart catheterization | ||
| Systolic pulmonary arterial pressure (mmHg) | 0.997 (0.986–1.009) | 0.633 |
| Mean pulmonary arterial pressure (mmHg) | 0.995 (0.977–1.013) | 0.569 |
| Diastolic pulmonary arterial pressure (mmHg) | 0.993 (0.972–1.015) | 0.517 |
| Right atrial pressure (mmHg) | 1.020 (0.975–1.067) | 0.392 |
| Mean right atrial pressure (mmHg) | 1.031 (0.973–1.093) | 0.303 |
| Cardiac output (L/min) | 1.025 (0.988–1.064) | 0.186 |
| Cardiac index (L/min/m2) | 1.052 (0.985–1.124) | 0.131 |
| Pulmonary vascular resistance (≥16.2 WU) | 2.356 (1.194–4.648) | 0.013 |
| Right ventricular end‐diastolic pressure (mmHg) | 1.026 (1.000–1.054) | 0.052 |
| Mean pulmonary artery wedge pressure (mmHg) | 1.023 (0.983–1.065) | 0.262 |
| Pulmonary artery oxygen saturation (%) | 0.965 (0.937–0.995) | 0.020 |
| Mixed vena cava oxygen saturation (%) | 0.977 (0.945–1.010) | 0.165 |
| Echocardiography | ||
| Right ventricular (mm) | 1.016 (0.979–1.054) | 0.406 |
| Right atrium (mm) | 1.015 (0.986–1.045) | 0.306 |
| Left atrium (mm) | 0.988 (0.940–1.039) | 0.646 |
| Left ventricular (mm) | 0.979 (0.937–1.024) | 0.355 |
| Ejection fraction (%) | 0.989 (0.944–1.036) | 0.640 |
| Systolic pulmonary arterial pressure (mmHg) | 0.999 (0.987–1.011) | 0.853 |
| Tricuspid annular plane systolic excursion (≤17.5 mm) | 0.482 (0.229–1.014) | 0.055 |
| Right ventricular S′ (cm/s) | 0.980 (0.857–1.119) | 0.761 |
| Tricuspid annular plane systolic excursion/systolic pulmonary arterial pressure (mm/mmHg) | 0.174 (0.007–4.042) | 0.276 |
| Right ventricular diameter/left ventricular diameter (≥0.62) | 3.884 (1.517–9.942) | 0.005 |
| Severe functional tricuspid regurgitation | 2.534 (1.343–4.784) | 0.004 |
| Pericardial effusion | 1.880 (0.862–4.100) | 0.113 |
Abbreviations: 95% CI, 95% confidence interval; WU, Wood units.
Multivariate Cox regression analysis. We first included the eligible parameters that could be associated with the outcomes. Severe FTR (HR 2.236, 95% CI 1.058–4.723, p = 0.035), WHO FC III/IV (HR 3.762, 95% CI 1.381–10.252, p = 0.010) and RVEDP (HR 1.039, 95% CI 1.013–1.065, p = 0.003) showed independent associations with the endpoint. Then, the mixed vena cava oxygen saturation, RV/LV, PAH monotherapy, combination targeted therapy, and RV function‐related variable TAPSE were adjusted, and RVEDP was the independent predictor (HR 1.042, 95% CI 1.015–1.070, p = 0.002) that was calculated by adding the predictive value of severe FTR (HR 2.485, 95% CI 1.148–5.381, p = 0.021) and WHO FC III/IV (HR 2.538, 95% CI 1.065–6.052, p = 0.036) for mortality. Last, we analyzed whether other characteristics (sex, age, TAPSE, RV/LV, and targeted therapy) affected the impact of severe FTR on mortality (HR 2.456, 95% CI 1.205–5.003, p = 0.013). WHO FC III/IV (HR 2.546, 95% CI 1.098–5.905, p = 0.029), and RVEDP (HR 1.040, 95% CI 1.015–1.067, p = 0.002) continued to demonstrate a significantly increased risk of mortality (Table 6).
Table 6.
Multivariate analysis of the association between severe TR and the all‐cause mortality.
| Hazard ratio (95% CI) | p Value | |
|---|---|---|
| Model 1 | ||
| Severe functional tricuspid regurgitation | 2.236 (1.058–4.723) | 0.035 |
| World Health Organization functional class III/IV | 3.762 (1.381–10.252) | 0.010 |
| Right ventricular end‐diastolic pressure (mmHg) | 1.039 (1.013–1.065) | 0.003 |
| Right ventricular diameter/left ventricular diameter (≥0.62) | 1.698 (0.602–4.793) | 0.317 |
| Elevated B‐type natriuretic peptide | 3.426 (0.413–28.425) | 0.254 |
| Model 2 | ||
| Severe functional tricuspid regurgitation | 2.485 (1.148–5.381) | 0.021 |
| World Health Organization functional class III/IV | 2.538 (1.065–6.052) | 0.036 |
| Right ventricular end‐diastolic pressure (mmHg) | 1.042 (1.015–1.070) | 0.002 |
| Mixed vena cava oxygen saturation, (%) | 0.992 (0.959–1.026) | 0.650 |
| Tricuspid annular plane systolic excursion (≤17.5 mm) | 1.412 (0.569–3.502) | 0.457 |
| Right ventricular diameter/left ventricular diameter (≥0.62) | 1.233 (0.426–3.565) | 0.700 |
| PAH monotherapy | 1.365 (0.524–3.554) | 0.524 |
| Combination targeted therapy | 0.803 (0.2988–2.162) | 0.664 |
| Model 3 | ||
| Severe functional tricuspid regurgitation | 2.456 (1.205–5.003) | 0.013 |
| World Health Organization functional class III/IV | 2.546 (1.098–5.905) | 0.029 |
| Right ventricular end‐diastolic pressure (mmHg) | 1.040 (1.015–1.067) | 0.002 |
| Mixed vena cava oxygen saturation, (%) | 0.986 (0.949–1.024) | 0.454 |
| Sex(male) | 0.988 (0.478–2.042) | 0.975 |
| Age (years) | 1.011 (0.989–1.034) | 0.338 |
| Tricuspid annular plane systolic excursion (≤17.5 mm) | 0.675 (0.279–1.634) | 0.393 |
| Targeted therapy | 2.510 (0.740–8.515) | 0.140 |
Abbreviations: 95% CI, 95% confidence interval; PAH, pulmonary arterial hypertension; TR, tricuspid regurgitation.
The Kaplan–Meier survival curve (Figure 1) showed higher mortality with severe FTR at 5 years. The prognosis of the patients with severe FTR was significantly worse than that of patients with mild or moderate FTR (log‐rank χ 2 8.950, p = 0.003. The patients with WHO FC III/IV had a significantly higher risk of mortality than the patients with WHO class I–II (log‐rank χ 2 10.111, p = 0.001). The patients were further divided into three groups: Group A—mild/moderate FTR and WHO FC I/II; Group C—both Severe FTR and WHO FC III/IV; Group B—the remaining. The prognosis of Group C was significantly worse than that of Group A (log‐rank χ 2 16.407, p < 0.001) and Group B (log‐rank χ 2 6.517, p = 0.011). Group B had a significantly higher risk of endpoint events than Group A (log‐rank χ 2 5.522, p = 0.019).
Figure 1.
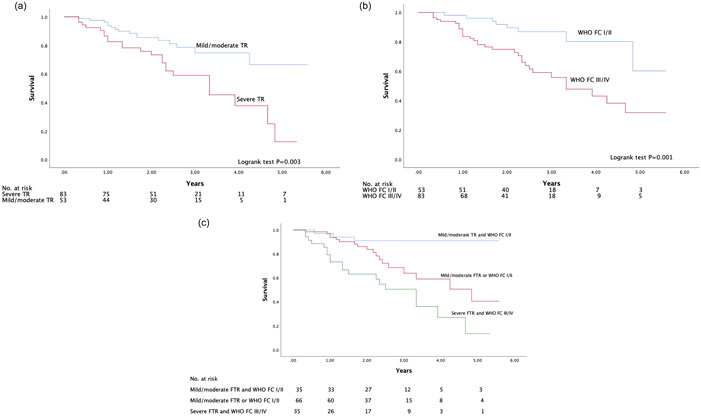
(a) Kaplan–Meier curves for the probability of mortality in patients with severe TR and mild/moderate FTR. (b) Kaplan–Meier curves for the probability of mortality in patients with WHO FC I/II and WHO FC III/IV. (c) Kaplan–Meier curves for the probability of mortality stratified by the combination of FTR and WHO FC. FTR, functional tricuspid regurgitation; TR, tricuspid regurgitation; WHO FC, World Health Organization functional class.
DISCUSSION
In this study, we evaluated the role of clinical, RHC, and echocardiographic parameters in the prognosis of patients with catheterization‐confirmed PH in western China. Our findings demonstrate that FTR severity remained an important prognostic marker and that severe FTR was strongly associated with mortality. This outcome was independent of the other risk factors significantly associated with mortality, such as RVEDP and WHO FC III/IV. FTR severity added value to WHO FC in improving the risk stratification of patients with PH. Severe FTR and WHO FC III/IV indicate patients at a particularly high risk of mortality.
Previous studies reported that TR is a prognostic marker for patients with PAH. Grapsa et al. was comprised of 777 patients with precapillary PH, and moderate or severe TR was an independent predictor of mortality. 11 Severe TR was previously associated with poor prognosis in patients with PAH. Among 727 newly diagnosed PAH patients, severe TR was shown to be an independent predictor for a greater 5‐year mortality risk (HR 1.83 95 CI 1.38–2.41; p < 0.001). 12 We also found that severe FTR was associated with poor survival (HR 2.634 in our cohort) in both precapillary and postcapillary PH patients independent of the clinical and RHC indexes. Schneider et al. found that moderate and severe FTR proved to be independent predictors of survival in PH patients. 13 However, the patients in their study were classified as having PH according to the respective maximal FTR velocity signal rather than being diagnosed according to RHC. The relationship of FTR grades with mortality in various populations of patients was assessed in a meta‐analysis. Wang et al. confirmed that moderate/severe FTR is associated with an increased mortality risk, which appears to be independent of pulmonary arterial pressures and RV dysfunction. 5 We also showed that severe FTR was closely related to all‐cause mortality even after adjusting for RV systolic function, WHO FC, and targeted therapy. According to our findings, baseline FTR may play an important role in prognosis for PH patients. Thus, the identification of established baseline risk parameters and also the assessment of FTR might be of prognostic value for the stratification of long‐term risk, regardless of whether they received either monotherapy or combination therapy.
However, left untreated, significant TR is associated with progressive RV dilation and dysfunction, heart failure, and increased mortality. 14 FTR represents an important unmet treatment need given its prevalence, adverse prognostic impact, and symptom burden associated with progressive right‐sided heart failure. 15 Patients with PH‐related FTR morphology may not all be appropriate annular repair candidates. Of note, correcting TR in patients with PAH or PH in (nonvalvular) left heart disease (LHD) with significantly elevated PVR and/or RV dysfunction must be considered with great caution, as this may be hazardous. 16 Patient selection appears crucial, and a comprehensive diagnostic approach integrating imaging modalities and invasive hemodynamic assessment is necessary in the evaluation process before tricuspid valve repair, particularly since echocardiography underestimates sPAP in the presence of severe TR. Hence, RHC is recommended in patients with severe TR with or without LHD before surgical or interventional valve repair in 2022 ESC/ERS guidelines. 17 Although severe FTR may be a relevant prognostic factor in patients with PH, the treatment decision need multidisciplinary teams to evaluate.
Xanthouli et al. enrolled 90 PAH patients without comorbidities and showed that WHO FC was significantly associated with survival. 18 In the Sildenafil for Improving Outcomes after Valvular Correction cohort, WHO FC III was related to mortality compared with WHO FC I or II. 19 We also demonstrated that the WHO FC was a significant predictor for survival in the entire sample of PH patients. However, Stadler et al. described that the WHO FC was not a predictor of survival in 259 PH patients. 20 This may be explained by the small sample size of this subgroup; most of our patients were PAH patients. Based on the combination of FTR and WHO FC, a three‐group risk‐stratification approach was built that enables for an individual patient to be allocated to a definitive risk group and makes it possible to identify a closed “cross talk” between the simply and widely available echocardiographic parameters and functional performance. In addition, it appears that baseline FTR with WHO FC III/IV is associated with vastly worse long‐term outcomes. Thus, our noninvasive risk‐stratification strategy of FTR and WHO FC could help to evaluate the prognosis of PH patients more accurately.
Our study proposed that RVEDP had an HR of 1.04 (CI: 1.015–1.067) for mortality in PH patients. The underlying mechanisms are that with increasing TR, the right ventricle dilates even further, and the RV systolic function progressively worsens, causing an increase in the RV diastolic pressure and an interventricular septum shift toward the left ventricle with the degree of distortion quantified by the LV eccentricity index, which was demonstrated to correlate with PH hospitalization 21 or composite adverse outcomes. 22 Bermejo et al. found that the catheterization variable mPAP was related to survival in valvular heart disease–PH. 19 Apitz et al. found that dPAP was not significantly predictive of death in children with PAH. 23 In our study, neither mPAP nor dPAP was associated with poor outcomes. This lack of association may be attributable to the fact that elevated PAP in PH is not an indicator of advanced RV systolic or diastolic dysfunction.
We found that the presence of severe FTR was closely related to the degree of RA/RV dilatation and RAP. The papillary muscles that support the TV are smaller than those that support the mitral valve, and they are often multiple and are variably subdivided. Each papillary muscle connects to the homolateral leaflet via the chordae tendineae, unlike the LV papillary muscles, each of which provides chordae tendineae to each mitral leaflet. This anatomical organization explains the greater ease with which the tricuspid annular expands as a result of right heart cavity enlargement. 24 Severe right ventricle dilation secondary to PH leads to lateral and apical papillary muscle displacement and subsequent leaflet tethering, generating functional valve insufficiency. 6 , 25 When RV dysfunction occurs, TAPSE might be pseudonormalized. Kessel et al. evaluated 57 patients with PH and RV dysfunction and pseudonormalized TAPSE values and showed that TAPSE was not a significant predictor of mortality. 26 In our cohort, most patients had dilated RVs with severely impaired systolic function, which may result in pseudonormalization of TAPSE. Therefore, in our multivariate analysis, we also did not find that TAPSE was associated with survival.
Several limitations in our study should be considered. First, the subjects in this study were all collected from a single center in western China, the sample size was limited, and validity should be ensured in a multicenter cohort. Second, the etiology may be an important prognostic factor and have some influence on the prognosis, the population included in the study comprises different forms of PH, but most of the patients were precapillary PH patients, and the sample size of postcapillary PH was relatively small. Third, the causes of death were not available, so the impact of FTR on cardiovascular mortality could not be assessed. Finally, we demonstrated the relationship between baseline FTR and mortality as well as clinical worsening. Further studies are required to determine whether changes in the FTR after therapy would be associated with adverse outcomes.
In conclusion, this study suggested that severe FTR at baseline was strongly associated with mortality in both precapillary and postcapillary PH patients, independent of the other risk factors as RVEDP, WHO FC III/IV, optimal PAH targeted therapy. Our results reinforce the value of echocardiography as a follow‐up tool.
AUTHOR CONTRIBUTIONS
Yunjing Yang, Huaqiao Chen, Qian Dong, and Kangla Liao contributed to study design, data collection, and writing of the manuscript. Huang Wei contributed to study design and manuscript revisions.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The permission for this study was got from local institutional review board.
ACKNOWLEDGMENTS
We would like to thank all patients who participated in the study. The study was funded by the Cultivation Foundation of The First Affiliated Hospital of Chongqing Medical University (PYJJ2020‐10).
Yang Y, Chen H, Dong Q, Liao K, Huang W. Severity of functional tricuspid regurgitation is associated with mortality in patients with pulmonary hypertension in long‐term follow‐up. Pulm Circ. 2023;13:e12222. 10.1002/pul2.12222
REFERENCES
- 1. Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. JACC. 2017;69(2):236–43. [DOI] [PubMed] [Google Scholar]
- 2. Travers A, Farber HW, Sarnak MJ. Pulmonary hypertension in chronic kidney disease. Cardiol Clin. 2021;39(3):427–34. [DOI] [PubMed] [Google Scholar]
- 3. Guazzi M, Ghio S, Adir Y. Pulmonary hypertension in HFpEF and HFrEF. JACC. 2020;76(9):1102–11. [DOI] [PubMed] [Google Scholar]
- 4. Haque A, Kiely DG, Kovacs G, Thompson AAR, Condliffe R. Pulmonary hypertension phenotypes in patients with systemic sclerosis. Eur Respir Rev. 2021;30(161):210053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang N, Fulcher J, Abeysuriya N, McGrady M, Wilcox I, Celermajer D, Lal S. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta‐analysis. Eur Heart J. 2019;40(5):476–84. [DOI] [PubMed] [Google Scholar]
- 6. Topilsky Y, Khanna A, Le Tourneau T, Park S, Michelena H, Suri R, Mahoney DW, Enriquez‐Sarano M. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5(3):314–23. [DOI] [PubMed] [Google Scholar]
- 7. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Noordegraaf AV, Beghetti, M , Ghofrani, A , MAG Sanchez, Hansmann, G , Klepetko W, Lancellotti, P , Matucci, M , McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 8. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14(7):611–44. [DOI] [PubMed] [Google Scholar]
- 9. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. [DOI] [PubMed] [Google Scholar]; quiz 786–8.
- 10. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- 11. Grapsa J, Pereira Nunes MC, Tan TC, Cabrita IZ, Coulter T, Smith BCF, Dawson D, Gibbs JSR, Nihoyannopoulos P. Echocardiographic and hemodynamic predictors of survival in precapillary pulmonary hypertension: seven‐year follow‐up. Circ Cardiovasc Imagin. 2015;8(6):e002107. [DOI] [PubMed] [Google Scholar]
- 12. Chen L, Larsen CM, Le RJ, Connolly HM, Pislaru SV, Murphy JG, McGoon MD, Frantz RP, Kane GC. The prognostic significance of tricuspid valve regurgitation in pulmonary arterial hypertension. Clin Respir J. 2018;12(4):1572–80. [DOI] [PubMed] [Google Scholar]
- 13. Schneider M, König A, Geller W, Dannenberg V, Winter MP, Binder T, Hengstenberg C, Mascherbauer J, Goliasch G. Severe tricuspid regurgitation: prognostic role of right heart remodelling and pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2022;23(2):246–54. [DOI] [PubMed] [Google Scholar]
- 14. Höke U, Auger D, Thijssen J, Wolterbeek R, van der Velde ET, Holman ER, Schalij MJ, Bax JJ, Delgado V, Marsan NA. Significant lead‐induced tricuspid regurgitation is associated with poor prognosis at long‐term follow‐up. Heart. 2014;100(12):960–8. [DOI] [PubMed] [Google Scholar]
- 15. Hahn RT, Waxman AB, Denti P, Delhaas T. Anatomic relationship of the complex tricuspid valve, right ventricle, and pulmonary vasculature: a review. JAMA Cardiol. 2019;4(5):478–87. [DOI] [PubMed] [Google Scholar]
- 16. Lurz P, Orban M, Besler C, Braun D, Schlotter F, Noack T, Desch S, Karam N, Kresoja KP, Hagl C, Borger M, Nabauer M, Massberg S, Thiele H, Hausleiter J, Rommel KP. Clinical characteristics, diagnosis, and risk stratification of pulmonary hypertension in severe tricuspid regurgitation and implications for transcatheter tricuspid valve repair. Eur Heart J. 2020;41(29):2785–95. [DOI] [PubMed] [Google Scholar]
- 17. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, Schwerzmann M, Dinh‐Xuan AT, Bush A, Abdelhamid M, Aboyans V, Arbustini E, Asteggiano R, Barberà JA, Beghetti M, Čelutkienė J, Cikes M, Condliffe R, de Man F, Falk V, Fauchier L, Gaine S, Galié N, Gin‐Sing W, Granton J, Grünig E, Hassoun PM, Hellemons M, Jaarsma T, Kjellström B, Klok FA, Konradi A, Koskinas KC, Kotecha D, Lang I, Lewis BS, Linhart A, Lip GYH, Løchen ML, Mathioudakis AG, Mindham R, Moledina S, Naeije R, Nielsen JC, Olschewski H, Opitz I, Petersen SE, Prescott E, Rakisheva A, Reis A, Ristić AD, Roche N, Rodrigues R, Selton‐Suty C, Souza R, Swift AJ, Touyz RM, Ulrich S, Wilkins MR, Wort SJ. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–731. [DOI] [PubMed] [Google Scholar]
- 18. Xanthouli P, Koegler M, Marra AM, Benjamin N, Fischer L, Eichstaedt CA, Harutyunova S, Nagel C, Grünig E, Egenlauf B. Risk stratification and prognostic factors in patients with pulmonary arterial hypertension and comorbidities a cross‐sectional cohort study with survival follow‐up. Respir Res. 2020;21(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bermejo J, González‐Mansilla A, Mombiela T, Fernández AI, Martínez‐Legazpi P, Yotti R, García‐Orta R, Sánchez‐Fernández PL, Castaño M, Segovia‐Cubero J, Escribano‐Subias P, Alberto San Román J, Borrás X, Alonso‐Gómez A, Botas J, Crespo‐Leiro MG, Velasco S, Bayés‐Genís A, López A, Muñoz‐Aguilera R, Jiménez‐Navarro M, González‐Juanatey JR, Evangelista A, Elízaga J, Martín‐Moreiras J, González‐Santos JM, Moreno‐Escobar E, Fernández‐Avilés F. Persistent pulmonary hypertension in corrected valvular heart disease: hemodynamic insights and long‐term survival. J Am Heart Assoc. 2021;10(2):e019949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stadler S, Mergenthaler N, Lange TJ. The prognostic value of DLCO and pulmonary blood flow in patients with pulmonary hypertension. Pulm Circ. 2019;9(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burkett DA, Patel SS, Mertens L, Friedberg MK, Ivy DD. Relationship between left ventricular geometry and invasive hemodynamics in pediatric pulmonary hypertension. Circ Cardiovasc Imaging. 2020;13(5):e009825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Critser PJ, Evers PD, Schneider K, Hirsch R, Taylor MD, Woods JC, Lang SM. Echocardiography‐derived septal curvature correlated with invasive hemodynamics in pediatric pulmonary hypertension. J Echocardiogr. 2022;20(1):24–32. [DOI] [PubMed] [Google Scholar]
- 23. Apitz C, Berger RMF, Ivy DD, Humpl T, Bonnet D, Beghetti M, Schranz D, Latus H. Hemodynamic and prognostic impact of the diastolic pulmonary arterial pressure in children with pulmonary arterial hypertension‐a registry‐based analysis. Cardiovasc Diagn Ther. 2021;11(4):1037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Condello F, Gitto M, Stefanini GG. Etiology, epidemiology, pathophysiology and management of tricuspid regurgitation: an overview. Rev Cardiovasc Med. 2021;22(4):1115–42. [DOI] [PubMed] [Google Scholar]
- 25. Spinner EM, Lerakis S, Higginson J, Pernetz M, Howell S, Veledar E, Yoganathan AP. Correlates of tricuspid regurgitation as determined by 3D echocardiography: pulmonary arterial pressure, ventricle geometry, annular dilatation, and papillary muscle displacement. Circ Cardiovasc Imaging. 2012;5(1):43–50. [DOI] [PubMed] [Google Scholar]
- 26. van Kessel M, Seaton D, Chan J, Yamada A, Kermeen F, Butler T, Sabapathy S, Morris N. Prognostic value of right ventricular free wall strain in pulmonary hypertension patients with pseudo‐normalized tricuspid annular plane systolic excursion values. Int J Cardiovasc Imaging. 2016;32(6):905–12. [DOI] [PubMed] [Google Scholar]


