Abstract
The repair and reconstruction of bone defects are still major problems to be solved in the field of orthopedics. Meanwhile, 3D-bioprinted active bone implants may provide a new and effective solution. In this case, we used bioink prepared from the patient’s autologous platelet-rich plasma (PRP) combined with polycaprolactone/β-tricalcium phosphate (PCL/β-TCP) composite scaffold material to print personalized PCL/β-TCP/PRP active scaffolds layer by layer through 3D bioprinting technology. The scaffold was then applied in the patient to repair and reconstruct bone defect after tibial tumor resection. Compared with traditional bone implant materials, 3D-bioprinted personalized active bone will have significant clinical application prospects due to its advantages of biological activity, osteoinductivity, and personalized design.
Keywords: Bone defect, 3D bioprinting, Personalized active bone, Platelet-rich plasma
1. Background
Bone defects caused by trauma, infection, tumor resection, and other factors are still a major problem that urgently needs to be solved in the field of orthopedics[1,2]. Conventional treatment methods include autologous bone, allogeneic bone, or artificial bone implantation, but these methods can only provide scaffold for the ingrowth of new cells; the repair and regeneration of bone also require the ingrowth of macrophages, osteoclasts, osteoblasts, and blood vessels, with the defects being completely replaced by new bone after crawling replacement, followed by reconstruction and shaping that ultimately completes the repair[3,4]. The traditional treatment method may lead to increased risk of surgical traumas after autologous bone transplantation as well as increased risk of infections transmitted by allogeneic bone transplantation and as a result of immune rejection. In addition, limited bone source is also another challenge facing traditional treatment method. Nevertheless, the 3D-printed personalized bioactive bone can outperform the traditional repair method by addressing the prevalent challenges of the latter method[5].
3D bioprinting is used to assemble biological materials through a layer-by-layer deposition method with computer assistance and at the same time accurately colonize active cells or cytokines on the biological scaffold, adjust the shape and size of the scaffold and porosity, and then regulate the interactions between cells, cytokines, and materials[6].
In this case, we used bioink prepared from the patient’s autologous platelet-rich plasma (PRP) to combine with polycaprolactone/β-tricalcium phosphate (PCL/β-TCP) composite scaffold material to print personalized PCL/β-TCP/PRP active scaffolds layer by layer with the help of digital medicine, autologous blood enrichment, and 3D bioprinting technology.
Compared with traditional bone implant materials, 3D-bioprinted personalized active bone not only completely matches the shape of the bone defect of the affected limb but also has a highly bionic microstructure. In addition, the activation of PRP in the active bone material can release a variety of bioactive factors. These advantages could facilitate the ingrowth of cells and blood vessels and accelerate the repair process[7].
2. Case presentation
A 16-year-old female patient was presented with intermittent pain in the left calf, which had lasted for 6 months. The patient developed proximal left calf pain after exercising, which was relieved by rest, followed by intermittent flare. A tumor was palpable in the proximal left calf, approximately 1 × 1 cm in size, with an unclear boundary, poor mobility, and mild tenderness. The X-ray and computed tomography (CT) scan of the left tibia and fibula showed that there was a mass occupying the proximal end of the left tibia (Figure 1A and B). MRI showed multiple mass-occupying masses in the proximal left tibia, which may be benign (Figure 1C). Pre-operative biopsy showed striated muscle and fibrous tissue, and there were proliferative capillaries and venules in the fibrous tissue. Thus, the preliminary diagnosis of the case was osteofibrous dysplasia. To promote the healing of bone defects after tibial tumor resection, we planned to use 3D-bioprinted active bone scaffolds for filling and repair. After obtaining informed consent from the patient, the physicians discussed the plan to perform left tibial tumor resection and implantation of bioprinted active bone for repair before surgery. The application of bioprinted active bone to repair bone defects was approved by the Ethics Committee of the Ninth People’s Hospital affiliated with Shanghai Jiao Tong University School of Medicine.
Figure 1.
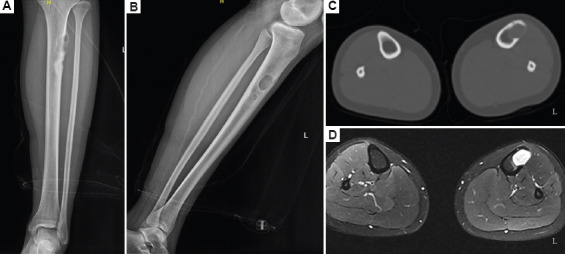
(A–D) Lower left extremity imaging examination.
The bioprinted active bone was designed and printed with the CT scan data obtained before the surgery (Figure 2), and the whole process of printing was carried out in a 10,000 level GMP laboratory. Approximately 35 mL of peripheral venous blood was drawn from the patient before surgery, and approximately 4 mL of PRP was prepared by two rounds of centrifugation. PCL/β-TCP composite material and autologous PRP gel were then printed layer by layer through dual channels to obtain bioprinted active bone with a porosity of approximately 55% (Figure 3A), which was then placed in normal saline for later use. During the operation, a longitudinal incision was made on the anterolateral side of the left proximal tibia, separated layer by layer. After exposing the tumor area of the proximal tibia, a hole was drilled and opened, and the lesions in the tibia were removed with a curette. The tumor lesions were approximately 3 cm × 2.5 cm × 2 cm in size as well as gray-white and medium in texture. The marginal tissue of the inner wall of the tibia was removed with a grinding drill and rinsed repeatedly with hydrogen peroxide and normal saline (Figure 3B). A PCL/β-TCP/PRP active bone scaffold was taken and implanted into the proximal tibial bone defect, and an 8-hole tibial locking plate was implanted externally, followed by screw fixation (Figure 3C and D). Post-operative pathological testing revealed that the condition was osteofibrous dysplasia; in addition, the patient had a good post-operative wound recovery and normal results in routine blood tests. After reviewing the X-ray and CT scan, we found that the plate screw was in place (Figure 4A–C). After 7 months, the implant was well integrated, and new bone formation was detectable by X-ray and CT (Figure 4D–F).
Figure 2.
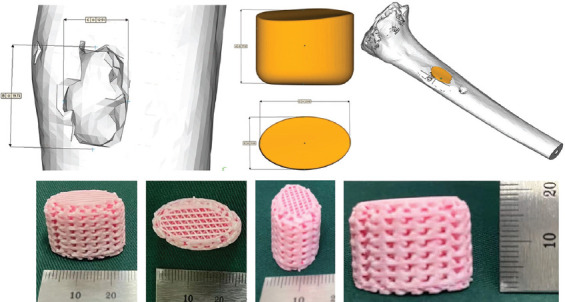
Design and printing of bioactive bone.
Figure 3.
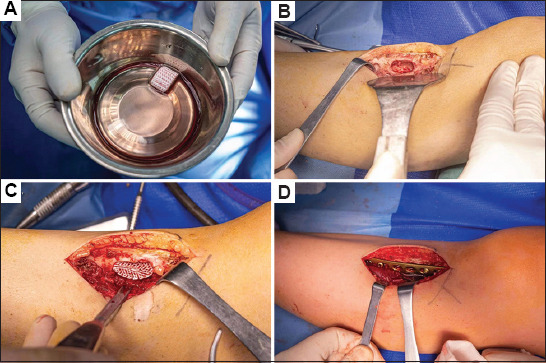
Tumor resection of the left tibia and implantation process of the bioprinted active bone.
Figure 4.
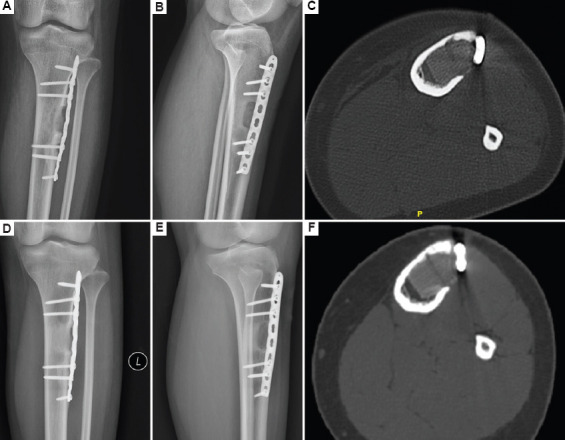
Post-operative X-ray and CT examination of the lower left extremity. (A–C) Two days after the operation. (D–F) Seven-month follow-up.
3. Discussion
The challenge in repair and functional reconstruction of bone defects is a long-standing problem in the field of bone repair and thus, it is a popular research hotspot[1,8]. Although bone tissue has a strong self-healing ability, only a certain range of defects can be repaired; once the critical point of self-healing is exceeded, complete healing is not possible without intervention. Conventionally, autologous bone transplantation is the gold standard treatment for bone defects. However, due to its limited source, additional surgery is required to obtain bone during the operation. This may give rise to complications, such as nerve damage and post-operative bone removal pain, infection, and fractures; therefore, autologous bone transplantation is limited in clinical practice. Allogeneic bone has the risk of inducing an immune response, integrating with host bone, slow remodeling, and spreading infection[9]. 3D bioprinting technology can fulfill the needs of anatomical structure remodeling and functional repair of the affected limb[10].
Traditional bone tissue repair requires macrophages, osteoclasts, osteoblasts, and blood vessels to continuously grow into the defect area from around the defect area, undergo processes such as crawling replacement, reconstruction and shaping, and finally complete the repair and reconstruction of the bone defect. However, these processes take a long time to complete. When 3D bioprinting technology is used for bone defect repair, it directly loads bioactive components such as stem cells or growth factors, which can directly act in situ in the defect area and rapidly develop into healthy, mature tissues. In this case report, the active bone repair scaffold prepared by 3D bioprinting not only completely matched the shape of the bone defect of the affected limb but also had a highly bionic microstructure. These advantages collectively promoted the ingrowth of cells and blood vessels and accelerated the repair process.
However, there are still many challenges and shortcomings in the application of bioprinting technology in clinical practice. In the bioprinting process, the selection of bioinks with suitable properties, such as the biocompatibility of materials, osteoinductive properties, and mechanical properties, is the first problem that needs to be addressed[11]. Second, from the perspective of printing technology, the material must be cured at room temperature without affecting the activity of cells or growth factors[12]. In addition, vascularization is a major challenge for tissue engineering at present. Vascularization is a complex physiological process that requires the participation of cells, scaffolds, and growth factors[13-15]. Therefore, bioinks that can support vascularization are in urgent need of development.
To quickly heal the bone defect formed after the patient’s limb tumor was removed, we designed and printed a PCL/β-TCP/PRP active bone scaffold based on the CT scan data of the affected limb. PCL is one of the most widely used biomaterials in bone tissue scaffolds. It has good biocompatibility and is non-toxic, non-immunogenic, and able to be degraded for absorption, making it a good implant material. However, studies have found that PCL degrades slowly and has no osteogenic activity as a replacement material for bone tissue filling[16]. Compared with calcium sulfate and hydroxyapatite, β-TCP has a moderate absorption rate in the body and is effective at stimulating osteogenesis, making it a good bone defect repair material[17]. Therefore, a composite material formed by melting and mixing PCL and β-TCP in a certain proportion has a suitable degradation rate, facilitates osteogenesis, and promotes bone fusion. However, due to the lack of biological activity of a single PCL/β-TCP composite scaffold, it has a limited effect on promoting the repair of bone defects. Therefore, it is necessary to add a safe and effective active factor to induce endothelial and mesenchymal stem cells so that they grow into the scaffold, proliferate, and differentiate, thereby accelerating the process of angiogenesis and the osteogenic repair of the bone defect site.
Osteogenesis-inducing factors such as bone morphogenetic protein (BMP) and angiogenesis-promoting growth factors such as vascular endothelial growth factor (VEGF) are expensive, unstable in their physical and chemical properties and have potential to trigger heterotopic ossification complications, such as tumor formation, limiting their application in the field of bone tissue engineering. It is extremely important to find an alternative, safe, and effective factor to regulate the growth, proliferation, and differentiation of cells that grow into 3D-bioprinted active bone. Therefore, to make the printed bone scaffold bioactive, we added the patient’s autologous PRP as another main component of the bioink. PRP is an extract of autologous whole blood containing a high concentration of platelets and small amounts of white blood cells and fibrin. Platelets are activated to release a variety of growth factors, including platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF), and play an important role in promoting bone formation and angiogenesis[18,19]. In addition, the preparation of PRP is simple, and only a small amount of blood will be taken from the peripheral vein of the patient, which significantly reduces the discomfort to the patient.
4. Conclusion
We successfully performed a left tibial tumor resection and implemented PCL/β-TCP/PRP bioprinting active bone implantation for the repair of bone defect. The wound recovered well after the operation, and we will continue to follow up with the patient regularly to evaluate the clinical efficacy of the bioprinted active bone. This report presents the first case of applying self-developed 3D-bioprinted active bone in the repair of bone defects, marking the advent of a new era of using 3D-bioprinted structures in clinical practice in China.
Acknowledgments
None.
Funding
The authors thank the funding support from the General program of NSFC (81972058) and Shanghai Key Clinical Specialty Construction Project – Biomedical Materials (shslczdzk06701), 3-year Action Plan of Shenkang Development Center (SHDC2020CR2019B), Huangpu District Industrial Support Fund (XK2020009) and National Key Science and Technology Infrastructure of Translational Medicine (Shanghai) Open Project (TMSZ-2020-207), Shanghai Engineering Research Center of Innovative Orthopedic Instruments and Personalized Medicine (19DZ2250200), Shanghai Science and Technology Commission Yangtze River Delta Science and Technology Innovation Community Project (21002411200), and Technical Standard Project of Shanghai Science and Technology Commission (21DZ2201500).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
Conceptualization: Yongqiang Hao
Investigation: All authors
Methodology: Yongqiang Hao
Writing – original draft: Bojun Cao
Writing – review and editing: Bojun Cao and Yongqiang Hao
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (SH9H-2021-T245-1). Informed consent was obtained from the patient.
Consent for publication
Signed consent was obtained from the patient and guardians for the publication of patient-related test results for this study.
Availability of data
Data will be made available on request.
References
- 1.Roddy E, DeBaun MR, Daoud-Gray A, et al. Treatment of critical-sized bone defects:Clinical and tissue engineering perspectives. Eur J Orthop Surg Traumatol. 2018;28:351–362. doi: 10.1007/s00590-017-2063-0. https://doi.org/10.1007/s00590-017-2063-0. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Lai Y, Li M, et al. Repair of infected bone defect with clindamycin-tetrahedral DNA nanostructure complex-loaded 3D bioprinted hybrid scaffold. Chem Eng J. 2022;435:134855. https://doi.org/10.1016/j.cej.2022.134855. [Google Scholar]
- 3.Agarwal R, Garcia AJ. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv Drug Deliv Rev. 2015;94:53–62. doi: 10.1016/j.addr.2015.03.013. https://doi.org/10.1016/j.addr.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruyt MC, van Gaalen SM, Oner FC, et al. Bone tissue engineering and spinal fusion:The potential of hybrid constructs by combining osteoprogenitor cells and scaffolds. Biomaterials. 2004;25:1463–1473. doi: 10.1016/s0142-9612(03)00490-3. https://doi.org/10.1016/s0142-9612(03)00490-3. [DOI] [PubMed] [Google Scholar]
- 5.Visscher DO, Farre-Guasch E, Helder MN, et al. Advances in bioprinting technologies for craniofacial reconstruction. Trends Biotechnol. 2016;34:700–710. doi: 10.1016/j.tibtech.2016.04.001. https://doi.org/10.1016/j.tibtech.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Ashammakhi N, Hasan A, Kaarela O, et al. Advancing frontiers in bone bioprinting. Adv Healthc Mater. 2019;8:1801048. doi: 10.1002/adhm.201801048. https://doi.org/10.1002/adhm.201801048. [DOI] [PubMed] [Google Scholar]
- 7.Menezes DJ, Shibli JA, Gehrke SA, et al. Effect of platelet-rich plasma in alveolar distraction osteogenesis:A controlled clinical trial. Br J Oral Maxillofac Surg. 2017;55:447–447. doi: 10.1016/j.bjoms.2015.09.027. https://doi.org/10.1016/j.bjoms.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Li SH, Liu YH, Tian TR, et al. Bioswitchable delivery of microRNA by framework nucleic acids:Application to bone regeneration. Small. 2021;17:2104359. doi: 10.1002/smll.202104359. https://doi.org/10.1002/smll.202104359. [DOI] [PubMed] [Google Scholar]
- 9.Masaeli R, Zandsalimi K, Rasoulianboroujeni M, et al. Challenges in three-dimensional printing of bone substitutes. Tissue Eng Part B Rev. 2019;25:387–397. doi: 10.1089/ten.TEB.2018.0381. https://doi.org/10.1089/ten.teb.2018.0381. [DOI] [PubMed] [Google Scholar]
- 10.Matai I, Kaur G, Seyedsalehi A, et al. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi: 10.1016/j.biomaterials.2019.119536. https://doi.org/10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 11.Wuest S, Godla ME, Mueller R, et al. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014;10:630–640. doi: 10.1016/j.actbio.2013.10.016. https://doi.org/10.1016/j.actbio.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Zheng X, Huang J, Lin J, et al. 3D bioprinting in orthopedics translational research. J Biomater Sci Polym Ed. 2019;30:1172–1187. doi: 10.1080/09205063.2019.1623989. https://doi.prg/10.1080/09205063.2019.1623989. [DOI] [PubMed] [Google Scholar]
- 13.Byambaa B, Annabi N, Yue K, et al. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv Healthc Mater. 2017;6:1700015. doi: 10.1002/adhm.201700015. 10.1002/adhm.201700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahabipour F, Ashammakhi N, Oskuee RK, et al. Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl Res. 2020;216:57–76. doi: 10.1016/j.trsl.2019.08.010. https://doi.org/10.1016/j.trsl.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Santos MI, Reis RL. Vascularization in bone tissue engineering:Physiology, current strategies, major hurdles and future challenges. Macromol Biosci. 2010;10:12–27. doi: 10.1002/mabi.200900107. https://doi.org/10.1002/mabi.200900107. [DOI] [PubMed] [Google Scholar]
- 16.Kamath MS, Ahmed SS, Dhanasekaran M, et al. Polycaprolactone scaffold engineered for sustained release of resveratrol:Therapeutic enhancement in bone tissue engineering. Int J Nanomed. 2014;9:183–195. doi: 10.2147/IJN.S49460. https://doi.org/10.2147/ijn.s49460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh WR, Vizesi F, Michael D, et al. Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials. 2008;29:266–271. doi: 10.1016/j.biomaterials.2007.09.035. https://doi.org/10.1016/j.biomaterials.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Wei L, Wu S, Kuss M, et al. 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering. Bioactive Mater. 2019;4:256–260. doi: 10.1016/j.bioactmat.2019.09.001. https://doi.org/10.1016/j.bioactmat.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma-Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. https://doi.org/10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


