Abstract
Toxicity with heavy metals has proven to be a significant hazard with several health problems linked to it. Heavy metals bioaccumulate in living organisms, pollute the food chain, and possibly threaten the health of animals. Many industries, fertilizers, traffic, automobile, paint, groundwater, and animal feed are sources of contamination of heavy metals. Few metals, such as aluminum (Al), may be eliminated by the elimination processes, but other metals like lead (Pb), arsenic (As), and cadmium (Ca) accumulate in the body and food chain, leading to chronic toxicity in animals. Even if these metals have no biological purpose, their toxic effects are still present in some form that is damaging to the animal body and its appropriate functioning. Cadmium (Cd) and Pb have negative impacts on a number of physiological and biochemical processes when exposed to sub-lethal doses. The nephrotoxic effects of Pb, As, and Cd are well known, and high amounts of naturally occurring environmental metals as well as occupational populations with high exposures have an adverse relationship between kidney damage and toxic metal exposure. Metal toxicity is determined by the absorbed dosage, the route of exposure, and the duration of exposure, whether acute or chronic. This can lead to numerous disorders and can also result in excessive damage due to oxidative stress generated by free radical production. Heavy metals concentration can be decreased through various procedures including bioremediation, pyrolysis, phytoremediation, rhizofiltration, biochar, and thermal process. This review discusses few heavy metals, their toxicity mechanisms, and their health impacts on cattle with special emphasis on the kidneys.
Keywords: lead, cattle, arsenic, cadmium, acute kidney failure, oxidative stress, geographic distribution
Introduction
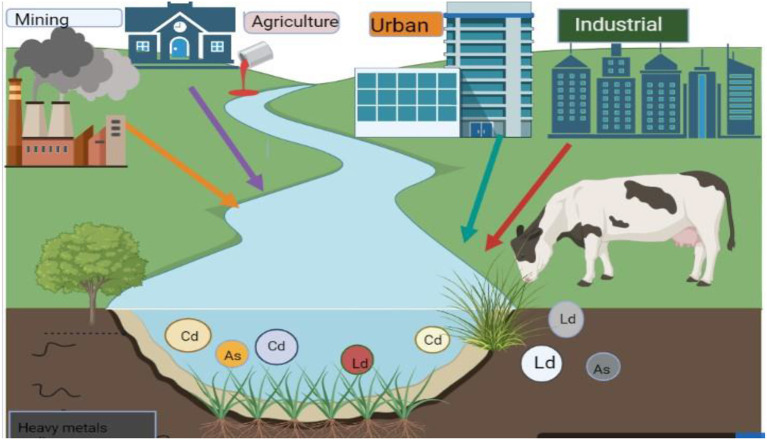
The existence of heavy metals in animal feed and water is injurious to animal health because of their bioaccumulation (1, 2). Few heavy metals including As, Cd, and Pb are well known for their toxicity, while others such as zinc (Zn), copper (Cu), cobalt (Co), manganese (Mn), iron (Fe), magnesium (Mg), and selenium (Se) are necessary for key physiological functions in trace amounts (3–6). Among all metals, Pb, As, and Cd have more negative effects on both animal and human health (7, 8). Mercury (Hg), Cd, and Pb are examples of toxic heavy metals that are dangerous even at very low doses and have no known biological benefits (9). Pb and Cd negatively impact several biochemical and physiological processes when exposed to sub-lethal doses (10, 11). Ruminants are often exposed to environmental poisons that are toxic at certain dosages in a number of areas (12, 13). However, they are particularly vulnerable to Cd, Pb, As, and Flouride (F−) environmental poisoning (14, 15). Domestic animals live in the same environment as people and are susceptible to heavy metals mostly through the plants, feed, soil, and water in their environment (16, 17). To a lesser extent, they are exposed through the air they breathe because of industrial and traffic pollution (18). The secondary cause of heavy metal contamination in animals is the use of pesticides, insecticides, and fertilizers in agricultural fields (19–21). Due to their many industrial, technological, domestic, medicinal, and agricultural applications, the risk of heavy metals exposure has significantly increased in the modern era as shown in Figure 1 (22–24). Animal feeds which might be one of the main sources of these heavy metal contaminations in animals have been found to contain higher amounts of heavy metals like Pb and As (25–27).
Figure 1.
Source of heavy metals toxicity in animals (derived from bio render).
Contaminants of heavy metals enter the food chain through agriculture and industry (28, 29). These substances have a significant potential for acute toxicity. Because they are hazardous metals, land toxic metals can enter plants and accumulate within them (30, 31). The susceptibility of animals and livestock to toxic metals is affected by many factors (32, 33), of which the mixture of necessary and harmful components is possibly one of the most significant (34–36). Toxicity varies according to the animal's trace element metabolic state, and toxic metals also have an impact on the metabolism of trace elements.
Heavy metals are toxicants for edible offal and meat (37, 38). They pose a risk to animal health since they can result in conditions that affect kidney function as well as the cardiovascular and nervous systems and damage different organs such as the reproductive system, nervous system, the respiratory system, the liver, the gastrointestinal tract, and the endocrine system (39, 40). The toxicants Pb, As, and Cd are common and have been linked to kidney damage at high exposure levels (41, 42). The nephrotoxic effects of Pb, As, and Cd are well established and have high amounts of naturally occurring environmental metals; in addition, occupational populations with a high level of exposure have an adverse relationship between kidney damage and toxic metal exposure (43, 44). Heavy metals have mutagenicity, teratogenicity, and carcinogenicity; they induce poor body conditions, reduced reproduction rate, and lead to immunosuppression in domestic animals even at lower dosages (45, 46) because heavy metals easily cross food chains and are not recognized to perform any vital biological functions (47, 48). Toxic elements like Cd, Pb, Hg, and As can contaminate milk (49, 50). Livestock production may be negatively impacted by exposure to either excessive levels of harmful metals like Pb and Cd or inadequate amounts of vital trace elements like molybdenum and selenium (51–54). Livestock is valued highly in different regions of the world (55, 56). Approximately 1.3 billion habitats worldwide live in developing countries where their source of income indirectly or directly depends on livestock (57–60).
Metals in their ionic form can interact with biological systems and toxicological targets in a wide range of ways, which chemically speaking can make them very reactive (24, 61–63). The main livestock species affected by metals poisoning in this context is cattle, which are mostly fed locally grown fodder (64, 65). To assess the potential impacts of pollutants on livestock themselves and to quantify contaminant consumption in people, it is crucial to be aware of the levels of hazardous metals in cattle (66–69). After the energy sector, agricultural production (mostly the manufacturing of ruminant milk and meat) is responsible for the greatest greenhouse gas emissions, which have a negative influence on the environment (70, 71). Because of the changing environment, there is a constant requirement for the supply of nutritious feed for animals, especially cattle (72–74). This review aimed to comprehensively present heavy metals toxicity mechanism and effects, with a special emphasis on the disorders of the kidney system and the prevention of heavy metal contamination in cattle exposed to heavy metals.
Heavy metals: Their toxicity mechanism and effects
Metals are entering the environment at an increasing rate due to industrialization. These metals are permanent because the environment cannot decay them. They eventually make their way into cattle tissue through the meal, where they first enter (51, 75).
Lead (Pb)
Lead is a chemical belonging to the carbon group of the periodic table with the symbol Pb and the Latin name Plumbum, which means “the liquid silver.” Pb has an atomic number of 82, and it was the first chemical with a specific type of toxicity. As one of the most dangerous and ubiquitous environmental contaminants, Pb affects all biological systems when it comes into contact with food, drink, and air (76). Exposure to Pb causes clinical pathological changes by raising toxicity in the endocrine system and the kidney (77).
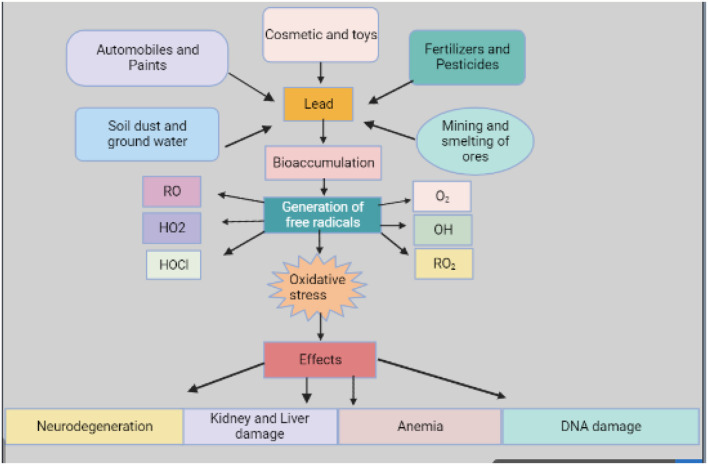
Pb is considered one of the major environmental toxins in industrial areas of the world and animals are frequently exposed to it (78, 79). Numerous environmental factors including industrial pollution, agricultural practices (80), cosmetics, automobiles, paints, and contaminated feed and soil (Figure 2) can cause Pb poisoning, which is especially common in animals (81, 82). Accumulated Pb is toxic in its chemical composition whether it is ingested or consumed in feed or water (83).
Figure 2.
Source and effects of lead (derived from bio render).
Orally administered Pb is only minimally absorbed by the host. However, continuous exposure to Pb, even at low levels, and due to its slower rate of removal, dangerous levels of Pb can accumulate in tissues (84), which is due to an increase in reactive oxygen species (ROS) such as superoxide radicals, hydroxyl radicals, lipid peroxides, and hydrogen peroxide (85). In mammals, ROS is produced either by nicotinamide adenine dinucleotide phosphate oxidase or the mitochondrial electron transport chain which plays a role in controlling genomic stability, cell proliferation, and differentiation (82, 86). Increased ROS production occurs in many pathologic situations, including premature senescence and hematopoietic stem cell and oxidative stress due to Pb exposure, though induced hematopoietic stem cell function remains unclear (87, 88). There are approximately forty heavy metals that can be combined with a broad variety of organic molecules and powerful enzyme inhibitors due to their interaction with the ligand present in the protein and inactivate the system cell of enzymes (83, 89).
Effect of lead on cattle
Numerous clinical signs of Pb exposure in cattle have been noted in toxicological studies (90). Young calves of beef cattle find automotive and other mineral oils extremely appealing; hence, they are more likely to be harmed (91). However, the risk of acute exposure in cattle owing to grease and Pb-contaminated engine oil has decreased as Pb use is limited in many countries (92). Acute Pb toxicity in buffaloes and cattle affecting essential trace mineral profiles was caused by contamination of vegetation and pastures near battery manufacturing smelters (battery recycling units) and lead zinc smelters (61, 93, 94). Due to their innate eating habits, cattle are more likely to get poisoned. Hungry cattle eat everything and their chances of ingesting objects containing lead are very high (95). It acts similar to calcium in the body and builds up in the kidney, the liver, and other tissues (96, 97). Clinically, poisoned cattle typically exhibit indications of malnutrition, emaciation, muscle loss, aberrant fetal development, opaque hair, and moderate anemia and thickening of phalange epiphyses (78, 98, 99). Additionally, according to other studies, Pb-poisoned cattle exhibit ataxia, paresis of the hypoglossal nerve, severe depression, muscle twitching, convulsions, coma, death, and respiratory failure (83, 100). Pb is a tissue toxin that accumulates over time and is stored throughout the body but especially in the bones, the liver, the kidney, and the brain (101, 102). A primary component of ingested blood Pb burden that raises blood Pb levels is stored Pb in the body (61). An additional significant source of Pb exposure in cattle is grease from machinery and empty paint cans (83, 103, 104).
Chelation therapy for mercury and lead poisoning can occasionally be fatal because the Pb deposit can cause an abrupt influx of lead into the blood, severely damaging the kidney and the brain (83, 96). Most cases of Pb poisoning are either acute or chronic (105, 106). The death rate from Pb poisoning might reach 100% in cases of acute Pb poisoning (107, 108). The indicators of acute Pb poisoning in cattle appear suddenly, and the animal may pass away in the pasture within 24 h (109, 110).
Mechanism of action of lead on kidney
Renal dysfunction may result from Pb exposure at high levels (>60 g/dL) (111, 112). Even a trace amount of Pb (<10 g/dL) can cause the same issue (113, 114). Chronic and acute nephropathy are two different forms of renal dysfunction. Nuclear enclosing bodies, which comprise Pb protein complexes and degenerative alterations in the tubular epithelium, can be used to classify acute nephropathy both visually and functionally as a mechanism of decreased tubular transport (8, 11). Acute nephropathy may produce an abnormal secretion of glucose amino acids and phosphates, a combination known as Fanconi's syndrome (115), although it is not the cause of protein appearing in the urine. Chronic nephropathy, on the other hand, is easier to treat but can result in permanent morphological and functional abnormalities. It causes hyperuricemia, hypertension, and renal breakdown but is classified by glomerular and tubulointerstitial variants (116, 117).
The oxidative stress that Pb exposure induces appears to have a detrimental effect on the kidneys of cattle, leading to the development of renal toxicity (118, 119). Cattle exposed to Pb have higher levels of lipid peroxidation in their kidneys (23, 120). Long-term Pb exposure causes the kidney to produce lipid peroxidation and free radicals, which lead to a loss of membrane permeability and the inactivation of components of tubular cells (121, 122). Pb affects the amount of Glutathione (GSH) and the function of antioxidant enzymes like catalase (CAT), glucose-6-phosphate dehydrogenase (G6PD), glutathione peroxidase (GPx), superoxide dismutase (SOD), and glutathione S-transferase (GST) in cattle (123). This indicates that a considerable decrease in the antioxidant enzyme activity in renal tissues is caused by continuous oral Pb exposure (64, 124, 125). The mechanism of the effects of Pb on enzymes can be complicated given that Pb can competitively hinder bio-element absorption or bind with the SH group of proteins (64). Oxidative stress as a mechanism of Pb toxicity in the kidney shows that Pb exposure causes an increase in apoptosis in the kidney (126). The frequency of apoptotic bodies inside proximal tubular cells increased after 12 weeks of continuous lead acetate therapy (23, 127). Therefore, it is conceivable that Pb poisoning affects the gene expression of proteins involved in apoptosis. Following absorption, Pb is transferred to a variety of bodily tissues. Pb exposure causes histopathological alterations in the renal proximal tubular epithelium, which result in interstitial nephritis typically associated with hypertension (128, 129). Pb gets collected in the renal cortex's proximal involuted tubules, which exhibit morphological and biochemical signs of Pb toxicity (130). Occult Pb nephropathy may not be detected as such because acute Pb-induced kidney damage can happen without acute overdose (131). Renal function impairment occurs as a result of persistent lead buildup in the body. It was concluded that the formation of renal toxicity due to environmental lead exposure results in major pathological lesions on the kidney of cattle that appears to be influenced by oxidative stress (23, 132, 133).
Arsenic (As)
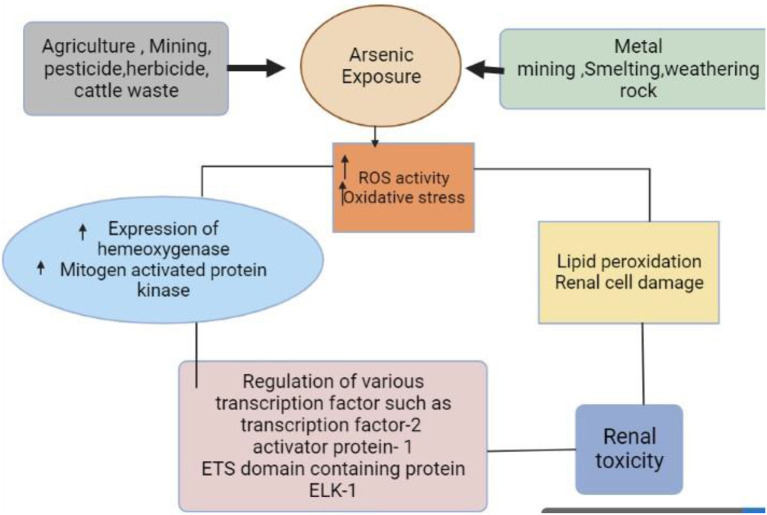
Arsenic is an environmental chemical substance of great significance to animal health (11, 134). Sodium arsenate, sodium and arsenic pentoxide, and disodium or monosodium acid are all deadly forms of As (107, 135), and their environmental contamination poses a serious health risk. Arsenic is a harmful element that is found everywhere and has become more concentrated in water and soil as shown in Figure 3 (136, 137). It can be found in inorganic, organic, pentavalent, and trivalent forms, and it can combine with a wide range of elements, including Pb, O, H, Cu, and S (31, 138). Similar to human exposure, cattle in As-affected areas are also exposed to hazardous quantities of the metal (6, 107). In places where As contamination is a problem, sources of As for animals other than drinking water include feed ingredients. Arsenic is frequently found in liquids used to dip and spray animals to control ectoparasites and cause toxicity (123). Arsenic-contaminated drinking water, feeds, vegetables, and grasses being fed to the large number of animals kept by the people severely affects the health of the animals (96). High levels of ingested As may remain in the feces, urine, blood, hair, and tissues of animals that are directly or indirectly consumed by humans. For instance, As levels of animal products are greater in polluted areas than in clean ones (17, 96). Similarly, when cattle are already As-affected, the use of their manure in agriculture and home settings causes As poisoning of the environment (139).
Figure 3.
Source and mechanism of arsenic exposure (derived from bio render).
In terms of human health, atmospheric emissions are typically the most concerning due to the amounts involved as well as the vast dispersion and exposure risk that frequently results (140). The intake of meat and other animal products from infected cattle could expose people to the hazardous effects of As (141). However, arsenic exposure is not only due to the presence of hazardous substances but also environmental contact, which is an important element in the exposure of As (31, 142). Depending on the kinds of food that animals eat, the level of accumulation of As in varying amounts is determined. Cattle exposed to As pass on this metal in their milk and meat. For instance, in contaminated locations, As has also been found in cow meat and milk (141, 143). The WHO puts a tolerable intake of As at 3.0 g/kg body weight (144).
Effects of arsenic on cattle
Compared to other species, cattle are more susceptible to As poisoning (145). Cattle arsenic toxicity symptoms range from gastrointestinal to nervous system symptoms (31), severe digestive tract inflammation, weight loss, severe gastrointestinal disease, unpredictable appetite, conjunctivitis, mucosal erythematous lesions, and decreased milk production (146, 147). Kidney hyperemia and severe parasite infestation in the abomasum walls were both discovered by microscopic inspection (148, 149). The affected cattle showed decreased superoxide dismutase and catalase activities, decreased plasma nitrite and erythrocyte levels, and an increased rate of lipid peroxidation, protein carbonyl, and blood As levels in comparison to those raised in As-free areas (150, 151).
Mechanism of action of arsenic on kidney
Cattle exposed to As evolve tubular necrosis, glomerular sclerosis, and increased N-acetyl beta-D-glucosaminidase (NAG) concentration in urine (152–154). They also experienced DNA oxidative damage and increased oxidative stress in the kidneys (155, 156). Arsenic is believed to cause endothelial dysfunction and promote inflammation and oxidative stress (157), which may cause kidney damage; however, these are rather general mechanisms (158, 159).
The higher lipid peroxidation in the kidney after As treatment may be caused by the formation of superoxide anion radical according to the decreased SOD activity in the kidney as shown in Figure 3 (160, 161). When molecular oxygen interacts with the dimethyl arsine metabolite of dimethyl As acid, free radicals are produced. These radicals are believed to be superoxide anion radicals, which are created when dimethyl arsine reduces molecular oxygen by one electron. Arsenic induced kidney lipid peroxidation and unchanged SOD activity point to no superoxide anion buildup (162, 163).
Inorganic arsenic is methylated by two distinct enzymatic processes (164). It has been shown that trivalent inorganic As has an inhibiting influence on the second methylation process that results in the creation of dimethyl As acid (165). Although one of the detoxication steps for As is methylation, the cellular methyl group intake results in DNA hypomethylation, which alters the gene expression and causes cellular change (166). Inorganic trivalent arsenic (AS3+) and pentavalent arsenic (As5+) exhibit significantly different acute toxicity and biological processes. The renal tubules actively transport arsenate (As5+), and a minor portion of this form is converted to AS3+, the molecule that is more acutely poisonous (138, 167). It was concluded that the formation of renal toxicity by As exposure causes major kidney problems in cattle influenced by oxidative stress and lipid peroxidation (167–169).
Cadmium (Cd)
The chemical element Cd has the atomic number 48. This silvery white and soft metal is chemically similar to the other two stable metals (zinc and mercury) in group 12 (170, 171). It is a heavy metal that is both naturally present and released as part of industrial pollution (172). Typically, it is found in minerals along with other elements like chlorine (cadmium chloride) (173–175), oxygen (cadmium oxide), or sulfur (cadmium sulfide, cadmium sulfate) (176). Although it is unknown how it operates biologically in either animals or people, it resembles the effects of other divalent metals that are crucial for a variety of biological processes (177, 178). In the aquatic environment, the presence of Cd is linked to Cd and other toxic metals being released from mining, sewage, and processing of toxic metals (179). The main sources of Cd include refined foods, water, coffee, water pipes, tea, burning coal, and chimneys (180, 181).
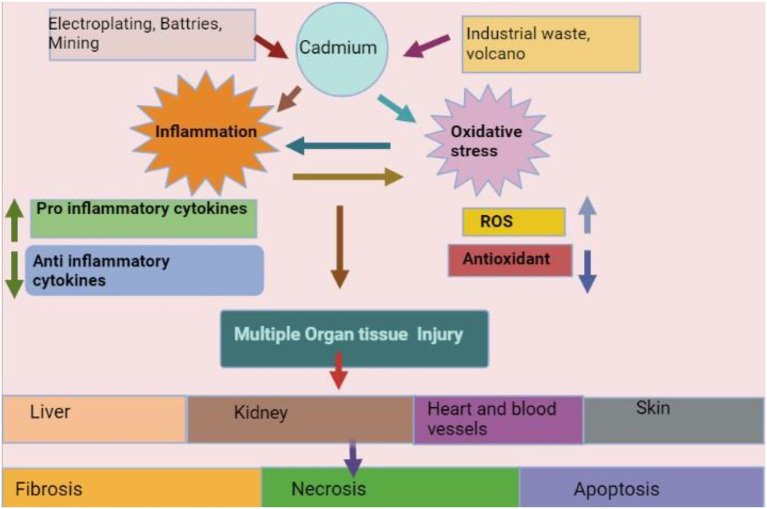
Commercial uses for Cd include TV screens, paint pigments, lasers, batteries, cosmetics, galvanizing steel, acting as a barrier in nuclear fission, and weld sealing in lead water pipes as shown in Figure 4 (182, 183). Cd exposure occurs from taking contaminated food (e.g., organ meat, crustaceans, rice from certain areas of China and Japan, leafy vegetables) or water (Cd and Zn sealed water pipe and industrial pollution) and can cause long-term health issues. Contaminated dietary supplements and drugs are also a source of contamination (176, 184, 185). Dietary consumption of Cd varies between 40 and 50 g per day (175, 186).
Figure 4.
Source, mechanism, and effects of cadmium (derived from bio render).
Effects of cadmium on cattle
Almost every system in the cattle body is affected by Cd toxicity (176, 186). The toxic effects of Cd include lung damage, hypertension, hepatic injury, and kidney dysfunction (187, 188). High amounts of Cd have been discovered in the kidneys, muscles, bones, and liver of cattle in Marrakech, Morocco (186, 189) where the sewage treatment fields have disrupted the normal metabolism of trace elements and reduced the levels of Zn and Cu (171, 190). Cattle slow the cellular clearance of Cd and the ineffectiveness of cellular export systems accounts for the element's prolonged retention in storage tissues such as the colon, the liver, and the kidneys (37, 191, 192). For instance, in Nigeria, cattle grazing in regions with high Cd contamination have been shown to have high amounts of the metal in their muscles, liver, and kidneys, which also reduced the quality of their meat (31, 193).
Mechanisms of toxicity of cadmium on kidney
Cadmium toxicity has been observed in many organs and Cd induces tissue damage through oxidative stress (194, 195), epigenetic alterations in DNA expression (196), and upregulation and inhibition of the transport pathway (197), especially in proximal S1 region in tubules of the kidney (198, 199). The kidney is the main organ affected by Cd toxicity (171), and the S1 portion of the proximal tubule is a prime target for Cd deposition. As a result of Cd-induced oxidative damage to carrier proteins and mitochondria (181), Fanconi syndrome is characterized by clinically visible defects in protein, bicarbonate, phosphate, and amino acid reabsorption (200, 201). Approximately thirty percent of body Cd is accumulated in kidney tubule segments, with tubular injury proportional to the amount of Cd that is not bound to metallothionein (202, 203). It was concluded that Cd toxicity damages the kidney through oxidative stress.
Geographical distribution of metal toxicity in cattle
Metal toxicity has been observed in different animals, but our focus is on cattle in this review. We found that metal toxicity is highly prevalent in cattle worldwide as detailed in Tables 1–3. Different metals have been examined in various studies among which one study measured Cd, As, and Pb concentrations in meat, kidney, and liver from 56 cattle and 438 calves slaughtered in Galicia, Spain in 1996. In cattle, the concentrations were observed as 0.057, 0.066, 0.017 mg/kg (Pb), 0.046, 0.068, 0.005 mg/kg (As), and 0.097, 0.458, 0.001 mg/kg (Cd) in meat, kidney, and liver, respectively. The concentrations of Cd, As, and Pb in cattle in Galicia infrequently exceeded the maximum acceptable limits that many nations have adopted (235).
Table 1.
Lead toxicity observed in different organs of cattle.
| Animal | Organ | Normal range | Toxicity level | Country | References |
|---|---|---|---|---|---|
| Cattle | Kidney | 0.1–0.35 ppm | >0.35 ppm | Canada | (99) |
| Cattle | Blood | 0.1 mg/kg | 1.30 mg/kg | Western Canada | (78) |
| Cattle | Liver | 0.1–1.0 mg/kg | 33.5 mg/kg | Western Canada | (78) |
| Cattle | Kidney | 0.2–1.0 mg/kg | 56.3 mg/kg | Western Canada | (78) |
| Cattle | Kidney | 30 ppm | 35.7–284.5 ppm | Paraná and São Paulo, Brazil | (105) |
| Cattle | Body | 0.48 mg/kg | 2.90 mg/kg | England | (100) |
| Cattle | Kidney | >10 mg/kg | >30 mg/kg | England | (100) |
| Cattle | Liver | 10 mg/kg | 23.2 mg/kg | America | (204) |
| Cattle | Kidney | 35 mg/kg | 62.8 mg/kg | America | (204) |
| Cattle | Liver | 4.99 mg/kg | 47.5 mg/kg | Spain | (107) |
| Cattle | Kidney | 5.30 mg/kg | 58.3 mg/kg | Spain | (107) |
| Cattle | Muscle | 7.12 mg/kg | 50.3 mg/kg | Spain | (107) |
| Cattle | Blood | 4.81 mg/kg | 34 mg/kg | Spain | (107) |
| Cattle | Kidney | 6.1 mg/kg | 59.7 mg/kg | Deza region (NW Spain) | (205) |
| Cattle | Blood | 0.01 μg/ml | 0.60 μg/ml | India | (76) |
| Bovine | Kidney | 0.5 mg/kg | 0.8 mg/kg | China | (206) |
| Bovine | Kidney | 0.5 mg/kg | 0.1 mg/kg | China | (206) |
| Bovine | Muscles | 0.2 mg/kg | 0.001 mg/kg | China | (206) |
| Cattle | Kidney | 0.05 μg/ml | 1.04 μg/ml | Kabwe, Zambia | (207) |
| Cattle | Kidney | 0.5 mg/kg | 0.52 mg/kg | Jamaica | (37) |
| cattle | Kidney | 0.5 mg/kg | 2.64 mg/kg | Netherland | (208) |
| Cattle | Muscle | 2.00 mg/kg | 1.95 mg/kg | Croatia | (209) |
| Cattle | Kidney | 0.172 mg/kg | 0.167 mg/kg | Croatia | (209) |
| Cattle | Kidney | 0.04–2.97 μg/g | 0.92 μg/g | Canada | (210) |
| Cattle | Kidney | 0.022–1.21 mg/kg | 0.006 mg/kg | Belgium | (211) |
| Cattle | Kidney | 0.05 ppm | 0.08 ppm | India | (120) |
| Cattle | Kidney | 0.006 mg/kg | 0.002 mg/kg | Italy | (212) |
| Cattle | Blood | 0.46 μg/g | 0.54 μg/g | Pakistan | (213) |
Table 3.
Cadmium toxicity observed in different organs of the cattle.
| Animal | Organ | Normal range | Toxicity level | Country | References |
|---|---|---|---|---|---|
| Cattle | Liver | 6.15 mg/kg | 23.4 mg/kg | Galicia, NW Spain | (107) |
| Cattle | Kidney | 5.91 mg/kg | 110 mg/kg | Spain | (107) |
| Cattle | Muscle | 9.46 mg/kg | 8.28 mg/kg | Spain | (107) |
| Cattle | Blood | 9.17 mg/kg | 1.65 mg/kg | Spain | (107) |
| Cattle | Kidney | 0.3 mg/kg | 59.7 mg/kg | Deza region (NW Spain) | (205) |
| Cattle | Kidney | 0.01 μg/ml | 0.05 μg/ml | India | (220) |
| Cattle | Kidney | 1.0 mg/kg | 2.15 mg/kg | China | (206) |
| Bovine | Liver | 0.5 mg/kg | 2.47 mg/kg | China | (206) |
| Bovine | Muscle | 0.01 mg/kg | 0.02 mg/kg | China | (206) |
| Bovine | Kidney | 0.05 μg/ml | 19.37 μg/ml | Kabwe, Zambia | (207) |
| Cattle | Kidney | 0.1 mg/kg | 9.58 mg/kg | Netherland | (208) |
| Cattle | Kidney | 0.1 mg/kg | 10.3 mg/kg | Morocco | (221) |
| Cattle | Kidney | 0.1 mg/kg | 33.1 mg/kg | Jamaica | (37) |
| Cattle | Liver | 0.1 mg/kg | 0.642 mg/kg | Belgium | (186) |
| Cattle | Kidney | 0.1 mg/kg | 4.22 mg/kg | Belgium | (186) |
| Cattle | Liver | 0.50 mg/kg | 2.655 mg/kg | Belgium | (186) |
| Cattle | Kidney | 0.1 mg/kg | 15.3 mg/kg | Belgium | (186) |
| Cattle | Liver | 0.50 mg/kg | 1.17 mg/kg | Belgium | (222) |
| Cattle | Kidney | 0.1 mg/kg | 7.99 mg/kg | Belgium | (222) |
| Cattle | Liver | 0.50 mg/kg | 0.061 mg/kg | Finland | (223) |
| Cattle | Kidney | 0.1 mg/kg | 0.35 mg/kg | Finland | (223) |
| Cattle | Kidney | 0.1 mg/kg | 0.036 mg/kg | Finland | (224) |
| Cattle | Kidney | 0.1 mg/kg | 8.63 mg/kg | Ireland | (225) |
| Cattle | Kidney | 0.1 mg/kg | 1.66 mg/kg | Netherlands | (208) |
| Cattle | Kidney | 0.1 mg/kg | 0.25 mg/kg | Poland | (226) |
| Cattle | Kidney | 0.1 mg/kg | 0.937 mg/kg | Poland | (227) |
| Cattle | Kidney | 0.1 mg/kg | 0.161 mg/kg | Spain | (228) |
| Cattle | Kidney | 0.1 mg/kg | 0.545 mg/kg | Spain | (229) |
| Cattle | Kidney | 0.1 mg/kg | 0.39 mg/kg | Sweden | (230) |
| Cattle | Kidney | 0.1 mg/kg | 0.373 mg/kg | Slovenia | (211) |
| Cattle | Kidney | 0.1 mg/kg | 0.65 mg/kg | Australia | (231) |
| Cattle | Kidney | 0.1 mg/kg | 38.3 mg/kg | China | (206) |
| Cattle | Kidney | 0.1 mg/kg | 7.92 mg/kg | Jamaica | (37) |
| Cattle | Kidney | 0.1 mg/kg | 0.1371 mg/kg | Iran | (232) |
| Cattle | Kidney | 0.1 mg/kg | 4.38 mg/kg | Morocco | (221) |
| Cattle | Muscle | 0.348 mg/kg | 0.341 mg/kg | Croatia | (209) |
| Cattle | Kidney | 0.544 mg/kg | 0.535 mg/kg | Croatia | (209) |
| Cattle | Kidney | 2.91 μg/g | 17.84 μg/g | Canada | (210) |
| Cattle | Kidney | 0.093–4.22 mg/kg | 0.002 mg/kg | Belgium | (211) |
| Cattle | Kidney | 0.05 ppm | 0.09 ppm | India | (120) |
| Cattle | Kidney | 0.001 mg/kg | 0.0008 mg/kg | Italy | (212) |
| Cattle | Kidney | 0.41 mg/kg | 11.50 mg/kg | Ethiopia | (233) |
| Cattle | Liver | 0.06 mg/kg | 0.5 mg/kg | Ethiopia | (233) |
| Cattle | Liver | 0.46 μg/g | 0.54 μg/g | Pakistan | (213) |
| Cattle | Kidney | 0.5 mg/kg | 0.34 mg/kg | Turkey | (234) |
In another study, the correlation between toxic As, Cd, and Pb was examined in the kidney, muscle, blood, and liver of 494 cattle from Galicia. These interactions are most likely a result of the effects that Cd has on the synthesis of metallothionein (107, 236). In the kidney, Pb and Zn were positively correlated; however, it is unclear how they interact. Overall, the levels of Pb and As in Galician cattle do not pose a threat to animal health. However, in some areas of Galicia, up to 20% of the cattle had toxic levels of Cd in their kidney (Table 3) (235, 237).
In a study in Belgium, trace element concentrations were found in the kidney, liver, and meat of cattle that had spent more than 18 months in areas that historically had been polluted by emissions from non-ferrous metal production or in areas with high levels of metals contamination (120, 238, 239). Trace element values were calculated using coupled plasma mass spectrometry. Concentrations of Cd, As, and Pb in meat were low in cattle (240). However, cattle from the polluted areas had kidney concentrations that were, respectively, 1.8, 2.2, and 2.5 times higher than those of animals from the reference locations. The European maximum level for Cd in cattle kidneys exceeded 75% in cattle from polluted environments and 47% of kidneys from reference sites. The levels of Cd, As, and Pb in cattle livers from polluted locations were 2.3 times higher. Cattle accumulated much more Cd in the kidneys and Pb in the liver and kidneys (Table 1) (120, 186).
In another study, metal detoxification and accumulation processes were determined in cattle from unpolluted and polluted areas of Italy. Dairy cattle from farms and free-ranging cattle from nature reserves were chosen as study animals (241). The concentration of Pb, Cd, and As were determined in the kidney, muscle, blood, and liver of cattle from reference and polluted areas. Cattle from contaminated areas had higher internal concentrations of Cd, Pb, and As than cattle from reference areas (Table 2) (206, 242). In another study, the results suggested Cd as the most important metal for MT induction in the kidney. Pb and Cd were significantly higher in both cattle from polluted and breed areas (243). While Cd concentration exceeded the European level by 85% in the kidney and 40% in the liver from sampled cattle, it was higher in the kidney and the liver of cows from contaminated areas (Table 3) (222, 244).
Table 2.
Arsenic toxicity observed in different organs of cattle.
| Animal | Organ | Normal range | Toxicity level | Country | References |
|---|---|---|---|---|---|
| Cattle | Liver | 4.57 mg/kg | 10.02 mg/kg | Spain | (107) |
| Cattle | Kidney | 5.03 mg/kg | 15.2 mg/kg | Spain | (107) |
| Cattle | Muscle | 3.34 mg/kg | 4.25 mg/kg | Spain | (107) |
| Cattle | Blood | 3.11 mg/kg | 2.92 mg/kg | Spain | (107) |
| Cattle | Muscle | 6.07 mg/kg | 5.87 mg/kg | Croatia | (209) |
| Cattle | Kidney | 0.033 mg/kg | 0.031 mg/kg | Croatia | (209) |
| Cattle | Kidney | 0.1 mg/kg | 0.1–0.5 mg/kg | Croatia | (214) |
| Cattle | Kidney | 0.02–0.20 μg/g | 0.17 μg/g | Canada | (210) |
| Cattle | Liver | 0.02–0.13 μg/g | 0.26 μg/g | Canada | (210) |
| Cattle | Kidney | 0.002 mg/kg | 0.048 mg/kg | Netherland | (210) |
| Cattle | Liver | 0.002 mg/kg | 0.013 mg/kg | Netherland | (210) |
| Cattle | Meat | 0.002 mg/kg | 0.004 mg/kg | Netherland | (210) |
| Cattle | Kidney | 0.002 mg/kg | 0.048 mg/kg | Netherland | (215) |
| Cattle | Kidney | 0.002 mg/kg | <0.02 mg/kg | Finland | (216) |
| Cattle | Kidney | 0.002 mg/kg | 0.034 mg/kg | Germany | (217) |
| Cattle | Kidney | 0.002 mg/kg | 0.03 mg/kg | Australia | (218) |
| Cattle | Kidney | 0.002 mg/kg | 0.018 mg/kg | Australia | (219) |
| Cattle | Kidney | 0.001–0.147 mg/kg | 0.030 mg/kg | Belgium | (211) |
| Cattle | Kidney | 0.002 mg/kg | 0.001 mg/kg | Italy | (212) |
In yet another study, concentrations of Cd, As, and Pb was determined in the kidney and the liver of cattle near a lead and zinc mine in Zambia, which was ranked among the top ten contaminated places in the world. The concentration of metals was measured in the kidney and liver of 51 cattle from Kabwe and other places in Zambia (79). Maximum metal concentrations expressed in the kidney and the liver were 0.05 As, 19.37 Cd, and 1.8 Pb. Concentrations of Cd and Pb in Kabwe cattle were high than the cattle from other parts of Zambia; the mean concentration of Cd exceeded the benchmark value (Table 3) (207, 245).
Pb poisoning is commonly detected in American cattle. In one of the studies, three groups of cattle were selected from various herds that had accidentally been in contact with discarded Pb batteries in the pasture (246). Blood samples were collected from cattle and monitored for changes in Pb concentration. The herds had Pb concentrations that were indicative of acute Pb exposure (>0.35 ppm) and asymptomatic Pb toxicities; between 7% and 40% of these asymptomatic cattle were in the high normal limit (0.1–0.35 ppm) (Table 1) (99).
One study evaluated the epidemiology of acute Pb poisoning in cattle in Canada over 16 years from 1998 to 2013. Over the duration of the study, there were 525 incidents of acute Pb poisoning. The toxic level of Pb was 11.2% in 2001, reduced to 9.9% in 2006, and rose to 15.6% in 2009 (78). Cattle calves six months of age were frequently poisoned (53.5%). The mean toxic Pb concentration in the kidney, the liver, and the blood was 56.3 ± 39.7 (n = 61), 33.5 ± 80.5 (n = 172), and 1.30 ± 1.70 (n = 301), respectively. The mean normal Pb concentration in the kidney, the liver, and the blood was 0.41 ± 0.62 mg/kg (n = 64), 0.16 ± 0.63 mg/kg (n = 382), and 0.036 ± 0.003 mg/kg (n = 1,081), respectively (78).
Toxicokinetics
The majority of industrial activities affect animals and the environment in both favorable and unfavorable ways. The energy usage mitigation measures are coupling desalination plants with renewable energy sources such as wind power, geothermal energy, tidal power and solar energy (247). Typically heavy metals removal techniques may depend on the reliability of the plants, cost, operation, concentration of competitive ions and concentration of heavy metals in water (248).
The total body kinetics that a chemical is subjected to in an organism is referred to as toxicokinetics (193, 249). A toxicant enters an organism through absorption. It is distributed throughout the organism through diffusion (250). The chemical is subsequently broken down into less dangerous metabolites, which the organism may expel or store in different regions of its body (251). A chemical toxicokinetic state can vary with prolonged exposure (252). In toxicokinetics, the type of chemical which will end up in the animal depends on the physicochemical composition of the metal and the biological makeup of the recipient organism (253, 254). Metals are absorbed into an organism either by conveyors or diffusion. Pb ion, an electrically charged particle, enters using conveyors or carriers like proteins (255). Other substances can enter intracellular compartments through damaged membranes. Another possibility is loss from the cells, which would result in a drop in intracellular concentration. The amount of toxicity felt by the organism directly depends on the uptake and reduction in intracellular concentration (256, 257).
Biological transformation
Biological transformation is the process of transforming substances within an organism (258). Biological transformation processes show how the organism's toxicant concentrations are decreased after being ingested (259, 260). The chemical breakdown within an organism is crucial to the biological transformation process because it creates new less dangerous compounds (261). In phase 1, enzymes convert a chemical toxin through the oxidative, reductive, and hydrolytic processes (262). In phase 2, transferase enzymes involve in the transformation of chemicals formed by toxicants. At this stage, the hydrophilicity of toxicants is increased (263).
Prevention and control of bioaccumulation of toxic metals
Physical danger could potentially injure an animal and its consumer physically; therefore, safe meat must be free of toxic metals. Soil remediation is employed to make soils more useful and therefore indirectly lower the susceptibility of animals to hazardous metals (123, 264).
Bioremediation
Techniques for restoring soil are dependent on chemical or biological principles. Toxic metals that damage the environment are removed from water and soil through bioremediation (53, 265). This entails using microbes and plants to biologically restore the utility values of polluted areas (266, 267). As a result, the hazardous metals in plants get immobilized, preventing their proliferation. These contaminants can be absorbed by bacteria that live in harmony with these plants (268).
Phytoremediation
Utilizing phytoremediation, landfill soils are recovered. This technique is based on the utilization of plants that take up metals from the soil or water and collect them (269, 270). The capacity of plants to store and absorb metals as well as their accessibility to the plants both affect the efficacy of phytoremediation (271, 272).
Rhizofiltration
Rhizofiltration is a type of phytoremediation in which wastewater, surface water, and contaminated groundwater are filtered by a dense network of roots to remove toxins or surplus nutrients (273, 274). The pollutants on the root undergo both adsorption and absorption during the process (275). Rhizofiltration is used for removing heavy metals from the environment.
Biochar
The term “biochar” refers to a material rich in carbon formed during the pyrolysis process, which is the thermochemical degradation of biomass at a temperature of roughly 700°C with little or no oxygen present (276). Biochar, which can be used in a variety of environmental applications, syngas, which is converted into electricity or heat (combined power and heat), and bio-oil, which can be used as a fuel or added to petroleum refining products, are all byproducts of pyrolysis (277–279). The best way to dispose of wastewater is biochar, which can also be utilized to enhance the soil's characteristics and fertility (280). Metals that are not eliminated during sewage treatment could be successfully decreased by adding biochar to sewage sludge (281). Pollution in wastewater is reduced by pyrolyzing it to create biochar and using it further. Biochar lowers the bioavailability of harmful metals and raises the pH of the soil (282). Additionally, biochar has the potential to enhance soil quality and drastically lower the bioavailability of hazardous metals (283). Biomass is pyrolyzed to make biochar (282).
Pyrolysis
Pyrolysis is the thermal breakdown of organic compounds at temperatures between 300 and 900°C in an oxygen-free atmosphere (284, 285). The technique of pyrolysis involves heating sewage sludge in an inactive environment to release organic material that can subsequently be recycled (286, 287). The heavy metals are concentrated by this mechanism around carbonaceous deposits (288).
Leachate
Any liquid that removes soluble or suspended particles and any other component of the matter it has passed through is known as leachate (289). Leachate is made up of different combinations of suspended and dissolved materials, heavy metals contaminants, inorganic and organic pollutants, and more (290, 291). To avoid the undesirable outcome of surface water and groundwater contamination, landfill leachate should be gathered and properly treated. Leachate is produced as a result of waste degradation or water access, and it can contaminate groundwater and soil (282, 283). A source of contamination in cattle is metal-containing leachate.
Thermal process
Manure has a similar energy value to wood waste, making it a suitable source of biomass for the production of energy (292, 293). Toxic metals are stabilized during the thermal processing of biomass for energy production, which lessens their toxicity (294). Most metals remain in their low-toxicity solid phase during combustion. This procedure generates energy while preventing metal contamination (294, 295).
Several preclinical and clinical research has examined the effects of heavy metal supplementation as chelating agents to facilitate pollutants elimination or as synthetic antioxidants to mitigate the oxidative stress caused by environmental pollutants to avoid or lessen toxicity (237, 296). These therapies and approaches themselves are believed to have a variety of safety and effectiveness issues.
Conclusion
We conclude that lead and cadmium have high toxicity in the kidney and thus lead to acute kidney disorders in cattle; however, arsenic also accumulates in the kidney but at low intensity. As regards other body parts, these heavy metals penetrate the liver and muscles, but with lower intensity compared to the kidney. Advanced technologies can reduce occupational exposure to heavy metals. Monitoring exposure and perhaps intervening to reduce subsequent exposure to heavy metals in the animals and environment can be a significant step toward prevention. There is an urgent need to decrease the concentration level of these heavy toxic metals through advanced scientific techniques such as biochar, bioremediation, and pyrolysis to minimize global economic losses. In the future, it will help develop advanced techniques to control heavy metals in cattle. Failure to reduce the exposure will lead to serious issues in the future due to the negative effects of heavy metals. National and international collaboration is essential for developing adequate heavy metal toxicity prevention strategies.
Author contributions
IT and KAA made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
The researchers would like to thank the Deanship of Scientific Research, Qassim University, Saudi Arabia for funding the publication of this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Agbugui MO, Abe GO. Heavy metals in fish: bioaccumulation and health. Br J Earth Sci Res. (2022) 10:47–66. 10.37745/bjesr.2013 [DOI] [Google Scholar]
- 2.Ghazzal M, Hussain MI, Khan ZI, Habib ur Rahman M, El-Habeeb AA, Yang HH. Chromium poisoning in buffaloes in the vicinity of contaminated pastureland, Punjab, Pakistan. Sustainability. (2022) 14:15095. 10.3390/su142215095 [DOI] [Google Scholar]
- 3.Sevostyanova O, Orobets V, Agarkov A, Fedota N, Klimanovich I. Aggregate-resistant vitamin-mineral complex based on selenium; comparative effectiveness in poultry farming against the technological stress. Int J Vet Sci. (2020) 9:141–4. Available online at: https://elibrary.ru/item.asp?id=45181041 [Google Scholar]
- 4.Pandey B, Agrawal M, Singh S. Coal mining activities change plant community structure due to air pollution and soil degradation. Ecotoxicology. (2014) 23:1474–83. 23: 1474–83. 10.1007/s10646-014-1289-4 [DOI] [PubMed] [Google Scholar]
- 5.Samy A, Hassan HMA, Elsherif HMR. Effect of nano zinc oxide and traditional zinc (oxide and sulphate) sources on performance, bone characteristics and physiological parameters of broiler chicks. Int J Vet Sci. (2022) 11:486–92. 11: 486–92. 10.47278/journal.ijvs/2022.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta S, Gorain B, Choudhury H, Roychoudhury S, Sengupta P. Environmental and occupational exposure of metals and female reproductive health. Environ Sci Pollut Res. (2022) 29:62067–92. 29: 62067–92. 10.1007/s11356-021-16581-9 [DOI] [PubMed] [Google Scholar]
- 7.Patwa D, Muigai HH, Ravi K, Sreedeep S, Kalita P. A novel application of biochar produced from invasive weeds and industrial waste in thermal backfill for crude oil industries. Waste Biomass Valor. (2022) 13:3025–42. 13: 3025–42. 10.1007/s12649-022-01694-0 [DOI] [Google Scholar]
- 8.Penticoff HB, Fortin JS. Toxic/metabolic diseases of the nervous system. In:Zigmond MJ, Wiley CA, Chesselet M-F, editors. Neurobiology of Brain Disorders. Cambridge, MA: Academic Press; (2023). p. 379–401. 379–401. 10.1016/B978-0-323-85654-6.00040-X [DOI] [Google Scholar]
- 9.Anjulo TK, Mersso BT. Assessment of dairy feeds for heavy metals. Am Acad Sci Res J Eng Technol Sci. (2015) 11:20–31. Available online at: https://asrjetsjournal.org/index.php/American_Scientific_Journal/article/view/541 [Google Scholar]
- 10.Elarabany NF, El-Batrawy OA. Physiological changes in the Cattle Egret, Bubulcus ibis, as a bioindicator of air pollution in New Damietta City, Egypt. African J Biol Sci. (2019) 15:13–31. 15: 13–31. 10.21608/ajbs.2019.63379 [DOI] [Google Scholar]
- 11.Wang X, Yasuda K, Zhang Y, Liu S, Watanabe K, Taniguchi T, et al. Interfacial ferroelectricity in rhombohedral-stacked bilayer transition metal dichalcogenides. Nat Nanotechnol. (2022) 17:367–71. 17: 367–71. 10.1038/s41565-021-01059-z [DOI] [PubMed] [Google Scholar]
- 12.Gensa U. Review on cyanide poisoning in ruminants. J Biol Agric Healthc. (2019). 9:6. 9:6. 10.7176/JBAH [DOI] [Google Scholar]
- 13.Guvvala N, Sarathi R, Vinu R. Effect of zeolite addition on partial discharge and dielectric behavior of thermally aged synthetic ester fluid under external magnetic field. IEEE Access. (2022) 10:46670–7. 10: 46670–7. 10.1109/ACCESS.2022.3171326 [DOI] [Google Scholar]
- 14.Mottet A, de Haan C, Falcucci A, Tempio G, Opio C, Gerber P, et al. Livestock: on our plates or eating at our table? A new analysis of the feed/food debate. Global Food Security. (2017) 14:1–8. 14: 1–8. 10.1016/j.gfs.2017.01.001 [DOI] [Google Scholar]
- 15.Khatoon E, Banik K, Harsha C, Sailo BL, Thakur KK, Khwairakpam AD, et al. Phytochemicals in cancer cell chemosensitization: current knowledge and future perspectives. In:Bishayee A, Sethi G, editors. Seminars in Cancer Biology, vol 80. Cambridge, MA: Academic Press (2022). p. 306–39. 306–39. 10.1016/j.semcancer.2020.06.014 [DOI] [PubMed] [Google Scholar]
- 16.Amadi EV, Venkataraman A, Papadopoulos C. Nanoscale self-assembly: concepts, applications and challenges. Nanotechnology. (2022) 33:132001. 10.1088/1361-6528/ac3f54 [DOI] [PubMed] [Google Scholar]
- 17.Nkhebenyane J, Tywabi-Ngeva Z. Heavy Metal Contamination in Food: The Perspective of the Sub-Saharan Informal Food Trade. London: IntechOpen Limited; (2023). [Google Scholar]
- 18.Sekar M, Mathimani T, Alagumalai A, Chi NT, Duc PA, Bhatia SK, et al. A review on the pyrolysis of algal biomass for biochar and bio-oil-bottlenecks and scope. Fuel. (2021) 283:119190. 283:119190. 10.1016/j.fuel.2020.119190 [DOI] [Google Scholar]
- 19.Maji S, Dwivedi DH, Singh N, Kishor S, Gond M. Agricultural waste: its impact on environment and management approaches. In:Arora NK, editor. Emerging Eco-Friendly Green Technologies for Wastewater Treatment. Singapore: Springer; (2020). p. 329–51. 10.1007/978-981-15-1390-9_15 [DOI] [Google Scholar]
- 20.Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. (2020) 6:e04691. 10.1016/j.heliyon.2020.e04691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa K, Imura T, Berndtsson R. Distribution of heavy metals and related health risks through soil ingestion in rural areas of western Japan. Chemosphere. (2022) 290:133316. 290:133316. 10.1016/j.chemosphere.2021.133316 [DOI] [PubMed] [Google Scholar]
- 22.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Mol Clin Environ Toxicol. (2012) 2012:133–64. 10.1007/978-3-7643-8340-4_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelmonem R, El-Enin HA, Abdelkader G, Abdel-Hakeem M. Formulation and characterization of lamotrigine nasal insert targeted brain for enhanced epilepsy treatment. Drug Deliv. (2023) 30:2163321. 30:2163321. 10.1080/10717544.2022.2163321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasseghian Y, Arunkumar P, Joo SW, Gnanasekaran L, Kamyab H, Rajendran S, et al. Metal-organic framework-enabled pesticides are an emerging tool for sustainable cleaner production and environmental hazard reduction. J Clean Prod. (2022) 2022:133966. 10.1016/j.jclepro.2022.133966 [DOI] [Google Scholar]
- 25.Nisbet C, Terzi G, Pilgir O, Sarac N. Determination of heavy metal levels in fish samples collected from the Middle Black Sea. Kafkas Univ Vet Fak Derg. (2010) 16:119–25. Available online at: https://www.researchgate.net/profile/Goknur-Terzi/publication/273275958_Orta_Karadeniz_Bolgesinden_Toplanan_Baliklarda_Agir_Metal_Duzeylerinin_Belirlenmesi/links/59eef45ea6fdcc32187de042/Orta-Karadeniz-Boelgesinden-Toplanan-Baliklarda-Agir-Metal-Duezeylerinin-Belirlenmesi.pdf [Google Scholar]
- 26.Dweba CC, Zishiri OT, El Zowalaty ME. Methicillin-resistant Staphylococcus aureus: livestock-associated, antimicrobial, and heavy metal resistance. Infect Drug Resist. (2018) 11:2497. 11:2497. 10.2147/IDR.S175967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hejna M, Moscatelli A, Onelli E, Baldi A, Pilu S, Rossi L, et al. Evaluation of concentration of heavy metals in animal rearing system. Ital J Anim Sci. (2019) 18:1372–84. 18: 1372–84. 10.1080/1828051X.2019.1642806 [DOI] [Google Scholar]
- 28.Tunegová M, Toman R, Tančin V. Heavy metals-environmental contaminants and their occurrence in different types of milk. Slovak J Anim Sci. (2016) 49:122–31. Available online at: https://office.sjasjournal.org/index.php/sjas/article/view/171 [Google Scholar]
- 29.Vanisree CR, Sankhla MS, Singh P, Jadhav EB, Verma RK, Awasthi KK, et al. Heavy Metal Contamination of Food Crops: Transportation via Food Chain, Human Consumption, Toxicity and Management Strategies. London: Intech Open; (2022). [Google Scholar]
- 30.Pouresmaieli M, Ataei M, Forouzandeh P, Azizollahi P, Mahmoudifard M. Recent progress on sustainable phytoremediation of heavy metals from soil. J Environ Chem Eng. (2022) 2022:108482. 2022:108482. 10.1016/j.jece.2022.108482 [DOI] [Google Scholar]
- 31.Verma N, Rachamalla M, Kumar PS, Dua K. Assessment and impact of metal toxicity on wildlife and human health. In:Kumar S, Kumar S, Mishra PK, editors. Metals in Water. Amsterdam: Elsevier; (2023). p. 93–110. 93–110. 10.1016/B978-0-323-95919-3.00002-1 [DOI] [Google Scholar]
- 32.Mustafa H, Khan WA, Kuthu ZH, Eui-Soo K, Ajmal A, Javed K, et al. Genome-wide survey of selection signatures in Pakistani cattle breeds. Pak Vet J. (2018) 38:214–8. 38: 214–8. 10.29261/pakvetj/2018.051 [DOI] [Google Scholar]
- 33.Gambushe SM, Zishiri OT, El Zowalaty ME. Review of Escherichia Coli O157, H7. Prevalence, pathogenicity, heavy metal and antimicrobial resistance. African Perspect Infect Drug Resist. (2022) 4645–73. 4645–73. 10.2147/IDR.S365269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goyer RA. Nutrition and metal toxicity. Am J Clin Nutr. (1995) 61:646S−50S. 61:646S−50S. 10.1093/ajcn/61.3.646S [DOI] [PubMed] [Google Scholar]
- 35.Tahir R, Ghaffar A, Abbas G, Turabi TH, Kausar S, Xiaoxia D, et al. Pesticide induced hematological, biochemical and genotoxic changes in fish: a review. Agrobiol Records. (2021) 3:41–57. 3: 41–57. 10.47278/journal.abr/2021.005 [DOI] [Google Scholar]
- 36.Menéndez-Pedriza A, Jaumot J, Bedia C. Lipidomic analysis of single and combined effects of polyethylene microplastics and polychlorinated biphenyls on human hepatoma cells. J Hazard Mater. (2022) 421:126777. 421:126777. 10.1016/j.jhazmat.2021.126777 [DOI] [PubMed] [Google Scholar]
- 37.Nriagu J, Boughanen M, Linder A, Howe A, Grant C, Rattray R, et al. Levels of As, Cd, Pb, Cu, Se and Zn in bovine kidneys and livers in Jamaica. Ecotoxicol Environ Saf. (2009) 72:564–71. 72: 564–71. 10.1016/j.ecoenv.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 38.Gidikova P, Deliradeva R, Chobanova S, Prakova G, Mihailova G. Heavy Metal Contents in Homemade Milk and Cheese From Villages With Possible Risk of Environmental Pollution. Zmeyovo and Borilovo: Agrobiological Science; (2016). [Google Scholar]
- 39.Volkov R, Ezhkova A. Migration of heavy metals in the system “soil-plant-animal-livestock products”. In:Guéhot S, editor. BIO Web of Conferences. Les Ulis Cedex: EDP Sciences (2020). (2020). 10.1051/bioconf/20202700068 [DOI] [Google Scholar]
- 40.Hossini H, Shafie B, Niri AD, Nazari M, Esfahlan AJ, Ahmadpour M, et al. Comprehensive review on human health effects of chromium: insights on induced toxicity. Environ Sci Pollut Res. (2022) 2022:1–20. 2022: 1–20. 10.1007/s11356-022-22705-6 [DOI] [PubMed] [Google Scholar]
- 41.Sanders Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN. Mechanism and health effects of heavy metal toxicity in humans. In: Poisoning in the Modern World-New Tricks for An Old Dog, Vol. 10. (2019). p. 70–90. [Google Scholar]
- 42.Egendorf SP, Li E, He E, Cheng Z, Spliethoff HM, Shayler HA, et al. Effectiveness of washing in reducing lead concentrations of lettuce grown in urban garden soils. (2022). [DOI] [PubMed] [Google Scholar]
- 43.Robles-Osorio ML, Sabath-Silva E, Sabath E. Arsenic-mediated nephrotoxicity. Ren Fail. (2015) 37:542–7. 10.3109/0886022X.2015.1013419 [DOI] [PubMed] [Google Scholar]
- 44.Butler-Dawson J, James KA, Krisher L, Jaramillo D, Dally M, Neumann N, et al. Environmental metal exposures and kidney function of Guatemalan sugarcane workers. J Expo Sci Environ Epidemiol. (2022) 32:461–71. 32: 461–71. 10.1038/s41370-021-00292-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bíreš J, Dianovský J, Bartko P, Juhásová Z. Effects on enzymes and the genetic apparatus of sheep after administration of samples from industrial emissions. BioMetals. (1995) 8:53–8. 8: 53–8. 10.1007/BF00156158 [DOI] [PubMed] [Google Scholar]
- 46.Dasharathy S, Arjunan S, Maliyur Basavaraju A, Murugasen V, Ramachandran S, Keshav R, et al. Mutagenic, carcinogenic, and teratogenic effect of heavy metals. Evid-Based Complement Altern Med. (2022) 2022:8011953. 10.1155/2022/8011953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friberg L, Nordberg GF, Vouk VB. Handbook on the Toxicology of Metals. Amsterdam: Elsevier/North-Holland Biomedical Press; (1979). [Google Scholar]
- 48.Biswal T. Phytoremediation of soils contaminated with heavy metals: techniques and strategies. In:Malik JA, editor. Advances in Bioremediation and Phytoremediation for Sustainable Soil Management. Cham: Springer; (2022). 10.1007/978-3-030-89984-4_3 [DOI] [Google Scholar]
- 49.Imran M, Cao S, Wan SF, Chen Z, Saleemi MK, Wang N, et al. Mycotoxins-a global one health concern: a review. Agrobiol Records. (2020) 2:1–6. 10.47278/journal.abr/2020.006 [DOI] [Google Scholar]
- 50.Rahman M, Rahaman M, Islam M, Rahman F, Mithi FM, Alqahtani T, et al. Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules. (2022) 27:233. 27:233. 10.3390/molecules27010233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baykov BD, Stoyanov MP, Gugova ML. Cadmium and lead bioaccumulation in male chickens for high food concentrations. Toxicol Environ Chem. (1996) 54:155–9. 54: 155–9. 10.1080/02772249609358308 [DOI] [Google Scholar]
- 52.Soriano A, Sánchez-García C. Nutritional composition of game meat from wild species harvested in Europe. Meat Nutr. (2021) 2021:77–100. 10.5772/intechopen.97763 [DOI] [Google Scholar]
- 53.Yan C, Qu Z, Wang J, Cao L, Han Q. Microalgal bioremediation of heavy metal pollution in water: recent advances, challenges, and prospects. Chemosphere. (2022) 286:131870. 286: 131870. 10.1016/j.chemosphere.2021.131870 [DOI] [PubMed] [Google Scholar]
- 54.Suttle NF. Mineral Nutrition of Livestock. Cambridge, MA: CABI; (2022). (2022). 10.1079/9781789240924.0000 [DOI] [Google Scholar]
- 55.Aerts L, Jenkerson MR, Nechayuk VE, Gailey G, Racca R, Blanchard AL, et al. Seismic surveys near gray whale feeding areas off Sakhalin Island, Russia: assessing impact and mitigation effectiveness. Environ Monit Assess. (2022) 194:1–7. 10.1007/s10661-022-10016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yun SW, Cho GJ. Genetic diversity of donkey (Equus asinus) by mitochondrial DNA (mt-DNA) analysis. Int J Vet Sci. (2022) 11:409–13. 10.47278/journal.ijvs/2022.139 [DOI] [PubMed] [Google Scholar]
- 57.Thornton PK, Jones PG, Alagarswamy G, Andresen J, Herrero M. Adapting to climate change: agricultural system and household impacts in East Africa. Agric Syst. (2010) 103:73–82. 103: 73–82. 10.1016/j.agsy.2009.09.003 [DOI] [Google Scholar]
- 58.Kebede T, Gadisa E, Tufa A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: a possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE. (2021) 16:e0249253. 10.1371/journal.pone.0249253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ayana GF, Megento TL, Kussa FG. The extent of livelihood diversification on the determinants of livelihood diversification in Assosa Wereda, Western Ethiopia. GeoJournal. (2022) 87:2525–49. 87: 2525–49. 10.1007/s10708-021-10379-5 [DOI] [Google Scholar]
- 60.Tella A, Balogun AL. GIS-based air quality modelling: spatial prediction of PM10 for Selangor State, Malaysia using machine learning algorithms. Environ Sci Pollut Res. (2022) 29:86109–25. 29: 86109–25. 10.1007/s11356-021-16150-0 [DOI] [PubMed] [Google Scholar]
- 61.Swarup D, Patra RC, Naresh R, Kumar P, Shekhar P. Blood lead levels in lactating cows reared around polluted localities; transfer of lead into milk. Sci Total Environ. (2005) 347:106–10. 347: 106–10. 10.1016/j.scitotenv.2004.12.055 [DOI] [PubMed] [Google Scholar]
- 62.Brandelli A. The interaction of nanostructured antimicrobials with biological systems: Cellular uptake, trafficking and potential toxicity. Food Sci. Hum. Wellness. (2020) 9:8–20. [Google Scholar]
- 63.Patel N, Chauhan D, Shahane S, Rai D, Ali Khan MZ, Mishra U, et al. Contamination and health impact of heavy metals. In: Water Pollution and Remediation: Heavy Metals. (2021) p. 259–80.36180568 [Google Scholar]
- 64.Wang SL, Xu XR, Sun YX, Liu JL, Li HB. Heavy metal pollution in coastal areas of South China: a review. Mar Pollut Bullet. (2013) 76:7–15. 10.1016/j.marpolbul.2013.08.025 [DOI] [PubMed] [Google Scholar]
- 65.Sharifian A, Gantuya B, Wario HT, Kotowski MA, Barani H, Manzano P, et al. Global principles in local traditional knowledge: a review of forage plant-livestock-herder interactions. J Environ Manage. (2023) 328:116966. 328:116966. 10.1016/j.jenvman.2022.116966 [DOI] [PubMed] [Google Scholar]
- 66.Koréneková B, Kottferová J, Korének M. Observation of the effects of nitrites and nitrates on yogurt culture. Food Res Int. (1997) 30:55–8. 30: 55–8. 10.1016/S0963-9969(97)00017-3 [DOI] [Google Scholar]
- 67.Tortajada C, Zhang H. When food meets BRI: China's emerging Food Silk Road. Global Food Secur. (2021) 29:100518. 10.1016/j.gfs.2021.100518 [DOI] [Google Scholar]
- 68.Raimi MO, Iyingiala AA, Sawyerr OH, Saliu AO, Ebuete AW, Emberru RE, et al. Leaving no one behind: impact of soil pollution on biodiversity in the global south: a global call for action. In:Izah SC, editor. Biodiversity in Africa: Potentials, Threats and Conservation. Singapore: Springer Nature Singapore; (2022). p. 205–37. 10.1007/978-981-19-3326-4_8 [DOI] [Google Scholar]
- 69.Ullah R, Tsui MT, Chow A, Chen H, Williams C, Ligaba-Osena A, et al. Micro (nano) plastic pollution in terrestrial ecosystem: emphasis on impacts of polystyrene on soil biota, plants, animals, and humans. Environ Monit Assess. (2023) 195:1–29. 10.1007/s10661-022-10769-3 [DOI] [PubMed] [Google Scholar]
- 70.Bonnet S. Why develop photoactivated chemotherapy? Dalton Trans. (2018) 47:10330–43. 47: 10330–43. 10.1039/C8DT01585F [DOI] [PubMed] [Google Scholar]
- 71.Abdi J, Sisi AJ, Hadipoor M, Khataee A. State of the art on the ultrasonic-assisted removal of environmental pollutants using metal-organic frameworks. J Hazard Mater. (2022) 424:127558. 424:127558. 10.1016/j.jhazmat.2021.127558 [DOI] [PubMed] [Google Scholar]
- 72.Nardone A, Ronchi B, Lacetera N, Ranieri MS, Bernabucci U. Effects of climate changes on animal production and sustainability of livestock systems. Livest Sci. (2010) 130:57–69. 130: 57–69. 10.1016/j.livsci.2010.02.011 [DOI] [Google Scholar]
- 73.Ritchie H, Roser M. Outdoor Air Pollution. Our World in Data (2019). [Google Scholar]
- 74.Basnet S, Wood A, Röös E, Jansson T, Fetzer I, Gordon L, et al. Organic agriculture in a low-emission world: exploring combined measures to deliver sustainable food system in Sweden. Sustain Sci. (2023) 2023:1–9. 2023: 1–9. 10.1007/s11625-022-01279-9 [DOI] [Google Scholar]
- 75.Hussain N, Bilal M, Iqbal HM. Carbon-based nanomaterials with multipurpose attributes for water treatment: greening the 21st-century nanostructure materials deployment. Biomater Polym Horiz. (2022) 1:1–1. 1: 1–1. 10.37819/bph.001.01.0131 [DOI] [Google Scholar]
- 76.Patra RC, Rautray AK, Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int. (2011) 2011:457327. 10.4061/2011/457327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Famurewa AC, Renu K, Eladl MA, Chakraborty R, Myakala H, El-Sherbiny M, et al. Hesperidin and hesperetin against heavy metal toxicity: insight on the molecular mechanism of mitigation. Biomed Pharmacother. (2022) 149:112914. 149:112914. 10.1016/j.biopha.2022.112914 [DOI] [PubMed] [Google Scholar]
- 78.Cowan V, Blakley B. Acute lead poisoning in western Canadian cattle—a 16-year retrospective study of diagnostic case records. Can Vet J. (2016) 57:421. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4790235/ [PMC free article] [PubMed] [Google Scholar]
- 79.Nakata H, Eguchi A, Nakayama SM, Yabe J, Muzandu K, Ikenaka Y, et al. Metabolomic alteration in the plasma of wild rodents environmentally exposed to lead: a preliminary study. Int J Environ Res Public Health. (2022) 19:541. 19:541. 10.3390/ijerph19010541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Badiei K, Mostaghni K, Nowrooziasl A, Naeini AT. Ameliorated effects of Allium sativum on subclinical lead toxicity in goats. Pak Vet J. (2006) 26:184. Available online at: http://www.pvj.com.pk/pdf-files/26_4/page%20184-186.pdf [Google Scholar]
- 81.Burki Z, Watkins S, Wilson R, Fenlon MA. Randomised controlled trial to investigate the effects of dehydration on tooth colour. J Dent. (2013) 41:250–7. 10.1016/j.jdent.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Shaheen SM, Azeem M, Zhang L, Feng C, Peng J, et al. Removal of lead (Pb+ 2) from contaminated water using a novel MoO3-biochar composite: performance and mechanism. Environ Pollut. (2022) 308:119693. 308:119693. 10.1016/j.envpol.2022.119693 [DOI] [PubMed] [Google Scholar]
- 83.Bernales GG, Calo CM, Dumago CM, Tibe A, Barbon M, Altar DM, et al. Analysis of heavy metal (Hg, Pb, Ni) content in Marsh Clam, Polymesoda expansa collected from Butuan Bay, Philippines. Int J Biol Phys Chem Stud. (2022) 4:14–23. 10.32996/ijbpcs.2022.4.1.3 [DOI] [Google Scholar]
- 84.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. (2001) 1:529–39. 1: 529–39. 10.2174/1568026013394831 [DOI] [PubMed] [Google Scholar]
- 85.Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. (2012) 5:47. 10.2478/v10102-012-0009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katsuyama E, Suarez-Fueyo A, Bradley SJ, Mizui M, Marin AV, Mulki L, et al. The CD38/NAD/SIRTUIN1/EZH2 axis mitigates cytotoxic CD8 T cell function and identifies patients with SLE prone to infections. Cell Rep. (2020) 30:112–23. 10.1016/j.celrep.2019.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishikawa Y, Vranka JA, Boudko SP, Pokidysheva E, Mizuno K, Zientek K, et al. Mutation in cyclophilin B that causes hyperelastosis cutis in American Quarter Horse does not affect peptidylprolyl cis-trans isomerase activity but shows altered cyclophilin B-protein interactions and affects collagen folding. J Biol Chem. (2012) 287:22253–65. 287: 22253–65. 10.1074/jbc.M111.333336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhutta ZA, Kulyar MF, Jahanzaib IS, Shabbir S, Boruah P, Bello A, et al. Evaluation of hematological, antioxidant enzymes and oxidative stress parameters in buffaloes infected with babesiosis. Continent Vet J. (2022) 2:29–34. Available online at: https://cvetj.com/upload/issue_paper/1656492093_4%20CVJ-22-0402%202(1)%20pp%2029-34.pdf [Google Scholar]
- 89.Pizzino G, Irrera N, Galfo F, Pallio G, Mannino F, D'amore A, et al. Effects of the antagomiRs 15b and 200b on the altered healing pattern of diabetic mice. Br J Pharmacol. (2018) 175:644–55. 175: 644–55. 10.1111/bph.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castañeda-Figueredo JS, Torralba-Dotor AI, Pérez-Rodríguez CC, Moreno-Bedoya AM, Mosquera-Vivas CS. Removal of lead and chromium from solution by organic peels: effect of particle size and bio-adsorbent. Heliyon. (2022) 8:e10275. 10.1016/j.heliyon.2022.e10275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narayanan Y. Mother Cow, Mother India: A Multispecies Politics of Dairy in India. RedWood City: Stanford University Press. 10.1515/9781503634381 [DOI] [Google Scholar]
- 92.Aslani MR, Heidarpour M, Najar-Nezhad V, Mostafavi M, Toosizadeh-Khorasani Y. Lead poisoning in cattle associated with batteries recycling: high lead levels in milk of nonsymptomatic exposed cattle. Iran J Vet Sci Technol. (2012) 4:47–52. Available online at: https://www.researchgate.net/profile/Vahid-Najarnezhad-2/publication/256838760_Lead_poisoning_in_cattle_associated_with_batteries_recycling_High_lead_levels_in_milk_of_nonsymptomatic_exposed_cattle/links/0deec53953baaab75b000000/Lead-poisoning-in-cattle-associated-with-batteries-recycling-High-lead-levels-in-milk-of-nonsymptomatic-exposed-cattle.pdf [Google Scholar]
- 93.Das Roy M, Sana SS. Inter-dependent lead-time and ordering cost reduction strategy: a supply chain model with quality control, lead-time dependent backorder and price-sensitive stochastic demand. Opsearch. (2021) 58:690–710. 58: 690–710. 10.1007/s12597-020-00499-w [DOI] [Google Scholar]
- 94.Dwivedi SK, Dey S. Medicinal herbs: a potential source of toxic metal exposure for man and animals in India. Arch Environ Health. (2002) 57:229–31. 10.1080/00039890209602941 [DOI] [PubMed] [Google Scholar]
- 95.Swarup D, Dwivedi SK. Environmental Pollution and Effects of Lead and Fluoride on Animal Health. New Delhi: Indian Council of Agricultural Research; (2002). [Google Scholar]
- 96.Das A. Nanotheranostics: the toxicological implications. Des Appl. (2023) 369. 369. 10.1016/B978-0-323-89953-6.00012-X [DOI] [Google Scholar]
- 97.Souza-Arroyo V, Fabián JJ, Bucio-Ortiz L, Miranda-Labra RU, Gomez-Quiroz LE, Gutiérrez-Ruiz MC, et al. The mechanism of the cadmium-induced toxicity and cellular response in the liver. Toxicology. (2022) 2022:153339. 2022:153339. 10.1016/j.tox.2022.153339 [DOI] [PubMed] [Google Scholar]
- 98.Cuomo D, Foster MJ, Threadgill D. Systemic review of genetic and epigenetic factors underlying differential toxicity to environmental lead (Pb) exposure. Environ Sci Pollut Res. (2022) 4:1–6. 4: 1–6. 10.1007/s11356-022-19333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waldner C, Checkley S, Blakley B, Pollock C, Mitchell B. Managing lead exposure and toxicity in cow–calf herds to minimize the potential for food residues. J Vet Diagn Investig. (2002) 14:481–6. 14: 481–6. 10.1177/104063870201400606 [DOI] [PubMed] [Google Scholar]
- 100.Payne SC, Bergman ME, Rodríguez JM, Beus JM, Henning JB. Leading and lagging: process safety climate-incident relationships at one year. J Loss Prevent Process Indus. (2010) 23:806–12. 23: 806–12. 10.1016/j.jlp.2010.06.004 [DOI] [Google Scholar]
- 101.Ernyasih AM, Palutturi S, Daud A. Calculating the potential risks of environmental and communities health due to lead contaminants exposure: a systematic review. J Pharm Negative Results. (2023) 14:68–76. 10.47750/pnr.2023.14.01.011 [DOI] [Google Scholar]
- 102.Satarug S, Vesey DA, Gobe GC, Phelps KR. Estimation of health risks associated with dietary cadmium exposure. Arch Toxicol. (2023) 2023:1–30. 10.1007/s00204-022-03432-w [DOI] [PubMed] [Google Scholar]
- 103.Uppal S, Liu T, Galvan E, Gomez F, Tittley T, Poliakov E, et al. An inducible amphipathic α-helix mediates subcellular targeting and membrane binding of RPE65. Life Sci Alliance. (2023) 6:1546. 6:1546. 10.26508/lsa.202201546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao M, Yin G, Xu J, Ge X, Li A, Mei Y, et al. Independent, combine and interactive effects of heavy metal exposure on dyslipidemia biomarkers: a cross-sectional study in northeastern China. Ecotoxicol Environ Saf. (2023) 250:114494. 10.1016/j.ecoenv.2022.114494 [DOI] [PubMed] [Google Scholar]
- 105.Marçal WS, Gaste L, Liboni M, Pardo PE, Do Nascimento MR, Hisasi CS, et al. Concentration of lead in mineral salt mixtures used as supplements in cattle food. Exp Toxicol Pathol. (2001) 53:7–10. 53: 7–10. 10.1078/0940-2993-00166 [DOI] [PubMed] [Google Scholar]
- 106.Viegas V, Burzlaff A, Brock III TO, Danzeisen R. A tiered approach to investigate the inhalation toxicity of cobalt substances. Tier 3: inflammatory response following acute inhalation exposure correlates with lower tier data. Regul Toxicol Pharmacol. (2022) 130:105127. 10.1016/j.yrtph.2022.105127 [DOI] [PubMed] [Google Scholar]
- 107.Alonso R, Aguirre A, Marzo F. Effects of extrusion and traditional processing methods on antinutrients and in vitro digestibility of protein and starch in faba and kidney beans. Food Chem. (2000) 68:159–65. 68: 159–65. 10.1016/S0308-8146(99)00169-7 [DOI] [Google Scholar]
- 108.Roy S, Edwards MA. Are there excess fetal deaths attributable to waterborne lead exposure during the Flint Water Crisis? Evidence from bio-kinetic model predictions and Vital Records. J Expos Sci Environ Epidemiol. (2022) 32:17–26. 10.1038/s41370-021-00363-z [DOI] [PubMed] [Google Scholar]
- 109.Borobia M, Villanueva-Saz S, Ruiz de Arcaute M, Fernández A, Verde MT, González JM, et al. Copper poisoning, a deadly hazard for sheep. Animals. (2022) 12:2388. Available online at: https://www.mdpi.com/2076-2615/12/18/2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gilbert P. Compassion: Conceptualisations, Research and Use in Psychotherapy. London: Routledge; (2005). [Google Scholar]
- 111.Chen F, Aqeel M, Maqsood MF, Khalid N, Irshad MK, Ibrahim M, et al. Mitigation of lead toxicity in Vigna radiata genotypes by silver nanoparticles. Environ Pollut. (2022) 308:119606. 308:119606. 10.1016/j.envpol.2022.119606 [DOI] [PubMed] [Google Scholar]
- 112.Cholin LK, Schold JD, Arrigain S, Poggio ED, Sedor JR, O'Toole JF, et al. Characteristics of potential and actual living kidney donors: a single-center experience. Transplantation. (2022) 10–97. 10–97. 10.1097/TP.0000000000004357 [DOI] [PubMed] [Google Scholar]
- 113.Grant AM. Does intrinsic motivation fuel the prosocial fire? Motivational synergy in predicting persistence, performance, and productivity. J Appl Psychol. (2008) 93:48. 93:48. 10.1037/0021-9010.93.1.48 [DOI] [PubMed] [Google Scholar]
- 114.Xue ZF, Cheng WC, Wang L, Hu W. Effects of bacterial inoculation and calcium source on microbial-induced carbonate precipitation for lead remediation. J Hazard Mater. (2022) 426:128090. 426:128090. 10.1016/j.jhazmat.2021.128090 [DOI] [PubMed] [Google Scholar]
- 115.Pyeritz RE. Heritable and polygenic inflammatory disorders. In:Pyeritz RE, Korf BR, Grody WW, editors. Emery and Rimoin's Principles and Practice of Medical Genetics and Genomics. Cambridge, MA: Academic Press; (2023). p. 321–6. 10.1016/B978-0-12-812534-2.00008-4 [DOI] [Google Scholar]
- 116.Baranowska-Bosiacka I, Gutowska I, Rybicka M, Nowacki P, Chlubek D. Neurotoxicity of lead. Hypothetical molecular mechanisms of synaptic function disorders. Neurol Neurochirur Polska. (2012) 46:569–78. 46: 569–78. 10.5114/ninp.2012.31607 [DOI] [PubMed] [Google Scholar]
- 117.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease?. Hypertension. (2003). 41:1183–90. 41: 1183–90. 10.1161/01.HYP.0000069700.62727.C5 [DOI] [PubMed] [Google Scholar]
- 118.Damiano S, Jarriyawattanachaikul W, Girolami F, Longobardi C, Nebbia C, Andretta E, et al. Curcumin supplementation protects broiler chickens against the renal oxidative stress induced by the dietary exposure to low levels of aflatoxin B1. Front Vet Sci. (2022) 8:1659. 10.3389/fvets.2021.822227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tian X, Shan X, Ma L, Zhang C, Wang M, Zheng J, et al. Mixed heavy metals exposure affects the renal function mediated by 8-OHG: a cross-sectional study in rural residents of China. Environ Pollut. (2023) 317:120727. 317:120727. 10.1016/j.envpol.2022.120727 [DOI] [PubMed] [Google Scholar]
- 120.Bhardwaj H, Singh C, Nayyar S. Assessment of adverse effects of lead, nickel and cadmium on biochemical parameters, antioxidants status and metallothionein expression in buffaloes slaughtered at local abattoir. Indian J Anim Res. (2022) 56:145–52. 10.18805/IJAR.B-4242 [DOI] [Google Scholar]
- 121.Ferreira G, Santander A, Chavarría L, Cardozo R, Savio F, Sobrevia L, et al. Functional consequences of lead and mercury exposomes in the heart. Mol Aspects Med. (2022) 87:101048. 87:101048. 10.1016/j.mam.2021.101048 [DOI] [PubMed] [Google Scholar]
- 122.Ilieva N, Tashkova D, Staykov D, Serteva D, Feodorova Y, Mehterov N, et al. Immunohistochemical expression of CK20, CK7, and CDX2 in colorectal carcinoma in correlation with pathomorphological characteristics. Folia Med. (2022) 64:214–20. 10.3897/folmed.64.e60950 [DOI] [PubMed] [Google Scholar]
- 123.Sharma P, Chaturvedi P, Chandra R, Kumar S. Identification of heavy metals tolerant Brevundimonas sp. from rhizospheric zone of Saccharum munja L. and their efficacy in in-situ phytoremediation. Chemosphere. (2022) 295:133823. 10.1016/j.chemosphere.2022.133823 [DOI] [PubMed] [Google Scholar]
- 124.Moneim AE, Dkhil MA, Al-Quraishy S. The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J Hazard Mater. (2011) 194:250–5. 194: 250–5. 10.1016/j.jhazmat.2011.07.097 [DOI] [PubMed] [Google Scholar]
- 125.Lakshmi D, Akhil D, Kartik A, Gopinath KP, Arun J, Bhatnagar A, et al. Artificial intelligence (AI) applications in adsorption of heavy metals using modified biochar. Sci Total Environ. (2021) 801:149623. 801:149623. 10.1016/j.scitotenv.2021.149623 [DOI] [PubMed] [Google Scholar]
- 126.Sujatha S, Bhat R, Kannan C, Balasimha D. Impact of intercropping of medicinal and aromatic plants with organic farming approach on resource use efficiency in arecanut (Areca catechu L.) plantation in India. Indus Crops Prod. (2011) 33:78–83. 10.1016/j.indcrop.2010.09.001 [DOI] [Google Scholar]
- 127.Huang Q, Yang Y, Zhao T, Chen Q, Liu M, Ji S, et al. Passively-targeted mitochondrial tungsten-based nanodots for efficient acute kidney injury treatment. Bioactive Mater. (2023) 21:381–93. 21: 381–93. 10.1016/j.bioactmat.2022.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aron AW, Krishnan N, Shirali AC. Pathogenesis of acute tubulointerstitial nephritis. In:Atta MG, Perazella MA, editors. Tubulointerstitial Nephritis. Cham: Springer; (2022). p. 21–47. 21–47. 10.1007/978-3-030-93438-5_3 [DOI] [Google Scholar]
- 129.Vervaet BA, Schreurs G, Nast CC, Santa-Cruz F, De Broe ME. Chronic interstitial nephritis in agricultural communities: a patient in paraguay. Kidney Int Rep. (2022) 7:1131–5. 7: 1131–5. 10.1016/j.ekir.2022.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ibrahim MA, Khalifa AM, Mohamed AA, Galhom RA, Korayem HE, Abd El-Fadeal NM, et al. Bone-marrow-derived mesenchymal stem cells, their conditioned media, and olive leaf extract protect against cisplatin-induced toxicity by alleviating oxidative stress, inflammation, and apoptosis in rats. Toxics. (2022) 10:526. 10:526. 10.3390/toxics10090526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Turner B, Devisscher T, Chabaneix N, Woroniecki S, Messier C, Seddon N, et al. The role of nature-based solutions in supporting social-ecological resilience for climate change adaptation. Annu Rev Environ Resour. (2022) 47:123–48. 10.1146/annurev-environ-012220-010017 [DOI] [Google Scholar]
- 132.Munir N, Jahangeer M, Bouyahya A, El Omari N, Ghchime R, Balahbib A, et al. Heavy metal contamination of natural foods is a serious health issue: a review. Sustainability. (2022) 14:161. 14:161. 10.3390/su14010161 [DOI] [Google Scholar]
- 133.Yoo MH, Lee SJ, Kim W, Kim Y, Kim YB, Moon KS, et al. Bisphenol A impairs renal function by reducing Na+/K+-ATPase and F-actin expression, kidney tubule formation in vitro and in vivo. Ecotoxicol Environ Saf. (2022) 246:114141. 10.1016/j.ecoenv.2022.114141 [DOI] [PubMed] [Google Scholar]
- 134.Ruidas BC, Pal DB. Biosorption of arsenic from wastewater. Biosorp Wastewater Contam. (2022) 2022:269–83. 10.1002/9781119737629.ch14 [DOI] [Google Scholar]
- 135.Algieri C, Pugliese V, Coppola G, Curcio S, Calabro V, Chakraborty S, et al. Arsenic removal from groundwater by membrane technology: advantages, disadvantages, and effect on human health. Groundwater Sustain Dev. (2022) 2022:100815. 10.1016/j.gsd.2022.100815 [DOI] [Google Scholar]
- 136.Aslam B, Javed I, Khan FH. Uptake of heavy metal residues from sewerage sludge in the milk of goat and cattle during summer season. Pak Vet J. (2011) 31:75–7. Available online at: https://web.p.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=02538318&AN=66655084&h=DH%2bECj9EQXHGeGHWa8SVX0STz0%2feVx2ECglmy4qn%2fJRWAjnwEFrLm8n4vfNCBBLaHavVtqSHdy9Kn%2bwI2A8NwA%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNoProfile&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d02538318%26AN%3d66655084 [Google Scholar]
- 137.Faizan M, Alam P, Rajput VD, Faraz A, Afzal S, Ahmed SM, et al. Nanoparticle mediated plant tolerance to heavy metal stress: what we know? Sustainability. (2023) 15:1446. 10.3390/su15021446 [DOI] [Google Scholar]
- 138.Valskys V, Hassan HR, Wołkowicz S, Satkunas J, Kibirkštis G, Ignatavičius GA, et al. review on detection techniques, health hazards and human health risk assessment of arsenic pollution in soil and groundwater. Minerals. (2022) 12:1326. 10.3390/min12101326 [DOI] [Google Scholar]
- 139.Chen Y, Zhang Y, Shi X, Shi E, Zhao T, Zhang Y, et al. The contribution of earthworms to carbon mineralization during vermicomposting of maize stover and cow dung. Bioresour Technol. (2023) 368:128283. 368:128283. 10.1016/j.biortech.2022.128283 [DOI] [PubMed] [Google Scholar]
- 140.Smichowski P, Gómez DR. An overview of natural and anthropogenic sources of ultrafine airborne particles: analytical determination to assess the multielemental profiles. Appl Spectrosc Rev. (2023) 1–27. 1–27. 10.1080/05704928.2023.2166522 [DOI] [Google Scholar]
- 141.Akanda R, Quraishi SB, Mamun S. Heavy metal content in beef collected from local markets of dhaka and assessing the health risk. Biores Commun. (2023) 9:1196–202. 10.3329/brc.v9i1.63599 [DOI] [Google Scholar]
- 142.Nielsen GH, Heiger-Bernays WJ, Levy JI, White RF, Axelrad DA, Lam J, et al. Application of probabilistic methods to address variability and uncertainty in estimating risks for non-cancer health effects. Environ Health. (2023) 21:1–3. 10.1186/s12940-022-00918-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Joardar A, Pandia S, Chakraborty H. Effect of polyunsaturated free fatty acids on membrane fusion mechanism. Soft Matter. (2023) 19:733–42. 10.1039/D2SM01474B [DOI] [PubMed] [Google Scholar]
- 144.Gomez-Delgado AI, Tibon J, Silva MS, Lundebye AK, Agüera A, Rasinger JD, et al. Seasonal variations in mercury, cadmium, lead and arsenic species in Norwegian blue mussels (Mytilus edulis L)-Assessing the influence of biological and environmental factors. J Trace Elem Med Biol. (2023) 76:127110. 76:127110. 10.1016/j.jtemb.2022.127110 [DOI] [PubMed] [Google Scholar]
- 145.Chopra S, Dhumal S, Abeli P, Beaudry R, Almenar E. Metal-organic frameworks have utility in adsorption and release of ethylene and 1-methylcyclopropene in fresh produce packaging. Postharvest Biol Technol. (2017) 130:48–55. 10.1016/j.postharvbio.2017.04.001 [DOI] [Google Scholar]
- 146.Rajawat NK, Bhardwaj K, Mathur N. Risk of Parkinson disease associated with pesticide exposure and protection by probiotics. Materials Today: Proceedings (2023). [Google Scholar]
- 147.Upadhyay K, Viramgami A, Balachandar R, Pagdhune A, Shaikh I, Sivaperumal P, et al. Development and validation of Graphite Furnace Atomic Absorption Spectrometry method and its application for clinical evaluation of blood lead levels among occupationally exposed lead smelting plant workers. Anal Sci. (2023) 2023:1–10. 10.1007/s44211-022-00260 [DOI] [PubMed] [Google Scholar]
- 148.Ashrafihelan J, Amoli JS, Alamdari M, Esfahani TA, Mozafari M, Nourian AR, et al. Arsenic toxicosis in sheep: the first report from Iran. Interdiscip Toxicol. (2013) 6:93. 6:93. 10.2478/intox-2013-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stefano T, Francesca M, Guendalina G, Michele B, Chiara F, Salvatore A, et al. Utility and diagnostic value of postmortem microbiology associated with histology for forensic purposes. Forensic Sci Int. (2023) 342:111534. 342:111534. 10.1016/j.forsciint.2022.111534 [DOI] [PubMed] [Google Scholar]
- 150.Rana T, Bera AK, Das S, Bhattacharya D, Bandyopadhyay S, Pan D, et al. Effect of chronic intake of arsenic-contaminated water on blood oxidative stress indices in cattle in an arsenic-affected zone. Ecotoxicol Environ Saf. (2010) 73:1327–32. 10.1016/j.ecoenv.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 151.Rana S, Biswas JP, Paul S, Paik A, Maiti D. Organic synthesis with the most abundant transition metal-iron: from rust to multitasking catalysts. Chem Soc Rev. (2021) 50:243–472. 50: 243–472. 10.1039/D0CS00688B [DOI] [PubMed] [Google Scholar]
- 152.Liu J, Liu Y, Habeebu SM, Waalkes MP, Klaassen CD. Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice. Toxicology. (2000) 147:157–66. 147: 157–66. 10.1016/S0300-483X(00)00194-3 [DOI] [PubMed] [Google Scholar]
- 153.Sabath E. Arsenic, kidney, and urinary bladder disorders. In:Flora SJS, editor. Handbook of Arsenic Toxicology. Cambridge, MA: Academic Press; (2015). p. 429–42. 429–42. 10.1016/B978-0-12-418688-0.00018-6 [DOI] [Google Scholar]
- 154.Luft FC. Biomarkers and predicting acute kidney injury. Acta Physiol. (2021) 231:e13479. 231:e13479. 10.1111/apha.13479 [DOI] [PubMed] [Google Scholar]
- 155.Li C, Zhou K, Qin W, Tian C, Qi M, Yan X, et al. Review on heavy metals contamination in soil: effects, sources, and remediation techniques. Soil Sediment Contam Int J. (2019) 28:380–94. 28: 380–94. 10.1080/15320383.2019.1592108 [DOI] [Google Scholar]
- 156.Hadj-Moussa H, Eaton L, Cheng H, Pamenter ME, Storey KB. Naked mole-rats resist the accumulation of hypoxia-induced oxidative damage. Comp Biochem Physiol Part A: Mol Integr Physiol. (2022) 273:111282. 273:111282. 10.1016/j.cbpa.2022.111282 [DOI] [PubMed] [Google Scholar]
- 157.Shaito A, Aramouni K, Assaf R, Parenti A, Orekhov A, El Yazbi A, et al. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Qatar: Frontier in Bioscience Landmark; (2022). 10.31083/j.fbl2703105 [DOI] [PubMed] [Google Scholar]
- 158.Escudero-Lourdes C, Alvarado-Morales I, Tokar EJ. Stem cells as target for prostate cancer therapy: opportunities and challenges. Stem Cell Rev Rep. (2022) 1–9. 1–9. 10.1007/s12015-022-10437-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS, et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. (2022) 11:552. 11:552. 10.3390/cells11030552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ugwu CN, Abali IO, Iwuoha CE, Chika-Igwenyi NM, Onyeaghala CA, Orji SF, et al. Ameliorative effect of Parkia biglobosa (African locust bean) seed against potassium bromateinduced oxidative stress. Merit Res J Med Med Sci. (2022) 10:213–9. 10.5281/zenodo.7013664 [DOI] [Google Scholar]
- 161.Radajewska A, Szyller J, Niewiadomska J, Noszczyk-Nowak A, Bil-Lula I. Punica granatum L polyphenolic extract as an antioxidant to prevent kidney injury in metabolic syndrome rats. Oxid Med Cellular Longevity. (2023) 2023:6144967. 10.1155/2023/6144967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang M, Choi SJ, Kim DW, Kim NY, Park CH, Yu SD, et al. Risk assessment of low-level cadmium and arsenic on the kidney. J Toxicol Environ Health Part A. (2009) 72:1493–8. 72: 1493–8. 10.1080/15287390903213095 [DOI] [PubMed] [Google Scholar]
- 163.Asare MO, Száková J, Tlustoš P. The fate of secondary metabolites in plants growing on Cd-, As-, and Pb-contaminated soils-a comprehensive review. Environ Sci Pollut Res. (2022) 2021:1–21. 2021: 1–21. 10.1007/s11356-022-24776-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fatoki JO, Badmus JA. Arsenic as an environmental and human health antagonist: a review of its toxicity and disease initiation. J Hazard Mater Adv. (2022) 2022:100052. 2022:100052. 10.1016/j.hazadv.2022.100052 [DOI] [Google Scholar]
- 165.El-Ghiaty MA, El-Kadi AO. The duality of arsenic metabolism: impact on human health. Annu Rev Pharmacol Toxicol. (2022) 63. 10.1146/annurev-pharmtox-051921-020936 [DOI] [PubMed] [Google Scholar]
- 166.Maiti S, Chatterjee AK. Differential response of cellular antioxidant mechanism of liver and kidney to arsenic exposure and its relation to dietary protein deficiency. Environ Toxicol Pharmacol. (2000) 8:227–35. 8: 227–35. 10.1016/S1382-6689(00)00046-6 [DOI] [PubMed] [Google Scholar]
- 167.Muzaffar S, Khan J, Srivastava R, Gorbatyuk MS, Athar M. Mechanistic understanding of the toxic effects of arsenic and warfare arsenicals on human health and environment. Cell Biol Toxicol. (2022) 2022:1–26. 2022: 1–26. 10.1007/s10565-022-09710-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Himeno S, Imura N. Selenium in Nutrition and Toxicology. Heavy Metals in the Environment. New York, NY: Marcel Dekker Inc. (2002). p. 587–629. 10.1201/9780203909300.ch17 [DOI] [Google Scholar]
- 169.García-Sevillano MA, Contreras-Acuña M, García-Barrera T, Navarro F, Gómez-Ariza JL. Metabolomic study in plasma, liver and kidney of mice exposed to inorganic arsenic based on mass spectrometry. Anal. Bioanal. Chem. (2014) 406:1455–69. [DOI] [PubMed] [Google Scholar]
- 170.Genchi G, Lauria G, Catalano A, Carocci A, Sinicropi MS. Arsenic: a review on a great health issue worldwide. Appl Sci. (2022) 12:6184. 10.3390/app12126184 [DOI] [Google Scholar]
- 171.Sreehari KS, Namratha WN, Joseph N, Balachandran M. Toxic heavy metal ion detection by fluorescent nanocarbon sensor derived from a medicinal plant. Results Chem. (2023) 5:100720. 5:100720. 10.1016/j.rechem.2022.100720 [DOI] [Google Scholar]
- 172.Bhattacharya S. Honey can obviate heavy metal toxicity: a review. J Environ Pathol Toxicol Oncol. (2023) 42:5311. 10.1615/JEnvironPatholToxicolOncol.2022045311 [DOI] [PubMed] [Google Scholar]
- 173.Yang XF, Zhang HT, Fan GY, Liu DY, Ge YM, Jiang JQ, et al. DNA damage of lung cells from immature cadmium-ingested mice. Pak Vet J. (2014) 34:73–7. Available online at: https://cvetj.com/upload/issue_paper/1639813158_3-CVJ-21-0805-Page-20-24.pdf23079988 [Google Scholar]
- 174.Gutyj B, Grymak Y, Drach M, Bilyk O, Matsjuk O, Magrelo N, et al. The impact of endogenous intoxication on biochemical indicators of blood of pregnant cows. Regul Mech Biosyst. (2017) 3:438–43. 3: 438–43. 10.15421/021769 [DOI] [Google Scholar]
- 175.García-Mayagoitia S, Torres-Gómez AP, Pérez-Hernández H, Patra JK, Fernández-Luqueño F. Collateral effects of nanopollution on human and environmental health. In:Patra JK, Fernández-Luqueño F, editors. Agricultural and Environmental Nanotechnology: Novel Technologies and their Ecological Impact. Singapore: Springer Nature Singapore; (2023). p. 619–45. 619–45. 10.1007/978-981-19-5454-2_23 [DOI] [Google Scholar]
- 176.Alonso Á. Previous stress causes a contrasting response to cadmium toxicity in the aquatic snail Potamopyrgus antipodarum: lethal and behavioral endpoints. Environ Sci Pollut Res. (2023) 11:1–1. 11: 1–1. 10.1007/s11356-022-24932-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ismail SA, Abolghait SK. Estimation of lead and cadmium residual levels in chicken giblets at retail markets in Ismailia city, Egypt. Int J Vet Sci Med. (2013) 1:109–12. 1: 109–12. 10.1016/j.ijvsm.2013.10.003 [DOI] [Google Scholar]
- 178.Otuechere CA, Adewuyi A, Neupane NP, Eromosele SO, Ibekwe OM, Ekozin A, et al. Action of vanillin-spiked zinc ferrite nanoparticles against cadmium-induced liver damage: computational insights with AKT 1, BCl-2 and TLR 8 proteins. J Drug Deliv Sci Technol. (2023) 2023:104139. 10.1016/j.jddst.2022.104139 [DOI] [Google Scholar]
- 179.Jabeen G, Manzoor F, Arshad M, Barbol BI. Effect of cadmium exposure on hematological and morphological changes in fresh water fish (Labeo rohita). Continent Vet J. (2021) 1:20–4. [Google Scholar]
- 180.Satarug S. Dietary cadmium intake and its effects on kidneys. Toxics. (2018) 6:15. 6:15. 10.3390/toxics6010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rahayuningsih CK, Puspitasari D, Puspitasari A. Differences in cadmium (Cd) levels in the blood of active smokers and passive smokers with type 2 diabetic in Puskesmas Kedungdoro Surabaya. Jurnal Teknokes. (2022) 15:69–75. 10.35882/jteknokes.v15i2.198 [DOI] [Google Scholar]
- 182.Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, et al. Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf. (2021) 211:111887. 211:111887. 10.1016/j.ecoenv.2020.111887 [DOI] [PubMed] [Google Scholar]
- 183.Zhou X, Wang B, Wang R. Insights into ion-imprinted materials for the recovery of metal ions: preparation, evaluation and application. Separat Purif Technol. (2022) 2022:121469. 2022:121469. 10.1016/j.seppur.2022.121469 [DOI] [Google Scholar]
- 184.Nordberg M, Duffus JH, Templeton DM. Explanatory dictionary of key terms in toxicology (IUPAC Recommendations 2007). Pure Appl. Chem. (2007) 79:1583–633. [Google Scholar]
- 185.Abernethy AP, Etheredge LM, Ganz PA, Wallace P, German RR, Neti C, et al. Rapid-learning system for cancer care. J Clin Oncol. (2010) 28:4268. 10.1200/JCO.2010.28.5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Waegeneers N, Pizzolon JC, Hoenig M, de Temmerman L. Accumulation of trace elements in cattle from rural and industrial areas in Belgium. Food Addit Contam A. (2009) 26:326–32. 26: 326–32. 10.1080/02652030802429096 [DOI] [PubMed] [Google Scholar]
- 187.Cobb GP, Sands K, Waters M, Wixson BG, Dorward-King E. Accumulation of heavy metals by vegetables grown in mine wastes. Environ. Toxicol. Chem. (2000) 19:600–7. 10.1002/etc.5620190311 [DOI] [Google Scholar]
- 188.Tsai KF, Hsu PC, Lee CT, Kung CT, Chang YC, Fu LM, et al. Association between enzyme-linked immunosorbent assay-measured kidney injury markers and urinary cadmium levels in chronic kidney disease. J Clin Med. (2022) 11:156. 10.3390/jcm11010156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McLaughlin MJ, Smolders E, Zhao FJ, Grant C, Montalvo D. Managing cadmium in agricultural systems. Adv Agron. (2021) 166:1–29. 10.1016/bs.agron.2020.10.004 [DOI] [Google Scholar]
- 190.Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. (2021) 12:643972. 12:643972. 10.3389/fphar.2021.643972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Akar Y, Ahmad N, Khalid M. The effect of cadmium on the bovine in vitro oocyte maturation and early embryo development. Intl J Vet Sci Med. (2018) 6:S73–7. 6: S73–7. 10.1016/j.ijvsm.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ghasemi-Soloklui AA, Didaran F, Kordrostami M, Al-Khayri JM. Plant mediation to tolerate cadmium stress with selenium and nano-selenium. In:Khayri JM, Alnaddaf LM, editors. Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications. Cham: Springer International Publishing; (2023). p. 455–70. 10.1007/978-3-031-20878-2_17 [DOI] [Google Scholar]
- 193.Wang J, Deng P, Wei X, Zhang X, Liu J, Huang Y, et al. Hidden risks from potentially toxic metal (loid) s in paddy soils-rice and source apportionment using lead isotopes: a case study from China. Sci Total Environ. (2023) 856:158883. 10.1016/j.scitotenv.2022.158883 [DOI] [PubMed] [Google Scholar]
- 194.Matović V, Buha A, Bulat Z, Dukić-Cosić D. Cadmium toxicity revisited: focus on oxidative stress induction and interactions with zinc and magnesium. Arh Hig Rada Toksikol. (2011) 62:65–75. 62: 65–75. 10.2478/10004-1254-62-2011-2075 [DOI] [PubMed] [Google Scholar]
- 195.Oraby MI, Baraka TA, Rakha GH. Impact of cadmium intoxication on health status, rumen and blood constituents in Egyptian Ossimi sheep. Int J Vet Sci. (2021) 10:102–6. 10: 102–6. 10.47278/journal.ijvs/2021.040 [DOI] [Google Scholar]
- 196.Wang B, Li Y, Shao C, Tan Y, Cai L. Cadmium and its epigenetic effects. Curr Med Chem. (2012) 19:2611–20. 10.2174/092986712800492913 [DOI] [PubMed] [Google Scholar]
- 197.Thévenod F. Catch me if you can! Novel aspects of cadmium transport in mammalian cells. Biometals. (2010) 23:857–75. 10.1007/s10534-010-9309-1 [DOI] [PubMed] [Google Scholar]
- 198.Vesey DA. Transport pathways for cadmium in the intestine and kidney proximal tubule: focus on the interaction with essential metals. Toxicol. Lett. (2010) 198:13–9. [DOI] [PubMed] [Google Scholar]
- 199.Nordberg M, Nordberg GF. Metallothionein and cadmium toxicology-Historical review and commentary. Biomolecules. (2022) 12:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thévenod F. Nephrotoxicity and the proximal tubule. Nephron Physiol. (2003) 93:87–93. 93: 87–93. 10.1159/000070241 [DOI] [PubMed] [Google Scholar]
- 201.Fujiwara Y, Dixon JA, O'Hara F, Funder ED, Dixon DD, Rodriguez RA, et al. Practical and innate carbon-hydrogen functionalization of heterocycles. Nature. (2012) 492:95–9. 10.1038/nature11680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nordberg G, Jin T, Bernard A, Fierens S, Buchet JP, Ye T, et al. Low bone density and renal dysfunction following environmental cadmium exposure in China. AMBIO: A Journal of the Human Environment. (2002) 31:478–81. [DOI] [PubMed] [Google Scholar]
- 203.Elsakka EG, Mokhtar MM, Hegazy M, Ismail A, Doghish AS. Megalin, a multi-ligand endocytic receptor, and its participation in renal function and diseases: a review. Life Sci. (2022) 120923. 120923. 10.1016/j.lfs.2022.120923 [DOI] [PubMed] [Google Scholar]
- 204.Humphreys DJ. Effects of exposure to excessive quantities of lead on animals. Br Vet J. (1991) 147:18–30. 10.1016/0007-1935(91)90063-S [DOI] [PubMed] [Google Scholar]
- 205.Blanco-Penedo I, López-Alonso M, Miranda M, Benedito JL, Shore RF. Organochlorine pesticide and polychlorinated biphenyl in calves from north-west Spain. Bull Environ Contam Toxicol. (2008) 81:583–7. 81: 583–7. 10.1007/s00128-008-9547-x [DOI] [PubMed] [Google Scholar]
- 206.Cai J, Kimura S, Wada M, Kuga S. Nanoporous cellulose as metal nanoparticles support. Biomacromolecules. (2009) 10:87–94. 10.1021/bm800919e [DOI] [PubMed] [Google Scholar]
- 207.Yabe Y, Galetin A, Houston JB. Kinetic characterization of rat hepatic uptake of 16 actively transported drugs. Drug Metab Dispos. (2011) 39:1808–14. 10.1124/dmd.111.040477 [DOI] [PubMed] [Google Scholar]
- 208.Spierenburg TJ, De Graaf GJ, Baars AJ, Brus DH, Tielen MJ, Arts BJ, et al. Cadmium, zinc, lead, and copper in livers and kidneys of cattle in the neighbourhood of zinc refineries. Environ Monit Assess. (1988) 11:107–14. 11: 107–14. 10.1007/BF00401723 [DOI] [PubMed] [Google Scholar]
- 209.Bilandžić N, Calopek B, Sedak M, Dokić M, Gajger IT, Murati T, et al. Essential and potentially toxic elements in raw milk from different geographical regions of Croatia and their health risk assessment in the adult population. J Food Compos Anal. (2021) 104:104152. 104:104152. 10.1016/j.jfca.2021.104152 [DOI] [Google Scholar]
- 210.Salisbury CD, Chan W, Saschenbrecker PW. Multielement concentrations in liver and kidney tissues from five species of Canadian slaughter animals. J Assoc Off Anal Chem. (1991) 74:587–91. 74: 587–91. 10.1093/jaoac/74.4.587 [DOI] [PubMed] [Google Scholar]
- 211.Doganoc DZ. Lead and cadmium concentrations in meat, liver and kidney of Slovenian cattle and pigs from 1989 to 1993. Food Addit Contam. (1996) 13:237–41. 13: 237–41. 10.1080/02652039609374402 [DOI] [PubMed] [Google Scholar]
- 212.Monteverde V, Camilleri G, Arfuso F, Pennisi M, Perillo L, Patitò G, et al. Heavy metal levels in milk and serum of dairy cows from different farms located near an industrial area. Animals. (2022) 12:2574. 12:2574. 10.3390/ani12192574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zaman MH, Mustafa G, Sheikh MA, Qadir A, Shahid SU, Abbasi NAA, et al. Multi-tissue biomonitoring investigation of toxic trace elements and their trophic transfer potential in a semi aquatic bird species, the Cattle Egret (Bubulcus ibis). Chemosphere. (2022) 300:134582. 300:134582. 10.1016/j.chemosphere.2022.134582 [DOI] [PubMed] [Google Scholar]
- 214.BilandŽić N, Sedak M, Dokić M, Šimić B. Wild boar tissue levels of cadmium, lead and mercury in seven regions of continental Croatia. Bull Environ Contam Toxicol. (2010) 84:738–743. 84: 738–743. 10.1007/s00128-010-9999-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vaessen HA, Ellen G. Arsenic, cadmium, mercury, lead and selenium in slaughter animals: a review of 10 years of research in the Netherlands. [Dutch]. Voeding. (1985). [Google Scholar]
- 216.Nuurtamo M, Varo P, Koivistoinen P, Saari E. Mineral element composition of Finnish foods [N, K, Ca, Mg, P, S, Fe, Cu, Mn, Zn, Mo, Co, Ni, Cr, F, Se, Si, Rb, Al, B, Br, Hg, As, Cd, Pb, ash], 5: Meat and meat products [incl. liver, kidney, heart, lung, stomach, brain, tongue, blood]. Acta Agriculturae Scandinavica. Supplementum (Sweden). (1980). [Google Scholar]
- 217.Horton GM, Holmes W. Compensatory growth by beef cattle at grassland or on an alfalfa-based diet. J Anim Sci. (1978) 46:297–303. 10.2527/jas1978.461297x [DOI] [Google Scholar]
- 218.Kramer HL, Steiner JW, Vallely PJ. Trace element concentrations in the liver, kidney, and muscle of Queensland cattle. Bull Environ Contam Toxicol. (1983) 30:588–94. [DOI] [PubMed] [Google Scholar]
- 219.Flanjak J, Lee HY. Trace metal content of livers and kidneys of cattle. J Sci Food Agric. (1979) 30:503–7. 10.1002/jsfa.2740300510 [DOI] [PubMed] [Google Scholar]
- 220.Patra AK, Abbadie L, Clays-Josserand A, Degrange V, Grayston SJ, Guillaumaud N, et al. Effects of management regime and plant species on the enzyme activity and genetic structure of N-fixing, denitrifying and nitrifying bacterial communities in grassland soils. Environ Microbiol. (2006) 8:1005–16. 8: 1005–16. 10.1111/j.1462-2920.2006.00992.x [DOI] [PubMed] [Google Scholar]
- 221.Sedki A, Lekouch N, Gamon S, Pineau A. Toxic and essential trace metals in muscle, liver and kidney of bovines from a polluted area of Morocco. Sci Total Environ. (2003) 317:201–5. 317: 201–5. 10.1016/S0048-9697(03)00050-0 [DOI] [PubMed] [Google Scholar]
- 222.Roggeman S, De Boeck G, De Cock H, Blust R, Bervoets L. Accumulation and detoxification of metals and arsenic in tissues of cattle (Bos taurus), and the risks for human consumption. Sci Total Environ. (2014) 466:175–84. 466: 175–84. 10.1016/j.scitotenv.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 223.Niemi RG, Craig SC, Mattei F. Measuring internal political efficacy in the 1988 National Election Study. Am Polit Sci Rev. (1991) 85:1407–13. 10.2307/1963953 [DOI] [Google Scholar]
- 224.Tahvonen R, Kumpulainen J. Lead and cadmium contents in pork, beef and chicken, and in pig and cow liver in Finland during 1991. Food Addit Contam. (1994) 11:415–26. 11: 415–26. 10.1080/02652039409374243 [DOI] [PubMed] [Google Scholar]
- 225.Canty MJ, Scanlon A, Collins DM, McGrath G, Clegg TA, Lane E, et al. Cadmium and other heavy metal concentrations in bovine kidneys in the Republic of Ireland. Science of the total environment. (2014) 485:223–31. 485: 223–31. 10.1016/j.scitotenv.2014.03.065 [DOI] [PubMed] [Google Scholar]
- 226.Włostowski T, Bonda E, Krasowska A. Free-ranging European bisons accumulate more cadmium in the liver and kidneys than domestic cattle in north-eastern Poland. Sci Total Environ. (2006) 364:295–300. 364: 295–300. 10.1016/j.scitotenv.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 227.Zasadowski A, Barski D, Markiewicz K, Zasadowski Z, Spodniewska A, Terlecka A, et al. Levels of cadmium contamination of domestic animals (cattle) in the region of Warmia and Masuria. Polish J Environ Stud. (1999) 8:443–6. [Google Scholar]
- 228.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. (2001) 5:62–71. 10.1006/niox.2000.0319 [DOI] [PubMed] [Google Scholar]
- 229.Miranda M, Lopez-Alonso M, Castillo C, Hernández J, Benedito JL. Effects of moderate pollution on toxic and trace metal levels in calves from a polluted area of northern Spain. Environ Int. (2005) 31:543–8. 31: 543–8. 10.1016/j.envint.2004.09.025 [DOI] [PubMed] [Google Scholar]
- 230.Jorhem L, Slorach S, Sundström B, Ohlin B. Lead, cadmium, arsenic and mercury in meat, liver and kidney of Swedish pigs and cattle in 1984-88. Food Additiv Contam. (1991) 8:201–11. 8: 201–11. 10.1080/02652039109373970 [DOI] [PubMed] [Google Scholar]
- 231.Langlands JP, Donald GE, Bowles JE. Cadmium concentrations in liver, kidney and muscle in Australian sheep and cattle. Aust J Exp Agric. (1988) 28:291–7. 10.1071/EA9880291 [DOI] [Google Scholar]
- 232.Rahimi E, Rokni N. Measurement of cadmium residues in muscle, liver and kidney of cattle slaughtered in Isfahan abattoir using grafite furnace atomic absorption spectrometry (GFAAS): a preliminary study. Iran J Vet Res. (2008) 9:174–7. Available online at: https://www.daneshir.ir/fileEssay/IJVR-vol9no2-87231-mm15.pdf [Google Scholar]
- 233.Akele ML, Desalegn SK, Asfaw TB, Assefa AG, Alemu AK, Oliveira RR, et al. Heavy metal contents in bovine tissues (kidney, liver and muscle) from Central Gondar Zone, Ethiopia. Heliyon. (2022) 20:e12416. 20:e12416. 10.1016/j.heliyon.2022.e12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bagda E, Altundag H, Keskin CS. Extraction and determination of cd, co, se, v and ag levels in cow's muscle, liver, kidney, lung, spleen and brain samples. Revue Roumaine Chim. (2022) 67:293–304. 10.33224/rrch.2022.67.4-5.08 [DOI] [Google Scholar]
- 235.Alonso ML, Benedito JL, Miranda M, Castillo C, Hernández J, Shore RF, et al. Interactions between toxic and essential trace metals in cattle from a region with low levels of pollution. Arch Environ Contam Toxicol. (2002) 42:165–72. 10.1007/s00244-001-0012-7 [DOI] [PubMed] [Google Scholar]
- 236.Okoye EA, Bocca B, Ruggieri F, Ezejiofor AN, Nwaogazie IL, Frazzoli C, et al. Arsenic and toxic metals in meat and fish consumed in Niger delta, Nigeria: employing the margin of exposure approach in human health risk assessment. Food Chem Toxicol. (2022) 159:112767. 10.1016/j.fct.2021.112767 [DOI] [PubMed] [Google Scholar]
- 237.Rahim S, Siano P. A survey and comparison of leading-edge uncertainty handling methods for power grid modernization. Expert Syst Appl. (2022) 2022:117590. 2022:117590. 10.1016/j.eswa.2022.117590 [DOI] [Google Scholar]
- 238.Cornelis WM, Corluy J, Medina H, Diaz J, Hartmann R, Van Meirvenne M, et al. Measuring and modelling the soil shrinkage characteristic curve. Geoderma. (2006) 137:179–91. 137: 179–91. 10.1016/j.geoderma.2006.08.02221230167 [DOI] [Google Scholar]
- 239.Yipel M, Türk E, Tekeli IÖ, Oguz H. Heavy metal levels in farmed and wild fishes of Aegean sea and assessment of potential risks to human health. Kafkas Univ Vet Fak Derg. (2016). 22:889–94. [Google Scholar]
- 240.Temmerman S, Govers G, Wartel S, Meire P. Spatial and temporal factors controlling short-term sedimentation in a salt and freshwater tidal marsh, Scheldt estuary, Belgium, SW Netherlands. Earth Surf Process Landforms. (2003) 28:739–55. 10.1002/esp.495 [DOI] [Google Scholar]
- 241.Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. (2004) 291:1900–1. 10.3390/ani12182388 [DOI] [PubMed] [Google Scholar]
- 242.Odongo M, Mutagaya SA, Wanasolo W, Oketch D. Investigation of levels of some selected heavy metals in raw bovine milk from Oyam District, Uganda and estimation of potential health risks. Am J Appl Indus Chem. (2022) 6:1–6. 10.11648/j.ajaic.20220601.11 [DOI] [Google Scholar]
- 243.Fritsch C, Cosson RP, Cœurdassier M, Raoul F, Giraudoux P, Crini N, et al. Responses of wild small mammals to a pollution gradient: host factors influence metal and metallothionein levels. Environ Pollut. (2010) 158:827–40. 10.1016/j.envpol.2009.09.027 [DOI] [PubMed] [Google Scholar]
- 244.Popov RG, Popova NV. Environmental compliance and nutritional value of Yakutian cattle products. In: IOP Conference Series: Earth and Environmental Science. Bristol: IOP Publishing; (2022). p. 042032 042032 10.1088/1755-1315/988/4/042032 [DOI] [Google Scholar]
- 245.Jaafarzadeh N, Tari K, Samarghandi MR, Fard MP, Jorfi S, Feizi R, et al. Non-carcinogenic risk assessment of cadmium and lead in raw milk from dairy production farms in Iran, using Monte Carlo simulation approach. J Food Compos Anal. (2023) 115:104864. 10.1016/j.jfca.2022.104864 [DOI] [Google Scholar]
- 246.Gudmundson P, Weilin Z. An analytic model for thermoelastic properties of composite laminates containing transverse matrix cracks. Int J Solids Struct. (1993) 30:3211–31. 30: 3211–31. 10.1016/0020-7683(93)90110-S [DOI] [Google Scholar]
- 247.Ishaq H, Dincer I, Naterer GF. Performance investigation of an integrated wind energy system for co-generation of power and hydrogen. Int J Hydro Energ. (2018) 43:9153–64. 10.1016/j.ijhydene.2018.03.139 [DOI] [Google Scholar]
- 248.de Namor AF, El Gamouz A, Frangie S, Martinez V, Valiente L, Webb OA. Turning the volume down on heavy metals using tuned diatomite. A review of diatomite and modified diatomite for the extraction of heavy metals from water. J Hazard Mater. (2012) 241:14–31. 10.1016/j.jhazmat.2012.09.030 [DOI] [PubMed] [Google Scholar]
- 249.Gestin O, Lacoue-Labarthe T, Delorme N, Garnero L, Geffard O, Lopes C, et al. Influence of the exposure concentration of dissolved cadmium on its organotropism, toxicokinetic and fate in Gammarus fossarum. Environ Int. (2023) 171:107673. 10.1016/j.envint.2022.107673 [DOI] [PubMed] [Google Scholar]
- 250.Alloy MM, Finch BE, Ward CP, Redman AD, Bejarano AC, Barron MG, et al. Recommendations for advancing test protocols examining the photo-induced toxicity of petroleum and polycyclic aromatic compounds. Aquatic Toxicol. (2023) 2023:106390. 10.1016/j.aquatox.2022.106390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Abdulqadir SZ, Aziz FM. Nickel nanoparticles induced nephrotoxicity in rats: influence of particle size. Pak Vet J. (2019) 39:548–52. 10.29261/pakvetj/2019.106 [DOI] [Google Scholar]
- 252.Haque MA, Quan H, Zuo Z, Khan A, Siddique N, He C, et al. Pathogenicity of feed-borne Bacillus cereus and its implication on food safety. Agrobiological Records. (2021) 3:1–6. 3: 1–6. 10.47278/journal.abr/2020.015 [DOI] [Google Scholar]
- 253.Coecke S, Pelkonen O, Leite SB, Bernauer U, Bessems JG, Bois FY, et al. Toxicokinetics as a key to the integrated toxicity risk assessment based primarily on non-animal approaches. Toxicol Invitro. (2013) 27:1570–7. 27: 1570–7. 10.1016/j.tiv.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 254.Pérez-Iglesias JM, Bach NC, Colombetti PL, Acuña P, Colman-Lerner JE, González SP, et al. Biomonitoring of alterations in fish that inhabit anthropic aquatic environments in a basin from semi-arid regions. Toxics. (2023) 11:73. 10.3390/toxics11010073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sruthi L, Asad M, Arshad MN, Asiri AM, Khan SS. Dual functionalized Ag plasmonic sensor for colorimetric detection of Cr (III)/Hg (II)-carboxyl complexes: mechanism and its sensing based on the interference of organic ligand. J Environ Chem Eng. (2023) 11:109147. 10.1016/j.jece.2022.109147 [DOI] [Google Scholar]
- 256.Melaram R, Newton AR, Chafin J. Microcystin contamination and toxicity: implications for agriculture and public health. Toxins. (2022) 14:350. 10.3390/toxins14050350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang M, Zhang T, Zhou L, Lou W, Zeng W, Liu T, et al. Soil microbial community assembly model in response to heavy metal pollution. Environ Res. (2022) 213:113576. 213:113576. 10.1016/j.envres.2022.113576 [DOI] [PubMed] [Google Scholar]
- 258.Ding X, Pu Y, Tang M, Zhang T. Environmental and health effects of graphene-family nanomaterials: potential release pathways, transformation, environmental fate and health risks. Nano Today. (2022) 42:101379. 42:101379. 10.1016/j.nantod.2022.101379 [DOI] [Google Scholar]
- 259.Sijm DT, Rikken MG, Rorije E, Traas TP, Mclachlan MS, Peijnenburg WJ. Transport, accumulation and transformation processes. In:Van Leeuwen CJ, Vermeire TG, editors. Risk Assessment of Chemicals. Dordrecht: Springer; (2007). p. 73–158. 10.1007/978-1-4020-6102-8_3 [DOI] [Google Scholar]
- 260.Xiang H, Min X, Tang CJ, Sillanpää M, Zhao F. Recent advances in membrane filtration for heavy metal removal from wastewater: a mini review. J Water Process Eng. (2022) 49:103023. 49:103023. 10.1016/j.jwpe.2022.103023 [DOI] [Google Scholar]
- 261.Boelsterli UA. Diclofenac-induced liver injury: a paradigm of idiosyncratic drug toxicity. Toxicol Appl Pharmacol. (2003) 192:307–22. 10.1016/S0041-008X(03)00368-5 [DOI] [PubMed] [Google Scholar]
- 262.Raj T, Chandrasekhar K, Morya R, Pandey AK, Jung JH, Kumar D, et al. Critical challenges and technological breakthroughs in food waste hydrolysis and detoxification for fuels and chemicals production. Bioresour Technol. (2022) 2022:127512. 10.1016/j.biortech.2022.127512 [DOI] [PubMed] [Google Scholar]
- 263.Krausová M, Braun D, Buerki-Thurnherr T, Gundacker C, Schernhammer E, Wisgrill L, et al. Understanding the chemical exposome during fetal development and early childhood: a review. Annu Rev Pharmacol Toxicol. (2022) 63. 63. 10.1146/annurev-pharmtox-051922-113350 [DOI] [PubMed] [Google Scholar]
- 264.Wuana RA, Okieimen FE. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Scholarly Res Notices. (2011) 2011:402647. 2011:402647. 10.5402/2011/402647 [DOI] [Google Scholar]
- 265.Yaashikaa PR, Kumar PS, Varjani S. Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: a critical review. Bioresour Technol. (2022) 343:126126. 343:126126. 10.1016/j.biortech.2021.126126 [DOI] [PubMed] [Google Scholar]
- 266.Amin A, Naik AR, Azhar M, Nayak H. Bioremediation of different waste waters-a review. Continent J Fish Aquat Sci. (2013) 7:7. Available online at: https://www.academia.edu/10700428/BIOREMEDIATION_OF_DIFFERENT_WASTE_WATERS_A_REVIEW [Google Scholar]
- 267.Riser-Roberts E. Remediation of Petroleum Contaminated Soils: Biological, Physical, and Chemical Processes. Boca Raton: CRC Press; (2020). 10.1201/9780367802547 [DOI] [Google Scholar]
- 268.Paul T, Saha NC.. Bioremediation of Heavy Metals. Biotechnology for Zero Waste: Emerging Waste Management Techniques. India: Biotechnology for Zero Waste: Emerging Waste Management Techniques; (2022). p. 67–81. 67–81. 10.1002/9783527832064.ch5 [DOI] [Google Scholar]
- 269.Khan SU, Khalid M, Hashim K, Jamadi MH, Mousazadeh M, Basheer F, et al. Efficacy of electrocoagulation treatment for the abatement of heavy metals: an overview of critical processing factors, kinetic models and cost analysis. Sustainability. (2023) 15:1708. 10.3390/su15021708 [DOI] [Google Scholar]
- 270.Shi L, Li J, Palansooriya KN, Chen Y, Hou D, Meers E, et al. Modeling phytoremediation of heavy metal contaminated soils through machine learning. J Hazard Mater. (2023) 441:129904. 441:129904. 10.1016/j.jhazmat.2022.129904 [DOI] [PubMed] [Google Scholar]
- 271.Raza H, Bibi T, Bibi H, Asim MJ, Bilal H, Rasheed A, et al. Role of phytoremediation in removing air pollutants: a review. Cross Curr Int J Agric Vet Sci. (2021) 3:54–9.34626947 [Google Scholar]
- 272.Gong J, Ouyang W, He M, Lin C. Heavy metal deposition dynamics under improved vegetation in the middle reach of the Yangtze River. Environ Int. (2023) 171:107686. 171:107686. 10.1016/j.envint.2022.107686 [DOI] [PubMed] [Google Scholar]
- 273.Mahey S, Kumar R, Sharma M, Kumar V, Bhardwaj RA. Critical review on toxicity of cobalt and its bioremediation strategies. SN Appl Sci. (2020) 2:1–2. 10.1007/s42452-020-3020-9 [DOI] [Google Scholar]
- 274.Borah G, Deka H. Crude oil associated heavy metals (HMs) contamination in agricultural land: Understanding risk factors and changes in soil biological properties. Chemosphere. (2023) 310:136890. 310:136890. 10.1016/j.chemosphere.2022.136890 [DOI] [PubMed] [Google Scholar]
- 275.Banerjee A, Roychoudhury A. Explicating the cross-talks between nanoparticles, signaling pathways and nutrient homeostasis during environmental stresses and xenobiotic toxicity for sustainable cultivation of cereals. Chemosphere. (2022) 286:131827. 10.1016/j.chemosphere.2021.131827 [DOI] [PubMed] [Google Scholar]
- 276.Basinas P, Rusín J, Chamrádová K, Kaldis SP. Pyrolysis of the anaerobic digestion solid by-product: characterization of digestate decomposition and screening of the biochar use as soil amendment and as additive in anaerobic digestion. Energy Convers Manage. (2023) 277:116658. 277:116658. 10.1016/j.enconman.2023.116658 [DOI] [Google Scholar]
- 277.Pecchi M, Baratieri M. Coupling anaerobic digestion with gasification, pyrolysis or hydrothermal carbonization: a review. Renew Sustain Energy Rev. (2019) 105:462–75. 105: 462–75. 10.1016/j.rser.2019.02.003 [DOI] [Google Scholar]
- 278.Tayibi S, Monlau F, Marias F, Thevenin N, Jimenez R, Oukarroum A, et al. Industrial symbiosis of anaerobic digestion and pyrolysis: performances and agricultural interest of coupling biochar and liquid digestate. Sci Total Environ. (2021) 793:148461. 10.1016/j.scitotenv.2021.148461 [DOI] [PubMed] [Google Scholar]
- 279.Dim EC, Akuru EA, Mgbor FN, Oyeagu CE, Falowo AB, Lewu FB, et al. Study of supplementation of various levels of biochar on health and production performance of growing local turkey (Meleagris gallopova) poults. Int J Vet Sci. (2022) 11:315–20. 10.47278/journal.ijvs/2021.116 [DOI] [Google Scholar]
- 280.Ghezzehei TA, Sarkhot DV, Berhe AA. Biochar can be used to capture essential nutrients from dairy wastewater and improve soil physico-chemical properties. Solid Earth. (2014) 5:953–62. [Google Scholar]
- 281.Kirchmann H, Börjesson G, Kätterer T, Cohen Y. From agricultural use of sewage sludge to nutrient extraction: a soil science outlook. Ambio. (2017) 46:143–54. 46: 143–54. 10.1007/s13280-016-0816-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Penido ES, Martins GC, Mendes TB, Melo LC, do Rosário Guimarães I, Guilherme LR. Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotoxicol Environ Saf. (2019) 172:326–33. 172: 326–33. 10.1016/j.ecoenv.2019.01.110 [DOI] [PubMed] [Google Scholar]
- 283.Elbehiry F, Darweesh M, Al-Anany FS, Khalifa AM, Almashad AA, El-Ramady H, et al. Using biochar and nanobiochar of water hyacinth and black tea waste in metals removal from aqueous solutions. Sustainability. (2022) 14:10118. 10.3390/su141610118 [DOI] [Google Scholar]
- 284.Malik N, Bulasara VK, Basu S. Surfactant induced ultrafiltration of heavy metal ions from aqueous solutions using a hybrid polymer-ceramic composite membrane. Chem Eng Res Des. (2022) 185:407–17. 185: 407–17. 10.1016/j.cherd.2022.07.013 [DOI] [Google Scholar]
- 285.Karthikeyan A, Joseph A, Nair BG. Promising bioactive compounds from the marine environment and their potential effects on various diseases. JGEB. (2022) 20:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kristanti RA, Mardarveran P, Almaary KS, Elshikh MS, AbdelGawwad MR, Tang DK. Phytoremediation of bauxite wastewater potentiality by Jatropa curcas. Bioprocess Biosyst Eng. (2022) 2022:1–7. 2022: 1–7. 10.1007/s00449-022-02745-5 [DOI] [PubMed] [Google Scholar]
- 287.Naz R, Khan MS, Hafeez A, Fazil M, Khan MN, Ali B, et al. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted industrial soils. Braz J Biol. (2022) 84:264473. 84:264473. 10.1590/1519-6984.264473 [DOI] [PubMed] [Google Scholar]
- 288.Pang YL, Quek YY, Lim S, Shuit SH. Review on phytoremediation potential of floating aquatic plants for heavy metals: a promising approach. Sustainability. (2023) 15:1290. 10.3390/su15021290 [DOI] [Google Scholar]
- 289.Atanes E, Cuesta-García B, Nieto-Márquez A, Fernández-Martínez F. A mixed separation-immobilization method for soluble salts removal and stabilization of heavy metals in municipal solid waste incineration fly ash. J Environ Manage. (2019) 240:359–67. 240: 359–67. 10.1016/j.jenvman.2019.03.122 [DOI] [PubMed] [Google Scholar]
- 290.Bera T, Kumar S, Devi MS, Kumar V, Behera BK, Das BK. JEB. J Environ Biol. (2022) 11:272–9. 10.1007/s11356-022-20573-8 [DOI] [PubMed] [Google Scholar]
- 291.Tavakoli S, Regenstein JM, Daneshvar E, Bhatnagar A, Luo Y, Hong H, et al. Recent advances in the application of microalgae and its derivatives for preservation, quality improvement, and shelf-life extension of seafood. Crit Rev Food Sci Nutr. (2022) 62:6055–68. 10.1080/10408398.2021.1895065 [DOI] [PubMed] [Google Scholar]
- 292.Kim SM, Eo KY, Park TM, Cho GJ. Evaluation of usefulness of infrared thermography for the detection of mastitis based on teat skin surface temperatures in dairy cows. Int J Vet Sci. (2023) 12:1–6. 10.47278/journal.ijvs/2022.151301 [DOI] [Google Scholar]
- 293.Masoud AM, El-Zahhar AA, El Naggar AM, Zahran AI, Al-Hazmi GA, Taha MH et al. Soya bean derived activated carbon as an efficient adsorbent for capture of valuable heavy metals from waste aqueous solution. Radiochimica Acta. (2023) 111:105–15. 10.1515/ract-2022-0098 [DOI] [Google Scholar]
- 294.Kumar A, Bhattacharya T. Biochar-based remediation of heavy metal polluted land. In:Panday VC, editor. Bio-Inspired Land Remediation. Cham: Springer International Publishing; (2023). p. 317–52. [Google Scholar]
- 295.Wang Y, Cai J, Tang C, Dong Z. Mitophagy in acute kidney injury and kidney repair. Cells. (2020) 9:338. 10.3390/cells9020338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bjørklund G, Rahaman MS, Shanaida M, Lysiuk R, Oliynyk P, Lenchyk L, et al. Natural dietary compounds in the treatment of arsenic toxicity. Molecules. (2022) 27:4871. [DOI] [PMC free article] [PubMed] [Google Scholar]