Key words: Coccidia, Eimeria, host–parasite interactions, life-history trade-offs, longitudinal sampling, nematodes, reproduction, sex differences, strongyles
Abstract
Within-year variation in infection is a ubiquitous feature of natural populations, but is determined by a complex interplay of environmental, parasitological and host factors. At the same time, co-infection is the norm in the wild. Longitudinal dynamics of co-infecting parasites may therefore be further complicated by covariation across multiple parasites. Here, we used fecal parasite egg and oocyst counts collected repeatedly from individually marked wild Soay sheep to investigate seasonal dynamics of six gastrointestinal parasite groups. Prevalence and abundance tended to be higher in spring and summer, and abundance was higher in lambs compared to adults. We found that within-year variation in highly prevalent strongyle nematode counts was dependent on adult reproductive status, where reproductive ewes had distinct dynamics compared to males and barren ewes. For similarly prevalent coccidia we found an overall peak in oocyst counts in spring but no differences among males, barren and pregnant ewes. Using multivariate mixed-effects models, we further show that apparent positive correlation between strongyle and coccidia counts was driven by short-term within-individual changes in both counts rather than long-term among-individual covariation. Overall, these results demonstrate that seasonality varies across demographic and parasite groups and highlight the value of investigating co-infection dynamics over time.
Introduction
Infection dynamics in wild animal populations are shaped by variation in the environment, which influences exposure and transmission, and variation in host physiology, which influences susceptibility and resistance (Stromberg, 1997; Acevedo-Whitehouse and Duffus, 2009; Cardon et al., 2011; Hawley and Altizer, 2011). Environmental influences incorporate short-term dynamic variation (e.g. seasonal and annual variation) and longer-term repeatable differences among hosts (e.g. influenced by host genotype or early-life environment; Smith et al., 1999; Altizer et al., 2006; Hayward et al., 2010). Wild populations are typically infected with diverse parasites, each potentially showing varying sensitivity to environmental pressures due to differences in their life cycles and vulnerability to different types of host immune response (Petney and Andrews, 1998; Cox, 2001; Pedersen and Fenton, 2007). Parasite burdens also vary with host age and sex (i.e. demographic group), and the exposure and susceptibility of these different host groups may respond in different ways to variation in the environment (Zuk and McKean, 1996; Hayward et al., 2009). Non-invasive, longitudinal studies of well-understood wild populations which monitor important parasite taxa repeatedly in individuals from different demographic groups (Clutton-Brock and Sheldon, 2010) can complement other important lines of evidence to better understand the complex parasite dynamics of natural systems.
Within-year variation in parasite dynamics of wild vertebrates depends on parasite and host population characteristics (Nelson and Demas, 1996; Altizer et al., 2006; Martin et al., 2008; Albery et al., 2018). Seasonal fluctuations in weather and resource availability can act directly on parasites and their vectors, for instance by influencing survival of vectors or environmental stages of parasites, limiting or enhancing exposure (Altizer et al., 2006). In seasonal breeders, the arrival of large numbers of highly susceptible, immunologically naïve juveniles into the population at a certain point in the year may also increase transmission and exposure across the population (Wilson et al., 2004). Environmental variation in a population can also impact the physiological state of hosts to impact their exposure, susceptibility and resistance to parasites. For example, reduced food availability in winter reduces the nutritional resources available to hosts, which is expected to reduce investment in immunity (Nelson and Demas, 1996; Zuk and Stoehr, 2002; Martin et al., 2008). Furthermore, host immunity can fluctuate within the year due to temporal variation in nutritional demands and constraints associated with growth and reproduction (Nelson and Demas, 1996; Martin et al., 2008). Reproductive costs can be sex-specific, particularly in polygynous species including several mammals (Zuk and McKean, 1996; Moore and Wilson, 2002). In such systems, males tend to have higher parasite burdens compared to females, and this is ascribed to the high costs of intrasexual competition for mates limiting investment in immunity in males (Zuk and McKean, 1996; Muehlenbein and Bribiescas, 2005; Ezenwa et al., 2012). However, there is also evidence that seasonal female investment in reproduction has costs which impact immunity and parasite burden (Zuk and McKean, 1996; Metcalf and Graham, 2018). Female mammals often show a peak in infections around birth (the so-called peri-parturient relaxation of immunity, PPRI) that is ascribed to reduced investment in immunity during late gestation and early lactation (Brunsdon, 1970; Houdijk et al., 2001; Ayalew and Gibbs, 2005; Houdijk, 2008). Thus, it is well-established that age and sex can impact parasite dynamics across wild and managed vertebrate systems in a season-dependent manner. However, whether longitudinal dynamics of multiple parasites present in the same host population is shaped by similar factors, and whether these parasites interact, is less clear.
When multiple parasites are endemic in a population, as is the norm in unmanaged systems, fluctuations across seasons and demographic groups may vary among co-infecting parasite species. The way the environment affects parasites with different transmission or life cycles may underlie this variation (Cable et al., 2017; Albery et al., 2018). Even among environmentally transmitted parasites inhabiting the same site within the host, differences in burdes among demographic groups and seasons can be observed. For example, gastrointestinal (GI) strongyle parasite burden in domestic and wild sheep are observed to peak around the breeding season in adult females (via PPRI), but a peak is observed later in summer and autumn in lambs (Vlassoff et al., 2001; Wilson et al., 2004; Hamer et al., 2019). In contrast, parasitic GI apicomplexans of the genus Eimeria (coccidian parasites) are found disproportionately in immature lambs and burdens across the population peak later in spring and summer aligned with increased transmission via the pasture after infections establish in young individuals (Chartier and Paraud, 2012). Furthermore, co-infecting parasites sharing host sites and resources can interact to impact infection dynamics (Lello et al., 2004; Fenton, 2008; Graham, 2008; Ezenwa, 2016). For instance, in wild wood mice removal of a key helminth species (Heligmosomoides polygyrus) resulted in a 15-fold increase in coccidia burdens via a hypothesized competitive release (Knowles et al., 2013; Rynkiewicz et al., 2015). Although interspecific parasite interactions are difficult to detect from observational data and correlation methods, longitudinal studies are among the more robust methods for reliably detecting interactions among parasites (Fenton et al., 2014). Monitoring environmental and demographic variations alongside longitudinal infection data for multiple parasites within host populations is important for determining whether factors shaping infection dynamics act similarly across parasites, and for determining processes underlying relationships within the parasite community.
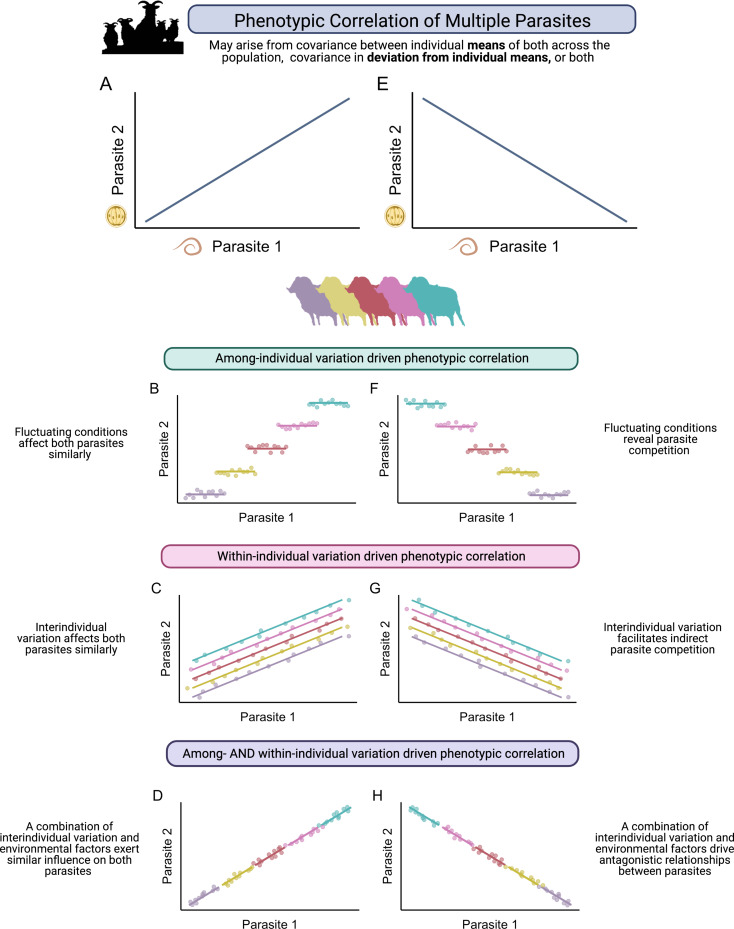
There are several biological processes occurring at different scales which might cause the prevalence or abundance of two (or more) parasites to significantly correlate on average, and which may be distinguishable by an appropriate model set-up. For instance, prevalence of two parasites might be positively correlated across hosts with overlapping space use because certain sites within the region are well suited to the transmission of both parasites while others are not. Here, the correlation would be driven by among-site, rather than within-site, covariation (Stutz et al., 2017). Within one host population, correlations among parasite groups could be driven by among- or within-individual host covariation, or by both (Fig. 1). A positive correlation between two parasite taxa at the population level (Fig. 1A) could result from only among-individual variation which influences exposure or susceptibility to both parasites in the same manner (Fig. 1B). For example, where genetically based differences in immunity confer resistance to multiple parasites, we would expect among-individual covariance to drive observed correlations across parasite abundances. Alternatively, solely within-individual covariation could be responsible (Fig. 1C). Here, short-term variation that influences deviations from a host's average abundance in the same direction for both parasites drives the overall correlation, and the host's average abundance over time for one parasite is not correlated with their average in the other parasite (Fig. 1C). For example, fine-scale spatial or temporal variation in resource availability might mean that some hosts had more resources to invest in immunity to all parasites at specific sampling points compared to others. The same conceptual framework would apply to an observed negative correlation between two parasites (Fig. 1E). For example, if genetically based variation in immunity was associated with resistance to only one type of parasite, allowing another parasite to consistently outcompete it, this would drive negative covariance at the among-individual level (Fig. 1F). Alternatively, some habitat types might be well suited for transmission of one parasite but limit transmission in another; if hosts regularly moved between such habitats this could generate negative covariation at the within-individual level (Fig. 1G). Due to the high degree of environmental and individual variation present in the wild, we might expect to observe contributions from both within- and between-individual levels to patterns of covariance in co-infection (Fig. 1D and H). Recent applications of multivariate hierarchical mixed-effects models to longitudinal data from wild animal systems demonstrate how this approach can separate these levels of covariance (Debeffe et al., 2016; Froy et al., 2019), but this approach has very rarely been applied to understand the patterns of co-infections (although see Stutz et al., 2017).
Fig. 1.
Decomposing relationships among co-infecting parasites in wild populations. Colours represent individual animals. (A and E) Positive and negative phenotypic correlations may be driven by multiple non-exclusive processes. (B and F) Correlation of two parasites driven entirely by within-individual processes result from covariance in deviations from means in the same (B) or opposite (F) direction for both parasites, likely induced by fluctuating environmental conditions. (C and G) Correlation of two parasites driven entirely by among-individual processes result from covariance in individual means of both, likely driven by interindividual variation in factors such as genotype or immunity which impact host susceptibility to both (C) or one (G) parasite, facilitating indirect interaction between parasites in competition. It is also likely in wild systems that multiple factors shape relationships among parasites, which can result in positive (D) or negative (H) correlations shaped by within- and between-host processes.
The Soay sheep living on the St Kilda archipelago represent a powerful system to disentangle the roles of long-term individual differences and short-term effects of environment, age and reproductive status on parasite dynamics in the wild. Environmentally transmitted GI nematodes and coccidian microparasites are the primary parasites infecting the Soay sheep (Wilson et al., 2004; Graham et al., 2016). Higher abundance of both parasite groups are expected in the spring and summer due to fecal–oral transmission cycles and the abundance of immunologically naïve lambs that harbour disproportionately high infection intensities which increase exposure in the environment (Wilson et al., 2004; Craig et al., 2007). In addition, for strongyle nematodes, there is evidence that males tend to have greater parasite abundance compared to females, and reproductively active females exhibit a strong peak in the spring due to PPRI (Wilson et al., 2004). To date, however, longitudinal parasitological studies in this system have been limited to spring and summer and have focused on the strongyle nematode group only. Here, we investigated longitudinal dynamics of six GI parasite groups by repeatedly fecal sampling known individuals belonging to different age and sex groups across five sampling points within a single year. We test the predictions that: (1) parasite abundances will be highest in warmest months and (2) in the youngest individuals; (3) males will have higher abundance compared to females across the year and (4) parasite abundance will be increased in spring among breeding females due to PPRI. We specifically test whether age and sex groups drive variation across sampling points, as well as estimate the individual repeatability of strongyle nematode and coccidian abundance and test whether correlations between these two highly prevalent parasite groups are driven by among- or within-individual level processes.
Materials and methods
Data collection and study system
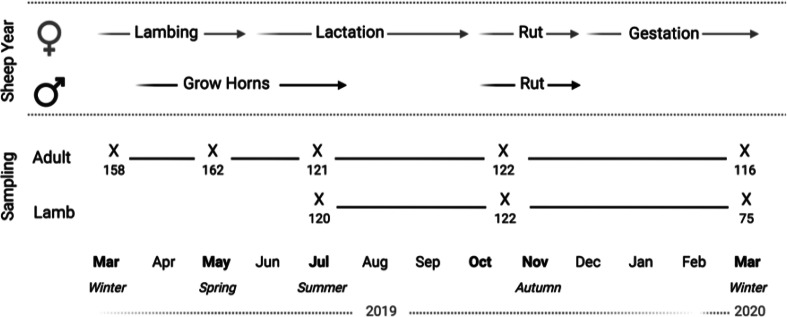
This study was conducted in an unmanaged population of Soay sheep on the St Kilda archipelago (57°49′ N, 08°34′ W, 65 km northwest of the Outer Hebrides, Scotland). A population of 107 Soay sheep was moved from the island of Soay to the largest island of the archipelago, Hirta, in 1934. The resultant unmanaged Soay sheep population living in Village Bay on Hirta has been part of a long-term individual-based study since 1985 (Clutton-Brock and Pemberton, 2004a). The annual reproductive cycle of the population is illustrated in Fig. 2. In April, most ewes give birth to one or two lambs (approximately 20% of lambs are twins), and neonates are captured within a few days of birth and marked with unique ear tags allowing them to be identified and monitored throughout life. Sheep gain condition and females lactate to their lambs over the months that follow, with most lambs weaned by approximately 4 months old. Males live in bachelor groups for much of the year and gain body condition through spring and summer in preparation for the autumn rut, during which males compete for mating opportunities with oestrous females. In the winter months that follow, the sheep are food limited and experience challenging climate conditions, and most natural mortality occurs late in the winter period (February and March).
Fig. 2.
Fecal sampling programme (‘sampling’) is illustrated alongside the major events of the annual cycle of Soay sheep on St Kilda (‘sheep year’). Each ‘X’ designates each sampling point for adults or lambs with the number of samples collected indicated. Wherever possible, the same individuals as initially selected were repeatedly sampled across the study period.
Fecal samples for parasitological analyses were collected seasonally in five sampling trips to capture major seasonal variation: winter 2019 (6 March–16 March 2019), spring 2019 (13 May–24 May 2019), summer 2019 (17 July–29 July 2019), autumn 2019 (27 October–8 November 2019) and winter 2020 (4 March–18 March 2020) (Fig. 2). We targeted individuals from two demographic groups for repeated sampling over this period: adults (aged 2 years or more, sampled from winter 2019 onwards) and lambs (sampled from summer 2019 onwards). Individuals were targeted for sampling based on having a home range centred within our core study area, with an effort made to distribute individuals as evenly as possible across age and sex groups, given the adult population is heavily female-biased (Wilson et al., 2004). Details of the total animals sampled are given in Table S1. Lambs were not sampled in spring 2019 as fecal samples were not consistently of sufficient size for parasitological analysis. Target individuals were observed closely until they defecated. The fecal sample produced was collected from the pasture within 2 min of defecation. For each sample, individual identity, collection date and time of day were recorded. Samples were weighed at the end of each collection day. A total of 2 g of fecal matter for adults and 1 g for lambs were stored in plastic bags, which were flattened and rolled tightly to provide anaerobic conditions, and refrigerated at 4°C until processing to minimize egg hatching. Parasite egg/oocyst counts were carried out upon return to the laboratory within 3 weeks of collection.
Parasitology
GI parasites were quantified using a modified salt-flotation method to enumerate eggs (helminths) or oocysts (protozoans) in the fecal samples (Hayward et al., 2019). Briefly, 2 g (adults) or 1 g (lambs) of fecal matter was mixed with 10 mL of water per gram and mechanically homogenized. A 10 mL aliquot of each sample suspension was filtered through a 1 mm sieve and washed with 5 mL of tap water. Filtrates were centrifuged in 15 mL polyallomer tubes for 2 min at 200 g. The supernatant was removed and the fecal pellet was re-suspended with 10 mL of saturated NaCl solution and centrifuged for another round of 2 min at 200 g. Tubes were clamped below the meniscus using medical forceps, leaving GI parasite eggs or oocysts which float on the salt solution in the fluid above the clamp. The fluid above the clamp was added to a cuvette in addition to approximately 1 mL of NaCl used to wash the upper chamber. The cuvette was added with NaCl solution and the entire cuvette surface was scanned to count parasite eggs and oocysts to a precision of 1 egg/oocyst per gram. Fecal egg counts (FECs) are a common proxy for worm burden (described as abundance) which typically show a positive correlation with worm number in Soay sheep as well as other wild animals and domestic sheep (McKenna, 1981; Cabaret et al., 1998; Wilson et al., 2004; Cringoli et al., 2008). High technical repeatability with the cuvette method described above has previously been previously established in Soay sheep (Albery et al., 2018; Hayward et al., 2019).
Statistical analysis
All statistical analyses were conducted using R version 3.6.1 (R Core Team, 2019). We fitted all models using the Bayesian modelling package ‘MCMCglmm’ (Hadfield, 2010). All models were run for 260 000 iterations with a 2000-iteration thinning interval and a 60 000-iteration burn-in period. We used generalized linear mixed-effects models (GLMMs) to test the effects of season and sex on egg/oocyst counts for each parasite group separately, and ran separate models for lambs and adults due to differences in the seasons sampled in each age group (Fig. 2; Table 1). In few instances, rarer parasites were not present at all time points so models were fit only to those time points containing meaningful infection data (Fig. 3; Table 1). Strongyle, coccidian, Capillaria and Nematodirus models were fitted with FECs/fecal oocyst counts (FOCs) as a response variable and Poisson error families. Strongyloides and Moniezia were scored as presence/absence and therefore modelled with a binomial error family (Table 1). Individual identity was included as a random effect in all models. In addition to model effect estimates which compared each level of season to the intercept category (Winter19), we assessed statistical support of pairwise differences between subsequent time points (e.g. spring vs summer) for each parasite, equivalent to a post-hoc difference of means, where significant values were determined using the proportion overlap of the posterior distributions of the Markov Chain Monte Carlo (MCMC) estimates for each level divided by half the number of stored iterations (Albery et al., 2018; Palmer et al., 2018).
Table 1.
Details of GLMMs fit to parasite data in this study
| Data | Response | Model family (link) | Season | NID | Nobs | Fixed effects | Random effects |
| Season and sex effects, univariate models | |||||||
| Adults | Strongyles (FEC) | Poisson (log) | All | 133 | 679 | Season + sex | ID |
| Coccidia (FOC) | Poisson (log) | All | |||||
| Capillaria (FEC) | Poisson (log) | Winter19–Spring19 | 129 | 320 | |||
| Strongyloides (P/A) | Binomial (logit) | All | 133 | 679 | |||
| Moniezia (P/A) | Binomial (logit) | Spring19–Autumn19 | 126 | 405 | |||
| Lambs | Strongyles (FEC) | Poisson (log) | Summer19–Winter20 | 124 | 317 | Season + sex | ID |
| Coccidia (FOC) | Poisson (log) | ||||||
| Nematodirus (FEC) | Poisson (log) | ||||||
| Strongyloides (P/A) | Binomial (logit) | Summer19–Autumn19 | 124 | 242 | |||
| Moniezia (P/A) | Binomial (logit) | Summer19–Autumn20 | 124 | 317 | |||
| Interactive effects of season and host factors, univariate and multivariate models | |||||||
| Adults | Strongyles (FEC) | Poisson (log) | All | 133 | 679 | Season + SexRepro + AgeScaled + SexRepro:Season | ID |
| Coccidia (FOC) | |||||||
FEC, fecal egg counts; FOC, fecal oocyst counts; P, presence; A, absence.
See text for further details.
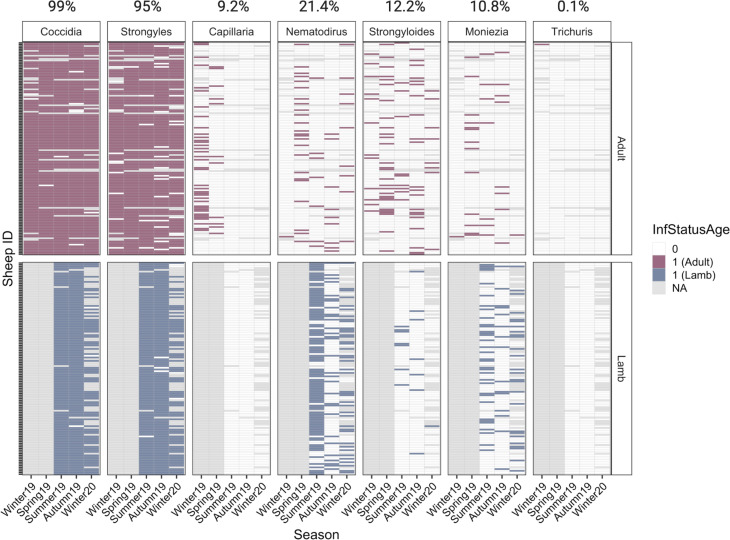
Fig. 3.
Infection status of individual sheep across parasite groups and seasons. Shading represents the presence of a parasite for each sample (filled: present; blank: absent; grey: not sampled). The top panel represents adult samples and the bottom panel represents lamb samples. Overall prevalence within the population is shown for each parasite at the top of the plot.
We then investigated whether and how observed seasonal dynamics in parasite counts were driven by particular demographic groups in the population. We fit interactive effects with sex and reproductive status in adult sheep. We used all seasons of strongyle egg and coccidian oocyst counts only, as these were the two most prevalent parasites in the population and other parasites had very low or no prevalence in some seasons (Fig. 3). To test for sex differences overall as well as differences among females that did or did not breed, we coded a ‘sex/reproductive status’ variable with the following categories: ‘male’, ‘female no lamb’ (did not breed in spring 2019) and ‘female with lamb’ (gave birth to at least one lamb in spring 2019). GLMMs with Poisson error families were fitted to both strongyle FECs and coccidian FOCs with the following fixed effects: sex/reproductive status (‘SexRepro’), Season (five level factor, as above), Age in years (continuous, scaled to a mean of zero and a standard deviation of 1) and a SexRepro-by-Season interaction (Table 1). Individual identity was included as a random effect in these models. We used posterior prediction to evaluate model performance by comparing predicted values to raw data, and to investigate whether and how SexRepro categories varied in their seasonal trajectories. In total, 1000 predicted values were generated for each SexRepro/Season category to calculate a distribution (Seasont+1 − Seasont) representing the relative change from season-to-season. These distributions were compared across each combination of SexRepro categories for each seasonal change to assess significant differences in seasonality for demographic groups, where significant values were calculated using proportional overlap of each pairwise set of distributions divided by half the number of predicted values (equivalent to a post-hoc test).
Next, we investigated repeatability of strongyle and coccidian counts for adults across sampling seasons. Finally, we estimated the among- and within-host covariance in strongyle and coccidian counts. We specified a multivariate Poisson model with strongyle and coccidian counts as response variables, and fixed and random effects identical to those in univariate models above (Table 1). Individual repeatability across seasons for both responses was derived using the individual variance estimate from each model divided by the total variance (VarID/(VarID + VarResidual + VarPoisson)) (Nakagawa and Schielzeth, 2010). This model also estimates the covariance at the among- and within-individual (residual) levels between the strongyle FEC and coccidian FOC. The covariance at the among-individual level (CovIndividiual) indicates the association between individuals' average strongyle FEC and coccidian FOC across the entire study period. The within-individual or residual covariance (CovResidual) reflects associations at the level of sample point having accounted for any association at the individual mean level. Overall (phenotypic) covariance between the two responses is then represented by Covphenotypic = CovIndividual + CovResidual.
Results
Strongyles (95% prevalence) and coccidian (99%) were by far the most prevalent parasites in the population, followed by Nematodirus (21.4%), Strongyloides (12.2%), Moniezia (10.8%) and Capillaria (9.2%; Fig. 3). Trichuris had a prevalence of <1% in the population (Fig. 3) and was not included in our analyses. Capillaria was found only in adults and therefore does not appear in lamb analysis, and Nematodirus were found almost exclusively in lambs and therefore does not appear in adult analysis. For parasites with associated count data (vs presence/absence), mean and range of counts for each age and season group are summarized in Table S2. There was a seasonal variation in the prevalence of all parasites (Fig. 3). Coccidia and strongyles were present at high prevalence in both adults and lambs for all seasons sampled. In contrast, other parasites were much less consistent in detection. Capillaria was found only in adults in the first two seasons only. Nematodirus was found primarily in lambs especially in the summer, but infrequently in adults across all seasons as well. Strongyloides was present primarily in adults and detected most frequently in spring and summer, whereas Moniezia was detected in low overall prevalence but more frequently in lambs. Finally, Trichuris was detected in only one adult and was dropped from further analysis.
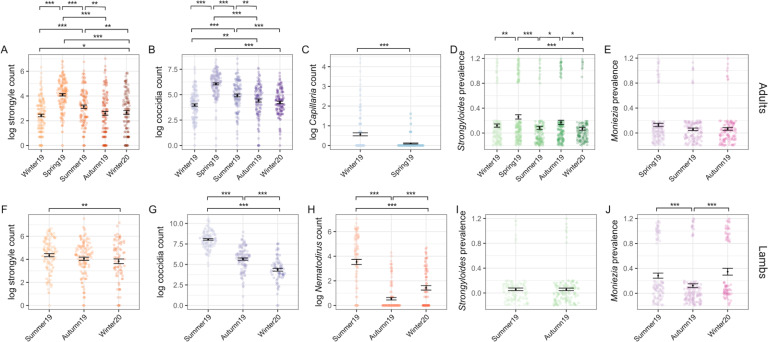
Our models of parasite counts likewise revealed seasonal variation for most parasite species groups (Fig. 4). In adults, parasite abundance was generally significantly higher in spring compared to in other seasons and, while it tended to be lower in summer compared to spring, summer counts tended to be higher compared to autumn or winter season (Fig. 4A–E; Tables S1 and S2). For strongyle and coccidian counts, there was a clear seasonal pattern where spring FECs/FOCs were greater than those of summer, and both spring and summer FECs/FOCs were greater than those of autumn and winter, which were more similar in magnitude (Fig. 4A and B; Table S2). Capillaria was detected only in winter and spring 2019 and had highest abundance in the winter (Fig. 4C). Probability of detection was highest in the spring for Strongyloides, although seasonal patterns for this parasite were less consistent compared to strongyles and coccidia (Fig. 4D). There was no difference in the probability of infection with Moniezia across seasons (Fig. 4E). In addition to seasonal effects, we found that males had higher strongyle egg counts compared to females [post mean = 0.97, 95% confidence interval (CI): 0.5–1.45, PMCMC < 0.001] and males had greater probability of infection with Strongyloides compared to females (post mean = 1.28, 95% CI: 0.21–2.38, PMCMC = 0.03). There were no sex differences in the other parasite groups for adults (Table S3). Although parasites were modelled within age groups, parasite abundance was higher in lambs compared to adults for all parasites except Capillaria (Table S2).
Fig. 4.
Seasonal dynamics of six GI taxa of Soay sheep spanning five sample trips. The top panel (A–E) represents data from adults (all sampling points). The bottom panel (F–J) represents data from lambs (Summer 2019 onwards). Coloured points represent raw data, where the width of the point spread is proportional to the density distribution. Black points and error bars represent the mean (for counts) or prevalence (for presence/absence) for each parasite ± s.e. Brackets above indicate pairwise comparisons across all season combinations, where significance was calculated as the proportional overlap between posterior distributions for pairs of Season levels divided by half the number of stored iterations. Significance is denoted by ***, ** and * for P < 0.001, P < 0.01 and P < 0.05, respectively. Some models were run in adults (Capillaria) or lambs (Nematodirus) or only run on subsets of data due to zero-extremely low prevalence within some time points. Plots and effect comparisons are limited to those categories represented in the models.
In lambs – for which we only had samples in summer and autumn 2019 and winter 2020 – we found that strongyle and Nematodirus FECs and coccidian FOCs were highest in summer (Fig. 4F–H; Table S3). The probability of detecting Moniezia eggs was lower in autumn compared to either in summer or winter (Fig. 4J), while there was no difference in the probability of detection of Strongyloides eggs between summer and autumn and this parasite was detected in only one lamb in winter 2020 (Fig. 4I; Table S3). We found no evidence for differences between male and female lambs in their average counts across seasons in any of these parasite groups (Table S3).
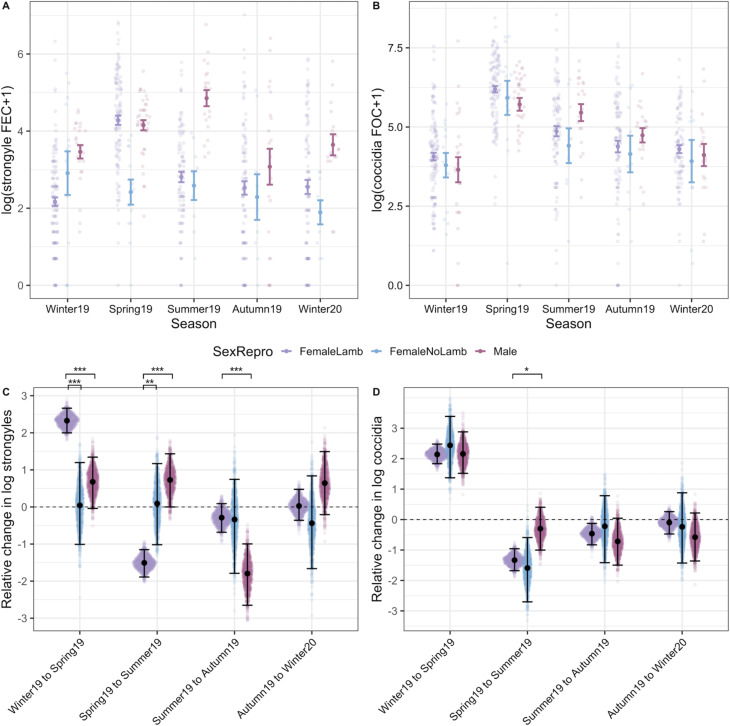
Models investigating interactive effects of sex/female reproductive status and season demonstrated much stronger interactions for strongyle FECs than in coccidian FOCs (Fig. 5; Tables S3 and S4). Strongyle counts increased significantly more from winter to spring in females that produced lambs than in males (Fig. 5A and C). Females that did not produce a lamb in contrast had a significantly lower magnitude of change from winter to spring and these barren ewes showed little seasonal variation in FEC overall (Fig. 5A and C). From spring to summer, strongyle FECs dropped in females with lambs to the levels similar to those observed in females without lambs (Fig. 5A and C), but male FECs remained high and actually increased slightly on average from spring to summer (Fig. 5A and C). Coccidian counts showed little evidence of a season-by-sex/reproductive status interaction in contrast: counts increased from winter to spring in a consistent manner across sex/reproductive status groups, and then fell from spring to summer, although this drop was less pronounced in males than in females (Fig. 5B and D). In both models, continuous age of adults was fit to account for potential ageing effects within the adult age class, but was insignificant for both strongyles and coccidia (Table S4).
Fig. 5.
Interactions with season and host sex and reproductive status in adult sheep. The top panel (A and B) represents raw data (coloured points) overlaid with mean and s.e. grouped according to SexRepro-by-Season groups for both (A) strongyle egg and (B) coccidian oocyst counts. Maroon indicates females that gave birth to at least one lamb in the spring, pink indicates females that did not give birth and blue indicates males. The bottom panel (C and D) represents the pairwise comparisons of seasonal changes calculated from 1000 predicted values for each SexRepro-by-Season group from GLMMs with (C) strongyle and (D) coccidian counts as a response. Sina plots of points represent each value from the predicted distribution (Seasont+1 − Seasont) for each group, overlaid with mean and 95% CIs. Significant differences between the SexRepro groups are indicated by brackets above plots, calculated from the proportional overlap of each pairwise set of distributions. Comparisons can be interpreted similarly to the following examples: panel C indicates that females with lambs show a much greater increase in FECs from winter to spring compared to both females without lambs and males, and panel D shows that females with lambs show a greater decrease from spring to summer compared to males. Significant differences between effects are indicated by ***, ** and * for P < 0.001, P < 0.01 and P < 0.05, respectively.
Bivariate GLMMs yielded nearly identical fixed effects estimates to the univariate models described above (Table S4). Repeatabilities of strongyle FECs and coccidian FOCs across season, after accounting for effects of sex and season, were 0.28 (95% CI: 0.19–0.36) and 0.20 (0.12–0.27), respectively. The overall phenotypic correlation between strongyle and coccidian counts was estimated as 0.27 (0.20–0.37, PMCMC < 0.001). Underlying this, we estimated a higher positive within-individual correlation (post mean = 0.46, 95% CI: 0.31–0.62) and a much weaker among-individual correlation which had credible intervals that overlapped zero (post mean = 0.15, 95% CI: −0.03 to 0.31).
Discussion
We found that most parasite groups varied in prevalence and abundance across the sampled seasons and, as predicted, these tended to be highest in the spring and summer. Strongyle nematodes and coccidian microparasites were highly prevalent across the entire study period in both lambs and adults, whereas other parasite groups were detected infrequently or only in certain age groups or seasons. These results largely agree with previous research in this system (Wilson et al., 2004; Craig et al., 2006, 2007), as well as in domestic sheep (Hamer et al., 2019; Evans et al., 2021). Generally, GI parasite communities differed between lambs and adults (Fig. 3) and strongyle FECs and coccidian FOCs were generally higher in lambs than in adults across seasons (Fig. 5; Table S2). In contrast to predictions, we only detected male bias across seasons in two out of the six parasites studied (strongyles and Strongyloides). Our in-depth analyses of the highly prevalent strongyle and coccidian groups in adults revealed striking interactions between season and reproductive group in the former but not in the latter, as well as a surprising increase in strongyle FECs for males during the summer. We also demonstrated that counts of both groups are moderately repeatable across our year-long study period, and that counts are positively correlated with each other overall. Bivariate modelling of these two parasite groups further revealed that this correlation was driven by short-term fluctuations in the same direction across seasons, rather than consistent differences in FECs and FOCs between individual sheep on average across the study period.
The seasonal epidemiology of the GI nematode parasites investigated here has been well-characterized in a study on domestic sheep (Vlassoff et al., 2001). These dynamics seem remarkably robust to management practices (Hamer et al., 2019), as well as being consistent with previous findings in the unmanaged population of Soay sheep on St Kilda (Wilson et al., 2004). Our results largely confirm these patterns, using a much larger dataset with finer-scale temporal resolution compared to previous studies on the Soay sheep. The widely observed increase in nematode FECs observed through spring and summer is associated with a surge in egg shedding onto pasture from immunologically naïve neonate lambs and ewes experiencing PPRI (Vlassoff et al., 2001). On St Kilda, strongyle eggs generally first appear in the feces of lambs around 6 weeks old and small-scale longitudinal studies of lambs suggest that while lamb FECs rise to a peak around August, they subsequently remain high and may continue to rise again through late winter (February–April; Wilson et al., 2004), whereas in domestic animals, egg shedding declines in the winter (Vlassoff et al., 2001). Although our sampling regime prohibited us from detecting the well-established increase in lamb FECs from spring to summer, our data confirm that lamb FECs remain high and do not decrease as markedly through autumn and winter as in adults (Fig. 5A and F). The reasons for this difference between the early-life dynamics in unmanaged Soay sheep and domestic sheep remain to be determined, and certainly warrant further investigation. One possibility is that on St Kilda lambs FECs remain high due to the food limitation experienced by Soay lambs during winter (Wilson et al., 2004; Craig et al., 2006), which delays the onset of immunity to the parasites relative to domestic lambs of higher nutritional status.
We found pronounced increases in strongyle FECs from the end of winter (March) to spring (May) among females that produced lambs, but not in barren ewes (Fig. 5A). This supports previous observations of reproduction-induced increases in FECs in Soay sheep (Hayward et al., 2019; Leivesley et al., 2019) as well as numerous other wild animals such as bighorn sheep (Festa-Bianchet, 1989), red deer (Albery et al., 2020), flying foxes (Plowright et al., 2008) and birds (Nordling et al., 1998; Knowles et al., 2009) and is a well-defined phenomenon in domesticated sheep flocks (Vlassoff et al., 2001; Hamer et al., 2019). The increased counts in reproductive ewes can be attributable to both relaxed immunity (PPRI) due to the demands of lactation and gestation (Festa-Bianchet, 1989; Sheldon and Verhulst, 1996) or due to higher exposure from increased foraging behaviour to compensate for resource demands (Hutchings et al., 2002). Previous evidence documenting decreased strongyle-specific immunoglobulin G antibody levels during the lambing season in Soay ewes suggests that the former mechanism is at least partially responsible for an increase in the FECs in reproductive ewes in this system (Hayward et al., 2019). The data from our study provide some further support for an immune-mediated explanation, as the FECs of reproductive ewes fall rapidly to the levels observed in barren ewes by summer (July; Fig. 5A). Breeding ewes are expected to experience high-nutritional demands through both spring and summer due to lactation, and therefore if increased exposure through altered foraging behaviour was responsible for increased egg shedding then we would expect reproductive females to have higher FECs compared to barren females through until the end of lactation in summer.
Our findings highlight the potential, and often overlooked, importance of males in the transmission dynamics of helminths in wildlife (Ferrari et al., 2004; Grear et al., 2009), where finer-scale sampling demonstrated that male FECs show a seasonal pattern distinct from that observed in lambs, pregnant or barren ewes. Adult males show a sustained increase in strongyle FECs through spring and summer, unlike reproductive ewes which show a peak in spring and a reduction in summer (Fig. 5A). Contrary to predictions for a persistent male bias in parasite abundance across ages and seasons, we found increased strongyle FECs in adult males relative to reproductive females only in summer. Although further investigation is required to understand the causes of this sex difference, it is likely that some combination of differences in resource allocation trade-offs and exposure to larvae on pasture in adult males compared with other demographic groups is responsible. Adult males invest their limited nutritional resources in horn growth and gaining weight and condition through spring and summer ahead of the autumn rut. The trade-off between testosterone production, investment in secondary sexual characteristics and immunity is hypothesized to predispose males to higher susceptibility to infection via an immunocompetence handicap (Zuk and McKean, 1996; Nunn et al., 2009; Ezenwa et al., 2012), and we might expect this to manifest as increased male parasite burdens during the peak in exposure to strongyle larvae on pasture in July and August. In the St Kilda population, adult males often spend time in ‘bachelor groups’ outside of the autumn rut (Clutton-Brock and Pemberton, 2004b). This social grouping of adult males through spring and summer may reinforce immunologically mediate sex differences, as these heavily infected males may be more likely to expose each other to larvae on shared grazing ranging. We note that high strongyle FECs in adult males during the rut have been associated with reduced time spent engaged in sexual activity, suggesting that the summer peak in male FECs may have fitness costs and impact sexual selection in this system (Wilson et al., 2004). Further research addressing the seasonal dynamics of mucosal immunity in adult males (as has been done for females recently on St Kilda, see Hayward et al., 2019), the relationship between home range sharing and parasite abundance and the effects of autumn infection on rut performance and reproductive success in males will help understand the causes and consequences of the male summer peak in strongyle FECs.
Coccidian parasites showed a seasonal pattern broadly consistent with expectations: a marked peak in spring in adults followed by a decline from summer onwards, and substantially higher FOCs in lambs compared to adults over summer and winter. However, in contrast to the patterns discussed above for strongyles, coccidian FOCs showed largely consistent seasonal dynamics across reproductive ewes, barren ewes and males (Fig. 5B). It seems that reproductive status and investment impact seasonal variation in abundance in quite different ways across these two groups of GI parasites. The main driver of seasonal variation in coccidia in domestic sheep is thought to be pasture oocyst contamination by immunologically naïve lambs through spring (Chartier and Paraud, 2012). Although we did not have lamb samples from May to confirm this in the current study, previous research on the Soay sheep supports the central role of lambs in transmission and shows that coccidian FOCs are, on average, an order of magnitude greater in lambs compared to adults in summer (Craig et al., 2007). Sheep develop immunity to coccidia over their first year of life, a response largely thought to be mediated by T helper type 1 (Th1) responses, and are thought to be largely resistant to pathology caused by these parasites after this point (Ovington et al., 1995; Jorg Reeg et al., 2005; Craig et al., 2007; Ozmen et al., 2012). One possible explanation for the lack of a PPRI effect or sex differences in coccidian FOCs, in contrast to strongyle FECs, may lie in differences in the way the immune response involved in controlling these parasite groups develops and trades off with investment in reproduction. Previous studies of mammals have observed that different parasite groups may be differentially impacted by reproduction (Rödel et al., 2016; Becker et al., 2018; Albery et al., 2020). Furthermore, resistance to helminths is dependent on T helper type 2 (Th2) responses, which can be constrained by immune commitment to the Th1 responses required for resistance to microparasites such as coccidia (Graham, 2008). Recent evidence in wild Soay sheep has shown that Th1-associated immune phenotypes predicted reduced coccidian FOCs, while Th2-associated phenotypes predicted reduced strongyle FECs (Corripio-Miyar et al., 2022). Although this supports the role of functionally distinct aspects of the immune response in controlling these two different parasite groups, we also found that sheep mounting strong Th1 responses also tended to mount strong Th2 responses (Corripio-Miyar et al. 2022 ). It is possible that the immune responses that develop in sheep to coccidia during the first year of life are more effective than those against strongyle and that varied resource costs of anti-coccidian vs anti-strongyle responses may account for the varied effect of reproductive status on severity of infection. However, further research testing the relationship between reproductive investment, T helper immunity and parasite abundnace would be required to test this hypothesis.
Strongyle FECs and coccidian FOCs showed a positive phenotypic correlation in this study. These results confirm previous reports of positive phenotypic correlations between these two parasite groups in both Soay and domestic sheep (Craig et al., 2008; Pacheco et al., 2021), but contrast with the previous experimental studies on wild rodents that have identified negative interactions between GI coccidia and strongyles, which appear linked to competition over shared host resources and competitive release (Knowles et al., 2013; Rynkiewicz et al., 2015). One plausible explanation, which also applies to observed positive correlations between Th1 and Th2 immune phenotypes in this population (Corripio-Miyar et al., 2021), is variation in resource acquisition and joint exposure to multiple parasites which is likely very common in natural systems. Sheep acquiring fewer resources, due to competitive ability or home range quality, may be less able to mount immune response to multiple parasites and may need to forage more widely and less selectively and risk greater exposure to multiple parasites. However, in addition to these environmental factors, the recent observation that FECs and FOCs are positively correlated at the genetic level in domestic Blackface lambs suggests that the correlation between strongyle and coccidian parasite abundances in this study could also have some genetic basis (Pacheco et al., 2021). In addition, strongyle FECs and coccidian FOCs were both moderately repeatable at the individual level over our study period. This shows that variation in parasite abundance across a year is driven, at least in part, by consistent among-host differences in some aspects of genotype or environment. Previous estimates in our study system put the heritability of August FEC and FOC measures at 10–20% (Beraldi et al., 2006), which suggests at least some of the among-individual variations observed in the current study may have a genetic basis.
Here, we have utilized multivariate hierarchical mixed-effects models to dissect whether the observed positive correlation between strongyle and coccidian counts is driven by among- or within-host processes (see Fig. 1). Although rarely used to date in the context of co-infection, previous studies have used this approach to dissect the role of among- and within-host processes in linking nematode parasite burdens in wild horses (Debeffe et al., 2016) and the role of among-host and among-site processes driving amphibian infection dynamics (Stutz et al., 2017). Our analyses demonstrate that the positive covariance between strongyle FECs and coccidian FOCs is predominantly a within-individual correlation. In other words, it results from short-term (season-to-season) deviations from an individual's mean parasite count in the same direction for both types of parasites, and not from some individuals having consistently higher or lower mean FEC and FOC. This highlights the utility of this approach for our understanding the drivers of associations among parasites within communities, and suggests that short-term environmental fluctuations impacting host resource availability – rather than long-term genetically or environmentally driven among-host differences – are responsible for the observed correlation between strongyles and coccidia. The addition of multivariate mixed models to larger-scale and longer-term studies could also allow researchers to go further and address the role that annual variation in environmental conditions (by e.g. random effect for year; Froy et al., 2019; Sweeny et al., 2021); host genetics (via pedigree-based ‘animal models’; Beraldi et al., 2006) and spatial structure (through inclusion of spatial field random terms; Albery et al., 2019) play in driving the patterns of co-infection in the wild.
Overall, these results demonstrate that seasonality varies across demographic and parasite groups and highlight the value of investigating fluctuating susceptibility and exposure over time for understanding epidemiology of a population. Although we focus here on strongyles and coccidia as broad taxonomic groups, there is considerable diversity of species within both groups on St Kilda (Wilson et al., 2004). Recent advances in characterizing the GI community with metabarcoding methods using noninvasive fecal samples for both nematodes and coccidia (Avramenko et al., 2015; Vermeulen et al., 2016; Evans et al., 2021) could offer higher resolution understanding of GI parasite community dynamics. Additionally, integration of multiple immune markers could provide further insights regarding how multiple components of reproductive effort influence different components of immunity. This will be a crucial link in understanding dynamic stressors affecting immunity and the outcome across multiple parasites in co-infected systems. Finally, results here using just 1 year of sampling demonstrate the value of repeated observations at the individual level for parsing among- and within- individual contributions to variance. From an epidemiological perspective, long-term individual sampling can inform processes underlying complex dynamics of multiparasite systems.
Acknowledgements
The authors acknowledge the National Trust for Scotland for support of this study on St Kilda, and QinetiQ and Kilda Cruises for logistical support. The authors acknowledge the Ecology Within Team for input in the analysis and manuscript. The authors thank Greg Albery, Josephine Pemberton, Robin Pakeman, Andy Fenton and Amy Pedersen for insightful discussion and comments on the manuscript. Figures 1 and 2 are created with Biorender.com. Sheep icons in Fig. 1 are based on photographs by Hannah Vallin and Martin Stoffel.
Author contributions
All authors contributed to the development and planning of the study. X. B. and J. G. P. collected samples. Y. C. M. and F. K. carried out parasitology. A. R. S. analysed the data and led the writing of the manuscript with assistance from D. H. N. and F. K. All authors contributed critically to manuscript drafts and analysis.
Financial support
This study was funded by a large NERC grant (NE/R016801/1), and the long-term study on St Kilda was funded principally by responsive mode grants from NERC.
Ethical standards
All sampling was carried out in accordance with UK Home Office regulations under Project License PP4825594.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021001980.
click here to view supplementary material
Data
Data for this study are available on GitHub: https://github.com/arsweeny/SoayLongitudinalPar.
Conflict of interest
The authors declare there are no conflicts of interest.
References
- Acevedo-Whitehouse K and Duffus ALJ (2009) Effects of environmental change on wildlife health. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albery GF, Kenyon F, Morris A, Morris S, Nussey DH and Pemberton JM (2018) Seasonality of helminth infection in wild red deer varies between individuals and between parasite taxa. Parasitology 85, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albery GF, Becker DJ, Kenyon F, Nussey DH and Pemberton JM (2019) The fine-scale landscape of immunity and parasitism in a wild ungulate population. Integrative and Comparative Biology 16, e2003538. [DOI] [PubMed] [Google Scholar]
- Albery GF, Watt KA, Keith R, Morris S, Morris A, Kenyon F, Nussey DH and Pemberton JM (2020) Reproduction has different costs for immunity and parasitism in a wild mammal. Functional Ecology 34, 229–239. [Google Scholar]
- Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M and Rohani P (2006) Seasonality and the dynamics of infectious diseases. Ecology Letters 9, 467–484. [DOI] [PubMed] [Google Scholar]
- Avramenko RW, Redman EM, Lewis R, Yazwinski TA, Wasmuth JD and Gilleard JS (2015) Exploring the gastrointestinal ‘Nemabiome’: deep amplicon sequencing to quantify the species composition of parasitic nematode communities. PLoS One 10, e0143559-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalew L and Gibbs HC (2005) Seasonal fluctuations of nematode populations in breeding ewes and lambs. Canadian Journal of Comparative Medicine 37, 79–89. [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Czirják GÁ, Volokhov DV, Bentz AB, Carrera JE, Camus MS, Navara KJ, Chizhikov VE, Fenton MB, Simmons NB, Recuenco SE, Gilbert AT, Altizer S and Streicker DG (2018) Livestock abundance predicts vampire bat demography, immune profiles and bacterial infection risk. Philosophical Transactions of the Royal Society of London B: Biological Sciences 373, 20170089-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraldi D, McRae AF, Gratten J, Pilkington JG, Slate J, Visscher PM and Pemberton JM (2006) Quantitative trait loci (QTL) mapping of resistance to strongyles and coccidia in the free-living Soay sheep (Ovis aries). International Journal for Parasitology: Parasites and Wildlife 37, 121–129. [DOI] [PubMed] [Google Scholar]
- Brunsdon RV (1970) The spring-rise phenomenon: seasonal changes in the worm burdens of breeding ewes and in the availability of pasture infection. New Zealand Veterinary Journal 18, 47–54. [DOI] [PubMed] [Google Scholar]
- Cabaret J, Gasnier N and Jacquiet P (1998) Faecal egg counts are representative of digestive-tract strongyle worm burdens in sheep and goats. Parasite 5, 137–142. [DOI] [PubMed] [Google Scholar]
- Cable J, Barber I, Boag B, Ellison AR, Morgan ER, Murray K, Pascoe EL, Sait SM, Wilson AJ and Booth M (2017) Global change, parasite transmission and disease control: lessons from ecology. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon M, Loot G, Grenouillet G and Blanchet S (2011) Host characteristics and environmental factors differentially drive the burden and pathogenicity of an ectoparasite: a multilevel causal analysis. Journal of Animal Ecology 80, 657–667. [DOI] [PubMed] [Google Scholar]
- Chartier C and Paraud C (2012) Coccidiosis due to Eimeria in sheep and goats, a review. Small Ruminant Research 103, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH and Pemberton JM (2004a) Individuals and populations. In Clutton Brock TH and Pemberton JM (eds), Soay Sheep. Cambridge: Cambridge University Press, pp. 1–13. [Google Scholar]
- Clutton-Brock TH and Pemberton JM (2004b) Soay Sheep: Dynamics and Selection in an Island Population. Cambridge: Cambridge University Press. [Google Scholar]
- Clutton-Brock T and Sheldon BC (2010) Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends in Ecology & Evolution 25, 562–573. [DOI] [PubMed] [Google Scholar]
- Corripio-Miyar Y, Hayward A, Lemon H, Sweeny AR, Bal X, Kenyon F, Pilkington JG, Pemberton JM, Nussey DH and McNeilly TN (2022) Functionally distinct T-helper cell phenotypes predict resistance to different types of parasites in a wild mammal. Scientific Reports 12, 3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox FEG (2001) Concomitant infections, parasites and immune responses. Parasitology 122, S23–S38. [DOI] [PubMed] [Google Scholar]
- Craig BH, Pilkington JG and Pemberton JM (2006) Gastrointestinal nematode species burdens and host mortality in a feral sheep population. Parasitology 133, 485–496. [DOI] [PubMed] [Google Scholar]
- Craig BH, Pilkington JG, Kruuk LEB and Pemberton JM (2007) Epidemiology of parasitic protozoan infections in Soay sheep (Ovis aries L.) on St Kilda. Parasitology 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Craig BH, Tempest LJ, Pilkington JG and Pemberton JM (2008) Metazoan-protozoan parasite co-infections and host body weight in St Kilda Soay sheep. Parasitology 135, 433–441. [DOI] [PubMed] [Google Scholar]
- Cringoli G, Rinaldi L, Veneziano V, Pennachio S, Morgoglione ME, Santaniello M, Schioppi M and Fedele V (2008) Gastrointestinal strongyle faecal egg count in goats: circadian rhythm and relationship with worm burden. Veterinary Research Communications 32, S191–S193. [DOI] [PubMed] [Google Scholar]
- Debeffe L, McLoughlin PD, Medill SA, Stewart K, Andres D, Shury T, Wagner B, Jenkins E, Gilleard JS and Poissant J (2016) Negative covariance between parasite load and body condition in a population of feral horses. Parasitology 143, 983–997. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Chaudhry UN, Costa-Júnior LM, Hamer K, Leeson SR and Sargison ND (2021) A 4 year observation of gastrointestinal nematode egg counts, nemabiomes and the benzimidazole resistance genotypes of Teladorsagia circumcincta on a Scottish sheep farm. International Journal for Parasitology 51, 393–403. [DOI] [PubMed] [Google Scholar]
- Ezenwa VO (2016) Helminth–microparasite co-infection in wildlife: lessons from ruminants, rodents and rabbits. Parasite Immunology 38, 527–534. [DOI] [PubMed] [Google Scholar]
- Ezenwa VO, Stefan Ekernas L and Creel S (2012) Unravelling complex associations between testosterone and parasite infection in the wild. Functional Ecology 26, 123–133. [Google Scholar]
- Fenton A (2008) Worms and germs: the population dynamic consequences of microparasite–macroparasite co-infection. Parasitology 135, 1545–1560. [DOI] [PubMed] [Google Scholar]
- Fenton A, Knowles SCL, Petchey OL and Pedersen AB (2014) The reliability of observational approaches for detecting interspecific parasite interactions: comparison with experimental results. International Journal for Parasitology 44, 437–445. [DOI] [PubMed] [Google Scholar]
- Ferrari N, Cattadori IM, Nespereira J, Rizzoli A and Hudson PJ (2004) The role of host sex in parasite dynamics: field experiments on the yellow-necked mouse Apodemus flavicollis. Ecology Letters 7, 88–94. [Google Scholar]
- Festa-Bianchet M (1989) Individual differences, parasites, and the costs of reproduction for Bighorn ewes (Ovis canadensis). The Journal of Animal Ecology 58, 785. [Google Scholar]
- Froy H, Sparks AM, Watt K, Sinclair R, Bach F, Pilkington JG, Pemberton JM, McNeilly TN and Nussey DH (2019) Senescence in immunity against helminth parasites predicts adult mortality in a wild mammal. Science (New York, N.Y.) 365, 1296–1298. [DOI] [PubMed] [Google Scholar]
- Graham AL (2008) Ecological rules governing helminth–microparasite coinfection. Proceedings of the National Academy of Sciences of the United States of America 105, 566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Nussey DH, Lloyd-Smith JO, Longbottom D, Maley M, Pemberton JM, Pilkington JG, Prager KC, Smith L, Watt KA, Wilson K, McNeilly TN and Brulisauer F (2016) Exposure to viral and bacterial pathogens among Soay sheep (Ovis aries) of the St Kilda archipelago. Epidemiology & Infection 144, 1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grear DA, Perkins SE and Hudson PJ (2009) Does elevated testosterone result in increased exposure and transmission of parasites? Ecology Letters 12, 528–537. [DOI] [PubMed] [Google Scholar]
- Hadfield JD (2010) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. Journal of Statistical Software 33, 1–22. [PMC free article] [PubMed] [Google Scholar]
- Hamer K, McIntyre J, Morrison AA, Jennings A, Kelly RF, Leeson S, Bartley DJ, Chaudhry U, Busin V and Sargison N (2019) The dynamics of ovine gastrointestinal nematode infections within ewe and lamb cohorts on three Scottish sheep farms. Preventive Veterinary Medicine 171, 104752. [DOI] [PubMed] [Google Scholar]
- Hawley DM and Altizer SM (2011) Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Functional Ecology 25, 48–60. [Google Scholar]
- Hayward AD, Wilson AJ, Pilkington JG, Pemberton JM and Kruuk LEB (2009) Ageing in a variable habitat: environmental stress affects senescence in parasite resistance in St Kilda Soay sheep. Proceedings of the Royal Society of London B: Biological Sciences 276, 3477–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward AD, Pilkington JG, Pemberton JM and Kruuk LEB (2010) Maternal effects and early-life performance are associated with parasite resistance across life in free-living Soay sheep. Parasitology 137, 1261–1273. [DOI] [PubMed] [Google Scholar]
- Hayward AD, Pilkington JG, Wilson K, McNeilly TN and Watt KA (2019) Reproductive effort influences intra-seasonal variation in parasite-specific antibody responses in wild Soay sheep. Functional Ecology 270, 1679–1614. [Google Scholar]
- Houdijk JGM (2008) Influence of periparturient nutritional demand on resistance to parasites in livestock. Parasite Immunology 30, 113–121. [DOI] [PubMed] [Google Scholar]
- Houdijk JGM, Jessop NS and Kyriazakis I (2001) Nutrient partitioning between reproductive and immune functions in animals. Proceedings of the Nutrition Society 60, 515–525. [DOI] [PubMed] [Google Scholar]
- Hutchings MR, Milner JM, Gordon IJ, Kyriazakis I and Jackson F (2002) Grazing decisions of Soay sheep, Ovis aries, on St Kilda: a consequence of parasite distribution? Oikos 96, 235–244. [Google Scholar]
- Jorg Reeg K, Gauly M, Bauer C, Mertens C, Erhardt G and Zahner H (2005) Coccidial infections in housed lambs: oocyst excretion, antibody levels and genetic influences on the infection. Veterinary Parasitology 127, 209–219. [DOI] [PubMed] [Google Scholar]
- Knowles SCL, Nakagawa S and Sheldon BC (2009) Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: a meta-regression approach. Functional Ecology 23, 405–415. [Google Scholar]
- Knowles SCL, Fenton A, Petchey OL, Jones TR, Barber R and Pedersen AB (2013) Stability of within-host–parasite communities in a wild mammal system. Proceedings of the Royal Society of London B: Biological Sciences 280, 20130598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivesley JA, Bussière LF, Pemberton JM, Pilkington JG, Wilson K and Hayward AD (2019) Survival costs of reproduction are mediated by parasite infection in wild Soay sheep. Ecology Letters 22, 1203–1213. [DOI] [PubMed] [Google Scholar]
- Lello J, Boag B, Fenton A, Stevenson IR and Hudson PJ (2004) Competition and mutualism among the gut helminths of a mammalian host. Nature 428, 840–844. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM and Nelson RJ (2008) Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philosophical Transactions of the Royal Society of London B: Biological Sciences 363, 321–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna PB (1981) The diagnosis value and interpretation of faecal egg counts in sheep. New Zealand Veterinary Journal 29, 129–132. [DOI] [PubMed] [Google Scholar]
- Metcalf CJE and Graham AL (2018) Schedule and magnitude of reproductive investment under immune trade-offs explains sex differences in immunity. Nature Communications 9, 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SL and Wilson K (2002) Parasites as a viability cost of sexual selection in natural populations of mammals. Science (New York, N.Y.) 297, 2015–2018. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP and Bribiescas RG (2005) Testosterone-mediated immune functions and male life histories. American Journal of Human Biology 17, 527–558. [DOI] [PubMed] [Google Scholar]
- Nakagawa S and Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biological Reviews of the Cambridge Philosophical Society 85, 935–956. [DOI] [PubMed] [Google Scholar]
- Nelson RJ and Demas GE (1996) Seasonal changes in immune function. The Quarterly Review of Biology 71, 511–548. [DOI] [PubMed] [Google Scholar]
- Nordling D, Andersson M, Zohari S and Lars G (1998) Reproductive effort reduces specific immune response and parasite resistance. Proceedings of the Royal Society of London B: Biological Sciences 265, 1291–1298. [Google Scholar]
- Nunn CL, Lindenfors P, Pursall ER and Rolff J (2009) On sexual dimorphism in immune function. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovington KS, Alleva LM and Kerr EA (1995) Cytokines and immunological control of Eimeria spp. International Journal for Parasitology: Parasites and Wildlife 25, 1331–1351. [DOI] [PubMed] [Google Scholar]
- Ozmen O, Adanir R and Haligur M (2012) Immunohistochemical detection of the cytokine and chemokine expression in the gut of lambs and kids with coccidiosis. Small Ruminant Research 105, 345–350. [Google Scholar]
- Pacheco A, McNeilly TN, Banos G and Conington J (2021) Genetic parameters of animal traits associated with coccidian and nematode parasite load and growth in Scottish Blackface sheep. Animal: An International Journal of Animal Bioscience 15, 100185. [DOI] [PubMed] [Google Scholar]
- Palmer WH, Hadfield JD and Obbard DJ (2018) RNA-interference pathways display high rates of adaptive protein evolution in multiple invertebrates. Genetics 208, 1585–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AB and Fenton A (2007) Emphasizing the ecology in parasite community ecology. Trends in Ecology & Evolution 22, 133–139. [DOI] [PubMed] [Google Scholar]
- Petney TN and Andrews RH (1998) Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. International Journal for Parasitology 28, 377–393. [DOI] [PubMed] [Google Scholar]
- Plowright RK, Field HE, Smith C, Divljan A, Palmer C, Tabor G, Daszak P and Foley JE (2008) Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proceedings of the Royal Society of London B: Biological Sciences 275, 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Rödel HG, Zapka M, Stefanski V and Holst D (2016) Reproductive effort alters immune parameters measured post-partum in European rabbits under semi-natural conditions. Functional Ecology 30, 1800–1809. [Google Scholar]
- Rynkiewicz EC, Pedersen AB and Fenton A (2015) An ecosystem approach to understanding and managing within-host parasite community dynamics. Trends in Parasitology 31, 212–221. [DOI] [PubMed] [Google Scholar]
- Sheldon BC and Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology & Evolution 11, 317–321. [DOI] [PubMed] [Google Scholar]
- Smith JA, Wilson K, Pilkington JG and Pemberton JM (1999) Heritable variation in resistance to gastro-intestinal nematodes in an unmanaged mammal population. Proceedings of the Royal Society of London, Series B: Biological Sciences 266, 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg BE (1997) Environmental factors influencing transmission. Veterinary Parasitology 72, 247–256, discussion 257–264. [DOI] [PubMed] [Google Scholar]
- Stutz WE, Blaustein AR, Briggs CJ, Hoverman JT, Rohr JR and Johnson PTJ (2017) Using multi-response models to investigate pathogen coinfections across scales: insights from emerging diseases of amphibians. Methods in Ecology and Evolution 9, 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeny AR, Albery GF, Venkatesan S, Fenton A and Pedersen AB (2021) Spatiotemporal variation in drivers of parasitism in a wild wood mouse population. Functional Ecology 35, 1277–1287. [Google Scholar]
- Vermeulen ET, Lott MJ, Eldridge MDB and Power ML (2016) Evaluation of next generation sequencing for the analysis of Eimeria communities in wildlife. Journal of Microbiological Methods 124, 1–9. [DOI] [PubMed] [Google Scholar]
- Vlassoff A, Leathwick DM and Heath A (2001) The epidemiology of nematode infections of sheep. New Zealand Veterinary Journal 49, 213–221. [DOI] [PubMed] [Google Scholar]
- Wilson K, Grenfell BT, Pilkington JG, Boyd HE and Gulland F (2004). Parasites and their impact. In Clutton Brock TH and Pemberton JM (eds), Soay Sheep. Cambridge, UK: Cambridge University Press, pp. 113–165. [Google Scholar]
- Zuk M and McKean KA (1996) Sex differences in parasite infections: patterns and processes. International Journal for Parasitology 26, 1009–1024. [PubMed] [Google Scholar]
- Zuk M and Stoehr AM (2002) Immune defense and host life history. The American Naturalist 160, S9–S22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021001980.
click here to view supplementary material
Data Availability Statement
Data for this study are available on GitHub: https://github.com/arsweeny/SoayLongitudinalPar.