Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature cells capable of inhibiting T-cell responses. MDSCs have a crucial role in the regulation of the immune response of the body to pathogens, especially in inflammatory response and pathogenesis during anti-infection. Pathogens such as bacteria and viruses use MDSCs as their infectious targets, and even some pathogens may exploit the inhibitory activity of MDSCs to enhance pathogen persistence and chronic infection of the host. Recent researches have revealed the pathogenic significance of MDSCs in pathogens such as bacteria and viruses, despite the fact that the majority of studies on MDSCs have focused on tumor immune evasion. With the increased prevalence of viral respiratory infections, the resurgence of classical tuberculosis, and the advent of medication resistance in common bacterial pneumonia, research on MDSCs in these illnesses is intensifying. The purpose of this work is to provide new avenues for treatment approaches to pulmonary infectious disorders by outlining the mechanism of action of MDSCs as a biomarker and therapeutic target in pulmonary infectious diseases.
Keywords: myeloid-derived suppressor cells, novel coronaviral pneumonia, tuberculosis, other infectious lung diseases, immunotherapy
1. Introduction
With the spread of novel coronavirus pneumonia (COVID-19), the resurgence of tuberculosis, and the emergence of antibiotic resistance in bacterial pneumonia, standard antiviral, anti-tuberculosis and antibiotic therapy against bacteria have reached a bottleneck. Immunotherapy for infectious illnesses has become one of the primary research foci as the hunt for therapies with fewer side effects and greater effectiveness has become imperative. In the inflammatory response to pathogens that assault the body and in the latter phases of chronic infection, the immune system is vital. Under normal circumstances, the innate immune system automatically recognizes and clears pathogens after they attack the body; however, when the innate immune system is compromised, pathogens are difficult to eliminate in a timely manner and are more likely to cause disease exacerbation or chronic infection. Myeloid-derived suppressor cells (MDSCs) are immunosuppressive cells, and the initial research on MDSCs focused mostly on malignancies, where MDSCs proliferate in the tumor microenvironment and may promote tumor proliferation and metastasis by mediating immune escape of tumor cells (1). Targeted immunotherapy against MDSCs promotes tumor regression by modulating the immune activity of T cells (2, 3). In addition, there is accumulating evidence that MDSCs also play a crucial role in regulating the immunological response of the body to infections. Numerous studies have also revealed that MDSCs may multiply and correlate with the severity of infectious lung disease, such as novel coronavirus pneumonia, tuberculosis, and bacterial pneumonia (4–10). Therefore, this research investigates the significance and mechanism of action of MDSCs in infectious lung illnesses, as well as MDSC immunotherapy.
2. Myeloid-derived suppressor cells
In the 1970s, a bone marrow-derived cell that suppressed T cells was identified in a mouse model of lung cancer and give the name “nature suppressor cells (NS)” due to its myeloid origin and immunosuppressive activity (11, 12). These cells were renamed “immature myeloid cells (IMCs)” or “myeloid suppressor cells (MSCs)” towards the beginning of the twenty-first century (13, 14). Due to the morphological, phenotypic, and functional heterogeneity of these cell populations, their nomenclature was controversial internationally until 2007, when they were unified as “myeloid-derived suppressor cells (MDSCs)” to describe heterogeneous cell populations of immature myeloid cells found in pathological settings (15). MDSCs are cells formed mostly from bone marrow hematopoietic precursor cells, which are progenitors of granulocytes, dendritic cells, or macrophages, and which are extensively dispersed in bone marrow, spleen, peripheral blood, cancers, and other tissues with significant heterogeneity and immunosuppressive activity (16, 17). Depending on their phenotypes, MDSCs may be divided into granulocytic/polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs or G-MDSCs) and monocytic myeloid-derived suppressor cells (M-MDSCs). MDSCs in mice are all expressed as CD11b and can be classified into PMN-MDSCs (CD11b+ Ly6Clow L6G+) and M-MDSCs (CD11b+ Ly6Chi Ly6G-) according to the expression levels of Ly6G and Ly6C (5, 18). In mice chronically infected with Staphylococcus aureus, Eo-MDSCs (CD11b+ SyglecF+ CCR3low IL-5Ralow SSC-Ahigh) with phenotypic characteristics of immature eosinophils were identified (16, 19). MDSC subtypes and phenotypic markers in human peripheral blood mononuclear cells (PBMC) include PMN-MDSCs (CD11b+ CD14- CD33+ CD15+ HLA-DR-/low) and M-MDSCs (CD11b+ CD14+ CD33+ CD15- HLA-DR-/low) (20, 21).
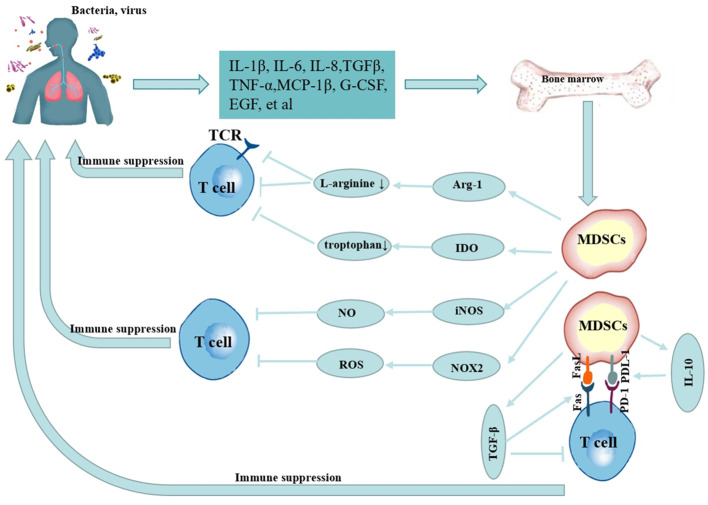
In a healthy state, immature myeloid cells may be produced in the bone marrow and develop into mature granulocytes, macrophages, or dendritic cells, which then penetrate the proper tissues and organs to execute typical immune tasks. However, their normal differentiation is hindered in pathological settings such as tumors, infectious diseases, and autoimmune diseases, and MDSCs can rapidly accumulate and be activated through injury-associated molecular patterns or pathogen-associated molecular patterns, etc., by promoting reactive oxygen species (ROS) release; expressing high levels of arginase-1 (Arg-1) (22) and inducible nitric oxide synthase (iNOS) (23), promoting the release of interleukin (IL)-10, IL-1β, IL-6, tumor necrosis factor (TNF)-α and other cytokine (10, 24–27), and the immunosuppressive activity of M-MDSCs was stronger than that of PMN-MDSCs (16, 28–30). The mechanism of MDSCs in infectious lung diseases is shown in Figure 1 . Despite the fact that the majority of data on MDSCs are generated from malignancies, pulmonary infectious illnesses have commonalities with their activity in tumors and are linked to poor clinical outcomes (5, 31, 32). Nevertheless, in infectious lung disorders, the behavior of MDSCs in infections seems to be dependent on the kind of invading pathogen and the disease stage (30, 33).
Figure 1.
Mechanism of MDSCs in infectious lung diseases.
3. MDSCs as a target for infectious lung diseases
3.1. Novel coronavirus pneumonia
Novel coronavirus pneumonia (COVID-19) remains a potentially life-threatening global pandemic acute infectious disease characterized by inflammatory storms, coagulation disorders, and organ damage (34). MDSCs with immunomodulatory activity were found to play an important role in mediating the excessive inflammation or inflammatory storm of COVID-19, and MDSCs can limit infection-induced excessive inflammation or inflammatory storm and protect host immunity (34); on the other hand, excessive inflammation or inflammatory storms also lead to accumulation of MDSCs in the peripheral blood of COVID-19 patients, participate in the pathological process of the disease, and correlate with the severity of the disease (8, 35–39). Significantly, a link between MDSCs and COVID-19 has been identified in a number of recent investigations ( Table 1 ). Compared with healthy subjects or patients with mild COVID-19, the frequency of PMN-MDSCs in the peripheral blood of COVID-19 increases with disease exacerbation, especially in severe instances and deceased patients, and proliferating PMN-MDSCs will further suppress T cells, resulting in a reduction in lymphocytes and further compromising the host immune response, so establishing a vicious cycle (7, 20, 44, 45). Comparison with indicators reflecting disease severity (levels of C-reactive protein, ferritin, and lactate dehydrogenase) demonstrated that these indicators were elevated with PMN-MDSCs in severe cases, especially immature PMN-MDSCs, confirming a positive correlation between PMN-MDSCs and COVID-19 severity (41). A 1% increase in PMN-MDSC frequency was independently related with a 3% increase in the probability of fatal outcomes, as determined by an age- and gender-adjusted Cox regression model (20). In contrast to these results, Japanese researchers found that the frequency of PMN-MDSCs (but not other MDSC subgroups) may be transiently elevated in patients surviving severe COVID-19 compared to patients dying from severe COVID-19, and the investigators suggest that PMN-MDSCs may reduce detrimental immune responses and be associated with genetic factors (36). In severely sick individuals, low levels of PMN-MDSCs may aid in survival (45). However, the majority of investigations have revealed that the frequency of M-MDSCs in peripheral blood following COVID-19 therapy is substantially linked with disease severity (8, 37). Analysis of SARS-CoV-2 viral RNA burden revealed a connection between M-MDSCs and viral load, indicating that SARS-CoV-2 infection may inhibit host immunological responses by encouraging the proliferation of M-MDSCs (43). M-MDSCs inhabit CD4+ and CD8+ T cell proliferation and IFN-γ production through an Arg-1-dependent mechanism, with downregulation of CD3ζ chain expression (8). Despite the fact that MDSCs and cytokine levels (such as IL-6, TNF, IL-1, etc.) remained persistently elevated during the recovery phase of COVID-19, this also suggests that MDSCs exerted an inhibitory T-cell recall response, that the suppressive activity of T cells persisted after recovery from infection (39, 42). It can be seen that M-MDSCs not only suppress the immune activity of T cells during the acute episode after SARS-CoV-2 infection, but also have a recall response to suppress T cells during the recovery period. In conclusion, the results of different studies may vary, but MDSCs exhibit different phenotypic characteristics and functional status with various stages of COVID-19, and MDSCs, as one of the key pathogenic factors of COVID-19 inflammation and immunosuppression, may be a major target for treatment (30, 46, 47).
Table 1.
Summary current studies on MDSCs in COVID-19 patients.
| Samples (COVID-19 patients and healthy donors (HD)) | Source of MDSCs | Subtypes and phenotypic markers of MDSCs | Frequency of MDSCs | Cytokines | Reference |
|---|---|---|---|---|---|
| N=68(COVID-19:48; HD:20) | PBMC | CD11b,CD14,HL (39)A-DR,CD33,CD88,CD56,CD19,CD3,CD15,CD45,DRAQ7 | MDSCs↑ (as compared to HD) | IL-1β, IL-6, IL-8 and TNF-α↑ | (40) |
| N=68(COVID-19:15 severe, Nosevere:26; HD:26) | PBMC | Total MDSCs: HLA-DR-CD11b+; Mature PMN-MDSCs: HLA-DR-CD11b+CD15+CD66b+CD14-CD16+; Immature PMN- MDSCs: HLA-DR-CD11b+CD15+CD66b+CD14-CD16-; M-MDSCs: HLA-DR-CD11b+CD15- CD66b-CD14+ |
Total MDSCs, mature PMN-MDSCs, immature PMN- MDSCs and M-MDSCs↑ (as compared to HD); mature PMN-MDSCs and immature PMN- MDSCs severe patients (as compared to nosevere) |
None | (41) |
| N=22(CoV2-=9, CoV2+=13) SARS Coronavirus 2 | PBMC | M-MDSCs;CD11b+CD33+CD14+HLA- DR-/lo |
M-MDSCs↑ | IL-6↑ | (39) |
| N=66(COVID-19 = 56, moderate: 45, severe: 11; HD=10); after 3 months (n=21) | EDTA-anticoagulated blood | PMN-MDSCs: CD11b+CD14-CD15+CD16+CD33-HLA-DR-; M-MDSCs: CD11b+CD14+CD15-/lowCD16-CD33+HLA-DR-/low | PMN-MDSCs: severe: moderate=10:1; M-MDSCs: severe: moderate=4:1 | IL-1β, IL-6, IL-7, EGF, HCF, PDGF-BB, et al. ↑ | (42) |
| N=158(COVID-19 = 96(ICU:32, non-ICU:96); HD:30); 59 patients recovered; 19 died |
PBMC | PMN-MDSCs: CD11b+CD14-CD33+CD15+ HLADR-/low; M-MDSC: CD11b+CD14+CD33+HLA-DR-/low |
PMN-MDSCs↑,especially in patients who required intensive care treatments (as compared to HDs) |
IL-1β, IL-6, IL-8, and TNF-α↑ | (20) |
| N=40(COVID-19 = 27(ICU:8, non-ICU:19; HD:13) | EDTA-anticoagulated peripheral blood | PMN-MDSCs: CD11b+CD33+CD15+HLA-DR+; M-MDSCs: CD11b+CD33+CD14+HLA-DRlo/neg or CD14+HLA-DRlo/neg; the new subset of MDSCs: CD14+HLA-DRlo/neg |
CD14+HLA-DRlo/neg MDSCs↑, especially in patients who required intensive care treatments (as compared to HD) |
None | (43) |
| N=71(COVID-19 = 62(ICU:31, non-ICU:31; HD:9)) | PBMC | PMN-MDSCs: HLA-DRlow/- CD11b+CD14-CD33+CD15+; M-MDSCs: HLA-DRlow/-CD11b+ CD14+CD33+ | PMN-MDSCs↑, especially in patients who required intensive care treatments (as compared to HD) |
None | (44) |
| N=26(COVID-19 = 18(mild:9; severe:9); HD=8) | PBMC | PMN-MDSCs: HLA-DR-Lin-CD33+CD11b+CD15+ | PMN-MDSCs↑, especially in severe (as compared to HD) | IL-6, IL-8, IL-1β, TNF-α↑; TGF-β↑ (in plasma from patients with severe disease, which decreased over time.) | (7) |
| N=224 (COVID-19 = 147; influenza A virus infected patients=44; HD=33) | PBMC and nasopharyngeal Aspirates (NPA) |
M-MDSCs : CD14+
Lin-(CD3-CD56-CD19-CD20-CD66-) HLA-DR-; PMN-MDSCs: CD56-CD14-CD3-CD19-HLA-DR-CD66abce LOX-1 |
M-MDSCs↑ (as compared to influenza A virus infected patients and HDs) | IL-6, IL-10↑ | (8) |
| N=80 (COVID-19 with ICU treatment) | Whole blood samples | M-MDSCs: CD45+CD11b+CD33+HLA-DRlow/−
CD14+CD15-, G-MDSCs: CD45+CD11b+CD33+HLA-DRlow/- CD14-CD15+ |
PMN-MDSCs ↓ (as compared to the ICU deceased) | None | (45) |
| N=47(COVID-19 = 40(mild:12; moderate I:7; moderate II:8; severe:13); HD=7) | PBMC | e-MDSCs : CD3-CD19+CD56-HLA-DR-CD11b+CD33+CD14-CD15-; M-MDSCs: D3-CD19-CD56-HLA-DR-CD11b+CD33+CD14+CD15-; PMN-MDSCs: CD3-CD19-CD56-HLA-DR-CD11b+CD33+CD14-CD15+ | PMN-MDSCs ↑in survivors of severe COVID-19 (as compared to mild, moderate, deceased and HDs) | IL-8↑ | (36) |
| N=32(excluded n=3; COVID-19 n=29(no secondary infection=17; secondary infection:12)) | EDTA anticoagulated blood | M-MDSCs: CD11b+HLA-DR- | M-MDSCs↑ | None | (31) |
| N=57(COVID-19 = 41(mild:21; severe:20); HD=16) | PBMC | PMN-MDSCs: Lin-HLA-DRlowCD11b+SSClowCD15+CD66b+; M-MDSCs: Lin-HLA-DRlowCD11b+SSClowCD14+; e-MDSCs: Lin-HLA-DRlowCD11b+SSClowCD15-CD66b-/CD14- | PMN-MDSCs and M-MDSCs↑ (as compared to HDs) | IL-6,IL-8,MCP-1,IL-18, TGF-β, IL-10↑ | (38) |
| N=26(ARDS COVID: 13; Moderate COVID:13) | PBMC | M-MDSCs: CD14+HLA-DR-; PMN-MDSCs: CD45+Lin-HLA-DR-CD15+CD11b+; e-MDSCs : CD45+CD3-Lin-HLA-DR-CD15+CD33+ | M-MDSCs and PMN-MDSCs↑ | IL-6, IL-10, MCP-1, CXCL9, CXCL10, ↑(as compared to HDs),and G-CSF↑ in ARDS | (37) |
↑ represents increased, ↓ represents decreased, PBMC, peripheral blood mononuclear cell; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10;IL-18, interleukin-18;TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β; EGF, Epidermal Growth Factor; PDGF-BB, Platelet derived growth factor-BB; MCP-1, monocyte chemotactic protein-1; G-CSF, granulocyte-colony stimulating factor; CXCL9, chemokine (C-X-C motif) ligand 9; CXCL10, chemokine (C-X-C motif) ligand 10.
Inflammatory cytokines are one of the primary mechanisms that induce expansion of MDSCs and are the focus of research on COVID-19-targeted therapy (40, 48). The pro-inflammatory cytokine IL-6 phosphorylates STAT3 via the gp130/JAK/STAT pathway, hence regulating M-MDSC differentiation, proliferation and survival in human and animal disease conditions including COVID-19 (49–51). In addition, studies have shown that IL-6 levels rise with the degree of illness in individuals with severe disease (7, 8, 52). Therapeutic modulation of IL-6 levels by anti-IL-6 receptor antagonists (tocilizumab, sarilumab) reduces the duration of COVID-19 and/or reduces the severity of the disease (53–55). In vitro culture of PBMC isolated from peripheral blood of COVID-19 patients revealed that 5-fluorouracil (5-FU) restored lymphocyte proliferation and propagated Th1-mediated immune response by decreasing levels of MDSCs and decreasing production of IL-10, IL-8, IL-17, and Th2 cytokines, while boosting production of IFN-γ and IL2 (38). In COVID-19 therapy, it has been proposed that 5-FU in conjunction with deoxyribonucleosides and deoxyribose may have antiviral effects (56). In addition, it has also been hypothesized that vitamin D deficiency increases the risk of developing ARDS in COVID-19 patients and that vitamin D supplementation may attenuate the inflammatory response caused by pulmonary macrophages and MDSCs in COVID-19 patients and reduce acute respiratory distress syndrome in COVID-19 patients (57). Despite the fact that the aforementioned studies demonstrated that targeting MDSCs for the treatment of COVID-19 may be more effective, these studies are still restricted to in vitro cell culture and clinical trials, and the particular therapeutic processes need more research.
3.2. Tuberculosis
Tuberculosis is a devastating infectious disease caused by Mycobacterium tuberculosis, as of the year 2020, it has superseded SARS-CoV-2 as the second most infectious disease killer, with roughly 1.3 million fatalities every year (58). Although BCG vaccination and antituberculosis chemotherapy have been extensively utilized for TB prevention and treatment, the consequences have been unsatisfactory (6, 59), thus, it has become necessary to investigate alternative antituberculosis treatment strategies. MDSCs have been found to make a significant contribution in the pathology of TB, and the majority of studies indicate that MDSCs provide ecological niches for the survival of Mycobacterium avium in the lungs of infected hosts and promote replication of Mycobacterium tuberculosis at the site of pulmonary infection (5, 6, 60, 61). Recent investigation has shown that Mycobacterium tuberculosis may employ the MPT64 protein to stimulate the creation of MDSCs, hence facilitating its survival and evasion of host immunological defenses (62). MDSCs not only accumulate in the peripheral blood of M. tuberculosis model mice (63), but also in the spleen of M. tuberculosis-infected mice (64). In addition, an increased frequency of MDSCs was observed in peripheral blood, bronchoalveolar lavage fluid, and pleural fluid specimens from patients with pulmonary or extrapulmonary tuberculosis, and the frequency of circulating MDSCs also decreased significantly at the end of antituberculosis treatment, indicating that MDSCs play an important role in the pathogenesis of tuberculosis (6, 65–69). In vitro granuloma model tests have shown that human MDSCs activate MAPK channels, hence boosting IL-1O production and Mycobacterium tuberculosis replication (60). The results of studies on TB patients also confirm the correlation between MDSCs and disease ( Table 2 ). The frequency of both subpopulations of MDSCs was elevated in PBMC of patients with active TB, was dominated by M-MDSCs, reduced the immunological function of lymphocytes in TB patients, and was proportional to the severity of the disease (67–70). Other investigations have shown that the levels of PMN-MDSCs are elevated in the peripheral blood and bronchoalveolar lavage fluid of patients with active TB, and that these levels correlate with plasma nitric oxide levels (6, 72). Grassi et al. established the link between PMN-MDSCs and TB severity by confirming by chest X-ray and experiment that PMN-MDSCs levels were higher in patients with milder disease severity than in those with more severe disease severity (71). Bindu et al. further found through studies on non-human primate TB granulomas that PMN-MDSCs levels were elevated in animal models of active TB (ATB) compared to latent TB-infected animals and were located in the lymphocyte cuffs surrounding the granuloma, thereby inhibiting T-cell entry into granuloma’s core (73). These results indicate that MDSCs may represent a novel target for TB host-directed treatment and a possible signal for detecting success.
Table 2.
Summary current studies on MDSCs in tuberculosis patients.
| Samples | Source of MDSCs | Subtypes and phenotypic markers of MDSCs | Frequency of MDSCs | Cytokines | Reference |
|---|---|---|---|---|---|
| N=62(TB=43(low responders (LR-TB):23, high responders (HR-TB):20); HD=19) | PBMC | M-MDSCs: CD14+CD33+CD11b+CD15-HLA-DR-/low; PMN-MDSCs: CD15+CD33+CD11b+CD14-HLA-DR-/low | M-MDSCs and PMN-MDSCs ↑ (M-MDSCs ↑ in LR-TB; PMN-MDSCs ↑ in HR-TB) | IFN-γ↓ (in LR-TB) | (70) |
| N=48 (active TB=38; HD=10) | PBMC | M-MDSCs: Lin-HLA-DR-/lowCD33+CD11b+CD14+CD15-; PMN-MDSCs: Lin-HLA-DR-/lowCD33+CD11b+CD14-CD15+; e-MDSCs: Lin- (CD3/CD14/CD15/CD19/56)HLA-DR-CD33+ | M-MDSCs↑ (as compared to recovered and HDs) | IL-6 ↑ | (67) |
| N=45 (active TB=35; HD=10) | PBMC and bronchoalveolar cells (BALc) | PMN-MDSCs: CD11b+CD14-CD33+CD15+HLA-DRlow; M-MDSCs : CD11b+CD14+CD33+ HLA-DRlow; MDSCs: HLA-DR-/lowCD11b+CD33+ | MDSCs ↑ (in PBMCs and BALc); PMN-MDSCs ↑ (as compared to HDs) | None | (6) |
| N=230 (active TB=110; latent TB infection (LTBI)=80; HD=40) | PBMC | PMN-MDSCs: CD14-CD15+CD11b+CD33+HLA-DRlow/−; M-MDSCs: CD14+CD15-CD11b+CD33+HLA-DRlow/-; MDSCs: CD33+ HLA-DR-/LOW | MDSCs↑ (as compared to HDs) | IFN-γ↓ | (68) |
| active TB and household contacts (HHC) | PBMC and/or pleural fluid | MDSCs: LIN-/lo HLA-DR+CD33+CD11b+; M-MDSCs: HLA-DR-/loCD11b+CD14+ or S100A9+; PMN-MDSCs: HLA-DR-/loCD11b+CD15+ | MDSCs↑ | IL-1β, IL-6, IL-8, G-CSF,MCP-1↑; GM-CSF and MIP-1β↓ | (65) |
| N=16 (standard TB treatment=8; standard TB treatment+ COX-2i=8); | PBMC | NDSCs: HLA-DRneg/lowCD14+CD33+CD11b+ | M-MDSCs↑ | None | (69) |
| N=49 (active TB=19; latent TB infection (LTBI)=18; HD=12) | PBMC | PMN-MDSCs: HLA-DR-/lowCD11b+CD14-CD15+/CD66b+; M-MDSCs: HLA-DR-/lowCD11b+CD33+ CD14+CD15-; e-MDSCs: HLA-DR-CD33+CD15-Lin(CD3-CD56-CD19-CD14-) | PMN-MDSCs ↑ (as compared to LTBI and HDs) | None | (71) |
| N=33 (active TB=23; latent TB infection (LTBI)=10) | PBMC | e-MDSCs: LIN1-HLA-DR-/lowCD11b+CD33+; PMN-MDSCs: HLA-DR-/lowCD14-CD15+CD33+/dim; M-MDSCs: HLA-DR-/lowCD14+CD15-CD33+ | PMN-MDSCs↑ (as compared to LTBI) | None | (72) |
IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-8, interleukin-8; G-CSF, granulocyte-colony stimulating facto; GM-CSF, granulocyte-macrophage colony stimulating factor; MCP-1, monocyte chemotactic protein-1; MIP-1β, macrophage inflammatory protein 1β.
↑ represents increased and ↓ represents decreased.
Host-directed therapy (HDT) is a novel approach to innovative host-specific therapies designed to reduce excessive inflammation or enhancing the host’s immune defense against pathogens, with the goal of shortening treatment regimens without inducing drug resistance (74). Several FDA-approved medicines, including all-trans retinoic acid (ATRA), cyclooxygenase-2 inhibitor (COX-2i), phosphodiesterase-5 inhibitor (PDE-5i), and sildenafil, have been validated in the treatment of tuberculosis (TB) (61, 69, 74–76). It was found that MDSCs levels were excessively elevated in the lungs of a mouse model of tuberculosis, which was related with increased mortality, and the frequency of MDSCs decreased while the number of T cells rose after host-directed therapy with all-formic retinoic acid (ARTA) (61). COX-2i has been demonstrated to reduce pathological lung damage caused by the host immunological response in tuberculosis patients (76). Combining COX-2i with anti-inflammatory effects with anti-tuberculosis basal treatment reduced cytokines that induce high levels of M-MDSCs, including IL-1, IL-10, IL-6, TNF, and S100A9 (69). Combining PDE-5i sildenafil with antituberculosis basal therapy improved treatment efficacy because PDE-5i sildenafil increased cyclic adenosine monophosphate (cGMP) in MDSCs, leading to a decrease in Arg-1 and nitric oxide synthase 2 (NOS2), thereby decreasing the mechanism of MDSCs-induced T-cell suppression (74). However, Vinzeigh N et al. demonstrated that sildenafil was incapable of reversing MDSCs-mediated T-cell suppression and had little effect on enhancing host immunity (77). The above findings for MDSCs-targeted therapy suggest that MDSCs may be a new target for anti-tuberculosis host-directed therapy, but the results are contradictory and additional investigation is required.
3.3. Other infectious lung diseases
Studies on the correlation between MDSCs and infectious lung diseases have included lung injury caused by pathogens such as Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Pneumocystis carinii, in addition to the two specific pathogens mentioned above. In a mouse model of Streptococcus pneumoniae pneumonia, MDSCs levels are elevated in model mice’s circulation and are associated with a choline-binding protein, although the specific mechanism remains unclear (78). However, the results of an animal experiment combined with clinical trials suggest that the macrolide antibiotic clarithromycin promotes elevated levels of MDSCs in the circulation of animals and humans through a particular mechanism that promotes the expansion of MDSCs (CD11b+Gr-1+) through the STAT3/Bv8 axis, decreases INF-γ, boosts IL-10 levels, and protects the organism from post-influenza Streptococcus pneumoniae infection (79). Staphylococcal enterotoxin B secreted by Staphylococcus aureus induces an increase in circulating levels of MDSCs in Staphylococcus aureus-infected mice, and treatment with resveratrol increases the proportion of circulating MDSCs because MDSCs can downregulate the body’s immune response to prevent tissue damage at the site of inflammation (80). MDSCs play a pivotal part in the efferocytosis of neutrophils following infection with Klebsiella pneumoniae, and elevated levels of MDSCs in animal models of Klebsiella pneumoniae pneumonia promote IL-10 production in the late stages of infection to facilitate the efferocytosis of apoptotic neutrophils and reduce lung injury (10, 81). It has also been hypothesized that the early expansion of M-MDSCs during an infection terminates the proinflammatory signaling essential for the clearance of Klebsiella pneumoniae, hence causing a chronic infection (82). While the particular mechanism of MDSCs in Klebsiella pneumoniae pneumonia remains a complicated process, it is known that MDSCs are involved (83). Both in clinical trials and in animal studies, P. aeruginosa infection leads to increased levels of circulating PMN-MDSCs in patients with chronic inflammatory diseases of the lung, including pulmonary cystic fibrosis, disrupting the host immune response (84). Levels of MDSCs in alveolar lavage fluid in animal models of Pneumocystis pneumonia (PcP) increase with increasing numbers of Pneumocystis carinii in the organism and with increasing lung inflammation; moreover, secondary transfer of MDSCs may directly cause lung damage in normal mice (85). Treatment with immunosuppressive drugs and antibiotics (all-trans retinoic acid combined with Primaquine) transforms MDSCs in the lung into alveolar macrophages capable of clearing Pneumocystis infection, enabling the host to successfully fight against infection (86). Further studies revealed that MDSCs are depleted of alveolar macrophage phagocytic activity during PcP via the PD-1/PD-L1 pathway (87). Despite the fact that the majority of the aforementioned studies on other pulmonary infectious diseases are limited to animal experiments, the fact that MDSCs are associated with the disease, for better or for worse, regardless of the type of pulmonary infectious disease may indicate that MDSCs are poised to become another therapeutic target for these diseases.
4. The role of traditional Chinese medicine in infectious lung diseases
Traditional Chinese medicine (TCM) refers to traditional medicine that studies the relationship between human physiology and pathology and the natural environment from a dynamic and holistic perspective under the guidance of the theory of yin-yang and the five elements, and explores effective methods to prevent and treat diseases, with a holistic view and discriminatory treatment as its main ideas. As traditional medicine, TCM is an important part of the medical field with a long history and rich experience in preventing and treating infectious diseases. Modern research results have also demonstrated the advantages of TCM in improving clinical symptoms, suppressing pathogens, promoting host immunity, and reducing side effects (88). Particularly, during COVID-19 pandemic, the vast majority of novel coronavirus pneumonia patients in China received TCM treatment, showing that TCM can significantly alleviate symptoms, reduce the inflammatory response, and promote recovery in patients with novel coronavirus pneumonia (89, 90). Several findings involving methods such as network pharmacology and molecular docking techniques have also pointed out that the active ingredients of single herbal medicines such as glycyrrhiza, scutellaria baicalensis, Coptis chinensis and lonicera japonica, and compound herbal medicines such as Yinqiaosan and LianhuaQingwen capsule can act on different targets and pathways of COVID-19, such as angiotensin-converting enzyme 2 (ACE2), TNF signaling pathway, T-cell receptor signaling pathway, Toll-like receptor signaling and MAPK signaling pathway (91–95). In vitro experiments have also demonstrated that LianhuaQingwen capsule inhibits the replication of SARS-CoV-2 and significantly reduces the production of pro-inflammatory cytokines (TNF-α, IL-6, CCL-2/MCP-1 and CXCL-10/IP-10) (96). In tuberculosis, the active ingredients of TCM not only modulate the cellular immune function of the body and promote the clearance of Mycobacterium tuberculosis, but also play a role in suppressing the inflammation of the body and inhibiting the development of drug resistance in Mycobacterium tuberculosis, such as gynostemma pentaphylla, luteolin and isoliquiritigenin (97–101). In addition, in pneumonia infected with P. aeruginosa, the active ingredients of TCM active ingredients not only inhibit the release of cytokines and chemokines in the organism, such as TNF-α, IL-6, IL-4, IL-8, and RANTES, to improve the lung infection, but also may inhibit the proliferation of P. aeruginosa through PI3K/AKT and Ras/MAPK pathways, selectively act on the QS (quorum sensing) of P. aeruginosa system to reduce bacterial virulence, and inhibition of P. aeruginosa biofilm formation (102–104).
5. Conclusions
MDSCs were discovered for the first time in oncological disorders, where they play unique immunomodulatory functions under pathological settings. There are increasing indications that MDSCs play a crucial role in regulating the immunological response of the organism, particularly in lung infectious illnesses. Depending on the disease state and research methodology, the percentage of MDSC subtypes might vary. In spite of the fact that the subtypes and levels of MDSCs in the host correlate with the severity of the disease, the exact mechanism of action of MDSCs in various diseases is still a matter of debate. MDSCs function as an immunosuppressive cell that inhibits the acute inflammatory response, promotes inflammation subsidence, and initiates the repair process of the organism, thereby ameliorating clinical symptoms, such as those caused by Streptococcus pneumoniae pneumonia and staphylococcal enterotoxin infection pneumonia. In COVID-19, tuberculosis, Pseudomonas aeruginosa infection, and Pneumocystis pneumonia, however, the levels of MDSCs in the circulation are positively correlated with the degree of inflammation of the disease, as MDSCs further impair the host’s immune response, resulting in persistent and recurrent bacterial or viral infections. In conclusion, there is a correlation between MDSCs and a variety of pulmonary infectious diseases, and the findings suggest that targeting MDSCs may reduce adverse drug reactions and resistance, and that MDSCs would be one of the important targets in the treatment of these pulmonary infectious diseases, with immune-targeted therapy against MDSCs being clearly proposed in the treatment of tuberculosis. These investigations imply that MDSCs create a pivotal regulatory function in lung infectious illnesses; nevertheless, due to the complexity of the disease, focused treatment in contemporary medicine has not yet been able to modify the disease’s overall development environment.
TMC, a traditional medicine with a holistic view and evidence-based treatment as the main ideas, is an important part of the medical field with a long history and rich experience in the prevention and treatment of infectious diseases. TMC plays a role in COVID-19, tuberculosis and other pulmonary infectious diseases by improving clinical symptoms, inhibiting pathogen proliferation, promoting pathogen clearance, regulating host immunity, reducing adverse effects and inhibiting pathogen resistance, highlighting the multi-target advantages of Chinese medicine in pulmonary infectious diseases. It can be seen that TCM may be an important available resource to target MDSCs for the treatment of pulmonary infectious diseases. There are no studies that have employed TCM to modify MDSCs and thereby affect lung infectious illnesses, according to a review of a broad body of research. Consequently, our future research will focus on advancing TCM research into the investigation of MDSCs in lung infectious illnesses. In addition, it is essential to integrate TCM with contemporary medicine in order to maximize the benefits of TCM in increasing and lowering toxicity, as well as in treating both symptoms and underlying causes in order to enhance patients’ quality of life.
Author contributions
M-NZ contributed to manuscript research and writing. Y-LY contributed to manuscript writing and review. S-HA contribute to manuscript supervision, writing, and review. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors are grateful to S-HA and Y-LY for generous help in critical revision of the manuscript.
Funding Statement
This work was supported by the funding of Southwest Medical University (Nos.2021ZKMS055 and 2021ZKQN115) and the Luzhou Science and Technology Bureau (No.2022-SYF-43).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol (2009) 182(1):240–9. doi: 10.4049/jimmunol.182.1.240 [DOI] [PubMed] [Google Scholar]
- 2. Hashimoto A, Sarker D, Reebye V, Jarvis S, Sodergren MH, Kossenkov A, et al. Upregulation of C/EBPα inhibits suppressive activity of myeloid cells and potentiates antitumor response in mice and patients with cancer. Clin Cancer Res (2021) 27(21):5961–78. doi: 10.1158/1078-0432.CCR-21-0986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Coaña YP, Wolodarski M, Poschke I, Yoshimoto Y, Yang Y, Nyström M, et al. Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma. Oncotarget. (2017) 8(13):21539–53. doi: 10.18632/oncotarget.15368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeisy-Scott V, Davis WG, Patel JR, Bowzard JB, Shieh WJ, Zaki SR, et al. Increased MDSC accumulation and Th2 biased response to influenza a virus infection in the absence of TLR7 in mice. PLoS One (2011) 6(9):e25242. doi: 10.1371/journal.pone.0025242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbosa Bomfim CC, Pinheiro Amaral E, Santiago-Carvalho I, Almeida Santos G, Machado Salles É, Hastreiter AA, et al. Harmful effects of granulocytic myeloid-derived suppressor cells on tuberculosis caused by hypervirulent mycobacteria. J Infect Diseases. (2021) 223(3):494–507. doi: 10.1093/infdis/jiaa708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El Daker S, Sacchi A, Tempestilli M, Carducci C, Goletti D, Vanini V, et al. Granulocytic myeloid derived suppressor cells expansion during active pulmonary tuberculosis is associated with high nitric oxide plasma level. Hoshino Y editor. PLoS One (2015) 10(4):e0123772. doi: 10.1371/journal.pone.0123772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agrati C, Sacchi A, Bordoni V, Cimini E, Notari S, Grassi G, et al. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19). Cell Death Differ (2020) 27(11):3196–207. doi: 10.1038/s41418-020-0572-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falck-Jones S, Vangeti S, Yu M, Falck-Jones R, Cagigi A, Badolati I, et al. Functional monocytic myeloid-derived suppressor cells increase in blood but not airways and predict COVID-19 severity. J Clin Invest (2021) 131(6):e144734. doi: 10.1172/JCI144734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rieber N, Brand A, Hector A, Graepler-Mainka U, Ost M, Schäfer I, et al. Flagellin induces myeloid-derived suppressor cells: Implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease. JI. (2013) 190(3):1276–84. doi: 10.4049/jimmunol.1202144 [DOI] [PubMed] [Google Scholar]
- 10. Peñaloza HF, Noguera LP, Ahn D, Vallejos OP, Castellanos RM, Vazquez Y, et al. Interleukin-10 produced by myeloid-derived suppressor cells provides protection to carbapenem-resistant klebsiella pneumoniae sequence type 258 by enhancing its clearance in the airways. Infection Immunity. (2019) 87(5):e00665–18. doi: 10.1128/IAI.00665-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young MR, Endicott RA, Duffie GP, Wepsic HT. Suppressor alveolar macrophages in mice bearing metastatic Lewis lung carcinoma tumors. J Leukoc Biol (1987) 42(6):682–8. doi: 10.1002/jlb.42.6.682 [DOI] [PubMed] [Google Scholar]
- 12. Young MR, Newby M, Wepsic HT. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res (1987) 47(1):100–5. [PubMed] [Google Scholar]
- 13. Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol (2001) 166(1):678–89. doi: 10.4049/jimmunol.166.1.678 [DOI] [PubMed] [Google Scholar]
- 14. van Cruijsen H, Hoekman K, Stam AGM, van den Eertwegh AJM, Kuenen BC, Scheper RJ, et al. Defective differentiation of myeloid and plasmacytoid dendritic cells in advanced cancer patients is not normalized by tyrosine kinase inhibition of the vascular endothelial growth factor receptor. Clin Dev Immunol (2007) 2007:17315. doi: 10.1155/2007/17315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res (2007) 67(1):425; author reply 426. doi: 10.1158/0008-5472.CAN-06-3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun (2016) 7:12150. doi: 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tcyganov E, Mastio J, Chen E, Gabrilovich DI. Plasticity of myeloid-derived suppressor cells in cancer. Curr Opin Immunol (2018) 51:76–82. doi: 10.1016/j.coi.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsiganov EN, Verbina EM, Radaeva TV, Sosunov VV, Kosmiadi GA, Nikitina IY, et al. Gr-1dimCD11b+ immature myeloid-derived suppressor cells but not neutrophils are markers of lethal tuberculosis infection in mice. J Immunol (2014) 192(10):4718–27. doi: 10.4049/jimmunol.1301365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldmann O, Beineke A, Medina E. Identification of a novel subset of myeloid-derived suppressor cells during chronic staphylococcal infection that resembles immature eosinophils. J Infect Dis (2017) 216(11):1444–51. doi: 10.1093/infdis/jix494 [DOI] [PubMed] [Google Scholar]
- 20. Sacchi A, Grassi G, Bordoni V, Lorenzini P, Cimini E, Casetti R, et al. Early expansion of myeloid-derived suppressor cells inhibits SARS-CoV-2 specific T-cell response and may predict fatal COVID-19 outcome. Cell Death Dis (2020) 11(10):921. doi: 10.1038/s41419-020-03125-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou L, Miao K, Yin B, Li H, Fan J, Zhu Y, et al. Cardioprotective role of myeloid-derived suppressor cells in heart failure. Circulation. (2018) 138(2):181–97. doi: 10.1161/CIRCULATIONAHA.117.030811 [DOI] [PubMed] [Google Scholar]
- 22. Yaseen MM, Abuharfeil NM, Darmani H, Daoud A. Mechanisms of immune suppression by myeloid-derived suppressor cells: the role of interleukin-10 as a key immunoregulatory cytokine. Open Biol (2020) 10(9):200111. doi: 10.1098/rsob.200111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aggen DH, Ager CR, Obradovic AZ, Chowdhury N, Ghasemzadeh A, Mao W, et al. Blocking IL1 beta promotes tumor regression and remodeling of the myeloid compartment in a renal cell carcinoma model: Multidimensional analyses. Clin Cancer Res (2021) 27(2):608–21. doi: 10.1158/1078-0432.CCR-20-1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber R, Groth C, Lasser S, Arkhypov I, Petrova V, Altevogt P, et al. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol (2021) 359:104254. doi: 10.1016/j.cellimm.2020.104254 [DOI] [PubMed] [Google Scholar]
- 25. Sade-Feldman M, Kanterman J, Ish-Shalom E, Elnekave M, Horwitz E, Baniyash M. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. (2013) 38(3):541–54. doi: 10.1016/j.immuni.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 26. Xiang H, Ramil CP, Hai J, Zhang C, Wang H, Watkins AA, et al. Cancer-associated fibroblasts promote immunosuppression by inducing ROS-generating monocytic MDSCs in lung squamous cell carcinoma. Cancer Immunol Res (2020) 8(4):436–50. doi: 10.1158/2326-6066.CIR-19-0507 [DOI] [PubMed] [Google Scholar]
- 27. Yang F, Li Y, Zou W, Xu Y, Wang H, Wang W, et al. Adoptive transfer of IFN-γ-induced m-MDSCs promotes immune tolerance to allografts through iNOS pathway. Inflammation Res (2019) 68(7):545–55. doi: 10.1007/s00011-019-01237-9 [DOI] [PubMed] [Google Scholar]
- 28. Ge Y, Cheng D, Jia Q, Xiong H, Zhang J. Mechanisms underlying the role of myeloid-derived suppressor cells in clinical diseases: Good or bad. Immune Netw (2021) 21(3):e21. doi: 10.4110/in.2021.21.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwak Y, Kim HE, Park SG. Insights into myeloid-derived suppressor cells in inflammatory diseases. Arch Immunol Ther Exp (Warsz). (2015) 63(4):269–85. doi: 10.1007/s00005-015-0342-1 [DOI] [PubMed] [Google Scholar]
- 30. Koushki K, Salemi M, Miri SM, Arjeini Y, Keshavarz M, Ghaemi A. Role of myeloid-derived suppressor cells in viral respiratory infections; hints for discovering therapeutic targets for COVID-19. Biomedicine Pharmacotherapy. (2021) 144:112346. doi: 10.1016/j.biopha.2021.112346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marais C, Claude C, Semaan N, Charbel R, Barreault S, Travert B, et al. Myeloid phenotypes in severe COVID-19 predict secondary infection and mortality: A pilot study. Ann Intensive Care (2021) 11(1):111. doi: 10.1186/s13613-021-00896-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donkor MK, Lahue E, Hoke TA, Shafer LR, Coskun U, Solheim JC, et al. Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int Immunopharmacol. (2009) 9(7–8):937–48. doi: 10.1016/j.intimp.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 33. Dorhoi A, Glaría E, Garcia-Tellez T, Nieuwenhuizen NE, Zelinskyy G, Favier B, et al. MDSCs in infectious diseases: regulation, roles, and readjustment. Cancer Immunol Immunother. (2019) 68(4):673–85. doi: 10.1007/s00262-018-2277-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perfilyeva YV, Ostapchuk YO, Tleulieva R, Kali A, Abdolla N, Krasnoshtanov VK, et al. Myeloid-derived suppressor cells in COVID-19: A review. Clin Immunol (2022) 238:109024. doi: 10.1016/j.clim.2022.109024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dean MJ, Ochoa JB, Sanchez-Pino M, Zabaleta J, Garai J, Del Valle L, et al. Transcriptome and functions of granulocytic myeloid-derived suppressor cells determine their association with disease severity of COVID-19. medRxiv [Preprint]. (2021) 29:2021. doi: 10.1101/2021.03.26.21254441 [DOI] [Google Scholar]
- 36. Takano T, Matsumura T, Adachi Y, Terahara K, Moriyama S, Onodera T, et al. Myeloid cell dynamics correlating with clinical outcomes of severe COVID-19 in Japan. Int Immunol (2021) 33(4):241–7. doi: 10.1093/intimm/dxab005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reizine F, Lesouhaitier M, Gregoire M, Pinceaux K, Gacouin A, Maamar A, et al. SARS-CoV-2-Induced ARDS associates with MDSC expansion, lymphocyte dysfunction, and arginine shortage. J Clin Immunol (2021) 41(3):515–25. doi: 10.1007/s10875-020-00920-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomić S, Đokić J, Stevanović D, Ilić N, Gruden-Movsesijan A, Dinić M, et al. Reduced expression of autophagy markers and expansion of myeloid-derived suppressor cells correlate with poor T cell response in severe COVID-19 patients. Front Immunol (2021) 12:614599. doi: 10.3389/fimmu.2021.614599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beliakova-Bethell N, Maruthai K, Xu R, Salvador LCM, Garg A. Monocytic-myeloid derived suppressor cells suppress T-cell responses in recovered SARS CoV2-infected individuals. Front Immunol (2022) 13:894543. doi: 10.3389/fimmu.2022.894543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bordoni V, Sacchi A, Cimini E, Notari S, Grassi G, Tartaglia E, et al. An inflammatory profile correlates with decreased frequency of cytotoxic cells in coronavirus disease 2019. Clin Infect Dis (2020) 71(16):2272–5. doi: 10.1093/cid/ciaa577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Emsen A, Sumer S, Tulek B, Cizmecioglu H, Vatansev H, Goktepe MH, et al. Correlation of myeloid-derived suppressor cells with c-reactive protein, ferritin and lactate dehydrogenase levels in patients with severe COVID-19. Scandinavian J Immunol (2022) 95(1):e13108. doi: 10.1111/sji.13108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schrijver IT, Théroude C, Antonakos N, Regina J, Le Roy D, Bart PA, et al. COVID-19 rapidly increases MDSCs and prolongs innate immune dysfunctions. Eur J Immunol (2022) 52(10):1676–9. doi: 10.1002/eji.202249827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xue G, Jiang M, Zhao R, Le A, Li J. Elevated frequencies of CD14+HLA-DRlo/neg MDSCs in COVID-19 patients. Aging. (2021) 13(5):6236–46. doi: 10.18632/aging.202571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sacchi A, Grassi G, Notari S, Gili S, Bordoni V, Tartaglia E, et al. Expansion of myeloid derived suppressor cells contributes to platelet activation by l-arginine deprivation during SARS-CoV-2 infection. Cells. (2021) 10(8):2111. doi: 10.3390/cells10082111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiménez-Cortegana C, Sánchez-Jiménez F, Pérez-Pérez A, Álvarez N, Sousa A, Cantón-Bulnes L, et al. Low levels of granulocytic myeloid-derived suppressor cells may be a good marker of survival in the follow-up of patients with severe COVID-19. Front Immunol (2021) 12:801410. doi: 10.3389/fimmu.2021.801410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li T, Zheng F, Cheng F. The function of myeloid-derived suppressor cells in COVID-19 lymphopenia. Int Immunopharmacol. (2022) 112:109277. doi: 10.1016/j.intimp.2022.109277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park SJ, Nam DE, Seong HC, Hahn YS. New discovery of myeloid-derived suppressor cell’s tale on viral infection and COVID-19. Front Immunol (2022) 13:842535. doi: 10.3389/fimmu.2022.842535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol (2009) 9(3):162–74. doi: 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res (2010) 70(11):4335–45. doi: 10.1158/0008-5472.CAN-09-3767 [DOI] [PubMed] [Google Scholar]
- 50. Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. (2010) 120(2):457–71. doi: 10.1172/JCI40483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kvedaraite E, Hertwig L, Sinha I, Ponzetta A, Hed Myrberg I, Lourda M, et al. Major alterations in the mononuclear phagocyte landscape associated with COVID-19 severity. Proc Natl Acad Sci USA. (2021) 118(6):e2018587118. doi: 10.1073/pnas.2018587118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe (2020) 27(6):992–1000. doi: 10.1016/j.chom.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet (2021) 397(10285):1637–45. doi: 10.1101/2021.02.11.21249258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. REMAP-CAP Investigators. Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med (2021) 384(16):1491–502. doi: 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med (2021) 9(5):522–32. doi: 10.1016/S2213-2600(21)00099-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ahmad SI. 5-fluorouracil in combination with deoxyribonucleosides and deoxyribose as possible therapeutic options for the coronavirus, COVID-19 infection. Med Hypotheses. (2020) 142:109754. doi: 10.1016/j.mehy.2020.109754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kloc M, Ghobrial RM, Lipińska-Opałka A, Wawrzyniak A, Zdanowski R, Kalicki B, et al. Effects of vitamin d on macrophages and myeloid-derived suppressor cells (MDSCs) hyperinflammatory response in the lungs of COVID-19 patients. Cell Immunol (2021) 360:104259. doi: 10.1016/j.cellimm.2020.104259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Linh NN, Viney K, Gegia M, Falzon D, Glaziou P, Floyd K, et al. World health organization treatment outcome definitions for tuberculosis: 2021 update. Eur Respir J (2021) 58(2):2100804. doi: 10.1183/13993003.00804-2021 [DOI] [PubMed] [Google Scholar]
- 59. John V, Kotze LA, Ribechini E, Walzl G, Du Plessis N, Lutz MB. Caveolin-1 controls vesicular TLR2 expression, p38 signaling and T cell suppression in BCG infected murine monocytic myeloid-derived suppressor cells. Front Immunol (2019) 10:2826. doi: 10.3389/fimmu.2019.02826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Agrawal N, Streata I, Pei G, Weiner J, Kotze L, Bandermann S, et al. Human monocytic suppressive cells promote replication of mycobacterium tuberculosis and alter stability of in vitro generated granulomas. Front Immunol (2018) 9:2417. doi: 10.3389/fimmu.2018.02417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Knaul JK, Jörg S, Oberbeck-Mueller D, Heinemann E, Scheuermann L, Brinkmann V, et al. Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am J Respir Crit Care Med (2014) 190(9):1053–66. doi: 10.1164/rccm.201405-0828OC [DOI] [PubMed] [Google Scholar]
- 62. Singh S, Maurya SK, Aqdas M, Bashir H, Arora A, Bhalla V, et al. Mycobacterium tuberculosis exploits MPT64 to generate myeloid-derived suppressor cells to evade the immune system. Cell Mol Life Sci (2022) 79(11):567. doi: 10.1007/s00018-022-04596-5 [DOI] [PubMed] [Google Scholar]
- 63. Dietlin TA, Hofman FM, Lund BT, Gilmore W, Stohlman SA, van der Veen RC. Mycobacteria-induced gr-1+ subsets from distinct myeloid lineages have opposite effects on T cell expansion. J Leukoc Biol (2007) 81(5):1205–12. doi: 10.1189/jlb.1006640 [DOI] [PubMed] [Google Scholar]
- 64. Ribechini E, Eckert I, Beilhack A, Du Plessis N, Walzl G, Schleicher U, et al. Heat-killed mycobacterium tuberculosis prime-boost vaccination induces myeloid-derived suppressor cells with spleen dendritic cell-killing capability. JCI Insight (2019) 5(13):e128664. doi: 10.1172/jci.insight.128664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Du Plessis N, Loebenberg L, Kriel M, von Groote-Bidlingmaier F, Ribechini E, Loxton AG, et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent Mycobacterium tuberculosis infection suppresses T-cell function. Am J Respir Crit Care Med (2013) 188(6):724–32. doi: 10.1164/rccm.201302-0249OC [DOI] [PubMed] [Google Scholar]
- 66. Rieber N, Hartl D. Eat and suppress: The two-faced role of myeloid-derived suppressor cells in tuberculosis. Am J Respir Crit Care Med (2014) 190(9):975–7. doi: 10.1164/rccm.201410-1764ED [DOI] [PubMed] [Google Scholar]
- 67. Kotze LA, Leukes VN, Fang Z, Lutz MB, Fitzgerald BL, Belisle J, et al. Evaluation of autophagy mediators in myeloid-derived suppressor cells during human tuberculosis. Cell Immunol (2021) 369:104426. doi: 10.1016/j.cellimm.2021.104426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhan X, Hu S, Wu Y, Li M, Liu T, Ming S, et al. IFN-γ decreased the suppressive function of CD33+HLA-DRlow myeloid cells through down-regulation of PD-1/PD-L2 signaling pathway. Mol Immunol (2018) 94:107–20. doi: 10.1016/j.molimm.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 69. Jøntvedt Jørgensen M, Jenum S, Tonby K, Mortensen R, Walzl G, Du Plessis N, et al. Monocytic myeloid-derived suppressor cells reflect tuberculosis severity and are influenced by cyclooxygenase-2 inhibitors. J Leukocyte Bio. (2021) 110(1):177–86. doi: 10.1002/JLB.4A0720-409RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Amiano NO, Pellegrini JM, Morelli MP, Martinena C, Rolandelli A, Castello FA, et al. Circulating monocyte-like myeloid derived suppressor cells and CD16 positive monocytes correlate with immunological responsiveness of tuberculosis patients. Front Cell Infect Microbiol (2022) 12:841741. doi: 10.3389/fcimb.2022.841741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grassi G, Vanini V, De Santis F, Romagnoli A, Aiello A, Casetti R, et al. PMN-MDSC frequency discriminates active versus latent tuberculosis and could play a role in counteracting the immune-mediated lung damage in active disease. Front Immunol (2021) 12:594376. doi: 10.3389/fimmu.2021.594376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Davids M, Pooran A, Smith L, Tomasicchio M, Dheda K. The frequency and effect of granulocytic myeloid-derived suppressor cells on mycobacterial survival in patients with tuberculosis: A preliminary report. Front Immunol (2021) 12:676679. doi: 10.3389/fimmu.2021.676679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Singh B, Singh DK, Ganatra SR, Escobedo RA, Khader S, Schlesinger LS, et al. Myeloid-derived suppressor cells mediate T cell dysfunction in nonhuman primate TB granulomas. Wormley FL editor. mBio. (2021) 12(6):e03189–21. doi: 10.1128/mbio.03189-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Leukes V, Walzl G, du Plessis N. Myeloid-derived suppressor cells as target of phosphodiesterase-5 inhibitors in host-directed therapeutics for tuberculosis. Front Immunol (2020) 11:451. doi: 10.3389/fimmu.2020.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Krug S, Parveen S, Bishai WR. Host-directed therapies: Modulating inflammation to treat tuberculosis. Front Immunol (2021) 12:660916. doi: 10.3389/fimmu.2021.660916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kroesen VM, Gröschel MI, Martinson N, Zumla A, Maeurer M, van der Werf TS, et al. Non-steroidal anti-inflammatory drugs as host-directed therapy for tuberculosis: A systematic review. Front Immunol (2017) 8:772. doi: 10.3389/fimmu.2017.00772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Leukes VN, Malherbe ST, Hiemstra A, Kotze LA, Roos K, Keyser A, et al. Sildenafil, a type-5 phosphodiesterase inhibitor, fails to reverse myeloid-derived suppressor cell-mediated T cell suppression in cells isolated from tuberculosis patients. Front Immunol (2022) 13:883886. doi: 10.3389/fimmu.2022.883886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Walker MM, Novak L, Widener R, Grubbs JA, King J, Hale JY, et al. PcP ptomotes higher levels of infection and modulates recruitment of myeloid-derived suppressor cells during modulates recruitment. J Immunol (2016) 196(5):2239–48. doi: 10.4049/jimmunol.1402518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Namkoong H, Ishii M, Fujii H, Yagi K, Asami T, Asakura T, et al. Clarithromycin expands CD11b+Gr-1+ cells via the STAT3/Bv8 axis to ameliorate lethal endotoxic shock and post-influenza bacterial pneumonia. PLoS Pathog (2018) 14(4):e1006955. doi: 10.1371/journal.ppat.1006955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rieder SA, Nagarkatti P, Nagarkatti M. Multiple anti-inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin b-induced lung injury: Therapeutic role of resveratrol in acute lung injury. Br J Pharmacol (2012) 167(6):1244–58. doi: 10.1111/j.1476-5381.2012.02063.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, Khare A, et al. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol (2013) 6(1):189–99. doi: 10.1038/mi.2012.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ahn D, Peñaloza H, Wang Z, Wickersham M, Parker D, Patel P, et al. Acquired resistance to innate immune clearance promotes klebsiella pneumoniae ST258 pulmonary infection. JCI Insight (2016) 1(17):e89704. doi: 10.1172/jci.insight.89704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wong Fok Lung T, Charytonowicz D, Beaumont KG, Shah SS, Sridhar SH, Gorrie CL, et al. Klebsiella pneumoniae induces host metabolic stress that promotes tolerance to pulmonary infection. Cell Metab (2022) 34(5):761–774.e9. doi: 10.1016/j.cmet.2022.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Öz HH, Zhou B, Voss P, Carevic M, Schroth C, Frey N, et al. Pseudomonas aeruginosa airway infection recruits and modulates neutrophilic myeloid-derived suppressor cells. Front Cell Infect Microbiol (2016) 6:167. doi: 10.3389/fcimb.2016.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang C, Lei GS, Shao S, Jung HW, Durant PJ, Lee CH. Accumulation of myeloid-derived suppressor cells in the lungs during pneumocystis pneumonia. Deepe GS editor. Infect Immun (2012) 80(10):3634–41. doi: 10.1128/IAI.00668-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lei GS, Zhang C, Shao S, Jung HW, Durant PJ, Lee CH. All-trans retinoic acid in combination with primaquine clears pneumocystis infection. Steele C editor. PLoS One (2013) 8(1):e53479. doi: 10.1371/journal.pone.0053479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lei GS, Zhang C, Lee CH. Myeloid-derived suppressor cells impair alveolar macrophages through PD-1 receptor ligation during pneumocystis pneumonia. Deepe GS editor. Infect Immun (2015) 83(2):572–82. doi: 10.1128/IAI.02686-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pang Z, Zhu Q. Traditional Chinese medicine is an alternative therapeutic option for treatment of pseudomonas aeruginosa infections. Front Pharmacol (2021) 12:737252. doi: 10.3389/fphar.2021.737252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Huang K, Zhang P, Zhang Z, Youn JY, Wang C, Zhang H, et al. Traditional Chinese medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacol Ther (2021), 225:107843. doi: 10.1016/j.pharmthera.2021.107843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ni L, Chen L, Huang X, Han C, Xu J, Zhang H, et al. Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm Sin B (2020) 10(7):1149–62. doi: 10.1016/j.apsb.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ren X, Shao XX, Li XX, Jia XH, Song T, Zhou WY, et al. Identifying potential treatments of COVID-19 from traditional Chinese medicine (TCM) by using a data-driven approach. J Ethnopharmacol (2020) 258:112932. doi: 10.1016/j.jep.2020.112932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Feng BJ. Investigation of the mechanism of coptis chinensis for the treatment of COVID-19 based on network pharmacology and molecular docking. J Jishou Univ (Natural Sci Edition) (2022) 43(5):64–73. doi: 10.13438/j.cnki.jdzk.2022.05.011 [DOI] [Google Scholar]
- 93. Liu C, Zhou Z, Yin ZG, Zhou Y. Explore mechanism of blood components lonicera japonica flos intervening COVID-19 based on network pharmacology and molecular docking technology. Drugs Clinic. (2022) 37(02):264–74. [Google Scholar]
- 94. Lin H, Wang X, Liu M, Huang M, Shen Z, Feng J, et al. Exploring the treatment of COVID-19 with yinqiao powder based on network pharmacology. Phytother Res (2021) 35(5):2651–64. doi: 10.1002/ptr.7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen X, Wu Y, Chen C, Gu Y, Zhu C, Wang S, et al. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm Sin B (2021) 11(1):222–36. doi: 10.1016/j.apsb.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res (2020) 156:104761. doi: 10.1016/j.phrs.2020.104761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sun J, Zhang Q, Yang G, Li Y, Fu Y, Zheng Y, et al. The licorice flavonoid isoliquiritigenin attenuates mycobacterium tuberculosis-induced inflammation through Notch1/NF-κB and MAPK signaling pathways. J Ethnopharmacol (2022) 294:115368. doi: 10.1016/j.jep.2022.115368 [DOI] [PubMed] [Google Scholar]
- 98. Chuang LP, Chu CM, Hu HC, Lin YK, Kao KC, Wu HP. Effects of curcuma longa l., eucommia ulmoides oliv. and gynostemma pentaphyllum (Thunb.) makino on cytokine production in stimulated peripheral blood mononuclear cells in patients with tuberculosis. Altern Ther Health Med (2022) 28(1):72–9. [PubMed] [Google Scholar]
- 99. Singh DK, Dwivedi VP, Singh SP, Kumari A, Sharma SK, Ranganathan A, et al. Luteolin-mediated Kv1.3 k+ channel inhibition augments BCG vaccine efficacy against tuberculosis by promoting central memory T cell responses in mice. PLoS Pathog (2020) 16(9):e1008887. doi: 10.1371/journal.ppat.1008887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fan X, Li N, Wang X, Zhang J, Xu M, Liu X, et al. Protective immune mechanisms of yifei tongluo, a Chinese herb formulation, in the treatment of mycobacterial infection. PLoS One (2018) 13(9):e0203678. doi: 10.1371/journal.pone.0203678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hung TC, Chen KB, Lee WY, Chen CY. The inhibition of folylpolyglutamate synthetase (folC) in the prevention of drug resistance in mycobacterium tuberculosis by traditional Chinese medicine. BioMed Res Int (2014) 2014:635152. doi: 10.1155/2014/635152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hou Y, Nie Y, Cheng B, Tao J, Ma X, Jiang M, et al. Qingfei xiaoyan wan, a traditional Chinese medicine formula, ameliorates pseudomonas aeruginosa-induced acute lung inflammation by regulation of PI3K/AKT and Ras/MAPK pathways. Acta Pharm Sin B (2016) 6(3):212–21. doi: 10.1016/j.apsb.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Luo J, Dong B, Wang K, Cai S, Liu T, Cheng X, et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS One (2017) 12(4):e0176883. doi: 10.1371/journal.pone.0176883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wei Q, Bhasme P, Wang Z, Wang L, Wang S, Zeng Y, et al. Chinese Medicinal herb extract inhibits PQS-mediated quorum sensing system in pseudomonas aeruginosa. J Ethnopharmacol (2020) 248:112272. doi: 10.1016/j.jep.2019.112272 [DOI] [PubMed] [Google Scholar]