Abstract
The low reproductive efficiency (RE) of geese limits their production in the poultry industry. To select ganders with high breeding potential, the effect of 3 sperm mobility ranks (SMRs; high-, medium-, and low-SMR) on the RE of naturally mating geese was determined. To exclude the confounding effect of social rank (SR) on RE in naturally mating flocks, a 2-factor nested experimental design was used to differentiate the effects of SMR and SR on RE. Twenty-seven ganders and 135 geese (Zi geese, Anser cygnoides L.) at approximately 1 yr of age were divided into 3 flocks, each of which included the 3 SMR groups. Each SMR group included 3 ganders and 15 female geese. Relative genetic contribution (RGC) is defined as the number of offspring sired by 1 male as a percentage of the entire goslings in each flock, and it was used to compare the differences in RE among ganders. The frequency of agonistic behavioral interactions (ABIs) among the ganders was video recorded in each SMR group, and the SR of each gander was determined. In total, 1,026 eggs were incubated, and 609 goslings hatched. Parent-offspring relationships among 771 individuals from the 2 generations were identified using 20 microsatellite markers, and the RGC was calculated. Results showed that the SMR and SR had significant effects on RGC in naturally mating geese (P = 0.001 and P = 0.000, respectively). Significant differences in RGC were observed among the high- and medium- and low-SMR groups, with average RGCs of 14.3, 10.6, and 8.4%, respectively. The high-SMR group had the highest RGCs in each flock, and the ganders with high SR had the highest RGCs among the 3 SMRs. The study showed that in a naturally mating geese population, selecting for the sperm mobility traits of a gander can effectively improve the RE.
Key words: Zi goose, sperm mobility rank, social rank, parent-offspring relationship, relative genetic contribution
INTRODUCTION
Geese have lower reproductive efficiency (RE) than other poultry species (Liu et al., 2008; Jerysz and Lukaszewicz, 2013; Zhang et al., 2021). Selecting ganders with high breeding potential is an effective approach to improving RE in geese (Gumułka and Rozenboim, 2015). Within a population, RE can be expressed by fertility and hatchability (Salamon, 2020). For individuals in a population, the relative genetic contribution (RGC) (Haberfeld et al., 1992) or paternity efficiency represents the RE (Donoghue et al., 1999).
RE measures male-to-male differences in fertility, and it is associated with interactions between sexes. Moreover, competition between individuals of the same sex is influenced by many factors, such as social rank (SR), BW, semen quality, and endocrine factors (Bilcik et al., 2005; Gumułka et al., 2021) in natural mating poultry population. Sperm quality and SR are 2 important determinants of RE in poultry (Zhang et al., 2021).
As an objective method of measuring sperm motility (Froman and Mclean, 1996), sperm mobility (SM) is an indicator of artificial insemination (AI) outcomes (Birkhead, 1998b; Birkhead et al., 1999; Manier et al., 2019). However, with natural mating, the RE of domestic fowl is not always correlated with the results obtained by AI. Bilcik et al. (2005) found that SM had no effect on roost paternity in a natural mating system. Farooq et al. (2018) found that sperm motility was partially successful in estimating fertility in Japanese quail and the correlation between sperm motility and egg fertility was affected by the genotype. In domestic Turkish geese, Boz et al. (2021) found that the semen quality factor (SQF) affected the fertility of 1- and 2-yr-old natural mating geese. In egg-laying chickens, Lin et al. (2021) confirmed that the sperm quality (sperm motility, sperm concentration, and semen volume) of males under natural mating was significantly related to the RGC. The effect of SM on RE in natural mating geese populations deserves careful study because it is the most representative indicator of semen quality (Liu et al., 2008).
Compared with AI, the SR of male birds has an important effect on RE under competitive mating conditions (Hobson and Dedeo, 2015). The frequency of aggressive behavioral interactions (ABIs) is typically used to determine the SR of chickens (Kim and Zuk, 2000). A high SR and related behaviors may increase access to limited resources, thereby increasing the likelihood of survival and reproductive success (Woog et al., 2012). Ganders with a higher SR have a higher RE in geese under competitive mating (Zhang et al., 2021).
Natural mating is the most common method for reproduction among commercial geese breeders (Gumułka and Rozenboim, 2015), and the key factors affecting individual RE have always been the focus of attention. Most studies have focused on the relationship between SM and RE under AI or harem mating (Liu et al., 2008; Łukaszewicz, 2010; Gumułka and Rozenboim, 2017) and not on natural mating. Male-to-male differences in RE have been estimated experimentally, although it is rarely performed by commercial breeders (Reddy and Sajadi, 1990). This study was performed to explore the influence of the sperm mobility rank (SMR) on the RGC in a natural mating commercial geese breeder.
MATERIALS AND METHODS
This study protocol was approved by the Ethics Committee for the Use and Care of Animals at Heilongjiang Bayi Agricultural University (Daqing, China).
Animals and Experimental Design
The experiments were conducted at the Institute of Animal Husbandry, Heilongjiang Academy of Agricultural Sciences, Qiqihar City, Heilongjiang Province, China (latitude: 47°19′; longitude: 123°45′; altitude: 155 m). The subjects were Zi geese (Anser cygnoides L., a small-type breed) that were approximately 1 yr in age, and they included 27 ganders and 135 female geese. The experiment consisted of 3 flocks. Each flock included 3 SMR groups, each with 3 ganders and 15 female geese (male:female = 1:5) (Yang et al., 2017).
Birds in flocks 1, 2, and 3 were 12-mo old. The experiments on flock 1 and flock 2 experiments were conducted from May 1st to July 1st, 2021, while those on flock 3 experiments were conducted from May 1st to July 1st, 2022.
Each group of geese was raised in a pen with a playground (0.5 geese per m2) and a no-door rain shelter (1 goose per m2). The nest was large enough (96 cm × 85 cm) to comfortably hold several standing geese and allowed them to turn around easily. At the start of the experiment, the geese were given natural light from 4:00 am to 19:30 pm. The light duration gradually changed based on natural sunshine. The ambient temperature was in the range of 15°C to 28°C. The geese were healthy, and BWs were measured using an electronic balance (Sartorius AZ212, Gottingen, Germany) with an accuracy of 0.1 g.
For the experimental diet, all geese were provided with the same formula and nutrient ingredients, as shown in Table 1. The geese had free access to food and water at all times.
Table 1.
Formula and nutrient components for geese.
| Ingredient | Content | Nutrient | Content |
|---|---|---|---|
| Corn | 61.00% | ME | 11.04 MJ/kg |
| Soybean meal | 21.00% | CP | 16.14% |
| Wheat bran | 10.00% | Crude fiber | 3.04% |
| Vegetable oil | 0.50% | NDF | 24.86% |
| Limestone powder | 3.00% | ADF | 14.35% |
| CaHPO4 | 0.23% | Ca | 0.8% |
| DL-Met | 0.27% | P | 0.5% |
| Premix compound | 4.00% | Lys | 0.8% |
| Total | 100% | Met | 0.45% |
Semen Analysis and Semen Mobility Rank Group
Semen samples were collected from each gander 3 times 20 d before mating. Eighty ganders in 2021 and 60 ganders in 2022 were preliminarily screened based on the results of semen quality analysis. A gander with white viscous semen was considered healthy and selected for subsequent analyses. SM was assessed using the Accudenz procedure, as described by Froman and McLean (1996) and Liu et al. (2023). We pipetted 300 µL of semen diluted to a standard concentration (1 × 106 sperm cells/mL) onto the surface of 3 mL of 6% Accudenz solution in a cuvette. After 5 min of incubation at 41°C, we measured the OD, which corresponds to the amount of sperm that actively penetrated the medium. Semen pipetting was performed using a positive-displacement pipette. The collection and analysis procedures were repeated 3 times for each male.
The ganders were further divided into high-, medium-, and low-SMR groups based on the SM in each flock. High- and low-SMR ganders were those whose sperm mobility ranked 1 standard deviation above or below the average mobility, respectively. Finally, 18 ganders in 2021 and 9 ganders in 2022 were selected for the experimental groups. The semen volume (SV), sperm concentration (SC), and SM of each gander are shown in Table 2.
Table 2.
Sperm mobility rank, semen volume, sperm concentration, sperm mobility, agonistic behavioral interactions, social rank, body weight, and relative genetic contribution in the 3 flocks ().
| Flock | SMR | ID1 | SV (mL) | SC (1 × 108/mL) | SM (absorbance units) | ABI (times/d) | SR2 | BW (kg) | RGC3 (%) |
|---|---|---|---|---|---|---|---|---|---|
| Flock 1 | High | 1-1-1 | 0.18± 0.03 | 5.48 ± 0.20 | 0.47 ± 0.02 | 0.94 ± 0.33 | Low | 4.05 | 5.45 |
| 1-1-2 | 0.23 ± 0.04 | 4.41 ± 0.05 | 0.42 ± 0.05 | 2.49 ± 0.77 | Medium | 5.40 | 19.09 | ||
| 1-1-3 | 0.23 ± 0.03 | 5.48 ± 0.08 | 0.42 ± 0.01 | 3.40 ± 0.86 | High | 4.85 | 22.27 | ||
| Medium | 1-2-1 | 0.24 ± 0.04 | 6.40 ± 0.25 | 0.28 ± 0.02 | 0.90 ± 0.31 | Low | 4.25 | 5.45 | |
| 1-2-2 | 0.34 ± 0.01 | 6.61 ± 0.52 | 0.26 ± 0.02 | 3.00 ± 0.59 | High | 4.95 | 17.27 | ||
| 1-2-3 | 0.30 ± 0.01 | 3.87 ± 0.41 | 0.22 ± 0.03 | 1.18 ± 0.49 | Medium | 3.90 | 5.45 | ||
| Low | 1-3-1 | 0.31 ± 0.01 | 5.90 ± 0.51 | 0.15 ± 0.02 | 1.50 ± 0.59 | Low | 4.30 | 4.09 | |
| 1-3-2 | 0.30 ± 0.01 | 5.28 ± 0.52 | 0.14 ± 0.02 | 3.18 ± 0.90 | High | 4.85 | 11.82 | ||
| 1-3-3 | 0.39 ± 0.01 | 4.42 ± 0.51 | 0.11 ± 0.03 | 2.27 ± 0.63 | Medium | 4.55 | 9.09 | ||
| Flock 2 | High | 2-1-1 | 0.32 ± 0.02 | 2.96 ± 0.07 | 0.31 ± 0.01 | 7.08 ± 2.04 | High | 3.65 | 21.82 |
| 2-1-2 | 0.23 ± 0.03 | 7.41 ± 0.50 | 0.31 ± 0.01 | 2.06 ± 0.38 | Low | 4.35 | 8.48 | ||
| 2-1-3 | 0.24 ± 0.03 | 6.78 ± 0.43 | 0.30 ± 0.03 | 4.01 ± 0.86 | Medium | 3.70 | 16.97 | ||
| Medium | 2-2-1 | 0.23 ± 0.01 | 6.16 ± 0.39 | 0.22 ± 0.01 | 1.17 ± 0.46 | Low | 4.10 | 7.27 | |
| 2-2-2 | 0.31 ± 0.01 | 5.13 ± 0.37 | 0.21 ± 0.01 | 2.75 ± 0.80 | Medium | 4.10 | 10.91 | ||
| 2-2-3 | 0.34 ± 0.01 | 4.67 ± 0.37 | 0.19 ± 0.02 | 4.42 ± 1.04 | High | 4.20 | 16.36 | ||
| Low | 2-3-1 | 0.10 ± 0.01 | 8.12 ± 0.50 | 0.10 ± 0.02 | 6.55 ± 1.58 | High | 3.70 | 10.30 | |
| 2-3-2 | 0.19 ± 0.01 | 6.15 ± 0.45 | 0.08 ± 0.01 | 4.18 ± 1.44 | Medium | 3.60 | 2.42 | ||
| 2-3-3 | 0.30 ± 0.02 | 4.90 ± 0.50 | 0.06 ± 0.01 | 1.09 ± 0.53 | Low | 3.40 | 5.45 | ||
| Flock 3 | High | 3-1-1 | 0.14 ± 0.03 | 6.15 ± 0.35 | 0.45 ± 0.02 | 2.24 ± 0.72 | Medium | 4.15 | 14.29 |
| 3-1-2 | 0.20 ± 0.04 | 6.26 ± 0.61 | 0.40 ± 0.02 | 3.18 ± 1.10 | High | 4.15 | 14.73 | ||
| 3-1-3 | 0.18 ± 0.01 | 7.13 ± 0.35 | 0.44 ± 0.04 | 1.24 ± 0.29 | Low | 4.10 | 5.36 | ||
| Medium | 3-2-1 | 0.31 ± 0.01 | 3.88 ± 0.15 | 0.23 ± 0.03 | 2.62 ±0.26 | Medium | 4.15 | 8.93 | |
| 3-2-2 | 0.34 ± 0.01 | 5.14 ± 0.55 | 0.24 ± 0.01 | 3.51 ± 0.39 | High | 4.05 | 17.41 | ||
| 3-2-3 | 0.32 ± 0.01 | 5.48 ± 0.50 | 0.26 ± 0.03 | 1.93 ± 0.28 | Low | 4.55 | 6.70 | ||
| Low | 3-3-1 | 0.17 ± 0.03 | 8.01 ± 0.19 | 0.16 ± 0.01 | 2.76 + 0.62 | Low | 4.75 | 6.25 | |
| 3-3-2 | 0.21 ± 0.02 | 6.27 ± 0.28 | 0.08 ± 0.02 | 3.62 ± 0.37 | Medium | 4.25 | 12.50 | ||
| 3-3-3 | 0.16 ± 0.01 | 6.72 ± 0.65 | 0.07 ± 0.01 | 5.93 ± 1.08 | High | 4.15 | 13.84 |
Abbreviations: ABI, agonistic behavioral interactions; ID, identification of gander; RGC, relative genetic contribution; SM, sperm mobility; SMR, sperm motility rank; SR, social rank.
Gander ID; for example, “1-2-3” indicates the third gander from the medium SMR of flock 1.
Social rank was determined by the ABI value for 3 ganders in each SMR group, and significant differences were observed in the ABI frequency among the SR groups.
The relative genetic contribution was the number of offspring sired by a gander as a percentage of the total number of goslings from a certain flock.
Ninety female geese of similar age, weight, and health status were selected from a flock of 350 female geese in 2021, and 45 identical female geese were selected from a flock of 200 female geese in 2022.
Recording of Behaviors and Social Rank
A video recording system (720p, Haikang Weishi, Jiangsu, China) was installed at the 4 corners of each experimental pen. The ABI frequency of ganders was recorded from 4:00 am to 19:30 pm at the beginning of the experiment on 21 May, and this period was gradually extended based on the sunshine duration until the end of the observation period on 3 June. The observation period was 14 d.
Different paint colors were used to mark the necks and backs of the ganders to distinguish and observe the individual behaviors. Flocks were named 1, 2, and 3, SMRs were named 1, 2, and 3, and ganders from the same group were numbered 1, 2, and 3 to obtain individual IDs. For example, the third generator from the medium-SMR group of flock 1 was named 1-2-3. The IDs of all ganders are listed in Table 2.
The frequency of ABI between the ganders in each SMR group was observed to determine the SR (Kim and Zuk, 2000). The SR values were classified as high, medium, or low based on at least 10 ABI per pair (SRs for ganders in each group are shown in Table 2).
Paternity Test and Relative Genetic Contribution
Eggs were collected daily, and the number of eggs collected in each group in each flock was equal to eliminate the effects of differences in incubated eggs on the RGCs in a flock. In total, 1,026 eggs were collected from the 9 SMR groups (417, 309, and 300 eggs in flocks 1, 2, and 3, respectively) and stored at 12°C and a relative humidity of 69%. Eggs hatched every 8 d. After 30 d of incubation, 609 goslings hatched in 9 SMR groups, resulting in 103, 62, and 55 goslings in the 3 SMRs of flock 1; 78, 57, and 30 goslings in the 3 SMRs of flock 2; and 77, 74, and 73 goslings in the 3 SMRs of flock 3.
Blood samples were collected from the parents and offspring and then analyzed using the TIANamp Blood DNA Kit (DP348; Tiangen, Beijing, China). DNA samples were marked to match the blood samples. Next, 2 µL of each DNA sample was added to 8 µL loading buffer (RT201, Tiangen). The samples were analyzed spectrophotometrically using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, New York, NY), and good quality was considered when the OD 260/280 ratio was 1.8 to 2.0. The DNA sample was diluted with double-distilled water (ddH2O) to obtain a concentration of 20 to 50 ng/µL for amplification.
DNA samples were used to evaluate the 20 microsatellite markers previously used for goose paternity testing (Zhang et al., 2021) using PCR (T100, Bio-Rad Laboratories Inc., Hercules, CA) (Table 3). The improved PCR amplification system (25 μL) based on Zhang et al. (2021) included 1 μL of forward primer (10 μM), 1 μL of reverse primer (10 μM), 1 μL of dNTP (mix) (10 μM), 2.5 μL of 10 × Taq Buffer (with MgCl2), 0.5 μL of Taq DNA polymerase (5 U/μL), 1 μL of template DNA (20–50 ng/μL), and ddH2O to 25 μL. The PCR conditions were as follows: predenaturation (95°C) for 3 min; denaturation for 10 cycles (denaturation (94°C) for 30 s, annealing (60°C) for 30 s, and extension (72°C) for 30 s); followed by 35 cycles (denaturation (94°C) for 30 s, annealing (55°C) for 30 s, and extension (72°C) for 30 s); and a final extension (72°C) for 7 min. Samples were stored at 4°C for no more than 2 d prior to sequencing. After mixing 990 μL HIDI and 10 μL LIZ500, the mixture was added to a 96-well reaction plate at 10 μL per well and centrifuged at 225 × g for 15 s. Then, 1 μL of each PCR sample was added to the corresponding wells of a 96-well plate and centrifuged at 225 × g for 15 s. The 96-well plate was sealed with sealing film, vortexed, and centrifuged at 225 × g for 30 s. The 96-well reaction plate was denatured at 98°C for 5 min, without heating the lid. After the program ended, the 96-well plate was placed in an ice-water mixture and cooled immediately. The 96-well plate was centrifuged at 225 × g for 15 s. These samples were named “STR samples” and detected using a DNA automatic sequencer (ABI3730; Applied Biosystems Inc., Boston, MA).
Table 3.
Microsatellite markers and primers used to identify parent-offspring relationships in Zi geese.
| Marker | Primer sequence (5′–3′) | Fragment (size/bp) |
|---|---|---|
| ans 2 | F: TTCTGTGCAGGGGCGAGTT R: AGGGAACCGATCACGACATG |
215 |
| ckw 11 | F: CTGAGTTGAACCTGATGCAGAC R: AACACCAAAGGAGAGCAGAGAC |
186 |
| ckw 12 | F: CATAAGTTCTCCCAAACAAGAGTG R: AGAAAGGGACACACAGCTAACC |
204 |
| ckw 21 | F: CAAGGTAGTCATAAACCCAGAAC R: AACAAAACTAATGGCAGGAAA |
351 |
| ckw 49 | F: TGAACACACATGCAGACTGG R: TTTGCGAGACAGAGCCTTTT |
204 |
| zaas 001 | F: TCTTCATGGTCTTTGGCAGA R: TTTTCGCAGATTGTGTGGAG |
172 |
| zaas 006 | F: GACACACTGCTGCCACTTTC R: TCCAGTGCGTAAGTGCTGTT |
197 |
| zaas 033 | F: GTTTGTGCCCTGCATTTGTA R: GGTGGGAGGTTTTGGAGAGT |
202 |
| zaas 036 | F: TCCCAGCTTCACTCCTTTTC R: GTGGTGTTCTTGCGGTGTAG |
199 |
| zaas 054 | F: CCCCACACCCCCAAAATA R: AAGGCTAGTTTGCCACAGGA |
176 |
| zaas 060 | F: GAATACAGCCCTGCATTGAAA R: TCTCCCACCAGTCCTCTGTT |
197 |
| zaas 061 | F: CTCCACAGCAGCAAGGTAGC R: CGTTAGCATCTCCCACATTG |
168 |
| zaas 071 | F: TGTAACTGCCATCCCAAACA R: AGGGAGTGACAGTGTAGGTGGT |
198 |
| zaas 144 | F: GGCCACATTCACTCGTCTTT R: TAGCACCTCGGAGGGTCA |
199 |
| zaas 154 | F: CGTGTCATGCTCAAAAGGAG R: TGCTTCTCATGGAAACAACG |
215 |
| zaas 169 | F: CGTGTCATGCTCAAAAGGAG R: TGCTTCTCATGGAAACAACG |
200 |
| zaas 175 | F: TGAGTAGTGGGGTCCCTGAA R: CCCATCTGCTAGTCCAGCTT |
203 |
| zaas 177 | F: GAAAGCTCCCTGATGCTGTT R: CCTCCATGGGAATGTTTTCT |
206 |
| zaas 178 | F: GTCATATGGCCTGCACTGTC R: TGGTCACTGTGCTTGACTTTG |
208 |
| zaas 181 | F: CAGAACAGGGAAGGGATGTG R: TTCACATCTGCAGGTTCAGG |
180 |
Gene Marker V1.91 (Beijing Huasheng Hengye Technology Co. Ltd., Beijing, China) was used to determine the microsatellite marker genotype of each sample. The STR genotype results were determined using Servus 3.0.7 (Amirian et al., 2019). The number of alleles per locus varied from 5 to 13, with a mean value of 7.05. The expected heterozygosity ranged from 0.437 to 0.803 (mean 0.612), and the total exclusion probability of the 20 microsatellite loci was 0.9958.
The number of hatched samples for each gander was recorded, and the individual RGC values were calculated as follows (Donoghue et al., 1999; Ely et al., 2017):
RGC (%) = number of offspring sired by a gander/total number of goslings from a certain flock × 100.
Statistical Methods
The SM and ABI frequencies for each gander were evaluated using the Kolmogorov-Smirnov test and Levene test for homogeneity of variances. A 1-way ANOVA of the SM and ABI frequency was performed. ABIs were classified as 1 (high social ranking), 2 (medium social ranking), and 3 (low social ranking) in each SMR group to evaluate the SR, and SM was classified as 1 (high-SM group), 2 (medium-SM group), and 3 (low-SM group) to evaluate the SMR.
Using the Kruskal-Wallis test (ɑ = 0.05), we tested the differences in ABI between the 3 SR groups.
The effects of SR and SMR on the RGC were analyzed using a 2-factor nested experimental design with the following formula:
We executed a Pearson partial correlation analysis to determine the potential relationships between BW, SV, SC, and RGC; and those of SM with BW, SV, SC, ABI. All data are shown as means ± SEM.
The data were analyzed using SPSS Statistics 26 (SPSS Inc., Chicago, IL), and P < 0.05 indicated a significant difference.
RESULTS
Relative Genetic Contribution of Different Sperm Mobility Ranks
The SM values of the ganders are presented in Table 2. SM differed significantly among the SMR groups (P = 0.000). The SM in the high-SMR ganders (0.39 ± 0.02) was higher than the estimates for the medium-SMR (0.23 ± 0.01) and low-SMR ganders (0.11 ± 0.01).
The RGCs of each gander are listed in Table 2. The SMR had a significant effect on RGCs (F = 10.084, P = 0.001). In particular, the RGC was higher for the high-SMR group (14.27 ± 2.18%) than for the medium-SMR (10.64 ± 1.69%) and low-SMR groups (8.42 ± 1.34%) (Table 4).
Table 4.
Nested ANOVA results for sperm mobility rank, social rank, and relative genetic contribution.
| Parameter | High | Medium | Low | P |
|---|---|---|---|---|
| SMR | 14.27 ± 2.18a | 10.64 ± 1.69b | 8.42 ±1.34b | 0.001 |
| SR | 16.20 ± 1.36a | 11.07 ± 1.77b | 6.06 ± 0.43c | 0.000 |
| Year | 0.998 | |||
| Model fit statistics | ||||
| R2 | 0.841 | |||
| Adj-R2 | 0.757 |
Abbreviations: RGC, relative genetic contribution (%); SMR, sperm mobility rank; SR, social rank.
Values within a row with different superscripts differ significantly at P < 0.05.
Agonistic Behavioral Interactions and Social Rank
The ABI frequencies and SRs of different ganders from the same flock are shown in Table 2. The Kruskal-Wallis test revealed a significant difference in the ABI frequency among the different SR groups (P = 0.000). The ABI frequency of the high-SR ganders (4.47 ± 0.54 times/d) was higher than that of the medium-SR (2.82 ± 0.32 times/d) and low-SR ganders (1.51 ± 0.21 times/d).
Relative Genetic Contribution and Social Rank
The RGCs of different ganders with different SRs are listed in Table 2. The RGCs differed significantly among the SRs (F = 11.667, P = 0.000). Significant differences were observed among the RGCs of the high-, medium-, and low-SR ganders, which were 16.20, 11.07, and 6.06%, respectively (Table 4).
Body Weight, Semen Volume, Sperm Concentration, and Relative Genetic Contribution
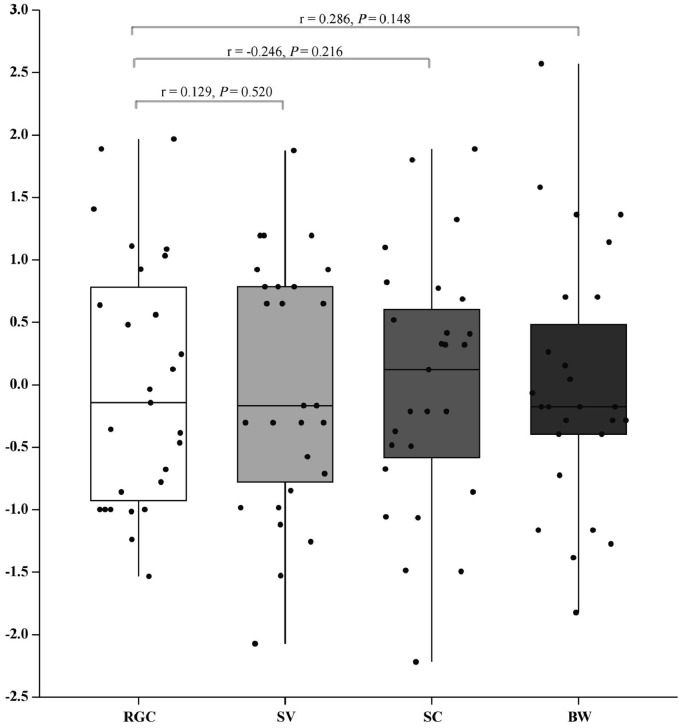
The BW, SV, and SC of the ganders are summarized in Table 2. Significant correlations were not observed between BW and RGC (P = 0.148), SV and RGC (P = 0.520), and SC and RGC (P = 0.216) (Figure 1).
Figure 1.
Pearson's partial correlation relationships among SV, SC, BW, and RGC. All data were normalized using the Z-score method. Abbreviations: BW, body weight; RGC, relative genetic contribution; SC, sperm concentration; SV, semen volume.
Association of Agonistic Behavioral Interactions and Semen Volume, Sperm Concentration, Body Weight, and Sperm Mobility
The ganders’ ABI data are summarized in Table 2. SM was not significantly correlated with ABI (P = 0.168), SV (P = 0.310), SC (P = 0.780), and BW (P = 0.202).
DISCUSSION
Sperm Mobility Rank on Relative Genetic Contribution
This is the first study to compare the RGCs of different SMRs in male geese under natural mating farm conditions, and it considers both reproductive performance and determines paternity using genetic markers. Bilcik et al. (2005) found that SM had no effect on roost paternity efficiency in a natural mating system in broiler breeders. Contrary to their results, we found that SM was significantly correlated with the RGC of ganders. There are 2 possible reasons for these conflicting results.
First, the different mating systems between chickens and geese are the main reason. Chickens are raised in a multimale and multifemale mating system (polygamy) (Pizzari et al., 2002), and females tend to mate with multiple males under natural mating conditions. Bilcik et al. (2005) observed that a high proportion of females (72%) had offspring sired by at least 2 of the 3 males in their group; thus, sperm competition within the oviduct (Birkhead, 1998a) may contribute to differential male RGCs. However, after paternity identification, we found that domestic geese share typical traits with wild species in which fixed pair bonds are common (Hirschenhauser et al., 2000; Poisbleau et al., 2005; Gumułka and Rozenboim, 2017; Kozak, 2019; Zhang et al., 2019), only 23% of females mate with 2 or more ganders (polyandry), 12% geese are monogamous, and 65% of females are polygamous and choose regular ganders to mate with (Wang et al., unpublished data). In this case, most geese do not have intense sperm competition within the oviduct, as observed in broilers. This is the reason the total RGC of the high SMR group had the highest RGC in each flock (Table 2).
Second, the accuracy of paternity testing may influence the accuracy of RGC assessments. Bilcik and Estevez (2005) determined the paternity of 83.0% of hatchlings, while our trials determined paternity for nearly 100% of hatchlings in the case of competitive mating.
Social Rank on Relative Genetic Contribution
Animal behavior is difficult to observe clearly in poultry groups under natural mating conditions. To reduce errors in the measurements of behavior, we designed replicates with 3 ganders in each SMR group.
SR can affect goose mating behavior, mating frequency, and effective mating times (Zhang et al., 2021), which directly affect the RGC in males with different SRs. From the RGC data shown in Table 2, we can derive the RGCs of high-ranking males within each flock. The RGCs of ganders with a high SR in their own flock were 51.36, 48.48, and 45.98% in the 3 flocks (average RGC, 48.61%), thus showing that a few males with a high SR contribute substantially to the reproductive efficiency of a flock. Therefore, the difference in RGCs among ganders in the SM group was mainly explained by SR.
SR has an important effect on RGC under competitive mating conditions. To exclude the effect of SR on the RGC of males within a flock, we adopted a 2-factor nested design. Jones and Mench (1991) found that the behaviors of different SR roosters were correlated with male mating success within a multisire flock and the frequencies of completed mating were positively correlated with fertility (P < 0.01). Zhang et al. (2021) found that the SR of ganders was significantly correlated with the frequency of successful copulation in a competitive mating system in geese and directly influenced paternity. Our results were consistent with these previous findings.
RE is the primary factor affecting geese productivity, and SMR and SR significantly affected the RE of naturally mating geese flocks. In commercial production, SM and aggressiveness should be considered in comprehensive evaluations of reproductive potential to improve RE. An individual's SR varies depending on its population, and SM is independent of time (Holsberger et al., 1998), therefore, SM is a reliable indicator of reproductive potential and can be selected before mating.
Body Weight, Semen Volume, Sperm Concentration, and Relative Genetic Contribution
BW differences will affect the aggressiveness among individuals, thus affecting SR and RE. In this study, to reduce the influence of BW on RE, individuals in each flock with small body weight differences were selected as much as possible when selecting experimental ganders, which may be the reason for the insignificant influence of BW on RE.
Boz et al. (2021) compared the effects of natural mating on the reproductive traits of 1- and 2-yr-old domestic Turkish geese and found that SV, SC, and fertility were not correlated; these results were similar to our results. Boz et al. (2021) also found that SQF is related to fertility because the SQF per ejaculation is calculated as follows: SQF = ejaculate volume (mL) × sperm concentration (×108/mL) × live and morphologically normal spermatozoa (%) (Liu et al., 2008). Moreover, SQF represents the total number of live sperm with a normal morphology in 1 ejaculation. Morphologically normal sperm is positively correlated with most sperm motility or mobility measures, and normally shaped sperm are motile, fast, and linear (Love, 2011). The mobility of sperm is determined by the motile sperm concentration and straight-line velocity (Froman and Feltmann, 2000). Although Boz et al. (2021) did not measure SM, we speculate that an intrinsic association occurs between the SQF and SM. This inference must be confirmed through further tests in geese.
Association of Agonistic Behavioral Interactions and Semen Volume, Sperm Concentration, Body Weight, and Sperm Mobility
The SM of ganders were measured individually using individual single pens before mixing male and female geese so that SM was not affected by ABI, which may be the reason why there was no significant correlation between SM and ABI. SV, SC, and SM indicate semen quality, and no significant correlations between SV, SC, and SM have been reported previously, thus the results of the present study are consistent with those of previous research (Froman et al., 2003). Ganders of uniform BW were selected in the experiment, thus the effect of BW on SM was not significant (Zhang et al., 2021).
CONCLUSIONS
The selection of male geese with high SM is important for improving the RE of goose flocks, and ganders with high SM can increase their RGC. This may lead to an increase in the RE of geese in commercial breeding.
ACKNOWLEDGMENTS
The authors thank Professor Zhiquan Wang of the University of Alberta, Canada, for his suggestions on the wording of this manuscript.
Funding: This work was supported by the Heilongjiang Provincial Natural Science Foundation Project [grant number LH2019C048], the earmarked fund for CARS (CARS-42-34), Heilongjiang Province construction project of corn-goose breeding ecological agriculture discipline support platform (2022), and the Research Project of Heilongjiang Bayi Agricultural University [No. ZDZX202103], Graduate Innovative Research Project of Heilongjiang Bayi Agriculture University [grant number YJSCX2021-Y03].
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Amirian A., Zafari Z., Sharifi Z., Kordafshari A., Karimipoor M., Zeinali S. Characterization and haplotype study of 6 novel STR markers related to the KCNQ1 gene in heterogeneous cardiovascular disorders in the Iranian population. Turk. J. Med. Sci. 2019;49:453–457. doi: 10.3906/sag-1805-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilcik B., Estevez I. Impact of male-male competition and morphological traits on mating strategies and reproductive success in broiler breeders. Appl. Anim. Behav. Sci. 2005;92:307–323. [Google Scholar]
- Bilcik B., Estevez I., Russek-Cohen E. Reproductive success of broiler breeders in natural mating systems: the effect of male-male competition, sperm quality, and morphological characteristics. Poult. Sci. 2005;84:1453–1462. doi: 10.1093/ps/84.9.1453. [DOI] [PubMed] [Google Scholar]
- Birkhead T.R. Sperm competition in birds. Rev. Reprod. 1998;3:123–129. doi: 10.1530/ror.0.0030123. [DOI] [PubMed] [Google Scholar]
- Birkhead T.R. Cryptic female choice: criteria for establishing female sperm choice. Evoluation. 1998;52:1212–1218. doi: 10.1111/j.1558-5646.1998.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Birkhead T.R., Martínez J.G., Burke T., Froman D.P. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. Biol. Sci. 1999;266:1759–1764. doi: 10.1098/rspb.1999.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boz M.A., Baş H., Sarica M., Erensoy K. The effects of natural mating and artificial insemination on reproductive traits of 1-and 2-year-old domestic Turkish geese. Vet. Res. Commun. 2021;45:211–221. doi: 10.1007/s11259-021-09802-6. [DOI] [PubMed] [Google Scholar]
- Donoghue A.M., Bakst M.R., Drummond P., Haqque S., Smith E.J., Donoghue D.J. Paternity efficiency in turkey differs extensively after heterospermic insemination. J. Appl. Poult. Res. 1999;8:214–221. [Google Scholar]
- Ely C.R., Wilson R.E., Talbot S.L. Genetic structure among greater white-fronted goose populations of the pacific flyway. Ecol. Evol. 2017;7:2956–2968. doi: 10.1002/ece3.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq U., Malecki I.A., Mahmood M., Martin G.B. Correlation between objective semen analysis and fertility in Japanese quail. Theriogenology. 2018;115:23–29. doi: 10.1016/j.theriogenology.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Froman D.P., Bowling E.R., Wilsont J.L. Sperm mobility phenotype not determined by sperm quality index. Poult. Sci. 2003;82:496–502. doi: 10.1093/ps/82.3.496. [DOI] [PubMed] [Google Scholar]
- Froman D.P., Feltmann A.J. Sperm mobility: phenotype in roosters (Gallus domesticus) determined by concentration of motile sperm and straight line velocity. Biol. Reprod. 2000;62:303–309. doi: 10.1095/biolreprod62.2.303. [DOI] [PubMed] [Google Scholar]
- Froman D.P., Mclean D.J. Objective measurement of sperm motility based upon sperm penetration of Accudenz. Poult. Sci. 1996;75:776–784. doi: 10.3382/ps.0750776. [DOI] [PubMed] [Google Scholar]
- Gumułka M., Avital-Cohen N., Rozenboim I. Determination of annual plasma hormone levels associated with reproduction in long-day breeding domestic geese. Animal. 2021;11:2363. doi: 10.3390/ani11082363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumułka M., Rozenboim I. Breeding period-associated changes in semen quality, concentrations of LH, PRL, gonadal steroid and thyroid hormones in domestic goose ganders (Anser anser f. domesticus) Anim. Reprod. Sci. 2015;154:166–175. doi: 10.1016/j.anireprosci.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Gumułka M., Rozenboim I. Effect of the age of ganders on reproductive behavior and fertility in a competitive mating structure. Ann. Anim. Sci. 2017;17:733–746. [Google Scholar]
- Haberfeld A., Dunnington E.A., Siegel P.B. Genetic distances estimated from DNA fingerprints in crosses of White Plymouth Rock chickens. Anim. Genet. 1992;23:167–173. doi: 10.1111/j.1365-2052.1992.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K., Möstl E., Péczely P., Wallner B., Dittami J., Kotrschal K. Seasonal relationships between plasma and fecal testosterone in response to GnRH in domestic ganders. Gen. Comp. Endocrinol. 2000;118:262–272. doi: 10.1006/gcen.2000.7463. [DOI] [PubMed] [Google Scholar]
- Hobson E.A., DeDeo S. Social feedback and the emergence of rank in animal society. PLoS Comput. Biol. 2015;11 doi: 10.1371/journal.pcbi.1004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsberger D.R., Donoghue A.M., Froman D.P., Ottinger M.A. Assessment of ejaculate quality and sperm characteristics in turkeys: sperm mobility phenotype is independent of time. Poult. Sci. 1998;77:1711–1717. doi: 10.1093/ps/77.11.1711. [DOI] [PubMed] [Google Scholar]
- Jerysz A., Łukaszewicz E. Effect of dietary selenium and vitamin E on ganders' response to semen collection and ejaculate characteristics. Biol. Trace Elem. Res. 2013;153:196–204. doi: 10.1007/s12011-013-9652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.E., Mench J.A. Behavioral correlates of male mating success in a multisire flock as determined by DNA fingerprinting. Poult. Sci. 1991;70:1493–1498. doi: 10.3382/ps.0701493. [DOI] [PubMed] [Google Scholar]
- Kim T., Zuk M. The effects of age and previous experience on social rank in female red junglefowl, Gallus gallus spadiceus. Anim. Behav. 2000;60:239–244. doi: 10.1006/anbe.2000.1469. [DOI] [PubMed] [Google Scholar]
- Kozak J. Variations of geese under domestication. World's Poult. Sci. J. 2019;75:247–259. [Google Scholar]
- Lin X., Lan F., Sun C., Yang N., Li J. Investigation on indexes characteristics related to genetic contribution of cocks and ratio of cocks and hens in colony cages. Chin. J. Anim. Sci. 2021;57:79–84. (in Chinese) [Google Scholar]
- Liu Y., Zhang J.Y., Gao Z.H., Wang J.D., Hao H.R., Song J.T., Wang X., Ma Z.G., Chen Z.F., Liu S.J. Relationship between sperm mobility and sperm motility parameters in geese and their effects on reproductive efficiency in natural mating geese. Chin. J. Anim. Sci. 2023;59:193–198. (in Chinese) [Google Scholar]
- Liu S.J., Zheng J.X., Yang N. Semen quality factor as an indicator of fertilizing ability for geese. Poult. Sci. 2008;87:155–159. doi: 10.3382/ps.2007-00300. [DOI] [PubMed] [Google Scholar]
- Love C.C. Relationship between sperm motility, morphology and the fertility of stallions. Theriogenology. 2011;76:547–557. doi: 10.1016/j.theriogenology.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Łukaszewicz E. Artificial insemination in geese. World's Poult. Sci. J. 2010;66:647–658. [Google Scholar]
- Manier M.K., Welch G., Van N.C., Bakst M.R., Long J. Low-mobility sperm phenotype in the domestic turkey: impact on sperm morphometry and early embryonic death. Reprod. Domest. Anim. 2019;54:613–621. doi: 10.1111/rda.13403. [DOI] [PubMed] [Google Scholar]
- Pizzari T., Froman D.P., Birkhead T.R. Pre- and post-insemination episodes of sexual selection in the fowl, Gallus g. domesticus. Heredity. 2002;88:112–116. doi: 10.1038/sj.hdy.6800014. [DOI] [PubMed] [Google Scholar]
- Poisbleau M., Fritz H., Guillon N., Chastel O. Linear social dominance hierarchy and corticosterone responses in male mallards and pintails. Horm. Behav. 2005;47:485–492. doi: 10.1016/j.yhbeh.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Reddy R.P.K., Sajadi R. In: Pages 47–59 in Control of Fertility in Domestic Birds. Brillard J.P., editor. INRA; Paris, France: 1990. Selection for growth and semen traits in the poultry industry: what can we expect in the future? [Google Scholar]
- Salamon A. Fertility and hatchability in goose eggs: a review. Int. J. Poult. Sci. 2020;19:51–65. [Google Scholar]
- Woog F., Schwarz K., Hulme M. All you can eat: do peck rates of Greylag Geese (Anser anser) vary with age and social status? J. Ornithol. 2012;153:1025–1029. [Google Scholar]
- Yang H.M., Wang Y., Wang Z.Y., Wang X.X. Seasonal and photoperiodic regulation of reproductive hormones and related genes in Yangzhou geese. Poult. Sci. 2017;96:486–490. doi: 10.3382/ps/pew340. [DOI] [PubMed] [Google Scholar]
- Zhang J.Y., Liu Y., Li E., Ning B.L., Zhang Y., Sun J.Y., Liu G.J., Liu S.J. The association of social rank with paternity efficiency in competitive mating flocks of Zi goose ganders (Anser cygnoides L.) Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yao Y., Wang M.M., Yang Y.Z., Gu T.T., Cao Z.F., Lu L., An C., Wang J.W., Chen G.H., Xu Q., Zhao W.M. Comparisons in geese of the courtship, mating behaviors and fertility of the Carlos and Sichuan breeds and the breed crosses. Anim. Reprod. Sci. 2019;204:86–94. doi: 10.1016/j.anireprosci.2019.03.008. [DOI] [PubMed] [Google Scholar]