Abstract
This study offered a possible systematic culinary approach to spent-laying ducks. Breast meat is suitable for processing due to its amount and completeness. Sous-vide cooking resulted in lower cooking loss than poaching, pan-frying (P < 0.05), and roasting. The sous-vide duck breast had higher gumminess, chewiness, and resilience than other culinary techniques (P < 0.05). Sous-vide cooking at 65°C had a lower cooking loss than 70°C (P < 0.05), and less than 1.5-h sous-vide could keep a lower cooking loss and WB shear value (P < 0.05) as the cooking period extended, the smaller (P < 0.05) quantity of myosin heavy chain and the destroyed sarcomere arrangement were observed. A condition at 65°C for 1.5 h could be the optimal sous-vide cuisine for spent-laying duck breast. These sous-vide products stored at 4°C were still safe for consumption due to no detectible microorganisms and unchangeable physicochemical properties within 7 d.
Key words: sous-vide condition, laying spent duck, breast, myofibrillar degradation, quality change of storage
GRAPHICAL ABSTRACT
INTRODUCTION
Because of the trend toward fitness and a healthy diet, the production and consumption of poultry meat steadily increase (Mottet and Tempio, 2017; OCED, 2020). According to a projection from OECD (2020), poultry meat production and consumption per capita will increase by 145 million tons and 70.3 kg in 2029 compared to 124 million tons and 69.3 kg on average in 2017 to 2019, respectively. Besides chicken production, a considerable increase in duck meat production enhanced approximately 1.6-folds from 1997 (2.5 million tons) to 2019 (4.6 million tons); meanwhile, more than 80% of production was calculated in Asia alone in 2017 (FAOSTAT, 2022). Compared with chicken meat, duck meat contains high levels of phospholipid/unsaturated fatty acids and heme iron, similarly characterized as those in red meat and marine meat (Baeza, 2006). The stronger flavor of duck meat than that of chicken meat results from the higher lipid contents (Biswas et al., 2019), so it may limit duck consumption as a replacement for chicken dependent on the dietary culture. According to the statistical result from the Council of Agriculture, Executive Yuan, Taiwan (2022), there were 400 laying duck farms in Taiwan, while the total number of raising ducks was up to 2.23 million in 2019. Laying ducks are weeded out at the age of 2 to 3 yr due to the poor duck-egg production efficacy, and it is estimated that there are at least 150,000 spent laying ducks yielded per year in Taiwan. Ordinarily, the usages of spent laying ducks are deficient, and only raw material of some traditional Taiwanese cuisines, such as ginger duck stew and duck essence, resulting from its tough texture and unflavored taste. Most of them are exported for broth or stock making in the food industry. However, the amount of exportation dramatically decreased owing to COVID-19. Hence, there is an urgent issue regarding expanding its exploration and diversifying its related products in markets.
Texture, flavor, and safety are the important characteristics of meat products; hence, the fulfillment of the mentioned criteria via culinary development may be a strategy for a surplus of spent-laying ducks (Tornberg, 2005). The dry heat method, that is, roasting and pan-frying, and the moist heat method, that is, boiling and poaching, are the common heat treatments for poultry meat (Baker et al., 1981; Wołoszyn et al., 2020). Zhang et al. (2019) reported that the higher heating temperature and longer heating period make Beijing roast duck have strong flavors due to the increased heterocyclic aromatic amine concentration. Pan-frying is a fundamental cooking technique that is easy to master and yields delicacy of meat products for the restaurant chef and many home cooks. In addition, poaching has become a more and more popular culinary technique for poultry meat because it is more beneficial to the health and fitness of people. The reason is that less fat in the meat results from the takeaway from hot water (Ge et al., 2021). They also reported that roasting and pan-frying are the ways that lead to meat dryness by the reduction of intramuscular juice, and boiled meat has a light flavor due to less lipid content. A modern culinary technique, sous vide, has recently given a new choice for heat treatment on meat products. Sous-vide cuisine puts the meat in a vacuum-sealed bag and is heated in a water bath under controlled temperature and time (Baldwin, 2012). This cooking technique gives better meat quality and increases the organoleptic characteristics so it could suit the tougher meat categories, such as beef, lamb, white-striping chicken breast, etc. (Baldwin, 2012; Roldán et al., 2014; Botinestean et al., 2016; Lee et al., 2021). The products prepared through sous vide could become not only ready-to-eat foods that satisfy consumers' convenience but also precooked foods that the consumers can shorten the cooking time and enjoy the delicacy. Therefore, the textural characteristics of spent-laying duck meat could be possibly improved by the sous-vide way.
Since a longer raising period and original breeding goal for laying eggs, not for meat production, the texture qualities of spent-laying duck meat are toughness and dryness. However, there is a lack of information on the optimal culinary technique for spent-laying duck meat. Therefore, the objectives of this study were to investigate 1) the suitable muscle part of spent-laying ducks for further production, 2) the optimal culinary technique and its optimal heating conditions for the applicable meat portion of spent-laying ducks, and 3) the shelf life of the product. It is expected that this research could offer a possible systematic sous-vide culinary approach of spent-laying duck and a new duck product to consumers.
MATERIALS AND METHODS
Spent-Laying Ducks
Forty slaughtered spent-laying ducks (approximately 25-mo old) were kindly offered from Fu-Hua Livestock Farm (Fangyuan Township, Changhua County, Taiwan). They were packaged in polyethylene bags with wax-coated corrugated boxes and transported at −20°C to the laboratory. The spent-laying ducks were manually cut into 5 parts: the head, wing, leg, breast, and skeleton, and afterward, the skin of the breast was removed. Each part of spent-laying ducks was weighed individually, and the portion of each part was calculated based on the regarding dressed carcass weight.
Sample Preparation
This study mainly aimed to look for the optimal culinary technique and its optimal heating conditions for spent-laying ducks. First, 24 slices of breast meat (approximately 1 cm thickness) selected from 40 spent-laying duck carcasses were randomly assigned to 4 culinary groups, that is, poaching, pan-frying, roasting, and sous-vide methods. Preliminary procedures for this experiment were followed. In the poaching group, the breast was placed in boiling water (breast: boiling water = 1:20, w:v) for 60 s, then the gas was turned off, and afterward, the breast was simmered in hot water for further 10 min. The internal temperature for poaching duck meat should reach 165°F (∼74°C). In the pan-frying group, 6 slices were heated at constant power (200°C) for 10 min by using an induction cooker (SAMPO KM-RB13, SAMPO Co., Taoyuan City, Taiwan), and meanwhile, each side of the breast was fried for 1 min. In the roasting group, 6 slices were placed in the prewarmed (200°C for 10 min) oven (CTOM320B, Whirlpool 32L oven, Benton Charter Township, MI), and each side of the breast was heated for 7 min. In the sous-vide group, the vacuum-packed breast was heated in preheated water (70°C) for 1.5 h (breast: water = 1:100, w:v), and the water temperature was controlled by the Laica Sous-vide Circulator (SVC107L1, LAICA S.p.A, Barbarano Vicentino (VI), Italy). The physical and chemical properties of breast meat in 4 groups (poaching, pan-frying, roasting, and sous vide) were assayed for determining the optimal culinary technique.
After sous-vide cooking was determined as the most optimal culinary technique for the breast of spent-laying ducks, the optimal cooking condition of sous vide was further investigated. First, regarding the suitable temperature, 12 slices of breasts were randomly selected from the remaining spent-laying duck carcasses and divided into 2 groups, that is, 65 and 70°C, and they were cooked for 1.5 h by using the Laica Sous-vide Circulator. After the optimal sous-vide temperature was determined, the optimal heating period (1, 1.5, and 2 h) was sequentially investigated by using 18 slices of breast selected from the remaining spent-laying duck carcasses. After the optimal condition of sous-vide technique were decided, 108 slices of spent-laying duck breast were kindly offered from Fu-Hua Livestock Farm again for the self-life evaluation of sous-vide duck breast meat. Herein, 1 slice per treatment combination of sous-vide period and storage day was used for the bacterial assay, and another slice was used to measure color parameters/TBARS assays.
Analysis Parameters
Cooking Loss (%) of Cooked Meat
The cooking loss of meat samples was measured according to the procedures of Ji et al. (2019) with a slight modification. Briefly, the sample of each culinary technique group was weighted before and after cooking, and the cooking loss (%) was calculated as [Weight before cooking (g) − Weight after cooking (g)]/Weight before cooking (g) × 100%.
Texture Profile and Warner-Bratzler Shear Force Measurements
The cooked breasts of spent-laying ducks were cut 1 × 1 × 1 cm3 for texture profile (TPA) and 1 × 3 × 1 cm3 for Warner-Bratzler (WB) shear force. The TPA and WB shear force indices of samples were determined using a texture analyzer (TA.Xtplus, Stable Micro Systems, Godalming, UK). For TPA analysis, the values were analyzed by using a P/50 probe (50 mm diameter cylinder, aluminum; Stable Micro Systems) under the following conditions: pretest speed, 5 mm/s; post-test speed, 5 mm/s; distance, 5 mm; trigger type, auto; trigger force, 5 g (Wu et al., 2021). The TPA parameters included hardness (N), springiness, cohesiveness, gumminess (N), chewiness (N), and resilience. The WB shear force values (N) of cooked meat samples were performed by Warner-Bratzler blade (Stable Micro Systems) at 180 mm/min speed.
Color Measurement
The color measurement on the breast sample's surface was analyzed using the color difference meter (Model NR-11, Nippon Denshoku Co., Tokyo, Japan). The heat-set samples were equilibrated to room temperature (25°C). The color difference meter was calibrated by using equipped white and black plates according to the manuals before measuring the color parameters. The color parameters included lightness (L*), redness (a*), and yellowness (b*). The whiteness was calculated by the following equation (Wu et al., 2022).
Thiobarbituric Acid Reactive Substances
The level of lipid peroxidation in cooked samples was evaluated by the thiobarbituric acid reactive substances (TBARS) assay (Ding et al., 2015). The TBARS was the reactant of thiobarbituric acid (TBA) and malondialdehyde (MDA) during the process of lipid oxidation in the sample. The breast sample was added to deionized water (1:9, w/v), homogenized, and centrifuged at 800 × g for 15 min. Then 60-µL supernatant was mixed with 90-µL TBA solution and 510-µL TCA-HCl reagent. The solution was boiled for 30 min at 95°C, cooled, and centrifuged at 9,000 × g for 3 min. The absorbance of the supernatant was measured at 535 nm and demonstrated as a TBARS value (mg MDA eq./kg of the sample) from the result.
Myofibril Fragmentation Index
Myofibrils would be fractured into small slices by a heating process. Hence, the myofibrillar fragmentation index (MFI), an indicator of degradation of myofibrillar proteins, was analyzed according to the procedure of Li et al. (2017) and USDA (2022) and with a slight modification. In determining the optimal sous-vide period under 65°C, 3 slices of cooked breast were randomly selected from 6 slices treated in each group. First, the 4 g samples without connective and fat tissues were put in 40 mL MFI buffer (KCl: 7.455 g, EDTA: 0.37224 g, Na2HPO4: 2.8392 g, NaH2PO4: 3.1202 g in 1 liter ddH2O), and homogenized for 1 min. Then the pellet of myofibrils was collected by centrifugation at 10,000 × g for 15 min. The pellet was resuspended and homogenized in a 40-mL MFI buffer. Then, the pellet was obtained again by centrifugation at 10,000 × g for 15 min and resuspended with 5-mL MFI buffer by the vortex. The resulting homogenate was filtrated by a plastic filter (80 µm pore) using a glass stirring rod. The protein concentration of the resulting homogenate was assayed by using a Bio-Rad protein assay kit (catalog #500-0006, Bio-Rad Laboratories, Inc., Hercules, CA). The 0.5 mg/mL suspension was adjusted using appropriate quantities of MFI buffer and resulting homogenate. The whole procedure was kept at 2°C. The absorbance of suspension (0.5 mg/mL) was obtained at 540 nm in triplicate. The MFI was calculated by the following equation.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
This analysis focused on observing the degree of myosin-heavy chain cleavage, which is approximately located at 230 kDa. In determining the optimal sous-vide period under 65°C, the remaining 3 slices of the cooked breast in each group were subjected for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. The procedure of SDS-PAGE was followed by the previous procedures (Wu et al., 2022). The prepared filtrate of homogenate (10%, w/w) with 5× loading dye [10% sodium dodecyl sulfate, β-mercaptoethanol, 20% glycerol, and 0.2% bromophenol blue in 3M Tris-HCl at pH 6.8] was heated at 95°C for 15 min. Then the aliquot (the same protein concentration) from each treatment was injected into a cell on a 5% polyacrylamide stacking gel and 10% polyacrylamide running gel. In contrast, the Dural Xtra Standards protein ladder (Precision Plus Protein, Cat#161-0377, Bio-Rad Laboratories, Inc.) was used as a molecular weight marker. The electrophoresis was set up in the following procedures, 80 V for 30 min and 110 V for approximately 120 min. Gels were subsequently stained using a solution including 0.06% (w/v) Coomassie Brilliant Blue G-250 in 10% acetic acid for 30 min and destained for 4 h in 10% acetic acid. Photomicrographs were taken with an imaging system (MUV21-312, Major Science Co., Saratoga, CA). The intensity of the myosin-heavy chain was quantified using ImageJ software (National Institutes of Health).
Transmission Electron Microscope
The transmission electron microscope (TEM) analysis was performed in the Electron Microscopy Room, Department of Veterinary Medicine, National Taiwan University. The 3 × 3 × 5 mm samples were cut along with the direction of myofibril and fixed by 2.5% glutaraldehyde, and then cut into 3 × 1 × 1 mm. The samples were washed with 0.1-M phosphate-buffered saline (PBS) 3 times while each wash lasted 10 min. They were fixed by 1% osmium tetroxide in 0.1-M PBS at 4°C for 80 min and then washed again. After that, the samples were dehydrated by using ethanol solution gradients (35, 35, 50, 60, 70, 85, 90, 95, 100, 100, and 100%) (ECHO Chemical Co., Ltd., Taipei, Taiwan) and each ethanol gradient lasted for 10 min. The dehydrated samples were dipped in resin for 6 h and then put into pure resin overnight. The samples were embedded in a mold at 70°C for 15 h. Successively, samples were cut into 3 µm slices using Reichert Jung (Leica) RM2165 slicer, and added water droplets to the glass slide. After dehydration at 60°C, the slices were dyed with 1% crystal violet solution for 30 s and then washed and dehydrated. The slices were cut into 70 nm thickness by a diamond knife and then put on the copper mesh. The thinner slices were dyed in a 5% uranyl acetate saturated 50% ethanol solution and lead citrate aqueous solution for 10 min and 15 min, respectively. The final sample was observed using TEM (Jeol, JEM-1200EX II, transmission electron microscope) at 20,000× magnification. Ten sarcomeres were taken randomly in each sample.
Microbiological Analyses
Two types of sheet media (Medi-Ca EC & Medi-Ca AC, Dai Nippon Printing Co., Ltd., Tokyo, Japan) were applied to detect the number of coliform/Escherichia coli (E. coli) and total plate count in sous-vide samples, respectively. Sous-vide breast samples were stored at 5°C for 1, 3, and 7 d, respectively, and were put in the aseptic bags (Whirl-pak Sterile bag, NASCO Whirl-Pak, Madison, WI) with a 1:10 ratio of sterilized saline solution (0.85%, w/w) and homogenized. The suspension was taken to be serially diluted by a decimal with sterilized saline. A total of 500-µL diluent was added to the sheet media for coliform/E. coli and total bacteria for 24 and 48 h at 35°C, respectively. Finally, the red colonies were considered the total aerobic bacterial count on the Medi-Ca AC sheet while the blue and purple colonies accounted for E. coli and coliform on the Medi-Ca EC.
Statistical Analyses
All analytical parameters, except cooking loss (%) and bacterial counts, were determined in 3 replications per meat sample. The total of 40 spent-laying ducks were used for the average weight and portion of each part. The 6 samples were assayed in cooking loss (%), meat juice %), textural properties, color parameters, TBARS, and bacterial counts, while 3 samples were used in SDS-PAGE/quantification of myosin heavy chain and MFI values. The experiment was conducted using a completely randomized design (CRD). When a significant difference (P < 0.05) among groups was detected by using ANOVA, the differences (P < 0.05) between treatments were further distinguished by using the least significant difference (LSD) assay. Besides, the effects of heating temperatures on physicochemical properties were distinguished by using the Student t test. All statistical data analyses were conducted via SAS (SAS Institute Inc., Cary, NC, 2002).
RESULTS
The Proportion of Slaughtered Spent-Laying Duck Carcasses
In comparison with other poultry categories, such as broiler chickens and meat ducks, which are bred for meat production and heavier than 2 kg, the average weight of spent-laying duck carcasses is only 865.25 g, where the skeleton (36.54%) is the largest part, followed by legs (21.51%), head (16.65%), breast (13.86%), and wings (11.44%) (Table 1). Although the proportion of meat in the spent-laying duck breast part is 13.86%, the breast skin even overpasses approximately 27% in the breast part, which means that the real applied part is only about 88.53 g on average. Between skin in the legs and wings, peeling off the complete meat from the breast part is easy. Hence, based on the accessibility and meat integrity, breast meat is an ideal part of a spent duck-product development in the future.
Table 1.
The average weight and proportion of each part in spent-laying ducks.
| Part | Weight (g) | Portion (%) |
|---|---|---|
| Head | 142.75 ± 2.29 | 16.65 ± 0.28 |
| Wing | 98.25 ± 2.26 | 11.44 ± 0.23 |
| Leg | 185.75 ± 4.58 | 21.51 ± 0.35 |
| Breast | 121.25 ± 3.74 | 13.86 ± 0.27 |
| Breast without skin | 88.53 ± 2.24 | 10.28 ± 0.24 |
| Skeleton | 317.25 ± 10.31 | 36.54 ± 0.56 |
| Total weight | 865.25 ± 18.54 | 100.00 |
The data were given as mean ± standard error of mean (SEM) (n = 40).
Comparison of Sous Vide and Common Culinary Techniques for Spent-Laying Duck Breast
In order to choose the suitable culinary technique for spent-laying duck breast, cooking loss (%), meat juice (%), TBARS value, and textural properties of cooked duck breast obtained from sous-vide cuisine and other 3 common cooking methods (poaching, pan-frying, and roasting) were investigated (Table 2). The sous-vide meat has the highest water-holding capacity due to the lowest cooking loss (29.20%) (P < 0.05) than the other 3 cooking methods. As for other culinary groups, similar (P > 0.05) results on cooking loss were observed among the poached, pan-fried, and roasted meat. TBARS value is applied to evaluate lipid peroxidation of cooked spent-laying duck breast during the processing. The sous-vide technique resulted in the lowest (P < 0.05) TBARS value which is approximately a 38% reduction compared to other products on average. Regarding the objective textural properties, the sous-vide and roasted products exhibited higher hardness, cohesiveness, gumminess, chewiness, and resilience values than other cooking methods (P < 0.05). Although no (P > 0.05) differences in springiness and WB shear values of products were measured, there was a lower tendency toward WB shear value in the sous-vide product than in other culinary products.
Table 2.
Effects of different culinary ways on cooking loss, expressible moisture, lipid oxidation, and texture profile and WB shear value of cooked duck breasts.
| Group | Poaching | Pan-frying | Roasting | Sous vide |
|---|---|---|---|---|
| Cooking loss (%) | 36.94 ± 1.26ab | 39.90 ± 5.54a | 36.05 ± 3.68ab | 29.20 ± 1.29b |
| TBARS (mg MDA eq./kg) | 3.90 ± 0.20a | 3.73 ± 0.20a | 4.02 ± 0.46a | 2.41 ± 0.20b |
| Textural properties | ||||
| Hardness (N) | 21.02 ± 2.20b | 28.47 ± 3.70b | 38.17 ± 4.12a | 39.37 ± 2.66a |
| Springiness | 0.66 ± 0.01a | 0.69 ± 0.01a | 0.68 ± 0.02a | 0.65 ± 0.02a |
| Cohesiveness | 0.63 ± 0.01b | 0.65 ± 0.01ab | 0.66 ± 0.01a | 0.68 ± 0.01a |
| Gumminess (N) | 13.19 ± 1.46c | 18.58 ± 2.67bc | 25.32 ± 2.80ab | 26.98 ± 2.02a |
| Chewiness (N) | 8.66 ± 0.98b | 12.88 ± 1.92ab | 17.42 ± 1.96a | 17.72 ± 1.64a |
| Resilience | 0.28 ± 0.00b | 0.28 ± 0.00b | 0.30 ± 0.01b | 0.36 ± 0.02a |
| WB shear value (N) | 48.00 ± 2.23a | 55.75 ± 3.95a | 47.12 ± 4.79a | 45.71 ± 3.18a |
The data are given as mean ± SEM (n = 6). Mean values in each parameter without a common letter were significantly different (P < 0.05).
Optimal Conditions of Sous-Vide Technique for Spent-Laying Duck Breast
To investigate the optimal cooking condition of the sous-vide technique for spent-laying duck breast, the 2 major key points (cooking temperature and period) were determined through cooking loss (%), TBARS value, textural properties, and degradation and microstructure of myofibrillar protein of cooked duck-breast products.
Optimal Sous-Vide Cooking Temperature for Spent-Laying Duck Breast
First, the optimal cooking temperature was determined. In comparison between 65°C and 70°C cooked products, the higher cooking loss was measured in 70°C ones (P < 0.05), but there was no (P > 0.05) significant difference in TBARS values (Table 3). About the textural properties, all parameters, except springiness, were not (P > 0.05) different between these 2 cooking temperatures, but 70°C cooking resulted in higher (P < 0.05) springiness. Furthermore, cooking temperatures did not (P > 0.05) affect the color parameters (whiteness, L*, a*, and b*) of final products, but a higher tendency of L* value probably resulted in higher whiteness (Suppl. Figure 1A). Based on the results collected above and energy saving, 65°C was determined as an optimal cooking temperature for spent-laying duck breast.
Table 3.
Effects of various cooking temperatures and periods on the physicochemical characteristics of sous-vide duck breasts.
| Test conditions | Cooking temperature (°C) |
Cooking period (h) |
|||
|---|---|---|---|---|---|
| 65 | 70 | 1 | 1.5 | 2 | |
| Cooking loss (%) | 23.10 ± 0.66 | 29.20 ± 1.01* | 19.55 ± 0.85b | 20.82 ± 1.26b | 24.56 ± 1.08a |
| TBARS (mg MDA eq./kg) | 2.30 ± 0.29 | 2.14 ± 0.31 | 3.11 ± 0.29a | 2.41 ± 0.33a | 3.05 ± 0.23a |
| Textural properties | Textural properties | ||||
| Hardness (N) | 34.20 ± 3.68 | 34.05 ± 1.97 | 37.19 ± 4.97a | 33.72 ± 2.47a | 35.10 ± 4.08a |
| Springiness | 0.59 ± 0.02 | 0.65 ± 0.01* | 0.61 ± 0.01a | 0.61 ± 0.01a | 0.58 ± 0.02a |
| Cohesiveness | 0.66 ± 0.01 | 0.67 ± 0.00 | 0.65 ± 0.01a | 0.66 ± 0.00a | 0.66 ± 0.01a |
| Gumminess (N) | 22.76 ± 2.59 | 22.77 ± 1.27 | 24.23 ± 3.45a | 22.37 ± 1.70a | 23.25 ± 2.90a |
| Chewiness (N) | 19.08 ± 5.16 | 14.94 ± 0.87 | 14.65 ± 1.86a | 13.71 ± 0.95a | 13.63 ± 1.74a |
| Resilience | 0.35 ± 0.01 | 0.34 ± 0.01 | 0.36 ± 0.00a | 0.35 ± 0.01a | 0.34 ± 0.01a |
| WB shear value (N) | 54.45 ± 7.76 | 52.96 ± 5.63 | 47.03 ± 6.10ab | 32.58 ± 2.63b | 54.67 ± 6.15a |
The data are given as mean ± SEM (n = 6). Mean values in each test parameter with mark (*) and without a common letter in various cooking temperatures and periods, respectively, were significantly different (P < 0.05). The assay for optimal cooking temperature was determined by setting for 1.5 h of the sous-vide period. The assay for the optimal cooking period was determined by setting 65°C of the sous-vide temperature.
Optimal Sous-Vide Cooking Period for Spent-Laying Duck Breast
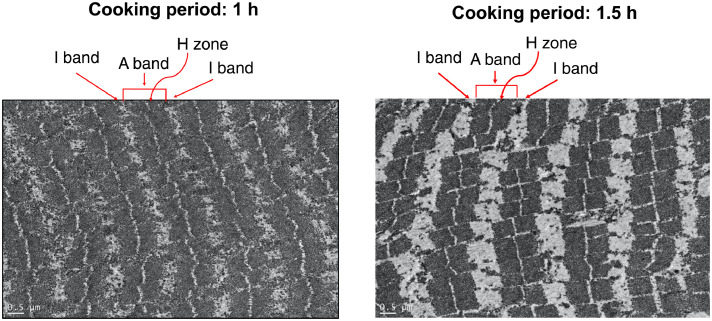
After the optimal cooking temperature (65°C) for spent-laying duck breast was determined, the optimal cooking period (1, 1.5, and 2 h) was further investigated. Regarding the physicochemical properties of sous-vide breast cooked at 65°C for various cooking periods, only differences were observed in cooking loss (%) and WB shear value among various cooking periods, respectively (Table 3). The highest (P < 0.05) cooking loss was obtained in 2-h cooking products than those in other cooking-period ones, while the highest (P < 0.05) WB shear value was detected in those of 2-h cooking as well, followed by those of 1-h and 1.5-h cooking. Although cooking periods did not (P > 0.05) influence TBARS values, generally 1.5-h cooking period resulted in the lowest TBARS value than other cooking periods. Moreover, the cooking periods also did not (P > 0.05) affect the color parameters (whiteness, L*, a*, and b*) of cooked products (Suppl. Figure 1B). In addition, the bands of myosin-heavy-chain (∼230 kDa) were getting lighter when the cooking period increased (Figure 1A), which results in the lower (P < 0.05) intensities of destruction to myosin heavy chain beyond 1.5-h cooking period. However, there were no (P > 0.05) differences in myofibrillar fragment indexes (MFIs) of various cooking-period products (Figure 1B). Via a microstructural observation of myofilaments under the TEM (Figure 2), the A band, H zone, and I band in sarcomeres were located in the dark area, thinner white line, and wider white zone in the photos. Generally, the Z lines of sarcomeres were destroyed by cooking at 65°C for 1 and 1.5 h in sous-vide spent-laying duck breasts. However, more destructive and unorganized sarcomeres in myofilaments were observed in 1.5-h cooking products. The arrangement of myofilaments seemed ruptured and loose, which might echo the lower WB shear value in 1.5-h cooking products than that in 1-h cooking ones (Table 3 and Figure 2). In addition, to mimic the sous-vide products available in the market, the sous-vide products prepared at 65°C for 1, 1.5, and 2 h were placed under a 4°C storage. The bacterial counts, that is, total plate account, E. coli, and coliform were not detectable within 7 d of storage, while the color of appearance and TBARS values in products were not (P > 0.05) changed (Table 4). Based on the above results, the optimal sous-vide technique for spent-laying duck breast could be prepared at 65°C for 1.5 h; meanwhile, the safety and quality of this sous-vide duck breast stored at 4°C could not be influenced within 7-day storage.
Figure 1.
Effects of cooking periods on myosin heavy-chain degradation of sous-vide duck breasts: (A) protein pattern illustration and quantifications of myosin heavy chain (∼230 kDa), and (B) myofibrillar fragment index (MFI). *The data bars were shown as mean ± SEM (n = 3). Data bars in each test parameter without a common letter were significantly different (P < 0.05).
Figure 2.
Effects of cooking periods (1 and 1.5 h) on the structures of myofilaments of 65°C sous-vide duck breasts. *TEM images at original magnifications of 20,000×.
Table 4.
Effects of various cooking periods on the bacterial counts, color parameters, and lipid oxidation of sous-vide duck breast cooked at 65°C and then stored at 4°C.
| Cooking period (h) | 1 |
1.5 |
2 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Storage day (d) | 0 | 3 | 7 | 0 | 3 | 7 | 0 | 3 | 7 | ||||||||
| Bacterial counts (CFU/g) | |||||||||||||||||
| Total plate count | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||
| E. coli | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||
| Coliform | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||
| Color parameters | |||||||||||||||||
| L* | 63.58 ± 1.95 | 62.87 ± 1.52 | 63.92 ± 0.62 | 66.81 ± 1.89 | 67.34 ± 2.10 | 64.68 ± 0.63 | 63.44 ± 2.41 | 67.71 ± 2.20 | 65.82 ± 0.61 | ||||||||
| a* | 9.46 ± 1.37 | 10.78 ± 0.75 | 11.56 ± 0.41 | 9.72 ± 1.24 | 9.89 ± 0.92 | 11.56 ± 0.44 | 10.90 ± 1.12 | 9.95 ± 0.97 | 10.05 ± 0.41 | ||||||||
| b* | 63.58 ± 1.95 | 62.87 ± 1.52 | 63.92 ± 0.62 | 66.81 ± 1.89 | 67.34 ± 2.10 | 64.68 ± 0.63 | 63.44 ± 2.41 | 67.71 ± 2.2 | 65.82 ± 0.61 | ||||||||
| Lipid oxidation | |||||||||||||||||
| TBARS (mg MDA eq./kg) | 3.33 ± 0.66 | 2.88 ± 0.88 | 2.92 ± 0.55 | 3.31 ± 0.26 | 3.95 ± 0.64 | 3.28 ± 0.26 | 3.57 ± 0.10 | 3.39 ± 0.53 | 3.10 ± 0.23 | ||||||||
The data were given as mean ± SEM (n = 6). Regarding the effects of cooking and storage periods, there are no (P > 0.05) differences in each test parameter. ND, not detectable, meaning the colonies per plate are less than 3.
DISCUSSION
Due to the tough and dried characteristics of spent-laying duck meat, there is only a limited usage, that is, ginger duck stew and duck broth/essence, in the market. Furthermore, COVID-19 results in decreased exportation, so it is urgent to diversify its related products in the market. Although spent-laying ducks have little amount of breast meat compared to those in Muscovy and Pekin ducks which are both common broiler ducks, accounted for higher than 20% is 550 and 480 g on average, respectively (Solomon et al., 2006), the breast meat is an ideal part for further laying duck product development due to the accessibility to peel skin and meat integrity. As we know, various cuisines produce different textures and flavors for meat products. It was reported that there is a positive relationship between the water-holding capacity and the juiciness or tenderness of meat products (Ding et al., 2018; Shen et al., 2022). It was reported that a long time and low cooking temperature resulted in lower cooking loss and toughness of meat products (Dominguez-Hernandez et al., 2018). Roasting, pan-frying, boiling, and poaching are the traditional culinary methods for poultry meat (Baker et al., 1981; Wołoszyn et al., 2020). The temperatures of poaching, pan-frying, and roasting used in this study were 100°C, 200°C, and 200°C, respectively, which are higher than that of sous vide used in this assay (70°C). Besides, vacuum packaging can prevent moisture evaporation, thus maintaining more juice in meat products during the sous-vide processing (Baldwin, 2012; Kilibarda et al., 2018; Rao et al., 2022). The lowest cooking loss measured in sous-vide products than in other culinary products echoes more juice preserved in the cooked meat samples (Ji et al., 2019). The TBARS is usually applied to detect lipid oxidation in meat (Ding et al., 2015; Rao et al., 2022). Either fluctuating cooking temperature or oxygen inclusion could cause protein and lipid denaturation, thus causing deterioration and rancidity of food products (Wang et al., 2019; Bıyıklı et al., 2020). The lower lipid oxidation in sous-vide products might be due to the vacuum packaging and lower cooking temperature than this study's other 3 traditional culinary ways. Hence, it is speculated that the sous vide an optimal cooking method for spent-laying duck breast due to lower cooking losses and lipid oxidation (Table 2).
Sous vide results in a more tender meat texture than other culinary techniques and is good for the elderly (Botinestean et al., 2016; Barido et al., 2022). Regarding textural profiles of cooked spent-laying duck breast, the hardness of sous-vide meat is not as anticipation in this study. It could be explained that the vacuum packaging before sous-vide cooking compresses spent-laying duck breast (approx. 1 cm thickness), which is tougher than meat-duck breast, thus making meat texture denser and tighter. Furthermore, the compression distance is 5 mm in the execution of texture profile analysis, so a higher strength (hardness) is required to compress the vacuum-packed meat. Cohesiveness is the ratio of active work under the second compression curve to that of the first, which is similar to hardness because of the higher strength of the second compression in vacuum-packed meat. Gumminess and chewiness are relevant to hardness because they are calculated as hardness × cohesiveness and hardness × cohesiveness × springiness, respectively. Hence, it is reasonable that the hardness, cohesiveness, gumminess, and chewiness of sous-vide breast are higher than those of other culinary ways. The WB shear force is also the most common assay to determine the meat tenderness globally, while it is used to determine the maximum strength required to shear through a meat sample of fixed cross-sectional area at right angles to the muscle fiber direction (Møller, 1981; Silva et al., 2015). It was reported that the tenderness evaluation by using the WB shear value highly correlates with consumers' tenderness ratings (Destefanis et al., 2008). Although the hardness and its corresponding values from texture profile analysis were higher in sous-vide breast than those of the other 3 culinary ways, the lowest tendency of WB shear values of sous-vide breast might result from the muscle fibers defragmented by a longer cooking period (1.5 h).
As we know, sous-vide cuisine is a cooking approach that focuses on minimal heat treatment of foods, while precise cooking temperatures and periods are chosen regarding the sensory, textural, nutritional, and safety characteristics of different food categories, that is, 50°C to 65°C, several hours or even days for seafood and meat; 90°C to 100°C, a few min for vegetable (Kilibarda et al., 2018; Ismail et al., 2019). Generally, the cooking temperatures of sous vide on poultry meat are about 60°C to 75°C (Baldwin, 2012; Bıyıklı et al., 2020; Park et al., 2020 ). Based on our preliminary experiment, the spent-laying duck breast that was heated at 60°C performed a relatively raw appearance, while a heat treatment of 75°C could cause overheating due to the thin thickness of the spent-laying duck breast. Therefore, this study set the cooking temperatures at 65°C and 70°C. It was mentioned that moisture loss occurs owing to muscle contraction, the transverse shrinkage is at 40°C to 60°C, and the parallel shrinkage is between 60°C and 90°C (Vaudagna et al., 2002). When the temperature increases, the muscle contracts and squeezes out water, which results in shortened sarcomere and declines cooking yield. The heat treatment also causes myoglobin to denature, which is related to the redness and yellowness of meat. The denaturation starts between 55°C and 65°C, and most protein denatures between 70°C and 80°C (Hunt et al., 1999). According to the results of physical characteristics of sous-vide breast between 65°C and 70°C (Table 3 and Suppl. Figure 1A), the 65°C might be the optimal sous-vide temperature for spent-laying duck breast.
Considering the variation in the thickness of the spent-laying duck breast, the cooking periods of sous vide were set for 1, 1.5, and 2 h, which was identical to Bıyıklı et al. (2020). The increased cooking period resulted in a higher cooking loss (Table 3). Furthermore, the collagen content in meat is an important factor in the tenderness under the mild cooking condition because the collagen starts to shrink and forms gelation at the denaturation temperature (60°C–70°C) (Light et al., 1985). Myofibril degradation also contributes to the tenderness improvement of meat, and myosin is the major component of myofibril protein. Myosin is made of 2 heavy chains (∼230 kDa) and 4 light chains (20 kDa) (Ertbjerg and Puolanne, 2017). Yin et al. (2020) observed that the quantity of myosin heavy chain (MHC) reduces when the sous-vide temperature goes up from 45°C to 65°C. Similarly, the cooking period increases and the MHC quantities lessen (Figure 1A). It was mentioned that the MFI, an indicator of degradation of myofibrillar proteins, is strongly associated with indices of meat tenderness, that is, WB shear force (Olson et al., 1976; Veiseth et al., 2001). Hence, it is speculated that the heat treatment of 65°C within 2 h is not enough to break down the myofibrillar proteins (Figure 1B). In addition, there is a protein domain, desmin, connecting Z lines of myofibrils vertically (Marzuca-Nassr et al., 2018). The desmin is susceptible to proteolysis by calpains, which are easily activated at mild temperatures (Elamrani et al., 1995). The continuous heating fractures the desmin and then discontinues the Z line, thus contributing to the meat tenderness. To summarize, 1.5 h could be a suitable cooking period for sous-vide spent-laying duck breast.
For safety concerns, it has been indicated that the meat heated at 65°C should cook for at least 40 min when the meat thickness is lower than 1.5 cm (Baldwin, 2012). Respective to storage temperature, the toxicology times of Clostridium botulinum in sous-vide chicken prepared at 65°C to 75°C for 25 to 30 min were 90, 16, 12, and 2 d at 4°C, 8°C, 12°C, and 30°C, respectively (Meng and Genigeorgis, 1994). Based on the data of bacterial and quality changes in sous-vide spent-duck breast prepared at 65°C for 1, 1.5, and 2 h under a 4°C storage, aerobic bacteria, E. coli, and coliform were not detectible while coloring parameters (L*, a*, and b*) and TBARS values were not affected. These results indicated that sous vide (65°C, 1.5 h) is a good culinary technique for spent-laying duck breast.
CONCLUSIONS
The amount spent-laying ducks in Taiwan causes a big problem for industry and government due to global COVID-19 status and trade barriers; hence, how to maximize their utilization is an emergent issue for the industrial chain, including laying duck farming, food industry as well academics. Due to the toughness characteristics of spent-laying duck breast, sous vide is such a good culinary technique that can improve water holding capacity and retard the lipid oxidation caused by heat treatment. Furthermore, the efficient condition of sous vide on the spent-laying duck breast meat is processed at 65°C for 1.5 h. In addition, the quality of this sous-vide spent-laying duck breast remains unchanged and safe consumption within 7 d of refrigerated storage (4°C). Eventually, we hope this analytical and theoretical study can offer a systematic sous-vide approach for spent-laying duck meat and assist in handling the excess spent-laying duck problem in the poultry market.
Acknowledgments
ACKNOWLEDGMENTS
This research was funded by the Council of Agriculture, Executive Yuan, Taiwan (Project: 111AS-2.2.2-AD-U1(1) and 112AS-2.2.2-AD-U1(1)) and Ministry of Science and Technology, Taiwan (Project: MOST 109-2313-B-002-007-MY3).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102636.
Appendix. Supplementary materials
REFERENCES
- Baeza E. Pages 79–82 in Proceedings of the 2006 Symposium COA/INRA Scientific Cooperation in Agriculture. Tainan, Taiwan. 2006. Effects of genotype, age and nutrition on intramuscular lipids and meat quality. [Google Scholar]
- Baker R.C., Darfler J.M., Rehkugler G.E. Electrical energy used and time consumed when cooking foods by various home methods: chickens. Poult. Sci. 1981;60:2062–2070. [Google Scholar]
- Baldwin D.E. Sous vide cooking: a review. Int. J. Gastron. Food Sci. 2012;1:15–30. [Google Scholar]
- Barido F.H., Jang A., Pak J.I., Kim Y.J., Lee S.K. Combined effects of processing method and black garlic extract on quality characteristics, antioxidative, and fatty acid profile of chicken breast. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S., Banerjee R., Bhattacharyya D., Patra G., Das A.K., Das S.K. Technological investigation into duck meat and its products – a potential alternative to chicken. Worlds Poult. Sci. J. 2019;75:609–620. [Google Scholar]
- Bıyıklı M., Akoğlu A., Kurhan Ş., Akoğlu İ.T. Effect of different sous vide cooking temperature-time combinations on the physicochemical, microbiological, and sensory properties of turkey cutlet. Int. J. Gastron. Food Sci. 2020;20 [Google Scholar]
- Botinestean C., KeenanJoe D.F., Kerry J.P., Hamill R.M. The effect of thermal treatments including sous vide, blast freezing and their combinations on beef tenderness of M. semitendinosus steaks targeted at elderly consumers. LWT - Food Sci. Technol. 2016;74:154–159. [Google Scholar]
- Council of Agriculture, Executive Yuan, Taiwan. 2022. Agriculture Production - 2. Livestock Production. Accessed May 2022. https://eng.coa.gov.tw/upload/files/eng_web_structure/2505611/02-2Livestock%20Production.pdf.
- Destefanis G., Brugiapaglia A., Barge M.T., Dal Molin E. Relationship between beef consumer tenderness perception and Warner-Bratzler shear force. Meat Sci. 2008;78:153–156. doi: 10.1016/j.meatsci.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Ding Y., Lin H.W., Yang D.Y., Yu Y.S., Chen J.W., Lin Y.L., Wang S.Y., Chen Y.C. Nutritional composition in the chia seed and its application on restructured ham-like products. J. Food Drug Anal. 2018;26:124–134. doi: 10.1016/j.jfda.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Wang S.Y., Yang D.J., Chang M.H., Chen Y.C. Alleviative effects of litchi (Litchi Chinensis Sonn.) flower on lipid peroxidation and protein degradation in emulsified pork meatballs. J. Food Drug Anal. 2015;23:501–508. doi: 10.1016/j.jfda.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Hernandez E., Salaseviciene A., Ertbjerg P. Low-temperature long-time cooking of meat: eating quality and underlying mechanisms. Meat Sci. 2018;143:104–113. doi: 10.1016/j.meatsci.2018.04.032. [DOI] [PubMed] [Google Scholar]
- Elamrani N., Brustis J.J., Dourdin N., Balcerzak D., Poussard S., Cottin P., Ducastaing A. Desmin degradation and Ca2+-dependent proteolysis during myoblast fusion. Biol. Cell. 1995;85:177–183. doi: 10.1016/0248-4900(96)85278-6. [DOI] [PubMed] [Google Scholar]
- Ertbjerg P., Puolanne E. Muscle structure, sarcomere length and influences on meat quality: a review. Meat Sci. 2017;132:139–152. doi: 10.1016/j.meatsci.2017.04.261. [DOI] [PubMed] [Google Scholar]
- FAOSTAT. 2022. Crops and livestock products. Accessed May 2022. http://www.fao.org/faostat/en/#data/QL.
- Ge X., Zhang L., Zhong H., Gao T., Jiao Y., Liu Y. The effects of various Chinese processing methods on the nutritional and safety properties of four kinds of meats. Innov. Food Sci. Emerg. Technol. 2021;70 [Google Scholar]
- Hunt M.C., Sorheim O., Slinde E. Color and heat denaturation of myoglobin forms in ground beef. J. Food Sci. 1999;64:847–851. [Google Scholar]
- Ismail I., Hwang Y.H., Joo S.T. Effect of different temperature and time combinations on quality characteristics of sous-vide cooked goat gluteus medius and biceps femoris. Food Bioprocess Technol. 2019;12:1000–1009. [Google Scholar]
- Ji D.S., Kim J.H., Yoon D.K., Kim J.H., Lee H.J., Cho W.Y., Lee C.H. Effect of different storage-temperature combinations on Longissimus dorsi quality upon sous-vide processing of frozen/thawed pork. Food Sci. Anim. Resour. 2019;39:240–254. doi: 10.5851/kosfa.2019.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilibarda N., Brdar I., Baltić B., Marković V., Mahmutović H., Karabasil N., Stanisic S. The safety and quality of sous vide food. Meat Technol. 2018;59:38–45. [Google Scholar]
- Lee B., Park C.H., Kong C., Kim Y.S., Choi Y.H. Muscle fiber and fresh meat characteristics of white-striping chicken breasts, and its effects on palatability of sous-vide cooked meat. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Li X., Gao X., Du M., Zhan D. Effect of inhibition of μ-calpain on the myofibril structure and myofibrillar protein degradation in postmortem ovine muscle. J. Sci. Food Agric. 2017;97:2122–2131. doi: 10.1002/jsfa.8018. [DOI] [PubMed] [Google Scholar]
- Light N., Champion A.E., Voyle C., Bailey A.J. The rôle of epimysial, perimysial and endomysial collagen in determining texture in six bovine muscles. Meat Sci. 1985;13:137–149. doi: 10.1016/0309-1740(85)90054-3. [DOI] [PubMed] [Google Scholar]
- Marzuca-Nassr G.N., Vitzel K.F., Mancilla-Solorza E., Márquez J.L. Sarcomere structure: the importance of desmin protein in muscle atrophy. Int. J. Morphol. 2018;36:576–583. [Google Scholar]
- Meng J., Genigeorgis C. Delaying toxigenesis of Clostridium botulinum by sodium lactate in 'sous-vide' products. Lett. Appl. Microbiol. 1994;19:20–23. [Google Scholar]
- Møller A.J. Analysis of Warner-Bratzler shear pattern with regard to myofibrillar and connective tissue components of tenderness. Meat Sci. 1981;5:247–260. doi: 10.1016/0309-1740(81)90015-2. [DOI] [PubMed] [Google Scholar]
- Mottet A., Tempio G. Global poultry production: current state and future outlook and challenges. Worlds Poult. Sci. J. 2017;73:245–256. [Google Scholar]
- OECD. 2020. OECD-FAO Agricultural Outlook 2020-2029-Table C.4 World meat projections. Accessed June 2021. https://www.oecd-ilibrary.org/agriculture-and-food/world-meat-projections_e481f6bb-en.
- Olson D.G., Parrish F.C., Jr., Stromer M.H. Myofibril fragmentation and shear resistance of three bovine muscles during postmortem storage. J. Food Sci. 1976;41:1036–1041. [Google Scholar]
- Park C.H., Lee B., Oh E., Kim Y.S., Choi Y.M. Combined effects of sous-vide cooking conditions on meat and sensory quality characteristics of chicken breast meat. Poult. Sci. 2020;99:3286–3291. doi: 10.1016/j.psj.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J.W., Meng F.B., Li Y.C., Chen W.J., Liu D.Y., Zhang J.M. Effect of cooking methods on the edible, nutritive qualities and volatile flavor compounds of rabbit meat. J. Sci. Food Agric. 2022;102:4218–4228. doi: 10.1002/jsfa.11773. [DOI] [PubMed] [Google Scholar]
- Roldán M., Antequera T., Hernández A., Ruiz J. Physicochemical and microbiological changes during the refrigerated storage of lamb loins sous-vide cooked at different combinations of time and temperature. Food Sci. Technol. Int. 2014;21:512–522. doi: 10.1177/1082013214552861. [DOI] [PubMed] [Google Scholar]
- Shen Y., Guo X., Li X., Wang W., Wang S., Pan J., Dong X., Li S. Effect of cooking temperatures on meat quality, protein carbonylation and protein cross-linking of beef packed in high oxygen atmosphere. LWT - Food Sci. Technol. 2022;154 [Google Scholar]
- Silva D.R.G., Torres Filho R.A.T., Cazedey H.P., Fontes P.R., Ramos A.L.S., Ramos E.M. Comparison of Warner–Bratzler shear force values between round and square cross-section cores from cooked beef and pork Longissimus muscle. Meat Sci. 2015;103:1–6. doi: 10.1016/j.meatsci.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Solomon J.K.Q., Austin R., Cumberbatch R.N., Gonsalves J., Seaforth E. A comparison of live weight and carcass gain of Pekin, Kunshan and Muscovy ducks on a commercial ration. Livest. Res. Rural Dev. 2006;18:154. [Google Scholar]
- Tornberg E. Effects of heat on meat proteins-Implications on structure and quality of meat products. Meat Sci. 2005;70:493–508. doi: 10.1016/j.meatsci.2004.11.021. [DOI] [PubMed] [Google Scholar]
- US Department of Agriculture (USDA) 2022. Myofibril Fragmentation Index.https://www.ars.usda.gov/ARSUserFiles/30400510/protocols/MFI.pdf Accessed Apr. 2022. [Google Scholar]
- Vaudagna S.R., Sánchez G., Neira M.S., Insani E.M., Picallo A.B., Gallinger M.M., Lasta J.A. Sous vide cooked beef muscles: effects of low temperature-long time (LT-LT) treatments on their quality characteristics and storage stability. Int. Food Sci. Technol. 2002;37:425–441. [Google Scholar]
- Veiseth E., Shackelford S.D., Wheeler T.L., Koohmaraie M. Technical note: comparison of myofibril fragmentation index from fresh and frozen pork and lamb longissimus. J. Anim. Sci. 2001;79:904–906. doi: 10.2527/2001.794904x. [DOI] [PubMed] [Google Scholar]
- Wang Y.Z., Wang S.Y., Fu S.G., Yang D.J., Yu Y.S., Chen J.W., Chen Y.C. Effects of rosemary (Rosmarinus officinalis L.) extracts and dry ice on physicochemical stabilities of omega-3 fatty-acid fortified surimi-like meat products. J. Sci. Food Agric. 2019;99:3843–3851. doi: 10.1002/jsfa.9606. [DOI] [PubMed] [Google Scholar]
- Wołoszyn J., Wereńska M., Goluch Z., Haraf G., Okruszek A., Teleszko M., Król B. The selected goose meat quality traits in relation to various types of heat treatment. Poult. Sci. 2020;99:7214–7224. doi: 10.1016/j.psj.2020.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.H.S., Lin Y.L., Wang S.Y., Lin D.Q., Chen J.W., Chen Y.C. Effects of washing step and salt-addition levels on textural and quality properties in the chicken-surimi products. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.H.S., Lin D.Q., Wang S.Y., Lin Y.L., Chen J.W., Nakthong S., Chen Y.C. Effects of wheat-fiber addition on emulsion and lipid/protein stabilities of an omega-3 fatty-acid fortified chicken-surimi product. Poult. Sci. 2021;100:1319–1327. doi: 10.1016/j.psj.2020.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Pereira J., Zhou L., Lorenzo J.M., Tian X., Zhang W. Insight into the effects of sous vide on cathepsin B and L activities, protein degradation and the ultrastructure of beef. Foods. 2020;9:1441. doi: 10.3390/foods9101441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Du H., Zhang P., Kong B., Liu Q. Heterocyclic aromatic amine concentrations and quality characteristics of traditional smoked and roasted poultry products on the northern Chinese market. Food Chem. Toxicol. 2019;135 doi: 10.1016/j.fct.2019.110931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.