Abstract
Approximately one-third of clinical trials fail to meet their recruitment goals, which can cause costly delays to sponsors and compromise the scientific integrity and generalizability of a trial. Inadequate recruitment and retention of patient groups who have the disease under investigation may produce insufficient medical knowledge about the therapeutic effects of drugs or products for the population at large. It is essential to address these issues to ensure that certain groups are not unduly subjected to disproportionate risks or denied the benefits of research. This commentary will present opportunities for clinical trialists to use emerging technologies and decentralized approaches to improve clinical trial recruitment, mitigate disparities, and improve individual and population-level outcomes within cardiovascular medicine.
Keywords: Health equity, Diversity and inclusion, Racial disparities, Cardiology
Key Summary Points
| Challenges in recruitment and retention of diverse study participants cause costly delays and inefficiencies for clinical trial sponsors and compromise trial generalizability. |
| Conducting clinical trials with non-representative study populations that lack diversity in racial identification, ethnicity, sex, age, geography, comorbidities, and socioeconomic status may exacerbate health disparities. |
| Opportunities exist to use emerging technologies and decentralized approaches to recruit and retain previously excluded or underrepresented groups in cardiovascular clinical research. |
| Recruiting a representative study population can mitigate disproportionate risks and health disparities and improve individual and population-level cardiovascular disease outcomes. |
Introduction
Approximately one-third of clinical trials are delayed because they fail to meet their recruitment goals [1]. Barriers to recruitment include financial constraints, travel time, inefficiencies in screening procedures, mistrust, miscommunication, and discrimination [1]. The number of times a patient has to visit a clinical trial site can vary depending on the specific trial protocol and the medical condition being studied. In general, patients may be required to make multiple visits to the clinical trial site for screening, baseline assessments, treatment administration, follow-up evaluations, and data collection. The frequency and duration of these visits can vary depending on the study design and the intervention being investigated. The median cost per patient visit in pivotal efficacy trials for new therapeutic agents approved by the U.S. Food and Drug Administration (FDA) between 2015 and 2016 was estimated to be US$3562 [2]. The median cost per patient through the duration of a clinical trial is over $41,000 [2]. Each day that a clinical trial is delayed, the sponsor can incur significant costs due to the need for continued funding, staff salaries, and other operational expenses. Trial delays also have significant implications for patients, including a longer wait for access to potentially life-saving treatments or therapies being studied in the trial; the inconvenience of rescheduling visits and any additional procedures, which can be particularly burdensome for patients who live far from the trial site or who have a disability.
Limitations in patient recruitment and retention are significant bottlenecks in clinical trials [3]. A review of 71 randomized controlled trials in four top medical journals found that dropout rates as high as 20% or more occurred in 18% of the trials [4]. Failure to address these limitations is not only inefficient, with sponsors often having to replace patients who drop out of a trial in order to maintain an appropriate sample size and statistical power for the study, but also compromises the scientific integrity of the results, hindering the development of effective treatments.
Having a representative study sample helps to ensure that the findings are applicable to the broader population that the treatment is intended for. A clinical trial that is underpowered may not detect a statistically significant difference between treatment groups, rendering the study findings inconclusive. Findings may also be susceptible to bias if there are issues with the study design, such as selection bias, measurement bias, or reporting bias, which can occur if the trial enrollment criteria and protocol are designed in a way that excludes or underrepresents certain groups. Non-representative samples and research bias can reinforce health disparities by perpetuating inequities in access to healthcare and limiting the generalizability of study findings to diverse patient populations [3].
In cardiovascular disease (CVD) trials, participant characteristics to consider include various disease risk factors, comorbidities, socioeconomic status (SES) (e.g., income, employment status, healthcare access) [4], demographic factors, and certain disease-specific factors (e.g., rare diseases and genetic subtypes of CVD) [5]. Important sensitivities and controversies related to the indistinct constructs of racial and ethnic categories are being increasingly acknowledged. However, these terms may be useful as a lens through which to study and view health disparities and inequities in health care, medical practice, education, and research [6]. It is key to consider the intersection of these characteristics in CVD trials because they affect the prevalence, incidence, and outcomes of CVD in different populations.
Historically, minoritized populations, such as women (and specifically, pregnant and lactating women) [7], people from rural areas, the elderly, people with comorbidities, those of lower SES [8], and certain racial and ethnic identifications, including indigenous groups [9], have been underrepresented in research. As a result, therapeutic guidelines may not be optimized for the needs of these populations and may not reflect their specific responses to treatment. For instance, there are well-known sex-specific differences in the etiology and pathophysiological mechanisms of CVD, as well as the way drugs are metabolized in the body. Studies have shown that women may have a lower clearance of certain beta-blockers, such as metoprolol and propranolol, compared to men. Women may also be at a higher risk of adverse effects from angiotensin-converting enzyme (ACE) inhibitors compared to men [10]. Despite this knowledge, women are largely underrepresented in trials studying stroke, arrhythmia, coronary heart disease, acute coronary syndrome, and heart failure (HF) [9]. Only a small percentage of HF therapy and lipid-lowering therapy trials have included women, with women comprising only 25% and 29% of these trials, respectively [9, 11]. Data suggest that the lower enrollment of women in certain CVD trials reflects the lower number of women referred for screening and indicates a need to address barriers to recruitment [7].
Similarly, racial disparities in hypertension and related events have been observed, particularly among Black men. The prevalence of hypertension among Black men was 41.2%, compared to 28% for non-Hispanic White men, 25.9% for Hispanic men, and 24.9% for non-Hispanic Asian men [12]. Only 3% of CVD trial participants between 2015 and 2019 were Black men aged younger than 65 years [13], indicating potential systematic bias in recruitment [14]. These findings underscore the need for greater diversity in clinical trial participation and standardized reporting of participant-level race and ethnicity data.
The U.S. FDA has recognized the importance of including minoritized populations in clinical trials with various initiatives, including designating 2016 as the “Year of Clinical Trial Diversity” [15]. In 2020, the FDA issued guidance for the industry titled “Enhancing the Diversity of Clinical Trial Populations: Eligibility Criteria, Enrollment Practices, and Trial Designs” [16]. The guidelines encourage the development of targeted outreach and recruitment strategies to increase participation and also suggest ways to mitigate participation, such as language barriers or cultural differences. While important, these documents and frameworks lack practical solutions to address the digital divide and may inadvertently reinforce health disparities that affect populations most at-risk for CVD [6]. It is crucial to closely examine participant recruitment and retention strategies to ensure that no groups are unfairly exposed to disproportionate risks or denied the potential benefits of participating in clinical trials [11]. The inclusion of diverse groups in clinical trials is essential to uphold the principles of justice and beneficence, which require clinical trialists and sponsors to prioritize maximizing benefits and minimizing harms in research.
Disruption, Innovation, and Opportunities
The COVID-19 pandemic caused significant disruptions in all areas of society, including the conduct of clinical trials. As a result, there has been a proliferation of rapid and innovative solutions, particularly in health technologies. These solutions have contributed to a novel clinical research infrastructure made possible by components of decentralization, digital health, electronic platforms, and other technologies which connect patients to health care in sites near where people live, beyond the four walls of a clinic. The FDA recognized the potential impact that these innovations could have on the conduct of clinical trials and issued guidance encouraging sponsors to consider alternate methods for research, such as virtual visits, telephone contact, and local laboratories when appropriate [17]. The FDA also recently proposed fully decentralized clinical trials (DCTs) as a solution to improving diversity in clinical research due to potentially easing barriers to participation [1]. DCTs are a form of clinical research in which data are collected from participants remotely to enable participants to complete study requirements from home, while still being closely monitored by healthcare professionals.
No large-scale phase three CVD trial has yet employed a fully decentralized protocol; however, trials integrating a range of individual digital health technologies for collecting heart health measurements have demonstrated feasibility. This commentary presents a range of opportunities to employ emerging technologies and decentralized approaches to eliminate bottlenecks in patient recruitment and retention in clinical trials and mitigate health disparities.
Clinical Trial Technologies for Improving Equity and Inclusion
Breakthrough technologies in clinical trials include synthetic biologies, digital twins, big data analytics, wearable devices and sensors, telemedicine, and electronic informed consent. These advancements have the potential to harness more comprehensive and accurate, real-world data (RWD) from participants and allow researchers to analyze a wider range of health metrics in real-time. Clinical trialists should thoughtfully select and implement technology solutions that improve the efficiency, accessibility, accuracy, and generalizability of their study, and ultimately better patient experiences and outcomes.
Selecting the appropriate technologies for CVD trials can be a complex process that involves several factors, including considering alignment with the research question, regulatory compliance, validity and reliability of the technologies themselves, appropriateness and user-friendliness, and cost. Technologies are the engine driving DCTs. However, technologies may also have unintended consequences that exclude certain groups of patients with limited access to the internet, smartphones, or other digital devices. For example, the Fitbit Heart Study targeted existing consumers who may have been concerned about heart rhythm abnormalities prior to study enrollment. This study design raises concerns about inclusive screening and generalizability [18]. Similarly, a recent DCT in atrial fibrillation patients reported success in rapid recruitment, high engagement, and physiologic reporting via the integration of digital technologies, but indicated a bias toward recruiting high-income, highly educated women who were more responsive to social media-based recruitment [19].
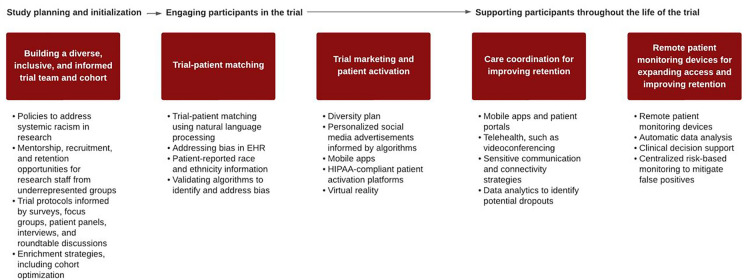
Cardiovascular clinical trials present a natural opportunity to leverage digital technologies due to the way CVD is monitored and managed. Patients with CVD monitor relevant biological signals continuously or near continuously, including heart rate, blood pressure, oxygen saturation, and electrocardiogram (ECG) readings. Non-invasive technologies are available on the consumer market, including smartwatches and other wearable devices and sensors, to collect physiological measurements from the comfort of the home. Non-invasive data collection and remote patient monitoring (RPM) are more accessible, comfortable, and appealing to participants, which may help to alleviate the former costs and inconveniences that prevented participation in clinical research. The following framework outlines practical “entry points” to incorporate innovative technologies and decentralized approaches, which, when paired with carefully planned research design and recruitment strategies, have the potential to enhance diversity in clinical research (Fig. 1).
Fig. 1.
Entry points and opportunities to increase clinical trial diversity using technology. EHR Electronic health record, HIPPA U.S. Health Insurance Portability and Accountability Act
Study Planning and Initialization
Building a Diverse, Inclusive, and Informed Trial Team and Cohort
Research leadership should institute policies to address systemic racism in research and develop mentorship, recruitment, and retention opportunities for research staff from underrepresented groups. The diversification of trial investigators and staff might help in the recruitment of participants from different backgrounds by connecting with participants in ways that are culturally competent [9]. Trial protocols should include patient-centered processes informed by surveys, focus groups, patient panels, interviews, and roundtable discussions, all of which can be conducted through cloud-based video conferencing platforms, online survey tools, automated chatbots, and other digital infrastructure. These methods allow for feedback on trial design to be collected from a diverse range of participants.
New statistical methods, such as cohort optimization and synthetic control arms, can be used to better define patient groups that are more likely to experience outcomes of clinical interest. This knowledge can be used to define inclusion and exclusion criteria and empower sponsors to achieve the same level of statistical significance with smaller trial arms and a shorter trial period.
The FDA defines three broad categories of enrichment strategies, such as cohort optimization, including:
Strategies to decrease variability: choosing patients with baseline measurements of a disease or biomarker characterizing the disease in a narrow range and excluding patients whose disease or symptoms improve spontaneously or whose measurements are highly variable
Prognostic enrichment strategies: choosing patients with a greater likelihood of having a disease-related endpoint event or a substantial worsening in condition
Predictive enrichment strategies: choosing patients who are more likely to respond to the drug treatment than other patients with the condition being treated based on a specific aspect of a patient’s physiology, a biomarker, or disease characteristic that is related to the study drug’s mechanism [20]
In recent years, prognostic enrichment strategies have been introduced in CVD trials. These strategies involve evaluating a patient's history of myocardial infarction (MI) or stroke; the presence of comorbidities, such as diabetes or hypertension; and the presence/level of certain blood markers, such as very high low-density lipoprotein (LDL) cholesterol, low high-density lipoprotein (HDL) cholesterol, and high C-reactive protein (CRP). This approach has been demonstrated in outcome studies involving ACE inhibitors for HF and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors for hyperlipidemia (for enalapril and statin trials, respectively) [20]. Similarly, synthetic control arms use statistical models to create a control group that closely matches the characteristics of the patients who are receiving the treatment. Synthetic control arms can be constructed by combining data from multiple sources, such as clinical trial databases, disease registries, and medical records, including RWD. These approaches can reduce the need for a traditional control group, which may be difficult to recruit from diverse populations due to eligibility criteria or other logistical challenges. Additionally, these approaches can reduce the number of patients needed to be recruited for the trial, and potentially make the study more accessible and inclusive.
Engaging Participants in the trial
Trial-Patient Matching
Trial–patient matching involves screening potential study participants to determine whether they meet the inclusion and exclusion criteria for the trial. This process often involves hours of labor-intensive review of medical records, conducting physical exams, and administering diagnostic tests to assess the patient's eligibility [21]. The goal of trial patient matching is to identify eligible patients who are likely to benefit from the study intervention while also minimizing the risk of adverse events.
Advancements in natural language processing (NLP) can enable clinical trial sites to markedly reduce the pool of potential candidates for enrollment and speed up the trial-patient matching process [21]. NLP can be used to extract information such as age, gender, medical history, medication use, laboratory results (e.g., cholesterol levels, and cardiac biomarkers), comorbidities, procedures (e.g., cardiac catheterization or surgery), adverse events, and other relevant clinical variables from electronic health records (EHRs). This information can be compared against the eligibility criteria for the clinical trial to identify potential candidates. However, the use of EHRs can introduce bias and errors since patients in EHR systems may not be representative of the general population due to factors such as informed presence bias, perceptions about the healthcare system that influence entry, and disparate access to health services [22]. Addressing bias requires better data capture and minimizing misclassification by staff labeling individuals based on their physical characteristics, name, or zip code [23]. Self-reported race and ethnicity information can also be collected directly from patients via patient portals or other digital platforms. Additionally, NLP algorithms should be validated to identify and address any potential bias or errors.
Trial Marketing and Patient Activation
Emerging technologies have the potential to revolutionize clinical trial marketing by providing new tools and methods to reach out to potential participants and improve recruitment and retention rates. The FDA will soon require sponsors seeking approval for late-stage clinical trials to submit a plan for ensuring diversity among trial participants. Diversity plans will require pre-specified demographic parameters for a representative study population, a plan to recruit underrepresented communities using appropriate marketing materials and patient activation strategies, and inclusion criteria that do not unnecessarily exclude diverse populations. Before the requirement takes effect, however, the FDA must first finalize its draft guidance and then offer the public an opportunity to comment. These steps could take more than 2 years.
Technologies that support trial marketing and patient activation include personalized social media advertisements informed by algorithms and mobile apps designed to educate potential participants about trials and provide information on eligibility criteria, study details, and the enrollment process. The Objective Randomized Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina (ORBITA) trial of a percutaneous coronary intervention (PCI) for patients with stable angina used social media advertising to recruit participants based on their demographic characteristics and social media activity. The advertisements were run on Facebook and included a brief description of the study and a link to a screening survey. The ORBITA trial reported that social media advertising was effective in recruiting a large number of participants from diverse demographic backgrounds [24].
Social media advertising connected with the U.S. Health Insurance Portability and Accountability Act (HIPAA)-compliant patient activation platforms can make for a more seamless experience in recruiting, consenting, and enrolling patients in clinical research. StudyPages is an example of a patient activation platform that helps patients find clinical trials that are relevant to their medical condition or interests and stay engaged and informed throughout the clinical trial process. The platform supports medication and symptom tracking, issues reminders about study consultations, and facilitates communication with study staff.
Additionally, virtual reality (VR) is beginning to be tested as a facilitator for clinical trial recruitment. VR can provide an immersive experience of what it is like to participate in a clinical trial, where potential participants can see what the trial involves, understand the benefits and risks, and ask questions in a safe and controlled environment. Emerging technologies that enable more personalized trial marketing and patient activation via a combination of targeted outreach, tailored educational resources, culturally sensitive communication, and patient-centered design (e.g., easy-to-use interfaces, personalized search criteria, and support for patients with disabilities or language barriers) have important implications for increasing awareness and access to trials among underrepresented patient populations.
Supporting Participants Throughout the Life of the Trial
Care Coordination for Improving Retention
Loss to follow-up (LTFU) is a common phenomenon in clinical trials. More than three-quarters of cardiovascular clinical trials have participants who are LTFU, and the statistical handling of these data varies widely [25]. In cardiovascular rehabilitation (CR) trials, dropout rates are as high as 39% [26]. The main factors related to dropout include clinical factors, logistical factors, and health system factors [27]. LTFU is problematic because it causes attrition bias and can drive either overestimation or underestimation of treatment effects [28].
Care coordination technologies can support retention and minimize LTFU. Examples of care coordination technologies include:
Mobile apps to support patient self-management, monitor medication adherence, and communication with their providers through secure messaging platforms
Patient portals that allow patients to access their health information, easily share information with their providers, and receive reminders about upcoming appointments or medication schedules
Telehealth technologies, such as videoconferencing alleviate the costs and inconveniences of having to travel to a clinic
Clinical decision support systems (CDSS) that provide evidence-based recommendations for treatment and care management to providers.
These solutions can help build trust among underrepresented populations by providing services in multiple languages or using images and messaging that resonate with different groups. However, it should be noted that care coordination technologies are often developed with minimal input from patients and should be revised to meet the needs of the end-user, including users with sight impairments, arthritis, impaired cognitive function, or other disabling conditions.
Clinical trial teams that include bilingual patient navigators have been shown to improve clinical outcomes for patients at risk of CVD [21]. Care coordination technologies that capitalize on sensitive communication and connectivity strategies may also encourage patient retention. Additionally, care coordination technologies can track and analyze patient data, and provide better insights into patient behavior to identify patterns that may impact retention. As a result, data analytics can be used to identify patients who are at risk of dropping out of a trial and initiate targeted interventions to keep them engaged.
RPM Devices for Expanding Access and Improving Retention
Remote patient monitoring devices make it possible to conduct clinical trials remotely and in new site locations, such as in pharmacies and via in-home nursing consultations. RPM devices commonly used in CVD trials include blood pressure monitors, ECG monitors, implantable cardioverter-defibrillators, continuous glucose monitors, and mHealth devices, such as mobile apps and wearable devices that can be used to monitor physical activity, sleep patterns, and other biometric monitors. These devices can provide real-time data, automatic data analysis, and clinical decision support to investigators receiving these signals from patients. RPM devices are being used to support an increasing number of clinical trials across therapeutic areas, including CVD, asthma, cancer, schizophrenia, and diabetes. However, more sophisticated machine learning approaches and sensor capabilities are needed to address nondiagnostic tracings and false positives. Furthermore, the usage of centralized risk-based monitoring (CRBM) can help to ensure the safety and quality of data being collected from RPM devices. CRBM uses statistical methods, algorithms, or other automated tools to detect anomalies and trends in data and mitigate the risk of false positives [29]. As RPM devices, sensor capabilities, and machine learning algorithms become more sophisticated and precise, the possibility of conducting fully decentralized trials becomes more feasible.
Some RPM devices and features are considered Software as a Medical Device (SaMD). SaMD products are subject to regulatory oversight and must meet certain standards for safety and effectiveness The FDA describes the control condition, a “sham,” as the medical device equivalent of a placebo control in pharmacological trials. A “sham” can be an ineffective device or a simulated procedure that used conditions designed to resemble the conditions of use under investigation. Given the general low-risk profile and potential of SaMD to improve access to health care, many trialists choose a less stringent minimal control or wait list control, in which the subjects in the control group do not receive the intervention during the initial phase of the study, rather they are put on a waiting list to receive the intervention at a later time, after the initial study period has ended. Alternatively, for efficacy studies conducted under controlled, artificial settings, minimal digital controls in the form of regular engagement with general digital content (known as digital diversions) may be appropriate [30]. There is an increasing number of clinical trials testing and validating RPM devices due to the growing interest in and adoption of RPM technology as a means of improving patient care and outcomes, particularly for CVD and other chronic conditions. These technologies can expand access to clinical trials and improve retention rates by enabling investigators to record physiological measurements without participants needing to travel to a clinic.
The Future of Inclusive Clinical Trial Design
Advancements in clinical trial technologies have initiated the transformation of research design and methods by enabling DCTs. The future state of clinical trial design has the potential to be more inclusive by incorporating technologies that address the needs and preferences of diverse patient populations, including underrepresented groups, and by utilizing patient-centered approaches that prioritize patient input and feedback throughout the research process. These approaches will make use of care coordination technologies and RPM devices, including smartwatches and other commercially available personal health devices, that streamline the patient experience. New statistical methods will reduce the need for traditional control groups and accelerate bench-to-bedside research timelines.
By learning from previous trials, we can identify the potential benefits and limitations of incorporating RPM devices and other decentralized technologies into clinical trial design and use this knowledge to inform a future of inclusive clinical trial design that maximizes patient participation and engagement while also ensuring scientific rigor and integrity.
The Decentralized Trial in Afib Patients (DeTAP) is one example that demonstrated how participants can successfully self-administer and transmit heart health data via RPM devices and remain highly engaged in a DCT. DeTAP was conducted over a period of 6 months with 100 atrial fibrillation (Afib) patients on oral anticoagulation (OAC) aged > 55 years. The trial evaluated four primary endpoints, including changes in pre- versus end-of-study OAC adherence, completion of tele-visits, surveys, and ECG and blood pressure measurements. These findings are consistent with trends observed in contemporary, foundational studies that employed RPM devices, including the Apple Heart Study and the Fitbit Heart Study. These single-arm trials employed smartwatches to screen for and detect CVD conditions in large populations.
The Apple Watch, Fitbit, and other smartwatches are generally considered to be wellness devices rather than medical devices, and they are not approved for use in clinical settings. However, some features have been cleared by the FDA. Specifically, advanced health monitoring features, including the Fitbit ECG app, are regulated by the FDA as Software as a Medical Device (SaMD) and are subject to requirements for validation and verification. Randomized controlled trials are an important method for gathering evidence in the field of SaMD, especially for those seeking regulatory approval. The strictness of the control conditions used may vary depending on factors such as the risk level and novelty of the intervention being tested.
Real-world and pragmatic study approaches are gaining popularity and have been recommended to support regulatory decisions [30]. Moving toward a future state of inclusive DCTs will involve building on the lessons learned from foundational studies and adapting to increasing regulatory oversight of SaMD.
Conclusion
Clinical trial technologies have the potential to improve research and address health disparities by making trials more accessible, engaging, and informative for participants, and by providing researchers with higher quality data and insights. The COVID-19 pandemic led to the rapid proliferation of innovative technologies, particularly technologies which connect patients to health care beyond the four walls of a clinical practice. Such technologies include synthetic biologies, digital twins, big data analytics, targeted advertising, VR, RPM devices, telemedicine, electronic informed consent, mobile apps/patient portals, CDSS, and new enrichment strategies (e.g., cohort optimization). By leveraging the power of new technologies, we can expect to see accelerated research and development (R&D) timelines, and improvements in patient outcomes and overall healthcare delivery in the years ahead. These efforts necessitate the incorporation of principles of health equity and inclusion into the pharmaceutical life-cycle from R&D to manufacturing and delivery of medicines. Thus, it is critical for sponsors and clinical trialists to capitalize on “entry points” and opportunities to incorporate innovative technologies and decentralized approaches, which, when paired with carefully planned research design and recruitment strategies, have the potential to enhance diversity in clinical research.
Acknowledgements
Funding
Fatima Rodriguez received support from the National Institutes of Health National Heart, Lung, and Blood Institute (1K01HL144607), the American Heart Association/Robert Wood Johnson Harold Amos Medical Faculty Development Program, and Grant #2022051 from the Doris Duke Charitable Foundation.
Author Contributions
All authors contributed to the study conception. Cassandra Broadwin conducted the literature search, data analysis, and original draft preparation. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Fatima Rodriguez serves as an advisor to HealthPals and has served as a consultant for Novartis, Novo Nordisk, and AstraZeneca. Cassandra Broadwin and Zahra Azizi have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Goodson N, Wicks P, Morgan J, Hashem L, Callinan S, Reites J. Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. NPJ Digit Med. 2022;5(1):58. doi: 10.1038/s41746-022-00603-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore TJ, Zhang H, Anderson G, Alexander GC. Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration, 2015–2016. JAMA Intern Med. 2018;178(11):1451–1457. doi: 10.1001/jamainternmed.2018.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habibzadeh F. Disparity in the selection of patients in clinical trials. Lancet. 2022;399(10329):1048. doi: 10.1016/S0140-6736(22)00176-3. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser P, Diez Roux AV, Mujahid M, et al. Neighborhood environments and incident hypertension in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2016;183(11):988–997. doi: 10.1093/aje/kwv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 6.Flanagin A, Frey T, Christiansen SL, AMA Manual of Style Committee Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 2021;326(7):621–627. doi: 10.1001/jama.2021.13304. [DOI] [PubMed] [Google Scholar]
- 7.Vasisht KP, Nugent BM, Woodcock J. Progress and opportunities for women in clinical trials: a look at recent data and initiatives from the US FDA. Med (New York, N.Y.) 2021;2(5):456–459. doi: 10.1016/j.medj.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Purnell TS, Calhoun EA, Golden SH, et al. Achieving health equity: closing the gaps in health care disparities, interventions, and research. Health Aff (Millwood) . 2016;35(8):1410–1415. doi: 10.1377/hlthaff.2016.0158. [DOI] [PubMed] [Google Scholar]
- 9.Michos ED, Van Spall HG. Increasing representation and diversity in cardiovascular clinical trial populations. Nat Rev Cardiol. 2021;18(8):537–538. doi: 10.1038/s41569-021-00583-8. [DOI] [PubMed] [Google Scholar]
- 10.Oertelt-Prigione S, Regitz-Zagrosek V. Gender aspects in cardiovascular pharmacology. J Cardiovasc Transl Res. 2009;2:258–266. doi: 10.1007/s12265-009-9114-9. [DOI] [PubMed] [Google Scholar]
- 11.Khan SU, Khan MZ, Subramanian CR, et al. Participation of women and older participants in randomized clinical trials of lipid-lowering therapies: a systematic review. JAMA Netw Open. 2020;3(5):e205202-e. doi: 10.1001/jamanetworkopen.2020.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferdinand KC, Yadav K, Nasser SA, et al. Disparities in hypertension and cardiovascular disease in blacks: the critical role of medication adherence. J Clin Hypertens. 2017;19(10):1015–1024. doi: 10.1111/jch.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whyte J. Racial and ethnic representation of participants in US clinical trials of new drugs and biologics. JAMA. 2022;327(10):985. doi: 10.1001/jama.2022.0875. [DOI] [PubMed] [Google Scholar]
- 14.Sarraju A, Maron DJ, Rodriguez F. Under-reporting and under-representation of racial/ethnic minorities in major atrial fibrillation clinical trials. Clin Electrophysiol. 2020;6(6):739–741. doi: 10.1016/j.jacep.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration Office of Minority Health. Clinical trial diversity stakeholder communications toolkit. 2016. https://www.census.gov/quickfacts/fact/table/US/PST045218. Accessed 14 Mar 2023.
- 16.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Enhancing the diversity of clinical trial populations—eligibility criteria, enrollment practices, and trial designs guidance for industry. 2020. https://www.fda.gov/media/127712/download. Accessed 14 Mar 2023.
- 17.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), et al. Conduct of clinical trials of medical products during the COVID-19 public health emergency. Guidance for industry, investigators, and institutional review boards. 2021. https://www.fda.gov/media/136238/download. Accessed 14 Mar 2023.
- 18.Lubitz SA, Faranesh AZ, Selvaggi C, et al. Detection of atrial fibrillation in a large population using wearable devices: the fitbit heart study. Circulation. 2022;146(19):1415–1424. doi: 10.1161/CIRCULATIONAHA.122.060291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarraju A, Seninger C, Parameswaran V, et al. Pandemic-proof recruitment and engagement in a fully decentralized trial in atrial fibrillation patients (DeTAP) NPJ Digit Med. 2022;5(1):80. doi: 10.1038/s41746-022-00622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products guidance for industry. 2019. https://www.fda.gov/media/121320/download. Accessed 14 Mar 2023.
- 21.Ni Y, Kennebeck S, Dexheimer JW, et al. Automated clinical trial eligibility prescreening: increasing the efficiency of patient identification for clinical trials in the emergency department. J Am Med Inform Assoc. 2015;22(1):166–178. doi: 10.1136/amiajnl-2014-002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bower JK, Patel S, Rudy JE, Felix AS. Addressing bias in electronic health record-based surveillance of cardiovascular disease risk: finding the signal through the noise. Curr Epidemiol Rep. 2017;4(4):346–352. doi: 10.1007/s40471-017-0130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breathett K, Spatz ES, Kramer DB, et al. The groundwater of racial and ethnic disparities research: a statement from circulation: cardiovascular quality and outcomes. Circ Cardiovasc Qual Outcomes. 2021;14(2):e007868. doi: 10.1161/CIRCOUTCOMES.121.007868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391(10115):31–40. doi: 10.1016/S0140-6736(17)32714-9. [DOI] [PubMed] [Google Scholar]
- 25.Fong LCW, Ford TJ, da Costa BR, Jüni P, Berry C. Bias and loss to follow-up in cardiovascular randomized trials: a systematic review. J Am Heart Assoc. 2020;9(14):e015361. doi: 10.1161/JAHA.119.015361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer CG, Jørgensen LB, Blume B, et al. Dropout during a 12-week transitional exercise-based cardiac rehabilitation programme: a mixed-methods prospective cohort study. Eur J Cardiovasc Nurs. 2022;21(6):578–586. doi: 10.1093/eurjcn/zvab119. [DOI] [PubMed] [Google Scholar]
- 27.Resurrección DM, Moreno-Peral P, Gómez-Herranz M, et al. Factors associated with non-participation in and dropout from cardiac rehabilitation programmes: a systematic review of prospective cohort studies. Eur J Cardiovasc Nurs. 2019;18(1):38–47. doi: 10.1177/1474515118783157. [DOI] [PubMed] [Google Scholar]
- 28.Valgimigli M, Garcia-Garcia HM, Vrijens B, et al. Standardized classification and framework for reporting, interpreting, and analysing medication non-adherence in cardiovascular clinical trials: a consensus report from the Non-adherence Academic Research Consortium (NARC) Eur Heart J. 2019;40(25):2070–2085. doi: 10.1093/eurheartj/ehy377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steve Y, Dan B, Vera P, Rowe J. The eradication of false signals in monitoring. 2022. https://www.appliedclinicaltrialsonline.com/view/anticipating-careless-responders-in-survey-design-and-analysis. Accessed 14 Mar 2023.
- 30.Lutz J, Offidani E, Taraboanta L, Lakhan SE, Campellone TR. Appropriate controls for digital therapeutic clinical trials: a narrative review of control conditions in clinical trials of digital therapeutics (DTx) deploying psychosocial, cognitive, or behavioral content. Front Digit Health. 2022;4:823977. 10.3389/fdgth.2022.823977. [DOI] [PMC free article] [PubMed]