Key words: Coevolution, evolution, parasite–host interaction, vector-borne diseases, vector ecology, virulence
Abstract
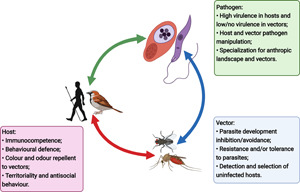
Transmission mode is a key factor that influences host–parasite coevolution. Vector-borne pathogens are among the most important disease agents for humans and wildlife due to their broad distribution, high diversity, prevalence and lethality. They comprise some of the most important and widespread human pathogens, such as yellow fever, leishmania and malaria. Vector-borne parasites (in this review, those transmitted by blood-feeding Diptera) follow unique transmission routes towards their vertebrate hosts. Consequently, each part of this tri-partite (i.e. parasite, vector and host) interaction can influence co- and counter-evolutionary pressures among antagonists. This mode of transmission may favour the evolution of greater virulence to the vertebrate host; however, pathogen–vector interactions can also have a broad spectrum of fitness costs to the insect vector. To complete their life cycle, vector-borne pathogens must overcome immune responses from 2 unrelated organisms, since they can activate responses in both vertebrate and invertebrate hosts, possibly creating a trade-off between investments against both types of immunity. Here, we assess how dipteran vector-borne transmission shapes the evolution of hosts, vectors and the pathogens themselves. Hosts, vectors and pathogens co-evolve together in a constant antagonistic arms race with each participant's primary goal being to maximize its performance and fitness.
Introduction
Symbionts live with or within their hosts and represent one of the most successful life-history strategies (Mestre et al., 2020). Due to their evolutionary success, virulent symbionts (i.e. pathogenic parasites) such as protozoans, helminths, bacteria and viruses probably account for over half of the world's biodiversity (Clayton et al., 2015). Indeed, a parasitic mode of life has evolved independently multiple times into variable life-history strategies that include fecal–oral, trophic transmission, airborne transmission and the use of vectors (i.e. mobile blood-feeding invertebrates involved in the transmission of pathogens to new potential hosts) (Weinstein and Kuris, 2016; Wilson et al., 2017). Vector-borne pathogens cause many of the most important infectious diseases that plague humans and animal hosts (Table 1) and will continue to do so in the next decades due to effects of climate change on arthropod vectors' abundance and distribution (Kelly-Hope et al., 2009; Garamszegi, 2011; Pérez-Rodríguez et al., 2014). Vector-borne pathogens include phylogenetically unrelated symbionts whose reliance on vectors emerged independently; hence, vector-borne pathogens present several distinct developmental strategies within their vectors that are reflected in many independent evolutionary histories among hosts, vectors and pathogens.
Table 1.
Pathogen name, pathogen and vector type and number of deaths and cases for the main human vector-borne diseases
| Disease | Pathogen name | Pathogen type | Vector type | Deaths per year | Cases per year | Geographical distribution |
|---|---|---|---|---|---|---|
| Malaria | Plasmodium spp. | Protozoa | Mosquito (Anopheles) | ~600 000 | 200–400 million | Africa, tropical and subtropical parts of Asia and Oceania, Latin America |
| Dengue | Dengue virus | Virus | Mosquito (Aedes) | ~40 000 | 50–100 million | Africa, tropical and subtropical parts of Asia and Oceania, Latin America |
| Leishmaniasis | Leishmania spp. | Protozoa | Sandfly | ~30 000 | ~1.3 million | Africa, tropical and subtropical parts of Asia, Americas |
| Yellow fever | Yellow fever virus | Virus | Mosquito (Aedes) | ~30 000 | ~120 000 | Africa and Latin America |
| Japanese encephalitis | Japanese encephalitis virus | Virus | Mosquito (Culex) | 10 000–20 000 | ~50 000 | East Asia |
| Chagas disease | Trypanosoma cruzi | Protozoa | Kissing bug | ~10 000 | ~7–8 million currently infected | Latin America |
| Lymphatic filariasis | Microfilaria | Nematode | Mosquito | <1000 | ~36 million currently infected | Africa, Asia, Americas and Pacific Islands |
| Chikungunya | Chikungunya virus | Virus | Mosquito (Aedes) | <1000 | 200 000–600 000 | Mostly Asia and Americas |
| Lyme disease | Borrelia bacteria | Bacteria | Tick | <1000 | 300 000–400 000 | North America, Europe and North Asia |
| Crimean-Congo haemorrhagic fever | Crimean-Congo haemorrhagic fever virus | Virus | Tick | 30% infected people | Variable | Africa, West Asia and East Europe |
Pathogens are subjected to several evolutionary selective pressures that are intrinsically dependent on their mode of transmission and dispersal ability (Ewald, 1995; Frank, 1996; Powell, 2019). For example, pathogens transmitted by mobile vectors might evolve towards phenotypes of higher virulence (i.e. extent of damage that a consumer inflicts to organisms being exploited) against the vertebrate host than related pathogens that rely on dispersal via a single host (Ewald, 1983; Day, 2002). Other differences in pathogen life cycle may also modulate host–parasite evolution (Frank, 1996; Powell, 2019; Mestre et al., 2020). For instance, many trophically transmitted parasites benefit from an infection-induced increase in their host vulnerability to predation (Moore, 2002; Poulin et al., 2005) as it enhances their chance of transmission to their next host. For this reason, evolution favoured trophically transmitted parasites that could manipulate their hosts' behaviour to specifically increase their risk of predation (Moore, 2002). Horizontally transmitted pathogens (i.e. pathogens transmitted among hosts outside the strict parent–offspring relationship) are subjected to a trade-off between increasing their reproduction and keeping their host alive, since increases in pathogen replication are generally associated with greater virulence through more aggressive exploitation of resources that can kill the host (Ewald, 1983; Giorgio, 1995; Frank, 1996; Davies et al., 2001). Therefore, pathogen selection should favour a balance between replication and virulence that leads to the highest lifetime transmission success.
Among horizontally transmitted pathogens, those transmitted by vectors face unique trade-offs because they must invade, escape immune defences and be transmitted between 2 phylogenetically distant organisms (i.e. hosts and vectors), with distinct immune systems. Here, we explore the evolutionary pressures and consequences of the use of dipteran vectors for the pathogens, hosts and the vectors themselves, hereby unifying these 3 components in a common framework. By applying this framework, we aim to identify potential gains and deleterious effects of co- and counter-evolution among the host–vector–pathogen triad to highlight trends in host–vector–pathogen evolution. We focus mostly on vertebrate hosts, such as mammals and birds, which sustain pathogen development and transmission via haematophagous dipteran vectors. Then, we discuss how each component of this triad influences the selective evolutionary pressures acting on the 2 other counterparts and propose new research directions.
Evolutionary consequences of vector transmission for vertebrate hosts
Certain host species’ traits and individual behaviours can be associated with infection risk by vector-borne pathogens, such as body size and preening behaviour (Bush and Clayton, 2018; Filion et al., 2020). For mosquitoes (family Culicidae) and sand flies (family Psychodidae), olfactory cues seem to be the main driver of host detection. Several studies have investigated the effects of odours and identified many odourants positively or negatively related to vector attraction (Lucas-Barbosa et al., 2021; Yan et al., 2021). In addition, carbon dioxide (CO2) has been recognized as one of the most important olfactory cues for host-seeking behaviour (Pinto et al., 2001; Müller et al., 2015; Yan et al., 2021). Larger body sizes emit greater volumes of carbon dioxide, increasing vector attractiveness (Daviews et al., 1991; Yan et al., 2018). Similar relationships exist for body temperature, as higher body temperatures also lead to higher emission of carbon dioxide (van Loon et al., 2015).
While vectors rely mainly on olfactory cues to locate hosts at long distances, visual cues are markedly important for short-range host choice (Cardé and Gibson, 2010). For example, coloration patterns can influence vector landing on their hosts, and there is evidence showing that darker colours are more attractive to mosquitos compared to lighter ones (Yan et al., 2021). Nonetheless, contrast against the background seems to be a more important cue for vector attraction than colours and intensity on their own (Yan et al., 2021). On the other hand, stripes seem to confer protection for the host. How et al. (2020) demonstrated that tabanid flies attempting to approach horses dressed in striped rugs remained more distant from the target and achieved lower landing success than flies approaching horses wearing black or grey rugs. However, the mechanism by which stripes protect zebras from these vectors is still poorly understood. Since vector attraction is shaped by host features that vary within species such as odour, size (Lucas-Barbosa et al., 2021) and colour (Yan et al., 2021), host individuals presenting traits less attractive or even repellent to vectors could benefit from lower pathogen exposure, potentially achieving higher fitness compared to their infected counterparts. Those traits (e.g. coloration and body size) are also subject to other selection pressures such as mating success, intraspecific competition and predator avoidance; therefore, selection for anti-vector traits should be balanced against selection for other fitness-enhancing functions.
Hosts from the same species may possess variable attractiveness to vectors. Prasadini (2019) suggested that Aedes aegypti mosquitoes fed preferably on people belonging to the blood type ‘O’ and that the blood type ‘A’ may confer protection against some diseases, such as dengue and zika, as people classified in this group incur the lowest biting rates. On the other hand, Goel et al. (2015) have shown that Plasmodium falciparum binds preferably to blood group ‘A’ cells, increasing formation of rosettes, severity of infection and potentially contributing to the heterogeneous distribution of ABO blood groups worldwide by favouring blood group ‘O’. Malaria is among the strongest evolutionary pressures in late human history (Hedrick, 2011) and, as a result, vectors and pathogens exert pressure on their vertebrate hosts through distinct pathways that drive the selection of distinct host phenotypes.
Anti-pathogen and anti-vector behaviours such as preening/grooming, scratching and nest maintenance are commonly observed in nature (Bush and Clayton, 2018; Sarabian et al., 2018; Poulin et al., 2020). Pathogen avoidance strategies can be costly to their hosts since they demand resources and may cause hosts to miss out on foraging and mating opportunities (Poulin et al., 2020). Preening/grooming behaviour is an effective strategy used by animals to control ectoparasite load and possibly avoid vector-borne infections. However, this does not necessarily reduce infection risk by pathogens transmitted by those vectors (Waite et al., 2014). At the same time, for animals socially organized into groups or colonies, preening/grooming of potential vectors (e.g. flies) might increase general pathogen prevalence. This may happen because host cleaning can induce vectors to move to new host individuals, increasing pathogen dissemination within the colony (Bush and Clayton, 2018). Moreover, the undue annoyance and vigorous swatting behaviour displayed by many animals are disproportionate to the amount of blood removed by the insect and the effect of the blood loss on fitness. In other words, the direct fitness loss associated with blood feeding by the occasional vector is often smaller than the indirect fitness loss associated with pathogen transmission; hence the latter is expected to exert a much stronger selective pressure for the host.
Hosts should evolve towards phenotypes of pathogen resistance (i.e. host capability to limit pathogen proliferation) or tolerance (i.e. host capability to reduce pathogenic effects of infection without controlling pathogen load/burden) depending on the cost of infection (Singh and Best, 2021). Indeed, introduction of the avian malaria parasite Plasmodium relictum has seemingly driven evolution of the Hawaiian honeycreeper amakihi Chlorodrepanis virens by selecting resistant/tolerant populations due to the strong selective pressure exerted by the parasite (Atkinson et al., 2013). In this case, immune-related genes were inferred to be under selection in areas with high rates of Plasmodium transmission (Cassin-Sackett et al., 2019). At the same time, avian malaria has been a major cause of extinction and population declines in the Hawaiian Islands (Van Riper et al., 1986; Lapointe et al., 2012), indicating that evolution of tolerance to this novel pathogen does not occur for all bird species. Since vector-borne pathogens are generally more virulent than other pathogens (Ewald, 1983, 1995; Frank, 1996), they may exert stronger selective pressures driving host evolution (Woolhouse et al., 2002). A classic example of host counter-evolution to vector-borne parasites is the high prevalence of the sickle cell haemoglobin gene in highly endemic human malaria regions in Africa (Hedrick, 2011). This gene induces malformation of red blood cells and, consequently, weakens the ability of cells to transport oxygen. In these regions, however, the benefit arising from malaria resistance surpasses the deleterious effects due to lower oxygen transport, which allows the maintenance of high frequencies of the sickle cell haemoglobin gene in human populations (Hedrick, 2011). Vector-borne pathogens should promote the evolution of protective host phenotypes (e.g. low vector attraction, high tolerance to infection) which are shaped by pathogens, vectors and other biotic and abiotic (i.e. interaction with other organisms and environmental conditions, respectively) selective pressures over evolutionary time.
Evolutionary consequences of vector transmission for vectors
As presented above, theoretical and empirical data support the notion that vector-borne pathogens can pose high costs to their vertebrate host. However, what are the pathogen replication/virulence trade-offs in relation to transmission success from the vector's perspective? Plasmodium parasites may reduce either vector survivorship (Ferguson and Read, 2002; Lambrechts and Scott, 2009) or fertility (Pigeault and Villa, 2018). Nevertheless, it is often difficult to estimate whether the presence of blood parasites decreases vector fitness and survivorship by direct deleterious effects or as a mere consequence of lower quality of the infected blood (Ferguson et al., 2003b; Kotepui et al., 2014; Pigeault et al., 2015). However, infection by some avian Plasmodium can increase vector survivorship (Vézilier et al., 2012; Gutiérrez-López et al., 2020), a phenotypic alteration that favours parasite transmission. Although mosquito-borne viruses can be pathogenic to their vectors (Girard et al., 2005) and may change their behaviour (Jackson et al., 2012), these effects are generally subtle (Halbach et al., 2017). Broadly, Alphaviruses with horizontal transmission (e.g. via blood feeding on infected hosts) are likely to increase mortality in vectors, whereas Bunyaviruses vertically transmitted within Aedes mosquitoes from females to their progeny do not induce mortality in the vector (Lambrechts and Scott, 2009). This happens due to the increased selective pressure that vertically transmitted pathogens face to not harm their vectors (Ebert, 2013).
Pathogens may directly harm their vectors by tissue damage, through activation of the immune system to fight off the infection or by subtracting resources for their own development and replication (Shaw et al., 2022). However, these effects were shown to be subtle at the transcriptome level in interactions between Culex and avian malaria parasites likely to occur in Hawaiʻi (Ferreira et al., 2022). Leishmania parasites cause structural damages in the sand fly (Lutzomyia longipalpis) gut (Schlein et al., 1992), reducing vector longevity without affecting its fecundity (Rogers and Bates, 2007). Therefore, vectors, similarly to vertebrate hosts, would benefit from the evolution of mechanisms that limit either pathogen multiplication (i.e. resistance) or the costs associated with response to the infection (i.e. tolerance). Alternatively, uninfected vectors could avoid feeding on infected hosts if the pathogen is costly to the vectors themselves. This parasite avoidance behaviour has been demonstrated in fewer studies (see Lalubin et al., 2012) when compared to a larger body of studies showing higher vector attraction to infected hosts (Cozzarolo et al., 2020; Santiago-Alarcon and Ferreira, 2020). Nonetheless, some earlier studies also suggest the absence of any effect of infection status on vector attraction (Cozzarolo et al., 2022).
There seems to be a threshold for parasite density within the host at which stochasticity determines the chances of a vector becoming infected (Alizon and van Baalen, 2008). In human malaria, few mosquitoes become infected with Plasmodium vivax and P. falciparum after taking an infectious blood meal, and infection rates are positively correlated with parasite density in the blood source (Nguitragool et al., 2017; Tadesse et al., 2018). Few highly susceptible mosquitoes of the same Anopheles species harbour high parasite burdens when infected with Plasmodium parasites, while most individuals carry only a few oocysts, creating the general overdispersed pattern with a low median number (1–4) of oocysts per mosquito (Bompard et al., 2020; Graumans et al., 2020). In the case of Leishmania parasites, hosts carrying the greatest parasitaemia levels are primarily responsible for vector (sand flies) infection, which in turn will be more likely to infect another vertebrate host (Miller et al., 2014). The overall variability in parasite infection rate and burden among vector specimens vary according to the amount of parasite ingested, which is a factor of blood meal size and parasitaemia (Da et al., 2015; Emami et al., 2017). However, little is known about how individual vector factors such as immune response affect parasite burden, individual mosquito susceptibility to parasite infection and the vector's ability to prevent pathogen development.
High parasite loads might result in vector death (Dawes et al., 2009). Consequently, vectors might evolve towards pathogen inhibition. For instance, mosquitoes can arrest the development of Plasmodium ookinetes and oocysts by melanotic encapsulation (i.e. deposition of melanin on the surface of invading pathogen) or by cell lyses as ookinetes cross the midgut (Beier, 1998; Hoffmann et al., 1999; Wen-Yue et al., 2007). Vectors can also constrain parasite development by degrading sporozoites when these migrate to the salivary glands through the haemolymph (Hillyer et al., 2007). At the same time, development of the pathogens in non-competent vectors can induce very high insect mortality rates. Valkiunas et al. (2014) showed that the avian malaria-like parasites Haemoproteus spp., whose vectors are Culicoides biting midges, kill mosquitoes that feed on birds with high parasite loads even in such abortive infections. However, low parasite burdens in the vertebrate host do not reduce mosquito survival. Therefore, vector avoidance towards hosts infected with deadly pathogens, or inhibition strategies against such pathogens within-vectors, should have been selected over the course of vector–host–pathogen evolution.
Although most research has focused on the impact of parasites on vector biology, vertebrate hosts also evolve behavioural responses and strategies to avoid or suppress vector blood meals (Billingsley et al., 2006). Therefore, vectors should evolve to minimize risks of being killed by the vertebrate host. Indeed, vectors have developed several mechanisms to avoid host defensive behaviours. Nocturnal vectors could benefit from feeding on diurnal hosts, while diurnal vectors would benefit from feeding on nocturnal hosts. For instance, Killeen et al. (2006) observed that about 80% of interactions between people and Anopheles mosquitoes occurred during peak sleeping hours. Feeding when hosts are not active is an advantageous behaviour for vectors because it allows the vectors to avoid behavioural defences. In addition, during blood ingestion, mosquitoes inject vasodilatory, antiplatelet and anti-inflammatory chemicals to reduce their detectability (Billingsley et al., 2006). Together with a blood meal, vectors ingest host immunoglobulins and proteins from the complement system which remain active from a couple of hours to days; these can have deleterious effects, causing reduction in fitness and survival or even death of vectors (Maitre et al., 2022). The host skin microbiome alters vector preference towards individual hosts and these microbes can also modulate host immune responses (Naik et al., 2012). Nevertheless, very little research has yet examined whether host defences are a driver of vector specialization/evolution. Additionally, future studies should investigate the potential association between vertebrate skin microbes and their role in host's immune response against vectors and vector fitness itself. Overall, vectors should benefit from and should evolve towards strategies to avoid pathogen infection, reduce infection damage, inhibit pathogen development and overcome host behavioural and immune defences.
Evolutionary consequences of vector transmission for pathogens
Pathogens can benefit from the use of vectors since it can increase pathogen transmissibility and spatial dispersal due to vector mobility. These advantages occur when the supply of vectors is greater than the supply of vertebrate hosts (Ewald, 1995; Auld and Tinsley, 2015) since mosquitoes can act as both reservoirs and vectors, maintaining and spreading the infection (Santiago-Alarcon et al., 2012). Pathogen evolution favours phenotypes that increase their transmission and fitness within both vectors and hosts (Powell, 2019). Increases in vector biting rates, for example, would benefit vector-borne pathogens by boosting the number of hosts to which the pathogen gets transmitted. Increases in vector feeding persistence (i.e. continued feeding attempts when prevented from feeding or disturbed by the host) should also promote transmission to multiple hosts by enhancing vector biting rates (Rogers and Bates, 2007). At the same time, these behaviours may benefit the vector as they may lead to enhanced resource acquisition from blood meals. Nonetheless, there are costs associated with increasing biting rates for vectors since this strategy should raise the probability of vector death from host defence behaviours. Therefore, strategies that reduce chances of vector death early during the infection by preventing blood meals and increase vector feeding behaviour after parasites reach the infective stages are advantageous for pathogens. Indeed, Cator et al. (2013) observed this pattern when investigating temporal changes in attraction towards hosts in mosquitoes following infection by Plasmodium.
Further, malaria parasites should also benefit from modulating densities of gametocytes (i.e. parasite sexual stage that precedes vector development) circulating in host blood (Churcher et al., 2015). Such adjustments to gametocyte densities and parasitaemia can be shaped by the biting behaviour of vectors in malaria parasites. For instance, the parasite cycle frequently matches the peak of activity of their vectors (e.g. malaria and microfilaria parasites), which favours parasite transmission (Hawking, 1967; Hawking et al., 1968). Cornet et al. (2013) have demonstrated that avian malaria parasites infecting birds exposed to mosquito bites achieve higher parasitaemia than non-exposed ones. Likewise, hosts previously subjected to vector bites are more likely to successfully infect new vectors (Isaïa et al., 2020), suggesting Plasmodium may increase gametocyte production in response to mosquito bites – enhancing their own transmission. Similarly, Leishmania-infected sand flies display increased feeding persistence when harbouring peak levels of the parasite's infective stage (Rogers and Bates, 2007). Infected sand flies usually take an incomplete blood meal, meaning they are likely to engage in further host seeking and feeding. These studies demonstrate how pathogens may evolve to manipulate vectors or change their own development schedule within hosts to increase their success of transmission and complete their life cycle.
According to the ‘parasite manipulation hypothesis’, pathogens often evolve to manipulate their hosts' and vectors' behaviour for increased transmission and performance (Moore, 2002; Poulin et al., 2005). It is advantageous for pathogens that uninfected vectors are particularly attracted to infected hosts, while infected vectors ‘should’ be more attracted to uninfected hosts, as these attraction patterns would lead to higher transmission rates. Pathogens can modify host attractiveness to vectors; however, there is evidence both in support and against the manipulation hypothesis (Santiago-Alarcon and Ferreira, 2020; Yan et al., 2021). Previous studies on human malaria have supported this hypothesis, showing that Anopheles mosquitos are more attracted to infected people (Yan et al., 2021). Likewise, Chelbi et al. (2021) observed that Leishmania-infected hosts are more attractive to sand flies. This phenomenon could be potentially explained by the increased emission of olfactory attractants from infected hosts (Yan et al., 2021). Nonetheless, for birds, contradictory results have been reported with existing research suggesting either an increase, decrease or no effect of host infection status on Culex mosquitos feeding or attraction to hosts (Santiago-Alarcon and Ferreira, 2020). However, avian and mammalian malaria are transmitted by distinct mosquito genera, which may also explain the difference in host attractiveness as a function of infection status.
Another important trait determining pathogen performance is pathogen load, which may follow an optimal developmental schedule in the host and within the vector (Frank, 1996; Elliot et al., 2003; Powell, 2019). Vector-borne pathogens rely on their vectors for transmission and dispersal instead of only relying on their host as do most horizontally transmitted pathogens. Therefore, these pathogens should evolve to have low virulence, or even avirulence, to their vectors because of their critical role in transmission (Elliot et al., 2003). At the same time, higher parasitaemia in the vertebrate hosts, which usually correlates with higher virulence, can be selected for as higher rates of pathogen replication generally enhance transmission to vectors (Ferguson et al., 2003a; Powell, 2019). The use of vectors uncouples pathogen transmission success from host fitness and therefore weakens selection against high virulence. Nonetheless, selection should prevent excessive virulence as the host must be kept alive long enough to pass the infection to new uninfected vectors (Ewald, 1995; Frank, 1996). Additionally, vectors may incur reduced mobility or even succumb to infection if pathogen loads in the vertebrate host are too high (Ferguson et al., 2003a; Gutiérrez-López et al., 2019). Vector-borne pathogens face a trade-off between maintaining high parasitaemia and the survival and performance of their host and vector. However, since parasitaemia is not the only predictor of virulence and vector performance, changes in other pathogen traits might also be selected (e.g. production of toxic metabolites). Pathogens face multiple evolutionary trade-offs; the maximization of their development and replication must be balanced against multiple behavioural (e.g. vector preference towards certain hosts), immune (e.g. haemolysis of infected and uninfected erythrocytes) and physiological (e.g. blood type) traits of their hosts and vectors.
Integrating selection across the host–vector–pathogen triad
Vector-borne transmission comes with multiple trade-offs for pathogens. While some of them can enhance their transmission and/or dispersal and increase their replication rates, they must overcome the challenge of infecting 2 distinct types of hosts. For this reason, any external factor impacting vector or host biology might disrupt pathogen development (e.g. insecticide use, see Box 1). Since vectors are ectothermic organisms that rely on precipitation and moderate/high temperatures for their own development (Forattini, 1995), pathogen development and transmission can be directly constrained by local climatic conditions (Lapointe et al., 2010). Vector-borne diseases such as human malaria and yellow-fever are more common in the tropics or subtropics, and, unlike other pathogens that require a single endothermic species for their transmission (e.g. SARS-CoV-2), the geographical expansion of vector-borne pathogens requires the presence of suitable vertebrate hosts, vectors and adequate climatic conditions. Naturally, populations of the same host species inhabiting different regions of the globe evolve under distinct disease pressures. One of the best-known examples of this phenomenon is the variation in the frequency of malaria resistance alleles among human populations; genes that confer protection can attain 100% prevalence among host populations in endemic areas and be absent from populations in temperate regions (Hedrick, 2011). Thus, vector-borne transmission has probably exerted distinct evolutionary pressures among distinct human (and potentially many other wildlife species) populations across the globe by constraining parasite expansion.
Box 1.
Effects of human activities on pathogen and vector evolution
Habitat modification (e.g. increases in temperature and environmental pollution) and scientific advances (e.g. vaccines and the development of antiparasitic medications) can directly alter vector-borne pathogen evolution by changing the taxonomic composition and abundance of mosquito communities (Forattini, 1995; Ferreira et al., 2016), or by altering pathogen circulation within wildlife populations, respectively (Bonneaud et al., 2009; Loiseau et al., 2010; Fecchio et al., 2021) (Fig. 2). Examples of human interventions that can impact pathogen and vector evolution are:
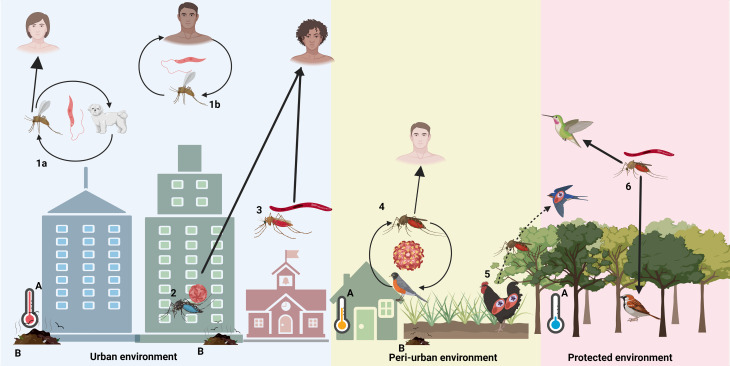
Composition of hosts and vectors in urbanized areas. Urban environments support high densities of hosts and adapted vector species that will inevitably promote pathogen specialization towards human, domestic and synanthropic wild animals under periurban conditions (Harhay et al., 2011; Kilpatrick, 2011; Santiago-Alarcon, 2022), and specific urban-adapted vectors (only 0.1% of all vector species occur in urban habitats (Powell, 2019; Figs 1C and 2).
Unique human habits. Certain human habits, such as housing and the use of mosquito nets and insecticides, can decrease opportunities for vectors to reach and infect hosts, further exacerbating the selective pressures acting on pathogens and vectors in human-modified environments (Fig. 2).
Development of drugs and vaccines. Access to medicine and vaccines creates an extra selective pressure for pathogens, which must overcome the effects of drugs and/or vaccine immunization to complete their life cycle. Those interventions tend to select pathogen strains that are resistant to the drugs or that make their hosts infectious before the onset of symptoms.
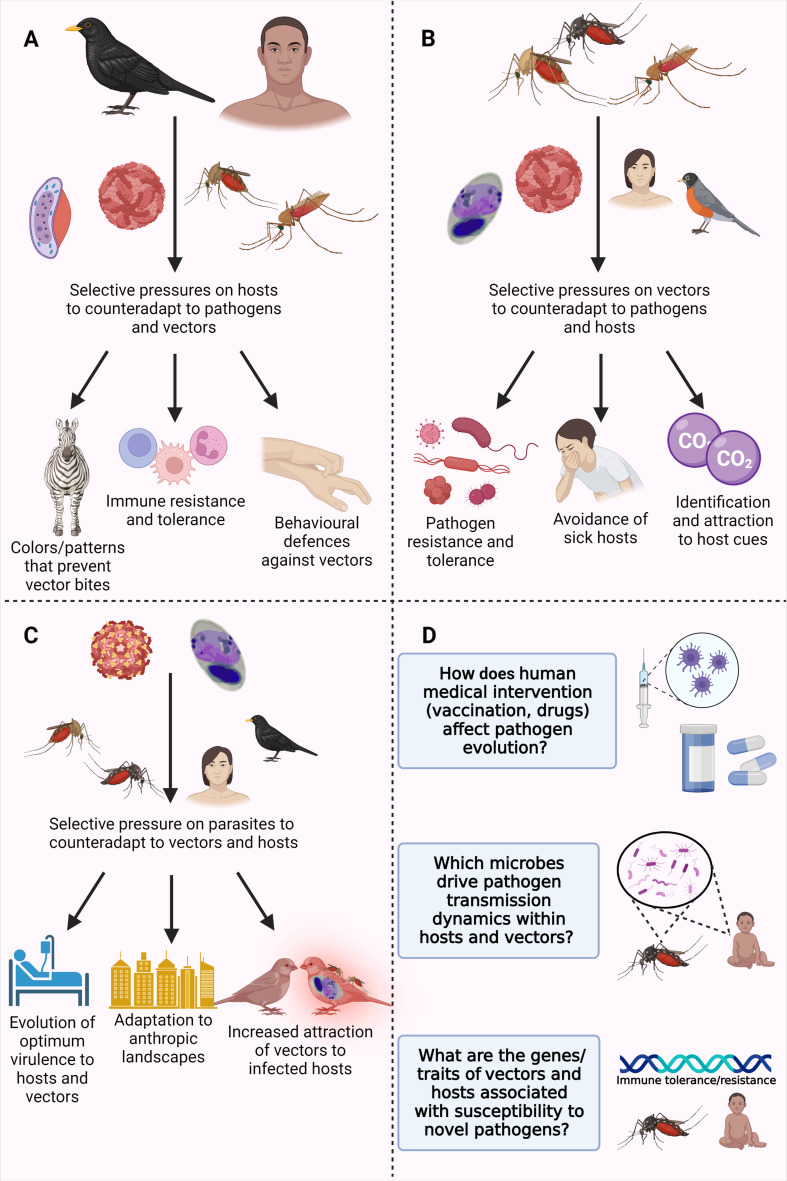
Hosts, vectors and pathogens impose distinct and, frequently, contrasting selective pressures on each other. For instance, vectors and parasites benefit from vectors' host-seeking behaviour and blood meal ingestion, whereas hosts may suffer from being exposed to these parasites. As a result, hosts have evolved mechanisms to avoid vectors, such as defensive and antisocial behaviours and colours and odours repellent to vectors, to escape the disease agents they carry (Fig. 1A). Vectors have evolved multiple sensory organs to detect and select their hosts based on the cues they emit (e.g. carbon dioxide detection, chemical receptors and visual stimuli) (Lucas-Barbosa et al., 2021) (Fig. 1B). Parasites may manipulate their hosts and vectors to increase both attraction of uninfected vectors towards infected hosts and the number of blood meals taken by an infected vector, thereby improving their own transmission (Fig. 1C). High virulence in vertebrate hosts also increases the susceptibility of infected hosts to vector feeding by minimizing defensive behaviours (Ewald, 1995). It is important to note that reductions in prevalence or parasite load among hosts and vectors can be advantageous for both to avoid the deleterious effects of infection. For example, both hosts and vectors benefit from the vectors' ability to distinguish and feed preferentially on uninfected hosts, as this can ultimately decrease the probability of infection among hosts. Thus, parasite manipulation must overcome both vector and host counter-adaptations (e.g. host resistance).
Fig. 2.
Vector-borne pathogens and their vertebrate hosts and dipteran vectors transmitted in human-modified habitat. (1) Leishmania spp. infects humans via sandfly bites in (a) zoonotic cycles (using domestic dogs as the main reservoirs) and in (b) anthroponotic cycles (i.e. human-to-human transmission). (2) Dengue virus infects mostly humans and is vectored by the mosquito Aedes aegypti. (3) Human malaria parasites are transmitted among humans by Anopheles mosquitoes in residential and agricultural areas. (4) West Nile virus circulates among birds and is vectored by Culex mosquitoes and infects humans mainly in residential and in agricultural areas. (5) Spillover of pathogen from domestic to wildlife animals, here illustrated by the spillover of Plasmodium juxtanucleare from domestic chickens to wild birds (Ferreira-Junior et al., 2018). (6) Avian haemosporidian prevalence has been positively and/or negatively associated with anthropization depending on the parasite genera (e.g. Plasmodium or Haemoproteus), the type of anthropic impact (e.g. farming, urbanization, pollution, etc.) and the geographic region of the study (e.g. Neotropics, Europe, etc.). Urbanization and landscape modifications driven by human activities can have several environmental effects, such as increases in (A) temperature and (B) environmental pollution. Figure created with BioRender.com.
Fig. 1.
Illustration of the main selective pressures acting on hosts (A), vectors (B) and parasites (C), and examples of research questions that still lack answers (D). Figure created with BioRender.com.
Currently, disease spread is a major threat to naïve wildlife (Daszak et al., 2000; Atkinson et al., 2014; Liao et al., 2017). For example, avian malaria and malaria-like parasites have excelled as one of the biggest threats to several bird species worldwide (Banda et al., 2013; Vanstreels et al., 2016; Ricklefs, 2017; McClure et al., 2020). This often happens due to the lack of coevolution, and thus coadaptation, between hosts and pathogens. However, pathogen tolerance can emerge in some populations of highly susceptible naïve species (Atkinson et al., 2013). Furthermore, past research has showed that facilitated adaptation (i.e. mediated by human intervention) can shape the odds of susceptible species/population extinctions in nature (Samuel et al., 2020). Hosts genetically modified to be resistant to infections could reduce a species' probability of extinction over time and place those hosts 1 step ahead in the evolutionary arms race against their pathogens (Samuel et al., 2020). Therefore, natural or facilitated adaptation leading to resistant or tolerant phenotypes might represent the main tool for susceptible hosts to persist when facing the introduction of new deleterious pathogens.
Immune response is an important mechanism deployed by both hosts and vectors to resist or tolerate infections (Hoffmann et al., 1999; Mendonça et al., 2013; Maitre et al., 2022). Vectors and hosts have evolved multiple immune pathways against infection, and both hosts and vectors would benefit indirectly from each other's defences against parasites if these defences were efficient enough to reduce pathogen circulation within a region. At the same time, vector pathogen inhibition tends to increase selective pressures for pathogens that can overcome the vector's immune system. Because vector-borne pathogens often induce lower virulence in their vectors (Elliot et al., 2003), the selective pressure to mount strong immune responses is certainly more evident among vertebrate hosts. For instance, humans use a robust combination of innate and adaptive immune responses against malaria parasites (Mourão et al., 2020). Nonetheless, the host's immune system can act against vectors as well, as some host immunoglobulins can remain active for days, targeting parasites within the vector's midgut and having deleterious effects on, or even causing the death of vectors (Maitre et al., 2022). For this reason, vaccines against vector-borne diseases could target vector survival to reduce pathogen transmission. Host immunoglobulins produced in response to immunization against commensal bacteria inhabiting the vector's midgut can alter the vector's gut microbiome, which can potentially reduce vector fitness and/or competence (Aželytė et al., 2022). Optimizing novel immunization strategies against vectors, pathogens and their microbiomes could exert new evolutionary pressures on vector-borne pathogens.
Vector and host microbiomes can also have profound effects on other facets of vector, host and pathogen interactions. This happens because the cascading effects of gut microbiome disruption can alter not just vector development but also the parasite cycle through indirect effects on co-occurring microbes and, indirectly, pathogen transmission rates (Dennison et al., 2014). For instance, vector microbiomes can alter vector competence due to resource competition or by mediating vector immune responses (Dennison et al., 2014). In addition, skin microbes are known to influence hosts' attractiveness to vectors (Fredrich et al., 2013; Verhulst et al., 2018) and a higher diversity of skin microbes seems associated with limited vector attractiveness, thereby providing protection against vector-borne diseases (Lucas-Barbosa et al., 2021). At the same time, the over exposure to antibiotics (due to direct medical intervention or indirect exposure to antibiotics used in farms and croplands) decreases gut and skin microbiome diversity and alters immune responses (Francino, 2016; Raymann et al., 2018). Hence, excessive use or exposure to antibiotics could increase human and wildlife attractiveness to vectors and their susceptibility to pathogens.
Different pathogens may compete or have synergistic interactions among them within their hosts and/or vectors (Clark et al., 2016, 2020). In the first case, competition might decrease fitness of 1 or more pathogens by limiting host resources available (Harvey et al., 2011; Clark et al., 2016). At the same time, prior infections might facilitate the development of new pathogens due to the weakening of the host immune system generated by the primary infection, favouring pathogens in secondary infections (Vaughan and Turell, 1996; Pollitt et al., 2015). Indeed, Clark et al. (2016) have observed altered heterophil/lymphocyte rates among birds coinfected by microfilaria and haemosporidians, indicating this nematode could facilitate protozoan infections as a result of immune modulation. Overall, there is increasing evidence that vector–host–pathogen interactions are mediated by several other players associated directly or indirectly with the pathogen's cycle (e.g. symbiotic microbes and other pathogens) (Vaughan and Turell, 1996; Dennison et al., 2014; Jupatanakul et al., 2014; Pollitt et al., 2015; Verhulst et al., 2018).
Environmental changes associated with human activities also represent a selective force-driving pathogen and vector evolution (see Box 1). For vector-borne pathogens, temperature, precipitation and distance to water bodies are major drivers of pathogen prevalence due to their direct effects on vector development (Ferraguti et al., 2018, 2020). Anthropogenic landscapes present a distinct microclimate, partly because they often attain higher temperatures, which can affect both the abundance and richness of vectors (Ferraguti et al., 2016) and, hence, favour the transmission of pathogens able to develop in the few vector species remaining. In addition, other human landscape interventions can shape vector evolution, such as larvicide treatments, which have been implemented in many areas to regulate mosquito populations. These interventions select for resistant/tolerant strains of mosquitoes and exert pressures on pathogens due to constraints on the numbers of available vectors (Ferraguti et al., 2020). However, ultimately, our unique human talent to create synthetic drugs and vaccines is probably the most promising weapon against pathogens and their vectors.
Scientists have developed drugs and vaccines that have greatly reduced the prevalence and even eradicated certain diseases, such as smallpox. However, vaccines have been successfully developed only for very few vector-borne diseases, such as yellow fever, dengue and Japanese encephalitis (Olajiga et al., 2021). There are current initiatives to develop and/or improve vaccines for other important human vector-borne diseases, such as leishmania and malaria (Lage et al., 2020; Datoo et al., 2021), but major advances have been few and far between. Remarkably, World Health Organization recommended in October 2021 the use of an RTS,S/AS01 malaria vaccine among children inhabiting regions of moderate-to-high transmission risk of P. falciparum malaria infection. Nonetheless, this vaccine confers only modest protection against malaria infections (Laurens, 2020). Furthermore, use of vaccines can promote increase of parasite virulence in naïve hosts over time due to relaxed selective evolutionary pressures on host mortality (Gandon et al., 2001). The development of new vaccines and drugs could become a strong tool to control or even eradicate vector-borne diseases. Because scientific advances may occur faster than biological evolution, they represent the best option to overcome pathogens and allow hosts to surge ahead in the coevolutionary arms race (Powell, 2019).
Conclusion
Here, we summarized the main evolutionary pressures faced by hosts, vectors and pathogens associated with vector-borne transmission (see Fig. 1A–C and Table 2). Pathogens and their hosts evolve in tandem and, consequently, adaptation by 1 antagonist should result in a counter-adaptation by its counterpart. In the specific case of vector-borne pathogens, 3 distinct ‘players’ coevolve together and are impacted by direct or indirect selective pressures from the others. Generally, vertebrate hosts and vectors should evolve traits allowing them to experience only reduced infection rates and infection-mediated fitness losses via increased resistance and/or tolerance to infections. Nevertheless, strategies towards less pathogenic interactions are highly variable between those 2 groups. While hosts are passively infected by parasites and should, therefore, evolve towards less attractive phenotypes, vectors would benefit from an active avoidance of infected hosts. Despite the fact pathogens are more virulent to their hosts than to their vectors, both have evolved immune/biochemical mechanisms to combat infections. On the other hand, parasites have evolved multiple mechanisms to increase their own transmission (e.g. behavioural manipulation, high rates of replication, etc.) and, due to their undoubtedly faster evolutionary rates compared to both hosts and vectors, parasites are unlikely to be overtaken naturally by either their vertebrate or vector hosts in this tripartite coevolutionary arms race. Thus, scientists should consider the evolutionary context encompassing hosts, vectors, pathogens and their microbiome to create new effective pathways for treatments and preventive interventions (see Fig. 1D), which could minimize pathogen burden for wildlife and human populations.
Table 2.
Examples of studies on adaptations and counter-adaptations of hosts, vectors and pathogens
| Host sp. | Vector sp. | Pathogen ID | Topic | Main results | Reference |
|---|---|---|---|---|---|
| Zebra (Equus quagga) | Horseflies (Haematopota pluvialis and Tabanus bromius) | None | Factors that protect hosts from vector bites | Stripes prevent landing and host approach by flies (i.e. protective effect) | How et al. (2020) |
| Humans (Homo sapiens) | None | Plasmodium falciparum | Host immune resistance and tolerance to pathogens | Blood group ‘O’ represents a protective phenotype against severe infections whereas ‘A’ blood group is associated with higher pathogenicity | Goel et al. (2015) |
| Rock pigeons (Columba livia) | Flies (Pseudolynchia canariensis) | Haemoproteus columbae | Host behavioural defences against vectors | Anti-vector behaviour and immune reaction decrease fly fitness and survival but do not affect pathogen prevalence | Waite et al. (2014) |
| None | Mosquitoes (Anopheles spp.) | Plasmodium spp. | Vector immune resistance and tolerance to pathogens | Vectors can constrain parasite development by degrading sporozoites when these migrate to the salivary glands through the haemolymph | Hillyer et al. (2007) |
| Great tits (Parus major) | Mosquitoes (Culex pipiens) | Plasmodium spp. | Vector avoidance of infected hosts | Vectors avoided infected hostsa | Lalubin et al. (2012) |
| Humans (H. sapiens) | Sandflies (Lutzomyia spp.) | None | Identification and attraction to host cues | Vectors were attracted to carbon dioxide and human odour | Pinto et al. (2001) |
| Mice | Mosquitoes (Anopheles stephensi) | Plasmodium chabaudi | Optimum virulence towards hosts and vectors | Pathogen benefits from high virulence in hosts but lower virulence among vectors | Ferguson et al. (2003a) |
| Dogs (Canis familiaris) | Sandflies (Phlebotomus perniciosus) | Leishmania infantum | Pathogen manipulation of hosts/vectors | Pathogen induces physiological modifications in the host that increase their attractiveness to their vectors | Chelbi et al. (2021) |
| Mice | Sandflies (Lutzomyia longipalpis) | Leishmania spp. | Pathogen manipulation of hosts/vectors | Pathogen induces increase in feeding persistence among infected vectors | Rogers and Bates (2007) |
Many studies on this topic show no or contrary effects.
Acknowledgements
We are grateful to Professor Diego Santiago-Alarcon for his comments on earlier version of this manuscript. His contribution certainly helped to improve the quality of our research. We also acknowledge the University of Otago, New Zealand for supporting Daniela Dutra with a doctoral scholarship.
Author contributions
D. d. A. D. and F. C. F. conceived and designed the study. D. d. A. D. wrote the manuscript with inputs from F. C. F. and R. P.
Financial support
Francisco Carlos Ferreira was supported, in part, by U.S. National Science Foundation (NSF), Ecology and Evolution of Infectious Diseases DEB 1717498. Daniela de Angeli Dutra was supported by a doctoral scholarship from the University of Otago.
Conflict of interest
The authors declare no conflicts of interest.
References
- Alizon S and van Baalen M (2008) Transmission-virulence trade-offs in vector-borne diseases. Theoretical Population Biology 74, 6–15. [DOI] [PubMed] [Google Scholar]
- Atkinson CT, Saili KS, Utzurrum RB and Jarvi SI (2013) Experimental evidence for evolved tolerance to avian malaria in a wild population of low elevation Hawai'i ‘amakihi (Hemignathus virens). EcoHealth 10, 366–375. [DOI] [PubMed] [Google Scholar]
- Atkinson CT, Utzurrum RB, Lapointe DA, Camp RJ, Crampton LH, Foster JT and Giambelluca TW (2014) Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands – an ongoing conservation crisis on the island of Kaua'i. Global Change Biology 20, 2426–2436. [DOI] [PubMed] [Google Scholar]
- Auld SK and Tinsley MC (2015) The evolutionary ecology of complex lifecycle parasites: linking phenomena with mechanisms. Heredity 114, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aželytė J, Wu-Chuang A, Žiegytė R, Platonova E, Mateos-Hernandez L, Maye J, Obregon D, Palinauskas V and Cabezas-Cruz A (2022) Anti-microbiota vaccine reduces avian malaria infection within mosquito vectors. Frontiers in Immunology 13, 841835. doi: 10.3389/fimmu.2022.841835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda ME, Howe L, Gartrell BD, McInnes K, Hunter S and French NP (2013) A cluster of avian malaria cases in a kiwi management programme. New Zealand Veterinary Journal 61, 121–126. [DOI] [PubMed] [Google Scholar]
- Beier JC (1998) Malaria parasite development in mosquitoes. Annual Review of Entomology 43, 519–543. [DOI] [PubMed] [Google Scholar]
- Billingsley PF, Baird J, Mitchell JA and Drakeley C (2006) Immune interactions between mosquitoes and their hosts. Parasite Immunology 28, 143–153. [DOI] [PubMed] [Google Scholar]
- Bompard A, Da DF, Yerbanga SR, Morlais I, Awono-Ambéné PH, Dabiré RK, Ouédraogo JB, Lefèvre T, Churcher TS and Cohuet A (2020) High Plasmodium infection intensity in naturally infected malaria vectors in Africa. International Journal for Parasitology 50, 985–996. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Sepil I, Milá B, Buermann W, Pollinger J, Sehgal RNM, Valkiūnas G, Iezhova TA, Saatchi S and Smith TB (2009) The prevalence of avian Plasmodium is higher in undisturbed tropical forests of Cameroon. Journal of Tropical Ecology 25, 439–447. [Google Scholar]
- Bush SE and Clayton DH (2018) Anti-parasite behaviour of birds. Philosophical Transactions of the Royal Society B: Biological Sciences 373, 20170196. doi: 10.1098/rstb.2017.0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardé R and Gibson G (2010) Host finding by female mosquitoes: mechanisms of orientation to host odours and other cues. In Takken W and Knols BGJ (eds), Olfaction in Vector–Host Interactions. Wageningen: Wageningen Academic Publishers, pp. 115–142. [Google Scholar]
- Cassin-Sackett L, Callicrate TE and Fleischer RC (2019) Parallel evolution of gene classes, but not genes: evidence from Hawai'ian honeycreeper populations exposed to avian malaria. Molecular Ecology 28, 568–583. [DOI] [PubMed] [Google Scholar]
- Cator LJ, George J, Blanford S, Murdock CC, Baker TC, Read AF and Thomas MB (2013) ‘Manipulation’ without the parasite: altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proceedings of the Royal Society B: Biological Sciences 280, 20130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelbi I, Maghraoui K, Zhioua S, Cherni S, Labidi I, Satoskar A, Hamilton JGC and Zhioua E (2021) Enhanced attraction of sand fly vectors of Leishmania infantum to dogs infected with zoonotic visceral leishmaniasis. PLoS Neglected Tropical Diseases 15, e0009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churcher TS, Trape JF and Cohuet A (2015) Human-to-mosquito transmission efficiency increases as malaria is controlled. Nature Communications 6, 6054. doi: 10.1038/ncomms7054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NJ, Wells K, Dimitrov D and Clegg SM (2016) Co-infections and environmental conditions drive the distributions of blood parasites in wild birds. Journal of Animal Ecology 85, 1461–1470. [DOI] [PubMed] [Google Scholar]
- Clark NJ, Owada K, Ruberanziza E, Ortu G, Umulisa I, Bayisenge U, Mbonigaba JB, Mucaca JB, Lancaster W, Fenwick A, Soares Magalhães RJ and Mbituyumuremyi A (2020) Parasite associations predict infection risk: incorporating co-infections in predictive models for neglected tropical diseases. Parasites & Vectors 13, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DH, Bush SE and Johnson KP (2015) Coevolution of Life on Hosts: Integrating Ecology and History. Chicago: University of Chicago Press. [Google Scholar]
- Cornet S, Nicot A, Rivero A and Gandon S (2013) Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecology Letters 16, 323–329. [DOI] [PubMed] [Google Scholar]
- Cozzarolo C-S, Glaizot O, Christe P and Pigeault R (2020) Enhanced attraction of arthropod vectors to infected vertebrates: a review of empirical evidence. Frontiers in Ecology and Evolution 8, 568140. doi: 10.3389/fevo.2020.568140 [DOI] [Google Scholar]
- Cozzarolo C-S, Pigeault R, Isaïa J, Wassef J, Baur M, Glaizot O and Christe P (2022) Experiment in semi-natural conditions did not confirm the influence of malaria infection on bird attractiveness to mosquitoes. Parasites & Vectors 15, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da DF, Churcher TS, Yerbanga RS, Yaméogo B, Sangaré I, Ouedraogo JB, Sinden RE, Blagborough AM and Cohuet A (2015) Experimental study of the relationship between Plasmodium gametocyte density and infection success in mosquitoes; implications for the evaluation of malaria transmission-reducing interventions. Experimental Parasitology 149, 74–83. [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA and Hyatt AD (2000) Emerging infectious diseases of wildlife – threats to biodiversity and human health. Science (New York, N.Y.) 287, 443–449. [DOI] [PubMed] [Google Scholar]
- Datoo MS, Magloire Natama H, Somé A, Traoré O, Rouamba T, Bellamy D, Yameogo P, Valia D, Tegneri M, Ouedraogo F, Soma R, Sawadogo S, Sorgho F, Derra K, Rouamba E, Orindi B, Ramos-Lopez F, Flaxman A, Cappuccini F, Kailath R, Elias SC, Mukhopadhyay E, Noe A, Cairns M, Lawrie A, Roberts R, Valéa I, Sorgho H, Williams N, Glenn G, Fries L, Reimer J, Ewer KJ, Shaligram U, Hill AVS and Tinto H (2021) High efficacy of a low dose candidate malaria vaccine, R21 in 1 adjuvant matrix-M™, with seasonal administration to children in Burkina Faso. SSRN Electronic Journal. doi: 10.2139/ssrn.3830681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CM, Webster JP and Woolhouse MEJ (2001) Trade-offs in the evolution of virulence in an indirectly transmitted macroparasite. Proceedings of the Royal Society of London, Series B: Biological Sciences 268, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviews CR, Ayres JM, Dye C and Deane LM (1991) Malaria infection rate of Amazonian primates increases with body weight and group size. Functional Ecology 5, 655. [Google Scholar]
- Dawes EJ, Churcher TS, Zhuang S, Sinden RE and Basáñez M-G (2009) Anopheles mortality is both age- and Plasmodium-density dependent: implications for malaria transmission. Malaria Journal 8, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T (2002) The evolution of virulence in vector-borne and directly transmitted parasites. Theoretical Population Biology 62, 199–213. [DOI] [PubMed] [Google Scholar]
- Dennison NJ, Jupatanakul N and Dimopoulos G (2014) The mosquito microbiota influences vector competence for human pathogens. Current Opinion in Insect Science 3, 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D (2013) The epidemiology and evolution of symbionts with mixed-mode transmission. Annual Review of Ecology, Evolution, and Systematics 44, 623–643. [Google Scholar]
- Elliot SL, Adler FR and Sabelis ML (2003) How virulent should a parasite be to its vector? Ecology 84, 2568–2574. [Google Scholar]
- Emami SN, Lindberg BG, Hua S, Hill SR, Mozuraitis R, Lehmann P, Birgersson G, Borg-Karlson A-K, Ignell R and Faye I (2017) A key malaria metabolite modulates vector blood seeking, feeding, and susceptibility to infection. Science (New York, N.Y.) 355, 1076–1080. [DOI] [PubMed] [Google Scholar]
- Ewald PW (1983) Host–parasite relations, vectors, and the evolution of disease severity. Annual Review of Ecology and Systematics 14, 465–485. [Google Scholar]
- Ewald PW (1995) The evolution of virulence: a unifying link between parasitology and ecology. Journal of Parasitology 81, 659–669. [PubMed] [Google Scholar]
- Fecchio A, Ribeiro RM, Ferreira FC, de Angeli Dutra D, Tolesano-Pascoli G, Alquezar RD, Khan AU, Pichorim M, Moreira PA, Costa-Nascimento MJ, Monteiro EF, Mathias BS, Guimarães LO, Simões RF, Braga ÉM, Kirchgatter K and Dias RI (2021) Higher infection probability of haemosporidian parasites in blue-black grassquits (Volatinia jacarina) inhabiting native vegetation across Brazil. Parasitology International 80, 102204. [DOI] [PubMed] [Google Scholar]
- Ferguson HM and Read AF (2002) Why is the effect of malaria parasites on mosquito survival still unresolved? Trends in Parasitology 18, 256–261. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Mackinnon MJ, Chan BH and Read AF (2003a) Mosquito mortality and the evolution of malaria virulence. Evolution 57, 2792–2804. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Rivero A and Read AF (2003b) The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology 127, S0031182003003287. [DOI] [PubMed] [Google Scholar]
- Ferraguti M, Martínez-de la Puente J, Roiz D, Ruiz S, Soriguer R and Figuerola J (2016) Effects of landscape anthropization on mosquito community composition and abundance. Scientific Reports 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti M, Martínez-de la Puente J, Bensch S, Roiz D, Ruiz S, Viana DS, Soriguer RC and Figuerola J (2018) Ecological determinants of avian malaria infections: an integrative analysis at landscape, mosquito and vertebrate community levels. Journal of Animal Ecology 87, 727–740. [DOI] [PubMed] [Google Scholar]
- Ferraguti M, Hernández-Lara C and Sehgal RNM (2020) Anthropogenic effects on avian haemosporidians and their vectors. In Santiago-Alarcon D and Marzal A (eds), Avian Malaria and Related Parasites in the Tropics. Switzerland: Springer US, pp. 451–485. [Google Scholar]
- Ferreira-Junior FC, de Angeli Dutra D, Silveira P, Pacheco RC, Witter R, de Souza Ramos DG, Pacheco MA, Escalante AA and Braga ÉM (2018) A new pathogen spillover from domestic to wild animals: Plasmodium juxtanucleare infects free-living passerines in Brazil. Parasitology 145, 1949–1958. [DOI] [PubMed] [Google Scholar]
- Ferreira FC, Rodrigues RA, Sato Y, Borges MAZ and Braga ÉM (2016) Searching for putative avian malaria vectors in a seasonally dry tropical forest in Brazil. Parasites & Vectors 9, 587. doi: 10.1186/s13071-016-1865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira FC, Videvall E, Seidl CM, Wagner NE, Kilpatrick AM, Fleischer RC and Fonseca DM (2022) Transcriptional response of individual Hawaiian Culex quinquefasciatus mosquitoes to the avian malaria parasite Plasmodium relictum. bioRxiv, 2022.02.10.479890. doi: 10.1101/2022.02.10.479890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion A, Eriksson A, Jorge F, Niebuhr CN and Poulin R (2020) Large-scale disease patterns explained by climatic seasonality and host traits. Oecologia 194, 723–733. [DOI] [PubMed] [Google Scholar]
- Forattini OP (1995) Principais mosquitos de importância sanitária no Brasil. Available at doi: 10.1590/S0102-311X1995000100027. [DOI]
- Francino MP (2016) Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Frontiers in Microbiology 6, 1543. doi: 10.3389/fmicb.2015.01543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA (1996) Models of parasite virulence. Quarterly Review of Biology 71, 37–78. [DOI] [PubMed] [Google Scholar]
- Fredrich E, Barzantny H, Brune I and Tauch A (2013) Daily battle against body odor: towards the activity of the axillary microbiota. Trends in Microbiology 21, 305–312. [DOI] [PubMed] [Google Scholar]
- Gandon S, Mackinnon MJ, Nee S and Read AF (2001) Imperfect vaccines and the evolution of pathogen virulence. Nature 414, 751–756. [DOI] [PubMed] [Google Scholar]
- Garamszegi LZ (2011) Climate change increases the risk of malaria in birds. Global Change Biology 17, 1751–1759. [Google Scholar]
- Giorgio S (1995) Moderna visão da evolução da virulência. Revista de Saúde Pública 29, 398–402. [DOI] [PubMed] [Google Scholar]
- Girard YA, Popov V, Wen J, Han V and Higgs S (2005) Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae). Journal of Medical Entomology 42, 429–444. [DOI] [PubMed] [Google Scholar]
- Goel S, Palmkvist M, Moll K, Joannin N, Lara P, Akhouri RR, Moradi N, Öjemalm K, Westman M, Angeletti D, Kjellin H, Lehtiö J, Blixt O, Ideström L, Gahmberg CG, Storry JR, Hult AK, Olsson ML, Von Heijne G, Nilsson I and Wahlgren M (2015) RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nature Medicine 21, 314–321. [DOI] [PubMed] [Google Scholar]
- Graumans W, Jacobs E, Bousema T and Sinnis P (2020) When is a Plasmodium-infected mosquito an infectious mosquito? Trends in Parasitology 36, 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-López R, Martínez-de la Puente J, Gangoso L, Yan J, Soriguer R and Figuerola J (2019) Experimental reduction of host Plasmodium infection load affects mosquito survival. Scientific Reports 9, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-López R, Martínez-de la Puente J, Gangoso L, Soriguer R and Figuerola J (2020) Plasmodium transmission differs between mosquito species and parasite lineages. Parasitology 147, 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbach R, Junglen S and van Rij RP (2017) Mosquito-specific and mosquito-borne viruses: evolution, infection, and host defense. Current Opinion in Insect Science 22, 16–27. [DOI] [PubMed] [Google Scholar]
- Harhay MO, Olliaro PL, Costa DL and Costa CHN (2011) Urban parasitology: visceral leishmaniasis in Brazil. Trends in Parasitology 27, 403–409. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Pashalidou F, Soler R and Bezemer TM (2011) Intrinsic competition between two secondary hyperparasitoids results in temporal trophic switch. Oikos 120, 226–233. [Google Scholar]
- Hawking F (1967) The 24-hour periodicity of microfilariae: biological mechanisms responsible for its production and control. Proceedings of the Royal Society of London, Series B. Biological Sciences 169, 59–76. [Google Scholar]
- Hawking F, Worms MJ and Gammage K (1968) 24- and 48-hour cycles of malaria parasites in the blood; their purpose, production and control. Transactions of the Royal Society of Tropical Medicine and Hygiene 62, 731–760. [DOI] [PubMed] [Google Scholar]
- Hedrick PW (2011) Population genetics of malaria resistance in humans. Heredity 107, 283–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer JF, Barreau C and Vernick KD (2007) Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito haemocoel. International Journal for Parasitology 37, 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JA, Kafatos FC, Janeway CA and Ezekowitz RAB (1999) Phylogenetic perspectives in innate immunity. Science (New York, N.Y.) 284, 1313–1318. [DOI] [PubMed] [Google Scholar]
- How MJ, Gonzales D, Irwin A and Caro T (2020) Zebra stripes, tabanid biting flies and the aperture effect: zebra stripes prevent biting flies. Proceedings of the Royal Society B: Biological Sciences 287, 20201521. doi: 10.1098/rspb.2020.1521rspb20201521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaïa J, Rivero A, Glaizot O, Christe P and Pigeault R (2020) Last-come, best served? Mosquito biting order and Plasmodium transmission: mosquito bite & Plasmodium transmission. Proceedings of the Royal Society B: Biological Sciences 287, 20202615. doi: 10.1098/rspb.2020.2615rspb20202615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BT, Brewster CC and Paulson SL (2012) La Crosse virus infection alters blood feeding behavior in Aedes triceratops and Aedes albopictus (Diptera: Culicidae). Journal of Medical Entomology 49, 1424–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupatanakul N, Sim S and Dimopoulos G (2014) The insect microbiome modulates vector competence for arboviruses. Viruses 6, 4294–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hope LA, Hemingway J and McKenzie FE (2009) Environmental factors associated with the malaria vectors Anopheles gambiae and Anopheles funestus in Kenya. Malaria Journal 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen GF, Kihonda J, Lyimo E, Oketch FR, Kotas ME, Mathenge E, Schellenberg JA, Lengeler C, Smith TA and Drakeley CJ (2006) Quantifying behavioural interactions between humans and mosquitoes: evaluating the protective efficacy of insecticidal nets against malaria transmission in rural Tanzania. BMC Infectious Diseases 6, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM (2011) Globalization, land use, and the invasion of West Nile virus. Science (New York, N.Y.) 334, 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotepui M, Phunphuech B, Phiwklam N, Chupeerach C and Duangmano S (2014) Effect of malarial infection on haematological parameters in population near Thailand–Myanmar border. Malaria Journal 13, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage DP, Ribeiro PAF, Dias DS, Mendonça DVC, Ramos FF, Carvalho LM, de Oliveira D, Steiner BT, Martins VT, Perin L, Machado AS, Santos TTO, Tavares GSV, Oliveira-da-Silva JA, Oliveira JS, Roatt BM, Machado-de-Ávila RA, Teixeira AL, Humbert MV, Coelho EAF and Christodoulides M (2020) A candidate vaccine for human visceral leishmaniasis based on a specific T cell epitope-containing chimeric protein protects mice against Leishmania infantum infection. NPJ Vaccines 5, 75. doi: 10.1038/s41541-020-00224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalubin F, Bize P, van Rooyen J, Christe P and Glaizot O (2012) Potential evidence of parasite avoidance in an avian malarial vector. Animal Behaviour 84, 539–545. [Google Scholar]
- Lambrechts L and Scott TW (2009) Mode of transmission and the evolution of arbovirus virulence in mosquito vectors. Proceedings of the Royal Society B: Biological Sciences 276, 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe DA, Goff ML and Atkinson CT (2010) Thermal constraints to the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawai'i. Journal of Parasitology 96, 318–324. [DOI] [PubMed] [Google Scholar]
- Lapointe DA, Atkinson CT and Samuel MD (2012) Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 1249, 211–226. [DOI] [PubMed] [Google Scholar]
- Laurens MB (2020) RTS,S/AS01 vaccine (Mosquirix™): an overview. Human Vaccines & Immunotherapeutics 16, 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Atkinson CT, LaPointe DA and Samuel MD (2017) Mitigating future avian malaria threats to Hawaiian forest birds from climate change. PLoS One 12, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau C, Iezhova T, Valkiūnas G, Chasar A, Hutchinson A, Buermann W, Smith TB and Sehgal RNM (2010) Spatial variation of haemosporidian parasite infection in African rainforest bird species. Journal of Parasitology 96, 21–29. [DOI] [PubMed] [Google Scholar]
- Lucas-Barbosa D, Degennaro M, Mathis A and Verhulst NO (2021) Parasitology skin bacterial volatiles: propelling the future of vector control. Trends in Parasitology 38, 1–8. doi: 10.1016/j.pt.2021.08.010 [DOI] [PubMed] [Google Scholar]
- Maitre A, Wu-Chuang A, Aželytė J, Palinauskas V, Mateos-Hernández L, Obregon D, Hodžić A, Valiente Moro C, Estrada-Peña A, Paoli JC, Falchi A and Cabezas-Cruz A (2022) Vector microbiota manipulation by host antibodies: the forgotten strategy to develop transmission-blocking vaccines. Parasites & Vectors 15, 4. doi: 10.1186/s13071-021-05122-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure KM, Fleischer RC and Kilpatrick AM (2020) The role of native and introduced birds in transmission of avian malaria in Hawaii. Ecology 101, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça VR, Queiroz AT, Lopes FM, Andrade BB and Barral-Netto M (2013) Networking the host immune response in Plasmodium vivax malaria. Malaria Journal 12, 69. doi: 10.1186/1475-2875-12-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre A, Poulin R and Hortal J (2020) A niche perspective on the range expansion of symbionts. Biological Reviews 95, 491–516. [DOI] [PubMed] [Google Scholar]
- Miller E, Warburg A, Novikov I, Hailu A, Volf P, Seblova V and Huppert A (2014) Quantifying the contribution of hosts with different parasite concentrations to the transmission of visceral leishmaniasis in Ethiopia. PLoS Neglected Tropical Diseases 8, e3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J (2002) Parasites and the Behavior of Animals. ed. May, R. M. and Harvey, P. H. New York: Oxford University Press. [Google Scholar]
- Mourão LC, Cardoso-Oliveira GP and Braga ÉM (2020) Autoantibodies and malaria: where we stand? Insights into pathogenesis and protection. Frontiers in Cellular and Infection Microbiology 10, 262. doi: 10.3389/fcimb.2020.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Hogsette JA, Kline DL, Beier JC, Revay EE and Xue RD (2015) Response of the sand fly Phlebotomus papatasi to visual, physical and chemical attraction features in the field. Acta Tropica 141, 32–36. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA and Belkaid Y (2012) Compartmentalized control of skin immunity by resident commensals. Science (New York, N.Y.) 337, 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguitragool W, Mueller I, Kumpitak C, Saeseu T, Bantuchai S, Yorsaeng R, Yimsamran S, Maneeboonyang W, Sa-angchai P, Chaimungkun W, Rukmanee P, Puangsa-art S, Thanyavanich N, Koepfli C, Felger I, Sattabongkot J and Singhasivanon P (2017) Very high carriage of gametocytes in asymptomatic low-density Plasmodium falciparum and P. vivax infections in western Thailand. Parasites & Vectors 10, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajiga O, Holguin-Rocha AF, Rippee-Brooks M, Eppler M, Harris SL and Londono-Renteria B (2021) Vertebrate responses against arthropod salivary proteins and their therapeutic potential. Vaccines 9, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Rodríguez A, de la Hera I, Fernández-González S and Pérez-Tris J (2014) Global warming will reshuffle the areas of high prevalence and richness of three genera of avian blood parasites. Global Change Biology 20, 2406–2416. [DOI] [PubMed] [Google Scholar]
- Pigeault R and Villa M (2018) Long-term pathogenic response to Plasmodium relictum infection in Culex pipiens mosquito. PLoS One 13, e0192315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeault R, Vézilier J, Cornet S, Zélé F, Nicot A, Perret P, Gandon S and Rivero A (2015) Avian malaria: a new lease of life for an old experimental model to study the evolutionary ecology of Plasmodium. Philosophical Transactions of the Royal Society B: Biological Sciences 370, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto MC, Campbell-Lendrum DH, Lozovei AL, Teodoro U and Davies CR (2001) Phlebotomine sandfly responses to carbon dioxide and human odour in the field. Medical and Veterinary Entomology 15, 132–139. [DOI] [PubMed] [Google Scholar]
- Pollitt LC, Bram JT, Blanford S, Jones MJ and Read AF (2015) Existing infection facilitates establishment and density of malaria parasites in their mosquito vector. PLoS Pathogens 11, e1005003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin R, Fredensborg BL, Hansen E and Leung TLF (2005) The true cost of host manipulation by parasites. Behavioural Processes 68, 241–244. [DOI] [PubMed] [Google Scholar]
- Poulin R, Bennett J, de Angeli Dutra D, Doherty JF, Filion A, Park E and Ruehle B (2020) Evolutionary signature of ancient parasite pressures, or the ghost of parasitism past. Frontiers in Ecology and Evolution 8, 1–7. [Google Scholar]
- Powell JR (2019) An evolutionary perspective on vector-borne diseases. Frontiers in Genetics 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasadini M (2019) Blood feeding preference of female Aedes aegypti mosquitoes for human blood group types and its impact on their fecundity: implications for vector control. American Journal of Entomology 3, 43. [Google Scholar]
- Raymann K, Bobay L and Moran NA (2018) Antibiotics reduce genetic diversity of core species in the honeybee gut microbiome. Molecular Ecology 27, 2057–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE (2017) Historical biogeography and extinction in the Hawaiian honeycreepers. The American Naturalist 190, 106–111. [DOI] [PubMed] [Google Scholar]
- Rogers ME and Bates PA (2007) Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathogens 3, e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MD, Liao W, Atkinson CT and LaPointe DA (2020) Facilitated adaptation for conservation – can gene editing save Hawaii's endangered birds from climate driven avian malaria? Biological Conservation 241, 108390. [Google Scholar]
- Santiago-Alarcon D (2022) 9. A meta-analytic approach to investigate mosquitoes’ (Diptera: Culicidae) blood feeding preferences from non-urban to urban environments. In Gutiérrez-López R, Logan JG and Martínez-de la Puente J (eds), Ecology of Diseases Transmitted by Mosquitoes to Wildlife. Wageningen: Wageningen Academic Publishers, pp. 161–177. doi: 10.3920/978-90-8686-931-2_9. [DOI] [Google Scholar]
- Santiago-Alarcon D and Ferreira FC (2020) Does Plasmodium infection affect mosquito attraction? Frontiers in Ecology and Evolution 8, 582943. doi: 10.3389/fevo.2020.582943 [DOI] [Google Scholar]
- Santiago-Alarcon D, Palinauskas V and Schaefer HM (2012) Diptera vectors of avian haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biological Reviews 87, 928–964. [DOI] [PubMed] [Google Scholar]
- Sarabian C, Curtis V and McMullan R (2018) Evolution of pathogen and parasite avoidance behaviours. Philosophical Transactions of the Royal Society B: Biological Sciences 373, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlein Y, Jacobson RL and Messer G (1992) Leishmania infections damage the feeding mechanism of the sandfly vector and implement parasite transmission by bite. Proceedings of the National Academy of Sciences 89, 9944–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw WR, Marcenac P and Catteruccia F (2022) Plasmodium development in Anopheles: a tale of shared resources. Trends in Parasitology 38, 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P and Best A (2021) Simultaneous evolution of host resistance and tolerance to parasitism. Journal of Evolutionary Biology 34, 1932–1943. [DOI] [PubMed] [Google Scholar]
- Tadesse FG, Slater HC, Chali W, Teelen K, Lanke K, Belachew M, Menberu T, Shumie G, Shitaye G, Okell LC, Graumans W, van Gemert G-J, Kedir S, Tesfaye A, Belachew F, Abebe W, Mamo H, Sauerwein R, Balcha T, Aseffa A, Yewhalaw D, Gadisa E, Drakeley C and Bousema T (2018) The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clinical Infectious Diseases 66, 1883–1891. [DOI] [PubMed] [Google Scholar]
- Valkiunas G, Kazlauskiene R, Bernotiene R, Bukauskaite D, Palinauskas V and Iezhova TA (2014) Haemoproteus infections (Haemosporida, Haemoproteidae) kill bird-biting mosquitoes. Parasitology Research 113, 1011–1018. [DOI] [PubMed] [Google Scholar]
- van Loon JJA, Smallegange RC, Bukovinszkiné-Kiss G, Jacobs F, De Rijk M, Mukabana WR, Verhulst NO, Menger DJ and Takken W (2015) Mosquito attraction: crucial role of carbon dioxide in formulation of a five-component blend of human-derived volatiles. Journal of Chemical Ecology 41, 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Riper C III, Van Riper SG, Goff ML and Laird M (1986) The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecological Monographs 56, 327–344. [Google Scholar]
- Vanstreels RET, Braga ÉM and Catão-Dias JL (2016) Blood parasites of penguins: a critical review. Parasitology 143, 931–956. [DOI] [PubMed] [Google Scholar]
- Vaughan JA and Turell MJ (1996) Facilitation of Rift Valley fever virus transmission by Plasmodium berghei sporozoites in Anopheles stephensi mosquitoes. The American Journal of Tropical Medicine and Hygiene 55, 407–409. [DOI] [PubMed] [Google Scholar]
- Verhulst NO, Boulanger N and Spitzen J (2018) Impact of skin microbiome on attractiveness to arthropod vectors and pathogen transmission. In Boulanger N (ed.), Skin and Arthropod Vectors. USA: Academic Press, pp. 55–81. doi: 10.1016/B978-0-12-811436-0.00003-4. [DOI] [Google Scholar]
- Vézilier J, Nicot A, Gandon S and Rivero A (2012) Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proceedings of the Royal Society B: Biological Sciences 279, 4033–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite JL, Henry AR, Owen JP and Clayton DH (2014) An experimental test of the effects of behavioral and immunological defenses against vectors: do they interact to protect birds from blood parasites? Parasites & Vectors 7, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SB and Kuris AM (2016) Independent origins of parasitism in Animalia. Biology Letters 12, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen-Yue X, Jian Z, Tao-Li Z, Fu-Sheng H, Jian-Hua D, Ying W, Zhong-Wen Q and Li-Sha X (2007) Plasmodium yoelii: contribution of oocysts melanization to natural refractoriness in Anopheles dirus. Experimental Parasitology 116, 433–439. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Morgan ER, Booth M, Norman R, Perkins SE, Hauffe HC, Mideo N, Antonovics J, McCallum H and Fenton A (2017) What is a vector? Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160085. doi: 10.1098/rstb.2016.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse MEJ, Webster JP, Domingo E, Charlesworth B and Levin BR (2002) Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nature Genetics 32, 569–577. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2014) Global status report on noncommunicable diseases 2014. Geneva, Switzerland: WHO. Available from http://www.who.int/nmh/publications/ncd-status-report-2014/en/. [Google Scholar]
- World Health Organization (2020) World malaria report 2020: 20 years of global progress and challenges. World Health Organization. Available from https://apps.who.int/iris/handle/10665/337660. [Google Scholar]
- Yan J, Broggi J, Martínez-de la Puente J, Gutiérrez-López R, Gangoso L, Soriguer R and Figuerola J (2018) Does bird metabolic rate influence mosquito feeding preference? Parasites & Vectors 11, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Gangoso L, Ruiz S, Soriguer R, Figuerola J and Martínez-de la Puente J (2021) Understanding host utilization by mosquitoes: determinants, challenges and future directions. Biological Reviews 96, 1367–1385. [DOI] [PubMed] [Google Scholar]