Abstract
306Three-dimensional (3D)-printed vascular models for cardiovascular surgery planning and endovascular procedure simulations often lack realistic biological tissues mimicking material properties, including flexibility or transparency, or both. Transparent silicone or silicone-like vascular models were not available for end-user 3D printers and had to be fabricated using complex and cost-intensive workarounds. This limitation has now been overcome by novel liquid resins with biological tissue properties. These new materials enable simple and low-cost fabrication of transparent and flexible vascular models using end-user stereolithography 3D printers and are promising technological advances toward more realistic patient-specific, radiation-free procedure simulations and planning in cardiovascular surgery and interventional radiology. This paper presents our patient-specific manufacturing process of fabricating transparent and flexible vascular models using freely available open-source software for segmentation and 3D post-processing, aiming to facilitate the integration of 3D printing into clinical care.
Keywords: 3D printing, Endovascular simulation, Flexible, Biological tissue, Resin
1. Introduction
Three-dimensional (3D) printing, also known as additive manufacturing or rapid prototyping, has been increasingly utilized in medicine over the past few decades. Many studies have documented the wide spectrum and increasing application of vascular and non-vascular 3D-printed models in clinical medicine and radiology for educational 307and clinical purposes1-4, and their suitability for numerous clinical scenarios has been graded in several guidelines5-7.
In contrast to two-dimensional (2D) images and digital volumetric renderings from computed tomography (CT) and magnetic resonance imaging (MRI) datasets displayed on 2D computer screens, 3D-printed models that are fabricated from the same data are able to provide a patient-specific understanding of anatomy and spatial relationships, and they are a step closer toward better personalized medicine and individualized patient care8.
In the field of cardiovascular surgery and interventional radiology (IR), vascular 3D printing is a technical challenge, especially when considering the complexity of vascular anatomy in addition to a large variety of available 3D printing technologies and materials9. Finding a 3D printing material with properties similar to biological tissues, which is ideally elastic and transparent, to simulate interventional procedures without the use of radiation is one of the greatest challenges thus far. Silicone or silicone-like materials seem to be ideal for this purpose, but they are not available for direct model fabrication with end-user 3D printers. Therefore, creating such models was managed amid complex, time- and cost-intensive workarounds, particularly silicone molding. However, with the introduction of novel liquid resins, this issue has been solved, enabling direct production of complex hollow vascular structures with low-cost stereolithography (SLA) 3D printing based on the imaging data of individual patients with high level of detail and accuracy. SLA technology allows the fabrication of models without internal support structures at printing resolutions of 50 to 100 μm, which is essential for printing vessels with small luminal diameter. In an appropriate simulation setting, these vascular models can be connected to a circulatory system with a peristaltic water pump for patient-specific, endovascular procedure simulations for both, training purpose and interventional procedure planning.
In this study, we present our manufacturing workflow of case-specific vascular 3D printing with flexible and transparent resin as well as demonstrate various applications in an illustrative case series.
2. Materials and methods
2.1. Image acquisition
Vascular models were fabricated from preprocedural CT angiography (CTA) scans (Somatom Definition Edge Plus, Siemens, Germany) using a bolus of 120 mL non-ionic contrast media at an injection rate of 3.5 to 5mL/s. An arterial phase was acquired with a delay of 30 s following contrast injection. Axial reconstructions with thin slices of 1-mm thickness were generated to ensure a high grade of detail and to prevent the formation of steps when reconstructing the 3D model. The Digital Imaging and Communications in Medicine (DICOM) images were exported from our picture archiving and communication system (PACS), and all patient identifiers were irreversibly removed. A positive ethics vote was obtained from the institutional review board (IRB-No.: 1004/2020).
2.2. Segmentation and post-processing
When 3D printing based on medical image data, a software is required for segmentation and 3D post-processing to create a printable stereolithography (STL) file. In this study, segmentation was done cost-effectively with a freely available open-source software, ImageJ (version 1.53, Laboratory for Optical and Computational Instrumentation, University of Wisconsin)10, while 3D post-processing was done with Blender (version 3.0, Blender Foundation)11. The CTA data were imported in ImageJ, and the inner lumen of the contrast-enhanced arteries was segmented using the “Window/Level” tool. Structures with similar Hounsfield units, like bones, were still included at this time point. In order to clear the stack from surrounding bones, the “Region of Interest (ROI) Manager” tool was used. Instead of defining the ROIs slice by slice, ROI interpolation was done to accelerate and simplify the segmentation workflow. All structures outside the borders of the defined ROIs, including the surrounding structures, such as bones, were removed by using a simple macro as defined in Table 1. Based on the segmented DICOM stack, the STL file was generated using the integrated and freely available 3D-Viewer plugin12. After being imported into Blender, the STL object was cleared from residual non-vascular structures. Modifiers were used to smooth the vessels and generate vessel walls with wall thickness of 1 mm. The endings of the aorta and procedure-relevant arteries were digitally cut open. The segmentation and 3D post-processing workflow is summarized step-by-step in Table 2.
Table 1.
Simple ImageJ macro for removing non-vascular structures using the ROI Manager
|
Especially in the abdomen, the spine can be in direct contact with the aorta via lumbar arteries, which causes difficulties in segmentation, as vascular structures and bones might fuse to one object. Using the ROI Manager in ImageJ, this macro automatically removes everything outside the ROI on each slice of the stack. The macro can be saved as ClearOutsideMacro.ijm and installed via Plugins → Macro → Install.
Table 2.
Workflow of segmentation and 3D post-processing
| Segmentation (ImageJ, version 1.53) | Description |
|---|---|
| 1. File → Import Image Sequence | Import the DICOM images as a 2D image stack. |
| 2. Image → Adjust → Window/Level (W&L) | Adjust the window and level to be focused on the vascular structures. |
| 3. Analyze → Tools → ROI Manager | Define the ROI for the whole stack using the “Polygon Selection” tool in combination with ROI interpolation (ROI Manager → More → Interpolate ROIs). |
| 4. Plugins → Macros → Clear Outside Macro | Using the macro defined in Table 1, bones and other high-density structures outside the ROI can be removed from the stack. |
| 5. Image → Adjust → Brightness/Contrast | (Optional) Contrast can be improved to remove residual non-vascular structures (i.e., early contrast-enhanced parenchyma). |
| 6. Paintbrush Tool | (Optional) Remove non-vascular structures slice-by-slice with a black paintbrush. |
| 7. Image → Transform → Flip Horizontally | The image stack has to be flipped horizontally before using the 3D Viewer plugin, caused by the triangulation algorithm. |
| 8. Plugins → 3D Viewer | Generate an STL file using the following parameters: Display as = Surface, Color = White, Threshold = 50, and Resampling factor = 2. After confirming the 8-bit conversion of the stack, a binary STL file can be exported (File → Export surfaces). |
| 3D post-processing (Blender, version 3.0) | Description |
| 1. File → Import → STL | Import the STL file into Blender. |
| 2. Object → Set Origin → Geometry to Origin | Move the object to the center of the scene. |
| 3. Edit mode: Select → Select Linked → Linked | Use the “Select Linked” tool to remove unconnected (unlinked) non-vascular parts of the object (i.e., residuals of parenchyma). |
| 4. Edit mode: Mesh → Bisect | Use the “Bisect” tool to cut the vasculature open (usually, at least the aorta and procedure relevant arteries). Without this step, a closed vascular model will be generated. |
| 5. Add modifier: Subdivision surface | Use the “Subdivision Surface Modifier” to smooth the object and prevent the formation of steps from triangulation. |
| 6. Add modifier: Solidify | Generate vessel walls using the “Solidify Modifier” with a wall thickness of 1 mm. |
The open-source software ImageJ was used for segmentation, while the open-source 3D software Blender was used for post-processing the vascular models.
2.3. Three-dimensional printing
308The vascular models were initially printed with Elastic 50A Resin (Formlabs, Somerville, Massachusetts, United States). After encountering problems such as material ruptures, the vascular models were printed with the newer, transparent Flexible 80A Resin with a shore hardness of 80A. For both materials, Form 3 SLA 3D printer was used (Formlabs, Somerville, Massachusetts, United States). No internal support structures were generated with the printer’s 3D printing software (PreForm, Formlabs, Somerville, Massachusetts, United States), as they cannot be removed from the vascular lumen of small vessels afterward; however, external support structures were generated automatically with a density of 0.8 and touching point size of 0.4 mm (raft type: “mini rafts”). The positioning on the build platform was carried out manually based on the recommendations made by the 3D printer’s software, which marked unprintable parts like overhangs in red. After 3D printing, the case-based models were finished by cleaning in isopropyl-alcohol for 10 min, flushing the small vessels with isopropyl-alcohol injection by hand, careful support structure removal, and ultraviolet light curing for 10 min at 60°C. The resin was used for patient-specific surgery planning in cardiovascular surgery and endovascular procedure simulations in interventional radiology. If specific cases were printed for endovascular procedure simulations, standard connectors were printed with rigid clear resin (Clear Resin, Formlabs) to connect the visceral artery models to a circulatory system with a peristaltic water pump via silicone tubes. The procedures were simulated by using interventional equipment, including catheters, guidewires, and microcatheters, as well as embolic agents (i.e., coils). Figure 1 and Videoclip S1 (1.2MB, MP4) demonstrate the transparent and flexible properties of the novel flexible resin.
Figure 1.
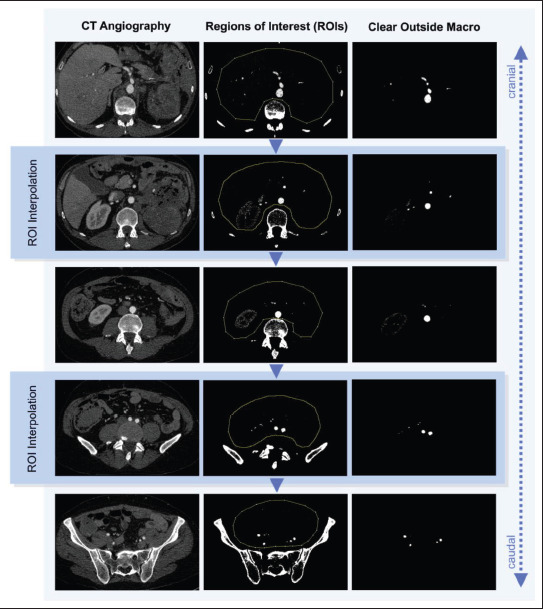
309 Process of ROI interpolation in segmenting vascular structures. Based on a DICOM stack of CTA images in arterial contrast phase, the regions of interest were interpolated from manually defined regions (yellow dotted lines). Using a macro, the structures outside these regions were removed, resulting in an image stack of the visceral arteries.
3. Results
3.1. Segmentation, post-processing, and 3D printing
The segmentation of patient-specific imaging data was successfully performed using ImageJ with standard image editing tools and ROI interpolation in combination with the macro (Table 1), as described in section 2.2. The results of using ROI interpolation and the macro in a real-life case are shown in Figure 1. The integrated 3D Viewer plugin was used to create triangulated surface models of the vascular segmentations and export them as STL files, while Blender was used to import and finalize the vascular models by smoothing the surface, cutting vessels open or reducing them, and generating vessel walls with 1-mm wall thickness. Figure 2 depicts the results of triangulation in 3D Viewer and post-processing in Blender in the same case. Printable vascular models were successfully created based on the patient’s CT data using the freely available open-source software packages under this workflow. 3D printing was successful using the end-user stereolithography 3D printer from Formlabs and the silicone-like Flexible 80A Resin. Videoclip S1 (1.2MB, MP4) demonstrates the flexible and transparent material properties of this novel 3D printing material with biological tissue mimicking characteristics.
Figure 2.
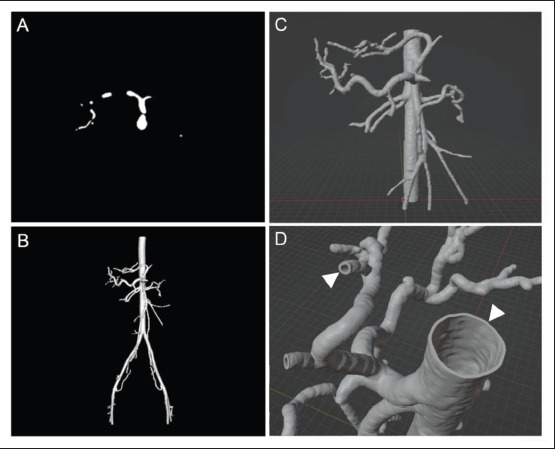
Results of segmentation and 3D post-processing. (A) Segmentation after removing all non-vascular structures and smoothing. (B) Surface model generated by stack triangulation using the integrated 3D Viewer plugin. (C) Vascular model after using the “Bisect” tool to remove unnecessary arteries and cut the vessels open in Blender. (D) Vascular model after generating vascular walls with 1-mm wall thickness (white arrow heads).
3.2. Practical applications in a case series
3.2.1. Aortic aneurysm
Case 1 (Figure 3) illustrates a case of thoracic aortic aneurysm in the right descending aorta associated with 310Kommerell’s diverticulum. The aneurysm was segmented with the open-source software based on the CTA data and successfully printed using the transparent, flexible resin. As the model was larger than the 3D printer’s build volume (14.5 × 14.5 × 18.5 cm), Blender was used to slice the model into two parts in order to print them separately. An ultraviolet light pen with a wavelength of 405 nm was used to fuse both parts into one aortic model using liquid resin from the resin tank. The life-size model was used by an interdisciplinary team of cardiovascular surgeons and interventional radiologists to gain a preprocedural impression of the actual dimension of the aorta, the aneurysm, as well as the location and course of the supra-aortic arteries in regard to the aneurysm, thereby assisting the team in planning the complex procedure. The combined surgical and endovascular treatment was successful in a total of three surgeries: the transposition of the left subclavian artery, a total aortic arch replacement with frozen elephant trunk (FET), and a thoracic endovascular aortic repair (TEVAR). The patient was discharged well from the hospital.
Figure 3.
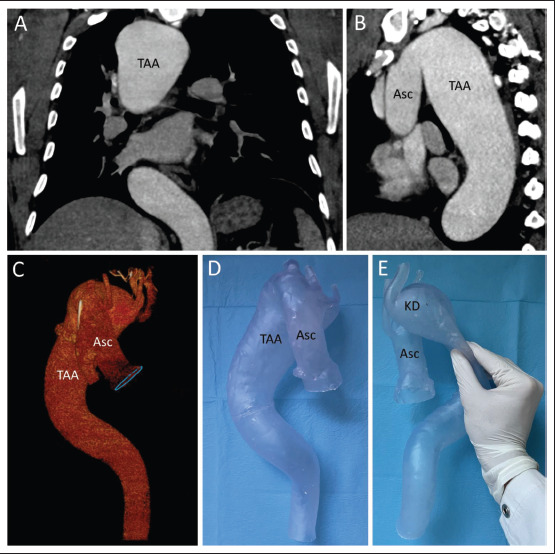
Thoracic aortic aneurysm in right descending aorta with Kommerell’s diverticulum. (A) Coronal projection of the CTA. (B) Parasagittal maximum intensity projection of the thoracic aorta. (C) 3D reconstruction of the thoracic aorta, representing the right descending course. (D) 3D-printed model of the thoracic aorta. (E) Proof of flexibility of the silicone-like resin. Abbreviations: Asc, ascending aorta; KD, Kommerell’s diverticulum; TAA, thoracic aortic aneurysm.
3.2.2. Active renal bleeding after percutaneous nephrostomy
Case 2 (Figure 4) illustrates a case of new onset life-threatening renal hemorrhage following percutaneous nephrostomy placement to treat hydronephrosis and renal failure in an 87-year-old gentleman. CTA demonstrated a nephrostomy catheter running along the segmental renal artery (Figure 3A) resulting in vascular injury and active arterial bleeding into the renal pelvis, thus causing macrohematuria. The patient was successfully treated with selective coil embolization of the affected segmental renal artery at our IR department, and the bleeding eventually stopped. The endovascular procedure was simulated retrospectively by a young interventional radiologist for hands-on training according to the real-life procedure using the 3D-printed model of the patient’s visceral arteries connected to a peristaltic pump (Guerbet KMP 2000, Villepinte, France).
Figure 4.
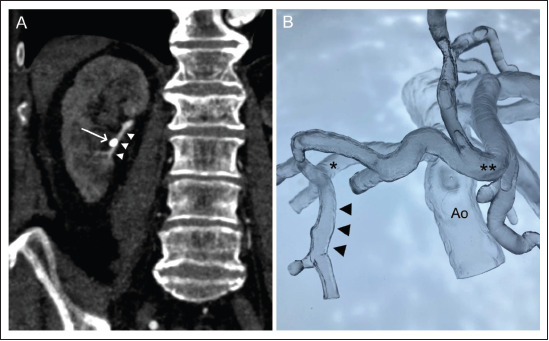
Segmental renal artery bleeding after percutaneous nephrostomy. (A) Coronal projection of the preprocedural CT scan in arterial phase (white arrow: nephrostomy catheter; white arrow heads: affected segmental renal artery). (B) Flexible resin vascular model (black arrow heads: segmental artery; single star: right renal artery; double star: proper hepatic artery). Abbreviation: Ao, infrarenal aorta.
3.2.3. Splenic pseudoaneurysm
Case 3 (Figure 5) illustrates a case of life-threatening acute arterial hemorrhage from splenic pseudoaneurysm following 311acute pancreatitis in a 36-year-old gentleman. The diagnosis was made using a CT protocol for gastrointestinal bleeding as a three-phase examination, including non-contrast, arterial, and venous phase imaging. The patient was successfully treated with an endovascular approach by selective coil embolization of the splenic artery. The visceral arteries, including the splenic artery with the pseudoaneurysm, were retrospectively fabricated with flexible resin and connected to a peristaltic water pump for endovascular simulation training (Figure 5C).
Figure 5.
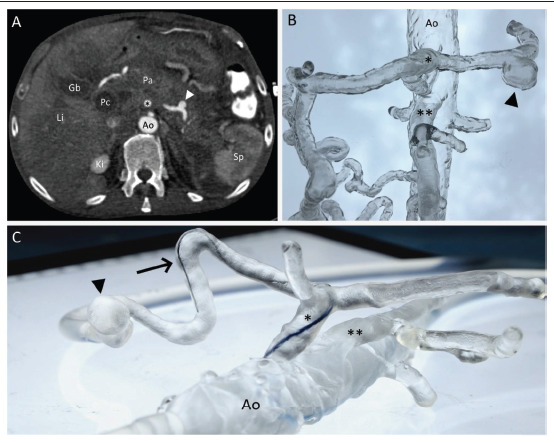
Splenic pseudoaneurysm with arterial hemorrhage from pancreatitis. (A) Axial projection of the preprocedural CT scan with pseudoaneurysm of the splenic artery (white arrow head). (B) 3D-printed vascular model of the visceral arteries, including the pseudoaneurysm (black arrow head). (C) Simulation setting of the 3D-printed model connected to a peristaltic water pump while performing microcatheter navigation in the splenic artery. Abbreviations: Ao, aorta; Gb, gall bladder; Ki, kidney; Li, Liver; Pa, pancreas; Pc, pseudocyst; Sp, spleen; *, celiac trunk; **, superior mesenteric artery
4. Discussion
Medical simulations are gaining popularity in student education, specialist training, and patient-specific procedure planning. In this context, 3D printing plays an important role for personalized simulations based on patient imaging data, particularly preprocedural CT or MRI scans. A broad spectrum of applications has been documented with the use of 3D printing for medical simulations, ranging from student teaching to tactile training of medical specialists and to the planning of complex procedures and operations. In liver surgery, for example, 3D-printed models are considered as tools for surgical or interventional planning and guiding of resections of hepatic lesions13. Similarly, 3D-printed models for surgical planning have been reported in cardiovascular surgery14. As another example, simulations with rapid prototyping enable the treatment planning of complex coronary artery disease; in that way, those involved would receive patient-specific training, gain confidence in treating the disease, and possibly predict the complications that might occur15. Moreover, in the field of IR, various applications have been documented. For instance, Shibata et al. used 3D-printed models for pre-interventional planning to establish optimal treatment plans and improve the safety and efficacy of selective embolizations of visceral artery aneurysms16.
312In addition to the continuously discovered applications of additive manufacturing in the field of medicine, novel 3D printing materials with biological tissue mimicking characteristics contribute to a more realistic setup of medical procedure simulations for hands-on trainings. In preceding studies, we have already proven the technical accuracy of SLA 3D printing with the novel flexible and transparent resin (Flexible 80A, Formlabs), as well as the simulation concept for emergency embolizations in a larger case series of acute internal bleedings17,18. In this technical follow-up study, we present our low-cost approach to segmentation and post-processing with a freely available open-source software in combination with an end-user 3D printer, aiming to improve the availability and feasibility of fabricating such patient-specific biological-tissue mimicking vascular models. ImageJ in combination with the presented macro and 3D Viewer plugin can be used for the segmentation of CTA data with slice thickness of at least 1 mm or less, while Blender enables the post-processing of the STL file, which includes the generation of an artificial vessel wall. Furthermore, the slicing of the vascular model to remove procedure-irrelevant arteries and the printing of objects larger than the 3D printer’s build volume are feasible with the use of Blender in combination with an ultraviolet light pen. The end-user 3D printer was thus able to print such biological tissue mimicking vascular structures based on the patient’s imaging data (Videoclip S1 (1.2MB, MP4) ).
This workflow, using an open-source software, has several technical limitations. The calcifications of arteries can be difficult to remove from the segmented vascular lumen. A threshold has to be defined to remove such calcifications from the vascular walls caused by atherosclerosis. Further investigations are needed to evaluate and determine an appropriate cutoff in Hounsfield units for such non-luminal structures. The same problem exists for external tubes and other medical devices, which might run close to arteries or cause blooming artifacts. In certain cases, non-vascular structures have to be removed by overpainting them slice-by-slice, which can be time-consuming. Moreover, the segmentation of models requires not only profound technical skills of 3D printing, but also a deep understanding of the anatomy, the underlying pathology, and the corresponding therapy. Hence, the segmentation of such models should be performed or at least checked by highly experienced medical professionals, especially when it comes to surgical planning. Therefore, one of the major limitations to implementing this technology as a routine in clinical practice is the lack of time that medical professionals have to employ this technically challenging and time-consuming process of fabricating such vascular models. Further investigations on using artificial intelligence to simplify and speed up segmentation and post-processing are needed to improve their feasibility.
5. Conclusion
313In conclusion, a workflow based on an open-source software and end-user 3D printer allowed for the fabrication of transparent vascular models with flexible biological tissue mimicking characteristics. Such vascular models can be used for case-based endovascular procedure simulations and pre-procedural planning in both, cardiovascular surgery and interventional radiology. Further investigations on artificial intelligence algorithms for the automatic generation of printable vascular models are needed to improve the efficiency and feasibility of this technology.
Acknowledgments
We would like to thank the company General Electric (GE, Boston, Massachusetts, United States) for the financial support of our in-house 3D print lab.
Funding
The fee for open-access publishing was paid from the General Electric (GE) Ancillary Research Grant (AR2021.275/G-6270) which aims to support patient-specific 3D printing and simulation training in interventional radiology.
Conflict of interest
The authors declare that they have no competing interests.
314Author contributions
Conceptualization: Reinhard Kaufmann
Investigation: Reinhard Kaufmann, Michael Deutschmann, Stefan Hecht
Funding acquisition: Klaus Hergan, Reinhard Kaufmann
Project administration: Matthias Meissnitzer, Klaus Hergan
Resources: Andreas Vötsch, Christian Dinges
Software: Reinhard Kaufmann
Visualization: Bernhard Scharinger, Reinhard Kaufmann
Writing – original draft: Reinhard Kaufmann, Stefan Hecht
Writing – review & editing: Bernhard Scharinger, Stefan Hecht
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of the Paracelsus Medical University Salzburg (IRB-No.: SS22-0017-0017).
Consent for publication
The consent for publication was given by the Institutional Review Board of the Paracelsus Medical University as all patient identifiers were irreversibly removed from this study.
Availability of data
Not applicable.
References
- 1.Jones T, Seckeler M. Use of 3D models of vascular rings and slings to improve resident education. Congen Heart Dis . 2017;12((5)):578–582. doi: 10.1111/chd.12486. https://doi.org/10.1111/chd.12486. [DOI] [PubMed] [Google Scholar]
- 2.White S, Sedler J, Jones, et al. Utility of threedimensional models in resident education on simple and complex intracardiac congenital heart defects. Congen Heart Dis . 2018;13((6)):1045–1049. doi: 10.1111/chd.12673. https://doi.org/10.1111/chd.12673. [DOI] [PubMed] [Google Scholar]
- 3.Borràs-Novell C, García Causapié M, Murcia M, et al. Development of a 3D individualized mask for neonatal noninvasive ventilation. Int J Bioprint . 2022;8((2)):516. doi: 10.18063/ijb.v8i2.516. https://doi.org/10.18063/ijb.v8i2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner M, Werther T, Unger E, et al. Development of a 3D printed patient-specific neonatal brain simulation model using multimodality imaging for perioperative management. Pediatr Res . 2021;91((1)):64–69. doi: 10.1038/s41390-021-01421-w. https://doi.org/10.1038/s41390-021-01421-w. [DOI] [PubMed] [Google Scholar]
- 5.Chepelev L, Wake N, Ryan J, et al. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG): Guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D Print Med . 2018;4((1)):11. doi: 10.1186/s41205-018-0030-y. https://doi.org/10.1186/s41205-018-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballard D, Wake N, Witowski J, et al. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG) clinical situations for which 3D printing is considered an appropriate representation or extension of data contained in a medical imaging examination: Abdominal, hepatobiliary, and gastrointestinal conditions. 3D Print Med . 2020;6((1)):13. doi: 10.1186/s41205-020-00065-6. https://doi.org/10.1186/s41205-020-00065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali A, Ballard D, Althobaity W, et al. Clinical situations for which 3D printing is considered an appropriate representation or extension of data contained in a medical imaging examination: Adult cardiac conditions. 3D Print Med . 2020;6((1)):24. doi: 10.1186/s41205-020-00078-1. https://doi.org/10.1186/s41205-020-00078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bastawrous S, Wu L, Liacouras P, et al. Establishing 3D printing at the point of care: Basic principles and tools for success. Radiographics . 2022;42((2)):451–468. doi: 10.1148/rg.210113. https://doi.org/10.1148/rg.210113. [DOI] [PubMed] [Google Scholar]
- 9.Alexander A, Wake N, Chepelev L, et al. A guideline for 3D printing terminology in biomedical research utilizing ISO/ASTM standards. 3D Print Med . 2021;7((1)):8. doi: 10.1186/s41205-021-00098-5. https://doi.org/10.1186/s41205-021-00098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rueden C, Schindelin J, Hiner M, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf . 2017;18((1)):529. doi: 10.1186/s12859-017-1934-z. https://doi.org/10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Community B.O. Blender - a 3D modelling and rendering package, Stichting Blender Foundation, Amsterdam. 2018 http://www.blender.org Available at: [Google Scholar]
- 12.Schmid B, Schindelin J, Cardona A, et al. A high- level 3D visualization API for Java and ImageJ. BMC Bioinf . 2010;11((1)):274. doi: 10.1186/1471-2105-11-274. https://doi.org/10.1186/1471-2105-11-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perica E, Sun Z. A systematic review of three-dimensional printing in liver disease. J Digit Imaging . 2018;31((5)):692–701. doi: 10.1007/s10278-018-0067-x. https://doi.org/10.1007/s10278-018-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang D, Park S, Lee K, et al. Applications of threedimensional printing in cardiovascular surgery: A casebased review. Cardiovasc Imaging Asia . 2018;2((4)):166. https://doi.org/10.22468/cvia.2018.00199. [Google Scholar]
- 15.Oliveira-Santos M, Oliveira Santos E, Marinho A, et al. Patient-specific 3D printing simulation to guide complex coronary intervention. Rev Port Cardiol . 2018;37((6)):541e1–541.e4. doi: 10.1016/j.repc.2018.02.007. https://doi.org/10.1016/j.repc.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Shibata E, Takao H, Amemiya S, et al. Embolization of visceral arterial aneurysms: Simulation with 3D-printed models. Vascular . 2020;28((3)):259–266. doi: 10.1177/1708538119900834. https://doi.org/10.1177/1708538119900834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann R, Zech C, Takes M, et al. Vascular 3D printing with a novel biological tissue mimicking resin for patient-specific procedure simulations in interventional radiology: A feasibility study. J Digit Imaging . 2022;35((1)):9–20. doi: 10.1007/s10278-021-00553-z. https://doi.org/10.1007/s10278-021-00553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann R, Zech C, Deutschmann M, et al. Endovascular embolization techniques in acute thoracic and abdominal bleedings can be technically reproduced and trained in a standardized simulation setting using SLA 3D printing: A 1-year single-center study. Insights Imaging . 2022;13((1)):72. doi: 10.1186/s13244-022-01206-7. https://doi.org/10.1186/s13244-022-01206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


