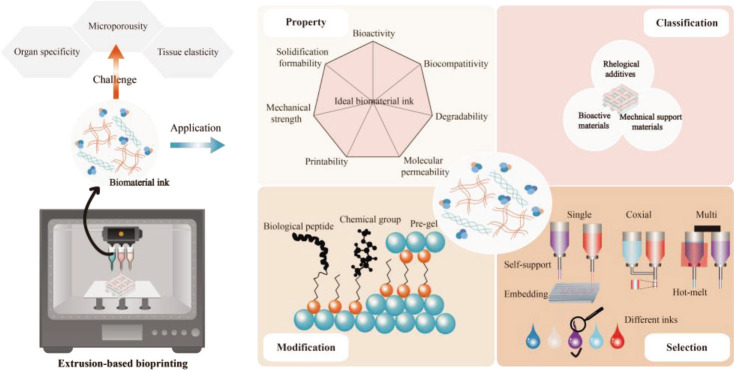
Abstract
Three-dimensional (3D) extrusion-based bioprinting is the most widely used bioprinting technology to fabricate bionic tissue or organ constructs by combining biomaterial ink and living cells for tissue engineering and regenerative medicine. One critical issue of this technique is the selection of suitable biomaterial ink to simulate extracellular matrix (ECM) that provides mechanical support for cells and regulates their physiological activities. Previous studies have demonstrated that it is an enormous challenge to form and maintain reproducible 3D constructs and eventually achieve the balance among biocompatibility, mechanical properties, and printability. This review highlights the properties of extrusion-based biomaterial inks and recent developments as well as details various biomaterial inks classified by their function. Key approaches related to their modification methods according to the functional requirements are also discussed, along with the selection strategies by varying extrusion paths and methods in extrusion-based bioprinting. This systematical review will assist researchers in identifying the most suitable extrusion-based biomaterial inks based on their requirements, as well as in elaborating current challenges and prospects of extrudable biomaterial inks in the field of bioprinting of in vitro tissue models.
Keywords: 3D bioprinting, Extrusion-based method, Bioink, Biomaterials, Tissue engineering
1. Introduction
1Three-dimensional (3D) bioprinting technology has been widely used to construct in vitro bionic functional tissues and organs with complex microarchitecture and physiological function for tissue repair and regeneration. This technique can automatically and accurately control the 3D microstructure and cell distribution as well as locate biological signals within scaffolds in tissue engineering. The application of engineered 3D vascularized tissue constructs derived from bioprinting can be further 2expanded by integrating it with organ-on-a-chip[1,2] and organoids biofabrication[3]. One representative work is that Grigoryan et al. fabricated a lung model with established multivascular networks and functional intravascular topologies by bioprinting with biocompatible hydrogels[4]. In another report, an engineered heart with cardiac ventricles, a product of co-bioprinting of collagen with cardiomyocytes, showed remarkable performance of synchronized contractions and directional action potential propagation[5]. The core of bioprinting is the bioink, which is the combination of living cells and biomaterial inks stored in a bioprinter cartridge[6]. The bioink determines the shape and function of printed constructs, which is closely related to the structure and function similarity of biomimetic tissue. The ideal bioinks require good bioactivity and printability as well as corresponding mechanical properties for 3D construction of tissue.
According to the working principle of bioprinter, traditional bioprinting technologies can be classified into four types: inkjet bioprinting, laser-assisted bioprinting, digital light processing, and extrusion-based bioprinting. Among the existing bioprinting modalities, extrusion-based bioprinting is one of the most widely used bioprinting technology with greatest flexibility to construct large scale tissues and in situ tissue or organ. The advantages of extrusion-based bioprinting include low cost, simple equipment, universality of biomaterials, and living cell-friendliness and compatibility, etc. Extrusion-based biomaterial inks (Figure 1) are biomaterial inks that can be extruded through the printing nozzle, and exhibit continuous filaments form during bioprinting process. Various biomaterials are compatible with extrusion-based bioprinter, such as biocompatible hydrogels, copolymers, and cell spheroids, thus multi-material complex 3D constructs can be engineered by using multi-nozzle extrusion-based bioprinting[7-9]. Extrusion-based biomaterial inks, including natural derived and synthetic polymers or their blends, have a wide range of viscosities, ranging from a minimum of 30 mPa·s to a maximum of 6 × 107 mPa·s[10].
Figure 1.
Definition of extrusion-based biomaterial inks.
Biomaterial ink plays the role of extracellular matrix (ECM) by providing mechanical support for cells and regulating their physiological activities. The selection of suitable biomaterial ink is an important aspect of bioprinting, and it is necessary to comprehensively consider the printing conditions and the functional requirements of the tissue constructs. Once the specific cell sources and tissues or organ types have been decided, different aspects of biomaterial ink should be taken into full consideration, such as bioactivity, biodegradability, printability, mechanical properties, and impact on the performance of bioprinted 3D constructs. There have been some reviews on bioinks[11-13] and extrusion-based bioprinitng[14-17]; for example, Panwar et al. reviewed bioinks for microextrusion-based bioprinting and focused on their printability[18]. Recently, a systematic review introduced the candidate of bioinks for extrusion-based bioprinting[19]. The lack of ideal bioinks presents a major challenge to extrusion-based bioprinting technology. However, there are a few systematic reviews that focused on extrusion-based biomaterial inks and their property, classification, modification, and selection strategy.
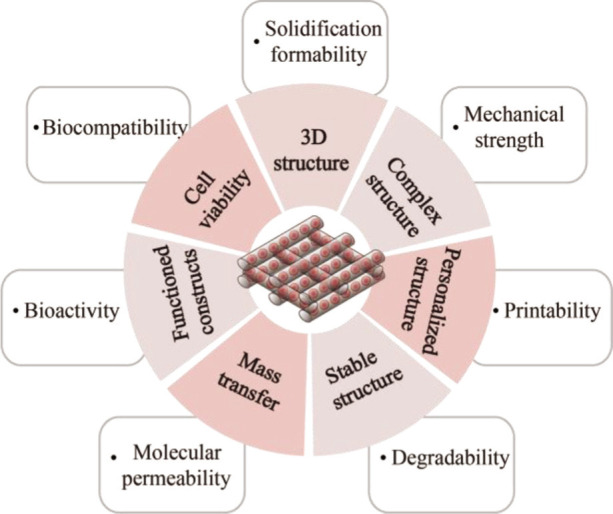
In this review, we systematically explain the properties of extrusion-based biomaterials inks, including biocompatibility, biodegradability, mechanical strength, printability, solidification formability, molecular permeability, and bionic bioactivity (Figure 2). Then, we detail the advantages and usable ranges of many 3commonly used biomaterial inks superiority by functional classification as bioactive materials, mechanical support materials, and rheological additives. The extrusion-based biomaterial inks are accurately designed by the biological and chemical modification and pre-gel formation to eventually achieve the balance between biocompatibility, mechanical property and printability, and a preferentially selected strategy is conceived by varying the extrusion strategies of single-nozzle, co-axial, and multiple-nozzle extrusion-based bioprinting. Finally, the challenges and prospects of extrudable biomaterial inks, mainly in the aspects of organ specificity, tissue elasticity and construct microporosity, are elaborated.
Figure 2.
Performance of extrusion-based biomaterial ink.
2. Properties
Biomaterial ink is a curable material that seeding cells within or on the constructed 3D scaffold. Biocompatibility and mechanical property are its basic performance requirements. Extrusion-based biomaterial ink needs the continuous deposition of extruded filaments through a suitable crosslinking mechanism. Certain swelling performance and short-term stability are also very critical to ensure the porosity and integrity of the constructed 3D structures. Furthermore, printed biomaterial inks that are mixed with living cells also require biological activity, molecular permeability and printability to ensure the delivery of nutrients and the adhesion and growth of cells. The cell-laden biomaterial inks provide structural support and allow transmission of signal molecule, cell adhesion, differentiation, and proliferation within the biomimetic ECM. This section introduces the biocompatibility, degradability, mechanical strength, printability, solidification formability, molecular permeability, and bionic bioactivity of extrusion-based biomaterial ink (Scheme 1).
2.1. Biocompatibility
Biocompatibility is one of the most basic properties of biomaterial ink. Biomaterial ink must be safe to use in the presence of endogenous tissues of the host in order to avoid immunological rejection or toxic effects. Ideally, the implant materials should perform biological functions and passively or actively produce the desired effects. Biocompatibility is reflected in the positive and controllable role of biomaterials in the biological safety and function of constructed 3D organisms. Biomaterials can support proper cell activity and promote molecular signaling or mechanical stimulation, which are critical to the success and function of transplantation. The biocompatibility of biomaterial inks in bioprinting 3D constructs is mainly reflected in the non-toxic effect of maintaining or enhancing cell proliferation and activity in in vitro drug screening application.
2.2. Degradability
Biomaterial ink scaffolds in bioprinted 3D tissue constructs will be gradually degraded by the proteases or other degrading substances and replaced by the new ECM produced by cells. The degradation rate of biomaterials needs to match the rate of cell proliferation and new ECM supplementation to ensure constant and steady substitution of ECM. The slow degradation rate increases the window period of potential foreign-body reaction or immune response, and creates a host tissue interface. The degradation products of biomaterials also need to be non-toxic and biocompatible with cells or host, as well as can be metabolized and quickly removed from the body. The fast degradation rate may affect the mechanical stability 4of 3D microstructure or scaffolds and cause collapse or deformation. The mechanical behavior like structure-property relationships should also be given attention as they could affect the degradability and degradation process of biomaterials ink.
Scheme 1. This systematical review summarizes the biomaterial inks for extrusion-based 3D bioprinting and their basic properties, functional classifications, selection principles, and biomimetic challenges.
2.3. Mechanical strength
Biomaterial inks possess suitable mechanical strength to maintain the structural stability of 3D-printed construct and balance the specific forces within the structure. It is very important to maintain the function of printed construct, which can be done by selecting biomaterial inks with corresponding mechanical and structural properties according to different tissue or organ types and the requirements of their elastic modulus. In this regard, bioprinting a scaffold-based or embedded hollow vessel with biomaterial inks will also affect the mechanical strength of the final printed structure. Therefore, it is necessary to reasonably optimize the design of 3D structure according to material properties and experimental requirements, especially to meet the mechanical properties of the native tissue.
2.4. Printability
Extrusion-based bioprinting renders biomaterial inks with a continuous linear shape, rather than a droplet shape, at the nozzle by extrusion, and directly stacks the inks into 3D structure. The printability of biomaterial inks relies on neither liquid nor solid state, but non-Newtonian fluids with certain viscosity. Generally, biomaterial inks with viscosity greater than 30 mPa·s are suitable for extrusion-based bioprinting[10]. The extrusion of biomaterial inks is a process of applying shear force, and the rheology and viscoelasticity of biomaterials affect its printability. The rheological properties of biomaterial inks are the decisive factor of printability in extrusion-based bioprinting[20]. The fluid viscoelasticity has two important parameters named viscosity modulus and elastic modulus. The viscosity modulus is also called storage modulus G’, which represents the solid property of fluid. The elastic modulus is also known as loss modulus G’’, which represents the liquid property of fluid. Extrusion-based printability reflects that the solid properties of biomaterial inks are not weaker than the liquid properties under printing conditions, that is, the viscosity modulus should be equal to or even higher than the elastic modulus to ensure the formation of 3D structures. Shear thinning performance is the basic performance of extrusion-based printability to form a continuous fluid; the apparent viscosity of biomaterial inks decreases with the increase of shear stress, and increases the fluidity during extrusion process.
The principles of extrusion-based printability are different for specific bioprinting strategies, such as embedded bioprinting and co-axial bioprinitng. For gel-bath embedded bioprinting, the properties of ink printability mentioned above are more applicable to supporting matrix. The supporting matrix should possess rheological properties, including yield stress, shear-thinning, and self-healing[21]. To easily allow nozzle movement, the yield stress should be lower than the shear stress, which is generated by the moving of nozzle inside the supporting matrix. This property allows the nozzle to insert, translate, and deposit bioinks inside the supporting matrix. In addition, the storage modulus G’ should be larger than that of supporting matrix, or else, the printed filaments would become discontinuous.
However, the printability performances of liquid-bath embedded bioprinted and co-axial bioprinted bioinks focus on fast curing, instead of rheological properties. Alginate is commonly used in co-axial bioprinting and liquid-bath bioprinting. Colosi et al. investigated the printability of core ink with different alginate concentrations and shell crosslinking solution with different calcium chloride concentrations in microfluidic-based co-axial bioprinting[22]. The printability of the bioinks was achieved by increasing the concentration of alginate and decreasing the concentration of the calcium chloride solution. The bioinks exhibited a Newtonian behavior in the range of shear rate and low viscosity, which are different from the general extrusion-based bioprinting inks.
2.5. Solidification formability
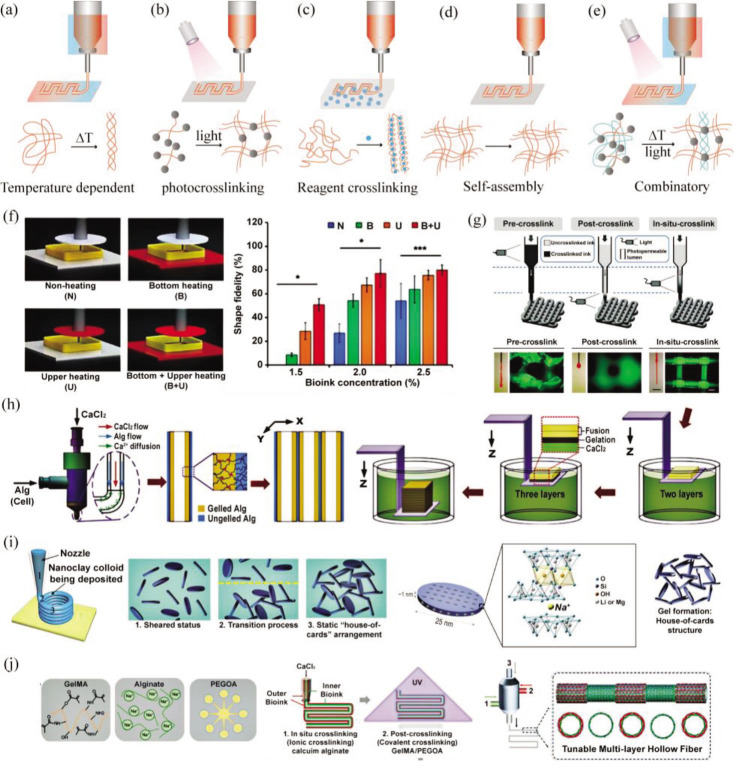
Solidification formability refers to the performance of biomaterial inks related to hydrogel forming or material curing, which is a prerequisite to construct 3D structure. The gel crosslinking method will affect the deposition of 3D structure and further affect its printability. According to the external action mode, extrusion-based bioprinting hydrogel can be divided into five types of crosslinking methods: temperature-dependent crosslinking, reagent AB crosslinking, photopolymerization crosslinking, self-assembly polymerization, and combinatory type, as shown in Figure 3.
Figure 3.
Common crosslinking methods and principles in extrusion-based printing. (a) Temperature-dependent crosslinking; (b) photocrosslinking; (c) reagent AB crosslinking; (d) self-assembly polymerization; (e) combinatory type; (f-j) examples of different crosslinking methods. (f) Printing dECM with different temperatures, reproduced under terms of the CC-BY license[23], copyright 2017, The Authors, published by Springer Nature. (g) Photocrosslinking effects before, during and after printing, adapted with permission from © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim[24]. (h) Printing alginate with CaCl2 crosslinking[25], adapted with permission from 2015 Elsevier Ltd . (i) Self-supporting printing with nanoclay, adapted with permission from © 2017 American Chemical Society[26]. (j) Printing alginate/GelMA/PEGOA with CaCl2 crosslinking and photocrosslinking, adapted with permission from © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim[27].
In temperature-dependent crosslinking, the printing temperature will affect printability, and the crosslinking of hydrogels or biomaterial inks can be achieved by controlling the temperature during or after printing process. 3D constructs based on decellularized extracellular matrix (dECM) were precisely stacked using a cell printing system equipped with heating modules[23]. Different heating conditions altered the saturated temperature, resulting in a change in the elastic modulus of the dECM bioink, affecting the gel formation, and ultimately causing an increase or decrease in printing fidelity. The crosslinked photopolymers, such 5as poly (ethylene glycol) diacrylate (PEGDA), refer to the biomaterial inks that require a certain intensity of light to form a crosslinked hydrogel under the action of a photoinitiator[28]. Reagent AB crosslinked type means that the biological material reagent A needs to be added to reagent B before it can form an ionically crosslinked hydrogel or covalently crosslinked hydrogel. The action time and concentration of reagent B will affect the solidification formability gel. For example, partially crosslinked alginate hydrogel was printed above the crosslinking reagent[29], and the suitable ratio of alginate to CaCl2 was 10:1 (w/w) to achieve suitable mechanical rigidity with best printing quality. The self-assembly polymer, such as nanoclay and nanocellulose, can be directly self-supporting printed without environment limitation. In the combinatory type, biomaterial inks can be processed using two or more gel crosslinking methods. For instance, biomaterial inks containing 1% alginate (w/v), 7% gelatin (w/v), and 5% Matrigel (v/v) pre-heated at 37°C were moved to cartridge for maintaining 35 minutes at room temperature to induce gelation in order to increase the yield stress[30]. The printed constructs were soaked in 100 mM CaCl2 for 1 minute to maintain the structure for prolonged incubation. It should be reminded that the corresponding hydrogel crosslinking method should be selected to meet the printability requirement of the biomaterial inks according to the performance of bioprinter and experimental requirements.
2.6. Molecular permeability
6The bioprinted 3D constructs need vascularized structure design to ensure the normal nutrient absorption and waste excretion by shortening the distance between mixed cells and culture medium. Biomaterial inks are also required to have certain molecular permeability to allow and maintain the transmission of macromolecular proteins, small molecule drugs, and other active factors across the gel network. The swelling rate of the biomaterial inks itself predicts the water content of the hydrogel and the microscopic porosity inside the hydrogel. Molecular permeability is very important for cell migration and nutrient transport as the absorption and transmission from cell culture medium to 3D construct could be affected.
2.7. Bionic bioactivity
The combination of biomimetic components in bioprinted constructs plays a good role in cell attachment, migration, proliferation, differentiation, and function. Bioactive materials provide a highly biomimetic environment that influences the size, shape, and adhesion of cells[31] and comprise many matrix nutriments essential for cellular activities. ECM is the 3D natural microenvironment of cells in vivo and have a positive effect on controlling cell proliferation and differentiation. The biomaterial inks with high similarity to ECM, and even tissue-specific ECM components, should be prioritized with the aim to improve their bionic biological activity.
3. Classification
Extrusion-based biomaterial ink can be a single component or a composite of multiple components, and its components can be classified into five types according to their sources. The first type is the natural dECM, such as commercialized Matrigel[30,32,3] and organ-derived dECM[23,34-39]. The second type is the natural polymer derivatives, which are divided into polysaccharides and proteins, such as collagen[40,43], hyaluronic acid[44,48], alginate[29,49-53], chitosan[54-60], fibrin[61-65], silk[66-68], gellan gum[69-73], guar gum[74,75], carrageenan[76-78], agarose[79-81], gelatin[82-88], gelatin methacrylate (GelMA)[22,89-94], cellulose derivatives[95-99], etc. The third type is the synthetic polymers, in either hydrogel form or hot melt type, such as polyethylene glycol (PEG)[100-103], PEGDA[104, 105], Pluronic[106-110], polycaprolactone (PCL)[111-113], polylactic acid (PLA)[114-116], poly (lactic co glycolic acid) (PLGA)[117,118], etc. The fourth type is the inorganic materials, such as hydroxyapatite[119-123] and nanoclay[26,124-126]. The fifth type is the semiconductor materials. Table 1 lists the commonly used biomaterial inks in extrusion-based bioprinting.7
Table 1. Common biomaterial inks for extrusion-based bioprinting.
| Material | Description | Crosslinking factor | Form | Function | Application |
|---|---|---|---|---|---|
| Matrigel | Native ECM | Temperature | Blending[30]/single[128] | Bioactivity | Bioprint tumor-derived gastric organoids for drug susceptibility test[30], bioprint bone tissue scaffolds for in vivo transplantation[33], bioprint breast tumor spheroids[128] |
| dECM | Temperature | Blending[38]/single[23] | Bioactivity | Bioprint cardiac patches for tissue repair[185], bioprint small intestine constructs with villi structure[186], fabricate vascularized airway chip[8] | |
| Collagen | Native protein | Temperature/pH | Blending[186]/single[5] | Bioactivity | Bioprint complex heart tissue constructs with gel support bath [5], print nanofiber scaffolds[110], coaxial print vascularized small intestine[187] |
| Gelatin | Temperature | Blending[188]/single[151] | Bioactivity > printability | Fabricate vascularized tissue constructs with sacrificial support printing method[131], bioprint human embryonic kidney cells for investigating the signaling pathways[137], bioprint cartilage tissue constructs[38] | |
| GelMA | Temperature /light | Blending[24]/single[189] | Bioactivity > printability > mechanical support | Directly write bioprinting cellularized scaffolds[94], microfluic-based bioprint heterogeneous tissue constructs[22], bioprint oxygenated cardiac tissue constructs[91] | |
| Fibrin | Thrombin/coagulation factors | Blending[46]/single[136] | Bioactivity | Bioprint peripheral nerve tissue constructs[62], microfluidic-based bioprint human induced pluripotent stem cells for directed neural differentiation [61], microfluidic-based bioprint glioma tissue constructs[63] | |
| Silk fibroin | Shear force | Blending[67]/single[68] | Printability > mechanical support | Bioprint bionic ears[82] | |
| Nanocellulose | Native polysaccharide | Self-assembly | Blending | Printability | Bioprint human induced pluripotent stem cells for directed osteogenic differentiation[190] |
| Methylcellulose | Self-assembly | Blending | Printability | Bioprint adipose-derived stem cells[47] | |
| Hydroxyethyl cellulose | Self-assembly | Blending | Printability | Bioprint breast cancer tumor tissue constructs for assessing drug structure-activity relationship[191] | |
| Hyaluronic acid | Temperature/light | Blending[190]/thiolat-ed[37]/methacrylated[24] | Bioactivity | Bioprint 3D scaffolds with high shape fidelity by in-situ crosslinking[24], bioprint liver tissue constructs with specific mechanical strength[37], bioprint thick skin tissue constructs for wound healing[46] | |
| Chitosan | Temperature/pH | Blending[54]/single[59] | Mechanical support | Bioprint skin tissue constructs[54] | |
| Alginate | Ca2+ | Blending[50]/single/[29]/ peptide modified[51] | Mechanical support > printability | Bioprint adipose-derived stem cells with nebulization crosslinking[49], bioprint liver tissue constructs for mimicking viral infection[36], coaxial bioprint vascularized bladder constructs[27] | |
| Gellan gum | Temperature/Ca2+ | Blending[70]/single[72] | Mechanical support > printability | Coaxial bioprint multi-layered brain tissues[72] | |
| Carrageenan | Temperature/Ca2+ | Blending[50]/single[78] | Mechanical support > printability | Bioprint oppositely charged hydrogel to form strong interface bonding[77] | |
| Agrose | Temperature | Blending[40]/single[81] | Mechanical support > printability | Bioprint bone marrow mesenchymal cells for osteogenic differentiation [40] | |
| Pluronic | Synthetic hydrogel | Temperature | Single[157]/blending[107] | Printability > mechanical support | Bioprint liver tissue constructs for assessing drug toxicity[107], fabricate perfusable vascularized constructs by sacrificial support printing method[108], bioprint thick vascularized constructs by sacrificial support printing method[9] |
| 8PEG | Synthetic hydrogel | Light | Blending[103]/single[101] | Mechanical support > printability | Bioprint multicell-laden scaffolds containing bone morphogenic pritein-4 for bone defect repair[103], bioprint human mesenchymal stem cells encapsulated with peptide-modified PEG microgels[101] |
| PEGDA | Light | Blending | Mechanical support > printability | Bioprint heart tissue constructs[105] | |
| PCL | Synthetic thermoplastic polymer | Temperature | Single[111]/blending[112] | Mechanical support | Bioprint conductive neural tissue constructs[112] |
| PLA | Temperature | Single[45]/blending[116] | Mechanical support | Bioprint cellularized scaffolds with bioactive glasses[115] | |
| PLGA | Temperature | Single[118]/blending[80] | Mechanical support | Bioprint multi-cellular scaffolds with high mechanical strength[80] | |
| Hydroxyapatite | Inorganic materials | - | Blending | Bioactivity > mechanical support | Bioprint bone scaffolds for in vivo implantation[123] |
| nanoclay | Self-assembly | Blending | Printability | Bioprint bone mineral tissue constructs[126] | |
| Polypyrrole | Semiconductor | - | Blending | Bioactivity | Bioprint conductive scaffolds for neural tissue constructs[87] |
| Carbon nanotube | - | Blending | Bioactivity | Bioprint conductive vascularized cardiac patches for subcutaneous implantation[147] |
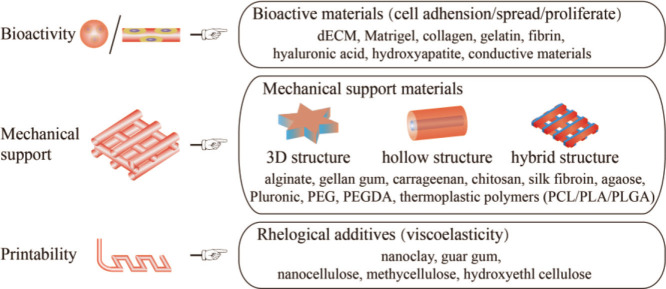
Different components perform different functions, mainly to provide bioactivity, maintain mechanical structure, and ensure printability. Two or more biomaterials are often combined and selected to fulfill the printability requirements of biomaterials inks and the needs of tissue bioactivity as well as achieve the balance among biocompatibility, mechanical properties, and printability. The addition of a certain concentration of PEGDA in GelMA can significantly increase the compression modulus of the printing structure and improve its mechanical property[127]. For example, adding nanoclay to gelatin and alginate improves their printing performance, and cells grow well on the printed nanoclay composite biomaterial scaffolds[26]. According to their main functions for biomaterial inks, biomaterial inks components can be divided into bioactive materials, mechanical support materials, and rheological additives, as shown in Figure 4. Biomaterial inks usually have a variety of basic functions; for instance, in addition to their bioactivity, gelatin biomaterials are used as mechanical supports to support tissue growth, and play a vital role in specific tissue type or printing process. Therefore, we classify the biomaterial inks according to their prominent functional characteristics, general preference of researchers, and previous literatures. Here, we mainly discuss the main function of materials used for extrusion-based biomaterial inks.
Figure 4.
The main functions of extrusion-based biomaterial inks.
3.1. Bioactive materials
Bioactive materials are used to simulate ECM, usually one or more components of ECM or their derivatives. They have great biocompatibility without cytotoxic effect and immune safety problems and can efficiently promote cell activity and biological function. This section introduces nine representative bioactive materials currently used in extrusion-based bioprinting.
3.1.1. Organ-derived dECM
Organ-derived dECM is considered the ideal biomaterial ink, which retains most of the active components in the ECM of tissues and organs. The physical crosslinking of dECM is temperature-dependent and irreversible. Despite its poor mechanical property, dECM solution forms gel in 30 minutes after an increase of the temperature from 4°C to 37°C. The structure of using dECM to perform printing alone is just a simple pattern with low resolution. Thus, combinatory dECM biomaterial inks are more reported to construct 3D structure[23]. For example, the rheological properties and shear thinning properties of human skin-dECM were improved through supplementation with fibrinogen hydrogel[34]. The basic alginate/gelatin bioink with 0.5 and 1 mg/mL human lung dECM showed the desirable viability and metabolic functions of the printed HepaRG cells[36].
3.1.2. Commercial dECMs
Matrigel, Geltrex, and BME are commercial decellularized extracellular matrix gel of Corning, Invitrogen, and R&D Systems, respectively. They are derived from the basement membrane matrix of Engelbreth Holm Swarm mouse sarcoma. These commercial dECMs contain a variety of protein components and active growth factors in ECM, such as collagen, laminin, glycan, epidermal growth factor, transforming growth factor, etc., and can promote cell proliferation and differentiation; these attributes justify why they are the first choice for 3D cell culture matrix gel. They are liquid at 4°C, reach a gel-like consistency at room temperature, and form a solidified gel at 37°C with a temperature-sensitive crosslinking mechanism similar to collagen. They can be used for extrusion-based bioprinting by controlling the temperature through the temperature-dependent gel crosslinking method. This requires the printer to be equipped with hardware equipment related to a temperature control system to ensure that the printing cartridge is at a relatively low temperature and facilitate the extrusion of matrix gel. Extrusion-based bioprinted individual cells can form spheroids in Matrigel ink with only a single-layer patterned structure[128]. It is difficult to form a 3D structure because the solidification time of matrix gel (from liquid to gel) takes about 30 minutes[16], resulting in the incomplete solidification between the printed layers and causing difficulties in stacking. Human mammary epithelial M10 cells encapsulated within Matrigel were successfully extruded at 0°C to 4°C[32], yet the construct structure was very simple and has the poor uniformity of filaments width. However, alginate/gelatin ink composited with low-concentration Matrigel has been bioprinted at room temperature to construct patient-derived gastric adenocarcinoma tissue models that support spheroid growth and expansion[30].
3.1.3. Collagen
Collagen, derived from ECM, is composed of three polypeptide chains that form a triple helix structure. Collagen, a bioactive material, contains some adhesion motifs as arginine-glycine-aspartic acid (RGD) for the interaction between cells and ECM. Collagen type I is the most abundant among the 27 types of collagen. Acidic collagen type I solution is neutralized by sodium hydroxide at low temperature and forms a gel when the temperature rises to 37°C. Collagen alone, which is used as biomaterial ink, cannot be easily patterned in a desired 3D shape. Blending it with other biomaterial inks can improve the printability. The printing fidelity and stability of bioprinted constructs can be improved by adding agarose into collagen[40]. Blending Pluronic with a low concentration of collagen improves the printability of collagen and makes the extrusion-based bioprinted constructs in the desired solid shape after thermal crosslinking[41]. Another way is to use a support bath to achieve extrusion-based bioprinting of collagen. For example, Lee et al. presented 9a method of bioprinting collagen to rebuild components of human heart by using freeform reversible embedding of suspended hydrogels[5]
3.1.4. Gelatin
Gelatin is a hydrolytic derivative of collagen that is widely used in tissue engineering. It is a mixture of peptides and proteins, and has good biocompatibility, high water absorption, and low immunogenicity. Gelatin is curly in solutions when the temperature is above 40°C, and it will reversibly form α-helical structure when the temperature drops to below 30°C[129]. One percent gelatin aqueous solution will produce chain association and 3D network. The reversibility of this helical structure depends on the concentration of gelatin and solution temperature[130]. The temperature-sensitive phase transition property of gelatin is helpful to maintain the 3D structure of printing at a certain printing temperature. Polypeptide sequences promote cell adhesion through integrin receptors and are widely used in extrusion-based bioprinting. However, it is difficult to optimize the printing temperature and viscosity due to its temperature-dependent, reversible sol-gel transition behavior. Therefore, gelatin is mostly composited with the other biomaterials as extrusion-based biomaterial inks, such as alginate[87,88]. In addition, gelatin can also be used as a sacrificial material to construct channels in 3D structure[131,132]. Gelatin derivatives that originate from gelatin through a variety of functional group modification are widely used in extrusion-based bioprinting due to their varied and better functions.
3.1.5. GelMA
GelMA is a photosensitive gelatin derivative, which is chemically modified by unsaturated methacrylamide side groups. It improves the physical and mechanical properties of gelatin and the mechanism of gel crosslinking, and is applied to extrusion-based bioprinting. GelMA has the temperature sensitivity similar to that of gelatin before photopolymerization, and forms stable covalent crosslinking after photopolymerization. The temperature-sensitive physical crosslinking is no longer reversible, and a fixed 3D structure is formed after bioprinting. The covalent crosslinking of GelMA requires the presence of photoinitiator, and its type and concentration will affect cell activity, which varies at different levels[13]. In addition, the amidation substitution degree and ultraviolet (UV) irradiation time of GelMA will affect the mechanical properties[133]. With the increase of substitution degree and UV irradiation time, the cell activity will decrease[134]; Moreover, the rheological properties will decrease with the increase of the degree of substitution[135], thus affecting the printability.
3.1.6. Fibrin
Fibrin is a component of the natural ECM. Fibrinogen is a glycoprotein composed of multiple pairs of polypeptide chains. It contains a cell signal domain, including protease degradation and cell adhesion sequences. Under the action of thrombin and coagulation factor VIIIa, fibrinogen is cleaved into fibrin polypeptide. These monomers spontaneously polymerize to form fibers, and subsequently form a fibrin gel. Therefore, fibrinogen has the potential to be used as a biomaterial ink in extrusion-based bioprinting due to the bioactivity of fibrin and the gelation mechanism of reagent AB. The cells can also adhered to and proliferated in the printed fibrin scaffolds[136]. However, fibrin gel is difficult to maintain the 3D structure due to its weak mechanical properties, and it is necessary to blend it with other polymers to compensate its mechanical defects[137,138]. The fibrin hydrogel was combined with gelatin, glycerol, and hyaluronic acid to generate a biomaterial ink that forms a robust gel for bioprinting full-thickness skin[46].
3.1.7. Hyaluronic acid
Hyaluronic acid, a component of ECM, plays an important role in influencing cell growth, migration, and 10differentiation. Hyaluronic acid, a linear glycosaminoglycan composed of repeating units of D-glucuronic acid and N-acetyl-D-glucosamine, is a highly hydrated polyanionic macromolecule that mainly exists in the form of sodium salt in nature. Sodium hyaluronate aqueous solution has high viscosity and good shear thinning property[139]. However, hyaluronic acid hydrogel has low gelation rate in the literature, and hyaluronic acid precursor solution was printed and crosslinked at 37°C for 4 hours[140]. In order to improve the hydrogel crosslinking rate, hyaluronic acid is often chemically modified to be photocrosslinkable[44,48,3]. For example, hydrogel precursor containing pentenoate-functionalized hyaluronic acid, dithiothreitol, and Irgacure 2959 was printed and then crosslinked after exposure to 312 nm UV light for 2 minutes. The poor mechanical strength of hydrogel results in simple pattern structure. Using hyaluronic acid blended with other hydrogels as biomaterial inks can improve printing fidelity to bioprint stable constructs. The compressive modulus of bioprinted hyaluronic acid/methylcellulose constructs increased with increasing methylcellulose contents[47]. Human articular chondrocytes encapsulated with hyaluronic acid/alginate were co-printed with PLA to engineer cartilage tissue[45]. The mechanical properties of the bioprinted constructs were comparable to those of human articular cartilage after 4 weeks of in vitro culture. Human glial cells were bioprinted with hyaluronic acid/alginate/gelatin for developing a brain matrix-mimetic microenvironment model, which simulated both mechanical and biological properties of human brain microenvironment[141].
3.1.8. Hydroxyapatite
Hydroxyapatite, which is the main inorganic component of bones, is mainly used to construct bone tissue by bioprinting. Although hydroxyapatite cannot provide natural binding sequences for cell attachment, it has excellent biocompatibility, osteoconductivity, and bioactivity, and it still belongs to category of bioactive material. Hybrid hydroxyapatite-containing biomaterials provide a promotive scaffold for chondrocytes, facilitating the proliferation and migration of chondrocytes as well as promoting the chondrogenic differentiation of stem cells[119]. As a heterologous material, hydroxyapatite is usually doped in other bioactive hydrogel materials, such as collagen, gelatin, GelMA, hyaluronic acid, and alginate, to form an extrusion-based biomaterial ink. For example, collagen/hydroxyapatite composite biomaterial ink was successfully used to print biomimic scaffolds seeded with bone marrow stromal cells for bone regeneration[123]. By doping nanosized hydroxyapatite into weak printable hydrogel, such as gelatin[119] and alginate[121], the fluidity, viscosity, and gelation time were modulated to allow more freedom in 3D structure designs. Although the bioprinted enzyme-crosslinked gelatin/hydroxyapatite scaffolds decreased the viability and proliferation of human umbilical cord blood-derived mesenchymal stem cells in vitro, they promoted the chondrogenetic differentiation both in vitro and in vivo in a pig model of cartilage repair. In addition to printing scaffolds for cell seeding, hydroxyapatite can also be printed with cells together using extrusion-based printing. For instance, adipose-derived stem cells-laden hydroxyapatite/GelMA/methacrylated hyaluronic acid inks were bioprinted for a stable grid structure at room temperature and cultured for 28 days[120]. The addition of hydroxyapatite showed positive effects on bone matrix production and remodeling. Hydroxyapatite is an important component for developing osteoinductive bioink and widely used in bone tissue bioprinting research.
3.1.9. Conductive materials
Conductive materials can be used as electrodes to promote signal transductions between biological tissues and electrical circuits. It is noteworthy to mention that conductive materials can also promote cell adhesion, proliferation, and differentiation by stimulation. Due to the potential, conductive materials have been used in smart biosensors, functional tissue engineering scaffolds, and implants. In extrusion-based bioprinting applications, conductive biomaterial inks can be formed by using different conductive materials including conductive polymers (e.g., polypyrrole[112], polyaniline[142], polythiophene[143], and polyethylene dioxythiophene[144]), conductive metal nanoparticles (e.g., gold[145] and silver[146]), conductive carbon-based materials (e.g., carbon nanotube[147] and graphene[148]), or ionic liquids[149]. Metal nanoparticles and carbon-based materials have long-term cytotoxicity, which can be a limitation for tissue engineering and regenerative medicine[112]. Electrical conductivity is a key to native tissue physiology and function of heart, brain, and nerve, so conductive hydrogels are often used for bioprinting cardiac and nervous tissues.
3.2. Mechanical support materials
Mechanical support materials are biocompatible, but they are generally biologically inert and not conducive to cell adhesion. They are usually used as auxiliary materials to support bioprinting 3D structures. This section introduces nine representative mechanical support materials currently used in extrusion-based bioprinting.
3.2.1. Alginate
Alginate is a natural polysaccharide extracted from brown algae or Sargassum species. It forms a hydrogel through the rapid exchange reaction of calcium ions and sodium ions, and is widely used in the field of regenerative medicine. The water-soluble, low-cost, and fast ionically crosslinked gel forming properties of naturally sourced alginate make 11it the first choice for cell embedding, and it is widely used in extrusion-based bioprinting to promote the rapid formation of 3D structures. Alginate is biologically inert with a low cell adhesion rate, and its corresponding calcium ion crosslinking reagents will adversely affect cell viability. However, alginate can be chemically modified by adding cell adhesion ligands to promote cell adhesion, stretching, and proliferation[150]. Benefited from its rapid hydrogel gelation rate, alginate is often combined with other hydrogels, such as gelatin[36], collagen[151], Matrigel[30], and Pluronic[107], to improve construct stability. Another main application of alginate is to directly fabricate hollow tubes by coaxial printing[25,27] so as to construct vascularized tissue for perfusion culture.
3.2.2. Gellan gum
Gellan gum is a natural polysaccharide gum obtained by the fermentation process of microorganism. Gellan gum, an anionic polysaccharide, like alginate, is capable of forming gels in the presence of Ca2+. Gellan gum is also used in co-axial bioprinting owing to the rapid crosslinking mechanism[72]. The addition of gellan gum to hydrogels, like GelMA[73], can significantly increase the viscosity due to the ionic crosslinking. In addition to the low production cost, gellan gum can achieve mechanical strength similar to that of gelatin at lower concentrations, which encourages increased use of the material[72]. On the other hand, the gel brittleness is also similar to gelatin, which restricts structural stability of printed constructs. The mechanical properties of gellan gum can be modified by blending it with other biomaterial inks, such as alginate[70], PEGDA[69], and even nanoparticles, such as graphene oxide[71].
3.2.3. Carrageenan
Carrageenan, a sulfated polysaccharide extracted from red algae, is composed of repeated galactose units, similar to natural glycosaminoglycans. Depending on the sulfate content, source of extraction and solubility, the carrageenan can be conventionally categorized into six basic forms: Kappa, Iota, Lambda, Mu, Nu, and Theta[152]. Kappa-carrageenan and Iota-carrageenan can perform thermogelation, that is, the polymer can form gels at low temperature. Blending carrageenan with other hydrogels can adjust rheological property due to the high viscosity. The addition of carrageenan to alginate hydrogels could increase rheological properties, such as shear shinning, thixotropic behavior, and viscoelasticity, which improve the printability and structure fidelity of printed constructs[50]. Carrageenans have negatively charged carboxyl and sulfate groups, which result in gelation through ionic crosslinking with specific cations, such as Ca2+ and K+. Due to the oppositely charged performance of GelMA, polyelectrolyte complexes are formed between Kappa-carrageenan and GelMA hydrogels, thereby forming strong interface bonding between different hydrogels and improving the adhesion of printed layers[77]. Carrageenan hydrogels are brittle and the mechanical stabilities are poor, resulting in the printed constructs structure unstable. To overcome this drawback, the polymer backbone is chemically modified. For example, methacrylated Kappa-carrageenan combined with NIH-3T3 cells was used as co-axial printing bioinks at room temperature, and the use of UV crosslinked hydrogel resulted in latticed constructs with high mechanical strength[78].
3.2.4. Chitosan
Chitosan, a linear polysaccharide composed of D-glucuronic acid and N-acetyl-D-glucosamine, is obtained from deacetylation of chitin. Chitosan powders are generally soluble at acidic pH lower than 6, and the dissolved positively charged chitosan solution has high viscosity and shear-rate shinning behavior for extrusion-based printing[59]. The mechanical integrity of chitosan hydrogel is weak; therefore, it is hardly used as biomaterial ink alone. Blending alginate with chitosan can improve the compression of printed constructs[58]. Chitosan has hemostasis, anti-bacterial, and antifungal activities, so it has great potential to be used in bioprinting skin tissue. A study reported that chitosan/PEG composite hydrogel-encapsulated keratinocytes and dermal fibroblasts were printed layer by layer to construct skin tissues for potential skin regeneration[54]. Although chitosan shows structural characteristics similar to those of hyaluronic acid, it is not conducive to cell adhesion and proliferation because it lacks cell binding domains. A study reported that blending gelatin with chitosan formed physical polyelectrolyte hydrogel at pH 6.3, which was extruded at room temperature to fabricate 3D constructs with high shape fidelity[57]. Neonatal human foreskin fibroblasts that are seeded onto the polyelectrolyte hydrogel could attach and proliferate better compared to the pure chitosan hydrogel.
3.2.5. Silk fibroin
Silk fibroin, a natural fibrous protein polymer, is commonly derived from silkworm silk and spider silk. Silk fibroin usually lacks cell binding domains[18]; however, silk from Philosamia ricini has the intrinsic presence of the cell-binding RGD tripeptide[82]. The sol-gel transition of silk is the change of secondary conformation from random coil to β—sheet structure. Silk solution can form gel under the action of shear force. Therefore, silk may cause frequent nozzle clogging when it is used as biomaterial ink alone[82]. Blending silk with other polymers, such as gelatin[82] and PEG[68], can improve injectability in the self-supporting printing process. The mechanical property of silk fibroin is poor under physiological condition and can be easily 12affected by β-sheet structure. Blends of hydroxy propyl methyl cellulose of methacrylation and silk fibroin formed double network hydrogel, and the fracture strength, breaking elongation, and compressive reproducibility of printed constructs increased significantly[67]. Silk fibroin solution is also used in freeform fabrication with nanoclay and PEG support bath[66]. Bioprinting of silk fibroin is more widely used in cartilage tissue engineering.
3.2.6. Agrose
Agrose, a natural polysaccharide, is obtained from the cell walls of red algae. Agarose solution has a sol-gel transition in the range from 32°C to 47°C, depending on the concentration, and physically polymerizes to form a gel within seconds[40]. Although agrose hydrogels lack cell binding sites and thus have limited bioactivity[153], adding agrose into other polymers can improve print fidelity and stability of printed structure[40,79]. Agrose can also be used as sacrificial material to construct hollow channel[81,154]. Besides, agrose can also serve as suspending hydrogel for the freeform fabrication[155].
3.2.7. Pluronic
Pluronic is a non-ionic triblock copolymer composed of polyoxyethylene-polyoxypropylene-polyoxyethylene. Due to the amphiphilicity caused by the hydrophobicity of polyoxypropylene and the hydrophilicity of polyoxyethylene, it can form soluble micelles as a carrier of nano drugs for drug delivery. Pluronic is a temperature-sensitive polymer and its critical micelle temperature is between 22°C and 37°C. It will self-associate and appear gelatinous above this temperature. Pluronic’s shear thinning performance and thixotropic performance are excellent, and the printing fidelity is extremely high in extrusion-based bioprinting. However, it is biologically inert with a low cell adhesion rate, and cannot be degraded by enzymes. Its printed cell activity is even as low as 50% when it is used as a biomaterial ink alone[156]. Pluronic is often used as a sacrificial material, that is, it is dissolved at low temperature after printing since it can perform temperature-sensitive and reversible gelation. Pluronic can be used to construct a mold loaded with matrix gel[157] or a vascular network channel in a 3D structure[9,106,3].
3.2.8. PEG
PEG is often used as pharmaceutic adjuvant. PEG-based gels can be formed by physical or covalent crosslinking, and used internally with FDA approval[158]. PEGDA is an acrylated derivative of PEG, which can be photopolymerized to form a gel with superior mechanical properties. PEGDA can be easily used to construct 3D scaffolds in extrusion-based bioprinting. Similar with Pluronic, synthetic polymer chains do not contain attachment points that enable interactions with cells, resulting in a lack of biological activity. Therefore, PEG-based gels are generally not printed with cells together because the cells cannot easily migrate and proliferate on the printed PEGDA scaffolds[16]; therefore, they are more likely to be used as carriers for loading bioactive materials to improve mechanical strength for maintaining the 3D structure[103]. Another method to improve the bioactivity of PEG is peptide modification. For example, PEG-based microgels were modified with the cell adhesive peptide and then printed together with human mesenchymal stem cells to form 3D constructs, which support cell spreading and proliferation[101].
3.2.9. Thermoplastic polymer
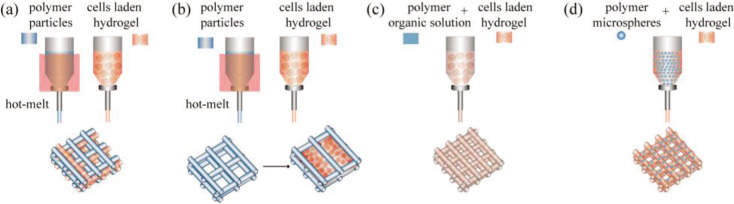
PCL, PLA, and PLGA are all thermoplastic polymers, which could serve as structural materials and are capable of resisting mechanical forces in hybrid constructs, as shown in Figure 5. Usually, thermoplastic polymers are deposited by hot melt approach at appropriate temperatures (e.g., PCL at 80°C[111], PLA at 200°C[45], and PLGA at 110°C[117]) to fabricate mechanical scaffolds, then cell-laden hydrogels are printed and deposited alongside the scaffold filaments[117] or injected into the scaffolds pores[45]. Thermoplastic polymers can also be dissolved in organic solvents for preparing extrudable ink by blending with other hydrogels[115,116]. For example, PLGA was dissolved in 13methyl ethyl ketone and successfully printed in a grid structure with a printing temperature of 20°C[118], thereby preventing high-temperature damage to the cells. Another low temperature printing strategy of thermoplastic polymers is blending printable hydrogels with polymers in the form of microspheres. The mechanical strength of printed constructs is greatly improved and up to more than 100 times after adding PLGA porous microspheres into agrose-collagen hydrogel[80].
Figure 5.
Thermoplastic polymers for extrusion-based bioprinting. (a) Cell-laden hydrogels are printed alongside the scaffold filaments. (b) Cell-laden hydrogels are injected into scaffolds pore. (c) Blending of cell-laden hydrogel and polymer organic solution. (d) Thermoplastic polymers are added into cell-laden hydrogel in microspheres form.
3.3. Rheological additives
Rheological additives are rheological control agents for coatings in the industrial field. The main function of rheological additives is to improve the viscosity of coatings, and then improve the anti-settlement during storage and anti-sagging during construction. Rheological additives are added to biomaterial inks to improve their rheological properties and printability so as to ensure the fidelity of complex 3D structure printing. This section introduces three representative rheological additives currently used in extrusion-based bioprinting.
3.3.1. Nanoclay
Nanoclay is a synthetic magnesium silicate clay, which is an inorganic material. It is widely used in the cosmetics industry and the coating industry as a rheology aid and film-forming additive[159]. The degradation products of nanoclay are non-toxic and even have a positive effect on bone metabolism and calcification[160], and have a great potential in tissue engineering applications. Nanoclay, which is sensitive to viscosity shearing, is able to be quickly sheared and thinned and to restore the structure after shearing. This good thixotropy endows it with good performance as an extrusion-based printing ink, and encourages extensive application of nanoclay in 3D bioprinting, even 4D printing[161,162]. However, nanoclay is a dispersion system in aqueous solution, not a solution system. The addition of low-concentration nanoclay to other polymer gels can cause deposition and result in blockage of the printing nozzle. Moreover, nanoclay existing as nanoparticles will fill the internal pores of gels, and further affect their swelling properties[124], reduce the permeability of active factors[163], and ultimately affect the nutrient delivery of embedded cells. Thus, nanoclay is not suitable to be used as cell embedding biomaterial inks, but is only applicable for printing scaffolds without cells.
3.3.2. Cellulose derivatives
Nanocellulose is a derivative of cellulose with high zero shear viscosity and strong shear thinning performance and is widely applied in extrusion-based bioprinting and 4D printing[161,164]. A problem with using nanocellulose is the nozzle blockage due to its colloidal water dispersion and the fact that it is undissolved in water at the molecular
level[165,166]. Besides, the activity of cells embedded in it is low and can only be maintained at about 70% due to the problem of mechanical force[167]. Therefore, similar to nanoclay, nanocellulose not suitable to be used as cell embedding biomaterial ink.
Hydroxyethyl cellulose and methylcellulose are both water-soluble non-ionic cellulose derivatives. They have been used in extrusion-based bioprinting to adjust the viscoelasticity of inks for improving the printability due to their shear thinning performance. For example, the shape fidelity of printed filament is improved by adding methylcellose into alginate[96]. Law et al. used blends of hyaluronic acid and methylcellose with different concentrations as biomaterial ink for bioprinting mesenchymal stem cells, and the cell viability was above 75% in bioprinted structures[47]. Hydroxyethyl cellulose is an environmentally friendly material and the most abundant biopolymer on Earth[168]. Hydroxyethyl cellulose has many hydroxyl groups, which determine hydrophilicity and capacity for chemical modification. In regards to bioprinting, hydroxyethyl cellulose seems to be more suitable than methylcellulose whose methyl groups are inert[169]. As a rheological additive, hydroxyethyl cellulose exhibited properties similar to those of nanoclay, and they can improve printability for self-supporting bioprinting[170].
3.3.3. Guar gum
Guar gum is a water-soluble natural polysaccharide produced from endosperms of leguminous plants, which comprise mannose and galactose[171]. Owing to extensive hydrogen bonding between galactose units and water, guar gum solution has high viscosity in cold water even with low concentrations. Compared to other natural gums, guar gum is cheaper. It is mainly used as thickener and stabilizer in industry. Guar gum forms a viscous colloidal dispersion in water and shows pseudoplastic and shear-thinning behavior, fulfilling the requirements of extrusion-based printing biomaterial ink. Blending guar gum with bioactive biomaterial inks can improve the printability. Blending of guar gum and chitosan at acid pH was printed in rectangular membrane structure at 37°C and then neutralized and gelled by immersing it in sodium hydroxide solution[74]. By adding guar gum into 10% gelatin solutions, the tanδ value, the ratio of G”/G’, increased over 0.151, which is an ideal requirement for the filament formation[75]. Meanwhile, the increased gel strength is able to control the structural integrity of the printed constructs.
4. Modification of biomaterial ink
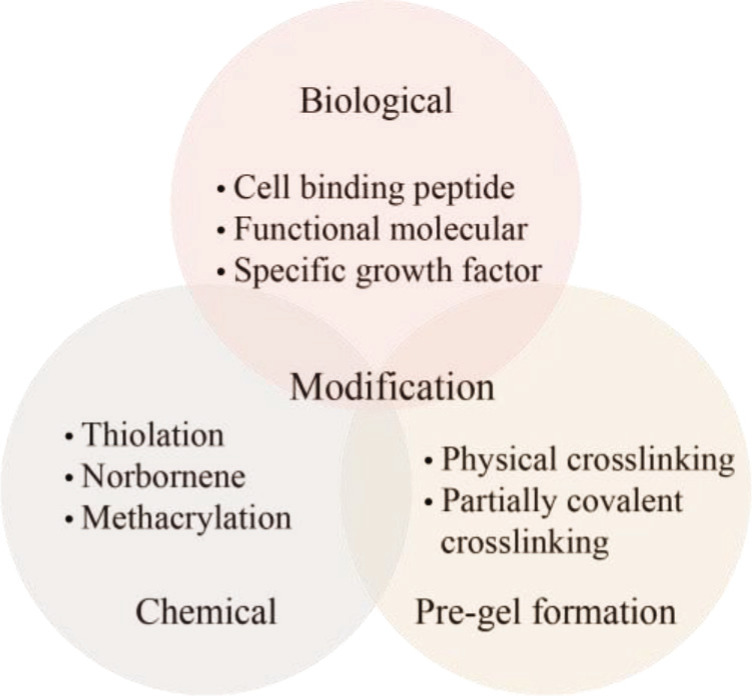
Although extrusion-based biomaterial ink can be used to generate structurally and mechanically well-integrated 14constructs, there are several general and specific challenges in the application of the ink. Mechanical properties usually need to be well tuned and matched to ensure specific functional requirements of various cells, tissues, or organs. Alginate can be printed into 3D tissue structure with relatively high printability by pre-crosslinking or coaxial nozzle-assisted crosslinking. Its surface modifications should be paid close attention as they can affect mechanical properties, which vary greatly according to solution concentration and curing strategies, and immobilize cell viability and interaction capabilities with the biomaterial matrix. As shown in Figure 6, the bioactivity, mechanical property and printability of biomaterial inks can be improved through molecular structure modification or physical modification, including biological modification, chemical modification, and pre-gel formation.
Figure 6.
Modification of biomaterial inks.
4.1. Biological modification
It is necessary to manipulate biological and biochemical environments of the bioprinted biological constructs, which are bioinert, for cell-cell and cell-ECM interactions, and to sustain the viability, spreading, and proliferation of living cell during long-term culture. Peptide modification of biomaterial ink that lacks cell-adhesion binding sites can influence cell viability, proliferation and differentiation[172]. The peptide modification is generally carried out with mechanical support materials. For example, peptide-modified alginate that serves as biomaterial ink was printed to fabricate bioactive constructs for cells adhesion[51], bone tissue engineering[173], nerve tissue engineering[174], and even tissue microvasculature[175]. In another study, primary cortical neurons and glial cells encapsulated in gellan gum were bioprinted in brain-like layer structure, and cortical neurons responded better in RGD-modified gellan gum constructs than in pure gellan gum[72]. Stem cells are seeded on printed PCL scaffolds for cartilage or bone tissue engineering according to the mechanical performance. To compensate for the absence of bioactive property, bioinert PCL is conjugated to tissue-specific peptides, such as bone morphogenetic protein mimetic peptide, glycine-histidine-lysine peptide, and osteogenic growth peptide, to promote chondrogenic or osteogenic differentiation of stem cells[176,177].
4.2. Chemical modification
Chemical modification of biomaterial ink is usually intended to form stronger intermolecular interactions that are related to its viscoelastic properties and perform more chemical functionalities, thereby improving the biocompatibility, printability, and mechanical properties[31]. The well-described chemical modifications include thiolation, norbornene, and methacrylation that could create more functional derivatives. Photocurable gelatin-based hydrogels, such as GelMA, are powerful light-responsive bioinks with adjustable hardness, excellent biocompatibility, and printability[178]. Norbornene-functionalized gelatin (GelNB) mixed with a thiolated crosslinker have recently gained increasing importance as thiol-ene functional hydrogel systems. Methacrylated hyaluronic acid and norbornene-functionalized hyaluronic acid were also used as inks in in situ photocrosslinking bioprinting[24]. In another case, thiolated hyaluronic acid-based bioink-encapsulated marrow-derived mesenchymal stromal cells were printed in scaffold structure with suitable mechanical property, and the constructs showed cartilaginous ECM deposition with good biological performance[179].
4.3. Pre-gel formation
To achieve high printability for complex layered constructs, the biomaterial inks are printed in the form of pre-gels that are partially crosslinked. The viscoelastic properties of pre-gels tend to exbibit more elastic (solid) behavior, rather than viscous (fluid) behavior, improving the structure stability. In most cases, crosslinking before printing is physical. The prepared dECM bioink from different tissue types may require different solubilized concentration and incubation at physiological temperature for pre-gel formation and gelation, and their printability is decided by the pre-gel consistency prior to gelation for retaining the generated 3D structure[180]. Calcium chloride solution[29] or calcium sulfate solution[50] was added into alginate-based biomaterial inks before printing to increase the fidelity of printed structure. Some biomaterial inks are partially covalent-crosslinked before printing to control viscosity. For example, thiolated hyaluronic acid and gelatin composites were spontaneously crosslinked with PEGDA through thiol-acrylate binding, forming a soft and extrudable biomaterial ink[37].
155. Selection of biomaterial ink
Many kinds of hydrogel polymers can be used in extrusion-based bioprinting for applying in the field of tissue engineering, and their properties and functions varies for ideal construct design and applied. However, a single type of hydrogel polymer can hardly fulfill all the performance requirements of extrusion-based bioprinting. Therefore, two or more biomaterial inks should be selected for blending to obtain the ideal extrusion-based bioink (Figure 7), according to the geometric shape to be constructed, the applicable printing method and the requirements of tissue function.
Figure 7.
Selection of biomaterial inks for specific bioprinting strategy. (a) Single-nozzle extrusion-based bioprinting. (b) Coaxial-nozzle extrusion-based bioprinting. (c) Multi-nozzle extrusion-based bioprinting.
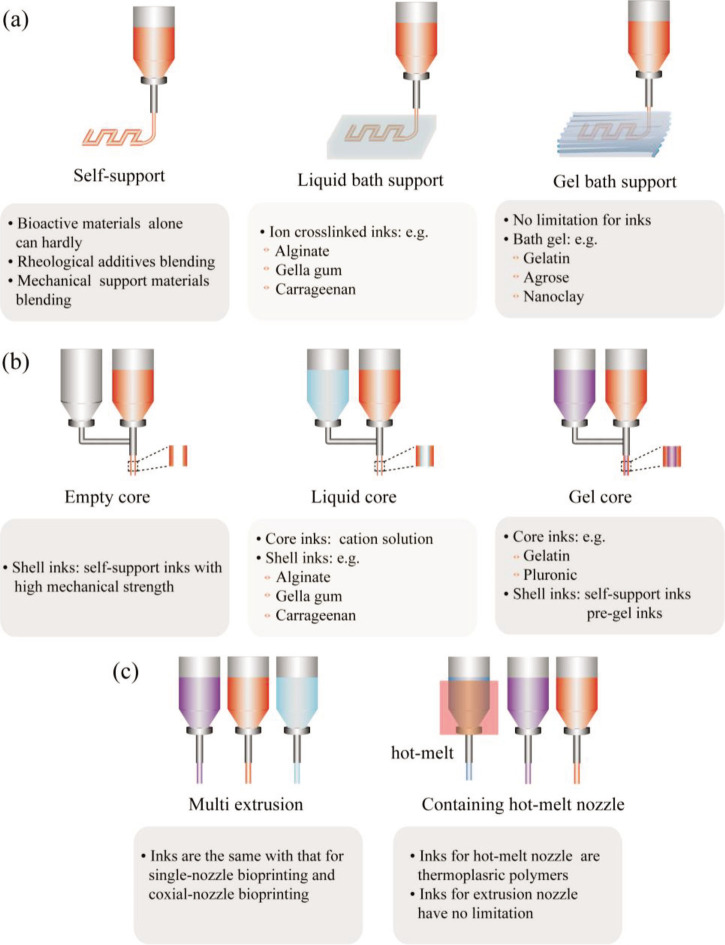
In addition to conventional single-nozzle/single-material bioprinting, the extrusion-based 3D bioprinting strategies also have some variations, such as embedding bioprinting, co-axial bioprinting, multi-nozzle/multi-material bioprinting, single-nozzle/multi-material bioprinting with the microfluidic nozzle[181], additional configurations of multi-material bioprinting and continuous chaotic bioprinting[15]. The basic molding unit 16of extrusion bioprinting is extruded filament; therefore, according to the type of filaments, these extrusion strategies can be classified into single-nozzle bioprinting, co-axial bioprinting, and multi-nozzle bioprinting.
5.1 Single-nozzle bioprinting
Micro-extrusion single-nozzle/multi-material bioprinting, additional configurations of multi-material bioprinting and continuous chaotic bioprinting are all defined as single-nozzle bioprinting. Only one filament is extruded from a single nozzle at the same time, and the composition and combination of multi-materials could vary. Specifically, in self-supporting bioprinting, biomaterial ink is directly printed in the air without structural support materials. The biomaterial inks for self-supporting bioprinting have excellent viscoelastic properties and mechanical properties sufficient to support the printed structure, thus compositing with rheological additives or structural auxiliary materials is essential. It is difficult to self-supporting bioprint bioactive materials alone, unless they have been chemically modified and pre-gelled.
Embedding bioprinting needs a rapid gelation profile after free deposition of the filament into a liquid or gelatinous coagulation support bath. For the liquid support bath, the liquid is usually a solution containing Ca2+, and the biomaterial inks are generally alginate-based inks or other hydrogels gelled through cations crosslinking, like gellan gum-based inks and carrageenan-based inks. For the gel support bath, the biomaterial ink can be selected as any compatible materials with extrusion-based bioprinting, the gels in the support bath should have shear-shinning viscosity behavior and thixotropic behavior to fulfill the self-supporting bioprinting requirements. More importantly, the gels in the support bath can be easily removed to ensure stability of printing structure and convenience of post-processing. So far, the use of gelled gelatin[5], gelled agarose[155], and nanoclay[182] as the gels in the support bath have been reported.
5.2. Co-axial bioprinting
The bioprinter corresponding to co-axial bioprinting is equipped with a coaxial nozzle performing continuous infusion with internal and external flows. The main purpose of co-axial bioprinting is usually aimed at printing hollow fiber directly in one step to fabricate blood vessels or other tubular structures. One scenario is that the core of the nozzle provides internal flow with ordinary fluid and non-curing molding after printing, then the shell inks must be self-supporting bioprinted with large mechanical strength. Otherwise, the shell structure may collapse, resulting in the failure to form hollow fibers. The other case is that the core of the nozzle provides internal flow with a cation solution, then the shell biomaterial inks are exactly corresponding to the inks printed for liquid support bath bioprinting. The third case is to use fugitive or sacrifice inks (e.g., gelatin and Pluronic) as the core ink, then the shell inks can be partially crosslinked pregels or self-support printed biomaterials that can hold out the structure shape. If the core and shell of co-axial nozzle were two phases of water and oil, the printing technique would help to fabricate uniform microbeads, such as structural color beads used for fluorescence detection[183].
5.3. Multi-nozzle bioprinting
Multi-nozzle extrusion-based bioprinting is a powerful tool to manufacture vascularized organs with hierarchical internal/external structures for biomimicing multiple physiological functions in vitro, such as bioartificial lungs and heart. It can also customize the 3D-printed bio-constructs with gradient material constituents by controlling the combination of multiple nozzles and corresponding biomaterials inks. The nozzles can be divided into two categories of hot melt nozzle and extrusion nozzle. The printing strategy can be selected as multi-extrusion nozzle, or the combination of hot melt nozzle and extrusion nozzle. The principles of multi-extrusion nozzle in the selection of biomaterial inks are similar to that of single-nozzle bioprinting. For the second strategy, the thermoplastic polymers in the form of wires are hot-melted and deposited into fibers to form scaffolds. Then, biomaterial inks are printed onto the scaffolds, resulting in hybrid constructs. The biomaterial inks can be extended for different hydrogels and their crosslinking agents to demonstrate the feasibility of this versatile multi-nozzle bioprinting method.
6. Outlook
Extrusion-based bioprinting has been successfully used to construct a variety of in vitro tissue and organ models, which are applied to the fields of drug screening, tissue engineering, and regenerative medicine. There are still some deficiencies and defects that warrant continuous improvement, especially the biomaterial inks, which represent the most important limitation. Biomaterial inks contain a wide range of printable biomaterials with different properties and functions. Nevertheless, their viscoelasticity and gel crosslinking mechanism mainly affect printing performance of the ideal design structures. Generally, the excellent printability and high shape fidelity can be achieved through different printing strategies. However, bioactivity and mechanical properties are limited by the biomaterial ink itself. Therefore, in the future, the extrudable biomaterial inks should be developed with good bioactivity and suitable mechanical property in regard to organ specificity, tissue elasticity, and construct microporosity, as shown in Figure 8.
Figure 8.
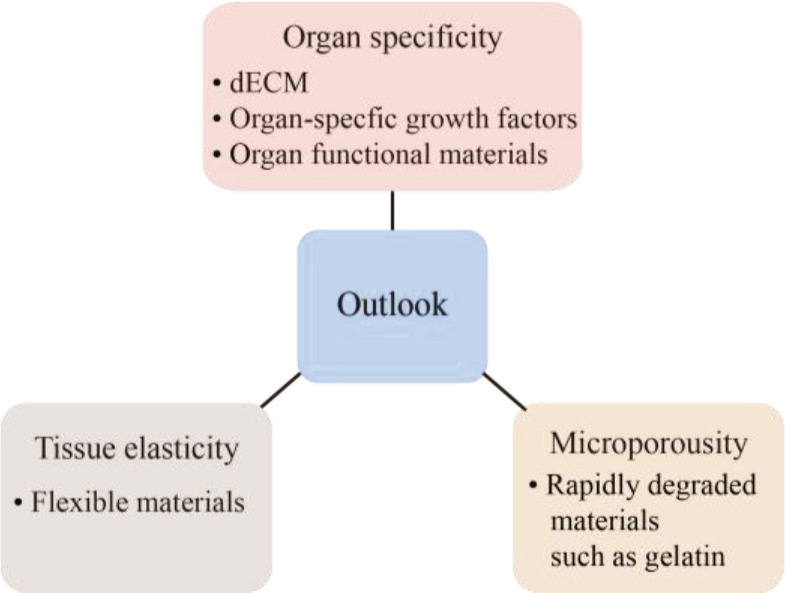
Future development of extrudable biomaterial inks.
17The ECM could vary among species, organs, and even individuals. The homogeneous biomaterial inks cannot reflect the specificity of tissue and organ ECM, and owing to the insufficient interaction between cells and ECM, the biological function of cells could be affected. In order to simulate the function and even pathological state of tissues and organs to the greatest extent, dECM should be selected as organ-specific biomaterial inks to build personalized constructs for realize the specific biological function of bioprinted tissues. Another strategy for organ-specific ink is conjugation of biomaterial inks and specific growth factor or bioactive molecules for maximum simulation of specific physiological microenvironment.
The biological soft tissues, other than bone tissues, require strength and elasticity for regular tissue function, such as stretching and contracting, and their geometric structure should be restored after the external force of tension and compression is removed. Extrusion-based biomaterial ink shows viscoelasticity and thixotropy during printing. However, fully crosslinked hydrogels after printing have greater rigidity with lower elasticity, and are brittle under mechanical action. In order to simulate the high elasticity of biological soft tissues, polymers with intrinsic elasticity and resilience can be applied to assist biomaterial inks for printing elastic constructs[184].
Bioprinted tissue constructs have macroscopic pore structures, thus culture media could diffuse into filaments, providing mass transfer for cells embedded in filaments. However, interconnections between cells are blocked by the gel matrix due to the lack of microporous structure. The microporous structure can provide large surface area for cell adhesion and promote vascularization of tissue constructs. Therefore, the preferred strategy is bioprinting constructs with microporous structure or adding rapidly degraded materials into biomaterial inks.
7. Conclusions
Extrusion-based bioprinting is the most widely used bioprinting technology to fabricate bionic tissue or organ constructs by combining biomaterial inks and living cells for drug screening, tissue engineering, and regenerative medicine. This paper reviews the properties of extrusion-based biomaterial inks and details various biomaterial inks classified by their functions, and presents the modifications that could achieve the balance between biocompatibility, mechanical properties, and printability. We also elaborate the challenges and prospects of extrudable biomaterial inks and introduce selected strategies based on different extrusion strategies, especially multi-materials and multiple-nozzle extrusion-based bioprinting. This systematical review also provides some guidance on selecting appropriate extrusion-based biomaterial inks and certainly contributes to new ideas and inspiration for bioprinting in vitro tissue models. We also firmly believe that the currently existing challenges of extrusion-based biomaterial inks can be addressed following the rapid development of technology in the near future.
Acknowledgments
None.
Funding
F.Y. Zheng acknowledges support by the Beijing Nova Program (Z201100006820038), National Natural Science Foundation of China (NSFC) Grants (No.82172110, No.32001015, No.21635001), Beijing Nova Program Cross Cooperation Project (Z211100002121015), and the Fundamental Research Funds for the Central Universities (YWF-21--BJ-J-1036, YWF-20-BJ-J-1035). L.Z. Wang acknowledges support by NSFC Grants (No.11822201) and Beijing Municipal Natural Science Foundation (No.7212205). Y.B. Fan acknowledges support by the NSFC Grants (No. U20A20390, No.11827803)
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions
Conceptualization: Xiaorui Li, Fuyin Zheng
Data curation: Xiaorui Li, Hui Liu, Xuezheng Geng, Yubing Leng
Funding acquisition: Fuyin Zheng, Lizhen Wang, Yubo Fan Project administration: Fuyin Zheng, Lizhen Wang, Yubo Fan
18Visualization: Xiaorui Li, Xudong Wang, Shudong Zhao, Dandan Dou
Writing - original draft: Xiaorui Li
Writing - review & editing: Fuyin Zheng, Xudong Wang, Xuezheng Geng, Shudong Zhao, Hui Liu, Dandan Dou, Yubing Leng, Lizhen Wang, Yubo Fan
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
Not applicable.
References
- 1.Zheng F, Fu F, Cheng Y, et al. Organ-on-a-chip systems: Microengineering to biomimic living systems. Small . 2016;12(17):2253–2282. doi: 10.1002/smll.201503208. https://doi.org/10.1002/smll.201503208. [DOI] [PubMed] [Google Scholar]
- 2.Zheng F, Xiao Y, Liu H, et al. Patient-specific organoid and organ-on-a-chip: 3D cell-culture meets 3D printing and numerical simulation. Adv Biol . 2021;5(6):2000024. doi: 10.1002/adbi.202000024. https://doi.org/10.1002/adbi.202000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawal P, Tripathi DM, Ramakrishna S, et al. Prospects for 3D bioprinting of organoids. Biodesign Manuf . 2021;4(3):627–640. https://doi.org/10.1007/s42242-020-00124-1. [Google Scholar]
- 4.Grigoryan B, Paulsen SJ, Corbett DC, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science . 2019;364(6439):458–464. doi: 10.1126/science.aav9750. https://doi.org/10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee A, Hudson AR, Shiwarski DJ, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science . 2019;365(6452):482–487. doi: 10.1126/science.aav9051. https://doi.org/10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 6.Groll J, Burdick JA, Cho D-W, et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication . 2019;11(1):013001. doi: 10.1088/1758-5090/aaec52. https://doi.org/10.1088/1758-5090/aaec52. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Kim S-W, Choi Y-J, et al. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater . 2019;95:188–200. doi: 10.1016/j.actbio.2019.04.026. https://doi.org/10.1016/j.actbio.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Park JY, Ryu H, Lee B, et al. Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication . 2019;11(1):015002. doi: 10.1088/1758-5090/aae545. https://doi.org/10.1088/1758-5090/aae545. [DOI] [PubMed] [Google Scholar]
- 9.Kolesky DB, Homan KA, Skylar-Scott MA, et al. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci USA . 2016;113(12):3179–3184. doi: 10.1073/pnas.1521342113. https://doi.org/10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol . 2014;32(8):773–785. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 11.Jungst T, Smolan W, Schacht K, et al. Strategies and molecular design criteria for 3D printable hydrogels. Chem Rev . 2016;116(3):1496–1539. doi: 10.1021/acs.chemrev.5b00303. https://doi.org/10.1021/acs.chemrev.5b00303. [DOI] [PubMed] [Google Scholar]
- 12.Bedell ML, Navara AM, Du Y, et al. Polymeric systems for bioprinting. Chem Rev . 2020;120(19):10744–10792. doi: 10.1021/acs.chemrev.9b00834. https://doi.org/10.1021/acs.chemrev.9b00834. [DOI] [PubMed] [Google Scholar]
- 13.Hospodiuk M, Dey M, Sosnoski D, et al. The bioink: A comprehensive review on bioprintable materials. Biotechnol Adv . 2017;35(2):217–239. doi: 10.1016/j.biotechadv.2016.12.006. https://doi.org/10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Pati F, Jang J, Lee JW, et al. Essentials of 3D Biofabrication and Translation . Elsevier; 2015. Extrusion bioprinting, in; pp. 123–152. [Google Scholar]
- 15.Zhang YS, Haghiashtiani G, Hübscher T, et al. 3D extrusion bioprinting. Nat Rev Methods Primers . 2021;1(1):75. https://doi.org/10.1038/s43586-021-00073-8. [Google Scholar]
- 16.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials . 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. https://doi.org/10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 17.Askari M, Afzali Naniz M, Kouhi M, et al. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater Sci . 2021;9(3):535–573. doi: 10.1039/d0bm00973c. https://doi.org/10.1039/d0bm00973c. [DOI] [PubMed] [Google Scholar]
- 18.Panwar A, Tan LP. Current status of bioinks for microextrusion-based 3D bioprinting. Molecules . 2016;21(6):685. doi: 10.3390/molecules21060685. https://doi.org/10.3390/molecules21060685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarassoli SP, Jessop ZM, Jovic T, et al. Candidate bioinks for extrusion 3D bioprinting—A systematic review of the literature. Front Bioeng Biotech . 2021;9:616753. doi: 10.3389/fbioe.2021.616753. https://doi.org/10.3389/fbioe.2021.616753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ojansivu M, Rashad A, Ahlinder A, et al. Wood-based nanocellulose and bioactive glass modified gelatin-alginate bioinks for 3D bioprinting of bone cells. Biofabrication . 2019;11(3):035010. doi: 10.1088/1758-5090/ab0692. https://doi.org/10.1088/1758-5090/ab0692. [DOI] [PubMed] [Google Scholar]
- 21.19Zeng X, Meng Z, He J, et al. Embedded bioprinting for designer 3D tissue constructs with complex structural organization. Acta Biomater . 2022;140:1–22. doi: 10.1016/j.actbio.2021.11.048. https://doi.org/10.1016/j.actbio.2021.11.048. [DOI] [PubMed] [Google Scholar]
- 22.Colosi C, Shin SR, Manoharan V, et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscositybioink. Adv Mater . 2016;28(4):677–684. doi: 10.1002/adma.201503310. https://doi.org/10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn G, Min K-H, Kim C, et al. Precise stacking of decellularized extracellular matrix based 3D cell-laden constructs by a 3D cell printing system equipped with heating modules. Sci Rep-Uk . 2017;7(1):8624. doi: 10.1038/s41598-017-09201-5. https://doi.org/10.1038/s41598-017-09201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang L, Highley CB, Sun W, et al. A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks. Adv Mater . 2017;29(8):1604983. doi: 10.1002/adma.201604983. https://doi.org/10.1002/adma.201604983. [DOI] [PubMed] [Google Scholar]
- 25.Gao Q, He Y, Fu J-z, et al. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials . 2015;61:203–215. doi: 10.1016/j.biomaterials.2015.05.031. https://doi.org/10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 26.Jin Y, Liu C, Chai W, et al. Self-supporting nanoclay as internal scaffold material for direct printing of soft hydrogel composite structures in air. ACS Appl Mater Interfaces . 2017;9(20):17456–17465. doi: 10.1021/acsami.7b03613. https://doi.org/10.1021/acsami.7b03613. [DOI] [PubMed] [Google Scholar]
- 27.Pi Q, Maharjan S, Yan X, et al. Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv Mater . 2018;30(43):1706913. doi: 10.1002/adma.201706913. https://doi.org/10.1002/adma.201706913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Z, Eglin D, Alini M, et al. Visible light-induced 3D bioprinting technologies and corresponding bioink materials for tissue engineering: A review. Engineering . 2021;7(7):966–978. https://doi.org/10.1016/j.eng.2020.05.021. [Google Scholar]
- 29.Tabriz AG, Hermida MA, Leslie NR, et al. Threedimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication . 2015;7(4):045012. doi: 10.1088/1758-5090/7/4/045012. https://doi.org/10.1088/1758-5090/7/4/045012. [DOI] [PubMed] [Google Scholar]
- 30.Flores-Torres S, Peza-Chavez O, Kuasne H, et al. Alginate-gelatin-Matrigel hydrogels enable the development and multigenerational passaging of patient-derived 3D bioprinted cancer spheroid models. Biofabrication . 2021;13(2):025001. doi: 10.1088/1758-5090/abdb87. https://doi.org/10.1088/1758-5090/abdb87. [DOI] [PubMed] [Google Scholar]
- 31.Parak A, Pradeep P, du Toit LC, et al. Functionalizing bioinks for 3D bioprinting applications. Drug Discov Today . 2019;24(1):198–205. doi: 10.1016/j.drudis.2018.09.012. https://doi.org/10.1016/j.drudis.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Snyder JE, Hamid Q, Wang C, et al. Bioprinting cellladen matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication . 2011;3(3):034112. doi: 10.1088/1758-5082/3/3/034112. https://doi.org/10.1088/1758-5082/3/3/034112. [DOI] [PubMed] [Google Scholar]
- 33.Fedorovich NE, Wijnberg HM, Dhert WJA, et al. Distinct tissue formation by heterogeneous printing of osteo- and endothelial progenitor cells. Tissue Eng A . 2011;17(15-16):2113–2121. doi: 10.1089/ten.TEA.2011.0019. https://doi.org/10.1089/ten.TEA.2011.0019. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen AM, Chou Z, Gillispie G, et al. Decellularized skin extracellular matrix (dsECM) improves the physical and biological properties of fibrinogen hydrogel for skin bioprinting applications. Nanomaterials Basel . 2020;10(8):1484. doi: 10.3390/nano10081484. https://doi.org/10.3390/nano10081484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toprakhisar B, Nadernezhad A, Bakirci E, et al. Development of bioink from decellularized tendon extracellular matrix for 3D bioprinting. Macromol Biosci . 2018;18(10):e1800024. doi: 10.1002/mabi.201800024. https://doi.org/10.1002/mabi.201800024. [DOI] [PubMed] [Google Scholar]
- 36.Hiller T, Berg J, Elomaa L, et al. Generation of a 3D liver model comprising human extracellular matrix in an alginate/gelatin-based bioink by extrusion bioprinting for infection and transduction studies. Int J Mol Sci . 2018;19(10):3129. doi: 10.3390/ijms19103129. https://doi.org/10.3390/ijms19103129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skardal A, Devarasetty M, Kang H-W, et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater . 2015;25:24–34. doi: 10.1016/j.actbio.2015.07.030. https://doi.org/10.1016/j.actbio.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 38.Visscher DO, Lee H, van Zuijlen PPM, et al. A photo- crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater . 2021;121:193–203. doi: 10.1016/j.actbio.2020.11.029. https://doi.org/10.1016/j.actbio.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao G, Lee JH, Jang J, et al. Tissue engineered bioblood-vessels constructed using a tissue-specific bioink and 3D coaxial cell printing technique: A novel therapy for ischemic disease. Adv Funct Mater . 2017;27(33):1700798. https://doi.org/10.1002/adfm.201700798. [Google Scholar]
- 40.Campos DFD, Blaeser A, Korsten A, et al. The stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward 20adipogenic and osteogenic lineages. Tissue Eng A . 2015;21(3-4):740–756. doi: 10.1089/ten.TEA.2014.0231. https://doi.org/10.1089/ten.tea.2014.0231. [DOI] [PubMed] [Google Scholar]
- 41.Moncal KK, Ozbolat V, Datta P, et al. Thermally- controlled extrusion-based bioprinting of collagen. J Mater Sci Mater M . 2019;30(5):55. doi: 10.1007/s10856-019-6258-2. https://doi.org/10.1007/s10856-019-6258-2. [DOI] [PubMed] [Google Scholar]
- 42.Skardal A, Mack D, Kapetanovic E, et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cell Transl Med . 2012;1(11):792–802. doi: 10.5966/sctm.2012-0088. https://doi.org/10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith CM, Stone AL, Parkhill RL, et al. Threedimensional bioassembly tool for generating viable tissue- engineered constructs. Tissue Eng . 2004;10(9-10):1566–1576. doi: 10.1089/ten.2004.10.1566. https://doi.org/10.1089/ten.2004.10.1566. [DOI] [PubMed] [Google Scholar]
- 44.Poldervaart MT, Goversen B, De Ruijter M, et al. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS One . 2017;12(6):e0177628. doi: 10.1371/journal.pone.0177628. https://doi.org/10.1371/journal.pone.0177628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antich C, de Vicente J, Jiménez G, et al. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater . 2020;106:114–123. doi: 10.1016/j.actbio.2020.01.046. https://doi.org/10.1016/j.actbio.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 46.Jorgensen AM, Varkey M, Gorkun A, et al. Bioprinted skin recapitulates normal collagen remodeling in fullthickness wounds. Tissue Eng A . 2020;26(9–10):512–526. doi: 10.1089/ten.tea.2019.0319. https://doi.org/10.1089/ten.TEA.2019.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Law N, Doney B, Glover H, et al. Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting. J Mech Behav Biomed . 2018;77:389–399. doi: 10.1016/j.jmbbm.2017.09.031. https://doi.org/10.1016/j.jmbbm.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 48.Kiyotake EA, Douglas AW, Thomas EE, et al. Development and quantitative characterization of the precursor rheology of hyaluronic acid hydrogels for bioprinting. Acta Biomater . 2019;95:176–187. doi: 10.1016/j.actbio.2019.01.041. https://doi.org/10.1016/j.actbio.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 49.Raddatz L, Lavrentieva A, Pepelanova I, et al. Development and application of an additively manufactured calcium chloride Nebulizer for alginate 3D-bioprinting purposes. J Funct Biomat . 2018;9(4):63. doi: 10.3390/jfb9040063. https://doi.org/10.3390/jfb9040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim MH, Lee YW, Jung W-K, et al. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. J Mech Behav Biomed . 2019;98:187–194. doi: 10.1016/j.jmbbm.2019.06.014. https://doi.org/10.1016/j.jmbbm.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Ooi HW, Mota C, Ten Cate AT, et al. Thiol-ene alginate hydrogels as versatile bioinks for bioprinting. Biomacromolecules . 2018;19(8):3390–3400. doi: 10.1021/acs.biomac.8b00696. https://doi.org/10.1021/acs.biomac.8b00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markstedt K, Mantas A, Tournier I, et al. 3D bioprinting human chondrocytes with nanocellulosealginate bioink for cartilage tissue engineering applications. Biomacromolecules . 2015;16(5):1489–1496. doi: 10.1021/acs.biomac.5b00188. https://doi.org/10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 53.Khalil S, Sun W. Bioprinting endothelial cells with alginate for 3D tissue constructs. J Biomech Eng-T Asme . 2009;131(11):111002. doi: 10.1115/1.3128729. https://doi.org/10.1115/1.3128729. [DOI] [PubMed] [Google Scholar]
- 54.Hafezi F, Shorter S, Tabriz AG, et al. Bioprinting and preliminary testing of highly reproducible novel bioink for potential skin regeneration. Pharmaceutics . 2020;12(6):550. doi: 10.3390/pharmaceutics12060550. https://doi.org/10.3390/pharmaceutics12060550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maturavongsadit P, Narayanan LK, Chansoria P, et al. Cell-laden nanocellulose/chitosan-based bioinks for 3D bioprinting and enhanced osteogenic cell differentiation. ACS Appl Bio Mater . 2021;4(3):2342–2353. doi: 10.1021/acsabm.0c01108. https://doi.org/10.1021/acsabm.0c01108. [DOI] [PubMed] [Google Scholar]
- 56.Ku J, Seonwoo H, Park S, et al. Cell-laden thermosensitive chitosan hydrogel bioinks for 3D bioprinting applications. Appl Sci Basel . 2020;10(7):2455. https://doi.org/10.3390/app10072455. [Google Scholar]
- 57.Ng WL, Yeong WY, Naing MW. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int J Bioprint . 2016;2(1):53–62. https://doi.org/10.18063/IJB.2016.01.009. [Google Scholar]
- 58.Liu Q, Li Q, Xu S, et al. Preparation and properties of 3D printed alginate-chitosan polyion complex hydrogels for tissue engineering. Polymers Basel . 2018;10(6):664. doi: 10.3390/polym10060664. https://doi.org/10.3390/polym10060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Q, Therriault D, Heuzey M-C. Processing and properties of chitosan inks for 3D printing of hydrogel microstructures. ACS Biomater Sci Eng . 2018;4(7):2643–2652. doi: 10.1021/acsbiomaterials.8b00415. https://doi.org/10.1021/acsbiomaterials.8b00415. [DOI] [PubMed] [Google Scholar]
- 60.Bergonzi C, Di Natale A, Zimetti F, et al. Study of 3D-printed chitosan scaffold features after different postprinting gelation processes. Sci Rep UK . 2019;9(1):362. doi: 10.1038/s41598-018-36613-8. https://doi.org/10.1038/s41598-018-36613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma R, Smits IPM, De La Vega L, et al. 3D bioprinting pluripotent stem cell derived neural tissues using a novel fibrin bioink containing drug releasing microspheres. Front Bioeng Biotech . 2020;8:57. doi: 10.3389/fbioe.2020.00057. https://doi.org/10.3389/fbioe.2020.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.21England S, Rajaram A, Schreyer DJ, et al. Bioprinted fibrin-factor XIII-hyaluronate hydrogel scaffolds with encapsulated Schwann cells and their in vitro characterization for use in nerve regeneration. Bioprinting . 2017;5:1–9. https://doi.org/10.1016/j.bprint.2016.12.001. [Google Scholar]
- 63.Lee C, Abelseth E, de la Vega L, et al. Bioprinting a novel glioblastoma tumor model using a fibrin-based bioink for drug screening. Mater Today Chem . 2019;12:78–84. https://doi.org/10.1016/j.mtchem.2018.12.005. [Google Scholar]
- 64.Ning L, Zhu N, Mohabatpour F, et al. Bioprinting Schwann cell-laden scaffolds from low-viscosity hydrogel compositions. J Mater Chem B . 2019;7(29):4538–4551. https://doi.org/10.1039/c9tb00669a. [Google Scholar]
- 65.Anil Kumar S, Alonzo M, Allen SC, et al. A visible light-cross-linkable, fibrin-gelatin-based bioprinted construct with human cardiomyocytes and fibroblasts. ACS Biomater Sci Eng . 2019;5(9):4551–4563. doi: 10.1021/acsbiomaterials.9b00505. https://doi.org/10.1021/acsbiomaterials.9b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez MJ, Dixon TA, Cohen E, et al. 3D freeform printing of silk fibroin. Acta Biomater . 2018;71:379–387. doi: 10.1016/j.actbio.2018.02.035. https://doi.org/10.1016/j.actbio.2018.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ni T, Liu M, Zhang Y, et al. 3D bioprinting of bone marrow mesenchymal stem cell-laden silk fibroin double network scaffolds for cartilage tissue repair. Bioconjug Chem . 2020;31(8):1938–1947. doi: 10.1021/acs.bioconjchem.0c00298. https://doi.org/10.1021/acs.bioconjchem.0c00298. [DOI] [PubMed] [Google Scholar]
- 68.Zheng Z, Wu J, Liu M, et al. 3D bioprinting of selfstanding silk-based bioink. Adv Healthc Mater . 2018;7(6):1701026. doi: 10.1002/adhm.201701026. https://doi.org/10.1002/adhm.201701026. [DOI] [PubMed] [Google Scholar]
- 69.Wu D, Yu Y, Tan J, et al. 3D bioprinting of gellan gum and poly (ethylene glycol) diacrylate based hydrogels to produce human-scale constructs with high-fidelity. Mater Design . 2018;160:486–495. https://doi.org/10.1016/j.matdes.2018.09.040. [Google Scholar]
- 70.Chen Y, Xiong X, Liu X, et al. 3D bioprinting of shearthinning hybrid bioinks with excellent bioactivity derived from gellan/alginate and thixotropic magnesium phosphatebased gels. J Mater Chem B . 2020;8(25):5500–5514. doi: 10.1039/d0tb00060d. https://doi.org/10.1039/d0tb00060d. [DOI] [PubMed] [Google Scholar]
- 71.Zhu S, Yao L, Pan C, et al. 3D printed gellan gum/ graphene oxide scaffold for tumor therapy and bone reconstruction. Compos Sci Technol . 2021;208:108763. https://doi.org/10.1016/j.compscitech.2021.108763. [Google Scholar]
- 72.Lozano R, Stevens L, Thompson BC, et al. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials . 2015;67:264–273. doi: 10.1016/j.biomaterials.2015.07.022. https://doi.org/10.1016/j.biomaterials.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 73.Mouser VHM, Melchels FPW, Visser J, et al. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication . 2016;8(3):035003. doi: 10.1088/1758-5090/8/3/035003. https://doi.org/10.1088/1758-5090/8/3/035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cleymand F, Poerio A, Mamanov A, et al. Development of novel chitosan/guar gum inks for extrusion-based 3D bioprinting: Process, printability and properties. Bioprinting . 2021;21:e00122. https://doi.org/10.1016/j.bprint.2020.e00122. [Google Scholar]
- 75.Indurkar A, Bangde P, Gore M, et al. Optimization of guar gum-gelatin bioink for 3D printing of mammalian cells. Bioprinting . 2020;20:e00101. https://doi.org/10.1016/j.bprint.2020.e00101. [Google Scholar]
- 76.Muthulakshmi L, Pavithra U, Sivaranjani V, et al. A novel Ag/carrageenan-gelatin hybrid hydrogel nanocomposite and its biological applications: Preparation and characterization. J Mech Behav Biomed . 2021;115:104257. doi: 10.1016/j.jmbbm.2020.104257. https://doi.org/10.1016/j.jmbbm.2020.104257. [DOI] [PubMed] [Google Scholar]
- 77.Li H, Tan YJ, Liu S, et al. Three-dimensional bioprinting of oppositely charged hydrogels with super strong interface bonding. ACS Appl Mater Interfaces . 2018;10(13):11164–11174. doi: 10.1021/acsami.7b19730. https://doi.org/10.1021/acsami.7b19730. [DOI] [PubMed] [Google Scholar]
- 78.Lim W, Kim GJ, Kim HW, et al. Kappa-carrageenan- based dual crosslinkable bioink for extrusion type bioprinting. Polymers Basel . 2020;12(10):2377. doi: 10.3390/polym12102377. https://doi.org/10.3390/polym12102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gong C, Kong Z, Wang X. The effect of agarose on 3D bioprinting. Polymers Basel . 2021;13(22):4028. doi: 10.3390/polym13224028. https://doi.org/10.3390/polym13224028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan YJ, Tan X, Yeong WY, et al. Hybrid microscaffoldbased 3D bioprinting of multi-cellular constructs with high compressive strength: A new biofabrication strategy. Sci Rep UK . 2016;6(1):39140. doi: 10.1038/srep39140. https://doi.org/10.1038/srep39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Massa S, Sakr MA, Seo J, et al. Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics . 2017;11(4):044109. doi: 10.1063/1.4994708. https://doi.org/10.1063/1.4994708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh YP, Bandyopadhyay A, Mandal BB. 3D bioprinting using cross-linker-free silk-gelatin bioink for cartilage tissue engineering. ACS Appl Mater Interfaces . 2019;11(37):33684–33696. doi: 10.1021/acsami.9b11644. https://doi.org/10.1021/acsami.9b11644. [DOI] [PubMed] [Google Scholar]
- 83.Schwartz R, Malpica M, Thompson GL, et al. Cell encapsulation in gelatin bioink impairs 3D bioprinting resolution. J Mech Behav Biomed . 2020;103:103524. doi: 10.1016/j.jmbbm.2019.103524. https://doi.org/10.1016/j.jmbbm.2019.103524. [DOI] [PubMed] [Google Scholar]
- 84.22Zehnder T, Sarker B, Boccaccini AR, et al. Evaluation of an alginate-gelatine crosslinked hydrogel for bioplotting. Biofabrication . 2015;7(2):025001. doi: 10.1088/1758-5090/7/2/025001. https://doi.org/10.1088/1758-5090/7/2/025001. [DOI] [PubMed] [Google Scholar]
- 85.Song K, Compaan AM, Chai W, et al. Injectable gelatin microgel-based composite ink for 3D bioprinting in air. ACS Appl Mater Interfaces . 2020;12(20):22453–22466. doi: 10.1021/acsami.0c01497. https://doi.org/10.1021/acsami.0c01497. [DOI] [PubMed] [Google Scholar]
- 86.Leucht A, Volz A-C, Rogal J, et al. Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents. Sci Rep UK . 2020;10(1):5330. doi: 10.1038/s41598-020-62166-w. https://doi.org/10.1038/s41598-020-62166-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kolan KCR, Semon JA, Bromet B, et al. Bioprinting with human stem cell-laden alginate-gelatin bioink and bioactive glass for tissue engineering. Int J Bioprint . 2019;5(2.2):204. doi: 10.18063/ijb.v5i2.2.204. https://doi.org/10.18063/ijb.v5i2.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yin J, Yan M, Wang Y, et al. In vitro and in vivo biocompatibility evaluation of a 3D bioprinted gelatin- sodium alginate/rat Schwann-cell scaffold. Mater Sci Eng C Mater . 2020;109:110530. doi: 10.1016/j.msec.2019.110530. https://doi.org/10.1016/j.msec.2019.110530. [DOI] [PubMed] [Google Scholar]
- 89.Yin J, Yan ML, Wang YC, et al. 3D bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl Mater Interfaces . 2018;10(8):6849–6857. doi: 10.1021/acsami.7b16059. https://doi.org/10.1021/acsami.7b16059. [DOI] [PubMed] [Google Scholar]
- 90.Rastin H, Ormsby RT, Atkins GJ, et al. 3D bioprinting of methylcellulose/gelatin-methacryloyl (MC/GelMA) bioink with high shape integrity. ACS Appl Biomater . 2020;3(3):1815–1826. doi: 10.1021/acsabm.0c00169. https://doi.org/10.1021/acsabm.0c00169. [DOI] [PubMed] [Google Scholar]
- 91.Erdem A, Darabi MA, Nasiri R, et al. 3D bioprinting of oxygenated cell-laden gelatin methacryloyl constructs. Adv Healthc Mater . 2020;9(15):1901794. doi: 10.1002/adhm.201901794. https://doi.org/10.1002/adhm.201901794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Colle J, Blondeel P, De Bruyne A, et al. Bioprinting predifferentiated adipose-derived mesenchymal stem cell spheroids with methacrylated gelatin ink for adipose tissue engineering. J Mater Sci Mater M . 2020;31(4):36. doi: 10.1007/s10856-020-06374-w. https://doi.org/10.1007/s10856-020-06374-w. [DOI] [PubMed] [Google Scholar]
- 93.Ning L, Mehta R, Cao C, et al. Embedded 3D bioprinting of gelatin methacryloyl-based constructs with highly tunable structural fidelity. ACS Appl Mater Interfaces . 2020;12(40):44563–44577. doi: 10.1021/acsami.0c15078. https://doi.org/10.1021/acsami.0c15078. [DOI] [PubMed] [Google Scholar]
- 94.Liu W, Heinrich MA, Zhou Y, et al. Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv Healthc Mater . 2017;6(12):1601451. doi: 10.1002/adhm.201601451. https://doi.org/10.1002/adhm.201601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu Y, Wenger A, Golzar H, et al. 3D bioprinting of bicellular liver lobule-mimetic structures via microextrusion of cellulose nanocrystal-incorporated shear-thinning bioink. Sci Rep UK . 2020;10(1):20648. doi: 10.1038/s41598-020-77146-3. https://doi.org/10.1038/s41598-020-77146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li H, Tan YJ, Leong KF, et al. 3D bioprinting of highly thixotropic alginate/methylcellulose hydrogel with strong interface bonding. ACS Appl Mater & Interfaces . 2017;9(23):20086–20097. doi: 10.1021/acsami.7b04216. https://doi.org/10.1021/acsami.7b04216. [DOI] [PubMed] [Google Scholar]
- 97.Frost BA, Sutliff BP, Thayer P, et al. Gradient poly(ethylene glycol) diacrylate and cellulose nanocrystals tissue engineering composite scaffolds via extrusion bioprinting. Front Bioeng Biotech . 2019;7:280. doi: 10.3389/fbioe.2019.00280. https://doi.org/10.3389/fbioe.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Z, Ramos A, Li M-C, et al. Improvement of cell deposition by self-absorbent capability of freeze-dried 3D-bioprinted scaffolds derived from cellulose materialalginate hydrogels. Biomed Phys Eng Express . 2020;6(4):045009. doi: 10.1088/2057-1976/ab8fc6. https://doi.org/10.1088/2057-1976/ab8fc6. [DOI] [PubMed] [Google Scholar]
- 99.Ji S, Abaci A, Morrison T, et al. Novel bioinks from UV-responsive norbornene-functionalized carboxymethyl cellulose macromers. Bioprinting . 2020;18:e00083. https://doi.org/10.1016/j.bprint.2020.e00083. [Google Scholar]
- 100.Wu Y, Heikal L, Ferns G, et al. 3D bioprinting of novel biocompatible scaffolds for endothelial cell repair. Polymers Basel . 2019;11(12):1924. doi: 10.3390/polym11121924. https://doi.org/10.3390/polym11121924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xin S, Chimene D, Garza JE, et al. Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater Sci . 2019;7(3):1179–1187. doi: 10.1039/c8bm01286e. https://doi.org/10.1039/C8BM01286E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hong S, Kim JS, Jung B, et al. Coaxial bioprinting of cell-laden vascular constructs using a gelatin-tyramine bioink. Biomater Sci . 2019;7(11):4578–4587. doi: 10.1039/c8bm00618k. https://doi.org/10.1039/C8BM00618K. [DOI] [PubMed] [Google Scholar]
- 103.Sun X, Ma Z, Zhao X, et al. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioactive Mater . 2021;6(3):757–769. doi: 10.1016/j.bioactmat.2020.08.030. https://doi.org/10.1016/j.bioactmat.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.23Hu D, Wu D, Huang L, et al. 3D bioprinting of cellladen scaffolds for intervertebral disc regeneration. Mater Lett . 2018;223:219–222. https://doi.org/10.1016/j.matlet.2018.03.204. [Google Scholar]
- 105.Shin YJ, Shafranek RT, Tsui JH, et al. 3D bioprinting of mechanically tuned bioinks derived from cardiac decellularized extracellular matrix. Acta Biomater . 2021;119:75–88. doi: 10.1016/j.actbio.2020.11.006. https://doi.org/10.1016/j.actbio.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 106.Kolesky DB, Truby RL, Gladman AS, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater . 2014;26(19):3124–3130. doi: 10.1002/adma.201305506. https://doi.org/10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 107.Gori M, Giannitelli SM, Torre M, et al. Biofabrication of hepatic constructs by 3D bioprinting of a cell-laden thermogel: An effective tool to assess drug-induced hepatotoxic response. Adv Healthc Mater . 2020;9(21):e2001163. doi: 10.1002/adhm.202001163. https://doi.org/10.1002/adhm.202001163. [DOI] [PubMed] [Google Scholar]
- 108.Suntornnond R, Tan EYS, An J, et al. A highly printable and biocompatible hydrogel composite for direct printing of soft and perfusable vasculature-like structures. Sci Rep UK . 2017;7(1):16902. doi: 10.1038/s41598-017-17198-0. https://doi.org/10.1038/s41598-017-17198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suntornnond R, Tan EYS, An J, et al. A mathematical model on the resolution of extrusion bioprinting for the development of new bioinks. Materials . 2016;9(9):756. doi: 10.3390/ma9090756. https://doi.org/10.3390/ma9090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee J, Kim G. Three-dimensional hierarchical nanofibrous collagen scaffold fabricated using fibrillated collagen and Pluronic F-127 for regenerating bone tissue. ACS Appl Mater Interfaces . 2018;10(42):35801–35811. doi: 10.1021/acsami.8b14088. https://doi.org/10.1021/acsami.8b14088. [DOI] [PubMed] [Google Scholar]
- 111.Kundu J, Shim J-H, Jang J, et al. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med . 2015;9(11):1286–1297. doi: 10.1002/term.1682. https://doi.org/10.1002/term.1682. [DOI] [PubMed] [Google Scholar]
- 112.Vijayavenkataraman S, Vialli N, Fuh JYH, et al. Conductive collagen/polypyrrole-b-polycaprolactone hydrogel for bioprinting of neural tissue constructs. Int J Bioprint . 2019;5(2.1):31–43. doi: 10.18063/ijb.v5i2.1.229. https://doi.org/10.18063/ijb.v5i2.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim BS, Jang J, Chae S, et al. Three-dimensional bioprinting of cell-laden constructs with polycaprolactone protective layers for using various thermoplastic polymers. Biofabrication . 2016;8(3):035013. doi: 10.1088/1758-5090/8/3/035013. https://doi.org/10.1088/1758-5090/8/3/035013. [DOI] [PubMed] [Google Scholar]
- 114.Narayanan LK, Huebner P, Fisher MB, et al. 3D-bioprinting of polylactic acid (PLA) nanofiber-alginate hydrogel bioink containing human adipose-derived stem cells. ACS Biomater Sci Eng . 2016;2(10):1732–1742. doi: 10.1021/acsbiomaterials.6b00196. https://doi.org/10.1021/acsbiomaterials.6b00196. [DOI] [PubMed] [Google Scholar]
- 115.Kolan KCR, Semon JA, Bindbeutel AT, et al. Bioprinting with bioactive glass loaded polylactic acid composite and human adipose stem cells. Bioprinting . 2020;18:e00075. https://doi.org/10.1016/j.bprint.2020.e00075. [Google Scholar]
- 116.Aydogdu MO, Oner ET, Ekren N, et al. Comparative characterization of the hydrogel added PLA/p-TCP scaffolds produced by 3D bioprinting. Bioprinting . 2019;13:e00046. https://doi.org/10.1016/j.bprint.2019.e00046. [Google Scholar]
- 117.Zamani Y, Mohammadi J, Amoabediny G, et al. Bioprinting of alginate-encapsulated pre-osteoblasts in PLGA/p-TCP scaffolds enhances cell retention but impairs osteogenic differentiation compared to cell seeding after 3D-printing. Regen Eng Transl Med . 2020;7(4):485–493. https://doi.org/10.1007/s40883-020-00163-1. [Google Scholar]
- 118.Naseri E, Butler H, Macnevin W, et al. Low-temperature solvent-based 3D printing of PLGA: A parametric printability study. Drug Dev Ind Pharm . 2020;46(2):173–178. doi: 10.1080/03639045.2019.1711389. https://doi.org/10.1080/03639045.2019.1711389. [DOI] [PubMed] [Google Scholar]
- 119.Huang J, Huang Z, Liang Y, et al. 3D printed gelatin/ hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater Sci . 2021;9(7):2620–2630. doi: 10.1039/d0bm02103b. https://doi.org/10.1039/D0BM02103B. [DOI] [PubMed] [Google Scholar]
- 120.Wenz A, Borchers K, Tovar GEM, et al. Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication . 2017;9(4):044103. doi: 10.1088/1758-5090/aa91ec. https://doi.org/10.1088/1758-5090/aa91ec. [DOI] [PubMed] [Google Scholar]
- 121.Bendtsen ST, Quinnell SP, Wei M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J Biomed Mater Res A . 2017;105(5):1457–1468. doi: 10.1002/jbm.a.36036. https://doi.org/10.1002/jbm.a.36036. [DOI] [PubMed] [Google Scholar]
- 122.Adhikari J, Perwez MS, Das A, et al. Development of hydroxyapatite reinforced alginate-chitosan based printable biomaterial-ink. Nanostruct Nanoobjects . 2021;25:100630. https://doi.org/10.1016/j.nanoso.2020.100630. [Google Scholar]
- 123.Lin K-F, He S, Song Y, et al. Low-temperature additive manufacturing of biomimic three-dimensional hydroxyapatite/collagen scaffolds for bone regeneration. ACS Appl Mater Interfaces . 2016;8(11):6905–6916. doi: 10.1021/acsami.6b00815. https://doi.org/10.1021/acsami.6b00815. [DOI] [PubMed] [Google Scholar]
- 124.Gao Q, Niu X, Shao L, et al. 3D printing of complex GelMA-based scaffolds with nanoclay. Biofabrication . 2019;11(3):035006. doi: 10.1088/1758-5090/ab0cf6. https://doi.org/10.1088/1758-5090/ab0cf6. [DOI] [PubMed] [Google Scholar]
- 125.24Hu C, Hahn L, Yang M, et al. Improving printability of a thermoresponsive hydrogel biomaterial ink by nanoclay addition. J Mater Sci . 2021;56(1):691–705. https://doi.org/10.1007/s10853-020-05190-5. [Google Scholar]
- 126.Cidonio 1 G, Glinka M, Kim Y-H, et al. Nanoclaybased 3D printed scaffolds promote vascular ingrowth ex vivo and generate bone mineral tissue in vitro and in vivo. Biofabrication . 2020;12(3):035010. doi: 10.1088/1758-5090/ab8753. https://doi.org/10.1088/1758-5090/ab8753. [DOI] [PubMed] [Google Scholar]
- 127.Zhu W, Cui H, Boualam B, et al. 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology . 2018;29(18):185101. doi: 10.1088/1361-6528/aaafa1. https://doi.org/10.1088/1361-6528/aaafa1. [DOI] [PubMed] [Google Scholar]
- 128.Swaminathan S, Hamid Q, Sun W, et al. Bioprinting of 3D breast epithelial spheroids for human cancer models. Biofabrication . 2019;11(2):025003. doi: 10.1088/1758-5090/aafc49. https://doi.org/10.1088/1758-5090/aafc49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carrow JK, Kerativitayanan P, Jaiswal MK, et al. In: Essentials of 3D Biofabrication and Translation . Atala A, and Yoo JJ, editors. Academic Press; Boston: 2015. Polymers for bioprinting, in; pp. 229–248. [Google Scholar]
- 130.Nijenhuis KT. Thermoreversible Networks: Viscoelastic Properties and Structure of Gels . 1 edn. Springer; Berlin, Heidelberg: 1997. [Google Scholar]
- 131.Lee VK, Lanzi AM, Ngo H, et al. Generation of multiscale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell Mol Bioeng . 2014;7(3):460–472. doi: 10.1007/s12195-014-0340-0. https://doi.org/10.1007/s12195-014-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao L, Lee VK, Yoo S-S, et al. The integration of 3-D cell printing and mesoscopic fluorescence molecular tomography of vascular constructs within thick hydrogel scaffolds. Biomaterials . 2012;33(21):5325–5332. doi: 10.1016/j.biomaterials.2012.04.004. https://doi.org/10.1016/j.biomaterials.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun Y, Yu K, Nie J, et al. Modeling the printability of photocuring and strength adjustable hydrogel bioink during projection-based 3D bioprinting. Biofabrication . 2021;13(3):035032. doi: 10.1088/1758-5090/aba413. https://doi.org/10.1088/1758-5090/aba413. [DOI] [PubMed] [Google Scholar]
- 134.Iliyana P, Katharina K, Thomas S, et al. Gelatin- methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering . 2018;5(3):55. doi: 10.3390/bioengineering5030055. https://doi.org/10.3390/bioengineering5030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee BH, Lum N, Seow LY, et al. Synthesis and characterization of types A and B gelatin methacryloyl for bioink applications. Materials . 2016;9(10):797. doi: 10.3390/ma9100797. https://doi.org/10.3390/ma9100797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cubo N, Garcia M, CaAizo JFD, et al. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication . 2017;9(11):2843–2854. doi: 10.1088/1758-5090/9/1/015006. https://doi.org/10.1088/1758-5090/9/1/015006. [DOI] [PubMed] [Google Scholar]
- 137.Ouyang L, Yao R, Chen X, et al. 3D printing of HEK 293FT cell-laden hydrogel into macroporous constructs with high cell viability and normal biological functions. Biofabrication . 2015;7(1):015010. doi: 10.1088/1758-5090/7/1/015010. https://doi.org/10.1088/1758-5090/7/1/015010. [DOI] [PubMed] [Google Scholar]
- 138.Testa S, Mozetic P, Barbetta A, et al. Microfluidic- enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials . 2017;131:98–110. doi: 10.1016/j.biomaterials.2017.03.026. https://doi.org/10.1016/j.biomaterials.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 139.Pescosolido L, Schuurman W, Malda J, et al. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules . 2011;12(5):1831–1838. doi: 10.1021/bm200178w. https://doi.org/10.1021/bm200178w. [DOI] [PubMed] [Google Scholar]
- 140.Park JY, Choi J-C, Shim J-H, et al. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication . 2014;6(3):035004. doi: 10.1088/1758-5082/6/3/035004. https://doi.org/10.1088/1758-5082/6/3/035004. [DOI] [PubMed] [Google Scholar]
- 141.Ma L, Li Y, Wu Y, et al. 3D bioprinted hyaluronic acid-based cell-laden scaffold for brain microenvironment simulation. Biodesign Manuf . 2020;3(3):164–174. https://doi.org/10.1007/s42242-020-00076-6. [Google Scholar]
- 142.Wibowo A, Vyas C, Cooper G, et al. 3D printing of polycaprolactone-polyaniline electroactive scaffolds for bone tissue engineering. Materials . 2020;13(3):512. doi: 10.3390/ma13030512. https://doi.org/10.3390/ma13030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao H, Xu J, Zhang E, et al. 3D bioprinting of polythiophene materials for promoting stem cell proliferation in a nutritionally deficient environment. ACS Appl Mater Interfaces . 2021;13(22):25759–25770. doi: 10.1021/acsami.1c04967. https://doi.org/10.1021/acsami.1c04967. [DOI] [PubMed] [Google Scholar]
- 144.Yuk H, Lu B, Lin S, et al. 3D printing of conducting polymers. Nat Commun . 2020;11(1):1604. doi: 10.1038/s41467-020-15316-7. https://doi.org/10.1038/s41467-020-15316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu K, Shin SR, van Kempen T, et al. Gold nanocomposite bioink for printing 3D cardiac constructs. Adv Funct Mater . 2017;27(12):1605352. doi: 10.1002/adfm.201605352. https://doi.org/10.1002/adfm.201605352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mannoor MS, Jiang Z, James T, et al. 3D printed bionic ears. Nano Lett . 2013;13(6):2634–2639. doi: 10.1021/nl4007744. https://doi.org/10.1021/nl4007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mehrotra S, Singh RD, Bandyopadhyay A, et al. Engineering microsphere-loaded non-mulberry silk-based 253D bioprinted vascularized cardiac patches with oxygenreleasing and immunomodulatory potential. ACS Appl Mater Interfaces . 2021;13(43):50744–50759. doi: 10.1021/acsami.1c14118. https://doi.org/10.1021/acsami.1c14118. [DOI] [PubMed] [Google Scholar]
- 148.Asulin M, Michael I, Shapira A, et al. One-step 3D printing of heart patches with built-in electronics for performance regulation. Adv Sci . 2021;8(9):2004205. doi: 10.1002/advs.202004205. https://doi.org/10.1002/advs.202004205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tian K, Bae J, Bakarich SE, et al. 3D printing of transparent and conductive heterogeneous hydrogelelastomer systems. Adv Mater . 2017;29(10):1604827. doi: 10.1002/adma.201604827. https://doi.org/10.1002/adma.201604827. [DOI] [PubMed] [Google Scholar]
- 150.Jia J, Richards DJ, Pollard S, et al. Engineering alginate as bioink for bioprinting. Acta Biomater . 2014;10(10):4323–4331. doi: 10.1016/j.actbio.2014.06.034. https://doi.org/10.1016/j.actbio.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kang D, Hong G, An S, et al. Bioprinting of multiscaled hepatic lobules within a highly vascularized construct. Small . 2020;16(13):1905505. doi: 10.1002/smll.201905505. https://doi.org/10.1002/smll.201905505. [DOI] [PubMed] [Google Scholar]
- 152.Yegappan R, Selvaprithiviraj V, Amirthalingam S, et al. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr Polym . 2018;198:385–400. doi: 10.1016/j.carbpol.2018.06.086. https://doi.org/10.1016/j.carbpol.2018.06.086. [DOI] [PubMed] [Google Scholar]
- 153.Nadernezhad A, Caliskan OS, Topuz F, et al. Nanocomposite bioinks based on agarose and 2D nanosilicates with tunable flow properties and bioactivity for 3D bioprinting. ACS Appl Biomater . 2019;2(2):796–806. doi: 10.1021/acsabm.8b00665. https://doi.org/10.1021/acsabm.8b00665. [DOI] [PubMed] [Google Scholar]
- 154.Bertassoni LE, Cecconi M, Manoharan V, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip . 2014;14(13):2202–2211. doi: 10.1039/c4lc00030g. https://doi.org/10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mirdamadi E, Muselimyan N, Koti P, et al. Agarose slurry as a support medium for bioprinting and culturing freestanding cell-laden hydrogel constructs. 3D Print Addit Manuf . 2019;6(3):158–164. doi: 10.1089/3dp.2018.0175. https://doi.org/10.1089/3dp.2018.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fedorovich NE, Swennen I, Girones J, et al. Evaluation of photocrosslinked lutrol hydrogel for tissue printing applications. Biomacromolecules . 2009;10(7):1689–1696. doi: 10.1021/bm801463q. https://doi.org/10.1021/bm801463q. [DOI] [PubMed] [Google Scholar]
- 157.Lewis PL, Yan M, Su J, et al. Directing the growth and alignment of biliary epithelium within extracellular matrix hydrogels. Acta Biomater . 2019;85:84–93. doi: 10.1016/j.actbio.2018.12.039. https://doi.org/10.1016/j.actbio.2018.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today . 2005;10(21):1451–1458. doi: 10.1016/S1359-6446(05)03575-0. https://doi.org/10.1016/s1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 159.Floody MC, Theng BKG, Reyes P, et al. Natural nanoclays: Applications and future trends—A Chilean perspective. Clay Miner . 2009;44(2):161–176. https://doi.org/10.1180/claymin.2009.044.2.161. [Google Scholar]
- 160.Xavier JR, Thakur T, Desai P, et al. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach. ACS Nano . 2015;9(3):3109–3118. doi: 10.1021/nn507488s. https://doi.org/10.1021/nn507488s. [DOI] [PubMed] [Google Scholar]
- 161.Gladman AS, Matsumoto EA, Nuzzo RG, et al. Biomimetic 4D printing. Nat Mater . 2016;15(4):413–418. doi: 10.1038/nmat4544. https://doi.org/10.1038/nmat4544. [DOI] [PubMed] [Google Scholar]
- 162.Liu J, Erol O, Pantula A, et al. Dual-gel 4D printing of bioinspired tubes. ACS Appl Mater Interfaces . 2019;11(8):8492–8498. doi: 10.1021/acsami.8b17218. https://doi.org/10.1021/acsami.8b17218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ahlfeld T, Cidonio G, Kilian D, et al. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication . 2017;9(3):034103. doi: 10.1088/1758-5090/aa7e96. https://doi.org/10.1088/1758-5090/aa7e96. [DOI] [PubMed] [Google Scholar]
- 164.Mulakkal MC, Trask RS, Ting VP, et al. Responsive cellulose-hydrogel composite ink for 4D printing. Mater Design . 2018;160:108–118. https://doi.org/10.1016/j.matdes.2018.09.009. [Google Scholar]
- 165.Zhang X, Zhou Y, Liu Y, et al. Effect of water soluble polymer on the dispersion stability of cellulose nanofibers in water. J Cell Sci Technol . 2021;28(1):26–33. https://doi.org/10.16561/j.cnki.xws.2020.01.07. [Google Scholar]
- 166.Lille M, Nurmela A, Nordlund E, et al. Applicability of protein and fiber-rich food materials in extrusion-based 3D printing. J Food Eng . 2018;220:20–27. https://doi.org/10.1016/j.jfoodeng.2017.04.034. [Google Scholar]
- 167.Wu Y, Lin ZYW, Wenger AC, et al. 3D bioprinting of liver-mimetic construct with alginate/cellulose nanocrystal hybrid bioink. Bioprinting . 2018;9:1–6. https://doi.org/10.1016/j.bprint.2017.12.001. [Google Scholar]
- 168.Giachini P, Gupta SS, Wang W, et al. Additive manufacturing of cellulose-based materials with continuous, multidirectional stiffness gradients. Sci Adv . 2020;6(8):eaay0929. doi: 10.1126/sciadv.aay0929. https://doi.org/10.1126/sciadv.aay0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gospodinova A, Nankov V, Tomov S, et al. Extrusion bioprinting of hydroxyethylcellulose-based bioink for cervical tumor model. Carbohydr Polym . 2021;260:117793. doi: 10.1016/j.carbpol.2021.117793. https://doi.org/10.1016/j.carbpol.2021.117793. [DOI] [PubMed] [Google Scholar]
- 170.26Li X, Deng Q, Wang S, et al. Hydroxyethyl cellulose as a rheological additive for tuning the extrusion printability and scaffold properties. 3D Print Addit Manuf . 2021;8(2):87–98. doi: 10.1089/3dp.2020.0167. https://doi.org/10.1089/3dp.2020.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mudgil D, Barak S, Khatkar BS. Guar gum: Processing, properties and food applications—A review. J Food Sci Technol Mysore . 2014;51(3):409–418. doi: 10.1007/s13197-011-0522-x. https://doi.org/10.1007/s13197-011-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hahn L, Beudert M, Gutmann M, et al. From thermogelling hydrogels toward functional bioinks: Controlled modification and cytocompatible crosslinking. Macromol Biosci . 2021;21(10):e2100122. doi: 10.1002/mabi.202100122. https://doi.org/10.1002/mabi.202100122. [DOI] [PubMed] [Google Scholar]
- 173.Daly AC, Cunniffe GM, Sathy BN, et al. 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv Healthc Mater . 2016;5(18):2353–2362. doi: 10.1002/adhm.201600182. https://doi.org/10.1002/adhm.201600182. [DOI] [PubMed] [Google Scholar]
- 174.Sarker MD, Naghieh S, McInnes AD, et al. Biofabrication of peptide-modified alginate scaffolds: Printability, mechanical stability and neurite outgrowth assessments. Bioprinting . 2019;14:e00045. https://doi.org/10.1016/j.bprint.2019.e00045. [Google Scholar]
- 175.Barrs RW, Jia J, Ward M, et al. Engineering a chemically defined hydrogel bioink for direct bioprinting of microvasculature. Biomacromolecules . 2021;22(2):275–288. doi: 10.1021/acs.biomac.0c00947. https://doi.org/10.1021/acs.biomac.0c00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Q, Yang X, Wang G, et al. Osteogenic growth peptide-loaded 3D-printed PCL scaffolds for the promotion of osteogenesis through the ERK pathway. Mater Design . 2020;193:108811. https://doi.org/10.1016/j.matdes.2020.108811. [Google Scholar]
- 177.Guo JL, Diaz-Gomez L, Xie VY, et al. Threedimensional printing of click functionalized, peptide patterned scaffolds for osteochondral tissue engineering. Bioprinting . 2021;22:e00136. doi: 10.1016/j.bprint.2021.e00136. https://doi.org/10.1016/j.bprint.2021.e00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang X, Ao Q, Tian X, et al. Gelatin-based hydrogels for organ 3D bioprinting. Polymers Basel . 2017;9(9):401. doi: 10.3390/polym9090401. https://doi.org/10.3390/polym9090401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hauptstein J, Boeck T, Bartolf-Kopp M, et al. Hyaluronic acid-based bioink composition enabling 3D bioprinting and improving quality of deposited cartilaginous extracellular matrix. Adv Healthc Mater . 2020;9(15):2000737. doi: 10.1002/adhm.202000737. https://doi.org/10.1002/adhm.202000737. [DOI] [PubMed] [Google Scholar]
- 180.Chameettachal S, Sasikumar S, Sethi S, et al. Tissue/ organ-derived bioink formulation for 3D bioprinting. J 3D Print Med . 2019;3(1):39–54. https://doi.org/10.2217/3dp-2018-0024. [Google Scholar]
- 181.Cheng Y, Zheng F, Lu J, et al. Bioinspired multicompartmental microfibers from microfluidics. Adv Mater . 2014;26(30):5184–5190. doi: 10.1002/adma.201400798. https://doi.org/10.1002/adma.201400798. [DOI] [PubMed] [Google Scholar]
- 182.Rajput S, Deo KA, Mathur T, et al. 2D nanosilicate for additive manufacturing: Rheological modifier, sacrificial ink and support bath. Bioprinting . 2022;25:e00187. https://doi.org/10.1016/j.bprint.2021.e00187. [Google Scholar]
- 183.Liu P, Mu Z, Ji M, et al. Robust carbonated structural color barcodes with ultralow ontology fluorescence as biomimic culture platform. Research . 2021;2021:9851609. doi: 10.34133/2021/9851609. https://doi.org/10.34133/2021/9851609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee S, Sani ES, Spencer AR, et al. Human-recombinantelastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv Mater . 2020;32(45):e2003915. doi: 10.1002/adma.202003915. https://doi.org/10.1002/adma.202003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Park B-W, Jung S-H, Das S, et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci Adv . 2020;6(13):eaay6994. doi: 10.1126/sciadv.aay6994. https://doi.org/10.1126/sciadv.aay6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim W, Kim GH. An intestinal model with a fingerlike villus structure fabricated using a bioprinting process and collagen/SIS-based cell-laden bioink. Theranostics . 2020;10(6):2495–2508. doi: 10.7150/thno.41225. https://doi.org/10.7150/thno.41225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim W, Kim G. Intestinal villi model with blood capillaries fabricated using collagen-based bioink and dual-cell-printing process. ACS Appl Mater Interfaces . 2018;10(48):41185–41196. doi: 10.1021/acsami.8b17410. https://doi.org/10.1021/acsami.8b17410. [DOI] [PubMed] [Google Scholar]
- 188.Wang Y, Shi W, Kuss M, et al. 3D bioprinting of breast cancer models for drug resistance study. ACS Biomater Sci Eng . 2018;4(12):4401–4411. doi: 10.1021/acsbiomaterials.8b01277. https://doi.org/10.1021/acsbiomaterials.8b01277. [DOI] [PubMed] [Google Scholar]
- 189.Bhise NS, Manoharan V, Massa S, et al. A liver- on-a-chip platform with bioprinted hepatic spheroids. Biofabrication . 2016;8(1):014101. doi: 10.1088/1758-5090/8/1/014101. https://doi.org/10.1088/1758-5090/8/1/014101. [DOI] [PubMed] [Google Scholar]
- 190.Nguyen D, Hagg DA, Forsman A, et al. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep UK . 2017;7:658. doi: 10.1038/s41598-017-00690-y. https://doi.org/10.1038/s41598-017-00690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li X, Deng Q, Zhuang T, et al. 3D bioprinted breast tumor model for structure-activity relationship study. Biodesign Manuf . 2020;3(4):361–372. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.