Abstract
At the cornerstone of the pelvis and spine, the sacrum may be fractured in patients of all ages. Sacral fractures range from high-energy injuries, with mortality rates of up to 18%, to low-energy insufficiency fractures. The intricate geometry of the sacrum, the breadth of morphotypes, and the presence of congenital anomalies all can complicate the treatment of these fractures. Agreement on the surgical indications for these injuries is limited. This narrative review aims to update orthopedic surgeons on the clinical evaluation and the non-surgical and surgical management of these fractures.
Keywords: sacrum, insufficiency fractures, sacral dysmorphism, sacral fracture classification, triangular osteosynthesis
Introduction
The diagnosis and treatment of sacral fractures requires a high degree of suspicion and clinical judgment. Sacral fractures have been known to occur in 30% to 45% of pelvic ring injuries [9,17]. The mechanisms range from high-energy trauma to low-energy falls in osteoporotic patients; 5% of sacral fractures have historically presented as isolated injuries [9,47].
Sacral fractures can be difficult to diagnose due to poor visualization on standard radiographs and distracting injuries in poly-traumatized patients. As a result, 30% of sacral fractures are identified late [63]. Nearly 25% of sacral fractures are also associated with neurologic injury, and missed fractures can result in neurologic compromise, including lower extremity, urinary, rectal, and sexual dysfunction [17]. Notably, in 1988, Denis et al found that a sacral fracture was missed in 24% of patients with an associated neurologic deficit and in 49% of patients who were neurologically intact [17].
Sacral fractures can be fatal in the elderly. Keil et al found an overall inpatient mortality rate of 10% after high-energy pelvic fractures, with a 3-fold higher mortality rate in patients over the age of 65 [30]. In the elderly, low-energy injuries may fracture the sacrum as a result of osteoporosis or may insidiously develop adjacent to spinal instrumentation and present as occult low back pain [11,66]. In their systematic review, Joaquim and Patel found the incidence of sacral and pelvic fractures after instrumented lumbar fusions to be nearly 2% with the major risk factors being elderly patients, multilevel surgery, long fusions stopping at L5 or S1 instead of the ilium, osteoporosis, obesity, and sagittal imbalance [26].
Anatomy of the Sacrum
The sacrum (Fig. 1) is an inverted triangle consisting of 5 vertebrae, which begin fusing around 18 years of age and complete fusion between 25 and 33 years [15]. It articulates with the lumbar spine proximally, the coccyx distally, and 2 innominate bones laterally [28]. The triangular shape allows for axial load transmission between the spine and the lower extremities through the sciatic buttress. The weight-bearing portion of the sacrum is through the S1–S2 segments. The gross geometry of the sacrum consists of a concave central vertebral body and promontory flanked by 2 sacral ala, which represent fused transverse processes, a central spinal canal, and a convex dorsal laminar surface. On the ventral and dorsal surfaces are 4-paired sets of foramina, which serve as conduits for the exit of neural structures. The L5 nerve root runs on the anterosuperior surface of the sacral ala, placing it at particular risk of sacral fracture. The ventral S1–S4 nerve roots exit the foramina superomedially and form the sacral plexus, as well as S1, S2, and S3 contributing to the sciatic nerve. Dorsally, the nerve roots are responsible for sensory transmission and form the cluneal nerves. The S1 and S2 nerve roots carry a higher rate of injury than the S3 and S4 nerve roots due to their larger caliber that fills a greater volume of their foramina [17].
Fig. 1.
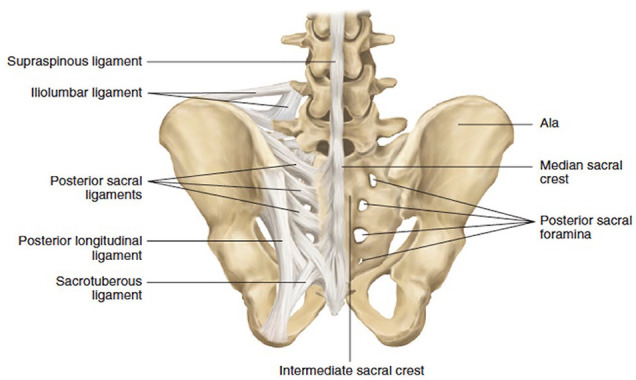
Bony and ligamentous lumbosacral anatomy. This figure was published in Surgical Anatomy and Techniques to the Spine (2nd ed) [37]. Copyright Elsevier (2013).
The sympathetic chain runs along the lateral lumbar spine with its preganglionic fibers investing the sacral roots as well as forming the superior hypogastric plexus. Branches of the ventral S2 to S4 nerve roots, or the pelvic splanchnic nerves, provide parasympathetic innervation to the inferior hypogastric plexus. The sympathetic input allows for anterograde ejaculation out of the urethra while the parasympathetic input allows for the vascular reflexes that maintain erectile functions. Injury to the sympathetic chain can result in ipsilateral lower extremity warmth and flushing in addition to retrograde ejaculation in men.
The sacroiliac (SI) joint is a synovial joint formed between the hyaline cartilage of the sacrum and the fibrocartilage of the ilium. The SI joint transfers load from the spine to the lower extremities [36]. Some of the strongest ligaments in the body serve to maintain lumbopelvic stability, including the anterior, interosseous, and posterior SI ligaments, which join the sacrum to the ilium. In combination with the ligaments of the pelvic floor (sacrotuberous, sacrospinous, and iliolumbar ligaments), this complex resists the forward tilt of the upper sacrum while preventing the posterior tilt of the inferior sacrum and countering the anterior translation of L5 over the sacral promontory [38]. The sacrotuberous ligament connects the anteroinferior sacrum to the ischial tuberosity while the sacrospinous ligament connects the lateral sacrum to the ischial spine. The iliolumbar ligament is a sleeve of connective tissue from the L5 transverse process to the inner aspect of the iliac crest.
The lumbosacral junction is highly variable. Lumbosacral transitional vertebrae are seen in up to 20% of the general population [62]. Depending on the number of total vertebrae in the spinal column and anatomic characteristics, a transitional segment can be a sacralized L5 or a lumbarized S1. A sacralized L5 segment is more common than the lumbarization of S1 and is defined by enlarged transverse processes, which either articulate or fuse with the first sacral segment. It is characterized by the presence of 4 rib-free lumbar-type vertebrae followed by a wedged vertebra or a vertebra with hypoplastic or absent facet joints or intervertebral disk. A lumbarized S1 demonstrates 6 rib-free lumbar-type vertebrae cranially and a subsequent segment with squaring of the vertebra, an abnormal transverse process, and/or facet joints and an intervertebral disk between S1 and S2.
Dysmorphism of the sacrum has also been identified in up to 40% of the population [29,43,50] (Fig. 2). It is essential to be aware of sacral dysmorphism, particularly if considering a construct with iliosacral screws. Seven important radiographic findings of patients with sacral dysmorphism include mammillary bodies, tongue-in-groove SI articulations, residual upper sacral disk space, collinearity, larger and noncircular sacral neural foramina, acute alar slope, and an iliac cortical density that is not coplanar with the alar slope [43,50].
Fig. 2.
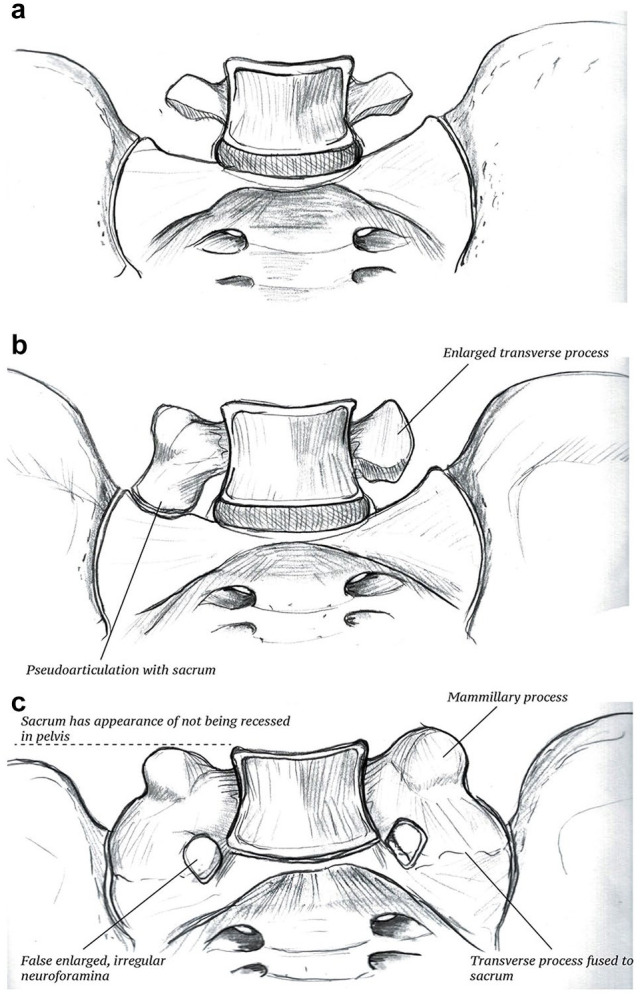
Sacral dysmorphism/lumbar sacralization are interchangeable terms used to describe morphotypes of the lumbar vertebrae: (a) Normal anatomy. (b) Partial sacralization where the lumbar transverse processes may be enlarged and articulate with the sacrum. (c) Full lumbar sacralization displays all the traditional findings of sacral dysmorphism described by Routt et al [50], including mamillary bodies, enlarged sacral foramina (which represents false foramina caused by fusion of the transverse process to the sacrum), and the sacrum having the appearance of not being recessed in the pelvis.
Surgically, the “safe zone” is a bony corridor in the sacrum where iliosacral screws can be placed intraosseously. Normally, this ellipsoid osseous area is bounded by the sacral alar cortical bone cranially and the first sacral neural foramen between the L5 and S1 nerve roots caudally and provides an interosseous corridor for iliosacral screw insertion. Similar corridors exist for transiliac-transsacral screws in the second sacral segment, bounded by the S1 and S2 osseous corridors.
However, with sacral dysmorphia, S1 transiliac-transsacral screw fixation may not be possible given the distorted and misshapen character of the upper sacral segment. In these cases, the “safe zone” for a SI screw is oriented obliquely from caudal to cranial and posterior to anterior or the S2 corridor may be used. Conflitti et al found the S2 segment provided a larger osseous site in patients with sacral dysmorphism for screw insertion and an instrumentation corridor amenable to transiliac-transsacral screws [16].
Clinical Evaluation
The complex anatomy of the sacrum and the frequency of concomitant and sometimes distracting injuries cause sacral fractures to often be diagnosed late or missed altogether. For trauma patients, a targeted examination for pelvic and sacral injuries should be conducted, in addition to the Advanced Trauma Life Support protocol.
History
To start, this should include a detailed history. Understanding the mechanism and possible injury pattern will help guide management and outcomes. Motor vehicle accidents or a fall from substantial height are common high-energy mechanisms, while low-energy repetitive stress in the setting of osteoporosis may cause insufficiency fractures in women >55 years of age [66]. Groin, low back, or buttock pain severe enough to render the patient non-ambulatory in the setting of no or low-impact trauma should raise concern for a sacral insufficiency fracture [61]. Neurologic deficits in this scenario are rare. However, urinary retention or rectal incontinence may indicate sacral nerve root compression.
Physical Examination
Trauma patients frequently present with peripelvic pain but must also be evaluated for neurologic compromise and soft tissue injuries. Sacral fractures may present with low back pain, and thus confuse clinicians when suspected lumbar fractures are not present on imaging. Subcutaneous tissues should be palpated to evaluate for fluid masses suggesting lumbosacral fascial degloving (Morel-Lavallee lesions). Vaginal and urologic examinations should be conducted to evaluate for open fractures and associated urologic injury. In addition to inspection for soft tissue trauma around the pelvis, pelvic ring stability may be assessed by internally and externally rotating the iliac wings in the hemodynamically stable patient without obvious radiographic instability.
Neurovascular Examination
The lower sacral nerve roots (S2–S5) function to control anal sphincter tone and voluntary contracture, the bulbocavernosus reflex, and perianal sensation [12]. Thus, in addition to evaluation of motor function, sensation, and reflexes of lower extremities, a comprehensive neurovascular examination should also include a digital rectal examination focusing on sphincter function, sensation, presence of blood, and the bulbocavernosus and cremasteric reflexes. Notably, unilateral preservation of S2–S5 is adequate for bowel and bladder control. Distal vascular status should be evaluated with palpation of the pulses or by obtaining an ankle-brachial index.
In their retrospective review of 44 patients with sacral fractures over a 2-year period, Gibbons et al found that fractures through the sacral ala only or involving the formina but not the central canal were less likely to cause nerve injury (24% and 29%, respectively) than vertical or transverse fractures involving the central canal (60% and 57%, respectively) [20]. Deficits caused by fractures of the ala or involving the formina but not the central canal were often unilateral radiculopathies, while deficits secondary to fractures of the central canal were generally bilateral, severe, and with concomitant bowel and/or bladder incontinence. Fortunately, they found that the deficits typically improved, particularly after surgical intervention [20].
Patients with sacral fractures may also present with traumatic dural tears. Bellabarba et al showed that 74% of patients with sacral fracture-dislocations and cauda equina syndrome had traumatic dural tears or sacral root avulsions noted during the index procedure [6]. In addition, 2 patients in their cohort required a return to the operating room for seroma exploration related to the traumatic dural tear.
Imaging
The standard radiographic trauma series—including an anteroposterior (AP) chest radiograph to evaluate for consecutive rib fractures, lung contusions, hemo-thoraces and pneumo-thoraces, or diaphragmatic injuries, and an AP pelvis radiograph to evaluate for pelvic ring injuries—should be obtained in all high-energy trauma patients. Additional pelvic views including a lateral, inlet, and outlet can be obtained to better evaluate the sacrum and characterize fracture patterns. The inlet view shows the sacral spinal canal, the sacral ala, and the pelvic ring, while the outlet provides a true AP of the sacrum, allowing for assessment of vertical displacement [63].
Schicho et al found the sensitivity of pelvic radiographs in identifying sacral fractures to be only 10.5% [54]. Although it may be difficult to diagnose a sacral fracture on plain radiographs, there are key findings associated with these injuries, including L4 or L5 transverse process fractures resulting from vertical displacement of the iliac wing or disruption of the iliolumbar ligaments, asymmetric foramina, and anterior pelvic ring disruptions [18,44,63]. A paradoxical inlet view of the sacrum on an AP radiograph should raise suspicions for lumbopelvic disassociation resulting in a kyphotic deformity of the sacral promontory. Bilateral sacral fractures should also raise suspicion for lumbopelvic disassociation [8]. The pelvic incidence measured on a lateral radiograph may assist the surgeon in surgical reduction of kyphotic fractures [23].
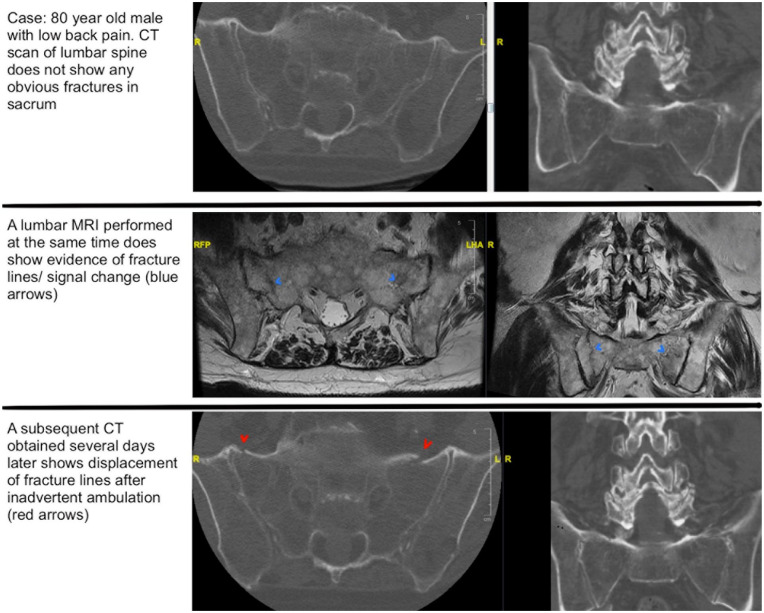
Given the low sensitivity of pelvic radiographs, particularly with the potential of obscuring bowel gas on plain films, 3-dimensional imaging is recommended to evaluate all suspected sacral fractures. Fine cut computed tomography (CT) allows accurate classification of the fracture and identification of any degree of central canal or foraminal involvement [48]. The coronal and sagittal plane images should be scrutinized for transverse fractures easily missed on axial images and could represent lumbopelvic dissociation. If there is concern for neurologic compromise or in cases of occult sacral insufficiency fracture, magnetic resonance imaging (MRI) is recommended [61,63]. If there is a neurological deficit, MRI may reveal sacral root avulsions, traction injuries, or evidence of a traumatic dural tear. On MRI, T2-weighted images with fat suppression or T2-weighted short tau inversion recovery images are helpful in demonstrating the fracture lines of sacral insufficiency fractures [61]. It is possible to have occult sacral fractures that are missed initially on CT, and thus the MRI axial cuts should be scrutinized for sacral alar edema (Fig. 3).
Fig. 3.
Case of occult sacral fractures. Top row: These are axial CT cuts of an 80-year-old man who presented with low back pain. The CT scan does not demonstrate any obvious sacral fractures. Middle row: A lumbar MRI was performed on the same day as the aforementioned CT and revealed evidence of fracture lines/signal change (blue arrows). Bottom row: These are similar axial cuts from a subsequent CT obtained several days later, which demonstrate displacement of fracture lines (red arrows). CT computed tomography, MRI magnetic resonance imaging.
In the setting of sacral insufficiency fractures, dual energy x-ray absorptiometry (DEXA) is a useful tool to evaluate bone density [61]. Bone density may also be obtained opportunistically by examining the Hounsfield units (HU) in the S1 body on CT [65]. Berger-Groch et al retrospectively reviewed the bone density measured with CT of 531 patients with sacral fractures [7]. They defined osteoporosis as less than 100 HU, osteopenia as 100 to 150 HU, and normal as above 150 HU, which follows the cutoffs established in the literature. Measuring the HU of the L5 vertebral body, they found that 75% of patients more than 65 years old had osteoporosis, and with each additional year of age, bone density decreased by 2.7 HU [7].
Fracture Classification
Location-Based Classifications
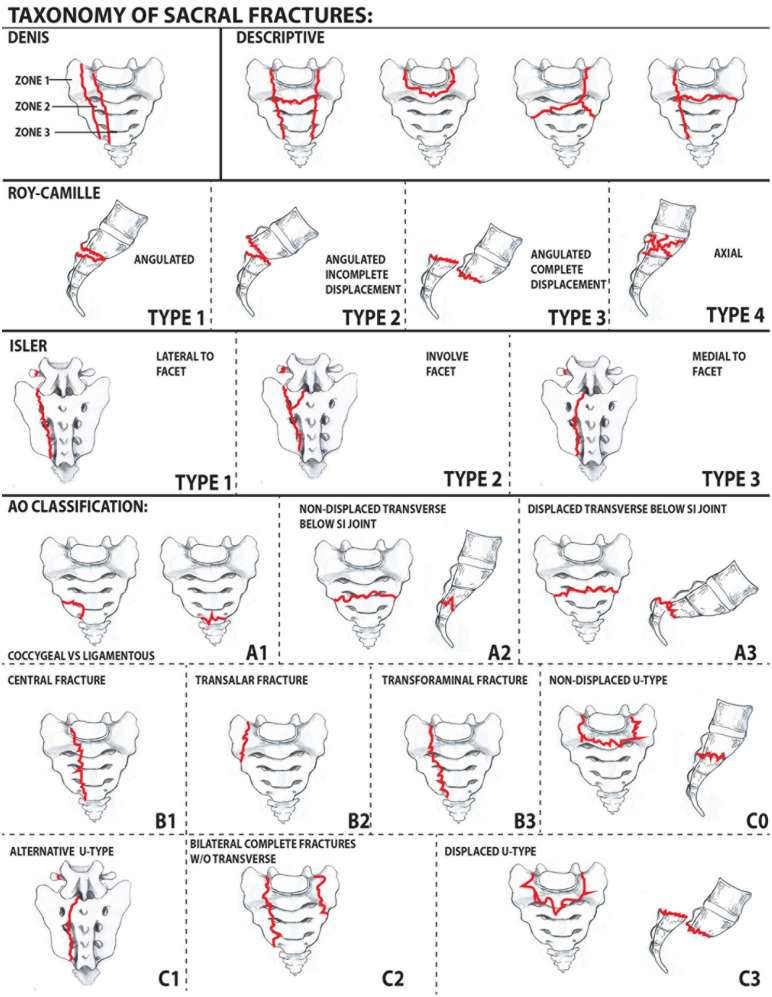
Many sacral fracture classification systems have been created to guide treatment and predict prognosis (Fig. 4). Denis et al created the first widely used system [17], which divides the sacrum into 3 anatomic zones prognostic for neurologic deficits: lateral to the foramina (Zone 1), through the foramina (Zone 2), and medial to the foramina (Zone 3). Rates of neurologic injury increase from Zone 1 (5.9%) to Zone 3 (57%) while incidence of the fracture pattern decreases. Zone 2 fractures may have a shear component creating instability, as well as an increased risk of non-union and poor function outcomes [63].
Fig. 4.
Taxonomy of sacral fractures. First row: Denis and descriptive classifications; second row: Roy-Camille subclassification of Denis Zone 3 fractures modified by Strange-Vognsen and Lebech; third row: Isler classification; bottom half: AO classification of sacral fractures.
In a larger study, Khan et al found similar decreasing rates of neurologic injury with increasing Denis zone. Although they noted the rate of nerve injury was significantly lower than originally reported by Denis (3.5% vs 21.6%, P < .001) [35]. The magnitude of fracture displacement serves as a prognostic factor as those more severely displaced have a higher incidence of neurologic deficit.
Transverse fractures can be considered a subtype of Denis Zone 3 injuries. As such, the Roy–Camille classification characterizes Zone 3 transverse fractures in the sagittal plane based on angulation and displacement [51]. Type 1 fractures are angulated but without translation. Type 2 fractures are angulated with incomplete translation. Type 3 fractures show both complete translation and displacement. As an additional modification, Strange-Vognsen and Lebech described the comminuted type 4 fracture pattern resulting from an axial-loading injury [59].
More recently, the Arbeitsgemeinschaft für Osteosyn-thesefragen (A O) classification attempted to comprehensively categorize injuries into 3 groups (types A, B, and C) according to location: type A injuries involve the lower portion of the sacrococcygeal region. Spinopelvic stability is unaffected by type A injuries, although higher injuries can have an associated neurological deficit. Type B injuries are posterior pelvic fractures characterized by unilateral, longitudinal sacral fractures in which the ipsilateral superior S1 facet is no longer continuous with the medial aspect of the sacrum, thus affecting the stability of the pelvis. Type C injuries are sacral fractures that result in instability of the spinopelvic region. These 3 broad categories can then be subdivided based on the AO hierarchy with additional modifiers and neurologic descriptors [5].
Descriptive Classification
Sacral fractures with a transverse component (so-called multiplanar fractures) can also be classified descriptively according to the letter they most closely resemble H-type, U-type, λ-type, and T-type fracture patterns. Notably, U-type sacral fractures represent spinopelvic dissociation because the upper portion of the sacrum remains attached to the lumbar spine while the lower portion remains attached to the pelvis. These fractures have a high incidence of neurologic injury, long-term pain, as well as bowel, bladder, and sexual impairments [24].
Stability-Based Classification
The Isler classification can be used for fractures that involve the lumbosacral articulation and is helpful in assessments of stability. Isler classified sacral fractures based on the involvement of the L5-S1 facet. Type 1 fractures occur lateral to the L5-S1 facet, type 2 fractures involve the facet, and type 3 fractures extend medially to the facet. Type 1 fractures are generally stable, but type 2 and 3 fractures are unstable and often benefit from operative management [25].
Clinical Decision-Making Classification
Lehman et al created the lumbosacral injury classification system (LSICS) based on injury morphology, posterior ligamentous complex integrity, and neurologic status. From this, an injury severity score can be calculated allowing for stratification into non-surgical and surgical treatment groups (Table 1). Furthermore, modifiers including injury burden, soft tissue status, and expected time to mobility allow for an algorithmic approach to operative technique selection [41].
Table 1.
Lumbosacral injury classification system (LSICS).
| Type | Points |
|---|---|
| Flexion compression • ≤20° kyphosis • >20° kyphosis |
1 2 |
| Axial compression (comminution of upper sacrum) • Without sacral canal or neuroforaminal encroachment • With sacral canal or neuroforaminal encroachment |
2 3 |
| Translational/rotational • Anterior or posterior translation of upper sacrum • Lumbosacral facet injury or dislocation • Vertical translation or instability |
3 |
| Blast/shear (severe comminution or segmental bone loss) | 4 |
This table was reproduced with permission from Lehman et al [41] https://www.thespinejournalonline.com/.
Sacral Insufficiency Fracture Classification
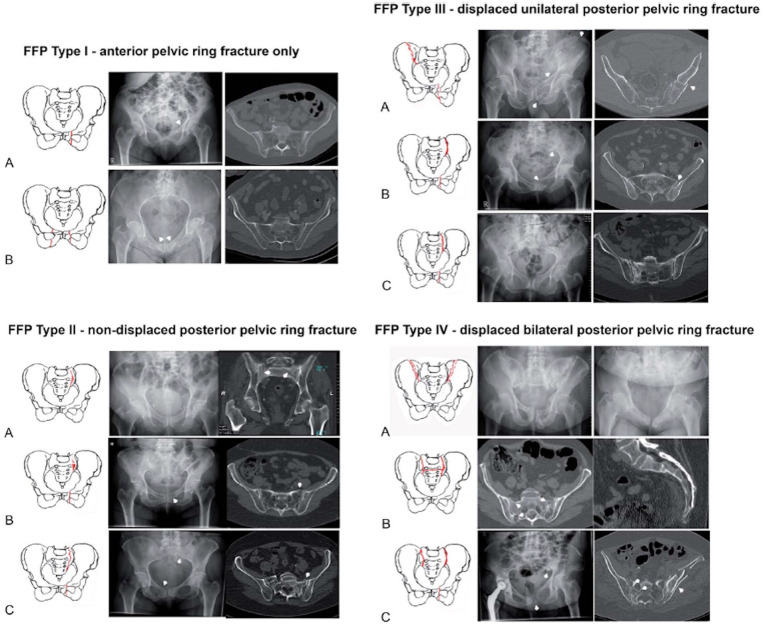
For low-energy pelvic fractures, Rommens et al devised a classification system according to fracture instability and morphology [49]. The authors noted that these fragility fractures are unique from high-energy pelvic injuries, and thus should have a separate framework for evaluation and management. Type I fragility fractures of the pelvis (FFP) involve only the anterior pelvic ring. Fragility fractures of the pelvis type II is defined by a non-displaced posterior pelvic ring fracture. Fragility fractures of the pelvis type III injuries are displaced unilateral posterior pelvic ring fractures, and FFP type IV injuries are displaced bilateral posterior pelvic ring fractures. Fragility fractures of the pelvis types I and II are generally stable and can be treated conservatively while FFP types III and IV should undergo surgical stabilization of the pelvic ring (Fig. 5) [49].
Fig. 5.
Fragility fractures of the pelvis. This figure was reproduced with permission from Rommens et al [49] https://journals.lww.com/jorthotrauma/pages/default.aspx.
To summarize, location-based and descriptive classifications can help predict neurologic compromise while the Isler and LSICS classifications can help guide operative or non-operative treatment choices. Providers should pay particular attention to the transverse and longitudinal morphology of the fracture as well as the location in relation to the foramina and facets.
Treatment Options
While classification systems clarify sacral fractures and potential associated injuries, treatment decisions should be made in conjunction with the patient’s overall status, particularly in the poly-traumatized patient. Surgical indications have been difficult to define in the literature and in practice. In their large prospective, observational study, Vallier et al demonstrated that surgical indications for sacral fractures vary widely among surgeons [64].
Non-Operative Management
Patients with stable sacral fractures without neurologic compromise can often be treated with focused rehabilitation. For patients with < 1 cm displacement (encompassing many sacral insufficiency fractures), a stable pelvis, and without neurologic deficits, a program of progressive weight bearing with or without an orthosis can be undertaken [57,63]. Non-surgical management of lumbopelvic disassociations is rare and reserved for patients who cannot tolerate surgery. In elderly, frail patients, bilateral sacral fractures may be managed with 6 weeks of non-weight bearing, followed by progressive weight bearing but attention to anticoagulation is critical as these patients are very prone to venous thrombosis. In these patients, non-operative management may include bedside transfers with wheelchair use.
In a multicenter, prospective study of 194 patients with unilateral sacral fractures displaced less than 5 mm, Tornetta et al found differences in pain scores within 24 hours but no differences at 3 months when comparing operatively and non-operatively treated fractures [60]. Furthermore, the differences seen were below the minimally clinically important difference for pain scores for other orthopedic conditions. Similarly, in a review of 281 patients with lateral compression type 1 and type 2 fractures with complete sacral injuries, Hagen et al found no difference in pain, opioid use, or post-injury mobilization between operatively and non-operatively treated patients [21].
Operative Management
Surgical intervention may be indicated for patients with displacement > 1 cm, lumbopelvic disassociation, spinopelvic instability, open fractures, posterior cortical displacement > 4 mm, positive stress examination under anesthesia, neurovascular compromise, progressive fracture displacement, or continued pain with non-operative treatment [64].
Unstable sacral fractures
Patients with unstable sacral fractures may benefit from posterior sacral stabilization with or without neural decompression. In addition to fracture reduction and stabilization, neurologic decompression, and soft tissue coverage, surgically treated patients can benefit from earlier mobilization.
Potential constructs for unstable sacral fractures or lumbopelvic dissociations include the following: open lumbopelvic and iliosacral screw fixation, percutaneous screw fixation, posterior tension band plate fixation, or posterior alar plate fixation depending on fracture pattern, soft tissue status, and surgeon preference [4,50,55].
Spinopelvic fixation is the most biomechanically stable approach. This includes a triangular construct with lumbar pedicle screws connected through longitudinal rods and transverse connectors to iliac screws parallel to the inclination angle of the outer table [1,52]. Such a construct bypasses the SI joint, allowing weight transmission [52].
In a retrospective review of 17 patients with spinopelvic dissociation, Williams et al found that their technique of L4 to pelvis bilateral percutaneous fixation was effective in reducing and stabilizing U-type and H-type injuries with minimal blood loss and a shorter operative time than in open fixation [68]. The authors also placed percutaneous iliosacral screws in fractures with a safe bony corridor needing a kyphotic reduction, which is now the mainstay of treatment as opposed to an open approach [58].
Percutaneous SI, transsacral, or transiliac-transsacral screws may be used for sagittal fractures. Under image-guidance or with the use of computer navigation, screws are placed percutaneously paying close attention to avoiding the neurovascular structures or overcompressing the fracture [19]. However, this approach does not allow for removal of loose bony fragments, which may be impinging on the neural elements [50].
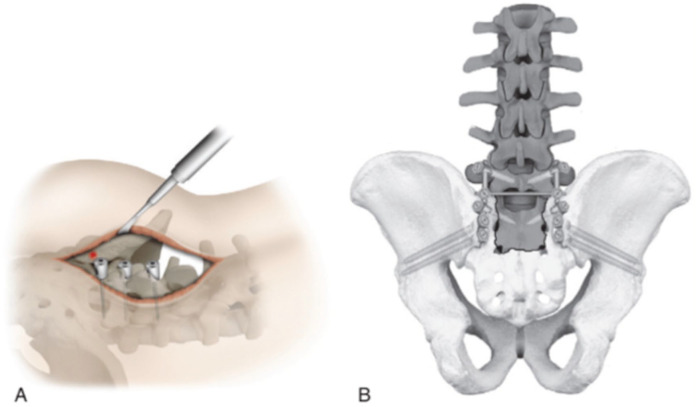
Posterior tension band plate fixation may be used in addition to iliosacral screws through a posterior 2-incision approach, which allows for direct visualization of the fracture [3]. In addition, the sacral lamina offers a novel target for open reduction internal fixation of transverse fractures [28]. However, given the incisions and limited soft tissue coverage of the sacrum, this surgical technique risks wound healing complications. In their systematic review including 109 patients with transverse sacral fractures, Bederman et al concluded posterior pelvic ring fixation (iliosacral screws, transiliac screws, transiliac screws with plating, posterior plating, or transiliac bar) was effective in the stabilization of transverse sacral fractures [4]. They recommended using spinopelvic fixation in the setting of additional lumbosacral instability (Fig. 6).
Fig. 6.
Lumbopelvic fixation. (a) Red dot represents starting point of screw into ilium. (b) Lumbopelvic stabilization. This figure was published in Surgical Anatomy and Techniques to the Spine (2nd ed) [37]. Copyright Elsevier (2013) as a reproduction from Vaccaro et al [63].
Sacral fractures with neurological compromise
A range of etiologies can cause neurologic deficits, from traction palsies to complete transection of the cauda equina. Thus, neural element decompression can be achieved through indirect reduction with axial traction or directly through a posterior approach followed by laminectomy and/or lumbosacral neurolysis, depending on the etiology of the deficit. In addition, several studies have demonstrated the importance of early decompression within the first 24 to 72 hours after injury for the return of neurologic function [17,56,69]. However, a systematic review by Kepler et al revealed early decompression (defined as before 72 hours) did not afford any benefit with respect to neurologic recovery [33]. Similarly, Kempen et al did not provide evidence of improved neurological recovery after surgical treatment of transverse sacral fractures compared with non-operative treatment in their systematic review of 521 patients [32]. Thus, the outcome of surgical decompression is difficult to discern given the overall improvement of 80% irrespective of treatment [56].
Sacral insufficiency fractures
Osteoporotic sacral insufficiency fractures are frequently treated non-operatively with protected weight-bearing, pain control, and exercise. However, in patients who continue to have limited ambulation or persistent posterior pain limiting mobility, image-guided sacroplasty may provide pain relief [13,42]. In the treatment of geriatric sacral U-type insufficiency fractures and posterior pain that prevents mobilization, Pulley et al found that percutaneous screw fixation permitted early mobilization, provided pain relief, and prevented progressive deformity in 16 patients with minimum 1-year follow-up [46]. Similarly, Walker et al demonstrated improved pain, ambulation, and rate of discharge home in elderly patients with isolated sacral fragility fractures undergoing percutaneous transiliac-transsacral screw fixation in their retrospective cohort study of 41 patients (16 treated operatively after failed non-operative management and 25 treated non-operatively) [10].
Periprosthetic sacral fractures
Wilde et al found that sacral fractures adjacent to pedicle screw-based lumbar spinal fusions to S1 have a characteristic pattern. They generally present within 3 months after lumbosacral fusion as transverse fractures through the sacral body and screw holes [67]. In their retrospective study of 116 patients who underwent instrumented lumbosacral fusion from L2 or above, Odate et al found female sex, higher pelvic incidence, and a larger lumbar lordosis-pelvic incidence mismatch were risk factors for developing a post-operative sacral fracture [45]. In general, indications for revision of periprosthetic sacral fractures include refractory pain, neurological deficit, fracture non-union with anterolisthesis or kyphotic angulation, L5-S1 pseudoarthrosis, and spinopelvic malalignment [11].
Outcomes and Complications
Given the wide array of treatment options, prospective studies evaluating operatively treated sacral fractures are limited. Most studies are confounded by small sample sizes, selection bias, a retrospective nature, and varying treatment techniques or outcome measures. In accordance with the patient’s overall condition, surgical stabilization and neural decompression should happen as early as safely possible. Latenser et al found patients with unstable pelvic fractures who underwent early fixation (within 8 hours of arrival) had decreased blood loss, a shorter hospital length of stay, less disability, fewer complications, and higher short-term survival rates than patients who had delayed pelvic stabilization [40]. Routt et al showed that unstable Denis Zone 1 and 2 fractures were more likely to be malreduced after closed reduction if surgery was delayed by more than 5 days [50].
Although the importance of early surgical intervention is supported by the literature, the choice of best surgical approach and construct is not clear; risks and benefits of each surgical intervention must be weighed. In their review of 31 isolated sacral fractures, Sathiyakumar et al found that patients who underwent percutaneous fixation had a significantly shorter length of stay without significant differences in complications or readmissions as compared with patients who underwent open reduction internal fixation [53]. Furthermore, Kelly et al described that while patients treated with lumbopelvic fusion of their U/H-type sacral fracture had increased operative time, they were also more likely to be discharged home instead of to a rehabilitation facility as compared with patients who underwent isolated iliosacral fixation [31].
After comparing SI screws, posterior sacral plating, triangular fixation, and spinopelvic fixation in 16 osteoporotic cadaver pelvis, Acklin et al concluded triangular fixation in unstable Denis Zone 2 fractures was the most biomechanically sound construct [1]. However, a prospective study by Sagi et al of 58 patients with vertically unstable pelvic ring injuries treated with triangular osteosynthesis demonstrated that while this technique allowed for early full weight-bearing and prevented the loss of reduction in the comminuted vertical shear transforaminal sacral fractures, there were significant complications observed at 1 year post-operatively [52]. Complications included iatrogenic nerve injury (13%), asymmetric L5 tilting with L5/S1 facet distraction (15%), and the need for re-operation to remove painful fixation in 95% of patients. The authors concluded that triangular osteosynthesis should be used selectively for comminuted transforaminal sacral fractures and only in cases where reliable iliosacral or transsacral screw fixation is not possible.
Venous thromboembolism may occur secondary to limited mobility in patients with sacral fractures. Complications of surgical intervention may also include infection, iatrogenic nerve injury from overcompression of the fracture or improper hardware placement, and malreduction, which is more common with vertically displaced fractures [50,56]. In a retrospective review of 19 consecutive patients with sacral fracture-dislocations and cauda equina syndrome treated with early surgical decompression and rigid segmental stabilization, Bellabarba et al found the most common complications were wound healing issues (26%), asymptomatic hardware failure (31%), and unplanned reoperations (42%) [5]. However, no patients had a loss of reduction at an average 25-month follow-up.
After following 28 patients with displaced sacral fractures and at least 8 years of follow-up, Adelved et al found that while neurological, urinary, and bowel deficits are all frequent after displaced sacral fractures, voiding issues and sexual dysfunction worsened overtime whereas neurological deficits and bowel function demonstrated no significant changes [2]. The authors thus suggested the importance of addressing this in the early rehabilitation period with a multi-disciplinary, long-term approach.
Advancements in Diagnosis and Treatment
Technological advancements in orthopedics such as artificial intelligence and CT guided navigation are changing the diagnosis and treatment of sacral fractures. Cheng devised a deep learning artificial intelligence algorithm that detects these fractures on pelvic x-rays. Reviewing 5204 pelvic x-rays, they found their algorithm demonstrates 94.4% accuracy, 90.8% sensitivity, and 93.2% specificity in detecting pelvic and hip fractures [14]. In addition, the algorithm detected 9% of pelvic fractures that were misdiagnosed by emergency medicine physicians and 5.5% of those misdiagnosed by consulting physicians, which included radiologists and orthopedic surgeons. Deep learning algorithms have been shown promise in medicine and may be the next frontier in trauma evaluation, particularly with aiding in the diagnosis of sacral fractures.
Another advancement comes in the form of CT-navigated instrumentation. The benefits of navigation and robotics have been well described within the spine and neurosurgery literature. These include decreased operative time, decreased radiation exposure to the surgeon and surgical staff, and increased accuracy [22]. The use of image-guided navigation systems may be particularly useful in patients with sacral dysmorphism and in percutaneous fixation, as described by Khan et al [34].
Summary
Sacral fractures are heterogeneous in mechanism, presentation, and morphology. They range from high-energy fractures to low-energy insufficiency fractures.
Early identification and management of sacral fractures can limit progressive deformity, pain, and loss of neurologic function. A careful examination should be made of the soft tissues, perineum, and nervous system in all cases.
Standard radiographs have low diagnostic sensitivity. Computer tomography should be obtained in all cases of suspected pelvic injury. Magnetic resonance imaging should be obtained in cases of suspected neurologic compromise.
Sacral anatomic variations are common. Transitional anatomy of the lumbosacral junction describes the morphological phenomenon of the last lumbar vertebrae taking on sacral traits or the first sacral segment taking on lumbar traits. The S1 corridor is limited to SI fixation in cases of full lumbar sacralization easily identified by radiographic findings.
The rate of neurologic injury can be high with detrimental long-term implications. Direct or indirect decompression may be used to treat progressive neurologic deficits.
Triangular osteosynthesis is the most biomechanically stable construct and is indicated in most cases of lumbopelvic dissociation.
Overall union rates in sacral fractures are good, but persistent pain and neurologic dysfunction can be disabling long-term [27,38,39,56].
Major advancements, including the use of machine learning and intra-operative image-guidance, have been made in the diagnosis and surgical treatment of sacral fractures.
Supplemental Material
Supplemental material, sj-docx-1-hss-10.1177_15563316221129607 for Sacral Fractures: A Review by Lauren A. Barber, Yoshihiro Katsuura and Sheeraz Qureshi in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-2-hss-10.1177_15563316221129607 for Sacral Fractures: A Review by Lauren A. Barber, Yoshihiro Katsuura and Sheeraz Qureshi in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-3-hss-10.1177_15563316221129607 for Sacral Fractures: A Review by Lauren A. Barber, Yoshihiro Katsuura and Sheeraz Qureshi in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Yoshihiro Katsuura, MD, reports relationships with Radius. Sheeraz Qureshi, MD, MBA, reports relationships with Stryker, K2M, Globus Medical, Paradigm Spine, AMOpportunities, RTI Surgical Inc., Tissue Differentiation Intelligence, and Vital 5. Lauren A. Barber, MD, declares no potential conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Informed consent was not required for this review article.
Required Author Forms: Disclosure forms provided by the authors are available with the online version of this article as supplemental material.
References
- 1.Acklin YP, Zderic I, Richards RG, Schmitz P, Gueorguiev B, Grechenig S. Biomechanical investigation of four different fixation techniques in sacrum Denis type II fracture with low bone mineral density. J Orthop Res. 2018;36(6):1624–1629. 10.1002/jor.23798. [DOI] [PubMed] [Google Scholar]
- 2.Adelved A, Tötterman A, Glott T, Madsen JE, Røise O. Functional outcome 10 years after surgical treatment of displaced sacral fractures. Spine. 2012;37(16):E1009–E1016. 10.1097/BRS.0b013e31823a0d83. [DOI] [PubMed] [Google Scholar]
- 3.Albert MJ, Miller ME, Macnaughton M, Hutton WC. Posterior pelvic fixation using a transiliac 4.5-mm reconstruction plate: a clinical and biomechanical study. J Orthop Trauma. 1993;7(3):226–232. 10.1097/00005131-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bederman SS, Hassan JM, Shah KN, Kiester PD, Bhatia NN, Zamorano DP. Fixation techniques for complex traumatic transverse sacral fractures: a systematic review. Spine. 2013;38(16):E1028–E1040. 10.1097/BRS.0b013e318297960a. [DOI] [PubMed] [Google Scholar]
- 5.Bellabarba C, Schildhauer TA, Vaccaro AR, Chapma n., JR.Complications associated with surgical stabilization of high-grade sacral fracture dislocations with spino-pelvic instability. Spine. 2006;31(11 Suppl.):S80–S88. 10.1097/01.brs.0000217949.31762.be. [DOI] [PubMed] [Google Scholar]
- 6.Bellabarba C, Schroeder GD, Kepler CK, et al. The AOSpine sacral fracture classification. Global Spine J. 2016;6(7):686–694. 10.1055/s-0036-1582696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger-Groch J, Thiesen DM, Ntalos D, et al. Determination of bone density in patients with sacral fractures via CT scan. Orthop Traumatol Surg Res. 2018;104(7):1037–1041. 10.1016/J.OTSR.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Bishop JA, Dangelmajer S, Corcoran-Schwartz I, Gardner MJ, Routt MLC, Castillo TN. Bilateral sacral ala fractures are strongly associated with lumbopelvic instability. J Orthop Trauma. 2017;31(12):636–639. 10.1097/BOT.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 9.Bonnin J. Sacral fractures and injuries to the Cauda Equina. J Bone Joint Surg. 1945;7(1):113–127. [Google Scholar]
- 10.Brock Walker J, Mitchell SM, Karr SD, Lowe JA, Jones CB. Percutaneous transiliac-transsacral screw fixation of sacral fragility fractures improves pain, ambulation, and rate of disposition to home. J Orthop Trauma. 2018;32(9):452–456. 10.1097/BOT.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 11.Buell TJ, Yener U, Wang TR, et al. Sacral insufficiency fractures after lumbosacral arthrodesis: salvage lumbopelvic fixation and a proposed management algorithm. J Neurosurg Spine. 2020; 2020:1–2. 10.3171/2019.12.spine191148. [DOI] [PubMed] [Google Scholar]
- 12.Bydon M, Fredrickson V, de la Garza-Ramos R, et al. Sacral fractures. Neurosurg Focus. 2014;37(1):1474. 10.3171/2014.5.FOCUS1474. [DOI] [PubMed] [Google Scholar]
- 13.Chandra V, Wajswol E, Shukla P, Contractor S, Kumar A. Safety and efficacy of sacroplasty for sacral fractures: a systematic review and meta-analysis. J Vasc Interv Radiol. 2019;30(11):1845. 10.1016/j.jvir.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CT, Wang Y, Chen HW, et al. A scalable physician-level deep learning algorithm detects universal trauma on pelvic radiographs. Nat Commun. 2021;12(1):1066. 10.1038/s41467-021-21311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng JS, Song JK. Anatomy of the sacrum. Neurosurg Focus. 2003;15(2):E3. 10.3171/foc.2003.15.2.3. [DOI] [PubMed] [Google Scholar]
- 16.Conflitti JM, Graves ML, Chip Routt ML. Radiographic quantification and analysis of dysmorphic upper sacral osseous anatomy and associated iliosacral screw insertions. J Orthop Trauma. 2010;24(10):630–636. 10.1097/BOT.0b013e3181dc50cd. [DOI] [PubMed] [Google Scholar]
- 17.Denis F, Davis S, Comfort T. Sacral fractures: an important problem. Retrospective analysis of 236 cases. Clin Orthop Rel Res. 1988;227:68–81. [PubMed] [Google Scholar]
- 18.Ebraheim NA, Biyani A, Salpietro B. Zone III fractures of the sacrum: a case report. Spine. 1996;21(20):2390–2396. 10.1097/00007632-199610150-00020. [DOI] [PubMed] [Google Scholar]
- 19.Ghisla S, Napoli F, Lehoczky G, et al. Posterior pelvic ring fractures: intraoperative 3D-CT guided navigation for accurate positioning of sacro-iliac screws. Orthop Traumatol Surg Res. 2018;104(7): 10631067. 10.1016/j.otsr.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Gibbons KJ, Soloniuk DS, Razack N. Neurological injury and patterns of sacral fractures. J Neurosurg. 1990;72(6):889–893. 10.3171/jns.1990.72.6.0889. [DOI] [PubMed] [Google Scholar]
- 21.Hagen J, Castill o R, Dubina A, Gaski G, Manson TT, O’Toole RV. Does surgical stabilization of lateral compression-type pelvic ring fractures decrease patients’ pain, reduce narcotic use, and improve mobilization? Clin Orthop Rel Res. 2016;474(6):1422–1429. 10.1007/s11999-015-4525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han X, Tian W, Liu Y, et al. Safety and accuracy of robot-assisted versus fluoroscopy-assisted pedicle screw insertion in thoracolumbar spinal surgery: a prospective randomized controlled trial. J Neurosurg: Spine. 2019;2019:1–8. 10.3171/2018.10.SPINE18487. [DOI] [PubMed] [Google Scholar]
- 23.Hart RA, Badra MI, Madala A, Yoo JU. Use of pelvic incidence as a guide to reduction of H-type spino-pelvic dissociation injuries. J Orthop Trauma. 2007;21(6):369–374. 10.1097/BOT.0b013e31806dd959. [DOI] [PubMed] [Google Scholar]
- 24.He L, Yi C, Hak DJ, Hou Z. Functional outcome of surgically treated U-shaped sacral fractures: experience from 41 cases. Eur Spine J. 2019;28(5):1146–1155. 10.1007/s00586-019-05900-x. [DOI] [PubMed] [Google Scholar]
- 25.Isler B. Lumbosacral lesions associated with pelvic ring injuries. J Orthop Trauma. 1990;4(1):1–6. 10.1097/00005131-199003000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Joaquim AF, Patel AA. Diagnosis, risk factors, and management of sacral and pelvic fractures after instrumented lumbar fusions: a systematic review. Global Spine J. 2019;9(5):540–544. 10.1177/2192568218779986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kabak S, Halici M, Tuncel M, Avsarogullari L, Baktir A, Basturk M. Functional outcome of open reduction and internal fixation for completely unstable pelvic ring fractures (Type C): a report of 40 cases. J Orthop Trauma. 2003;17(8):555–562. 10.1097/00005131-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Katsuura Y, Chang E, Sabri SA, Gardner WE, Doty JF. Anatomic parameters for instrumentation of the sacrum and pelvis. J Am Acad Orthop Surg Glob Res Rev. 2018;2(8):e034. 10.5435/jaaosglobal-d-18-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katsuura Y, Lorenz E, Gardner W. Anatomic parameters of the sacral lamina for osteosynthesis in transverse sacral fractures. Surg Radiol Anat. 2018;40(5):521–528. 10.1007/s00276-017-1955-3. [DOI] [PubMed] [Google Scholar]
- 30.Keil DS, Gross S, Seymour RB, Sims S, Karunakar MA. Mortality after high-energy pelvic fractures in patients of age 65 years or older. J Orthop Trauma. 2018;32(3):124–128. 10.1097/BOT.0000000000001041. [DOI] [PubMed] [Google Scholar]
- 31.Kelly M, Zhang J, Humphrey CA, Gorczyca JT, Mesfin A. Surgical management of U/H type sacral fractures: outcomes following iliosacral and lumbopelvic fixation. J Spine Surg. 2018;4(2):361–367. 10.21037/jss.2018.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempen DHR, Delawi D, Altena MC, et al. Neurological outcome after traumatic transverse sacral fractures: a systematic review of 521 patients reported in the literature. JBJS Rev. 2018;6(6):e1. 10.2106/JBJS.RVW.17.00115. [DOI] [PubMed] [Google Scholar]
- 33.Kepler CK, Schroeder GD, Hollern DA. Do formal laminectomy and timing of decompression for patients with sacral fracture and neurologic deficit affect outcome? J Orthop Trauma. 2017;31(Suppl. 4):S75–S80. 10.1097/BOT.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 34.Khan JM, Lara DL, Marquez-Lara A, Rosas S, Hasty E, Pilson HT. Intraoperative CT and surgical navigation for iliosacral screws: technique for patients with sacral dysmorphism. J Orthop Trauma. 2018;32(Suppl. 1):S24–S25. 10.1097/BOT.0000000000001213. [DOI] [PubMed] [Google Scholar]
- 35.Khan JM, Marquez-Lara A, Miller AN. Relationship of sacral fractures to nerve injury: is the Denis classification still accurate? J Orthop Trauma. 2017;31(4):181–184. 10.1097/BOT.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 36.Kiapour A, Joukar A, Elgafy H, Erbulut DU, Agarwal AK, Goel VK. Biomechanics of the sacroiliac joint: anatomy, function, biomechanics, sexual dimorphism, and causes of pain. Int J Spine Surg. 2020;14(Suppl. 1):3. 10.14444/6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D, Henn J, Vacarro AR. Surgical anatomy and techniques to the spine. Philadelphia, PA: Elsevier; 2006. [Google Scholar]
- 38.Korovessis P, Baikousis A, Stamatakis M, Katonis P. Medium- and long-term results of open reduction and internal fixation for unstable pelvic ring fractures. Orthopedics. 2000;23(11):1165–1171. 10.3928/0147-7447-20001101-15. [DOI] [PubMed] [Google Scholar]
- 39.Kricun ME. Fractures of the pelvis. Orthop Clin North Am. 1990;21(3):573–590. 10.5005/jp/books/12118_5. [DOI] [PubMed] [Google Scholar]
- 40.Latenser BA, Gentilello LM, Tarver AA, Thalgott JS, Batdorf JW. Improved outcome with early fixation of skeletally unstable pelvic fractures. J Trauma. 1991;31(1):28–31. 10.1097/00005373-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Lehman RA, Kang DG, Bellabarba C. A new classification for complex lumbosacral injuries. Spine J. 2012;12(7):612–628. 10.1016/j.spinee.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Mahmood B, Pasternack J, Razi A, Saleh A. Safety and efficacy of percutaneous sacroplasty for treatment of sacral insufficiency fractures: a systematic review. J Spine Surg. 2019;5(3):365–371. 10.21037/jss.2019.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller AN, Routt MLC. Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8–16. 10.5435/JAAOS-20-01-008. [DOI] [PubMed] [Google Scholar]
- 44.Nork SE, Jones CB, Hardin g SP, Mirza SK, Routt MLC. Percutaneous stabilization of U-shaped sacral fractures using iliosacral screws: technique and early results. J Orthop Trauma. 2001;15(4):238–246. 10.1097/00005131-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Odate S, Shikata J, Kimura H, Soeda T. Sacral fracture after instrumented lumbosacral fusion: analysis of risk factors from spinopelvic parameters. Spine. 2013;38(4):E223–E229. 10.1097/BRS.0b013e31827dc000. [DOI] [PubMed] [Google Scholar]
- 46.Pulley BR, Cotman SB, Fowler T. Surgical fixation of geriatric sacral U-type insufficiency fractures: a retrospective analysis. J Orthoptrauma. 2018;32(12):617–622. 10.1097/BOT.0000000000001308. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues-Pinto R, Kurd MF, Schroeder GD, et al. Sacral fractures and associated injuries. Global Spine J. 2017;7(7):609–616. 10.1177/2192568217701097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rommens PM, Vanderschot PM, Broos PL. Conventional radiography and CT examination of pelvic ring fractures. A comparative study of 90 patients. Unfallchirurg. 1992;95(8):387–392. [PubMed] [Google Scholar]
- 49.Rommens PM, Wagner D, Hofmann A. Do we need a separate classification for fragility fractures of the pelvis? J Orthop Trauma. 2019;33(Suppl 2):S55–S60. 10.1097/BOT.0000000000001402. [DOI] [PubMed] [Google Scholar]
- 50.Routt MLC. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584–589. 10.1097/00005131-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Roy-Camille R, Saillant G, Gagna G, Mazel C. Transverse fracture of the upper sacrum: suicidal jumper’s fracture. Spine. 1985;10(9):838–845. 10.1097/00007632-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Sagi HC, Militano U, Caron T, Lindvall E. A comprehensive analysis with minimum 1-year follow-up of vertically unstable transforaminal sacral fractures treated with triangular osteosynthesis. J Orthop Trauma. 2009;23(5):313–319. 10.1097/BOT.0b013e3181a32b91. [DOI] [PubMed] [Google Scholar]
- 53.Sathiyakumar V, Shi H, Thakore RV, et al. Isolated sacral injuries: postoperative length of stay, complications, and readmission. World J Orthop. 2015;6(8):629–635. 10.5312/wjo.v6.i8.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schicho A, Schmidt SA, Seeber K, Olivier A, Richter PH, Gebhard F. Pelvic X-ray misses out on detecting sacral fractures in the elderly—importance of CT imaging in blunt pelvic trauma. Injury. 2016;47(3):707–710. 10.1016/j.injury.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 55.Schildhauer TA, Ledoux WR, Chapman JR, Henley MB, Tencer AF, Routt CJL. Triangular osteosynthesis and iliosacral screw fixation for unstable sacral fractures: a cadaveric and biomechanical evaluation under cyclic loads. J Orthop Trauma. 2003;17(1):22–31. 10.1097/00005131-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Schmidek HH, Smith DA, Kristiansen TK. Sacral fractures. Neurosurgery. 1984;15(5):735–746. 10.1227/00006123-198411000-00021. [DOI] [PubMed] [Google Scholar]
- 57.Sembler Soles GL, Lien J, Tornetta P. Nonoperative immediate weightbearing of minimally displaced lateral compression sacral fractures does not result in displacement. J Orthop Trauma. 2012;26(10):2610–2567. 10.1097/BOT.0b013e318251217b. [DOI] [PubMed] [Google Scholar]
- 58.Shah DS, Bates T, Fowler J, Osborn P, Jorgensen AY. Minimally invasive lumbopelvic fixation for unstable U-type sacral fractures. Cureus. 2019;11(9):e5621. 10.7759/cureus.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strange-Vognsen HH, Lebech A. An unusual type of fracture in the upper sacrum. J Orthrop Trauma. 1991;5(2):200–203. [DOI] [PubMed] [Google Scholar]
- 60.Tornetta P, Lowe JA, Agel J. Does operative intervention provide early pain relief for patients with unilateral sacral fractures and minimal or no displacement? J Orthop Trauma. 2019;33(12):614–618. 10.1097/BOT.0000000000001578. [DOI] [PubMed] [Google Scholar]
- 61.Tsiridis E, Upadhyay N, Giannoudis PV. Sacral insufficiency fractures: current concepts of management. Osteoporosis Int. 2006;17(12):1716–1725. 10.1007/s00198-006-0175-1. [DOI] [PubMed] [Google Scholar]
- 62.Uçar D, Uçar BY, Coşar Y, et al. Retrospective cohort study of the prevalence of lumbosacral transitional vertebra in a wide and well-represented population. Arthritis. 2013;2013:461425. 10.1155/2013/461425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaccaro AR, Kim DH, Brodke DS, et al. Diagnosis and management of sacral spine fractures. Instr Course Lect. 2004;53:375–385. [PubMed] [Google Scholar]
- 64.Vallier HA, Lowe JA, Agel J, et al. Surgery for unilateral sacral fractures: are the indications clear? J Orthop Trauma. 2019;33(12):619–625. 10.1097/BOT.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 65.Wagner D, Kamer L, Sawaguchi T, Richards RG, Noser H, Rommens PM. Sacral bone mass distribution assessed by averaged three-dimensional CT models. J Bone and Joint Surg Am. 2016;98(7):584–590. 10.2106/JBJS.15.00726. [DOI] [PubMed] [Google Scholar]
- 66.Weber M, Hasler P, Gerber H. Insufficiency fractures of the sacrum: twenty cases and review of the literature. Spine. 1993;18(16):2507–2512. 10.1097/00007632-199312000-00021. [DOI] [PubMed] [Google Scholar]
- 67.Wilde GE, Miller TT, Schneider R, Girardi FP. Sacral fractures after lumbosacral fusion: a characteristic fracture pattern. Am J Roentgenol. 2011;197(1):184–188. 10.2214/AJR.10.5902. [DOI] [PubMed] [Google Scholar]
- 68.Williams SK, Quinnan SM. Percutaneous lumbopelvic fixation for reduction and stabilization of sacral fractures with spinopelvic dissociation patterns. J Orthop Trauma. 2016;30(9):e318–e324. 10.1097/BOT.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zelle BA, Gruen GS, Hunt T, Speth SR. Sacral fractures with neurological injury: is early decompression beneficial? Int Orthopaed. 2004;28(4):244–251. 10.1007/s00264-004-0557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-hss-10.1177_15563316221129607 for Sacral Fractures: A Review by Lauren A. Barber, Yoshihiro Katsuura and Sheeraz Qureshi in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-2-hss-10.1177_15563316221129607 for Sacral Fractures: A Review by Lauren A. Barber, Yoshihiro Katsuura and Sheeraz Qureshi in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-3-hss-10.1177_15563316221129607 for Sacral Fractures: A Review by Lauren A. Barber, Yoshihiro Katsuura and Sheeraz Qureshi in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery