Abstract
Urban-dwelling species present feeding and behavioural innovation that enable them to adjust to anthropogenic food subsidies available in cities. In 2020, the SARS-CoV-2 virus outbreak resulted in unprecedented reduction in the human activity worldwide associated with the human lockdown. This situation opened an excellent opportunity to investigate the capability of urban wildlife to cope with this anthropopause event. Here, we investigated the effects of the COVID-19 lockdown on the feeding strategies of the urban yellow-legged gull (Larus michahellis) population inhabiting the highly dense city of Barcelona (NE Spain). We compared the diet of chicks (through stomach content and stable isotope analyses) sampled randomly around the city of Barcelona before (2018 and 2019), during (2020) and after (2021) the COVID-19 lockdown. The results revealed that the anthropopause associated with the lockdown had an effect on the diet of this urban-dwelling predator. The diversity of prey consumed during the lockdown was lower, and consumption of urban birds (pigeons and parakeets) and marine prey (fishery discards and natural prey) decreased during the year of lockdown. Although it was not analysed, these diet changes probably were associated with variations in the availability of these resources due to the decrease in human activity during the lockdown. These results demonstrate the trophic flexibility of urban-dwelling species to cope with the changes in the availability of human-related anthropogenic resources in urban marine ecosystems.
Keywords: anthropopause, COVID-19, feeding ecology, marine predator, urban wildlife, urban ecology
1. Introduction
Human activity causes alterations in the functioning of natural ecosystems at global scale [1,2]. Among the different human impacts, the process of urbanization has notably affected biodiversity [3], displacing species from their natural habitats and reducing the size of their populations [4,5]. However, although human activity may negatively affect a large number of species, others are able to adjust and thrive in these urban environments [1]. Overall, urban-dwelling species tend to present high rates of feeding and behavioural innovation that enable them to exploit novel food resources present in these human-impacted habitats, which then could increase their survival and fitness [6,7]. This behavioural plasticity allows these species to adjust to new anthropogenic food resources present in the cities [7,8]. These flexible species are able to adjust their behaviours in human-altered landscapes successfully and can live in sympatry with humans in highly dense cities [6,9], sometimes providing ecosystem services, but very often disservices to society [10].
Among urban-dwelling wildlife, opportunistic gulls are clear examples of successful species that have become very common in urban areas around the world [11–14]. The high behavioural plasticity of these large seabirds has allowed them to exploit efficiently a great variety of novel trophic opportunities available in cities and surrounding habitats, including prey of human, marine, freshwater and terrestrial origin [13,15–18]. This is the example of the yellow-legged gull (Larus michahellis), a large-size gull with a widespread distributed along the Mediterranean region [19]. It is well adapted to urban life, efficiently preying on abundant urban birds such as rock pigeons (Columba livia) or monk parakeets (Myiopsitta monachus) and other resources associated with human activity such as human garbage and fishery discards [13,20–23]. Under scenarios of reduction of fishing activity or the closure of landfills or fisheries, natural populations of large gulls respond by changing their foraging strategies [24–26].
In 2020, the outbreak of the SARS-CoV-2 virus resulted in unprecedented reduction in human activity worldwide. In Spain, between March and May 2020, severe lockdown (COVID-19 lockdown) restrictions forced humans to confine themselves to their homes [27] and, similarly to other European countries, drastically reduced human activity to the essentials [28]. As a result, COVID-19 lockdown produced a reduction of human presence in the streets [29] and an alteration in the availability of human-sourced resources for opportunistic urban species [30,31]. This unique scenario offered an excellent opportunity to investigate the ability of urban wildlife to cope with this drastic anthropopause event [31]. Research in this area has revealed how the COVID-19 lockdown affected the behavioural patterns of urban mammals and birds associated with changes in food availability or human presence [32–35]. Overall, these studies evidenced how successful species inhabiting human-related habitats rapidly adjusted to the novel environmental conditions, directly related to their behavioural plasticity [33].
Here, we aimed to investigate the effects of the COVID-19 lockdown on the feeding behaviour of the urban yellow-legged gull population inhabiting the city of Barcelona (NE Spain), a highly populated European city severely affected by the COVID-19 anthropopause [29]. For this, we compared the diet (stomach content and stable isotope values) of chicks sampled randomly around the city of Barcelona before, during, and after the COVID-19 lockdown. Due to the high dependence on human resources of this species [13,36–38], we expected that the reduction in the human activity associated with the COVID-19 lockdown would affect the feeding behaviour of this urban gull population. For example, during the COVID-19 lockdown, the availability of fishery discards at sea, an important resource for this breeding colony [13,39], was reduced notably, associated with the reduction of the fishing activity in the waters close to Barcelona [40,41]. To compensate for the lack of fishing discards, we expected that urban yellow-legged gulls would increase the use of terrestrial habitats, increasing the consumption of resources such as urban birds (for example, rock pigeons), also an important part of their diet [13,21].
2. Methods
2.1. Fieldwork procedures
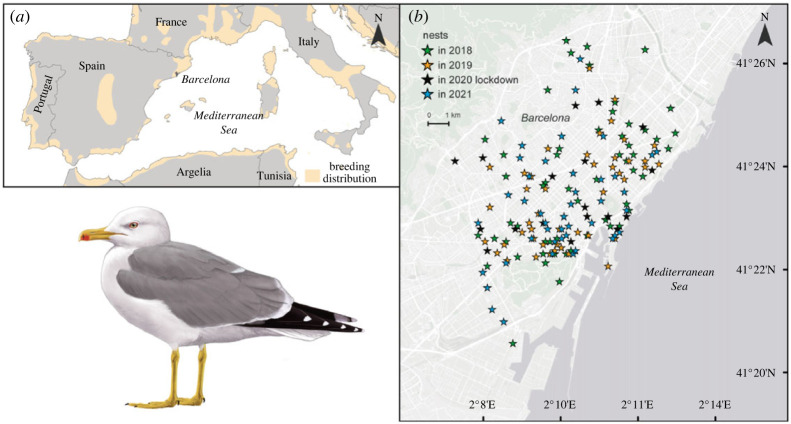
This study was developed in the city of Barcelona (NE Spain, figure 1) during the 2 years before the COVID-19 lockdown (2018 and 2019), the COVID-19 lockdown year (2020) and the year after (2021). Barcelona is a coastal urban area considered the second and eighth largest city of Spain and Europe regarding the number of habitants, respectively. The breeding population of yellow-legged gull of Barcelona has experienced a remarkable increment from one to five breeding pairs in the 1980s to around 300 pairs nowadays, distributed along the entire urban area [42,43]. Barcelona has different features that make it attractive to this opportunistic gull. The existence of elevated buildings provides rooftops that offer protection against predators or human disturbances. Furthermore, the urban environment of this city is surrounded by important fishing harbours, freshwater/river habitats, agricultural areas and waste management installations offerings a high variety of potential prey [13,20]. A significant activity is conducted in the fishing harbour of Barcelona and close coastal cities, with almost 3500 tons of fish caught every year [44]. This activity generates a large amount of fishing discards [45], being an important feeding resource for the yellow-legged gulls in this and other breeding areas [13,38,39]. In addition to marine prey, abundant urban birds such as rock pigeon and monk parakeet have been identified as key prey for this species [13,21].
Figure 1.
(a) General map showing breeding distribution of the yellow-legged gull (Larus michahellis) in Europe. (b) Distribution of the nests sampled in Barcelona during the pre-lockdown years (2018, 2019), the COVID-19 lockdown year (2020) and the year after (2021). The image of the yellow-legged gull was made by Martí Franch.
To investigate the trophic ecology of yellow-legged gull, we analysed the stomach content and the stable isotope values of the new formed scapular feathers of 209 chicks collected and sacrificed along the city of Barcelona (only one chick per nest, and when we found more than one in a nest, we analysed the larger one; figure 1 for the distribution of nests) during the chick-rearing period (April–June). The Public Health Agency of Barcelona provided all these chicks. This is the responsible institution for the surveillance and control of wildlife species in the city (Legislative Decree 2/2008 of 15 April, DOGC; experimental permits 56L789 of Generalitat de Catalunya, Spain). To avoid potential differences in the diet between size/age [21], all chicks analysed showed similar tarsus length (ANOVA tests, F3,205 = 2.24, p = 0.91; table 1) and body mass (F3,205 = 0.72, p = 0.54; table 1). Moreover, all chicks were between one and three weeks of age, based on the principal component analysis (PCA) scores of the tarsus length and body mass, as these PCA scores increase linearly with the age of yellow-legged gull chicks [46]. We also discarded a potential effect on the diet in relation to the localization of the nests within the city, since unpublished GPS data of yellow-legged gull adults breeding in Barcelona during 2018, 2019 and 2021 showed similar foraging locations regardless of where the nest was placed within the city.
Table 1.
Mean and s.d. of the body mass and tarsus length, and the stomach contents of yellow-legged gull chicks sampled in Barcelona before (2018, n = 82; 2019, n = 59), during the COVID lockdown (2020, n = 22) and the year after (2021, n = 46). %FO = frequency of occurrence of each prey in the stomachs. %N = contribution by number of each prey in the stomachs.
| 2018 |
2019 |
2020 lockdown |
2021 |
|||||
|---|---|---|---|---|---|---|---|---|
| body mass (g) | 662.27 ± 207.54 |
666.54 ± 194.72 |
710.51 ± 129.46 |
707.13 ± 235.506 |
||||
| tarsus length (cm) | 62.83 ± 7.05 |
61.81 ± 5.28 |
66.16 ± 7.18 |
63.58 ± 6.94 |
||||
| prey | %FO | %N | %FO | %N | %FO | %N | %FO | %N |
| demersal marine prey | 31.71 | 22.75 | 42.37 | 43.56 | 27.27 | 44.44 | 26.09 | 25.00 |
| Boops boops | 19.51 | 11.38 | 27.12 | 32.67 | 27.27 | 44.44 | 17.39 | 11.36 |
| Phycis blennoides | 0 | 0 | 5.08 | 2.97 | 0 | 0 | 4.35 | 6.82 |
| Microchirus variegatus | 0 | 0 | 1.69 | 0.99 | 0 | 0 | 0 | 0 |
| Trisopterus capelanus | 2.44 | 1.20 | 3.39 | 1.98 | 0 | 0 | 0 | 0 |
| Nezumia aequalis | 3.66 | 1.80 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pagellus acarne | 1.22 | 1.20 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pagellus erythrinus | 1.22 | 0.60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Micromesistius poutassou | 2.44 | 1.20 | 1.69 | 0.99 | 0 | 0 | 6.52 | 5.68 |
| Coelorinchus coelorinchus | 2.44 | 1.20 | 3.39 | 1.98 | 0 | 0 | 0 | 0 |
| Gaidropsarus biscayensis | 0 | 0 | 1.69 | 0.99 | 0 | 0 | 0 | 0 |
| Lesueurigobius sp. | 1.22 | 0.60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diplodus sp. | 2.44 | 1.20 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trisopterus sp. | 2.44 | 1.20 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gadiformes | 1.22 | 0.60 | 1.69 | 0.99 | 0 | 0 | 2.17 | 1.14 |
| pelagic marine prey | 8.54 | 5.99 | 18.64 | 12.87 | 0 | 0 | 4.35 | 4.55 |
| Gadiculus argenteus | 0 | 0 | 5.08 | 3.96 | 0 | 0 | 4.35 | 3.41 |
| Spicera smaris | 0 | 0 | 3.39 | 1.98 | 0 | 0 | 0 | 0 |
| Engraulis encrasicolus | 1.22 | 0.60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sardina pilchardus | 1.22 | 0.60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachinotus ovatus | 1.22 | 0.60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachurus mediterranius | 0 | 0 | 1.69 | 0.99 | 0 | 0 | 0 | 0 |
| Trachurus trachurus | 1.22 | 0.60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachurus sp. | 6.10 | 4.19 | 0 | 0 | 0 | 0 | 2.17 | 1.14 |
| Spicera sp. | 0 | 0 | 3.39 | 1.98 | 0 | 0 | 0 | 0 |
| Sparidae | 0 | 0 | 6.78 | 3.96 | 0 | 0 | 0 | 0 |
| other marine prey | 28.05 | 13.77 | 6.78 | 3.96 | 9.09 | 11.11 | 21.74 | 12.50 |
| cephalopods | 6.10 | 2.99 | 0 | 0 | 4.55 | 5.56 | 4.35 | 3.41 |
| marine crustaceans | 4.88 | 2.40 | 0 | 0 | 0 | 0 | 2.17 | 1.14 |
| unidentified fish | 17.07 | 8.38 | 6.78 | 3.96 | 4.55 | 5.56 | 15.22 | 7.95 |
| urban birds | 67.07 | 34.73 | 61.02 | 35.64 | 27.27 | 33.33 | 76.09 | 40.91 |
| Columba livia | 19.51 | 9.58 | 40.68 | 23.76 | 13.64 | 16.67 | 36.96 | 20.45 |
| Myiopsitta monachus | 10.98 | 5.39 | 1.69 | 0.99 | 0 | 0 | 4.35 | 2.27 |
| unidentified bird | 40.25 | 19.76 | 20.34 | 10.89 | 13.64 | 16.67 | 34.78 | 18.18 |
| other terrestrial prey | 23.53 | 8.78 | 1.98 | 9.09 | 11.11 | 23.91 | 14.67 | 1.14 |
| Mus musculus | 1.22 | 0.60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rattus rattus | 1.22 | 0.60 | 0 | 0 | 0 | 0 | 0 | 0 |
| unidentified mammal | 28.05 | 13.77 | 3.39 | 1.98 | 9.09 | 11.11 | 23.91 | 12.50 |
| invertebrate | 7.32 | 4.19 | 0 | 0 | 0 | 0 | 2.17 | 1.14 |
| garbage | 7.32 | 3.59 | 3.39 | 1.98 | 0 | 0 | 6.52 | 3.41 |
| Gallus gallus domesticus | 6.10 | 2.99 | 1.69 | 0.99 | 0 | 0 | ||
| unidentified meat | 0 | 0 | 1.69 | 0.99 | 0 | 0 | 2.17 | 1.14 |
| unidentified vegetal | 1.22 | 0.60 | 0 | 0 | 0 | 0 | 4.35 | 2.27 |
| inorganic items | 53.66 | 0 | 64.41 | 0 | 59.09 | 0 | 69.57 | 0 |
| plastic items | 47.56 | 0 | 55.93 | 0 | 54.55 | 0 | 56.52 | 0 |
| metallic items | 0 | 0 | 1.69 | 0 | 4.55 | 0 | 8.70 | 0 |
| textile items | 0 | 0 | 0 | 0 | 4.55 | 0 | 4.35 | 0 |
| others | 19.51 | 0 | 25.42 | 0 | 0 | 0 | 19.57 | 0 |
By combining the stomach content analysis with the determination of stable isotopes in feathers, we were able to benefit from the advantages and solve some of the biases associated with each methodology [38,47]. For example, stomach content analysis allows identifying the diet with a high taxonomic precision [48] but this methodology presents certain bias towards the overestimation of prey tissues/structures that are difficult to digest or towards preys ingested shortly before the sampling period [49]. The analysis of stable isotopes of δ15N (a proxy of trophic position; δ15N are positively related with the trophic position), δ13C (a proxy of the inshore, i.e. low δ13C values, and offshore habitats, i.e. high δ13C values) and δ34S (related to the terrestrial-marine origin of the prey; high δ34S values in terrestrial prey), is a complementary tool to infer the trophic niche of consumers during the period of the tissue analysed [50,51]. In our case, as the new scapular feathers are metabolically inert after synthesis, the feathers from yellow-legged chicks integrate the diet consumed and assimilated by the chicks during the feather growth (along two–three weeks) during the chick-rearing period [52].
2.2. Stomach content analysis
The stomach contents were identified and classified to the lowest possible taxonomic level, and counted to the lowest possible number of items. To determine the dietary importance of each prey type, for each year, we calculated two trophic metrics: %FO (frequency of occurrence of each prey in relation to the total number of stomachs analysed) and %N (contribution by number of each prey in relation to the total number of preys analysed). In addition to the trophic resources, we also calculated the %FO of plastic, textile fibres and metallic items. As a measure of trophic diversity, the Shannon's diversity index [53] was calculated for each year. Shannon's diversity index for the years 2018 (82 stomachs analysed), 2019 (59 stomachs analysed) and 2021 (46 stomachs analysed) was estimated by 1000 resamples of 22 random stomachs per year, 22 being the lowest number of stomachs analysed in the year 2020. Resampling procedure was done using the R package mosaic [54].
2.3. Stable isotope analysis
Newly formed scapular feathers were cleaned, dried and the entire feathers were powdered and between 0.28 and 0.33 mg for δ13C and δ15N analysis and around 1.5 mg for δ34S analysis were packed into tin capsules and sent to the Stable Isotopes Lab of the Estación Biológica de Doñana (EBD-CSIC; www.ebd.csic.es/lie/index.html), where stable isotopic analyses were performed. The samples were combusted at 1020°C using a continuous flow isotope ratio mass spectrometry system (Thermo Electron) by means of a Flash HT Plus elemental analyser interfaced with a Delta V Advantage mass spectrometer which applies international standards. Stable isotope ratios were expressed in the standard δ-notation (‰) relative to troilite from the Canyon Diablo Meteorite (δ34S), atmospheric N2 (δ15N) and Vienna Pee Dee Belemnite (δ13C). The measurement error (± s.d.) was ±0.1, ±0.1 and ±0.2‰ for δ34S, δ13C and δ15N, respectively.
δ13C, δ15N and δ34S values from feathers were used to infer the isotopic niche volume (NR) of the yellow-legged gulls for each sampling year. The NR is defined as a three-dimensional volume contained in a multi-variate space that contains the probability of finding a specific individual from a certain year in 40% of the core isotopic niche volume of that specific year. We used 40% of the total niche volume because it is a percentage commonly associated with the core NR of a species [55], and in this case, the core NR of the gulls for each year was calculated using a Bayesian probabilistic method implemented in the R package nicheROVER [56]. NR estimation was based on 1000 random projections generated by the Bayesian probabilistic method, and they were used to calculate the mean NR and overlap between years. Ten of these random projections of the bivariate niches were used for plotting in order to ease the visualization. As NR was estimated with Bayesian statistics, we calculated the probability that the posterior distribution of 2020 is smaller than the rest of the years.
2.4. Statistical analysis
We applied PERMANOVA and pairwise tests based on a Bray–Curtis distance matrix and square-root transformed data to compare the %N, %FO, δ15N, δ13C and δ34S values between the years before (2018 and 2019), the COVID-19 lockdown year (2020) and the year after (2021). The method calculates a pseudo-F statistic, analogue to the traditional F statistic of ANOVA tests, using permutation procedures to obtain the p-values [57]. In the case of differences among years, a similarity percentage analysis (SIMPER tests) with 999 permutations was performed [57] to identify which prey type contributed most to the observed diet differences among years. PERMANOVA and SIMPER tests were conducted with PRIMER-E v. 6 software [57].
3. Results
3.1. Stomach content
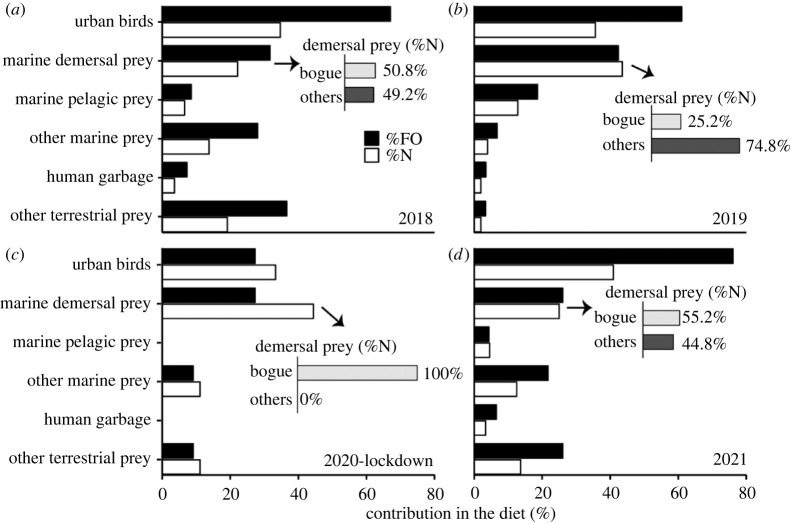
Overall for all years, the stomach content of yellow-legged gull chicks was mainly composed of urban birds, followed by marine demersal prey, with less importance of marine pelagic prey, other marine prey, other terrestrial prey and human garbage (table 1, figure 2). However, we found differences among years in prey found in the stomachs (%N; pseudo-F3,207 = 3.27, p = 0.006; %FO; pseudo-F3,207 = 1.02, p = 0.02; table 1, figures 2 and 3) and in the Shannon diversity index (figure 3). In particular, during the COVID-19 lockdown year, urban birds such as pigeons (Columba livia) or monk parakeets (Myiopsitta monachus) decreased in both %N and %FO in comparison with the previous years (2018 and 2019) and the year after the lockdown (2021) (SIMPER tests comparing %N and %FO between 2020 and the other years always present p < 0.05; table 1, figure 2). Marine pelagic prey, although overall were not an important part of the yellow-legged gull diet, during the COVID-19 lockdown, this group was not detected in any of the 22 stomachs analysed this year (table 1, figure 2). In relation to the other groups, a decrease in the %N of marine demersal prey between the 2019 and the COVID-19 lockdown year (SIMPER tests, p = 0.01) was found (table 1, figure 2). Within marine demersal prey, the predominant demersal species during all years was the bogue (Boops boops), but it was the only marine prey found in the stomach content of the yellow-legged gull chicks during the COVID-19 lockdown year (table 1, figure 2). Other items associated with anthropogenic origin such as plastic remains were also found in the stomachs, with elevated %FO values that ranged between 53% and 69%, but without differences among years (table 1).
Figure 2.
Prey categories found in the stomach content of yellow-legged gull chicks sampled in Barcelona before ((a) 2018: n = 82 chicks; (b) 2019: n = 59 chicks), during the COVID lockdown ((c) 2020: n = 22 chicks) and the year after ((d) 2021: n = 46) calculated as the percentage of stomachs (%FO) and the percentage of preys (%N) for each category. The (%N) of the bogue (Boops boops) in relation to the other demersal fish prey is also indicated.
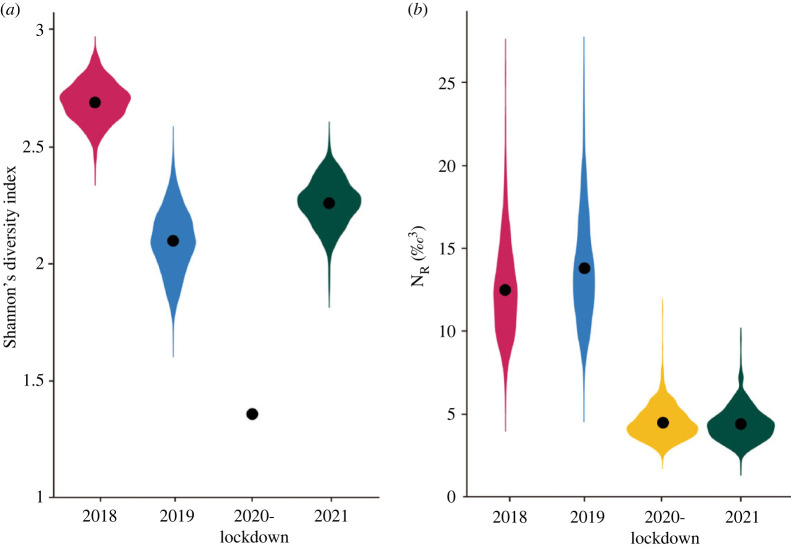
Figure 3.
(a) Shannon index values based on the stomach contents and (b) trophic niche volume (NR ‰3) inferred from δ13C, δ15N and δ34S isotopic values of newly formed scapular feathers of yellow-legged gull chicks sampled in Barcelona before (2018 and 2019), during the COVID lockdown (2020) and the year after (2021). For the years 2018, 2019 and 2021, the Shannon index was estimated from 1000 resamples of 22 random stomachs (the number of stomachs analysed in 2020).
Regarding the Shannon diversity index, the COVID-19 lockdown year showed the lowest values (1.52) whereas higher diversity values were found in 2018 (mean ± s.d. = 2.23 ± 0.36), followed by 2021 (2.01 ± 0.34) and 2019 (1.79 ± 0.45) (figure 3a).
3.2. Stable isotopes
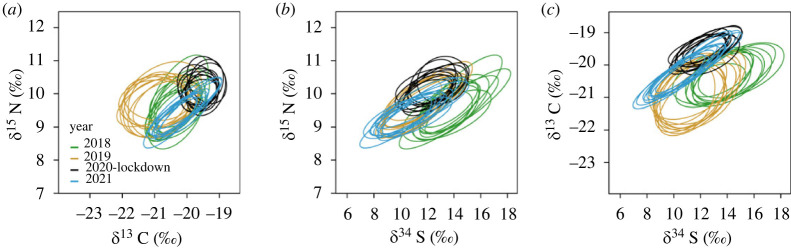
We found significant differences among years for the three stable isotopes analysed (δ15N, pseudo-F3,115 = 2.14, p < 0.0001; δ13C, pseudo-F3,115 = 5.63, p < 0.0001; δ34S, pseudo-F3,115 = 1.94, p < 0.0001; table 2, electronic supplementary material, table S1). Specifically, SIMPER tests indicated that during the COVID-19 lockdown year, yellow-legged gull chicks showed higher δ15N and δ13C values than during the 2 previous years and the year after (p < 0.05; table 2). In the case of the δ34S values, yellow-legged gull chicks showed significantly higher values during 2018 in comparison with the other years (p < 0.05; table 2).
Table 2.
Number of samples (n), mean and s.d. of δ13C, δ15N, δ34S and the niche volume (NR) values of newly formed scapular feathers of yellow-legged gull chicks sampled in Barcelona before (2018 and 2019), during the COVID lockdown (2020) and the year after (2021). See electronic supplementary material, table S1 for more information.
| year | n | δ15N (‰) | δ13C (‰) | δ34S (‰) | NR (‰3) |
|---|---|---|---|---|---|
| 2018 | 29 | 9.52 ± 0.88a | −20.35 ± 0.89a | 13.72 ± 2.67a | 12.48 ± 2.99 |
| 2019 | 29 | 9.68 ± 0.71a | −20.82 ± 1.09b | 11.35 ± 2.88b | 14.01 ± 3.14 |
| 2020 lockdown | 32 | 10.16 ± 0.61b | −19.57 ± 0.57c | 12.23 ± 2.09b | 4.43 ± 0.99 |
| 2021 | 29 | 9.43 ± 0.72a | −20.21 ± 0.87a | 10.71 ± 2.94b | 4.37 ± 1.00 |
Values with the same subscript indicate no significant differences between years based on SIMPER tests.
The isotopic niche volume (NR) showed higher values during the 2 years before the year of the COVID-19 lockdown and the year after (figure 3b, table 2). Specifically, we found that 2020 had a greater than 99% probability of being smaller than 2018 and 2019, and a 47.8% probability of being smaller than 2021 (figure 3b). In addition, NR volume showed a partial segregation between the COVID-19 lockdown and the years before and after (table 3, figure 4).
Table 3.
NR overlap of yellow-legged gull chicks sampled in Barcelona before (2018 and 2019), during the COVID lockdown (2020) and the year after (2021). As the volume is different depending on the year, the overlap is asymmetric (i.e. if you compare for example 2018 with the volume of 2019 or 2019 with the volume of 2018).
| overlap (%) | 2018 | 2019 | 2020 lockdown | 2021 |
|---|---|---|---|---|
| 2018 | 16.8 [7.0–31.5] | 3.3 [0.8–7.1] | 5.6 [2.0–10.8] | |
| 2019 | 13.6 [4.7–26.7] | 6.3 [2.7–11.7] | 9.6 [5.2–15.3] | |
| 2020 lockdown | 5.9 [0.4–20.8] | 20.1 [3.9–46.7] | 16.4 [5.9–30.7] | |
| 2021 | 16.9 [3.8–38.0] | 36.5 [15.3–60.8] | 18.5 [9.2–30.3] |
Figure 4.
Two-dimensional isotopic niche based on ellipse areas (‰2) from the (a) δ15N and δ13C values, (b) δ15N and δ34S values and (c) δ13C and δ34S values of newly formed scapular feathers of yellow-legged gull chicks sampled in the city of Barcelona before (2018: n = 29 chicks; 2019: n = 29 chicks), during the COVID lockdown (2020: n = 32 chicks) and the year after (2021: n = 21 chicks).
4. Discussion
The present study revealed how the interruption of human activity associated with the strict COVID-19 lockdown in Barcelona, which overlapped with the chick-rearing period of the urban yellow-legged gulls, forced a change in their feeding behaviour. During the COVID-19 lockdown year, the diet of yellow-legged gull chicks showed smaller trophic niche and prey diversity, less importance of urban birds, marine pelagic prey and human garbage, and an increase of marine demersal prey in their diet, and an enrichment in δ13C and δ15N values in their feathers in comparison with the previous and the subsequent years. These results provided evidence of how this opportunistic predator is able to adjust and modify its diet to cope with the constraint associated with a drastic change in the availability of different resources mainly associated with humans in an urban marine ecosystem.
During the COVID-19 lockdown, the fishing activity along the northwestern Mediterranean Sea, an important feeding ground for yellow-legged gulls inhabiting Barcelona [13,39], was reduced considerably [40,41]. This situation affected the abundance and availability of fishery discards provided by trawlers and purse-seiners [40], important human-related resources for opportunistic seabirds [13,46,58]. For this reason, we expected a clear reduction in the presence of marine resources in the diet of the yellow-legged gull chicks during the COVID-19 lockdown year. This was evident in the absence of marine pelagic fish in the stomachs of the chicks during the lockdown year, reflecting the low availability due to the reduction of purse-seine activity, a fishing activity that provides a high amount of pelagic fish via discards for Mediterranean seabirds [59,60]. However, although a similar pattern was expected for marine demersal prey, only available for surface feeder seabirds through trawling discards [61,62], here we found that during the lockdown, demersal prey group was an important food item for yellow-legged gulls. Specifically, the bogue, the main demersal prey identified during all the years analysed and considered one of the most discarded species by the trawling fleet in the western Mediterranean [45], was the only demersal prey identified in the stomachs during the lockdown period. Although bogue is present in Mediterranean deep waters up to 350 m, it is a species that can also be found close to the sea surface in shallow waters [63], where gulls are able to capture them. Moreover, it is possible that the presence of the bogue in shallow waters increased during the lockdown associated with the reduction in human mobility [64,65], increasing the availability of this prey in particular habitats such as beaches or harbours, commonly visited by urban yellow-legged gulls [13]. Both hypotheses could explain why the bogue is the main demersal prey in the diet of yellow-legged gull and it was the only demersal fish present in the stomachs during the COVID-19 lockdown year. Moreover, we could interpret this result as the necessity of adults to provide fish of better nutritional quality than terrestrial resources to the chicks to compensate for the lack of other marine resources often available in other years.
Isotopically, the enriched δ13C and δ15N values are related to diets with a high proportion of demersal and/or nearshore species [66–68]. For this, the enriched δ13C and δ15N values during the lockdown reflected the absence of marine pelagic prey and the relatively high consumption of bogue showed in the stomach contents. Alternatively, the increase of δ15N values during the COVID-19 lockdown year could be reflecting the use of their protein stores by the chicks during the feather formation [52] associated with a potential reduction in the quantity of food to the chicks provided by their parents due to the reduction of easy-to-catch resources such fishery discards [39].
To compensate for the lack of fishing discards during the lockdown [40], we expected that urban yellow-legged gulls increased the consumption of alternative resources such as urban birds, considered one of the main resources consumed by urban yellow-legged gulls in Barcelona [13,21]. The reason for this initial prediction was that urban birds such as pigeons or invasive monk parakeets might have become more accessible to be captured by the gulls during the lockdown due to the reduction of human and vehicle presence in Barcelona [27,28]. However, based on our results, urban birds were consumed in a lower proportion during the lockdown than during the other years. In fact, it is important to remark that during the lockdown, the proportion of urban birds found in the stomachs was lower than the proportion of marine resources. Thus, why did urban bird consumption not increase during the COVID-19 lockdown year? Two complementary explanations directly related to the availability of these prey for the urban yellow-legged gulls could explain this behaviour. The reduction in the number of vehicles associated with the lockdown [27,28] probably reduced the number of dead urban birds associated with vehicle collisions [69,70], reducing the availability of bird carcases for urban gulls [15]. In addition to the scavenging behaviour, the yellow-legged gull also preys on rock pigeons when they are concentrated in large groups of hundreds of individuals feeding on food provided by citizens [71,72]. The lack of human food provisioning in urban habitats during the lockdown probably caused a reduction in the presence of these large aggregations of rock pigeons, dispersing them throughout the city [30]. Thus, this change in the aggregation patterns of urban birds during the lockdown probably reduced the success of capture by yellow-legged gulls [73]. However, the similar δ34S values between the lockdown and the year before and after, suggest that despite the low consumption of urban birds, their diet assimilation did not change [51,74]. It is also important to mention that the predation of pigeons and parakeets explains the presence of some pathogens [75] or seeds of plants [76] carried by gulls that potentially could be spread to humans [77–79] or to natural habitats [21], respectively. For this, indirectly, the low consumption of urban birds in the diet of yellow-legged gull during the lockdown could reduce the potential spread of both pathogens and seeds during 2020.
Regarding the diet diversity metrics, we found some differences between the Shannon diversity index estimated with the stomach content and the isotopic niche (NR). During the COVID-19 lockdown year, the Shannon index using the stomach content information showed a lower value, clearly reflecting that during this year only one species of demersal and pelagic marine prey was detected in the stomachs. By contrast, NR metric indicated that in both the lockdown and the following year, the yellow-legged chicks showed smaller trophic niche volumes, a clear reflection that the diet was less diverse than during the years prior to lockdown [80].
In conclusion, as we predicted, the anthropopause associated with the COVID-19 lockdown apparently had an effect on the feeding ecology of this urban-dwelling predator. The diversity of prey consumed during the lockdown was lower than in the years before and after. In addition, the predation on urban birds and marine prey decreased during the year of lockdown, associated with changes in the availability of these resources due to a drastic decrease in human activity and mobility. In addition to the observed change in stomach contents, stable isotope values in yellow-legged gulls' feathers also reflects the feeding changes produced by the anthropopause. The results of this study, therefore, demonstrate the trophic flexibility of this species to cope with the changes in the availability of human-related anthropogenic resources in urban marine ecosystems.
Acknowledgements
Thanks to Míriam Gimeno, Teresa Militão, Raül Ramos, Jacob Gonzaléz-Solís and Santi Mañosa for their very useful feedback and suggestions in previous drafts.
Ethics
All fieldwork was conducted in accordance with the Spanish and EU legislation on the protection of animals used for scientific purposes (Legislative Decree 2/2008 of 15 April, DOGC).
Data accessibility
The raw stable isotope data is provided in the electronic supplementary material.
The data are provided in the electronic supplementary material [81].
Authors' contributions
M.V.-G.: formal analysis, investigation, methodology and writing—review and editing; J.G.: data curation, formal analysis, methodology, resources, supervision and writing—review and editing; A.S.-M.: formal analysis, investigation and writing—review and editing; T.M.: investigation, methodology and writing—review and editing; J.N.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, supervision, validation and writing—original draft.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no competing interests.
Funding
This study is a contribution of the ICM-TEF (Trophic Ecology Facility of the Institut de Ciències del Mar—CSIC), of the BCN-Gulls project and the Master's thesis of M.V.-G. M.V.-G. and J.G. were supported by a JAE-Intro grant of CSIC (JAEIntro2020, CSIC) and a contract of the Spanish National Program Juan de la Cierva-Formación (FJC2019-040016-I), respectively. This work acknowledges the accreditation of the ‘Severo Ochoa Center of Excellence’ (CEX2019-000928-S). Open Access funding provided thanks to the CRUE-CSIC agreement with Royal Society.
References
- 1.McKinney ML, Lockwood JL. 1999. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450-453. ( 10.1016/S0169-5347(99)01679-1) [DOI] [PubMed] [Google Scholar]
- 2.Worm B, et al. 2006. Impacts of biodiversity loss on ocean ecosystem services. Science (1979) 314, 787-790. ( 10.1126/science.1132294) [DOI] [PubMed] [Google Scholar]
- 3.McKinney ML. 2006. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247-260. ( 10.1016/j.biocon.2005.09.005) [DOI] [Google Scholar]
- 4.Cardillo M, Purvis A, Sechrest W, Gittleman JL, Bielby J, Mace GM. 2004. Human population density and extinction risk in the world's carnivores. PLoS Biol. 2, e197. ( 10.1371/journal.pbio.0020197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal A, Redford K. 2009. Conservation and displacement: an overview. Conserv. Soc. 7, 1. ( 10.4103/0972-4923.54790) [DOI] [Google Scholar]
- 6.Lowry H, Lill A, Wong BBM. 2013. Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537-549. ( 10.1111/brv.12012) [DOI] [PubMed] [Google Scholar]
- 7.Tuomainen U, Candolin U. 2011. Behavioural responses to human-induced environmental change. Biol. Rev. 86, 640-657. ( 10.1111/j.1469-185X.2010.00164.x) [DOI] [PubMed] [Google Scholar]
- 8.Sih A, Ferrari MCO, Harris DJ. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367-387. ( 10.1111/j.1752-4571.2010.00166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.di Giovanni J, Fawcett TW, Templeton CN, Raghav S, Boogert NJ. 2022. Urban gulls show similar thermographic and behavioral responses to human shouting and conspecific alarm calls. Front. Ecol. Evol. 10, 891985. ( 10.3389/fevo.2022.891985) [DOI] [Google Scholar]
- 10.Soulsbury CD, White PCL. 2015. Human–wildlife interactions in urban areas: a review of conflicts, benefits and opportunities. Wildl. Res. 42, 541. ( 10.1071/WR14229) [DOI] [Google Scholar]
- 11.Rock P. 2005. Urban gulls. Br. Birds 98, 338-355. [Google Scholar]
- 12.Spelt A, Williamson C, Shamoun-Baranes J, Shepard E, Rock P, Windsor S. 2019. Habitat use of urban-nesting lesser black-backed gulls during the breeding season. Sci. Rep. 9, 10527. ( 10.1038/s41598-019-46890-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Méndez A, Montalvo T, Aymí R, Carmona M, Figuerola J, Navarro J. 2020. Adapting to urban ecosystems: unravelling the foraging ecology of an opportunistic predator living in cities. Urban Ecosyst. 23, 1117-1126. ( 10.1007/s11252-020-00995-3) [DOI] [Google Scholar]
- 14.Washburn BE, Bernhardt GE, Kutschbach-Brohl L, Chipman RB, Francoeur LC. 2013. Foraging ecology of four gull species at a coastal–urban interface. Condor 115, 67-76. ( 10.1525/cond.2013.110185) [DOI] [Google Scholar]
- 15.Schwartz ALW, Williams HF, Chadwick E, Thomas RJ, Perkins SE. 2018. Roadkill scavenging behaviour in an urban environment. J. Urban Ecol. 4, juy006. ( 10.1093/jue/juy006) [DOI] [Google Scholar]
- 16.Belant JL. 1997. Gulls in urban environments: landscape-level management to reduce conflict. Landsc. Urban Plan. 38, 245-258. ( 10.1016/S0169-2046(97)00037-6) [DOI] [Google Scholar]
- 17.Belant JL, Ickes SK, Seamans TW. 1998. Importance of landfills to urban-nesting herring and ring-billed gulls. Landsc. Urban Plan. 43, 11-19. ( 10.1016/S0169-2046(98)00100-5) [DOI] [Google Scholar]
- 18.Oro D, Genovart M, Tavecchia G, Fowler MS, Martínez-Abraín A. 2013. Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 16, 1501-1514. ( 10.1111/ele.12187) [DOI] [PubMed] [Google Scholar]
- 19.Keller V, et al. 2020. European breeding bird atlas 2: distribution, abundance and change. Barcelona, Spain: European Bird Census Council & Lynx Edicions. [Google Scholar]
- 20.Carmona M, Aymí R, Navarro J. 2021. Importance of predictable anthropogenic food subsidies for an opportunistic gull inhabiting urban ecosystems. Eur. J. Wildl. Res. 67, 9. ( 10.1007/s10344-020-01446-2) [DOI] [Google Scholar]
- 21.Martín-Vélez V, Montalvo T, Afán I, Sánchez-Márquez A, Aymí R, Figuerola J, Lovas-Kiss Á, Navarro J. 2022. Gulls living in cities as overlooked seed dispersers within and outside urban environments. Sci. Total Environ. 823, 153535. ( 10.1016/j.scitotenv.2022.153535) [DOI] [PubMed] [Google Scholar]
- 22.Coccon F, Vanni L, Dabalà C, Giunchi D. 2022. The abundance of yellow-legged gulls Larus michahellis breeding in the historic centre of Venice, Italy and the initial effects of the new waste collection policy on the population. Urban Ecosyst. 25, 643-656. ( 10.1007/s11252-021-01175-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pais de Faria J, Paiva VH, Veríssimo S, Gonçalves AMM, Ramos JA. 2021. Seasonal variation in habitat use, daily routines and interactions with humans by urban-dwelling gulls. Urban Ecosyst. 24, 1101-1115. ( 10.1007/s11252-021-01101-x) [DOI] [Google Scholar]
- 24.Langley LP, Bearhop S, Burton NHK, Banks AN, Frayling T, Thaxter CB, Clewley GD, Scragg E, Votier SC. 2021. GPS tracking reveals landfill closures induce higher foraging effort and habitat switching in gulls. Mov. Ecol. 9, 56. ( 10.1186/s40462-021-00278-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zorrozua N, Aldalur A, Herrero A, Diaz B, Delgado S, Sanpera C, Jover L, Arizaga J. 2020. Breeding yellow-legged gulls increase consumption of terrestrial prey after landfill closure. Ibis 162, 50-62. ( 10.1111/ibi.12701) [DOI] [Google Scholar]
- 26.Wilhelm SI, Rail JF, Regular PM, Gjerdrum C, Robertson GJ. 2016. Large-scale changes in abundance of breeding herring gulls (Larus argentatus) and great black-backed gulls (Larus marinus) relative to reduced fishing activities in Southeastern Canada. Waterbirds 39, 136-142. ( 10.1675/063.039.sp104) [DOI] [Google Scholar]
- 27.Ponce-de-Leon M, et al. 2021. COVID-19 flow-maps an open geographic information system on COVID-19 and human mobility for Spain. Sci. Data 8, 310. ( 10.1038/s41597-021-01093-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santamaria C, Sermi F, Spyratos S, Iacus SM, Annunziato A, Tarchi D, Vespe M. 2020. Measuring the impact of COVID-19 confinement measures on human mobility using mobile positioning data: a European regional analysis. Saf. Sci. 132, 104925. ( 10.1016/j.ssci.2020.104925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz J, Ruiz M, Jara JA. 2021. Seismic monitoring of urban activity in Barcelona during the COVID-19 lockdown. Solid Earth 12, 725-739. ( 10.5194/se-12-725-2021) [DOI] [Google Scholar]
- 30.Soh MCK, Pang RYT, Ng BXK, Lee BPYH, Loo AHB, Er KBH. 2021. Restricted human activities shift the foraging strategies of feral pigeons (Columba livia) and three other commensal bird species. Biol. Conserv. 253, 108927. ( 10.1016/j.biocon.2020.108927) [DOI] [Google Scholar]
- 31.Rutz C, et al. 2020. COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nat. Ecol. Evol. 4, 1156-1159. ( 10.1038/s41559-020-1237-z) [DOI] [PubMed] [Google Scholar]
- 32.Bedoya-Pérez MA, Ward MP, Loomes M, McGregor IS, Crowther MS. 2021. The effect of COVID19 pandemic restrictions on an urban rodent population. Sci. Rep. 11, 12957. ( 10.1038/s41598-021-92301-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warrington MH, Schrimpf MB, des Brisay P, Taylor ME, Koper N. 2022. Avian behaviour changes in response to human activity during the COVID-19 lockdown in the United Kingdom. Proc. R. Soc. B 289, 20212740. ( 10.1098/rspb.2021.2740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilby BL, et al. 2021. Potentially negative ecological consequences of animal redistribution on beaches during COVID-19 lockdown. Biol. Conserv. 253, 108926. ( 10.1016/j.biocon.2020.108926) [DOI] [Google Scholar]
- 35.Gordo O, Brotons L, Herrando S, Gargallo G. 2021. Rapid behavioural response of urban birds to COVID-19 lockdown. Proc. R. Soc. B 288, 20202513. ( 10.1098/rspb.2020.2513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vidal E, Medail F, Tatoni T. 1998. Is the yellow-legged gull a superabundant bird species in the Mediterranean? Impact on fauna and flora, conservation measures and research priorities. Biodivers. Conserv. 7, 1013-1026. ( 10.1023/A:1008805030578) [DOI] [Google Scholar]
- 37.Bosch M, Oro D, Ruiz X. 1994. Dependence of yellow-legged gulls (Larus cachinnans) on food from human activity in two Western Mediterranean colonies. Avocetta 18, 135-139. [Google Scholar]
- 38.Ramos R, Ramírez F, Sanpera C, Jover L, Ruiz X. 2009. Feeding ecology of yellow-legged gulls Larus michahellis in the western Mediterranean: a comparative assessment using conventional and isotopic methods. Mar. Ecol. Prog. Ser. 377, 289-297. ( 10.3354/meps07792) [DOI] [Google Scholar]
- 39.Gimeno M, García JA, Afán I, Aymí R, Montalvo T, Navarro J. In press. Age-related differences in foraging behaviour at sea and interactions with fishing vessels in an opportunistic urban gull. ICES J. Mar. Sci. ( 10.1093/icesjms/fsac120) [DOI] [Google Scholar]
- 40.Coll M, Ortega-Cerdà M, Mascarell-Rocher Y. 2021. Ecological and economic effects of COVID-19 in marine fisheries from the Northwestern Mediterranean Sea. Biol. Conserv. 255, 108997. ( 10.1016/j.biocon.2021.108997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sala MM, et al. 2022. COVID-19 lockdown moderately increased oligotrophy at a marine coastal site. Sci. Total Environ. 812, 151443. ( 10.1016/j.scitotenv.2021.151443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anton M, Herrando S, García D, Ferrer X, Cebrian R. 2017. Atles dels ocells nidificants de Barcelona. Barcelona, Spain: Ajuntament de Barcelona / ICO / UB / Zoo. [Google Scholar]
- 43.Galimany E, Navarro J, Martino I, Aymí R, Cermeño P, Montalvo T, 2023. Gulls as potential sentinels for urban litter: combining nest and GPS-tracking information. Environ. Monit. Assess. 195, 521. ( 10.1007/s10661-023-11133-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Idescat. 2019. Anuari estadístic de Catalunya. Pesca. See https://www.idescat.cat/indicadors/?id=aec. [Google Scholar]
- 45.Blanco M, Nos D, Lombarte A, Recasens L, Company JB, Galimany E. 2022. Characterization of discards along a wide bathymetric range from a trawl fishery in the NW Mediterranean. Fish. Res. 258, 106552. ( 10.1016/j.fishres.2022.106552) [DOI] [Google Scholar]
- 46.Ramos R, Ramírez F, Sanpera C, Jover L, Ruiz X. 2008. Diet of yellow-legged gull (Larus michahellis) chicks along the Spanish Western Mediterranean coast: the relevance of refuse dumps. J. Ornithol. 150, 265-272. ( 10.1007/s10336-008-0346-2) [DOI] [Google Scholar]
- 47.Polito MJ, Trivelpiece WZ, Karnovsky NJ, Ng E, Patterson WP, Emslie SD. 2011. Integrating stomach content and stable isotope analyses to quantify the diets of pygoscelid penguins. PLoS ONE 6, e26642. ( 10.1371/journal.pone.0026642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrett RT, et al. 2007. Diet studies of seabirds: a review and recommendations. ICES J. Mar. Sci. 64, 1675-1691. ( 10.1093/icesjms/fsm152) [DOI] [Google Scholar]
- 49.Duffy DC, Jackson S. 1986. Diet studies of seabirds: a review of methods. Colonial Waterbirds 9, 1. ( 10.2307/1521138) [DOI] [Google Scholar]
- 50.Inger R, Bearhop S. 2008. Applications of stable isotope analyses to avian ecology. Ibis 150, 447-461. [Google Scholar]
- 51.Krouse HR, Herbert HK. 1988. Sulphur and carbon isotope studies of food webs. In Diet and subsistence: current archaeological perspectives (eds Kennedy BV, LeMoine GM), pp. 315-322. Calgary, Canada: University of Calgary Archaeology Association. [Google Scholar]
- 52.Cherel Y, Hobson KA, Bailleul F, Groscolas R. 2005. Nutrition, physiology, and stable isotopes: new information from fasting and molting penguins. Ecology 86, 2881-2888. [Google Scholar]
- 53.Beisel JN, Moreteau JC. 1997. A simple formula for calculating the lower limit of Shannon's diversity index. Ecol. Modell. 99, 289-292. ( 10.1016/S0304-3800(97)01954-6) [DOI] [Google Scholar]
- 54.Pruim R, Kaplan DT, Horton NJ. 2017. The mosaic package: helping students to think with data using R. R J. 9, 77. ( 10.32614/RJ-2017-024) [DOI] [Google Scholar]
- 55.Jackson A, Inger R, Parnell AC, Bearhop S. 2011. Comparing isotopic niche widths among and within communities: SIBER – stable isotope Bayesian ellipses in R. J. Anim. Ecol. 80, 595-602. ( 10.1111/j.1365-2656.2011.01806.x) [DOI] [PubMed] [Google Scholar]
- 56.Swanson HK, Lysy M, Power M, Stasko AD, Johnson JD, Reist JD. 2015. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 96, 318-324. ( 10.1890/14-0235.1) [DOI] [PubMed] [Google Scholar]
- 57.Anderson M, Gorley R, Clarke K. 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth, UK: PRIMER-E Ltd. [Google Scholar]
- 58.Bartumeus F, Giuggioli L, Louzao M, Bretagnolle V, Oro D, Levin SA. 2010. Fishery discards impact on seabird movement patterns at regional scales. Curr. Biol. 20, 215-222. ( 10.1016/j.cub.2009.11.073) [DOI] [PubMed] [Google Scholar]
- 59.Arcos JM, Oro D. 2002. Significance of nocturnal purse seine fisheries for seabirds: a case study off the Ebro Delta (NW Mediterranean). Mar. Biol. 141, 277-286. ( 10.1007/s00227-002-0828-3) [DOI] [Google Scholar]
- 60.Ruiz J, Louzao M, Oyarzabal I, Arregi L, Mugerza E, Uriarte A. 2021. The Spanish purse-seine fishery targeting small pelagic species in the Bay of Biscay: landings, discards and interactions with protected species. Fish. Res. 239, 105951. ( 10.1016/j.fishres.2021.105951) [DOI] [Google Scholar]
- 61.Votier SC, et al. 2004. Changes in fisheries discard rates and seabird communities. Nature 427, 727-730. ( 10.1038/nature02315) [DOI] [PubMed] [Google Scholar]
- 62.Bicknell AWJ, Oro D, Camphuysen KCJ, Votier SC. 2013. Potential consequences of discard reform for seabird communities. J. Appl. Ecol. 50, 649-658. ( 10.1111/1365-2664.12072) [DOI] [Google Scholar]
- 63.Froese R, Pauly D. 2023. Fishbase. World Wide Web electronic publication. See https://www.scienceopen.com/document?vid=dc419213-0ca3-48cc-901c-2934ecf4441e. [Google Scholar]
- 64.China V, Zvuloni A, Roll U, Belmaker J. 2021. Reduced human activity in shallow reefs during the COVID-19 pandemic increases fish evenness. Biol. Conserv. 257, 109103. ( 10.1016/j.biocon.2021.109103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patterson Edward JK, et al. 2021. COVID-19 lockdown improved the health of coastal environment and enhanced the population of reef-fish. Mar. Pollut. Bull. 165, 112124. ( 10.1016/j.marpolbul.2021.112124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hobson KA, Piatt JF, Pitocchelli J. 1994. Using stable isotopes to determine seabird trophic relationships. J. Anim. Ecol. 63, 786-798. [Google Scholar]
- 67.Bugoni L, McGill RAR, Furness RW. 2010. The importance of pelagic longline fishery discards for a seabird community determined through stable isotope analysis. J. Exp. Mar. Biol. Ecol. 391, 190-200. ( 10.1016/j.jembe.2010.06.027) [DOI] [Google Scholar]
- 68.Navarro J, Oro D, Bertolero A, Genovart M, Delgado A, Forero MG. 2010. Age and sexual differences in the exploitation of two anthropogenic food resources for an opportunistic seabird. Mar. Biol. 157, 2453-2459. ( 10.1007/s00227-010-1509-2) [DOI] [Google Scholar]
- 69.García-Martínez-de-Albéniz Í, Ruiz-de-Villa JA, Rodriguez-Hernandez J. 2022. Impact of COVID-19 lockdown on wildlife-vehicle collisions in NW of Spain. Sustainability 14, 4849. ( 10.3390/su14084849) [DOI] [Google Scholar]
- 70.Bíl M, et al. 2021. COVID-19 related travel restrictions prevented numerous wildlife deaths on roads: a comparative analysis of results from 11 countries. Biol. Conserv. 256, 109076. ( 10.1016/j.biocon.2021.109076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones DN, James Reynolds S. 2008. Feeding birds in our towns and cities: a global research opportunity. J. Avian Biol. 39, 265-271. ( 10.1111/j.0908-8857.2008.04271.x) [DOI] [Google Scholar]
- 72.Senar JC, Montalvo T, Pascual J, Peracho V. 2017. Reducing the availability of food to control feral pigeons: changes in population size and composition. Pest Manag. Sci. 73, 313-317. ( 10.1002/ps.4272) [DOI] [PubMed] [Google Scholar]
- 73.Scharf I, Ovadia O, Foitzik S. 2012. The advantage of alternative tactics of prey and predators depends on the spatial pattern of prey and social interactions among predators. Popul. Ecol. 54, 187-196. ( 10.1007/s10144-011-0286-1) [DOI] [Google Scholar]
- 74.Ouled-Cheikh J, Morera-Pujol V, Bahillo Á, Ramírez F, Cerdà-Cuéllar M, Ramos R. 2021. Foraging in the Anthropocene: feeding plasticity of an opportunistic predator revealed by long term monitoring. Ecol. Indic. 129, 107943. ( 10.1016/j.ecolind.2021.107943) [DOI] [Google Scholar]
- 75.Vázquez B, Esperón F, Neves E, López J, Ballesteros C, Muñoz M. 2010. Screening for several potential pathogens in feral pigeons (Columba livia) in Madrid. Acta Vet. Scand. 52, 45. ( 10.1186/1751-0147-52-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borray-Escalante NA, Mazzoni D, Ortega-Segalerva A, Arroyo L, Morera-Pujol V, González-Solís J, Senar JC. 2020. Diet assessments as a tool to control invasive species: comparison between monk and rose-ringed parakeets with stable isotopes. J. Urban Ecol. 6, juaa005. ( 10.1093/jue/juaa005) [DOI] [Google Scholar]
- 77.Migura-Garcia L, Ramos R, Cerdà-Cuéllar M. 2017. Antimicrobial resistance of Salmonella Serovars and Campylobacter spp. Isolated from an opportunistic gull species, yellow-legged gull (Larus michahellis). J. Wildl. Dis. 53, 148-152. ( 10.7589/2016-03-051) [DOI] [PubMed] [Google Scholar]
- 78.Navarro J, Grémillet D, Afán I, Miranda F, Bouten W, Forero MG, Figuerola J. 2019. Pathogen transmission risk by opportunistic gulls moving across human landscapes. Sci. Rep. 9, 10659. ( 10.1038/s41598-019-46326-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vergara A, et al. 2017. Prevalence of extended-spectrum-β-lactamase- and/or carbapenemase-producing Escherichia coli isolated from yellow-legged gulls from Barcelona, Spain. Antimicrob. Agents Chemother. 61, e02071-16. ( 10.1128/AAC.02071-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petta JC, Shipley ON, Wintner SP, Cliff G, Dicken ML, Hussey NE. 2020. Are you really what you eat? Stomach content analysis and stable isotope ratios do not uniformly estimate dietary niche characteristics in three marine predators. Oecologia 192, 1111-1126. ( 10.1007/s00442-020-04628-6) [DOI] [PubMed] [Google Scholar]
- 81.Vez-Garzón M, Giménez J, Sánchez-Márquez A, Montalvo T, Navarro J. 2023. Changes in the feeding ecology of an opportunistic predator inhabiting urban environments in response to COVID-19 lockdown. Figshare. ( 10.6084/m9.figshare.c.6534041) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw stable isotope data is provided in the electronic supplementary material.
The data are provided in the electronic supplementary material [81].