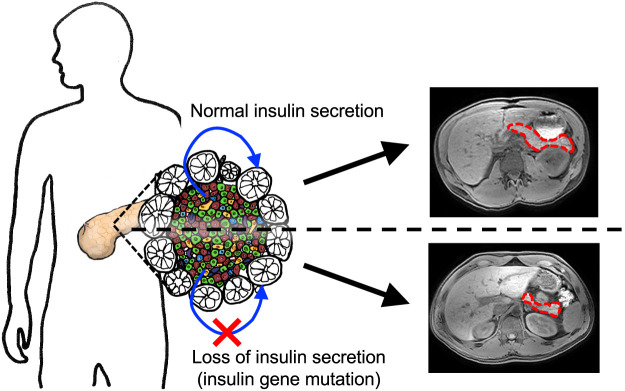
Abstract
OBJECTIVE
To determine the mechanism of reduced pancreas size in type 1 diabetes and the significance of islet-derived insulin in pancreatic growth.
RESEARCH DESIGN AND METHODS
Using a validated and standardized MRI protocol, we measured pancreas volume and shape in a family with an autosomal-dominant insulin gene mutation that results in insulin deficiency similar in severity to that of type 1 diabetes but without autoimmunity. DNA sequencing confirmed the mutation in all four affected individuals and none of the four control family members. Insulin secretory capacity was determined by measuring postprandial urinary C-peptide.
RESULTS
Family members with this form of monogenic diabetes had a markedly smaller pancreas and a severely impaired postprandial C-peptide level than family members without diabetes.
CONCLUSIONS
These results suggest that severe insulin deficiency, rather than islet-directed autoimmunity, leads to reduced pancreas size in type 1 diabetes and that insulin is a major trophic factor for the exocrine pancreas.
Graphical Abstract
Introduction
Individuals with new-onset type 1 diabetes have a smaller pancreas than age- and weight-matched control individuals, with pancreas size continuing to decline in the first years after diagnosis (1,2), likely because of a reduction in pancreatic acinar cell number (3). Whether the insulin deficiency that defines type 1 diabetes or the underlying autoimmunity that drives β-cell loss is responsible for the smaller pancreas and reduced acinar cell number in type 1 diabetes is not known. To investigate the role of islet-derived insulin in determining pancreas size, we quantified pancreas volume by MRI in a family with an INS mutation that causes severe insulin deficiency without autoimmunity.
Research Design and Methods
Pancreas Imaging and Analysis
Informed consent was obtained and documented in accordance with the institutional review boards at Vanderbilt University Medical Center and The University of Chicago. Participants underwent pancreas imaging using a standardized and validated MRI protocol developed as part of the MAP-T1D (Multicenter Assessment of the Pancreas in Type 1 Diabetes) program (4) as previously described (5). The pancreas volume (mL) was divided by the individual’s weight (kg) to yield the pancreas volume index (PVI). Pancreas fat was quantified using a multi-echo Dixon sequence. Image analysis and three-dimensional reconstruction were performed in MATLAB (R2021A; MathWorks, Natick, MA).
Genetic Analysis
Saliva samples were collected using the Oragene self-collection kit (DNA Genotek, Inc., Ottawa, Ontario, Canada) to isolate genomic DNA. Sanger sequencing and PCR amplification were performed for the INS gene with exon 2 forward (5′ CTG CCT GTC TCC CAG ATC AC 3′) and reverse (5′ CCA GGT CAC CCA GGA CTT TA 3′) primers to confirm the presence of the c.94G>C (p.Gly32Arg) variant in all family members.
Insulin Secretory Capacity
Two hours after emptying their bladder and consuming a large mixed meal, participants collected a urine sample using a self-collection kit. Urine C-peptide was measured by the Roche Elecsys C-peptide electrochemiluminesence immunoassay on the cobas e 801 analyzer. Urine creatinine was measured by the Roche Elecsys creatinine plus (version 2) enzymatic assay on the cobas e 702 analyzer. C-peptide and creatinine concentrations were converted to appropriate units for calculation of urinary C-peptide–to–creatinine ratio (UCPCR) (nmol/mmol), as previously reported (6).
Results
Study Participants
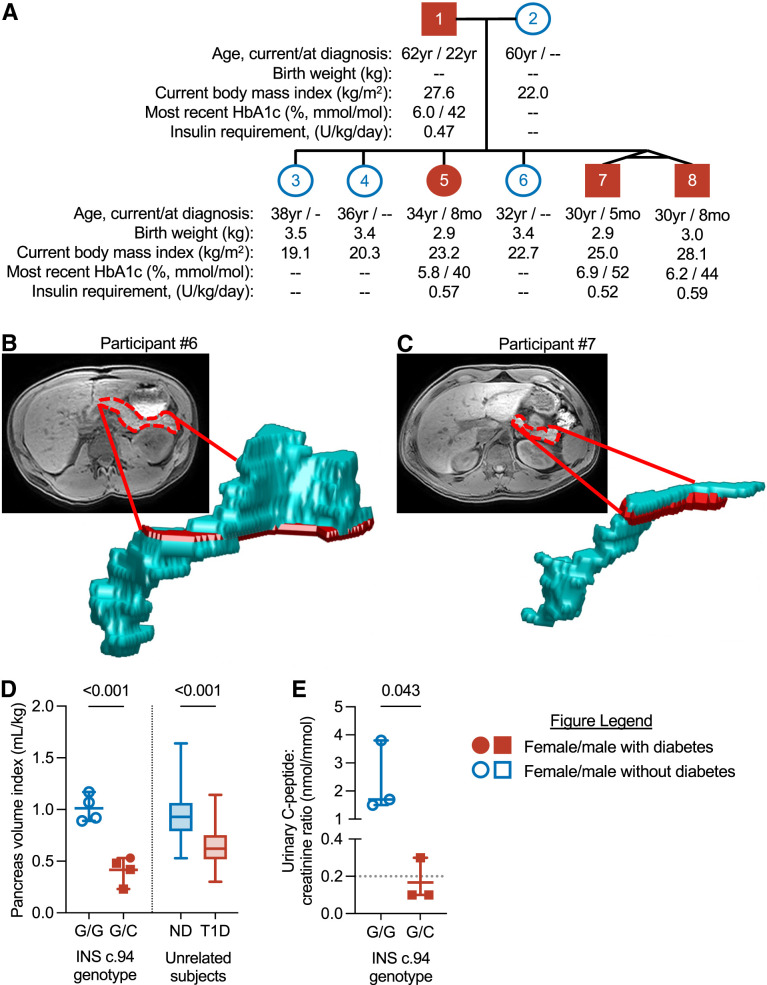
The family, carrying a heterozygous c.94G>C (p.Gly32Arg) INS mutation, was identified as part of the Monogenic Diabetes Registry at The University of Chicago (7). The father, now age 62 years, was diagnosed with insulin-dependent diabetes at age 22 years. Three of six children, now ages 30–38 years, were diagnosed with insulin-requiring and ketosis-prone diabetes before age 8 months (Fig. 1A), as previously reported (7). All children were born at or near term with normal birth weight. Affected family members all have characteristics of insulin-deficient diabetes, including lean body habitus and relatively low daily insulin requirements, and are being treated similarly to individuals with type 1 diabetes (e.g., insulin pumps, glucose sensors). All participants with diabetes currently have an HbA1c below the American Diabetes Association–recommended target of 7%. They have no clinical evidence of exocrine insufficiency or autoimmune disease. All family members, including the mother and the three children without diabetes, are otherwise healthy.
Figure 1.
Individuals with monogenic diabetes resulting from insulin gene mutation have a smaller pancreas and reduced urinary C-peptide. A: Pedigree showing current age, age at diagnosis, birth weight, BMI, HbA1c, and insulin requirement of the study participants. B and C: Representative MRI images with pancreas outlined in red and three-dimensional reconstruction of pancreas in a participant without (participant 6) (B) and with (participant 7) (C) diabetes. D: Individual PVIs of family members without diabetes (INS c.94 G/G) or with monogenic diabetes (INS c.94 G/C) compared with unrelated individuals with no diabetes (ND) (n = 57) or type 1 diabetes (T1D) (n = 54), as previously reported (1). E: Postprandial UCPCRs of indicated participants; two family members (participants 4 and 5) were unavailable to complete urinary C-peptide measurement. Dotted line represents the published cutoff value for T1D (5). Error bars show mean and range. Indicated P values were by Student t test.
Pancreas Shape, Pancreas Volume, and UCPCR
The family members with diabetes had a narrower pancreas (Fig. 1B and C) and a markedly smaller PVI compared with those of family members without diabetes (0.42 mL/kg; 95% CI 0.21, 0.62 vs. 1.01; 95% CI 0.8, 1.22) (Fig. 1D). The PVI of family members with diabetes was similar to that seen in previously studied cohorts (1) of individuals with type 1 diabetes (n = 54; mean PVI 0.65; 95% CI 0.6, 0.7). The PVI of family members without diabetes was similar to that of unrelated control individuals (1) (n = 57; mean PVI 0.97; 95% CI 0.9, 1.03). The alteration in pancreas shape was similar to that seen in type 1 diabetes; no changes in pancreas fat were seen (P = 0.78), and the pancreatic border seemed unaffected. Family members with diabetes had marked insulin deficiency, as measured by a 2-h postprandial UCPCR, whereas unaffected individuals had a normal UCPCR (Fig. 1E). Both twin sons with diabetes diagnosed in infancy who had postprandial C-peptide measured (participants 7 and 8) had severe insulin deficiency (UCPCR ≤0.1 nmol/mmol) similar to reported levels in type 1 diabetes (6). The father, who was diagnosed with diabetes as a young adult, had low, but detectable, insulin levels (UCPCR 0.3 nmol/mmol). The sample size was insufficient to determine correlation between PVI and UCPCR.
Conclusions
Our findings in this unique family demonstrate that individuals with an INS mutation causing severe insulin deficiency without autoimmunity have a smaller pancreas than family members without diabetes. These findings support the hypothesis that insulin deficiency is principally responsible for the reduction in exocrine compartment size seen in type 1 diabetes, although we cannot exclude an additional effect of the autoimmune process in type 1 diabetes on pancreatic size.
Studying this family with the same mutation and unique features, including having monozygotic twins and later onset of diabetes in the father, helps isolate insulin deficiency from other variables that may regulate pancreas size. The reduced pancreas size in the offspring and the father, who was diagnosed in adulthood (presumably because of mosaic expression of the mutation that became complete when passed on to the next generation), suggests that adult pancreas size depends mostly on postnatal insulin production. The birth timing and weights of the individuals with and without insulin deficiency were similar and were not compared directly because of differences in gestational age and the presence of twins; none had growth restriction that would be reflective of significant intrauterine insulin deficiency. The study of a single family may limit the generalizability of our findings. Such studies in families with other INS mutations or types of monogenic diabetes are needed to clarify the role of insulin signaling in determining pancreas size and broaden the applicability of these findings.
These results strongly suggest that an intraislet product is trophic for acinar cells and influences exocrine pancreas size. Whether an islet-derived product, like insulin, influences the surrounding exocrine tissue through vascular (8) or paracrine mechanisms is not clear, although a local effect could lead to diminished exocrine pancreas size in type 1 diabetes before obvious systemic insulin insufficiency leading to elevated glucose. In the nondiabetic state, exocrine pancreatic tissue is thought to be exposed to local insulin concentrations that are much higher than plasma concentrations (9). Therefore, although exogenous insulin therapy can normalize peripheral glucose, it does not normalize pancreatic tissue exposure to insulin. Whether the effect of insulin on pancreas volume is mediated by binding to the insulin receptor or the IGF-I receptor, both of which are expressed in human acinar cells (10,11), is unknown.
This study of a unique family answers a fundamental question regarding the effect of insulin on pancreas growth and suggests that the mechanism for reduced pancreas size in type 1 diabetes is likely insulin deficiency.
Article Information
Acknowledgments. The authors thank the family involved in this study for their participation in The University of Chicago Monogenic Diabetes Registry (https://monogenicdiabetes.uchicago.edu) and for allowing their story to be part of the discovery process. The authors thank Dr. John A. Williams at the University of Michigan and our colleagues in the MAP-T1D program, especially Dr. Andrea Steck (University of Colorado School of Medicine, Denver, CO) and Dr. Thomas Kay (St. Vincent’s Institute of Medical Research, Melbourne, Australia), for their valuable discussion and input.
Funding. These studies were performed with assistance from the Vanderbilt University Institute of Imaging Sciences (National Institutes of Health [NIH] project 1S10OD021771-01), the Vanderbilt Institute for Clinical and Translational Research (UL1-TR000445), and the Institute for Translational Medicine (UL1-TR000430) and with the support of The Leona M. and Harry B. Helmsley Charitable Trust, the Juvenile Diabetes Research Foundation, the Doris Duke Charitable Foundation, the NIH (DK104942, DK129979), and the Vanderbilt and Chicago Diabetes Research and Training Centers (DK20593, DK20595).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.J.W. wrote the original manuscript draft. J.J.W., J.M.W., L.P., S.A.W.G., A.C.P., J.V., and D.J.M. reviewed and edited the manuscript. J.J.W., S.A.W.G., A.C.P., J.V., and D.J.M. were responsible for study conceptualization. J.J.W. and J.V. performed formal analyses. J.M.W., L.R.L.-F., B.K., D.R., A.G.K., and M.A.H. performed investigations. L.P. and S.A.W.G. provided resources. S.A.W.G. and J.V. were responsible for methodology. A.C.P., J.V., and D.J.M. acquired funding. J.V. and D.J.M. curated data. All authors provided final review and approval of the manuscript. J.V. and D.J.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Virostko J, Williams J, Hilmes M, et al. Pancreas volume declines during the first year after diagnosis of type 1 diabetes and exhibits altered diffusion at disease onset. Diabetes Care 2019;42:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campbell-Thompson ML, Filipp SL, Grajo JR, et al. Relative pancreas volume is reduced in first-degree relatives of patients with type 1 diabetes. Diabetes Care 2019;42:281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wright JJ, Saunders DC, Dai C, et al. Decreased pancreatic acinar cell number in type 1 diabetes. Diabetologia 2020;63:1418–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Multicenter Assessment of the Pancreas in Type 1 Diabetes . About MAP-T1D. Accessed 27 January 2023. Available from https://www.map-t1d.com
- 5. Virostko J, Craddock RC, Williams JM, et al. Development of a standardized MRI protocol for pancreas assessment in humans. PloS One 2021;16:e0256029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Besser RE, Shepherd MH, McDonald TJ, et al. Urinary C-peptide creatinine ratio is a practical outpatient tool for identifying hepatocyte nuclear factor 1-alpha/hepatocyte nuclear factor 4-alpha maturity-onset diabetes of the young from long-duration type 1 diabetes. Diabetes Care 2011;34:286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edghill EL, Flanagan SE, Patch A-M, et al.; Neonatal Diabetes International Collaborative Group . Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes 2008;57:1034–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dybala MP, Kuznetsov A, Motobu M, et al. Integrated pancreatic blood flow: bidirectional microcirculation between endocrine and exocrine pancreas. Diabetes 2020;69:1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakagawa A, Stagner JI, Samols E. In situ binding of islet hormones in the isolated perfused rat pancreas: evidence for local high concentrations of islet hormones via the islet-acinar axis. Diabetologia 1995;38:262–268 [DOI] [PubMed] [Google Scholar]
- 10. Karlsson M, Zhang C, Méar L, et al. A single-cell type transcriptomics map of human tissues. Sci Adv 2021;7:eabh2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tosti L, Hang Y, Debnath O, et al. Single-nucleus and in situ RNA-sequencing reveal cell topographies in the human pancreas. Gastroenterology 2021;160:1330–1344.e11 [DOI] [PubMed] [Google Scholar]