Abstract
OBJECTIVE
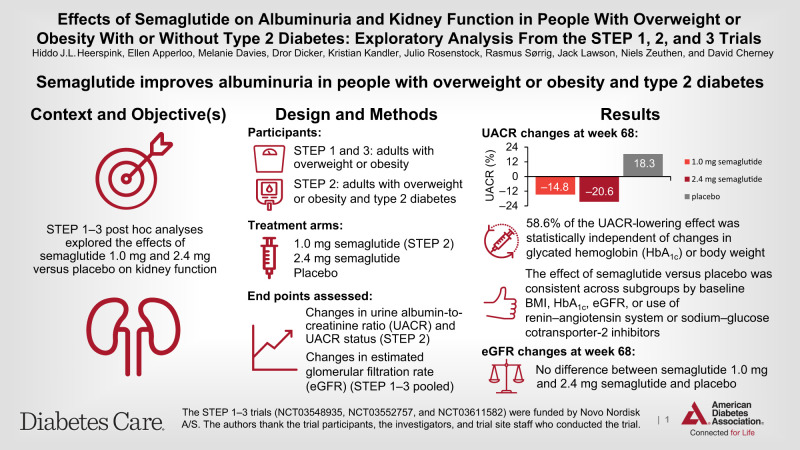
These post hoc analyses of the Semaglutide Treatment Effect in People with obesity (STEP) 1–3 trials (NCT03548935, NCT03552757, and NCT03611582) explored the effects of semaglutide (up to 2.4 mg) on kidney function.
RESEARCH DESIGN AND METHODS
STEP 1–3 included adults with overweight/obesity; STEP 2 patients also had type 2 diabetes. Participants received once-weekly subcutaneous semaglutide 1.0 mg (STEP 2 only), 2.4 mg, or placebo for 68 weeks, plus lifestyle intervention (STEP 1 and 2) or intensive behavioral therapy (STEP 3). Changes in urine albumin-to-creatinine ratio (UACR) and UACR status from baseline to week 68 were assessed for STEP 2. Changes in estimated glomerular filtration rate (eGFR) were assessed from pooled STEP 1–3 data.
RESULTS
In STEP 2, 1,205 (99.6% total cohort) patients had UACR data; geometric mean baseline UACR was 13.7, 12.5, and 13.2 mg/g with semaglutide 1.0 mg, 2.4 mg, and placebo, respectively. At week 68, UACR changes were −14.8% and −20.6% with semaglutide 1.0 mg and 2.4 mg, respectively, and +18.3% with placebo (between-group differences [95% CI] vs. placebo: −28.0% [−37.3, −17.3], P < 0.0001 for semaglutide 1.0 mg; −32.9% [−41.6, −23.0], P = 0.003 for semaglutide 2.4 mg). UACR status improved in greater proportions of patients with semaglutide 1.0 mg and 2.4 mg versus placebo (P = 0.0004 and P = 0.0014, respectively). In the pooled STEP 1–3 analyses, 3,379 participants had eGFR data; there was no difference between semaglutide 2.4 mg and placebo in eGFR trajectories at week 68.
CONCLUSIONS
Semaglutide improved UACR in adults with overweight/obesity and type 2 diabetes. In participants with normal kidney function, semaglutide did not have an effect on eGFR decline.
Graphical Abstract
Introduction
Clinical practice guidelines for patients with type 2 diabetes recommend lifestyle interventions, including increased physical activity and weight loss, glucose-lowering agents to optimize glycemic control, and pharmacological treatment with renin–angiotensin system (RAS) inhibitors and sodium–glucose cotransporter 2 (SGLT2) inhibitors to slow progression of kidney function loss and reduce the incidence of cardiovascular disease (CVD) events (1,2). Despite the fact that clinical trials have demonstrated the efficacy of these lifestyle and pharmacological interventions (3–10), residual cardiovascular (CV) and kidney risk remains present even with guideline-recommended treatment (8,10). Part of this high residual risk of kidney function decline is associated with the biomarker of persistently elevated levels of albuminuria (11). New treatment strategies that further reduce body weight, improve glycemic control, and directly or indirectly reduce albuminuria are therefore needed.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) are recommended by clinical practice guidelines for the treatment of type 2 diabetes and obesity to reduce CV risk (1,12). CV outcomes trials with GLP-1RAs, including semaglutide, have demonstrated that these agents reduce CV risk and slow kidney function decline. These benefits may be partially due to improvements in glycemic control but are likely also mediated by other effects, such as reductions in blood pressure, body weight, and albuminuria, beneficial effects on endothelial function, and inhibition of proinflammatory mediators (13).
The body weight–lowering effects of GLP-1RAs are clinically important as the prevalence of obesity continues to rise. In people with type 2 diabetes, once-weekly subcutaneous semaglutide doses of 1.0 and 2.0 mg reduce body weight (14). A higher dose (2.4 mg once weekly) has been approved for the treatment of overweight and obesity (15,16). The Semaglutide Treatment Effect in People with obesity (STEP) clinical trial program examined the effect of semaglutide 2.4 mg (administered subcutaneously once weekly) on body weight compared with placebo in people with overweight or obesity (17). The STEP 1, 2, 3, and 4 trials demonstrated that semaglutide 2.4 mg compared with placebo, as adjunct to lifestyle management or intensive behavioral therapy, led to mean weight losses of 6–15% in participants with and without type 2 diabetes (14,18–20). The effects of semaglutide 2.4 mg on albuminuria and estimated glomerular filtration rate (eGFR) are unknown, as are the potential benefits of semaglutide in patients using and not using SGLT2 inhibitors. It is also unknown whether the effects on albuminuria can be explained by concomitant changes in glycated hemoglobin (HbA1c), body weight, and blood pressure.
The aims of these post hoc analyses were to first explore the effect of semaglutide compared with placebo on albuminuria in patients with type 2 diabetes, as albuminuria was only measured in the STEP 2 trial, and second, to assess the effects of semaglutide on eGFR in a pooled analysis of the STEP 1–3 trials.
Research Design and Methods
Trial Designs
The current study is a post hoc analysis of the STEP 1–3 trials. The full methods, trial profile and patient flow, and primary results for the STEP 1–3 trials have previously been described (14,18,19). In brief, STEP 1–3 (NCT03548935, NCT03552757, and NCT03611582) were all phase 3a, double-blind, placebo-controlled, multicenter trials. In STEP 1 and 3, participants were randomized 2:1 to escalating doses of semaglutide up to 2.4 mg/week or placebo for 68 weeks as an adjunct to either lifestyle intervention (counseling on diet and physical activity; STEP 1) or an initial meal-replacement diet plus intensive behavioral therapy (low-calorie diet and intensive counseling on diet and physical activity followed by randomization to semaglutide or placebo to assess maintenance and/or further weight loss; STEP 3) (18,19). In STEP 2, participants were randomized 1:1:1 to 68 weeks of semaglutide 2.4 mg, 1.0 mg, or placebo, all plus lifestyle intervention (similar to that in STEP 1) (14). In all trials, semaglutide was initiated at 0.25 mg once weekly and escalated every 4 weeks until the target dose was achieved (2.4 mg over 16 weeks, or 1.0 mg over 8 weeks for participants assigned that dose in STEP 2). At the end of the 68-week double-blind treatment period, participants proceeded to a 7-week off-drug follow-up period in each trial. All three trials were conducted according to the International Conference on Harmonisation Good Clinical Practice Guideline and the Declaration of Helsinki; all participants provided written informed consent (14,18,19). STEP 4 was not included in this analysis because key differences in trial design, including its 20-week run-in period in which all participants received semaglutide, made it unsuitable to pool the results with the STEP 1–3 trials.
Participants
Male or female adults (≥18 years of age) with a stable body weight (≤5 kg weight change within 90 days before screening) and a history of at least one self-reported unsuccessful dietary effort to lose body weight were eligible for the STEP 1–3 trials (14,18,19). In addition, participants in STEP 1 and 3 were required to have either a BMI of ≥30 kg/m2 or ≥27 kg/m2 plus at least one weight-related comorbidity (excluding type 2 diabetes) (18,19). In STEP 2, participants were required to have a BMI of ≥27 kg/m2, a diagnosis of type 2 diabetes that was managed by diet and physical activity or ≤3 oral glucose-lowering therapies, and an HbA1c of 7–10% (53–86 mmol/mol) (14). Participants were excluded from the trials if they had an eGFR of <15 mL/min/1.73 m2 in STEP 1 and 3 (18,19) and of <30 mL/min/1.73 m2 (<60 mL/min/1.73 m2 in those receiving SGLT2 inhibitors) in STEP 2 (14).
Outcomes
The coprimary outcome in STEP 1–3 was the percent change from baseline in body weight (alongside the achievement of ≥5% weight loss) as reported previously (14,18,19). Changes in albuminuria and eGFR were assessed as exploratory post hoc outcomes (14,18,19). Albuminuria was expressed as urine albumin-to-creatinine ratio (UACR). Urinary albumin and creatinine were measured in a central laboratory at weeks 0, 20, and 68 in STEP 2 only; UACR was not measured in STEP 1 or 3. The albuminuria-related outcomes assessed from baseline to week 68 were: mean percent change in urinary albumin concentration, mean percent change in UACR in the overall population and in patient subgroups; proportions of patients with normoalbuminuria (UACR <30 mg/g), microalbuminuria (UACR ≥30 to ≤300 mg/g), and macroalbuminuria (UACR >300 mg/g); and proportions of patients whose UACR status improved or worsened. For the subgroup analyses of the change in UACR, patients were grouped according to the following variables: baseline UACR (<30 or ≥30 mg/g), baseline systolic blood pressure (<130 or ≥130 mmHg), baseline eGFR (<90 or ≥90 mL/min/1.73 m2), baseline HbA1c (<8.0 or ≥8.0%), baseline BMI (<35 or ≥35 kg/m2), SGLT2 inhibitor use (yes/no), and RAS inhibitor use (yes/no). For the analysis of change in UACR status, an improvement was defined as regression from microalbuminuria at baseline to normoalbuminuria, or macroalbuminuria at baseline to micro-/normoalbuminuria by week 68, while a worsening was defined as either progression from normoalbuminuria at baseline to micro-/macroalbuminuria, or microalbuminuria at baseline to macroalbuminuria by week 68. A mediation analysis was performed to investigate whether the effect of semaglutide in reducing UACR was mediated by concomitant changes in HbA1c, body weight, and blood pressure from baseline to week 68.
Serum creatinine was measured at screening and at weeks 20, 52, and 68 in STEP 1–3. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration 2009 creatinine equation (21). A sensitivity analysis was performed using the race-free Chronic Kidney Disease Epidemiology Collaboration 2021 creatinine equation (22). The change from baseline to week 68 in eGFR was assessed in STEP 1–3.
Statistical Analysis
Statistical analyses were based on the trial product estimand (the secondary estimand in the STEP trials), which assessed the treatment effect in all randomized participants when each drug was taken as intended. The analyses only included data from all randomized assigned participants until first treatment discontinuation or use of a rescue intervention (initiation of other antiobesity medications or bariatric surgery). Observed data from the on-treatment observation period (during treatment with trial product; any dose of trial medication administered within the previous 2 weeks [i.e., any period of temporary treatment interruption with trial product was excluded]) are reported for the following outcomes (STEP 2 only unless otherwise stated): urinary albumin by week, UACR by week, proportions of patients by UACR status, proportions of patients with an improvement or worsening in UACR status, and eGFR by week (STEP 1–3). In a sensitivity analysis, the effects of semaglutide versus placebo were analyzed according to the treatment policy estimand, which assessed the effect in all randomized participants irrespective of discontinuation of randomized treatment or use of rescue medication. The proportions of patients whose UACR status improved were compared by treatment using a Chi-square test.
The changes from baseline to week 68 in urinary albumin concentration (STEP 2), UACR (STEP 2), and eGFR (STEP 1–3) were assessed using a mixed model for repeated measurements with randomized treatment, randomization stratification groups (background type 2 diabetes medication and HbA1c; STEP 2 only), and the interaction between stratification groups (STEP 2 only) as factors and baseline value of the outcome measure of interest as a covariate, all nested within visit. For the subgroup analysis of the change in UACR, subgroup and the interaction between treatment and subgroup were also included in the model as factors. None of the analyses were adjusted for multiplicity.
The mediation analysis was performed using the medflex package in R (23). A natural effects model was fitted using an imputation-based procedure (24), allowing for decomposition of the treatment effect estimates into natural direct and indirect effect estimates. The percent mediated was then calculated as the natural indirect effect divided by the total treatment effect and the CI obtained using Fieller’s method (25).
Urinary albumin and UACR were analyzed on a log scale as estimated ratios to baseline (within treatment groups) and estimated treatment ratios (between treatment groups). For interpretation, data are expressed as relative percent changes and estimated relative percent differences between groups, respectively, calculated using the formula (estimated ratio − 1) × 100. P values <0.05 were considered to indicate statistical significance. Analyses were performed with R version 4.20/SAS version 9.4. No adjustment for multiple comparisons was made, and as such, all results should be considered exploratory.
Data and Resource Availability
Data will be shared with bona fide researchers who submit a research proposal approved by the independent review board. Individual patient data will be shared in data sets in a de-identified and anonymized format. Data will be made available after research completion and approval of the product and product use in the European Union and U.S. Information about data access request proposals can be found at https://www.novonordisk-trials.com.
Results
Participants
In STEP 2, the baseline characteristics were well balanced among the three treatment groups (Table 1). The geometric mean (coefficient of variation) for UACR at baseline was 13.2 (199.8) mg/g in the placebo group and 13.7 (249.6) mg/g and 12.5 (225.1) mg/g in the semaglutide 1.0 mg and 2.4 mg treatment groups, respectively. A total of 248 participants had UACR of ≥30 mg/g at baseline. In STEP 2, the mean ± SD for eGFR at baseline was 95.4 ± 18.1 and 96.3 ± 18.5 mL/min/1.73 m2 in the semaglutide 1.0 mg and 2.4 mg groups and 94.6 ± 19.3 mL/min/1.73 m2 in the placebo group.
Table 1.
Baseline demographics and clinical characteristics (STEP 2)
| Semaglutide 1.0 mg (n = 403) | Semaglutide 2.4 mg (n = 404) | Placebo (n = 403) | |
|---|---|---|---|
| Age, years, mean (SD) | 56 (10) | 55 (11) | 55 (11) |
| Sex | |||
| Male | 200 (49.6) | 181 (44.8) | 213 (52.9) |
| Female | 203 (50.4) | 223 (55.2) | 190 (47.1) |
| Race | |||
| American Indian or Alaska Native | 0 | 4 (1.0) | 2 (0.5) |
| Asian | 97 (24.1) | 112 (27.7) | 108 (26.8) |
| Black or African American | 28 (6.9) | 35 (8.7) | 37 (9.2) |
| White | 272 (67.5) | 237 (58.7) | 242 (60.0) |
| Other | 6 (1.5) | 16 (4.0) | 13 (3.2) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 1 (0.2) |
| Duration of diabetes, years [no.*], mean (SD) | 7.7 (5.9) | 8.2 (6.2) | 8.2 (6.2) [402] |
| HbA1c, mmol/mol, mean (SD) | 65.4 (8.5) | 65.3 (8.7) | 65.3 (9.0) |
| HbA1c, % | 8.1 (0.8) | 8.1 (0.8) | 8.1 (0.8) |
| Body weight, kg, mean (SD) | 99.0 (21.1) | 99.9 (22.5) | 100.5 (20.9) |
| BMI, kg/m2, mean (SD) | 35.3 (5.9) | 35.9 (6.4) | 35.9 (6.5) |
| eGFR, CKD-EPI, mL/min/1.73 m2 | |||
| Mean (SD) | 95.4 (18.1) | 96.3 (18.5) | 94.6 (19.3) |
| Distribution† [no.*] | [402] | [403] | [402] |
| ≥90 | 265 (65.9) | 270 (67.0) | 259 (64.4) |
| <90 | 137 (34.1) | 133 (33.0) | 143 (35.6) |
| UACR, mg/g† [no.*] | [402] | [403] | [402] |
| Geometric mean (CV) | 13.7 (249.6) | 12.5 (225.1) | 13.2 (199.8) |
| Normal albuminuria (UACR <30) | 306 (77.5) | 318 (80.5) | 317 (79.4) |
| Microalbuminuria (UACR ≥30 to <300) | 72 (18.2) | 64 (16.2) | 71 (17.8) |
| Macroalbuminuria (UACR ≥300) | 17 (4.3) | 13 (3.3) | 11 (2.8) |
| Blood pressure, mmHg, mean (SD) | |||
| Systolic blood pressure | 130 (14) | 130 (13) | 130 (13) |
| Diastolic blood pressure | 80 (9) | 80 (9) | 80 (9) |
| History of CV | 90 (22.3) | 58 (14.4) | 69 (17.1) |
| SGLT2 inhibitor use | 90 (22.3) | 95 (23.5) | 99 (24.6) |
| Agents acting on the RAS | 244 (60.5) | 216 (53.5) | 241 (59.8) |
Data are n (%) and are for the full analysis set unless otherwise indicated. n is number of patients. Novo Nordisk data published in Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021;397:971–984.
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CV, coefficient of variation.
[no.] is number of participants analyzed (where different from the number in the full analysis set).
Data are for the safety analysis set.
Baseline characteristics for STEP 1 and STEP 3 are presented in Supplementary Tables 1 and 2. The mean ± SD for eGFR at baseline in STEP 1 was 97.9 ± 16.9 mL/min/1.73 m2 for semaglutide 2.4 mg and 97.4 ± 16.7 mL/min/1.73 m2 for placebo. In STEP 3, the mean ± SD for eGFR at baseline was 98.6 ± 19.3 mL/min/1.73 m2 for semaglutide 2.4 mg and 98.5 ± 19.3 mL/min/1.73 m2 for placebo.
Effect of Semaglutide Versus Placebo on UACR (STEP 2)
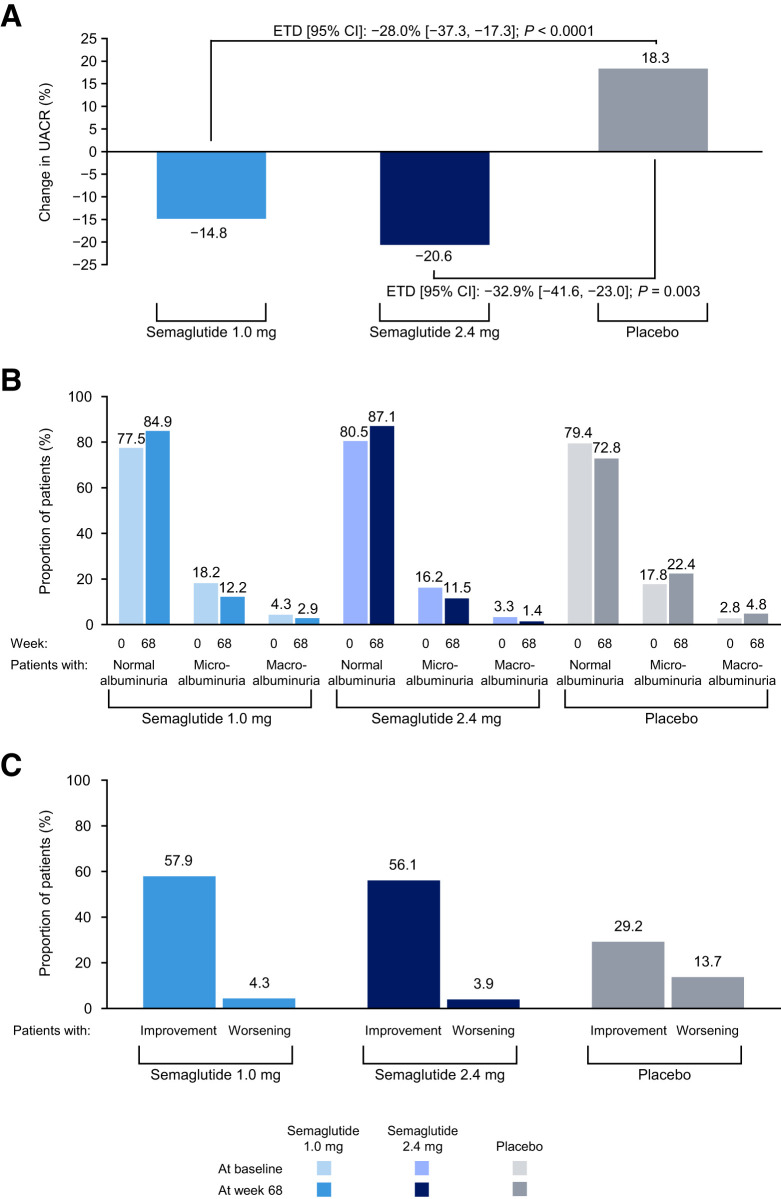
Figure 1A shows the albuminuria change from baseline to week 68, and Supplementary Fig. 1 shows the change over time in UACR by week. In the placebo group, UACR increased by 18.3%. In the semaglutide 1.0 mg and 2.4 mg dose groups dose groups, UACR changed from baseline by −14.8% and −20.6%, respectively, at week 68. Accordingly, mean percentage UACR difference compared with placebo at week 68 was −28.0% (95% CI −37.3, −17.3) (P < 0.0001) and −32.9% (95% CI −41.6, −23.0) (P = 0.003) in the semaglutide 1.0 mg and 2.4 mg groups, respectively. Changes in urinary albumin concentration from baseline to week 68 were −8.6% in the semaglutide 1.0 mg treatment group (estimated treatment difference, −22.6 [95% CI −34.1, −9.1]) and −12.6% in the semaglutide 2.4 mg treatment group (estimated treatment difference, −26.0 [95% CI −37.0, −13.1]) versus 18.1% in the placebo group. The change over time in urinary albumin by week is shown in Supplementary Fig. 2. Results were essentially similar when we repeated the analysis according to the treatment policy estimand. Mean percentage UACR difference compared with placebo at week 68 was −27.2% (95% CI −37.6, −15.0) (P < 0.0001) and −30.5% (95% CI −40.3, −19.1) (P < 0.0001) in the semaglutide 1.0 mg and 2.4 mg groups, respectively.
Figure 1.
A: Percent change in UACR from baseline to week 68. B: Distribution of patients by UACR status at baseline and week 68. Proportions are based on the number of patients in each UACR status category at the visit over the total number of patients with a UACR observation at the visit. The left-hand bar in each pair refers to baseline and the right-hand bar to week 68. C: Patients with changes in UACR status from baseline to week 68. Proportions are based on the number of patients with an improvement in UACR status from baseline to week 68 over the total number of patients with a UACR observation at both time points; a large proportion of patients were not included in this analysis because of missing data at baseline (n = 7 for semaglutide 1.0 mg, n = 8 for semaglutide 2.4 mg, and n = 3 for placebo) or week 68 (n = 57 for semaglutide 1.0 mg, n = 55 for semaglutide 2.4 mg, and n = 67 for placebo). Improvement is regression of baseline macroalbuminuria to microalbuminuria/normal or microalbuminuria to normal at week 68. Worsening is progression of baseline normal albuminuria to microalbuminuria/macroalbuminuria or microalbuminuria to macroalbuminuria at week 68. ETD, estimated treatment difference.
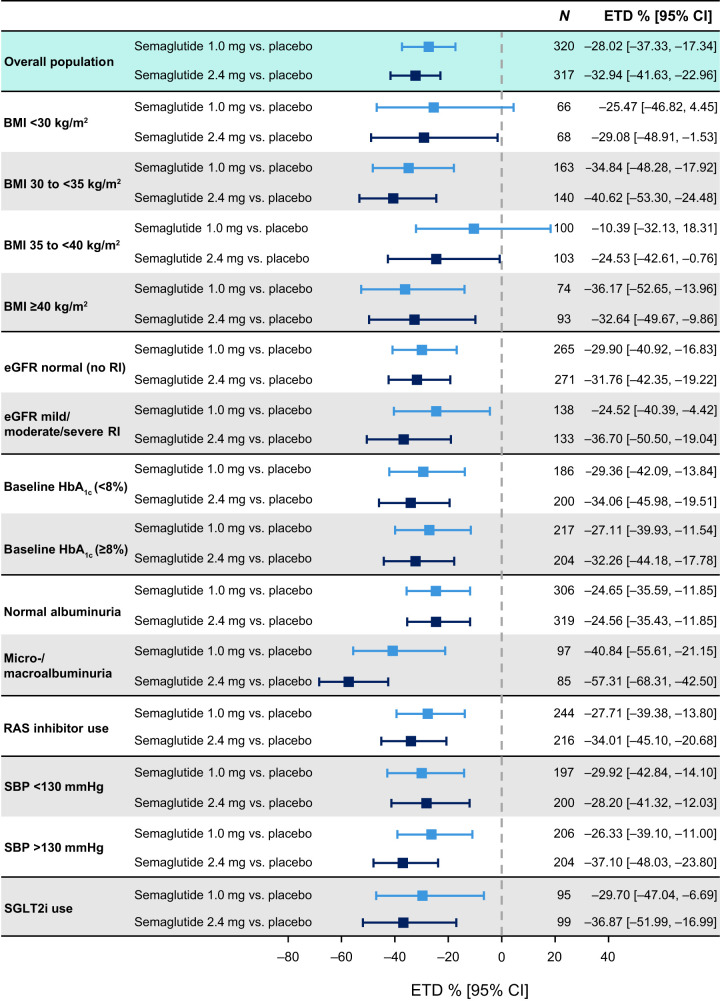
Estimated changes in UACR by subgroups of patients are shown in Fig. 2. The effect of semaglutide compared with placebo was consistent in subgroups by baseline BMI, HbA1c, eGFR, or use of RAS or SGLT2 inhibitors. The reduction in UACR with semaglutide 2.4 mg compared with placebo was more pronounced in patients with microalbuminuria or macroalbuminuria compared with patients with normoalbuminuria (P = 0.0009).
Figure 2.
Change in UACR by subgroup analyses. Data are for the trial product estimand (assesses treatment effect if all patients adhered to treatment without rescue intervention) unless otherwise stated. RI, renal impairment; SBP, systolic blood pressure; SGLT2i, SGLT2 inhibitor.
A higher proportion of patients on semaglutide 2.4 mg and semaglutide 1.0 mg had normoalbuminuria by week 68 compared with those receiving placebo (87.1% vs. 84.9% vs. 72.8%, respectively) (Fig. 1B). The percentage of patients with microalbuminuria and macroalbuminuria at week 68 was lower in the semaglutide 2.4 mg and 1.0 mg treatment groups compared with placebo (11.5% and 1.4% vs. 12.2%, and 2.9% vs. 22.4% and 4.8%, respectively).
The proportion of patients with a >30% reduction in UACR at week 68 in the semaglutide 1.0 mg and 2.4 mg groups was 37.4% (P = 0.0014 vs. placebo) and 38.0% (P = 0.0008 vs. placebo), respectively, compared with 25.9% in the placebo group. The corresponding proportions of patients who improved from macroalbuminuria or microalbuminuria categories to a lower category (microalbuminuria or normoalbuminuria) were 57.9% for semaglutide 1.0 mg (P = 0.0004) and 56.1% for semaglutide 2.4 mg (P = 0.0014) versus 29.2% for placebo (Fig. 1C). The proportions of patients who showed progression from normoalbuminuria or microalbuminuria to a higher category for semaglutide 1.0 mg and 2.4 mg were 4.3% and 3.9% (P < 0.0001 for both doses vs. placebo), respectively, versus 13.7% with placebo (Fig. 1C).
Effect of Semaglutide on UACR Explained by Changes in HbA1c, Body Weight, and Systolic Blood Pressure (STEP 2)
At week 68, patients receiving semaglutide 2.4 mg had placebo-corrected changes in HbA1c of −1.5% (95% CI −1.7, −1.4). The corresponding changes in body weight and systolic blood pressure were −7.6% (95% CI −8.6, −6.6) and −4.8 mmHg (95% CI −6.7, −2.9), respectively, for semaglutide 2.4 mg versus placebo (14). To assess the extent to which the effect of semaglutide on albuminuria could be explained by concomitant changes in HbA1c, body weight, or systolic blood pressure, the main analysis of change in UACR was repeated with adjustments for week 68 changes in these parameters (Supplementary Fig. 3). The effect of semaglutide 2.4 mg compared with placebo after adjustment for HbA1c, body weight, and systolic blood pressure was 58.6% (95% CI 26.5, 97.8), suggesting that more than half of the effect of semaglutide on UACR may be related to its HbA1c, body weight, and systolic blood pressure–lowering effects (Supplementary Fig. 3). Repeating the analysis for semaglutide 1.0 mg revealed similar results (Supplementary Fig. 4).
Effects on Overall eGFR Change (STEP 1–3)
To characterize the effect of semaglutide on kidney function, we pooled the data from the STEP 1–3 clinical trials, involving 3,379 participants with overweight or obesity with or without type 2 diabetes (data for the semaglutide 1.0 mg treatment arm from STEP 2 were not included in this analysis, but are included for baseline data). Of these participants, 1,262 were assigned to placebo and 2,117 to semaglutide 2.4 mg. Mean ± SD eGFR at baseline was 99.3 ± 17.0 mL/min/1.73 m2 for the total population, and eGFR was <60 mL/min/1.73 m2 in 85 (2.5%) participants. At week 68, no between-group differences in eGFR were observed. During the total 68 weeks of treatment, the rate of change in eGFR from baseline did not differ between the semaglutide (−0.70% [95% CI −1.17, −0.22]) and placebo group (−0.21% [95% CI −0.85, 0.42]; P = 0.2351) (Supplementary Fig. 5). Results were similar for semaglutide 1.0 mg treatment versus placebo in the STEP 2 trial (Supplementary Fig. 6). Results were also similar when the Chronic Kidney Disease Epidemiology Collaboration 2021 equation was used to estimate eGFR (Supplementary Fig. 7).
Safety
Frequency of adverse events potentially related to kidney function (i.e., renal and urinary disorders, acute kidney injury, and renal impairment) were similar across the STEP 1 and 2 trials and were relatively low. In STEP 3, there were no events of acute renal failure reported (Table 2). Overall, semaglutide was well tolerated with no increase in adverse events leading to study drug discontinuation. Nausea and vomiting occurred more frequently with semaglutide than placebo.
Table 2.
Kidney-related adverse events (STEP 1–3)
| STEP 1 | STEP 2 | STEP 3* | |||||
|---|---|---|---|---|---|---|---|
| Semaglutide 2.4 mg (n = 1,306) | Placebo (n = 655) | Semaglutide 1.0 mg (n = 402) | Semaglutide 2.4 mg (n = 403) | Placebo (n = 402) | Semaglutide 2.4 mg (n = 407) | Placebo (n = 204) | |
| Renal and urinary disorders | 3 (0.2) | 2 (0.3) | 2 (0.5) | 4 (1.0) | 2 (0.5) | 0 | 0 |
| Acute kidney injury | 3 (0.2) | 1 (0.2) | 2 (0.5) | 3 (0.7) | 2 (0.5) | 0 | 0 |
| Renal impairment | 1 (<0.1) | 1 (0.2) | 0 | 1 (0.2) | 0 | 0 | 0 |
Data are n (%) and are for the safety analysis set unless otherwise indicated. N is number of patients experiencing at least one event and % is percentage of patients experiencing at least one event. Adverse events with onset date during on-treatment period. A time point is considered as on-treatment if any dose of trial product has been administered within the prior 49 days. Sorted in descending order by System Organ Class and Preferred Term based, respectively, on the percentage of patients in the semaglutide 2.4 mg arm experiencing at least one event.
There were no events of acute renal failure identified by the Medical Dictionary for Regulatory Activities terminology search in either of the treatment groups.
Conclusions
In the STEP phase 3a trials, semaglutide has been shown to markedly reduce body weight and improve glycemic control in adults with overweight or obesity with or without type 2 diabetes (14,18–20). In these post hoc exploratory analyses from the STEP program, semaglutide significantly reduced albuminuria and increased the likelihood of improvement from a high to lower albuminuria category in the population with type 2 diabetes (14). Semaglutide did not affect the rate of change in eGFR over time in the pool of participants from the three trials, of whom the vast majority had normal kidney function.
In patients with type 2 diabetes who participated in the SUSTAIN 6 trial, semaglutide 1.0 mg reduced albuminuria and the risk of new-onset macroalbuminuria (26). This current analysis is the first from the STEP program to assess the effects of the semaglutide 2.4 mg dose on kidney outcomes. Numerically, a higher UACR reduction was observed with semaglutide 2.4 mg compared with the 1.0 mg dose, which seems to confirm the albuminuria-lowering effect of semaglutide. Moreover, both doses of semaglutide increased the probability of regression in albuminuria categories and reduced the likelihood of progression to worsening albuminuria categories. These effects are clinically relevant because therapies that reduce albuminuria by 25–30% are likely to confer significant benefits in reducing the risk of long-term clinical kidney outcomes (27,28). These analyses also report for the first time that the albuminuria-lowering effects of semaglutide were consistent in patients using or not using SGLT2 inhibitors. This is clinically relevant since SGLT2 inhibitors are now guideline-recommended therapies for kidney protection in people with type 2 diabetes, and guidelines suggest that semaglutide can be prescribed as an adjunct to RAS inhibitors and SGLT2 inhibitors to further reduce albuminuria (1,2).
The effects of semaglutide in reducing albuminuria in STEP 2 were also consistent in patients with various degrees of glycemic control and overweight or obesity, supporting the generalizability of our findings. The albuminuria-lowering effects of semaglutide are more pronounced in patients with micro- or macroalbuminuria at higher risk, which may translate into larger relative and absolute treatment effects on CV and kidney benefits, but this hypothesis requires confirmation in future studies.
The mechanism by which semaglutide reduces albuminuria may involve both direct intrarenal and indirect effects outside of the kidneys. The reductions in HbA1c, body weight, and blood pressure with semaglutide may account in part for lowering albuminuria. Of these clinical parameters, the reduction in HbA1c appears to explain the largest part of the albuminuria-lowering effect. However, some of the benefit in lowering albuminuria does not appear to be explained or accounted for by changes in these parameters alone. Semaglutide may also reduce albuminuria through direct means that may lead to beneficial effects on endothelial function or the endothelial glycocalyx, and increased natriuresis, either through inhibition of the sodium–hydrogen transporter or through anti-inflammatory or antifibrotic effects (13,29). Mechanistic studies are ongoing to study potential mechanisms for how semaglutide may confer kidney protection in patients with type 2 diabetes (REMODEL; NCT04865770) and in patients with overweight or obesity (SMART; NCT04889183).
Semaglutide 2.4 mg had no appreciable effect on eGFR over time compared with placebo. Since kidney function was in the normal range in the vast majority of participants at baseline, eGFR was only modestly decreased in the placebo group, and thus, it was not unexpected that there was no effect with semaglutide in slowing progressive kidney function loss, given the relatively short trial duration. The FLOW trial (NCT03819153) in patients with type 2 diabetes and established chronic kidney disease (CKD) will determine whether semaglutide 1.0 mg slows eGFR over time and reduces the risk of kidney failure (30). For the eGFR profile over time, treatment with semaglutide 2.4 mg reduced eGFR at 20 weeks. This initial reduction in eGFR may suggest a favorable hemodynamic effect on kidney function since eGFR did not further decline during the subsequent 48 weeks. However, a relatively large decrease in eGFR was also seen for placebo at week 20. An acute reduction in eGFR occurs with other classes of kidney-protective therapies, such as RAS inhibitors and SGLT2 inhibitors, and has been associated with long-term kidney protection (6,11,31,32). Dedicated mechanistic studies, such as the REMODEL and SMART studies, are required to confirm if the observed initial reduction in eGFR with semaglutide reflects a salutary hemodynamic effect or can be explained by other factors.
The effects of semaglutide on albuminuria and eGFR observed in the present STEP analysis are consistent with post hoc analyses of other GLP-1RA clinical trials. Dulaglutide and efpeglenatide reduced albuminuria and the risk of a composite kidney end point in two CV outcomes trials involving patients with type 2 diabetes at early stages of CKD (33,34). Dulaglutide, compared with insulin glargine, also reduced albuminuria and slowed the decline in kidney function in patients with type 2 diabetes and CKD (35). A post hoc analysis of the SUSTAIN 1–7 trials reported that semaglutide was associated with initial reductions in eGFR that plateaued during chronic treatment (26). Similarly, in a post hoc analysis of the SUSTAIN-6 and LEADER trials, treatment with semaglutide or liraglutide slowed eGFR decline and reduced the risk of substantial loss of kidney function in patients with type 2 diabetes, with greater effects among those with preexisting CKD (36). More pronounced effects of GLP-1RAs in slowing kidney function decline among patients with type 2 diabetes and established CKD were also observed in the EXSCEL trial (37). This pattern of effect may potentially explain why an effect was not observed with semaglutide on eGFR in the pooled STEP 1–3 analysis, since the vast majority of participants did not have CKD or were in very early stages of CKD.
These exploratory analyses have limitations. First, the study was a post hoc analysis of randomized controlled trials. Our results are therefore hypothesis generating. Second, we recognize that by design, the STEP 2 trial was not a dedicated kidney outcome trial, and relatively few participants had established CKD. Accordingly, since many participants had baseline clinical kidney parameters in the normal range, effects on albuminuria cannot be directly generalized to people with more advanced stages of CKD. Third, there is considerable uncertainty around the estimates of the proportion of the albuminuria-lowering effect of semaglutide that is mediated by changes in HbA1c, body weight, and blood pressure. Furthermore, the mediation analysis requires an assumption that no other variables confound either the effect of semaglutide on albuminuria, HbA1c, or body weight, or the associations among these. As a result, the results of the mediation analysis should be interpreted with caution. Fourth, albuminuria was not measured in the STEP 1 or STEP 3 trials, and we are therefore unable to generalize the albuminuria-lowering effects of semaglutide to people with overweight or obesity but without diabetes. Finally, semaglutide reduces body weight, which may affect serum creatinine and eGFR. Additional studies measuring cystatin (38,39), an alternative filtration marker less influenced by changes in body weight, would provide more insight about the effects of semaglutide on eGFR over time and to what extent serum creatinine–based eGFR estimates are influenced by body weight changes.
In conclusion, in people with type 2 diabetes and overweight or obesity, semaglutide reduced UACR relative to placebo, including in patients using RAS inhibitors or SGLT2 inhibitors at baseline. These results support ongoing trials examining the impact of semaglutide on kidney protection in individuals at high risk of kidney disease progression.
Article Information
Acknowledgments. The authors thank the trial participants, investigators, and site staff who conducted the trial. The authors also thank Thomas Idorn (Medical & Science, Novo Nordisk A/S, Søborg, Denmark) for important discussions on the scientific rationale for this post hoc analysis.
Funding. Medical writing support was provided by Casey McKeown of Axis, a division of Spirit Medical Communications Group Limited, and funded by Novo Nordisk A/S, in accordance with Good Publication Practice 3 guidelines (www.ismpp.org/gpp3). The STEP 1, 2, and 3 trials (NCT03548935, NCT03552757, and NCT03611582) were funded by Novo Nordisk A/S.
Duality of Interest. H.J.L.H. is a consultant for AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook Therapeutics, CSL Pharma, Gilead Sciences, Inc., Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novo Nordisk, and Travere Therapeutics; and has received research support for conducting investigator-initiated clinical trials from AbbVie, AstraZeneca, Boehringer Ingelheim, Janssen, and Novo Nordisk. M.D. received research funding from AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk, and Sanofi-Aventis; served as consultant, advisory board member, and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi-Aventis; served as advisory board member and speaker for AstraZeneca; is an advisory board member for Gilead Sciences, Inc., Janssen, and Lexicon Pharmaceuticals; and is a speaker for Napp Pharmaceuticals and Takeda Pharmaceuticals International Inc., cofunded by the NIHR Leicester Biomedical Research Centre. D.D. received research funding from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi-Aventis; and served as consultant, advisory board member, and speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi-Aventis. K.K. and J.L. are employees of Novo Nordisk A/S. J.R. received scientific advisory board fees, honoraria, consulting fees, and grants/research support from Novo Nordisk, Applied Therapeutics, Boehringer Ingelheim, Eli Lilly, Intarcia Therapeutics, Novo Nordisk, Oramed Pharmaceuticals, and Sanofi; received honoraria or consulting fees from Zealand Pharma; and received grants/research support from Genentech, Novartis, Pfizer, REMD Biotherapeutics, and vTv Therapeutics. R.S. and N.Z. are employees and shareholders of Novo Nordisk A/S. D.C. has received honoraria from AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim-Lilly, Bristol-Myers Squibb, CSL-Behring, Gilead Sciences, Inc., Janssen, Lexicon Pharmaceuticals, Maze Therapeutics, Merck, Mitsubishi Tanabe, Novartis, Novo Nordisk, Otsuka, Prometic Pharma, Sanofi, and Youngene Therapeutics and has received operational funding for clinical trials from AstraZeneca, Boehringer Ingelheim-Lilly, CSL-Behring, Janssen, Merck, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. H.J.L.H. was involved in the design of this post hoc analysis, provided input on the interpretation of the results, and wrote the first draft of the article. E.A. provided input on the interpretation of the results and revision of the manuscript. M.D. was involved in the study design and concept and provided input on the interpretation of the results and revision of the manuscript. D.D. provided input on the interpretation of the results and revision of the manuscript. K.K. was involved in the study conception and design, analysis and interpretation of results, and manuscript preparation. J.R. was involved in the study design and concept and provided input on the interpretation of the results and revision of the manuscript. R.S. was involved in the study conception and design, analysis and interpretation of results, and manuscript preparation. J.L. was involved in the analysis and interpretation of results and manuscript preparation. N.Z. was involved in the analysis and interpretation of results and manuscript preparation. D.C. provided input on the interpretation of the results and revision of the manuscript. H.J.L.H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at Zoom Forward 2022, Maastricht, the Netherlands, 4–7 May 2022, and at the World Congress of Nephrology 2022, Kuala Lumpur, Malaysia, 24–27 February 2022.
Footnotes
Clinical trial reg. nos. NCT03548935, NCT03552757, and NCT03611582, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.21893931.
References
- 1. de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int 2020;98:839–848 [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association Professional Practice Committee . 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S113–S124 [DOI] [PubMed] [Google Scholar]
- 3. Wing RR, Bolin P, Brancati FL, et al.; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma M. The RENAAL study investigation. Clin Diabetes 2002;20:19–20 [Google Scholar]
- 5. Rodby RA, Rohde RD, Clarke WR, et al.; Collaborative Study Group . The Irbesartan Type II Diabetic Nephropathy Trial: study design and baseline patient characteristics. Nephrol Dial Transplant 2000;15:487–497 [DOI] [PubMed] [Google Scholar]
- 6. Jardine MJ, Mahaffey KW, Neal B, et al.; CREDENCE study investigators . The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 2017;46:462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Persson F, Rossing P, Vart P, et al.; DAPA-CKD Trial Committees and Investigators . Efficacy and safety of dapagliflozin by baseline glycemic status: a prespecified analysis from the DAPA-CKD trial. Diabetes Care 2021;44:1894–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bakris GL, Agarwal R, Anker SD, et al.; FIDELIO-DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–2229 [DOI] [PubMed] [Google Scholar]
- 9. Lewis EJ, Hunsicker LG, Clarke WR, et al.; Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 10. Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 11. Oshima M, Neuen BL, Li J, et al. Early change in albuminuria with canagliflozin predicts kidney and cardiovascular outcomes: a post hoc analysis from the CREDENCE Trial. J Am Soc Nephrol 2020;31:2925–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garvey WT, Mechanick JI, Brett EM, et al.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines . American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 2016;22(Suppl. 3):1–203 [DOI] [PubMed] [Google Scholar]
- 13. Muskiet MHA, Tonneijck L, Smits MM, et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 2017;13:605–628 [DOI] [PubMed] [Google Scholar]
- 14. Davies M, Færch L, Jeppesen OK, et al.; STEP 2 Study Group . Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021;397:971–984 [DOI] [PubMed] [Google Scholar]
- 15. Wegovy: highlights of prescribing information, 2021. Accessed 7 September 2022. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215256s000lbl.pdf
- 16. Wegovy: product information, 2022. Accessed 7 September 2022. Available from https://www.ema.europa.eu/en/documents/product-information/wegovy-epar-product-information_en.pdf
- 17. Kushner RF, Calanna S, Davies M, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity (Silver Spring) 2020;28:1050–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilding JPH, Batterham RL, Calanna S, et al.; STEP 1 Study Group . Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384:989–1002 [DOI] [PubMed] [Google Scholar]
- 19. Wadden TA, Bailey TS, Billings LK, et al.; STEP 3 Investigators . Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA 2021;325:1403–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rubino D, Abrahamsson N, Davies M, et al.; STEP 4 Investigators . Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA 2021;325:1414–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inker LA, Eneanya ND, Coresh J, et al.; Chronic Kidney Disease Epidemiology Collaboration . New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steen J, Loeys T, Moerkerke B, Vansteelandt S. medflex: an R package for flexible mediation analysis using natural effect models. J Stat Softw 2017;76:1–4636568334 [Google Scholar]
- 24. Vansteelandt S, Bekaert M, Lange T. Imputation strategies for the estimation of natural direct and indirect effects. Epidemiol Methods 2012;1:131–158 [DOI] [PubMed] [Google Scholar]
- 25. Fieller EC. The biological standardization of insulin. J R Stat Soc 1940;7:1–64 [Google Scholar]
- 26. Mann JFE, Hansen T, Idorn T, et al. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post-hoc analysis of the SUSTAIN 1-7 randomised controlled trials. Lancet Diabetes Endocrinol 2020;8:880–893 [DOI] [PubMed] [Google Scholar]
- 27. Heerspink HJL, Greene T, Tighiouart H, et al.; Chronic Kidney Disease Epidemiology Collaboration . Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 2019;7:128–139 [DOI] [PubMed] [Google Scholar]
- 28. Coresh J, Heerspink HJL, Sang Y, et al.; Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration . Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol 2019;7:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cherney DZI, Udell JA, Drucker DJ. Cardiorenal mechanisms of action of glucagon-like-peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors. Med (N Y) 2021;2:1203–1230 [DOI] [PubMed] [Google Scholar]
- 30. Perkovic V, Baeres F, Bakris G, et al. FC 123: baseline characteristics of the FLOW trial population: kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant 2022;37(Suppl. 3):gfac126.002 [Google Scholar]
- 31. Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 2011;80:282–287 [DOI] [PubMed] [Google Scholar]
- 32. Heerspink HJL, Cherney D, Postmus D, et al.; DAPA-CKD Trial Committees and Investigators . A pre-specified analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial on the incidence of abrupt declines in kidney function. Kidney Int 2022;101:174–184 [DOI] [PubMed] [Google Scholar]
- 33. Gerstein HC, Colhoun HM, Dagenais GR, et al.; REWIND Investigators . Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019;394:131–138 [DOI] [PubMed] [Google Scholar]
- 34. Gerstein HC, Sattar N, Rosenstock J, et al.; AMPLITUDE-O Trial Investigators . Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med 2021;385:896–907 [DOI] [PubMed] [Google Scholar]
- 35. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018;6:605–617 [DOI] [PubMed] [Google Scholar]
- 36. Shaman AM, Bain SC, Bakris GL, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation 2022;145:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Aart-van der Beek AB, Clegg LE, Penland RC, et al. Effect of once-weekly exenatide on estimated glomerular filtration rate slope depends on baseline renal risk: a post hoc analysis of the EXSCEL trial. Diabetes Obes Metab 2020;22:2493–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Odutayo A, Cherney D. Cystatin C and acute changes in glomerular filtration rate. Clin Nephrol 2012;78:64–75 [DOI] [PubMed] [Google Scholar]
- 39. Bjornstad P, Cherney DZ, Maahs DM. Update on estimation of kidney function in diabetic kidney disease. Curr Diab Rep 2015;15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]