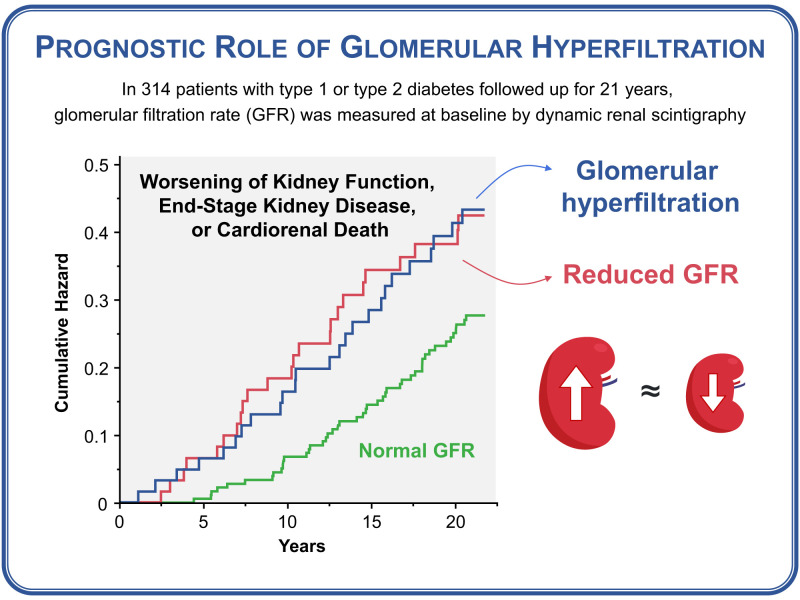
Abstract
OBJECTIVE
To evaluate the prognostic value of glomerular hyperfiltration on long-term kidney-related outcomes and mortality in patients with diabetes.
RESEARCH DESIGN AND METHODS
We retrospectively analyzed 21-year longitudinal data from 314 patients with long-standing type 1 or type 2 diabetes. Glomerular hyperfiltration was identified based on the age- and sex-specific distribution of measured glomerular filtration rate (mGFR) by 99mTc-DTPA dynamic renal scintigraphy. The primary outcome was a composite of doubling of serum creatinine, end-stage kidney disease (ESKD), or cardiorenal death. The kidney-specific outcome was a composite of doubling of serum creatinine, ESKD, or renal death.
RESULTS
Over a median of 21.0 years, the primary composite outcome occurred in 25 (39.7%), 24 (38.1%), and 46 (24.5%) participants with high mGFR (H-mGFR) (n = 63), low mGFR (L-mGFR) (n = 63), or normal mGFR (N-mGFR) (n = 188), respectively. Compared with N-mGFR, the hazard ratio (HR) for the primary composite outcome was 2.09 (95% CI 1.25–3.49) in H-mGFR and 1.81 (1.05–3.16) in L-mGFR. The HR for the kidney-specific composite outcome was 4.95 (2.21–11.09) in H-mGFR and 3.81 (1.70–8.56) in L-mGFR. The HRs for doubling of serum creatinine and cardiorenal death were 4.86 (2.18–10.90) and 2.18 (1.24–3.83) in H-mGFR and 4.04 (1.77–9.20) and 2.26 (1.27–4.01) in L-mGFR, respectively.
CONCLUSIONS
Glomerular hyperfiltration, similar to hypofiltration, increases the combined risk of worsening kidney function and mortality from cardiovascular or renal causes in patients with diabetes. These findings encourage the active screening of these patients to optimize risk stratification and treatment of subclinical kidney disease.
Graphical Abstract
Introduction
Diabetic nephropathy is a leading cause of end-stage kidney disease (ESKD), with an estimated prevalence of 30–40% in patients with type 2 diabetes (T2D) and type 1 diabetes (T1D) (1). In the earliest stage of diabetic nephropathy, a supraphysiologic elevation in glomerular filtration rate (GFR), defined as glomerular hyperfiltration, can lead to an increase in glomerular capillary pressure and tensile stress that, in turn, may initiate and facilitate the progression of kidney damage (2,3). Glomerular hyperfiltration has been associated with nephropathy development and progression over short observation periods in patients with T2D and T1D (4–7), but findings were not confirmed in all cohorts (8,9). On the other hand, a reduction in glomerular hyperfiltration via pharmacological inhibition of the renin-angiotensin-aldosterone system (RAAS) (10–13) and possibly sodium–glucose cotransporter 2 (SGLT2) (14–17) can eventually translate into long-term benefits on kidney outcomes. Glomerular hyperfiltration was also linked to increased mortality in both healthy people (18) and patients with T2D (19) in large population-based studies. However, these studies relied only on estimated GFR (eGFR) to identify individuals with hyperfiltration, which is known to be inaccurate (20).
The correct identification of glomerular hyperfiltration is hampered by the high discordance (±30%) between eGFR and directly measured GFR (mGFR), especially for the extreme values characteristic of patients with glomerular hyperfiltration (20). In a cohort of 600 patients with T2D, the majority (64%) of patients with mGFR-confirmed glomerular hyperfiltration were not identified by any eGFR creatinine-based equation (21), whereas another cross-sectional study showed that both the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) equations systematically underestimate values >90 mL/min/1.73 m2 in people with diabetes (22). Moreover, validated eGFR equations are adjusted for established risk factors for kidney disease and all-cause mortality, including age, sex, and race, which would eventually lead to an over- or underestimation of the prognostic value of eGFR-based glomerular hyperfiltration (21). Therefore, there is a need for studies with appropriate diagnostic measures of GFR to better define the long-term prognostic value of this condition, which remains underdiagnosed and undertreated.
This longitudinal study aimed to evaluate the long-term impact of measured glomerular hyperfiltration on kidney function decline and mortality in a well-characterized cohort of patients with either T2D or T1D. To overcome previous study limitations, we identified patients with glomerular hyperfiltration by using age- and sex-specific percentiles of mGFR assessed using dynamic renal scintigraphy with 99mTc-diethylenetriaminepentaacetic acid (DTPA) and compared the performance of this classification with eGFR-based assessments. The time-dependent prognostic impact of glomerular hyperfiltration on kidney-related outcomes was evaluated over a long follow-up period, extending over 20 years.
Research Design and Methods
Study Protocol
The Chronic Diabetes Complications and All-Cause Mortality in Pisa From 1999 Onwards (CHAMP1ON) study is a single-center, observational study involving 497 consecutive patients who were referred to the outpatient clinic for the management of diabetes at the University Hospital of Pisa between 1999 and 2000. The main inclusion criteria were age between 18 and 75 years, female or male sex, and history of diabetes or prediabetes (either impaired fasting glucose or impaired glucose tolerance). Exclusion criteria were concomitant acute or chronic diseases associated with reductions in life expectancy, including ESKD, lung, hepatic, neoplastic or inflammatory diseases, and cardiovascular disease (CVD) events in the previous 12 months.
At baseline, a detailed clinical history was obtained from all participants through standardized questionnaires, blood and urine samples were taken for biochemical examinations, and a physical examination was performed by a physician. Additionally, participants underwent a comprehensive screening for the presence of diabetes-related microvascular complications, including chronic kidney disease (CKD), cardiovascular autonomic neuropathy (CAN), peripheral neuropathy, and retinopathy.
After enrollment, participants periodically attended the clinic in relation to their clinical needs and were treated for the control of major cardiovascular risk factors according to the best clinical practice in those years. Relevant kidney outcomes and mortality on 30 April 2021 were retrieved from local administrative and clinical health records. Cardiovascular death was defined as any death resulting from an acute myocardial infarction, sudden cardiac death, heart failure, stroke, cardiovascular procedures, cardiovascular hemorrhage, and other cardiovascular causes. Renal death was defined as any death in patients with ESKD who died prior to initiating renal replacement therapy (RRT) or after withdrawal from RRT if there was no evidence of other major diseases leading to death or RRT withdrawal (23).
In this retrospective longitudinal study, we included CHAMP1ON study participants with either T2D or T1D who were screened for CKD using dynamic renal scintigraphy and overnight urine collection and with complete longitudinal data on survival and relevant kidney outcomes, for a total of 314 participants (Supplementary Fig. 1). The study was approved by the local human ethics committee and conducted in accordance with the principles expressed in the Declaration of Helsinki. All participants provided written informed consent prior to enrollment.
Dynamic Renal Scintigraphy
The mGFR was determined using dynamic renal scintigraphy with 99mTc-DTPA, a glomerular-specific radiotracer (24). Patients were hydrated with 500 mL water 30 min prior to examination. After an intravenous injection of 200 MBq/m2 of 99mTc-DTPA, image acquisition was performed while the participant was in the posterior position, with the regions of kidneys and bladder placed in the center view of the gamma camera. The values of mGFR were calculated by the Gates method (24). The eGFR was determined using the CKD-EPI creatinine equation (25).
Overnight Urine Collection
Urinary albumin excretion was measured via radioimmunoassay using timed overnight urine collection. Albuminuria was defined by a urinary albumin excretion rate between 20 and 200 μg/min (microalbuminuria) or >200 μg/min (macroalbuminuria), excluding urine samples that were indicative of significant urinary tract infection or hematuria.
CAN
A validated battery of cardiovascular tests for the measurement of heart rate variability in response to lying to standing, standing to lying, and deep breathing was performed using a portable computerized system (Cardionomic; Medimatica, Martinsicuro, Italy). Orthostatic hypotension was defined as a ≥20-mmHg reduction in systolic blood pressure within 3 min of standing. CAN was defined as the presence of at least two cardiovascular tests showing reduced heart rate variability and/or orthostatic hypotension.
Peripheral Neuropathy
A standardized questionnaire for symptoms of diabetic peripheral neuropathy (DPN), physical examination, and monofilament testing were used for the screening of DPN. If suspected, DPN was subsequently confirmed by electroneurography and electromyography.
Diabetic Retinopathy
Dilated fundus oculi examination was performed by a trained ophthalmologist. Retinopathy was defined by the presence of any characteristic lesions, including intraretinal microvascular abnormalities, microaneurysms, hemorrhages, cotton wool spots, hard exudates, venous beading or dilation, and new vessels.
Outcomes
Primary and secondary outcomes, assessed in time-to-event analyses, were defined according to recent kidney outcome trials (26,27). The primary composite outcome was the first occurrence of any of the following: a doubling of serum creatinine levels from baseline, the onset of ESKD (defined as the initiation of dialysis, kidney transplantation, or an eGFR <15 mL/min/1.73 m2), or death from renal or cardiovascular causes. Secondary outcomes were the kidney-specific composite outcome of a doubling of serum creatinine level, ESKD, or renal death; doubling of serum creatinine level; incident ESKD; cardiorenal death; and all-cause death.
Statistical Analysis
Glomerular hyperfiltration (or high glomerular filtration [H-GFR]), normal glomerular filtration (N-GFR), and low glomerular filtration (L-GFR) were defined as a GFR value greater than the 80th age- and sex-specific percentile, between the 20th and 80th percentile, or lower than the 20th percentile, respectively. Cohen κ-coefficient was used to estimate the agreement between different classifications. The best cutoff for eGFR to predict mGFR–based glomerular hyperfiltration in women and men stratified by age groups was identified using the Youden method among cut points providing at least 60% sensitivity.
Variables were tested for normality using the Shapiro-Wilk test. Continuous normally and nonnormally distributed variables are presented as mean ± SD or median (interquartile range), respectively. Categorical variables are presented as count (percentage).
Differences between groups were tested using Kruskal-Wallis test or Fisher exact test, followed by post hoc pairwise comparisons as appropriate. Group differences in dichotomous variables were also tested using the Cochran-Armitage test for trends.
Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% CIs for the primary and secondary outcomes according to the mGFR-based grouping at baseline. Multivariable models were used to account for potential confounders, including age, sex, BMI, diabetes type and duration, HbA1c, systolic blood pressure, and log-transformed albuminuria as covariates.
Sensitivity analyses were performed defining H-mGFR and L-mGFR as the extreme quartiles (>75% and <25%, respectively) or deciles (>90% and <10%) of the age- and sex-specific distribution of mGFR values and by using mGFR values not adjusted by body surface area. Sensitivity analyses were also conducted by adjusting models for the competing risk of death from causes unrelated to each specific outcome, for history of previous CVD events, and for active treatment with RAAS inhibitors at baseline. The effect of albuminuria was examined by removing this covariate from multivariable models. The effect of diabetes type was examined by adding an interaction term between the mGFR-based grouping and type of diabetes in all the multivariable models. Statistical analysis was performed using JMP Pro version 16 software (SAS Institute, Cary, NC), with significance set at a two-sided α-level of 0.05.
Data and Resource Availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Results
Study Population
The study population included 314 participants (age 55.1 ± 12.5 years, BMI 29.2 ± 6.1 kg/m2, HbA1c 8.9 ± 2.2%) of whom 235 (74.8%) had T2D and 79 (25.2%) had T1D. Women (52.2%) and men (47.8%) were evenly represented. Most patients had long diabetes duration (>5 years; 65.6%) and poor glycemic control (HbA1c >7.5%; 69.7%), and there was a high prevalence of associated metabolic risk factors, including obesity (39.0%) and hypertension (48.1%).
Identification of Patients With Glomerular Hyperfiltration
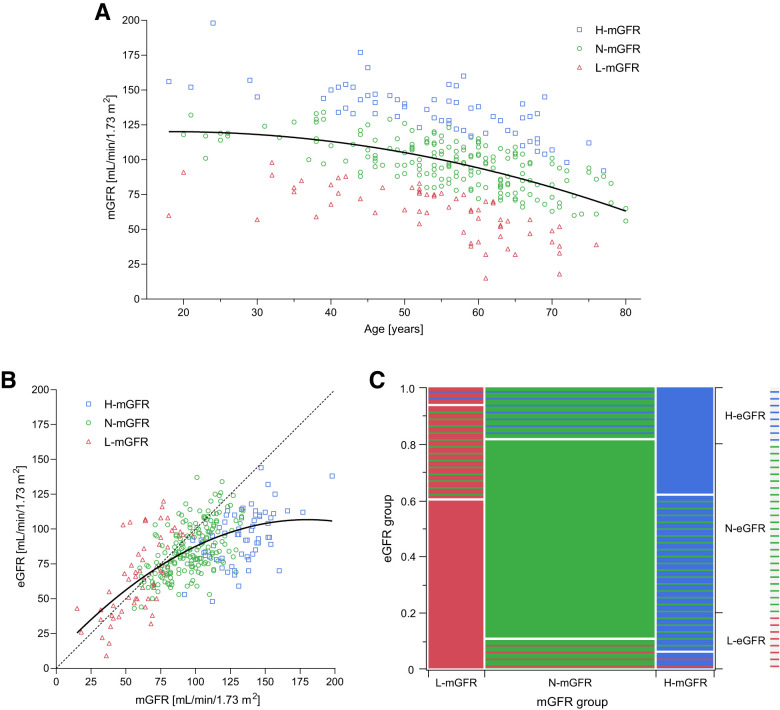
A total of 63 (20.1%) patients were classified as H-mGFR, 188 (59.9%) as N-mGFR, and 63 (20.1%) as L-mGFR (Fig. 1A). mGFR and eGFR using the CKD-EPI equation showed a good correlation up to ∼100 mL/min/1.73 m2 (Fig. 1B). After this threshold, increases in mGFR were not followed by proportional increases in eGFR. The classification of participants by eGFR showed a weak level of agreement with the classification based on mGFR (Cohen κ-coefficient 0.319), with >50% of H-mGFR misclassified as N-mGFR (Fig. 1C). The optimal thresholds of eGFR for predicting mGFR-based glomerular hyperfiltration in women and men stratified by age groups are reported in Supplementary Table 1.
Figure 1.
A and B: Distribution of mGFR by 99mTc-DTPA dynamic renal scintigraphy across ages (A) and its relationship with eGFR by the creatinine-based CKD-EPI equation (B). C: Distribution of participants classified as having H-GFR, N-GFR, or L-GFR according to either mGFR or eGFR. H-GFR, N-GFR, and L-GFR were defined as a GFR value greater than the 80th age- and sex-specific percentile, between the 20th and 80th percentile, or lower than the 20th percentile, respectively. The best fit lines (quadratic) of data are shown as continuous lines, and an ideal linear line of perfect fit is shown as a dashed line. The proportions on the x-axis and the right y-axis of the mosaic plot represent the number of observations for each mGFR and eGFR group, respectively. The proportions on the left y-axis represent the number of observations of each eGFR group within each mGFR group.
Baseline Characteristics of Patients With Glomerular Hyperfiltration
The clinical and metabolic characteristics of the study population stratified by mGFR are presented in Table 1. The three groups had similar age, sex, disease duration, history of CVD, and albuminuria, although the L-mGFR group showed the highest prevalence of macroalbuminuria. Participants with T1D were equally represented across groups. Participants in the H-mGFR group had lower BMI and systolic blood pressure than those in the L-mGFR group and worse glucose control compared with both the L-mGFR and N-mGFR groups. Serum creatinine increased progressively from H-mGFR to N-mGFR to L-mGFR, while both mGFR and eGFR decreased. The absolute difference between average mGFR and eGFR was greater in H-mGFR (41 mL/min/1.73 m2) than N-mGFR and L-mGFR (14 and −4 mL/min/1.73 m2, respectively). The prevalence of diabetic retinopathy increased progressively from N-mGFR to H-mGFR to L-mGFR (32.1%, 35.6%, and 52.1%, respectively; P = 0.045; P for trend = 0.018), while the three groups were similarly affected by CAN (P = 0.576) and DPN (P = 0.347).
Table 1.
Baseline characteristics of study participants stratified by mGFR
| Characteristic | H-mGFR (n = 63) | N-mGFR (n = 188) | L-mGFR (n = 63) | P |
|---|---|---|---|---|
| Age, years | 56 (45–65) | 57 (50–64) | 57 (46–63) | 0.326 |
| Women | 33 (52.4) | 99 (52.7) | 32 (50.8) | 0.987 |
| BMI, kg/m2 | 26.3 (23.9–30.3) | 28.1 (24.7–32.0) | 30.0 (26.2–36.6)a | 0.011 |
| Body surface area, m2 | 1.84 (1.73–1.97) | 1.88 (1.71–1.99) | 1.94 (1.73 –2.10) | 0.081 |
| Diabetes type | 0.122 | |||
| T1D | 18 (28.6) | 40 (21.3) | 21 (33.3) | |
| T2D | 45 (71.4) | 148 (78.7) | 42 (66.7) | |
| Duration of diabetes, years | 11 (7–20) | 11 (3–23) | 10 (4–20) | 0.721 |
| History of CVD | 16 (25.4) | 41 (21.8) | 17 (27.0) | 0.782 |
| Smoking status | 0.279 | |||
| Active | 23 (36.5) | 57 (30.3) | 12 (19.1) | |
| Former | 10 (15.9) | 36 (19.2) | 14 (22.2) | |
| Blood pressure, mmHg | ||||
| Systolic | 139 (120–150) | 140 (124–154) | 142 (132–160)a | 0.039 |
| Diastolic | 80 (80–90) | 82 (78–90) | 85 (75–90) | 0.863 |
| Fasting plasma glucose, mg/dL | 178 (147–232) | 155 (12–210) | 154 (121–187)a | 0.022 |
| HbA1c, % | 9.3 (8–10.6) | 8.6 (7–10.1)a | 8.0 (6.9–9.3)a | 0.003 |
| Cholesterol, mg/dL | ||||
| Total | 211 (184–237) | 210 (180–252) | 210 (188–241) | 0.832 |
| HDL | 50 (39–59) | 43 (38–52) | 47 (42–58)b | 0.017 |
| LDL | 131 (109–148) | 134 (107–166) | 124 (105–152) | 0.695 |
| Triglycerides, mg/dL | 122 (89–163) | 143 (101–199) | 147 (114–227) | 0.182 |
| Creatinine, mg/dL | 0.78 (0.70–0.91) | 0.88 (0.76–1.00)a | 1.12 (0.87–1.47)a,b | <0.0001 |
| mGFR, mL/min/1.73 m2 | 136 (122–146) | 98 (85–109)a | 64 (49–76)a,b | <0.0001 |
| mGFR, mL/min | 145 (128–159) | 104 (89–118)a | 72 (52–89)a,b | <0.0001 |
| eGFR, mL/min/1.73 m2 | 95 (81–110) | 84 (73–98)a | 68 (45–89)a,b | <0.0001 |
| Albuminuria, μg/min | 6.1 (3.3–11.25) | 6.4 (3.0–15.3) | 9.0 (2.0–14.6) | 0.498 |
| Microalbuminuria | 7 (11.1) | 27 (14.4) | 8 (12.7) | 0.847 |
| Macroalbuminuria | 1 (1.6) | 7 (3.7) | 9 (14.3)a,b | 0.005 |
| Oral glucose-lowering drugs | 31 (49.0) | 86 (45.7) | 15 (23.8)a,b | 0.003 |
| Insulin | 25 (39.7) | 74 (39.4) | 28 (44.4) | 0.877 |
| Insulin total daily dose, IU | 42 (32–51) | 40 (30–48) | 39 (30–50) | 0.620 |
| Statins | 3 (5.0) | 16 (8.5) | 8 (12.7) | 0.287 |
| ACEi/ARB | 22 (35.0) | 77 (41.0) | 26 (41.3) | 0.695 |
| β-Blockers | 0 (0) | 11 (5.9) | 4 (6.4) | 0.111 |
| Calcium antagonists | 7 (11.1) | 38 (20.2)a | 19 (30.2)a | 0.030 |
| α-1 antagonists | 2 (3.2) | 20 (11.0)a | 12 (19.0)a | 0.016 |
| α-2 agonists | 0 (0) | 4 (2.0) | 3 (5.0) | 0.216 |
| Diuretics | 6 (10) | 23 (12) | 7 (11) | 0.910 |
Data are median (interquartile range) or n (%). Differences were tested using Kruskal-Wallis test or Fisher exact test, followed by post hoc pairwise comparisons as appropriate. ACEi/ARB, ACE inhibitor/angiotensin receptor blocker.
P < 0.05 vs. H-mGFR.
P < 0.05 vs. N-mGFR.
Prognostic Value of Glomerular Hyperfiltration on the Primary Composite Outcome
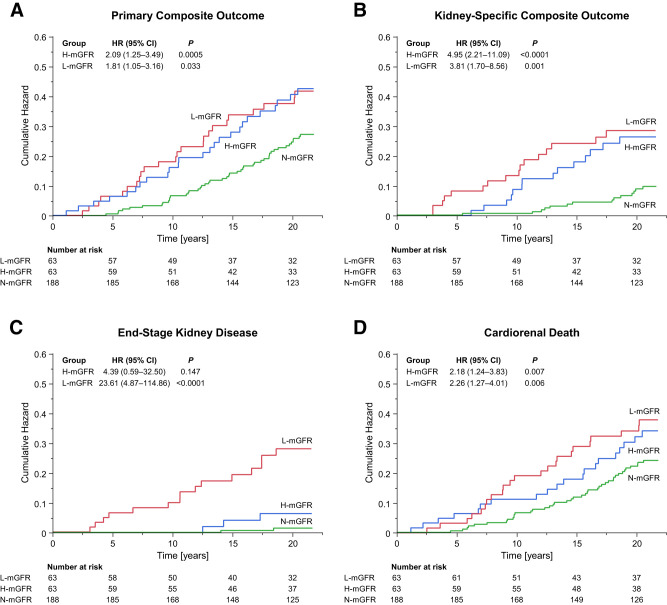
After a median follow-up of 21.0 years (range 1.1–22.0 years), the primary composite outcome of doubling of serum creatinine level, ESKD, or cardiorenal death occurred in 95 (30.3%) participants (16.9 events per 1,000 person-years). The event rate of the primary composite outcome was comparably higher in the H-mGFR and L-mGFR groups than in the N-mGFR group (23.9, 24.3, and 13.5 per 1,000 patient-years, respectively), which resulted in a 109% and 81% higher adjusted relative risk (HR 2.09 [95% CI 1.25–3.49, P = 0.005] and 1.81 [1.05–3.16, P = 0.033], respectively) (Table 2 and Fig. 2A). These estimates were consistent in unadjusted models (Supplementary Table 2) and in sensitivity analyses using different group classifications (Supplementary Tables 3–5) or accounting for competing risks (Supplementary Table 6), history of CVD (Supplementary Table 7), or treatment with RAAS inhibitors (Supplementary Table 8). Not accounting for albuminuria increased the relative risk of the primary composite outcome in the L-mGFR group but not in the H-mGFR group (Supplementary Table 9). There was no interaction between type of diabetes and mGFR group in predicting the primary composite outcome (P = 0.953).
Table 2.
Primary and secondary outcomes
| H-mGFR (n = 63) | N-mGFR (n = 188) | L-mGFR (n = 63) | H-mGFR vs. N-mGFR | L-mGFR vs. N-mGFR | H-mGFR vs. L-mGFR | ||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | n (%)/event ratea | n (%)/event ratea | n (%)/event ratea | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Primary composite outcome | 25 (39.7)/23.9 | 46 (24.5)/13.5 | 24 (38.1)/24.3 | 2.09 (1.25–3.49) | 0.005 | 1.81 (1.05–3.16) | 0.033 | 1.15 (0.62–2.15) | 0.653 |
| Kidney-specific composite outcome | 14 (22.2)/13.4 | 14 (7.5)/4.1 | 16 (25.4)/16.2 | 4.95 (2.21–11.09) | <0.0001 | 3.81 (1.70–8.56) | 0.001 | 1.30 (0.54–3.11) | 0.555 |
| Doubling of serum creatinine level | 14 (22.2)/13.6 | 14 (7.5)/4.2 | 16 (25.4)/16.9 | 4.86 (2.18–10.90) | 0.0001 | 4.04 (1.77–9.20) | 0.0009 | 1.20 (0.50–2.89) | 0.678 |
| ESKD | 3 (4.8)/2.7 | 2 (1.1) /0.6 | 15 (23.8)/14.7 | 4.39 (0.59–32.50) | 0.147 | 23.61 (4.87–114.86) | <0.0001 | 0.19 (0.04–0.91) | 0.038 |
| eGFR <15 mL/min/1.73 m2 | 2 (3.2)/1.9 | 1 (0.5)/0.3 | 13 (20.6)/13.7 | 4.97 (0.30–82.98) | 0.265 | 46.51 (5.57–388.30) | 0.0004 | 0.11 (0.01–0.92) | 0.042 |
| Dialysis initiated or kidney transplantation | 2 (3.2)/1.8 | 2 (1.1)/0.6 | 12 (19.1)/10.5 | 4.91 (0.61–39.58) | 0.135 | 17.31 (3.18–94.07) | 0.001 | 0.28 (0.05–1.48) | 0.135 |
| Cardiorenal death | 20 (31.8)/18.1 | 41 (21.8)/11.9 | 23 (36.5)/21.4 | 2.18 (1.24–3.83) | 0.007 | 2.26 (1.27–4.01) | 0.006 | 0.97 (0.50–1.89) | 0.920 |
| Renal death | 1 (1.6)/0.9 | 0 (0)/0 | 3 (4.8)/2.8 | NA | NA | NA | NA | 0.39 (0.02–8.17) | 0.542 |
| Cardiovascular death | 19 (30.2)/17.2 | 41 (21.8)/11.9 | 20 (31.8)/18.6 | 2.05 (1.16–3.63) | 0.014 | 1.98 (1.09–3.60) | 0.026 | 1.04 (0.52–2.08) | 0.921 |
| Death from any cause | 26 (41.3)/23.5 | 66 (35.1)/19.1 | 29 (46.0)/27.0 | 1.62 (1.00–2.61) | 0.049 | 1.65 (1.02–2.68) | 0.041 | 0.98 (0.54–1.75) | 0.938 |
The primary outcome was a composite of a doubling of serum creatinine levels from baseline, incident ESKD (defined as the initiation of dialysis, kidney transplantation, or an eGFR <15 mL/min/1.73 m2), or death from cardiovascular or renal causes. The kidney-specific outcome was a composite of a doubling of serum creatinine level, ESKD, or death from renal cause. Cox proportional hazards models adjusted for age, sex, BMI, diabetes type and duration, HbA1c, systolic blood pressure, and log-transformed albuminuria were used to estimate HRs and 95% CIs for the primary and secondary outcomes according to the mGFR-based grouping at baseline. NA, not applicable.
Number of events per 1,000 person-years.
Figure 2.
A–D: Cumulative hazard of the primary composite outcome, kidney-specific composite outcome, ESKD, or cardiorenal death in participants with H-mGFR or L-mGFR compared with those with N-mGFR. The primary outcome was a composite of a doubling of serum creatinine levels from baseline, incident ESKD (defined as the initiation of dialysis, kidney transplantation, or an eGFR <15 mL/min/1.73 m2), or death from cardiovascular or renal causes. The kidney-specific outcome was a composite of a doubling of serum creatinine level, ESKD, or death from renal cause. Cox proportional hazards regression models adjusted for age, sex, BMI, diabetes type and duration, HbA1c, systolic blood pressure, and log-transformed albuminuria were used to calculate HRs 95% CIs for the H-mGFR and L-mGFR groups vs. the N-mGFR group.
Prognostic Value of Glomerular Hyperfiltration on the Kidney-Specific Composite Outcome
The kidney-specific composite outcome occurred in 44 (14.0%) patients. The event rate was higher in both the H-mGFR and L-mGFR groups than in the N-mGFR group (13.4, 16.2, and 4.1 per 1,000 patient-years, respectively), which resulted in a 395% and 281% higher relative risk (HR 4.95 [95% CI 2.21–11.09, P < 0.0001] and 3.81 [1.70–8.56, P = 0.001], respectively) (Table 2 and Fig. 2B). These effects were confirmed in all the sensitivity analyses (Supplementary Tables 2 and 4–9), except when a more conservative definition of H-mGFR was adopted (Supplementary Table 3). There was no significant effect of the interaction between type of diabetes and mGFR group (P = 0.654).
Prognostic Value of Glomerular Hyperfiltration on Individual Outcome Components
Doubling of serum creatinine level occurred in 44 (13.7%) participants, who showed a median 68.8% (interquartile range 59.2–86.8%) reduction in eGFR. The event rate was higher in both the H-mGFR and L-mGFR groups than in the N-mGFR group (13.6, 16.9, and 4.2 per 1,000 patient-years, respectively), which resulted in a 386% and 304% higher adjusted relative risk (HR 4.86 [95% CI 2.18–10.90, P = 0.0001] and 4.04 [1.77–9.20, P = 0.0009], respectively) (Table 2).
ESKD occurred in 20 (6.4%) participants (Table 2). The event rate and relative risk of ESKD in the H-mGFR group were significantly lower compared with the L-mGFR group (2.7 and 14.7 per 1,000 patient-years, respectively; HR 0.19 [95% CI 0.04–0.91, P = 0.038]) but was numerically higher than N-mGFR (0.6 per 1,000 patient-years; HR 4.39 [0.59–32.50, P = 0.147]) (Table 2 and Fig. 2C).
Cardiorenal and all-cause death occurred in 84 (26.8%) and 121 (38.5%) participants, respectively (Table 2). The event rate of cardiorenal death was higher in both the H-mGFR and L-mGFR groups than in the N-mGFR group (18.1, 21.4, and 11.9 per 1,000 patient-years, respectively), which resulted in a 118% and 126% higher adjusted relative risk (HR 2.18 [95% CI 1.24–3.83, P = 0.007] and 2.26 [1.27–4.01, P = 0.006], respectively) (Table 2 and Fig. 2D). The event rate of all-cause death was also higher in both the H-mGFR and L-mGFR groups than in the N-mGFR group (23.5, 27.0, and 19.1 per 1,000 patient-years, respectively), which resulted in a 62% and 65% higher adjusted relative risk (HR 1.62 [1.00–2.61, P = 0.049] and 1.65 [1.02–2.68, P = 0.041], respectively) (Table 2).
The effects of the mGFR group on doubling of serum creatinine level and ESKD were confirmed in all the sensitivity analyses, while the effects on cardiorenal and all-cause death showed some discrepancies (Supplementary Tables 2–9). There were no significant effects of the interaction between type of diabetes and mGFR group on doubling of serum creatinine levels (P = 0.743), ESKD (P = 0.544), cardiorenal death (P = 0.951), and all-cause death (P = 0.535).
Conclusions
In this 21-year-long longitudinal study, glomerular hyperfiltration was identified in patients with long-standing diabetes by using dynamic renal scintigraphy for GFR measurement. More than 50% of these participants would have been misdiagnosed as having normofiltration based on the CKD-EPI–calculated eGFR, which underestimates high GFR values. Compared with participants with normal kidney function matched for competing risk factors, those with glomerular hyperfiltration showed worse glucose control, greater prevalence of diabetic retinopathy, and an increased relative risk for both the primary and kidney-specific composite outcomes over time, with faster decline of kidney function and increased cardiorenal mortality independent of potential confounders. Overall, these findings demonstrate that glomerular hyperfiltration 1) is a pathological condition associated with markedly impaired glucose control and diabetic retinopathy; 2) cannot be accurately diagnosed using eGFR, which is, however, valuable for screening purposes; and 3) has a long-term prognostic role on kidney function and mortality. Thus, the identification of patients with glomerular hyperfiltration may improve risk stratification and refine kidney outcome–directed prevention strategies.
We used a prespecified age- and sex-adjusted percentile of mGFR distribution to define glomerular hyperfiltration while accounting for important sex-based differences and the physiological GFR decline in elderly patients (28), avoiding fixed thresholds (4,29). This method, supported by other groups (30,31), allowed us to identify a priori a group of individuals with glomerular hyperfiltration whose prevalence was similar to that reported in 600 patients with hypertension and T2D and by using iohexol-measured GFR (4). Of note, the 80th age- and sex-specific percentile of mGFR, herein proposed as a conservative cutoff point in patients at high risk for glomerular hyperfiltration, was graphically comparable to the 92nd age-specific percentile of the distribution of mGFR in a cohort 633 healthy kidney donors (32), where the prevalence of glomerular hyperfiltration was expectedly lower. Different thresholds may be adopted without affecting the main study findings (Supplementary Tables 3 and 4). Given that glomerular hyperfiltration is a predictor of accelerated GFR decline, the N-mGFR group may have included some participants with advanced glomerular hyperfiltration, having already progressed to apparent normofiltration because of a numerical reduction in hyperfiltrating nephrons. Nevertheless, considering that this error should reduce the differences between the H-mGFR and N-mGFR groups, the lower risk for the primary and kidney-specific outcomes observed in the latter reassures that this possible misclassification did not significantly affect the results. Early identification of glomerular hyperfiltration through repeated GFR measurements should have minimized the possible dilution effect in these participants, further amplifying the important differences between H-mGFR and N-mGFR.
We highlighted the challenges in the identification of glomerular hyperfiltration and the value of direct measurement of GFR. Several cross-sectional studies evaluated the performance of eGFR versus mGFR over a wide spectrum of kidney function in patients with diabetes and obesity (20,33), showing a ±30% difference (20) and poor concordance in the identification of glomerular hyperfiltration (21). Consistently, the creatinine-based CKD-EPI equation for eGFR, though widely used and supported by current guidelines (1), failed to reflect extreme mGFR values in our cohort, leading to a 50% misclassification of participants with glomerular hyperfiltration, with potential clinical consequences. Although dynamic renal scintigraphy with 99mTc-DTPA provides an accurate measure of GFR (34,35), this technique is expensive and requires advanced equipment and expertise. More recently, other techniques based on the clearance of exogenous filtration markers (e.g., inulin, iohexol) have become reference methods for direct measurement of GFR. These techniques, still labor intensive and unfeasible in clinical practice and population-based studies, show potential for screening in selected individuals with high pretest probability of having glomerular hyperfiltration. Importantly, our findings suggest that preferential candidates for direct GFR measurement are patients with high eGFR, worse glycemic control, and retinopathy, who can be identified by using readily available clinical data.
Hyperglycemia is involved in the pathogenesis of glomerular hyperfiltration (2). Thus, finding that glycemic control was worse in patients with glomerular hyperfiltration, despite similar diabetes duration and intensity of the glucose-lowering therapy, was expected. On the other hand, we demonstrated that the negative long-term effects of glomerular hyperfiltration on GFR decline and mortality are not solely explained by glucose control at baseline. Chronic hyperglycemia contributes also to the progression of diabetic microvascular complications and may explain the increased prevalence of diabetic retinopathy in the H-mGFR group. Given the synergistic, negative prognostic role of diabetes-related retinopathy and nephropathy (36,37), this novel observation should encourage the screening for diabetic retinopathy in patients with high-normal glomerular filtration for risk refinement.
Persistent glomerular hyperfiltration despite intensified treatment has been associated with kidney function decline during shorter observation periods (up to 10 years) in most (4–7) but not all (8,9) previous studies. Furthermore, glomerular hyperfiltration assessed by eGFR increased the risk of cardiovascular disease (31,38) and reduced life expectancy (18,19) in large-scale studies. Our findings extend previous evidence with an accurate definition of glomerular hyperfiltration based on mGFR and a follow-up period of >20 years, which is at least twice as long as previous observations. This long observation period, approaching the typical life expectancy of adult patients referred for complicated T2D, allowed us to appreciate the time-dependent negative effects of glomerular hyperfiltration on GFR decline and mortality, which become increasingly evident after 5 years of observation.
There is a U-shaped relationship between GFR and cardiovascular events, which remain the leading cause of death associated with kidney disease (31,38). Consistently, the higher mortality rate observed in both the H-mGFR and L-mGFR groups was largely driven by an increased incidence of cardiovascular deaths, being that renal death is a rare event (at least according to the current definition [23]) and non-cardiorenal deaths are possibly unrelated to the GFR.
The effect of glomerular hyperfiltration was inconsistent across individual components of the prespecified primary outcome, with the associated relative risk greater for kidney function decline than for cardiorenal death and not significant for ESKD. The lack of statistical difference in incident ESKD between the H-mGFR and N-mGFR groups could be explained by the limited statistical power in relation to the low event rate of ESKD, which occurred in fewer than five participants in each of these two groups.
Glomerular hyperfiltration more than doubles the risk for incident albuminuria (6), which may directly contribute to the progression of kidney disease. Thus, multivariable models were adjusted for baseline albuminuria to dissect the prognostic role of glomerular hyperfiltration from that of albuminuria. We also reported HRs not adjusted for albuminuria to provide additional mechanistic insight. Remarkably, accounting for albuminuria attenuated the relative risks of the primary and secondary outcomes associated with L-mGFR but not with H-mGFR. This observation, which may be explained by the relatively low prevalence of clinically meaningful micro- and macroalbuminuria in H-mGFR in our cohort, does not substantiate the hypothesis that the kidney damage associated with glomerular hyperfiltration is mediated by albuminuria, at least in the early phase of the disease.
Two classes of drugs, namely RAAS inhibitors and SGLT2 inhibitors, have shown beneficial actions against glomerular hyperfiltration, leading to a transient acute dip in GFR followed by a slower decline in kidney function and delayed progression toward ESKD (10–17). Therefore, identifying patients with glomerular hyperfiltration can be relevant in clinical practice not only to improve risk stratification but also to tailor medical therapy to the individual patient’s needs. In our cohort, <40% of study participants were treated with RAAS inhibitors at enrollment, and none could benefit from SGLT2 inhibitors, which were approved for the treatment of T2D 14 years later (39). Suboptimal therapy, along with long disease duration and poor metabolic control, compared with current standards, can partly justify the high mortality rate, which nonetheless is in line with other studies performed in those years (40).
There are some limitations to the current study mostly inherent to its retrospective design. Baseline assessments were not repeated regularly over the follow-up; thus, we could not evaluate the time course of clinical parameters and biochemical variables after enrollment or track treatment changes that may influence study findings. Statistical power was limited when assessing the relative incidence of rare events, including ESKD and renal death; thus, negative findings should be interpreted with caution. Although multivariable models were implemented to account for relevant confounders, the models were not adjusted for all the available variables (e.g., medications, microvascular complications) to avoid model overfitting. Nonetheless, this study also had several strengths, being the first, to our knowledge, to demonstrate the effect of glomerular hyperfiltration on kidney-related outcomes and mortality in people with T2D and T1D over such an extended follow-up period. Furthermore, the characterization of participants using a direct method for GFR measurement allowed us to overcome the limitations of estimation equations of eGFR, which can lead to misclassification of participants with hyperfiltration.
In conclusion, this study demonstrates that measured glomerular hyperfiltration associated with long-standing diabetes is a pathological condition with lifelong negative effects on kidney function and mortality, independent from traditional risk factors. Identifying glomerular hyperfiltration in populations at risk via direct methods can help to refine risk stratification and prompt the therapeutic management of subclinical kidney disease, using widely available pharmacological options, to prevent a rapid GFR decline and progression toward overt CKD.
Article Information
Acknowledgments. The authors are grateful to Dr. Simone Leonetti (Sant’Anna School of Advanced Studies, Pisa, Italy) for statistical advice. The authors are also grateful to many former research and clinical team members of the Section of Dietology and the Unit of Internal Medicine 1, University Hospital of Pisa, for valuable contributions to this project.
Funding. D.T. is supported by the European Foundation for the Study of Diabetes through a Rising Star Fellowship and the Future Leaders Mentorship Programme for clinical diabetologists.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.M. contributed to the study design, longitudinal data collection, data analysis, interpretation of results, and drafting of the manuscript. L.S., M.C., and L.N. contributed to the longitudinal data collection, interpretation of results, and editing of the manuscript. G.F. contributed to the study design and original data collection. A.N. and A.S. contributed to the study design, longitudinal data collection, interpretation of results, and critical revision of the manuscript. D.T. contributed to the study design, funding, data analysis, interpretation of results, and drafting and final revision of the manuscript. D.M. and D.T. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21932301.
A.S. and D.T. contributed equally to this work.
References
- 1. de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022;45:3075–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cortinovis M, Perico N, Ruggenenti P, Remuzzi A, Remuzzi G. Glomerular hyperfiltration. Nat Rev Nephrol 2022;18:435–451 [DOI] [PubMed] [Google Scholar]
- 3. Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M. Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 2019;143:38–42 [DOI] [PubMed] [Google Scholar]
- 4. Ruggenenti P, Porrini EL, Gaspari F, et al.; GFR Study Investigators . Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 2012;35:2061–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moriya T, Tsuchiya A, Okizaki S, Hayashi A, Tanaka K, Shichiri M. Glomerular hyperfiltration and increased glomerular filtration surface are associated with renal function decline in normo- and microalbuminuric type 2 diabetes. Kidney Int 2012;81:486–493 [DOI] [PubMed] [Google Scholar]
- 6. Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 2009;52:691–697 [DOI] [PubMed] [Google Scholar]
- 7. Yip JW, Jones SL, Wiseman MJ, Hill C, Viberti G. Glomerular hyperfiltration in the prediction of nephropathy in IDDM: a 10-year follow-up study. Diabetes 1996;45:1729–1733 [DOI] [PubMed] [Google Scholar]
- 8. Chaiken RL, Eckert-Norton M, Bard M, et al. Hyperfiltration in African-American patients with type 2 diabetes. Cross-sectional and longitudinal data. Diabetes Care 1998;21:2129–2134 [DOI] [PubMed] [Google Scholar]
- 9. Nelson RG, Bennett PH, Beck GJ, et al.; Diabetic Renal Disease Study Group . Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med 1996;335:1636–1642 [DOI] [PubMed] [Google Scholar]
- 10. Lewis EJ, Hunsicker LG, Clarke WR, et al.; Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 11. Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011;4:685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 2011;80:282–287 [DOI] [PubMed] [Google Scholar]
- 13. Apperloo AJ, de Zeeuw D, de Jong PE. A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int 1997;51:793–797 [DOI] [PubMed] [Google Scholar]
- 14. Cherney DZI, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–597 [DOI] [PubMed] [Google Scholar]
- 15. Adamson C, Docherty KF, Heerspink HJL, et al. Initial decline (dip) in estimated glomerular filtration rate after initiation of dapagliflozin in patients with heart failure and reduced ejection fraction: insights from DAPA-HF. Circulation 2022;146:438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kraus BJ, Weir MR, Bakris GL, et al. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int 2021;99:750–762 [DOI] [PubMed] [Google Scholar]
- 17. Oshima M, Jardine MJ, Agarwal R, et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int 2021;99:999–1009 [DOI] [PubMed] [Google Scholar]
- 18. Park M, Yoon E, Lim YH, Kim H, Choi J, Yoon HJ. Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol 2015;26:1426–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Penno G, Orsi E, Solini A, et al.; Renal Insufficiency and Cardiovascular Events (RIACE) Study Group . Renal hyperfiltration is independently associated with increased all-cause mortality in individuals with type 2 diabetes: a prospective cohort study. BMJ Open Diabetes Res Care 2020;8:e001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Porrini E, Ruggenenti P, Luis-Lima S, et al. Estimated GFR: time for a critical appraisal. Nat Rev Nephrol 2019;15:177–190 [DOI] [PubMed] [Google Scholar]
- 21. Gaspari F, Ruggenenti P, Porrini E, et al.; GFR Study Investigators . The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int 2013;84:164–173 [DOI] [PubMed] [Google Scholar]
- 22. MacIsaac RJ, Ekinci EI, Premaratne E, et al. The Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation does not improve the underestimation of glomerular filtration rate (GFR) in people with diabetes and preserved renal function. BMC Nephrol 2015;16:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herrington WG, Staplin N, Wanner C, et al.; EMPA-KIDNEY Collaborative Group . Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gates GF. Split renal function testing using Tc-99m DTPA. A rapid technique for determining differential glomerular filtration. Clin Nucl Med 1983;8:400–407 [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 27. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 28. Premaratne E, Macisaac RJ, Tsalamandris C, Panagiotopoulos S, Smith T, Jerums G. Renal hyperfiltration in type 2 diabetes: effect of age-related decline in glomerular filtration rate. Diabetologia 2005;48:2486–2493 [DOI] [PubMed] [Google Scholar]
- 29. Tomaszewski M, Charchar FJ, Maric C, et al. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int 2007;71:816–821 [DOI] [PubMed] [Google Scholar]
- 30. Sun ZJ, Yang YC, Wu JS, Wang MC, Chang CJ, Lu FH. Increased risk of glomerular hyperfiltration in subjects with impaired glucose tolerance and newly diagnosed diabetes. Nephrol Dial Transplant 2016;31:1295–1301 [DOI] [PubMed] [Google Scholar]
- 31. Reboldi G, Verdecchia P, Fiorucci G, et al. Glomerular hyperfiltration is a predictor of adverse cardiovascular outcomes. Kidney Int 2018;93:195–203 [DOI] [PubMed] [Google Scholar]
- 32. Pottel H, Delanaye P, Weekers L, et al. Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J 2017;10:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. López-Martínez M, Luis-Lima S, Morales E, et al. The estimation of GFR and the adjustment for BSA in overweight and obesity: a dreadful combination of two errors. Int J Obes 2020;44:1129–1140 [DOI] [PubMed] [Google Scholar]
- 34. Perrone RD, Steinman TI, Beck GJ, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. The Modification of Diet in Renal Disease study. Am J Kidney Dis 1990;16:224–235 [DOI] [PubMed] [Google Scholar]
- 35. Lewis R, Kerr N, Van Buren C, et al. Comparative evaluation of urographic contrast media, inulin, and 99mTc-DTPA clearance methods for determination of glomerular filtration rate in clinical transplantation. Transplantation 1989;48:790–796 [DOI] [PubMed] [Google Scholar]
- 36. Sabanayagam C, Chee ML, Banu R, et al. Association of diabetic retinopathy and diabetic kidney disease with all-cause and cardiovascular mortality in a multiethnic Asian population. JAMA Netw Open 2019;2:e191540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sacchetta L, Chiriacò M, Nesti L, et al. Synergistic effect of chronic kidney disease, neuropathy, and retinopathy on all-cause mortality in type 1 and type 2 diabetes: a 21-year longitudinal study. Cardiovasc Diabetol 2022;21:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dupuis M-E, Nadeau-Fredette AC, Madore F, Agharazii M, Goupil R. Association of glomerular hyperfiltration and cardiovascular risk in middle-aged healthy individuals. JAMA Netw Open 2020;3:e202377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiriacò M, Tricò D, Solini A. Mechanisms of cardio-renal protection of sodium-glucose cotransporter-2 inhibitors. Curr Opin Pharmacol 2022;66:102272. [DOI] [PubMed] [Google Scholar]
- 40. Jensen MH, Dethlefsen C, Hejlesen O, Vestergaard P. Association of severe hypoglycemia with mortality for people with diabetes mellitus during a 20-year follow-up in Denmark: a cohort study. Acta Diabetol 2020;57:549–558 [DOI] [PubMed] [Google Scholar]