Abstract
Study Objective
Early deployment of extracorporeal cardiopulmonary resuscitation (ECPR) is critical in treating refractory out‐of‐hospital cardiac arrest (OHCA) patients who are potential candidates for ECPR. The effect of prehospital advanced life support (ALS), including epinephrine administration or advanced airway, compared with no ALS in this setting remains unclear. This study's objective was to determine the association between any prehospital ALS care and outcomes of patients who received ECPR with emergency medical services‐treated OHCA.
Methods
This was a secondary analysis of data from the Study of Advanced Cardiac Life Support for Ventricular Fibrillation with Extracorporeal Circulation in Japan (SAVE‐J) II study. Patients were separated into 2 groups—those who received prehospital ALS (ALS group) and those did not receive prehospital ALS (no ALS group). Multiple logistic regression analysis was used to investigate the association between prehospital ALS and favorable neurological outcomes (defined as Cerebral Performance Category scores 1–2) at hospital discharge.
Results
A total of 1289 patients were included, with 644 patients in the ALS group and 645 patients in the no ALS group. There were fewer favorable neurological outcomes at hospital discharge in the ALS group compared with the no ALS group (10.4 vs 19.8%, p <0.001). A multiple logistic regression analysis revealed that any prehospital ALS care (adjusted odds ratios 0.47; 95% confidence interval 0.34–0.66; p <0.001) was associated with unfavorable neurological outcomes at hospital discharge.
Conclusion
Prehospital ALS was associated with worse neurological outcomes at hospital discharge in patients treated with ECPR for OHCA. Further prospective studies are required to determine the clinical implications of these findings.
1. INTRODUCTION
1.1. Background
Out‐of‐hospital cardiac arrest (OHCA) remains a considerable global challenge with unfavorable neurological outcomes, despite advances in its management. 1 Implementation of veno‐arterial extracorporeal membrane oxygenation (ECMO), often referred to as extracorporeal cardiopulmonary resuscitation (ECPR), during cardiac arrest has surfaced as a suitable therapeutic strategy for adult OHCA. 2 Although ECPR may be a promising treatment, special considerations regarding indications for ECPR should be given in terms of cost‐effectiveness, resource use, and ethical issues. 3 To date, it is still unclear who would most benefit from ECPR. 4 , 5 Previous research demonstrated that time matters greatly in ECPR: shorter time span from collapse to ECPR initiation was associated with better outcomes. 6 , 7
1.2. Importance
Meanwhile, early prehospital advanced life support (ALS), including epinephrine administration or advanced airway, was associated with higher survival rates after OHCA. 8 , 9 In Japan, only specially trained emergency medical services (EMS) personnel are authorized to perform ALS under real‐time medical direction by physicians. 10 As such, collaboration and integration of prehospital and in‐hospital management are crucial when activating the ECPR team and immediately establishing ECMO support for appropriate candidates. At present, however, whether there is a beneficial effect of prehospital ALS rather than prompt transport without ALS on outcomes in patients, particularly those who receive ECPR, is unknown.
1.3. Goals of this investigation
We sought to examine the association of any prehospital ALS care provided by EMS personnel with neurological outcomes in adult OHCA patients who underwent ECPR.
2. MATERIALS AND METHODS
This work was a secondary analysis of the Study of Advanced Cardiac Life Support for Ventricular Fibrillation with Extracorporeal Circulation in Japan (SAVE‐J) II study, which is registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000036490). 11 SAVE‐J II was a retrospective, multicenter registry study conducted at 36 investigational sites in Japan to describe the complete real‐world clinical practice of adult ECPR. The present study was approved by the Okayama University Hospital Ethics Committee (K2203‐002).
2.1. Study population
The registry included patients aged 18 years old or older for whom ECPR was given to treat refractory OHCA from January 2013 to December 2018. To assess the effect of prehospital ALS by EMS personnel, we excluded patients whose prehospital medical care was provided by dispatched physicians. Patients who were on ECMO support longer than 60 min after hospital arrival; those whose cause of cardiac arrest was accidental hypothermia, suffocation, drowning, or traumatic injuries; those transferred from another hospital; and those with missing prehospital information were also excluded.
2.2. EMS system in Japan
An in‐depth description of the EMS system in Japan has been previously published. 10 EMS personnel are obligated to resuscitate all OHCA patients, unless obvious signs of death are present. Indeed, 95% of all OHCA patients were transported to the hospital based on the the All‐Japan Utstein Registry database. 10 A vast majority of the patients are transported by a ground ambulance, which is sent from the nearest fire station. Each ambulance is staffed by 3 or 4 EMS personnel. Among them, at least 1 crew member is a highly trained staff member known as an emergency life‐saving technician (ELST) who is authorized to provide ALS, including insertion of supraglottic airway devices. Specially trained ELSTs are permitted to perform procedures including tracheal intubation and intravenous administration of epinephrine under online supervision by a medical consultant, who normally is an emergency physician at a receiving hospital. ELSTs at the scene are required to seek the advice of the medical consultant. Basically, every patient with OHCA is able to receive ALS, but the procedure needs approval by emergency physicians under real‐time medical direction. All EMS personnel are allowed to deliver defibrillation as appropriate. Although there are local‐specific protocols, the final decision of whether to permit ELSTs to perform ALS, including the choice of advanced airway devices is completely left to the discretion or preference of individual medical director based on the case‐by‐case basis.
2.3. ECPR implementation
ECPR was performed for selected patients, such as those being unresponsive to conventional ALS in the hospital, those whose time interval from collapse to ECMO initiation was considered to be short, and those whose cause of arrest was potentially reversible. The final decision on whether or not to perform ECPR was made based on the individual hospital's protocol or at the physician's discretion. Although ECPR was available 24/7 at all sites, the annual number of patients resuscitated with ECPR varied according to the hospital: 20–29 cases, 6 hospitals; 10–19 cases, 6 hospitals; 5–9 cases, 11 hospitals; and <5 cases, 13 hospitals. 11 According to a nationwide database between June 2014 to December 2019, 890 out of 50,135 patients received ECPR, which accounted for 1.8% of all adult OHCA patients. 12
2.4. Main exposure and outcomes
The primary exposure of interest was prehospital ALS, including intravenous epinephrine or use of advanced airway devices provided by ELSTs. The primary outcome was favorable neurological outcome at hospital discharge defined as Cerebral Performance Category score of 1 or 2. 13 The secondary outcome was survival at hospital discharge.
2.5. Statistical analysis
Participants were compared between the ALS group and the no ALS group based on whether or not they had received any prehospital ALS care. Continuous data were expressed as medians with interquartile range (IQR) and categorical data were expressed as counts and percentages. Comparisons between the 2 groups were made using the Mann–Whitney U test for continuous variables and chi‐square test for categorical variables. A multiple logistic regression model was applied to estimate adjusted odds ratios (ORs) with their 95% confidence intervals (CIs) for the primary and secondary outcomes. Covariates included age, sex, witnessed status (whether or not the patient collapse was witnessed), provision of bystander CPR, initial rhythm at the scene [shockable rhythm vs pulseless electrical activity vs asystole], public‐access automated external defibrillator use, and any prehospital ALS care (either epinephrine administration and/or advanced airway). These variables were selected based on previous studies and information available at the time of medical direction by physicians. 7 , 14 , 15 , 16 Total prehospital time, defined as interval from EMS activation to hospital arrival, was not entered as a covariate because total prehospital time could theoretically be prolonged by the provision of ALS in the prehospital setting. As a sensitivity analysis, additional models were developed after excluding those who achieved return of spontaneous circulation (ROSC) at hospital arrival and excluding those who achieved ROSC during the period between hospital arrival and ECMO support to deal with unmeasured confounding related to the intractability of cardiac arrest. ROSC was defined as a transient return of palpable pulse for at least 1 min. Several subgroup analyses were performed according to witnessed status, initial rhythm at the scene, and etiologies of cardiac arrest. Moreover, because institutional variation in the proportion of patients who received prehospital ALS care was assumed, we conducted another subgroup analysis according to the institutional distribution for the proportion of patients who received prehospital ALS care (≥50% or <50%). Finally, to examine the effects of specific components of ALS care on neurological outcome at hospital discharge, prehospital epinephrine administration and prehospital advanced airway (bag‐valve‐mask ventilation vs supraglottic airway devices or tracheal intubation) were included as a variable in the model, instead of any ALS care. All tests were 3 tailed and a p value of <0.05 was considered statistically significant. Analyses were performed using Stata SE version 17 statistical software (Stata‐Corp LP, College Station, TX, USA).
The Bottom Line
Extracorporeal cardiopulmonary resuscitation (ECPR) is available for many out‐of‐hospital cardiac arrest patients in Japan. Advanced life support (ALS) care is dictated by the receiving hospitals. Use of ALS care and ECPR are not standardized. This secondary evaluation of an existing registry of ECPR patients demonstrated worse outcomes with those patients who received ALS interventions.
3. RESULTS
Of the 2157 SAVE‐J II study patients, 1579 were identified as those without prehospital involvement by dispatched physicians. After excluding 290 patients who met other exclusion criteria, 1289 patients were included in the main analysis, 644 patients who received prehospital ALS and 645 patients who did not receive prehospital ALS (Figure 1).
FIGURE 1.
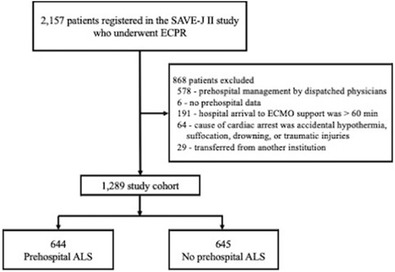
Study participant flow chart. ALS, advanced life support; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; SAVE‐J, Study of Advanced Cardiac Life Support for Ventricular Fibrillation with Extracorporeal Circulation in Japan.
3.1. Patient characteristics
Study participants’ demographic and clinical characteristics are shown in Table 1. The median age was 60 years old (IQR, 49–69 years), 1069 patients (82.9%) were men, and 220 patients (17.1%) were women. Of the 1289 patients, 372 (28.9%) received epinephrine administration and 506 (39.2%) received advanced airway. More than three quarters of the patients had cardiac etiologies. Patients who received prehospital ALS had longer total prehospital times than those not receiving ALS (32 vs 29 min, p < 0.001). Time from hospital arrival to ECPR support did not differ between the groups.
TABLE 1.
Patient characteristics of the study population.
| All (n = 1289) | ALS (n = 644) | No ALS (n = 645) | p value | |
|---|---|---|---|---|
| Age, y, median (IQR) | 60 (49–69) | 59 (48–69) | 61 (50–68) | 0.642 |
| Sex | ||||
| Men, n (%) | 1069 (82.9) | 546 (84.8) | 523 (81.1) | 0.078 |
| Women, n (%) | 220 (17.1) | 98 (15.2) | 122 (18.9) | 0.078 |
| Witnessed arrest, n (%) | 1016 (78.8) | 494 (76.7) | 522 (80.1) | 0.027 |
| Unknown, n (%) | 8 (0.6) | 1 (0.1) | 7 (1.0) | |
| Bystander CPR , n (%) | 708 (54.9) | 335 (52.0) | 373 (57.8) | 0.018 |
| Unknown, n (%) | 26 (2.0) | 10 (1.5) | 16 (2.4) | |
| Initial rhythm at the scene | ||||
| VF/pVT, n (%) | 804 (62.4) | 405 (62.9) | 399 (61.9) | 0.898 |
| Pulseless electrical activity, n (%) | 353 (27.4) | 165 (25.6) | 188 (29.1) | 0.123 |
| Asystole, n (%) | 117 (9.1) | 70 (10.9) | 47 (7.3) | 0.029 |
| Unknown, n (%) | 15 (1.1) | 4 (0.6) | 11 (1.7) | |
| AED use, n (%) | 748 (58.0) | 407 (63.2) | 341 (52.9) | <0.001 |
| Unknown, n (%) | 9 (0.7) | 2 (0.3) | 7 (1.1) | |
| Prehospital ALS care | ||||
| Epinephrine administration, n (%) | 372 (28.9) | 372 (57.8) | 0 (0) | <0.001 |
| Advanced airway management, n (%) | 506 (39.2) | 506 (78.6) | 0 (0) | <0.001 |
| Tracheal intubation, n (%) | 78 (6.1) | 78 (12.1) | 0 (0) | <0.001 |
| Supraglottic airway devices, n (%) | 428 (33.2) | 428 (66.5) | 0 (0) | <0.001 |
| Cardiac rhythm on hospital arrival | ||||
| VF/pVT, n (%) | 561 (43.5) | 302 (46.9) | 259 (40.2) | 0.014 |
| Pulseless electrical activity, n (%) | 415 (32.2) | 200 (31.1) | 215 (33.3) | 0.392 |
| Asystole, n (%) | 270(20.9) | 126 (19.6) | 144 (22.3) | 0.229 |
| ROSC, n (%) | 40 (3.1) | 14 (2.1) | 26 (4.0) | 0.055 |
| Unknown, n (%) | 3 (0.2) | 2 (0.3) | 1 (0.2) | |
| Time from EMS activation to hospital arrival, median (IQR), min | 31 (25–36) | 32 (26–38) | 29 (24–35) | <0.001 |
| Time from hospital arrival to ECPR support, median (IQR), min | 24 (16–34) | 25 (17–34) | 23 (16–33) | 0.358 |
| Etiologies of arrest | ||||
| Cardiac a , n (%) | 1009 (78.2) | 511 (73.0) | 498 (77.2) | 0.352 |
| Pulmonary embolism, n (%) | 52 (4.0) | 32 (5.0) | 20 (3.1) | 0.088 |
| Aortic dissection, n (%) | 74 (5.7) | 27 (4.2) | 47 (7.3) | 0.017 |
| Primary cerebral disorder, n (%) | 22 (1.7) | 11 (1.7) | 11 (1.7) | 0.997 |
| Others b , n (%) | 132 (8.8) | 63 (9.8) | 69 (10.7) | 0.588 |
| ROSC after hospital arrival, n (%) | 1001 (77.6) | 470 (72.9) | 531 (82.3) | <0.001 |
| Before ECPR support, n (%) | 243 (18.9) | 114 (17.7) | 129 (20.0) | 0.975 |
| After ECPR support, n (%) | 757 (58.7) | 356 (55.2) | 401 (62.2) | 0.975 |
| Unknown, n (%) | 1 (0.0) | 0 (0.0) | 1 (0.1) | |
| Favorable neurological outcomes at hospital discharge c , n (%) | 195 (15.1) | 67 (10.4) | 128 (19.8) | <0.001 |
| Unknown, n (%) | 1 (0.0) | 0 (0.0) | 1 (0.1) | |
| Survival at hospital discharge, n (%) | 362 (28.1) | 160 (24.8) | 202 (31.3) | 0.009 |
| Unknown, n (%) | 1 (0.1) | 0 (0.0) | 1 (0.1) | |
Abbreviations: AED, automated external defibrillator; ALS, advanced life support; CPR, cardiopulmonary resuscitation; ECPR, extracorporeal cardiopulmonary resuscitation; EMS, emergency medical services; IQR, interquartile range; pVT, pulseless ventricular tachycardia; ROSC, return of spontaneous circulation; VF, ventricular fibrillation.
Includes acute coronary syndrome, arrhythmia, cardiomyopathy, myocarditis, and other cardiac origin.
Includes infectious diseases, other non‐cardiac origin, and unknown etiologies.
Defined as Cerebral Performance Category of 1 or 2 at hospital discharge.
3.2. Clinical outcomes
In univariate analysis, the ALS group had worse neurological outcomes (10.4 vs 19.8%, p < 0.001) and survival at hospital discharge (24.8 vs 31.3%, p = 0.009) compared with the no ALS group (Table 1). Table 2 summarizes the results of multiple logistic regression analysis. After adjusting covariates, any prehospital ALS care (adjusted ORs 0.47; 95% CI 0.34–0.66; p < 0.001) were associated with unfavorable neurological outcomes at hospital discharge. We obtained similar results for survival at hospital discharge (adjusted ORs 0.73; 95% CI 0.56–0.94; p = 0.019). In a sensitivity analysis after excluding those who achieved ROSC at hospital arrival and excluding those who achieved ROSC during the period between hospital arrival and ECMO support, similar findings were observed (Tables S1 and S2).
TABLE 2.
A multiple logistic regression analysis for the primary and secondary outcomes.
| Variables | Favorable neurological outcomes | Survival | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| Age, y | 0.97 (0.96–0.98) | <0.001 | 0.98 (0.97–0.99) | <0.001 |
| Male | 0.70 (0.46–1.07) | 0.107 | 0.66 (0.47–0.93) | 0.018 |
| Witnessed arrest | 0.98 (0.63–1.52) | 0.936 | 1.22 (0.87–1.73) | 0.239 |
| Bystander CPR | 1.49 (1.05–2.11) | 0.015 | 1.07 (0.82–1.40) | 0.596 |
| Initial rhythm on the scene | ||||
| VF/pVT | Reference | Reference | ||
| Pulseless electrical activity | 0.41 (0.25–0.68) | <0.001 | 0.46 (0.32–0.67) | <0.001 |
| Asystole | 0.40 (0.19–0.83) | 0.015 | 0.46 (0.27–0.78) | 0.005 |
| AED use | 0.91 (0.62–1.36) | 0.675 | 1.03 (0.56–1.41) | 0.819 |
| Any ALS care | 0.47 (0.34–0.66) | <0.001 | 0.73 (0.56–0.94) | 0.019 |
Abbreviations: AED, automated external defibrillator; ALS; advanced life support; CI, confidence interval; CPR, cardiopulmonary resuscitation; OR, odds ratio; pVT, pulseless electrical activity; VF, ventricular fibrillation.
3.3. Subgroup analyses for the primary outcome
In subgroup analyses, any prehospital ALS care was associated with unfavorable neurological outcomes regardless of witness status (Table 3) and in those whose initial rhythm was shockable at the scene (Table 4). Further, any prehospital ALS care was associated with unfavorable neurological outcomes in the subgroup of those whose cause of arrest was cardiac and non‐cardiac (Table 5). Institutional variation in the proportion of patients who received prehospital ALS care is shown in Figure S1. We observed similar results regardless of this variation (Table 6).
TABLE 3.
A multiple logistic regression analysis for the primary outcome according to the witnessed status.
| Variables | Witnessed arrest (n = 1016) | Unwitnessed arrest (n = 265) | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| Age, y | 0.96 (0.95–0.98) | <0.001 | 0.98 (0.95–1.00) | 0.172 |
| Male | 0.67 (0.41–1.09) | 0.114 | 0.84 (0.33–2.10) | 0.712 |
| Bystander CPR | 1.83 (1.22–2.75) | 0.006 | 0.81 (0.32–2.03) | 0.661 |
| Initial rhythm on the scene | ||||
| VF/pVT | Reference | Reference | ||
| Pulseless electrical activity | 0.31 (0.17–0.55) | <0.001 | 1.60 (0.59–4.33) | 0.347 |
| Asystole | 0.18 (0.05–0.61) | 0.006 | 1.15 (0.42–3.16) | 0.773 |
| AED use | 1.04 (0.66–1.64) | 0.864 | 0.51 (0.22–1.17) | 0.113 |
| Any ALS care | 0.48 (0.33–0.70) | <0.001 | 0.42 (0.19–0.91) | 0.029 |
Abbreviations: AED, automated external defibrillator; ALS, advanced life support; CI, confidence interval; CPR, cardiopulmonary resuscitation; OR, odds ratio; pVT, pulseless electrical activity; VF, ventricular fibrillation.
TABLE 4.
A multiple logistic regression analysis for the primary outcome according to the initial rhythm on the scene.
| Variables | Shockable rhythm on the scene (n = 804) | Non‐shockable rhythm on the scene (n = 353) | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| Age, y | 0.97 (0.95–0.98) | <0.001 | 0.97 (0.94–0.99) | 0.044 |
| Male | 0.52 (0.31–0.88) | 0.016 | 1.13 (0.47–2.68) | 0.777 |
| Witnessed arrest | 1.69 (0.96–3.00) | 0.068 | 0.32 (0.12–0.83) | 0.020 |
| Bystander CPR | 1.68 (1.12–2.52) | 0.011 | 0.91 (0.40–2.10) | 0.841 |
| AED use | 1.13 (0.70–1.81) | 0.602 | 0.36 (0.10–1.25) | 0.109 |
| Any ALS care | 0.42 (0.28–0.63) | <0.001 | 0.79 (0.34–1.70) | 0.559 |
Abbreviations: AED, automated external defibrillator; ALS, advanced life support; CI, confidence interval; CPR, cardiopulmonary resuscitation; OR, odds ratio.
TABLE 5.
A multiple logistic regression analysis for the primary outcome, stratifying the patients into cardiac origin or non‐cardiac origin.
| Variables | Cardiac origin (n = 1009) | Non‐cardiac origin (n = 280) | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| Age, y | 0.96 (0.95–0.98) | <0.001 | 0.97 (0.95–1.00) | 0.070 |
| Male | 0.86 (0.52–1.43) | 0.587 | 0.36 (0.16–0.84) | 0.019 |
| Witnessed arrest | 1.10 (0.67–1.82) | 0.680 | 0.58 (0.20–1.64) | 0.308 |
| Bystander CPR | 1.63 (1.10–2.42) | 0.019 | 0.95 (0.41–2.19) | 0.914 |
| Initial rhythm on the scene | ||||
| VF/pVT | Reference | Reference | ||
| Pulseless electrical activity | 0.45 (0.25–0.79) | 0.006 | 0.32 (0.11–0.93) | 0.036 |
| Asystole | 0.36 (0.15–0.84) | 0.019 | 0.49 (0.11–2.19) | 0.358 |
| AED use | 1.02 (0.70–1.72) | 0.657 | 0.43 (0.17–1.08) | 0.074 |
| Any ALS care | 0.47 (0.32–0.69) | <0.001 | 0.41 (0.18–0.93) | 0.034 |
Abbreviations: AED, automated external defibrillator; ALS, advanced life support; CI, confidence interval; CPR, cardiopulmonary resuscitation; OR, odds ratio; pVT, pulseless ventricular tachycardia; VF, ventricular fibrillation.
TABLE 6.
A multiple logistic regression analysis for the primary outcome, stratifying the patients according to an institutional proportion of patients who received prehospital ALS care (≥50% or <50%).
| Variables | ≥50% (n = 723) | <50% (n = 566) | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| Age, y | 0.96 (0.95–0.97) | <0.001 | 0.97 (0.96–0.99) | 0.024 |
| Male | 0.90 (0.51–1.58) | 0.730 | 0.49 (0.25–0.96) | 0.038 |
| Witnessed arrest | 0.80 (0.46–1.38) | 0.436 | 1.67 (0.75–3.72) | 0.208 |
| Bystander CPR | 1.57 (0.98–2.49) | 0.057 | 1.44 (0.83–2.49) | 0.184 |
| Initial rhythm on the scene | ||||
| VF/pVT | Reference | Reference | ||
| Pulseless electrical activity | 0.51 (0.26–0.96) | 0.040 | 0.24 (0.11–0.54) | 0.036 |
| Asystole | 0.42 (0.18–0.98) | 0.045 | 0.13 (0.17–1.06) | 0.058 |
| AED use | 0.68 (0.38–1.18) | 0.177 | 1.12 (0.62–2.01) | 0.691 |
| Any ALS care | 0.55 (0.35–0.87) | 0.011 | 0.16 (0.06–0.39) | <0.001 |
Abbreviations: AED, automated external defibrillator; ALS, advanced life support; CI, confidence interval; CPR, cardiopulmonary resuscitation; OR, odds ratio; pVT, pulseless ventricular tachycardia; VF, ventricular fibrillation.
3.4. Exploratory analyses for the primary outcome
When we examined specific components of ALS care, both prehospital epinephrine administration (adjusted ORs 0.52; 95% CI 0.34–0.80; p = 0.003) and prehospital advanced airway (adjusted ORs 0.68; 95% CI 0.47–0.99; p = 0.046) were associated with unfavorable neurological outcomes at hospital discharge (Table S3).
4. LIMITATIONS
This study has several limitations. First, the registry included only those who underwent ECPR. Presumably, there would be many potential candidates for ECPR who were not actually resuscitated with ECPR for reasons such as successful resuscitation by persistent conventional ALS, unavailability of ECMO teams or equipment, and its futility. Remarkably, prior randomized controlled trials showed that prehospital epinephrine administration or tracheal intubation increased the proportion of ROSC, 17 , 18 which would potentially make a difference in the “severity” of cardiac arrest between the groups, particularly among the population who underwent ECPR. Second, who would receive prehospital ALS care was not protocolized, but depended on the physicians' discretion. Hence, our results might be affected by unknown confounders. Third, the registry lacks several relevant data including number of defibrillations, prehospital epinephrine doses in the ALS group, and in‐hospital treatment data regarding ALS before ECMO establishment. Fourth, the timing of prehospital epinephrine administration or advanced airway in the ALS group was unknown. Given resuscitation time bias, the timing of these interventions may have been taken into account. 19 Fifth, the registry did not collect data on transport time, which could affect medical directors’ decisions. Some medical directors might advise EMS personnel to perform ALS because a longer transport time was expected. However, a previous study reported that transport time did not have an impact on neurological outcomes in patients with shockable rhythm. 20 Lastly, our findings may not be generalized outside of EMS systems where prehospital ALS is mainly provided by ELSTs under direct medical control by a physician.
5. DISCUSSION
In this exploratory post hoc analysis of SAVE‐J II study, we found that prehospital ALS, including epinephrine administration or advanced airway provided by EMS personnel, was associated with unfavorable neurological outcomes at hospital discharge in OHCA patients who received ECPR.
To our knowledge, there have been no prior studies investigating the effect of prehospital ALS, specifically among those who underwent ECPR after hospital arrival for refractory OHCA. A small observational study demonstrated that lower epinephrine doses during CPR were associated with good neurological outcomes in patients treated with ECPR. 21 However, this study did not provide any data regarding prehospital management. As for prehospital advanced airway, a registry study from Korea reported that patients with shorter time from arrest to ECPR had received prehospital advanced airway less frequently compared to patients with longer times from arrest to ECPR. 22 This study showed that patients with shorter time from arrest to ECPR had better survival; however, it failed to perform multivariable analysis for neurologically intact survival, owing to its limited number of outcomes.
Of note, major guidelines suggest that selection criteria for ECPR include shorter time to establishing ECPR or shorter “low flow time,” as well as younger age or witnessed arrest, based on extensive literature showing that the earlier the initiation of ECPR, the better the prognosis. 5 , 23 To start ECMO support as soon as possible, it would be reasonable for a medical director or code leader at a receiving hospital to request EMS personnel to immediately transport patients who meet the candidacy criteria for ECPR without performing ALS. Several studies have observed an association between prehospital epinephrine administration or advanced airway and increase in EMS scene time or total prehospital time. 24 , 25 Although the ALS group had prolonged total prehospital time compared with the no ALS group, merely 3‐min delay would not result in worse outcomes among patients who received prehospital ALS. Rather, a plausible interpretation for the adverse association of prehospital ALS in this study is that the patients in the ALS group were possibly more intractable to treatment because they did not respond to “early” ALS, indicating that prehospital ALS would be an unfavorable prognostic predictor among patients who underwent ECPR.
In subgroup analysis, the association between prehospital ALS care and unfavorable neurological outcomes at hospital discharge was not found in patients with non‐shockable rhythm on the scene. This observation might be attributed to the fact that prehospital advanced airway was associated with better survival among patients with non‐shockable rhythm but not among patients with shockable rhythm. 26
ECPR should be considered as an alternative intervention despite 10–15 min of conventional ALS. 23 Practically, ECMO support can take as little as 10 min or even much longer to be established. We observed that ECMO support was achieved after a median of 25 min of hospital arrival in both groups. Although detailed records of in‐hospital conventional ALS before ECMO support were unavailable, this would be a reasonable length of time. 27 Indeed, however, optimal timing for initiating ECPR remains undefined. A recent study documented a dilemma between early transport for ECPR and continuing on‐scene ALS. 28 Our study's strength is being a pragmatic, large‐scale registry study, in which we discovered the prehospital ALS was associated with unfavorable neurological outcomes in patients who received ECPR. We do not conclude that immediate transport strategy without performing ALS is superior if the patients would inevitably be eligible for ECPR; however, our study indicates that among patients resuscitated with ECPR, receiving prehospital ALS could be a predictor associated with worse neurological outcomes.
In conclusion, among patients who underwent ECPR for OHCA, patients with prehospital ALS compared to those without ALS were associated with higher unfavorable neurological outcomes at hospital discharge. These findings should be further investigated in future prospective studies to explore the clinical significance of prehospital ALS care among patients who are potential candidates for ECPR.
AUTHOR CONTRIBUTIONS
Tetsuya Yumoto, Toru Hifumi, Akihiko Inoue, and Hiromichi Naito designed the concept of the study. Tetsuya Yumoto, Toru Hifumi, Akihiko Inoue, Tetsuya Sakamoto, Yasuhiro Kuroda, and Hiromichi Naito conducted the study and performed the data acquisition. Tetsuya Yumoto, Takashi Hongo, and Takashi Yorifuji assessed the quality of the study and performed the analysis and interpretation. Tetsuya Yumoto, Atsunori Nakao, and Hiromichi Naito wrote the manuscript, and the other authors made substantial revisions and edits. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
We thank all members of the SAVE‐J II study group who participated in this study: Hirotaka Sawano, MD, PhD (Osaka Saiseikai Senri Hospital), Yuko Egawa, MD, Shunichi Kato, MD (Saitama Red Cross Hospital), Kazuhiro Sugiyama MD (Tokyo Metropolitan Bokutoh Hospital), Naofumi Bunya, MD, Takehiko Kasai, MD (Sapporo Medical University), Shinichi Ijuin, MD, Shinichi Nakayama, MD, PhD (Hyogo Emergency Medical Center), Jun Kanda, MD, PhD, Seiya Kanou, MD (Teikyo University Hospital), Toru Takiguchi, MD, Shoji Yokobori, MD, PhD (Nippon Medical School), Hiroaki Takada, MD, Kazushige Inoue, MD (National Hospital Organization Disaster Medical Center), Ichiro Takeuchi, MD, PhD, Hiroshi Honzawa, MD (Yokohama City University Medical Center), Makoto Kobayashi, MD, PhD, Tomohiro Hamagami, MD (Toyooka Public Hospital), Wataru Takayama, MD, Yasuhiro Otomo, MD, PhD (Tokyo Medical and Dental University Hospital of Medicine), Kunihiko Maekawa, MD (Hokkaido University Hospital), Takafumi Shimizu, MD, Satoshi Nara, MD (Teine Keijinkai Hospital), Michitaka Nasu, MD, Kuniko Takahashi, MD (Urasoe General Hospital), Yoshihiro Hagiwara, MD, MPH (Imperial Foundation Saiseikai, Utsunomiya Hospital), Shigeki Kushimoto, MD, PhD (Tohoku University Graduate School of Medicine), Reo Fukuda, MD (Nippon Medical School Tama Nagayama Hospital), Takayuki Ogura, MD, PhD (Japan Red Cross Maebashi Hospital), Shin‐ichiro Shiraishi, MD (Aizu Central Hospital), Ryosuke Zushi, MD (Osaka Mishima Emergency Critical Care Center), Norio Otani, MD (St. Luke's International Hospital), Migaku Kikuchi, MD, PhD (Dokkyo Medical University), Kazuhiro Watanabe, MD (Nihon University Hospital), Takuo Nakagami, MD (Omihachiman Community Medical Center), Tomohisa Shoko, MD, PhD (Tokyo Women's Medical University Medical Center East), Nobuya Kitamura, MD, PhD (Kimitsu Chuo Hospital), Takayuki Otani, MD (Hiroshima City Hiroshima Citizens Hospital), Yoshinori Matsuoka, MD, PhD (Kobe City Medical Center General Hospital), Makoto Aoki, MD, PhD (Gunma University Graduate School of Medicine), Masaaki Sakuraya, MD, MPH (JA Hiroshima General Hospital Hiroshima), Hideki Arimoto, MD (Osaka City General Hospital), Koichiro Homma, MD, PhD (Keio University School of Medicine), Shunichiro Nakao, MD, PhD (Osaka University Graduate School of Medicine), Tomoya Okazaki, MD, PhD (Kagawa University Hospital), Yoshio Tahara, MD, PhD (National Cerebral and Cardiovascular Center), Hiroshi Okamoto, MD, MPH (St. Luke's International Hospital), Jun Kunikata, MD, PhD, and Hideto Yokoi, MD, PhD (Kagawa University Hospital). The authors thank Christine Burr for editing the manuscript as well.
Biography
Tetsuya Yumoto, MD, PhD, practices medicine and performs clinical research in the Department of Emergency Medicine, Critical Care, and Disaster Medicine in the Faculty of Medicine, Dentistry, and Pharmaceutical Sciences at Okayama University in Okayama, Japan.

Yumoto T, Hongo T, Hifumi T, et al. Association between prehospital advanced life support by emergency medical services personnel and neurological outcomes among adult out‐of‐hospital cardiac arrest patients treated with extracorporeal cardiopulmonary resuscitation: A secondary analysis of the SAVE‐J II study. JACEP Open. 2023;4:e12948. 10.1002/emp2.12948
Supervising Editor: Karl Sporer, MD.
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist
REFERENCES
- 1. Yan S, Gan Y, Jiang N, et al. The global survival rate among adult out‐of‐hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta‐analysis. Crit Care. 2020;24(1):8‐13. doi: 10.1186/s13054-020-2773-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inoue A, Hifumi T, Sakamoto T, Kuroda Y. Extracorporeal cardiopulmonary resuscitation for out‐of‐hospital cardiac arrest in adult patients. J Am Heart Assoc. 2020;9(7):1‐12. doi: 10.1161/JAHA.119.015291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abrams D, MacLaren G, Lorusso R, et al. Extracorporeal cardiopulmonary resuscitation in adults: evidence and implications. Intensive Care Med. 2022;48(1):1‐15. doi: 10.1007/s00134-021-06514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. 2020;142(16_suppl_2):S366‐S468. doi: 10.1161/CIR.0000000000000916 [DOI] [PubMed] [Google Scholar]
- 5. Soar J, Böttiger BW, Carli P, et al. European Resuscitation Council Guidelines 2021: adult advanced life support. Resuscitation. 2021;161:115‐151. doi: 10.1016/j.resuscitation.2021.02.010 [DOI] [PubMed] [Google Scholar]
- 6. Wengenmayer T, Rombach S, Ramshorn F, et al. Influence of low‐flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care. 2017;21(1):1‐6. doi: 10.1186/s13054-017-1744-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bougouin W, Dumas F, Lamhaut L, et al. Extracorporeal cardiopulmonary resuscitation in out‐of‐hospital cardiac arrest: a registry study. Eur Heart J. 2020;41(21):1961‐1971. doi: 10.1093/eurheartj/ehz753 [DOI] [PubMed] [Google Scholar]
- 8. Ewy GA, Bobrow BJ, Chikani V, et al. The time dependent association of adrenaline administration and survival from out‐of‐hospital cardiac arrest. Resuscitation. 2015;96:180‐185. doi: 10.1016/j.resuscitation.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 9. Izawa J, Iwami T, Gibo K, et al. Timing of advanced airway management by emergency medical services personnel following out‐of‐hospital cardiac arrest: a population‐based cohort study. Resuscitation. 2018;128:16‐23. doi: 10.1016/J.RESUSCITATION.2018.04.024 [DOI] [PubMed] [Google Scholar]
- 10. Naito H, Yumoto T, Yorifuji T, et al. Improved outcomes for out‐of‐hospital cardiac arrest patients treated by emergency life‐saving technicians compared with basic emergency medical technicians: a JCS‐ReSS study report. Resuscitation. 2020;153:251‐257. doi: 10.1016/j.resuscitation.2020.05.007 November 2019 [DOI] [PubMed] [Google Scholar]
- 11. Inoue A, Hifumi T, Sakamoto T, et al. Extracorporeal cardiopulmonary resuscitation in adult patients with out‐of‐hospital cardiac arrest: a retrospective large cohort multicenter study in Japan. Crit Care. 2022;26(1):1‐11. doi: 10.1186/s13054-022-03998-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe M, Matsuyama T, Miyamoto Y, Kitamura T, Komukai S, Ohta B. The impact of different targeted temperatures on out‐of‐hospital cardiac arrest outcomes in patients receiving extracorporeal membrane oxygenation: a nationwide cohort study. Crit Care. 2022;26(1):1‐9. doi: 10.1186/s13054-022-04256-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perkins GD, Jacobs IG, Nadkarni VM, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry templates for out‐of‐hospital cardiac arrest: a statement for healthcare professionals from a task force of the international liaison committee. Circulation. 2015;132(13):1286‐1300. doi: 10.1161/CIR.0000000000000144 [DOI] [PubMed] [Google Scholar]
- 14. Lee SW, Han KS, Park JS, Lee JS, Kim SJ. Prognostic indicators of survival and survival prediction model following extracorporeal cardiopulmonary resuscitation in patients with sudden refractory cardiac arrest. Ann Intensive Care. 2017;7(1):87. doi: 10.1186/s13613-017-0309-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Debaty G, Babaz V, Durand M, et al. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out‐of‐hospital refractory cardiac arrest. A systematic review and meta‐analysis. Resuscitation. 2017;112:1‐10. doi: 10.1016/j.resuscitation.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 16. Okada Y, Kiguchi T, Irisawa T, et al. Development and validation of a clinical score to predict neurological outcomes in patients with out‐of‐hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation. JAMA Netw open. 2020;3(11):e2022920. doi: 10.1001/jamanetworkopen.2020.22920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perkins GD, Ji C, Deakin CD, et al. A randomized trial of epinephrine in out‐of‐hospital cardiac arrest. N Engl J Med. 2018;379(8):711‐721. doi: 10.1056/nejmoa1806842 [DOI] [PubMed] [Google Scholar]
- 18. Jabre P, Penaloza A, Pinero D, et al. Effect of bag‐mask ventilation vs endotracheal intubation during cardiopulmonary resuscitation on neurological outcome after out‐of‐hospital cardiorespiratory arrest a randomized clinical trial. JAMA ‐ J Am Med Assoc. 2018;319(8):779‐787. doi: 10.1001/jama.2018.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andersen LW, Grossestreuer AV, Donnino MW. “Resuscitation time bias”—A unique challenge for observational cardiac arrest research. Resuscitation. 2018;125:79‐82. doi: 10.1016/j.resuscitation.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chien CY, Tsai SL, Tsai LH, et al. Impact of transport time and cardiac arrest centers on the neurological outcome after out‐of‐hospital cardiac arrest: a retrospective cohort study. J Am Heart Assoc. 2020;9(11):1‐9. doi: 10.1161/JAHA.119.015544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shin YS, Kim YJ, Ryoo SM, et al. Promising candidates for extracorporeal cardiopulmonary resuscitation for out‐of‐hospital cardiac arrest. Sci Rep. 2020;10(1):1‐9. doi: 10.1038/s41598-020-79283-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park JH, Song KJ, Do ShinS, Ro YS, Hong KJ. Time from arrest to extracorporeal cardiopulmonary resuscitation and survival after out‐of‐hospital cardiac arrest. EMA ‐ Emerg Med Australas. 2019;31(6):1073‐1081. doi: 10.1111/1742-6723.13326 [DOI] [PubMed] [Google Scholar]
- 23. Richardson AC, Tonna JE, Nanjayya V, et al. Extracorporeal cardiopulmonary resuscitation in adults. interim guideline consensus statement from the extracorporeal life support organization. ASAIO J. 2021:221‐228. doi: 10.1097/MAT.0000000000001344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park SY, Lim D, Kim SC, et al. Effect of prehospital epinephrine use on survival from out‐of‐hospital cardiac arrest and on emergency medical services. J Clin Med. 2022;11(1). doi: 10.3390/jcm11010190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Zhang Q, Qu GB, et al. Effects of prehospital management in out‐of‐hospital cardiac arrest: advanced airway and adrenaline administration. BMC Health Serv Res. 2022;22(1):1‐10. doi: 10.1186/s12913-022-07890-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Izawa J, Komukai S, Gibo K, et al. Pre‐hospital advanced airway management for adults with out‐of‐hospital cardiac arrest: nationwide cohort study. BMJ. 2019;364. doi: 10.1136/bmj.l430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SJ, Jung JS, Park JH, Park JS, Hong YS, Lee SW. An optimal transition time to extracorporeal cardiopulmonary resuscitation for predicting good neurological outcome in patients with out‐of‐hospital cardiac arrest: a propensity‐matched study. Crit Care. 2014;18(5):1‐15. doi: 10.1186/s13054-014-0535-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alm‐Kruse K, Sørensen G, Osbakk SA, et al. Outcome in refractory out‐of‐hospital cardiac arrest before and after implementation of an ECPR protocol. Resuscitation. 2021;162:35‐42. doi: 10.1016/j.resuscitation.2021.01.038 September 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


