Abstract
In Japan, a legal framework has been established for the safe and effective application of regenerative medicine. After eight years of the Act on the Safety of Regenerative Medicine (RM Act), discussions have been underway in the Ministry of Health, Labor and Welfare of Japan to revise the law owing to numerous novel technologies and inappropriate case reports not anticipated when the law was enacted. Therefore, in this review article, we have reviewed the regenerative medicine provision plans and the contribution of platelet-rich plasma (PRP) therapy, a regenerative medicine technique widely used in Japan post RM Act implementation, to these plans.
As of January 2022, 97.2% of the regenerative medicine provided under the RM Act had been for private practice, and most of them were Class Ⅲ regenerative medicine. Notably, PRP was the most used processed cell under the RM Act. PRP therapy accounted for approximately 66% of the regenerative medicine provision plans in clinical research or private practice and was the most provided regenerative medicine technology in Japan. PRP therapy was primarily used in dentistry to regenerate periodontal tissue (approximately 50%), followed by orthopedics, where it is used to treat osteoarthritis. We suggest that further discussion is essential to determine the factors that should be addressed by the RM act to evaluate the efficacy and safety of PRP therapy.
Keywords: Platelet-rich plasma, Regenerative medicine, Dental, Orthopedic, The Act on the Safety of Regenerative Medicine
Highlights
-
•
PRP therapy is the most widely provided regenerative medicine in Japan.
-
•
Approximately 50% of PRP therapy is offered in dentistry, followed by orthopedics.
-
•
PRP therapy is mostly used to treat periodontal tissue loss and osteoarthritis.
1. Introduction
In 2012, the receipt of the Nobel Prize in Physiology or Medicine by Professor Shinya Yamanaka, Kyoto University, for discovering induced pluripotent stem (iPS) cell lines, has increased public attention to regenerative medicine technology. It has further caused a breakthrough in regenerative medicine research in Japan. In addition, regenerative medicine is a crucial field for medical strategy in Japan, given that this country is one of the largest aging populations in the world and is predicted to have an increasingly aging population in the future.
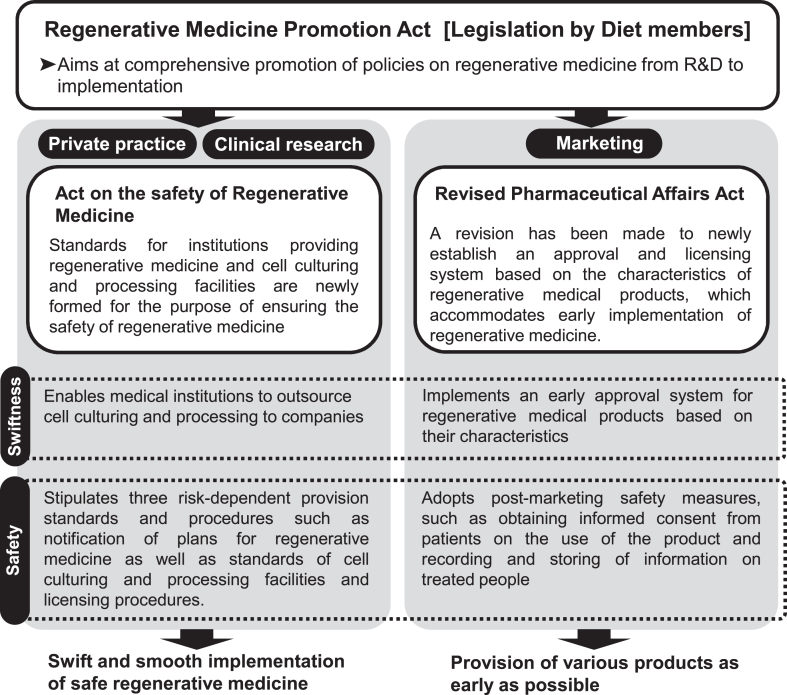
For these reasons, a framework was established to promote the safe and appropriate provision of regenerative medicine in Japan. In 2013, the “Act on Comprehensive Promotion of Measures for Ensuring Prompt and Safe Access to Regenerative Medicine for the Public (Regenerative Medicine Promotion Act)" was promulgated to promote measures from research and development to the practical application of regenerative medicine. As a result of this legislated law, two regenerative medicine bills, “the Act on the Safety of Regenerative Medicine (RM Act)" and “Pharmaceuticals, Medical Devices, and Other Therapeutic Products Act (PMD Act)," came into effect on November 25, 2014 [1,2]. The RM Act stipulates items the matters that physicians and dentists must comply with when providing regenerative medicine, and it is positioned under the Medical Care Act and the Medical Practitioner's Act, to regulate the safety and appropriateness of regenerative medical technologies using processed cells. In contrast, the PMD Act stipulates items that companies must comply with when manufacturing and selling regenerative medical products. Furthermore, the newly approval process for regenerative medical products was also introduced (conditional time-limited approval system), under the system, if the clinical trials predict likely efficacy and confirm safety, the regenerative medicine products will be given conditional, time-limited approval (Fig. 1).
Fig. 1.
Institutional framework for promoting the future implementation of regenerative medicine [2].
Eight years have passed since the enactment of the RM Act, and discussions are underway in the Ministry of Health, Labor and Welfare (MHLW) to revise the law in response to the advent of numerous technologies and inappropriate case reports that were not anticipated when the law was enacted [3,4]. In this review article, we have summarized the regenerative medicine provision plans. Furthermore, we have elucidated the contribution of platelet-rich plasma (PRP) therapy, a regenerative medicine technique widely used in Japan post RM act implementation, to these provision plans.
2. Act on the safety of regenerative medicine
The RM Act stipulates regulations for regenerative medicine procedures performed in medical institutions under the responsibility of physicians or dentists. Specifically, the act ensures the safety of regenerative medicine therapy in clinical research and private practice by stipulating sampling procedures, standards for medical institutions, and cell culturing facilities.
Medical institutions providing regenerative medicine therapy can have a cell processing facility. The manufacture of specific processed cells is subjected to a permit system (or notification in the case of medical institutions). A medical institution may only outsource cell processing to an external facility that has obtained a manufacturing permit.
Once a cell processing facility is accredited, each plan for regenerative medicine provision must be reviewed by the Certified Committee for Regenerative Medicine according to their risk classification. Based on the risk to humans, regenerative medicines are classified into three levels: Class Ⅰ (high risk), Class Ⅱ (medium risk), and Class Ⅲ (low risk) [2]. Class Ⅰ regenerative medicine includes therapies that use iPS, transfected, or allogeneic cells. Class Ⅱ regenerative medicine includes therapies that use cultured or uncultured autologous stem cells. Class Ⅲ regenerative medicine includes therapies that use autologous somatic cells (such as processed cells without culture and cancer immunotherapy using activated autologous lymphocytes).
After the examination and approval by the Certified Committee for Regenerative Medicine, the provision plan must be submitted to the Japanese government, and periodic reports on the status (such as the implementation status) must also be updated. The government can monitor their actual status through the licensing, review, approval, and periodic reports of cell processing facilities.
3. Actual situation of providing regenerative medicine under the RM act
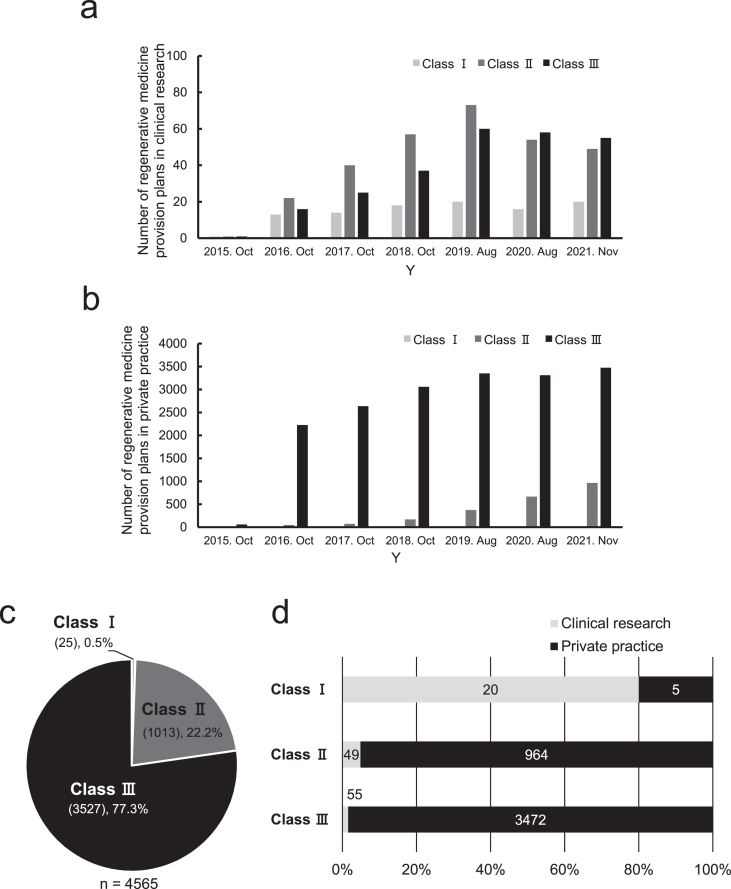
We determined that the annual trend of the number of regenerative medicine provision plans in clinical research had gradually increased from 2016 to 2019 and gradually decreased from 2019 to 2021 after the implementation of the RM Act (Fig. 2a). This is mainly attributed to a substantial increase in provision plans for Classes Ⅱ and Ⅲ (Fig. 2a). In private practice, the number of Class Ⅲ regenerative medicine provision plans had markedly increased and accounted for approximately 95% of all the provision plans in 2018 (Fig. 2b). Subsequently, the number of Class Ⅱ regenerative medicine provision plans had gradually increased from 2019 to 2021 and accounted for approximately 20% of all the provision plans as of November 2021 (Fig. 2b).
Fig. 2.
Current status of regenerative medicine provision plans under the RM Act in Japan. a, Annual transition of the number of regenerative medicine provision plans on research. b, Annual transition of the number of regenerative medicine provision plans on private practice. c, Ratio of the number of plans by type to the total number of provision plans as of November 2021. d, Ratio of research and private practice in each class of provision plans as of November 2021.
Total cases as of November 2021 had been 4565 (Fig. 2c). Among these, class Ⅲ and Class Ⅱ provision plans accounted for 77.3% (3527 cases) and 22.2% (1013 cases), respectively. Further breakdown of the classification revealed that class I provision plans accounted for 80% of total provision plans in clinical research, and class Ⅱ and Ⅲ provision plans accounted for over 95% of total provision plans in private practice. (Fig. 2d).
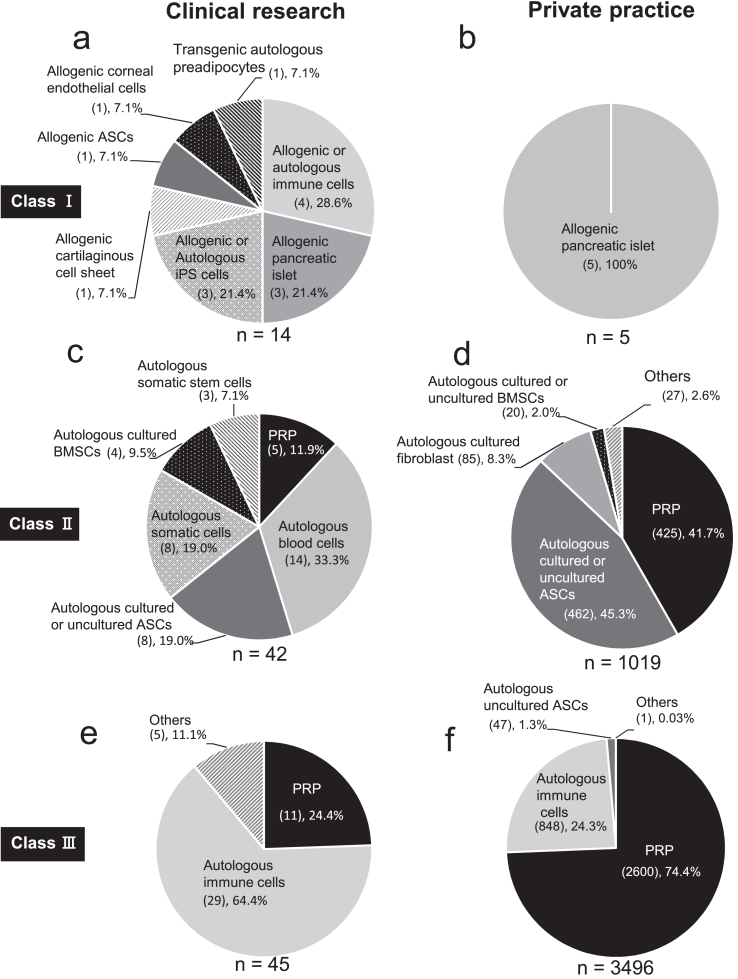
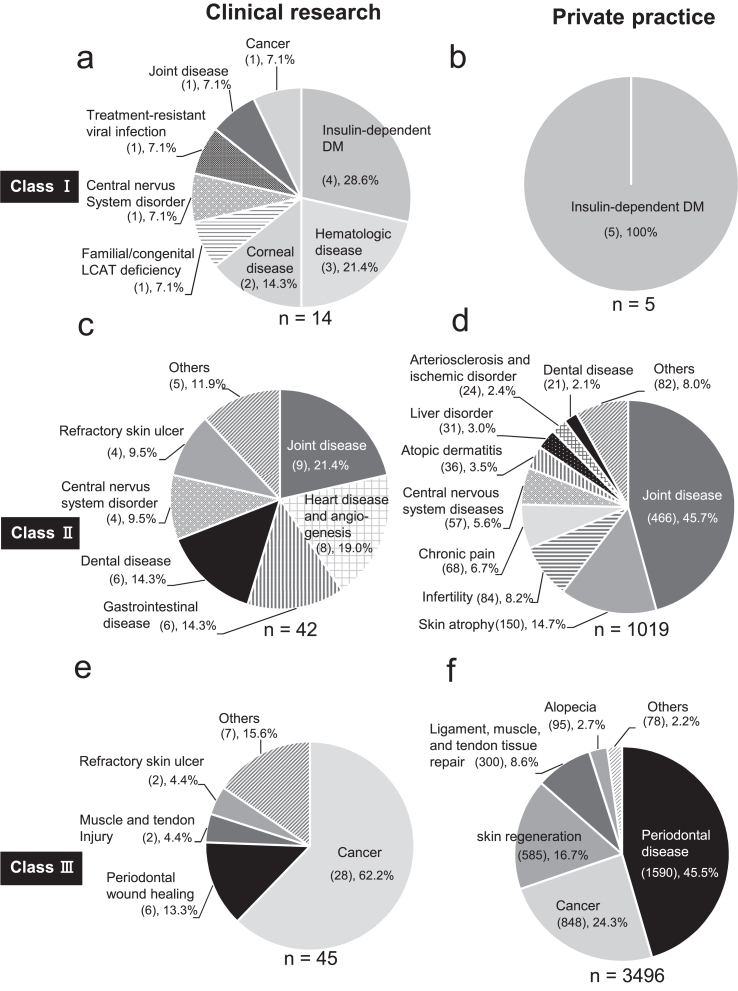
Next, we examined the specific processed cells that had been used to target diseases in both clinical research and private practice under the RM Act as of January 2022 (Fig. 3, Fig. 4).
Fig. 3.
Types of specific processed cells used in regenerative medicine under the RM act in Japan. We used the information on the regenerative medicine provision plans published by the Ministry of Health, Labor and Welfare as of Jan 2022. a, c, e, types of specific processed cells for regenerative medicine in clinical research. b, d, f, types of specific processed cells for regenerative medicine in private practice. PRP, platelet-rich plasma. ASCs, adipose-derived stem cells. BMSCs, bone marrow-derived stem cells.
Fig. 4.
Types of diseases targeted by regenerative medicine in private practice under the RM act in Japan. We used the information on the regenerative medicine provision plans published by the Ministry of Health, Labor and Welfare as of Jan 2022. a, c, e, target disease of regenerative medicine on clinical research. b, d, f, Target disease of regenerative medicine in private practice. DM, diabetes mellitus.
Under Class Ⅰ, allogenic or autologous immune cells, donor-derived islets, iPS cells, and transgenic patient's cells were used in clinical research (Fig. 3a). In contrast, only donor-derived islets were used in private practice (Fig. 3b). In target diseases in Class Ⅰ, insulin-dependent diabetes mellitus (Fig. 4 a and b) was the most common target disease in clinical research (4 out of 14 cases, 28.6%) and only target disease in private practice (all 5 cases, 100%). Under Class Ⅱ, allogenic blood cells, mesenchymal stem cells (adipose-derived stem cells (ASCs), and somatic cells were the most commonly used cells in clinical research (Fig. 3c). In contrast, autologous PRP (425 out of 1019 cases, 41.7%) and ASCs (462 out of 1019 cases, 45.3%) were the most commonly used cells used in private practice (Fig. 3d). Joint disease such as osteoarthritis (Fig. 4 c and d) was the most common target disease in Class Ⅱ in both clinical research (9 out of 42 cases, 21.4%) and private practice (466 out of 1019 cases, 45.7%).
Under Class Ⅲ, autologous immune cells (29 out of 45 cases, 64.4%) and PRP (2600 out of 3496 cases, 74.4%) were the most commonly used cells in clinical research (Fig. 3e) and private practice (Fig. 3f), respectively. Cancer (28 out of 45 cases, 62.2%) and periodontal disease tissue loss (1590 out of 3496 cases, 45.5%) were the most common target diseases in clinical research (Fig. 4e) and private practice (Fig. 4f), respectively.
4. Actual situation of providing PRP therapy under the RM act
PRP is the portion of the plasma recovered by centrifuging the autologous blood. As PRP is rich in multiple growth factors and cytokines, it is used for wound healing, pain relief, skincare, and anti-aging treatment. Furthermore, PRP treatment is used in various fields, such as dentistry, orthopedics, plastic surgery, and dermatology [[5], [6], [7], [8]]. Plasma rich in growth factor (PRGF) [9,10] is obtained by centrifuging the anticoagulant-added blood at a lower speed and for a shorter time than in PRP centrifugation. Concentrated growth factor (CGF) [11] and platelet-rich fibrin (PRF) [12], which are produced by the centrifugation of blood without adding an anticoagulant, are also used for the treatment. In addition, PRP treatment under the RM act is one of the most popular regenerative medicine techniques in Japan owing to its simple and short time (without culturing) manufacturing process.
As of January 2022, among 4621 regenerative medicine provision plans in clinical research and private practice in Japan, 3041 provision plans (65.8%) had been for PRP therapy, contributing to the maximum number of provision plans.
Subsequently, we classified the regenerative medicine provision plans for PRP therapy by risk. We observed that PRP therapy was provided as Class Ⅱ and Class Ⅲ regenerative medicine. In clinical research, provision plans for PRP therapy were provided as Class II for 5 out of 42 cases (11.9%) (Fig. 3c) and Class Ⅲ for 11 out of 45 cases (24.4%) (Fig. 3e). In private practice, provision plans for PRP therapy were provided as Class Ⅱ for 425 out of 1019 cases (41.7%) (Fig. 3d) and Class Ⅲ for 2600 out of 3496 cases (74.4%) (Fig. 3f). As of January 2022, most PRP therapies had been provided as Class III in private practice.
The breakdown of PRP-related therapies in Class Ⅲ private practice (2600 out of 3496 cases, 74.4%) suggested that the treatment for 1200, 1139, 150, 108, and 3 cases were with PRP, CGF, PRF, PRGF, and others, respectively. Almost all CGF, PRF, and PRGF therapies were provided in dentistry.
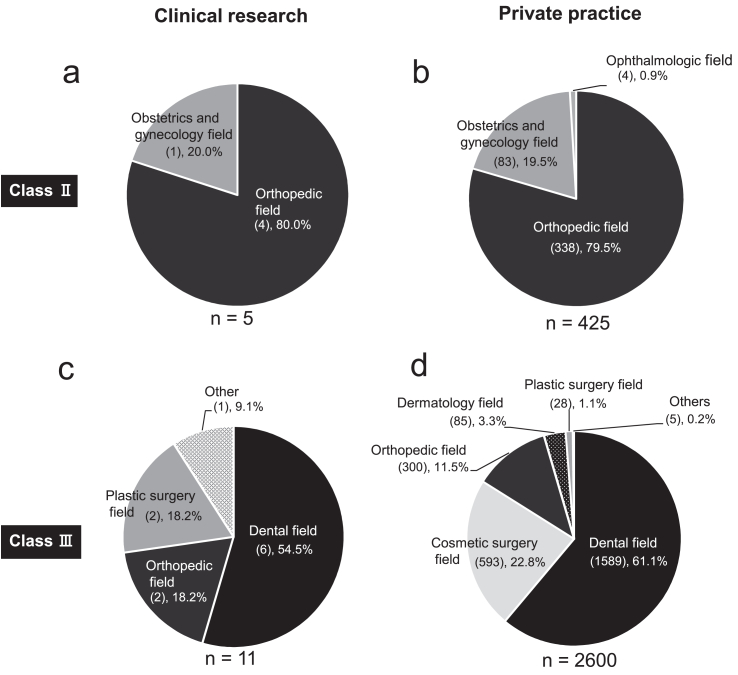
Next, PRP therapy was categorized by its percentage utilization in a department (Fig. 5). PRP therapy is mainly used in dentistry (e.g., tissue regeneration in periodontal disease), orthopedics (e.g., treatment of osteoarthritis and other joint diseases), plastic surgery (e.g., treatment of intractable skin ulcers), and cosmetic surgery (e.g., anti-aging). In Class Ⅱ, PRP therapy contributed to 80% and 79.5% of orthopedic disease treatment (intra-articular administration) in clinical research and private practice, respectively. It contributed to 20% and 19.5% of obstetrics and gynecology (infertility) treatment in clinical research and private practice, respectively. (Fig. 5a and b). Approximately 50% of Class Ⅲ PRP therapies in both clinical research and private practice were provided for diseases in the dental field, which contributed to the maximum utilization of PRP therapy in Japan (Fig. 5c and d).
Fig. 5.
Purpose of using PRP in clinical research and private practice under the RM act in Japan. We used the information on the regenerative medicine provision plans published by the Ministry of Health, Labor and Welfare as of Jan 2022. a, class Ⅱ, research. b, class Ⅱ, private practice. c, class Ⅲ, research. d, class Ⅲ, private practice.
5. Conclusions
In this review article, we have summarized the regenerative medicine applications based on eight years of data generated after the enactment of the RM act in Japan. As of January 2022, 97.2% of the regenerative medicine provided under the RM act had been for private practice, and most of them were for Class Ⅲ regenerative medicines. Notably, PRP was the most substantially used type of all specific processed cells.
PRP therapy accounted for approximately 66% of regenerative medicine provision plans in clinical research or private practice and was the most provided regenerative medicine technology in Japan. PRP therapy was significantly utilized in orthopedics (as class Ⅱ) and dentistry (as class Ⅲ) for treating osteoarthritis and periodontal tissue loss, respectively. PRP can be manufactured as PRF, CGF, and PRGF; each type is applied depending on the requirement for target disease treatment in dentistry.
Although a safety reporting requirement exists under the RM Act, serious adverse events have not been observed thus far in Japan. In contrast, in other countries, a case of local Staphylococcus aureus infection after PRP injection to treat ruptured gastrocnemius muscle was reported in Turkey [13]. In addition, clinical studies in Iran, Egypt, France, and Spain have reported cases of infection after PRP therapy [[14], [15], [16], [17], [18]]. The causal relationship between PRP administration and infection is controversial. Furthermore, in PRP therapy for anti-aging, severe cases of serum sickness after PRP administration in Poland [19] and a case of blindness after PRP administration in an American spa facility [20] have been reported. Therefore, further careful examination is required to ascertain whether PRP and other regenerative therapies performed in Japan are truly safe as a treatment.
Currently, MHLW is considering legal revisions to the RM Act in response to several new technologies and inappropriate case reports that were not anticipated when the law was enacted. Strategies for the efficient evaluation of efficacy and ensuring the safety of PRP therapy are currently ongoing. In regenerative medicine therapy, collecting treatment results and adequately evaluating the efficacy and safety is crucial. Therefore, an improved system, including the development of a treatment registry, may be required.
Declaration of competing interest
All the authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank editage for the English language review.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Konomi K., Tobita M., Kimura K., Sato D. New Japanese initiatives on stem cell therapies. Cell Stem Cell. 2015;16:350–352. doi: 10.1016/j.stem.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Tobita M., Konomi K., Torashima Y., Kimura K., Taoka M., Kaminota M. Japan's challenges of translational regenerative medicine: act on the safety of regenerative medicine. Regen Ther. 2016;4:78–81. doi: 10.1016/j.reth.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The materials of "the 74th council on health sciences, evaluation subcommittee on regenerative medicine, etc.", https://www.mhlw.go.jp/content/10808000/000910650.pdf [Accessed 22 April 2022].
- 4.The Materials of "the 76th council on health sciences, evaluation subcommittee on regenerative medicine, etc.". , https://www.mhlw.go.jp/content/10808000/000933837.pdf [Accessed 28 April 2022].
- 5.Xu J., Gou L., Zhang P., Li H., Qiu S. Platelet-rich plasma and regenerative dentistry. Aust Dent J. 2020;65:131–142. doi: 10.1111/adj.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connell B., Wragg N.M., Wilson S.L. The use of PRP injections in the management of knee osteoarthritis. Cell Tissue Res. 2019;376:143–152. doi: 10.1007/s00441-019-02996-x. [DOI] [PubMed] [Google Scholar]
- 7.Merchán W.H., Gómez L.A., Chasoy M.E., Alfonso-Rodríguez C.A., Muñoz A.L. Platelet-rich plasma, a powerful tool in dermatology. J Tissue Eng Regen Med. 2019;13:892–901. doi: 10.1002/term.2832. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Ghname A., Perdanasari A.T., Davis M.J., Reece E.M. Platelet-rich plasma, principles and applications in plastic surgery. Semin Plast Surg. 2019;33:155–161. doi: 10.1055/s-0039-1693400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–535. [PubMed] [Google Scholar]
- 10.Anitua E. The use of plasma-rich growth factors (PRGF) in oral surgery. Pract Proced Aesthetic Dent PPAD. 2001;13:487–493. [PubMed] [Google Scholar]
- 11.Corigliano M., Sacco L., Baldoni E. CGF-una proposta terapeutica per la medicina rigenerativa. Odontoiatria. 2010;1:69–81. [Google Scholar]
- 12.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J., et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Dincer D., Tanacan E., Cakir Akay G.A., Atac G.K., Evrin T. Localized infection and leg ulcer after platelet-rich plasma injection. Dermatol Ther. 2020;33 doi: 10.1111/dth.13948. [DOI] [PubMed] [Google Scholar]
- 14.Senet P., Bon F.X., Benbunan M., Bussel A., Traineau R., Calvo F., et al. Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers. J Vasc Surg. 2003;38:1342–1348. doi: 10.1016/s0741-5214(03)00908-x. [DOI] [PubMed] [Google Scholar]
- 15.Anitua E., Aguirre J.J., Algorta J., Ayerdi E., Cabezas A.I., Orive G., et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater. 2008;84:415–421. doi: 10.1002/jbm.b.30886. [DOI] [PubMed] [Google Scholar]
- 16.Ghaffarpasand F., Shahrezaei M., Dehghankhalili M. Effects of platelet rich plasma on healing rate of long bone non-union fractures: a randomized double-blind placebo controlled clinical trial. Bull Emerg Trauma. 2016;4:134–140. [PMC free article] [PubMed] [Google Scholar]
- 17.Mehrabi Bahar M., Ali Akbarian M., Azadmand A. Investigating the effect of autologous platelet-rich plasma on pain in patients with pilonidal abscess treated with surgical removal of extensive tissue. Iran Red Crescent Med J. 2013;15 doi: 10.5812/ircmj.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gohar M.M., Ali R.F., Ismail K.A., Ismail T.A., Nosair N.A. Assessment of the effect of platelet rich plasma on the healing of operated sacrococcygeal pilonidal sinus by lay-open technique: a randomized clinical trial. BMC Surg. 2020;20:212. doi: 10.1186/s12893-020-00865-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owczarczyk-Saczonek A., Wygonowska E., Budkiewicz M., Placek W. Serum sickness disease in a patient with alopecia areata and Meniere' disease after PRP procedure. Dermatol Ther. 2019;32 doi: 10.1111/dth.12798. [DOI] [PubMed] [Google Scholar]
- 20.Kalyam K., Kavoussi S.C., Ehrlich M., Teng C.C., Chadha N., Khodadadeh S., et al. Irreversible blindness following periocular autologous platelet-rich plasma skin rejuvenation treatment. Ophthalmic Plast Reconstr Surg. 2017;33:S12–S16. doi: 10.1097/IOP.0000000000000680. [DOI] [PubMed] [Google Scholar]