Abstract
Introduction
Immunoadsorption (IA) of isohemagglutinins is an often-crucial procedure in preparation of major ABO blood group-incompatible living donor kidney transplantation (ABOi LDKT). Standard citrate-based anticoagulation during the procedure has potential disadvantages for distinct patient groups. In this study, we report our experience with an alternative anticoagulation scheme using heparin during IA for selected patients.
Methods
We conducted a retrospective analysis of all patients who underwent IA with heparin anticoagulation between February 2013 and December 2019 at our institution with focus on the safety and efficacy of the adapted procedure. For further validation, we compared graft function, graft survival, and overall survival with those of all recipients of living donor kidney transplants with or without pretransplant desensitizing apheresis for ABO antibodies at our institution during the same period.
Results
In thirteen consecutive patients prepared for ABOi LDKT with IA with heparin anticoagulation, no major bleeding or other significant complications were observed. All patients achieved sufficient isohemagglutinin titer reduction to proceed to transplant surgery. Graft function, graft survival, and overall survival did not significantly differ from patients treated with standard anticoagulation for IA or ABO compatible recipients of living donor kidneys.
Conclusion
IA with heparin in preparation of ABOi LDKT is safe and feasible for selected patients after internal validation.
Keywords: Apheresis, Immunoadsorption, Heparin, Citrate, ABO-incompatible kidney transplant
Introduction
Since the first successful kidney transplantation in 1954, many patients have benefited from this procedure, living longer and with a better quality of life at less healthcare costs [1, 2, 3, 4]. However, the number of patients qualifying for a kidney transplant still exceeds the number of available organs by far, resulting in increased morbidity and mortality for patients on a waiting list [5]. The main obstacle for living donor transplantation is the additional immunohematological barrier caused by the ABO-blood group system [6], hindering a regular transplant because of potential immediate humoral rejection in case of major ABO incompatibility [7, 8]. ABO-blood group antigens are highly expressed on the endothelium in renal arteries, glomerular and peritubular capillaries, and veins [9]. The chance that a willing living donor has a mismatched ABO blood group is about 30% [10]. For many patients with end-stage renal failure, ABO-incompatible living donor kidney transplantation (ABOi LDKT) remains the only available option to avoid renal replacement therapy.
In the past decades, progress in pretransplant ABO antibody (i.e., isohemagglutinins) removal and immunosuppression has markedly improved outcomes after ABOi LDKT and therefore has become a standard procedure in many centers [11, 12]. Essential for a successful ABOi LDKT is the sufficient lowering of isohemagglutinin titers at the time of transplantation and ideally during the few following weeks until tolerance is established [13]. Immunosuppression alone may be sufficient in patients with low initial ABO antibody titers, and the pheresis procedure may be omitted [14].
Immunoadsorption (IA) is a standard procedure for antibody removal before ABOi LDKT [15]. Due to the specificity of the columns for a single or few antibodies, other plasma proteins including coagulation factors are largely unaffected, and exogenous replacement fluids are not needed. Compared to plasma exchange (PEX), larger plasma volumes can be processed with IA maintaining coagulation factors [16]. In therapeutic apheresis, effective anticoagulation to prevent clotting of extracorporeal blood is mandatory [17]. For most therapeutic apheresis procedures, acid-citrate-dextrose solution A (ACD-A) is the standard anticoagulation. ACD-A is effective at low costs, generally well-tolerated, and rapidly metabolized if hepatic and renal functions are adequate [17, 18]. One main disadvantage of ACD-A is the common side effect of hypocalcemia with its risk for dysesthesias, tetany, seizures, cardiac arrhythmias, or metabolic alkalosis [17, 18, 19]. Hence, in many centers, calcium infusions, which have shown to be superior to oral administration [20], are given during the procedure to minimize side effects. This measure can be disadvantageous, especially in patients with end-stage renal disease at risk of volume overload. Moreover, in renal disease, reduced capacity to excrete bicarbonate also hampers clearance of byproducts of citrate metabolism and may further increase the risk of metabolic alkalosis. Therefore, it remains unclear whether ACD-A is the best option for anticoagulation during IA. For some apheresis procedures, for example, extracorporeal photopheresis, heparin anticoagulation is established as an alternative to ACD-A [17]. With heparin anticoagulation, smaller volumes and electrolyte shifts occur during apheresis compared to apheresis with ACD-A anticoagulation and calcium infusion. This represents a potential advantage for patients at risk of clinically relevant volume overload. Thus, additional hemodialysis for volume adjustment may be avoided in the days immediately preceding transplantation surgery. Heparin clearance is reduced in severe kidney disease, especially at higher doses, but can be safely used in these patients [21]. Anticoagulation effects are completely reversible with protamine [22]. The most severe adverse effects of heparin are bleeding [23] and the less common but equally feared heparin-induced thrombocytopenia (HIT) [24]. The prevalence of HIT due to procedural heparin anticoagulation during apheresis is not known, and data are limited to case reports [25]. Following this, an alternative strategy for large-volume IA with heparin anticoagulation in selected patients was developed and established at our center in 2013.
In this retrospective case series, we aim to describe the feasibility and safety of heparin anticoagulation as an alternative to ACD-A anticoagulation for preoperative IA in patients scheduled for ABOi LDKT. Further, we compare the main outcomes of patients after living donor kidney transplant with or without IA with both ACD-A and heparin anticoagulation.
Material and Methods
Study Design and Population
A protocol for IA with heparin anticoagulation was introduced at our institution in February 2013 with continuous prospective and standardized documentation of procedural measures including coagulation assays and safety outcomes. All adult patients undergoing ABOi LDKT were evaluated for the eligibility of IA with heparin by the treating nephrologist. Selection was based on the risk of volume overload. Patients already on hemodialysis or with a daily urine output of less than 1,000 mL qualified for heparin IA. The definite allocation to the heparin anticoagulation was a joint decision between the treating nephrologist and the responsible physician of the apheresis unit.
For this retrospective, single-center study, we included all patients who underwent IA with heparin anticoagulation since the introduction of the procedure in February 2013 until December 2019. The main goal was to assess the safety, feasibility, and efficiency of the newly introduced anticoagulation scheme during apheresis. Recorded safety events included major bleeding > WHO grade 2 and coagulation assays out of target range; feasibility was assessed with clotting in the filter system of the apheresis machine. Efficiency of IA was assessed by the reduction of isohemagglutinin titer to a defined threshold to allow transplantation. In addition to the procedural documentation, patient, donor, and transplant characteristics were collected by chart review and through the electronic database of our institution. Besides this, we analyzed the total number of procedures for achieving target isohemagglutinin titers as indicated below, the need for early posttransplant interventions in case of rebound of isohemagglutinins, or the need for treating immediate rejection.
All patients undergoing a kidney transplant are routinely followed up monthly for the first 6 months and then every 3 months for the first year followed by yearly follow-up in the nephrology outpatient clinic. All complications were prospectively recorded at each clinical visit.
For further intrainstitutional validation of the newly introduced procedural anticoagulation scheme, short-term and long-term outcomes were compared to all 222 remaining recipients of LDKT at our institution during the study period divided into two groups. The first comparison group consists of all ABOi LDKT recipients who were not treated with IA with heparin anticoagulation (ABOi nonheparin) because they either received ACD-A anticoagulation during apheresis (n = 26) or did not need any apheresis procedure due to low isohemagglutinin titers (n = 12). The second group consists of all LDKT recipients with matched ABO blood group (ABOc) (n = 184). Review of baseline characteristics was done as for our main cohort (ABOi heparin). For further analyses, we assessed graft function by creatinine and eGFR at 1 month, 1 year, 3 years, and 5 years after transplantation as well as graft survival and overall survival. Follow-up was current as of January 2022. The study was performed according to the Declaration of Helsinki and approved by the Local Ethics Committee (EKNZ: 2016-01967).
Patient Preparation and Desensitization Protocol
All patients undergoing major ABOi LDKT were prepared and desensitized as previously described [26]. In short, basic immunosuppressive therapy including tacrolimus, mycophenolate mofetil, and prednisone was started 2 weeks before transplantation. A single intravenous infusion of rituximab (375 mg/m2) was given 4 weeks before transplantation in an outpatient setting. The transplantation was carried out after achieving the target isohemagglutinin titer (see below). Additional 20 mg of intravenous (IV) basiliximab was administered on days 0 and 4.
Immunoadsorption
IA was performed on a cell separator (Spectra Optia; Terumo BCT, Lakewood, CO, USA) using a low-molecular carbohydrate column containing A and/or B blood group antigens linked to a Sepharose matrix (Glycosorb; Glycorex Transplantation, Lund, Sweden). The number of planned procedures was depending on initial isohemagglutinin titers and adjusted according to isohemagglutinin titer reduction, based on the estimation that one IA session was able to lower isohemagglutinins by one titer level. For logistical reasons, transplant surgery was scheduled on Mondays (hospital admission of the living donor on Sundays) with the aim to reach the target isohemagglutinin titer by Friday, continuing daily IA during the weekend. For example, if the initial isohemagglutinin titer is 1:128, five titer reductions are necessary to reach the target titer level of 8 by Friday. Hence, seven IA sessions are planned before surgery on Monday. With each IA session, at least two plasma volumes (calculated with the formula of Nadler [27]) were processed. This was increased up to four plasma volumes per treatment if the titer reduction was insufficient. IA was performed daily until the isohemagglutinin titers for both IgG and IgM were 1:8 or less at the beginning of the last intended IA. If IgG or IgM remained higher than 1:8, IA was continued until the target titer was achieved. Priming of the cell separator was done with 500 mL ACD-A and 10,000 IU heparin. Then 10,000 IU heparin diluted in 500 mL normal saline (NaCl 0.9%) was given as a perfusion of 40 IU/min with the aim to maintain thrombin time 1 and 2 (TT1 and TT2) >120 s, and anti-factor X activity (Anti-FXa) between 1.0 and 2.0 IU/mL. The filter system was regularly controlled visually by the apheresis nurse for clots. Isohemagglutinins were measured before and after each IA procedure in patient's blood samples. The efficacy of the Glycosorb column was monitored daily, measuring isohemagglutinin titers in a plasma probe taken from the apheresis system after the passage of the patient's plasma in the Glycosorb ABO column 10 min prior to the end of the procedure.
Laboratory Measurements
Accuracy of anticoagulation was monitored by activated partial TT (aPTT), TT1 (i.e., time to thrombus formation after adding 6 units of thrombin per milliliter to citrated plasma), TT2 (i.e., addition of 20 U/mL thrombin), and Anti-FXa. These assays were performed before each procedure, after the heparin bolus, and every 30–60 min during the procedure.
Isohemagglutinin titers were measured with the conventional tube method until June 1, 2017 and the validation of the ID gel system (Bio-Rad, Hercules, CA, USA) which showed reproducible results. Thereafter, the gel system was used. For the tube method, recipient serum was serially diluted and incubated with aliquots of a 5% suspension of red blood cells of the kidney donor in a test tube for approximately 15 min at room temperature. After centrifugation, macroscopic agglutinations of red blood cells were evaluated for anti-A or anti-B IgM antibodies or both. For IgG detection, IgM in patient serum was inactivated (Neutr-AB II, Medion Grifols Diagnostics Ag) before testing for agglutination. Titers were determined as the highest dilution that still produced macroscopic agglutination. With the gel system, IgM inactivation was not needed. The agglutination on the ID card was checked manually. After transplantation, IgM and IgG titers against the donor blood group were measured daily in the first week, weekly until day 28, and then at 3, 6, and 12 months.
Outcomes
Main outcomes as clotting in the filter system or any other procedural problems as well as patient-reported symptoms and observed clinical signs including bleeding events were routinely documented during each procedure. Other outcomes as graft survival and overall survival were compared to patients who underwent living donor kidney transplantation (ABO-compatible or ABO-incompatible with nonheparin or no apheresis procedure) during the same observation period at the University Hospital Basel, Switzerland.
Statistics
Descriptive statistics were performed for baseline characteristics reporting frequencies and proportions. The main cohort included assessments of 13 patients undergoing kidney transplantation and treated with heparin for procedural anticoagulation during preparative IA. The focus was on the safety endpoint of clinically significant bleeding (i.e., >WHO grade 2); feasibility endpoints of clumping events in the filter system; and coagulation assays TT1, TT2, and Anti-FXa during different time points of the procedure. Efficiency was assessed with isohemagglutinin titer reduction. Further outcome measures included eGFR and creatinine at different treatment times. Differences in creatinine and eGFR values among the three treatment groups were assessed using nonparametric Kruskal-Wallis test. Moreover, we estimated unadjusted Kaplan-Meier survival functions for overall survival and graft survival. Furthermore, we performed a competing risk analysis with death as a competing risk for graft failure. Patients entered the analysis from date of transplant to date of graft failure, censored for patient death with a functioning allograft or at the date of last known function on their last clinical follow-up. Statistical significance was established at p < 0.05.
Results
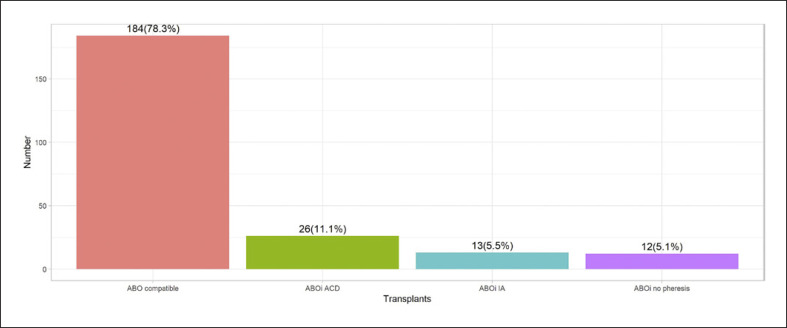
During the study period, a total of 235 living donor kidney transplantations were performed at our institution. Fifty-one patients (21.7%) had an ABO blood group incompatibility and were treated with the described desensitization protocol. Twelve patients (23.5% of all ABOi LDKT) did not need IA due to low baseline isohemagglutinin titers after immunosuppressive therapy alone. Thirty-nine patients (76.5% of ABOi LDKT) needed an apheresis procedure to lower isohemagglutinin titers before transplantation. Of these 39 patients, twenty-six (66.7%) received IA or PEX with standard anticoagulation and 13 (33.3%) received IA with heparin anticoagulation (Fig. 1).
Fig. 1.
Proportions of all 235 living donor kidney transplant recipients between 2013 and 2019 at the University Hospital Basel. 184 major ABO blood group compatible transplantations were performed. From the 51 ABOi transplantations, 26 patients received apheresis procedures with standard ACD-A anticoagulation and 13 patients received the newly introduced heparin anticoagulation during IA. Eight patients who received an ABOi kidney did not receive any apheresis procedure due to low isohemagglutinin titers.
Patient Characteristics
The median age of the 13 patients treated with IA and heparin anticoagulation in preparation for major ABOi LDKT was 54 years (range from 23 to 69 years), and recipients were predominantly male (77%). The underlying disease categories varied; most patients had a glomerulopathy. Eleven (84.6%) patients were on a regular dialysis program before kidney transplantation, while the other two were scheduled for a preemptive kidney transplant. Four (31%) of the patients received aspirin 100 mg at least once during the 7 days prior to the first procedure. Further patient and disease characteristics are described in Table 1.
Table 1.
Baseline characteristics of LDKT recipients
| Characteristics, n [percentage] | ABOi heparin (n = 13) | ABOi nonheparin (n = 38) | ABOc (n = 184) |
|---|---|---|---|
| Male sex | 10 [77] | 24 [63] | 109 [59] |
| Female sex | 3 [23] | 14 [37] | 75 [41] |
| Recipient age, years, median (range) | 54 [23–69] | 57 [21–79] | 51.5 [13–77] |
| Donor age, years, median (range) | 53 [38–73] | 54.5 [33–78] | 57.5 [27–79] |
| Underlying disease | |||
| Glomerulonephritis/glomerulopathy | 6 [46] | 10 [26] | 66 [36] |
| Diabetic nephropathy | 2 [15] | 2 [5] | 14 [8] |
| ADPKD | 1 [8] | 13 [34] | 37 [20] |
| Interstitial nephritis | 1 [8] | 0 | 5 [3] |
| Other | 1 [8] | 5 [13] | 33 [18] |
| Unknown | 2 [15] | 4 [11] | 18 [10] |
| Vascular/arterial hypertension | 0 | 4 [11] | 11 [6] |
| Second or third transplant, n (%) | 0 | 6 [16] | 15 [8] |
| HLA identical, n (%) | 0 | 4 [11] | 13 [7] |
| Preemptive kidney transplant | 2 [15] | 25 [66] | 82 [45] |
| Donor category, n (%) | |||
| Partner | 7 [54] | 17 [45] | 70 [38] |
| Friend | 3 [23] | 1 [3] | 11 [6] |
| Parent | 1 [8] | 5 [13] | 45 [25] |
| Child | 0 | 1 [3] | 0 |
| Sibling | 0 | 12 [32] | 44 [24] |
| Other relative | 2 [15] | 2 [5] | 14 [8] |
| Other risk factor | 1 [8] | 4 [11] | 37 [20] |
| Husband to wife | 1 [8] | 1 [3] | 8 [4] |
| Donor-specific antibody (DSA) | 0 | 3 [8] | 25 [14] |
| Other risk factor | 0 | 0 | 4 [2] |
| ABO blood group recipient, n (%) | |||
| O | 11 [85] | 20 [53] | n/a |
| A | 2 [15] | 7 [18] | n/a |
| B | 0 | 11 [29] | n/a |
| ABO blood group donor/recipient, n (%) | |||
| A/O | 8 [62] | 14 [37] | n/a |
| AB/O | 2 [15] | 0 | n/a |
| B/O | 1 [8] | 6 [16] | n/a |
| B/A | 1 [8] | 2 [5] | n/a |
| AB/A | 1 [8] | 5 [13] | n/a |
| A/B | 0 | 8 [21] | n/a |
| AB/B | 0 | 3 [8] | n/a |
Baseline characteristics of all patients undergoing LDKT between 2013 und 2019 divided in three groups. The first group (ABOi heparin) consists of all 13 patients who underwent IA with heparin anticoagulation. Group two (ABOi nonheparin) consists of the remainder of all ABOi recipients who underwent either IA or PEX without heparin (i.e., ACD-A) or no apheresis. The third group includes all ABOc LDKT recipients. ABOi, major ABO blood group incompatible; ABOc, major ABO blood group compatible; ADPKD, autosomal dominant polycystic kidney disease; HLA, human leukocyte antigen.
Procedures
Between 3 and 10 IA sessions with heparin were conducted per patient before transplant surgery (median 5). A median of 2.5 times the calculated plasma volume was processed per procedure with a maximum of 4 plasma volumes in one procedure. This correlated to a median amount of 13.9 L and a maximum amount of 22.7 L processed in a single procedure. Further details of the IA treatment plan are indicated in Table 2. In the ABOi nonheparin group, 3–16 procedures were performed per patient (median 4). The number of procedures was similar between heparin and ACD-A anticoagulation groups. In the heparin group, 4 patients had IA and/or PEX after kidney transplantation. Two of the 13 patients (15.4%) had increased isohemagglutinin titers (highest titer 1:64) and early biopsy proven humoral rejection. Both were treated successfully in a multimodal approach with steroids, anti-thymoglobulin (ATG), and apheresis. In another patient, thrombotic microangiopathy (TMA) was found but no increase in isohemagglutinin. A last patient had TMA and possible humoral rejection on biopsy without increase of isohemagglutinin titer above 1:8. Of the 26 patients desensitized by apheresis in the nonheparin group, 3 patients (11.5%) were treated for humoral rejection with elevated isohemagglutinins including an apheresis procedure. One patient received additional two IA sessions, as isohemagglutinin titer reduction against B antigen was borderline at 1:8. No humoral rejection was observed. Two patients who did not qualify for pretransplant apheresis received PEX for TMA post-LDKT.
Table 2.
Immunoadsorption treatment plan
| Patient number | Baseline titer |
IA sessions planned | IA sessions performed | Mean PV treated per IA session | Titer at transplantation |
||
|---|---|---|---|---|---|---|---|
| IgG | IgM | IgG | IgM | ||||
| 1 | 128 | 32 | 6 | 6 | 2.5 | 2 | 2 |
| 2 | 32 | 32 | 5 | 5 | 2.2** | neg | 4 |
| 3 | 32 | 32 | 5 | 5 | 2.5 | 4 | 4 |
| 4 | 16 | 16 | 5 | 5 | 2.5 | neg | 2 |
| 5 | 32 | 32 | 5 | 5 | 2.5 | neg | 2 |
| 6 | 256 | 16 | 7 | 5 | 2.5 | 2 | 1 |
| 7 | 32 | 8 | 5 | 5 | 2.4 | neg | 1 |
| 8* | 16 | 2 | 4 | 6 | 3.3 | 2 | 1 |
| 9 | 4 | 16 | 4 | 4 | 3 | neg | 4 |
| 10* | 256 | 16 | 6 | 10 | 3.4 | 8 | 1 |
| 11 | 256 | 32 | 6 | 6 | 3.3 | 4 | neg |
| 12* | 128 | 16 | 6 | 3*** | 4 | 4 | 1 |
| 13 | 64 | 16 | 6 | 4**** | 2.5 | 4 | neg |
Isohemagglutinin titers for IgG and IgM at baseline were considered to plan the number of IA sessions based on the estimation that one IA session was able to lower isohemagglutinins by one titer level. For logistical reasons, a margin was used at the discretion of the treating nephrologist. Generally, 2.5 patient's plasma volumes (PV) were processed per IA session. This was increased up to 4 plasma volumes per session if the titer reduction was insufficient. IA, immunoadsorption; PV, plasma volumes (calculated with the formula of Nadler).
In 3 patients, in response to insufficient titer lowering the processed PV per IA session was increased and the transplant postponed.
Last IA was interrupted because of clumping in the filter.
Because of low blood pressure during IA and hemodialysis, anticoagulation was switched from ACD-A to heparin after 7 IA sessions to facilitate higher flow rates. Sessions with citrate anticoagulation are not included in the analysis.
Two plasma exchange sessions (not included in the analysis) were performed before switching to IA heparin for logistical reasons as the Glycosorb column was not available on time.
Safety and Feasibility
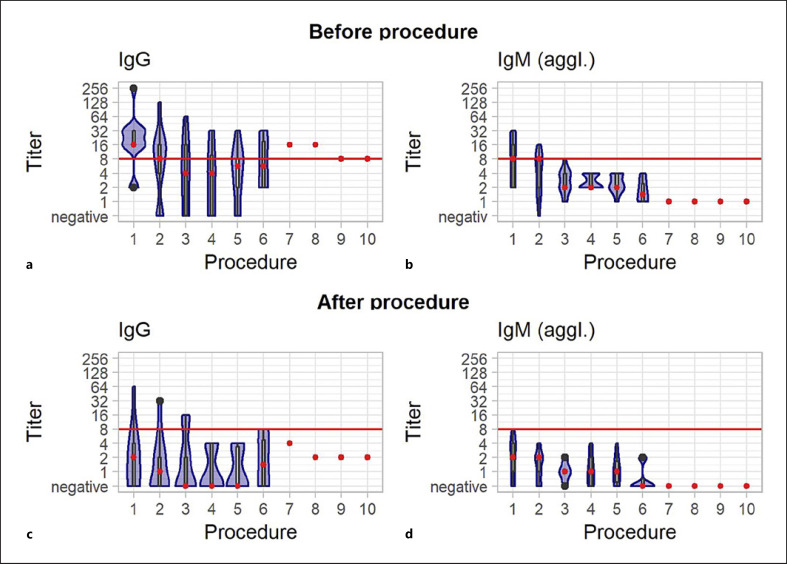
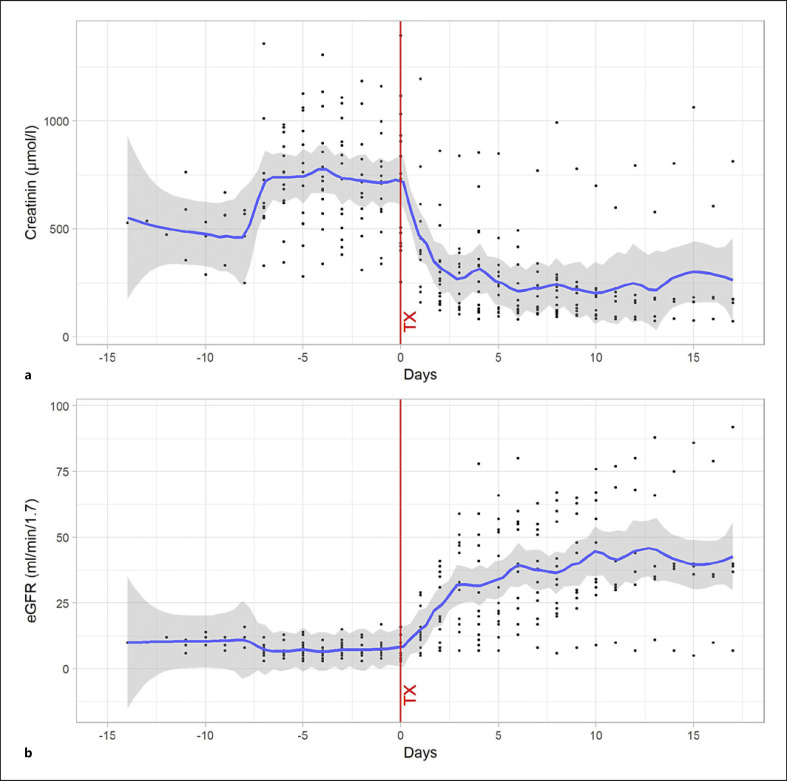
Clumping in the filter system was observed during 3 (4%) out of the overall 69 IA procedures with heparin. In the first instance, the procedure was terminated early after processing one plasma volume. In the other two instances, a heparin bolus (2,000 IU i.v.) was given, and the procedure could be completed as planned. In the 3 involved patients, TT1 and TT2 were in the expected range in all measurements during the whole procedures in which the clumping in the filter was observed. In these 3 patients, TTs were in the range of 95.2% of a total of 63 TT1 and 56 TT2 measurements during all procedures. Anticoagulation assays were not routinely done before surgery. On the day of transplant surgery, all measured TT1 (n = 9) were <120 s and Anti-FXa ≤0.1 IU/mL (n = 2). No bleeding was observed in the patients during heparin anticoagulation for IA and until the first day after transplantation. No other significant adverse events were reported. All 13 patients had sufficient isohemagglutinin titer lowering to proceed to surgery (Fig. 2), 9 (69.2%) had negative IgG titer, and 6 (46%) had negative IgM after the last procedure. Cumulative creatinine measurements during hospital stay for kidney transplantation before and after surgery as first proof of graft function is shown in Figure 3.
Fig. 2.
Cumulative isohemagglutinin course (IgG and IgM) before (a/b) and after (c/d) IA with heparin anticoagulation in the sequence of procedures. The number of procedures varied between 3 and 10 per patient. The red line marks the target cut off (1:8) for continuation to transplant surgery. The red dot represents the mean. Within the violin plot, a boxplot showing the 25th and 75th percentile is included.
Fig. 3.
a, b Cumulative creatinine and glomerular filtration rate values of the 13 patients treated with heparin during IA are shown 15 days before and after the kidney transplantation (during the hospital stays). The point cloud is centered to the day of transplantation (= day 0). A nonparametric adjustment curve (lowess smoother) shows the mean values.
Anticoagulation
The number of TT measurements per procedure was between 1 and 9 per patient. Overall, 351 measurements of TT 1 and 340 of thrombin 2 were performed. 98% of TT1 measurements were >120 s as were 81.8% of TT2 (mean and IQR >120 s)
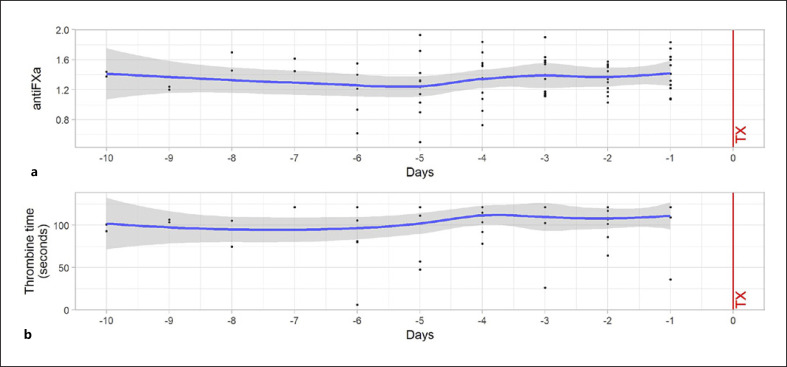
Of a total of 407 Anti-FXa measurements, 274 (67.3%) were in the range of >1 IU/mL and ≤ 2 IU/mL. Ninety-three (22.9%) of measurements were below the target range; 40 (9.8%) measurements were above the target (>2 IU/mL). Average Anti-FXa and TT2 during IA over the course of procedures are shown in Figure 4. As described previously, no correlation between clumping within the filter system was observed even when coagulation assays indicated suboptimal anticoagulation.
Fig. 4.
Cumulative Anti-FXa levels (a) and TT 2 (b) during IA sessions on days −10 to −1 of the 13 patients treated with heparin during IA are shown. A nonparametric adjustment curve (lowess smoother) shows the mean values.
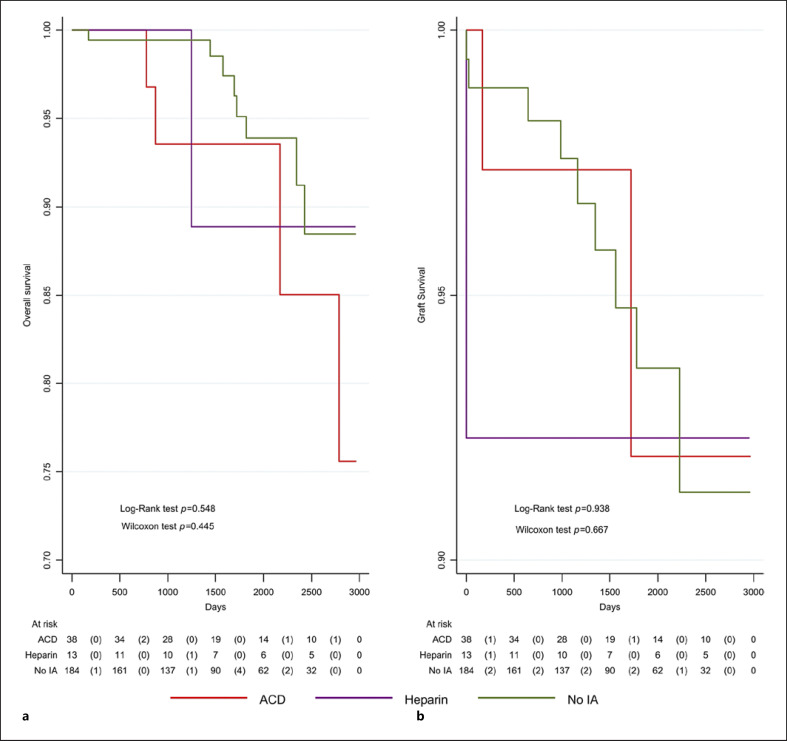
Graft Function and Graft Survival
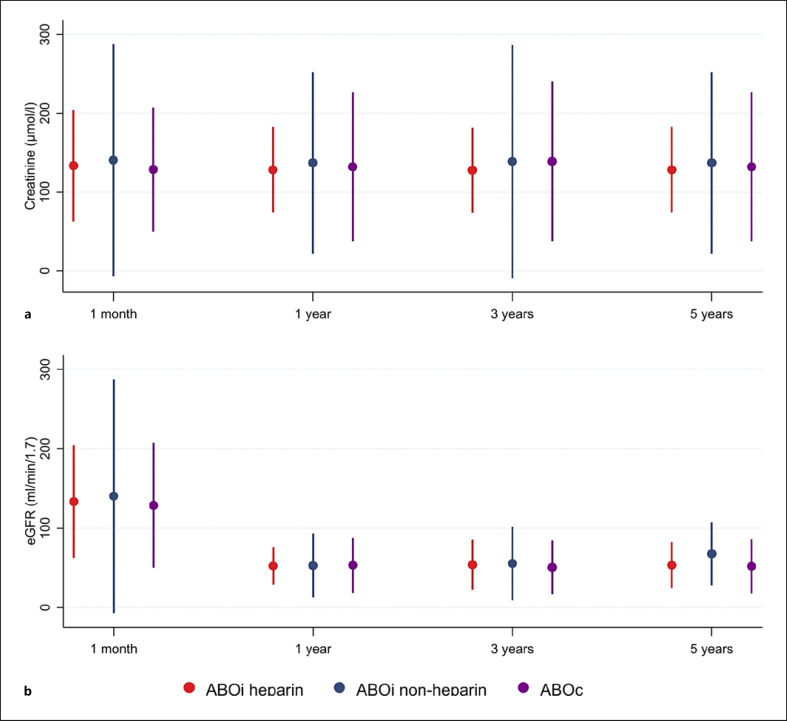
Overall, 235 patients were included in the analysis and were 116.6 years at risk of graft failure. Among all patients, twelve grafts were lost (Fig. 5). Graft survival was not significantly different between treatment groups (log-rank test p = 0.938). Competing risk analysis with death as a competing risk yielded a similar result with no significant difference between the three groups (Pepe and Mori test p = 0.616, p = 0.568, p = 0.909 for ACD vs. heparin, no IA vs. heparin, and no IA vs. ACD, respectively). Among the 13 patients treated with heparin anticoagulation, one graft was lost on the day of transplantation due to biopsy-confirmed TMA unrelated to humoral rejection or the heparin anticoagulation. The cause of the TMA remained unclear upon extensive investigation. In the other 12 patients, no delayed graft function was observed (Fig. 5). Follow-up was between 1 and 9 years (median 4 years). As described in Figure 6, mean creatinine values after 1 month were 133.5 ± 35.4 μmol/L in the ABOi heparin group and 140.4 ± 73.5 μmol/L and 128.7 ± 39.2 μmol/L in the ABOi nonheparin and ABOc groups, respectively. The corresponding eGFRs did not differ significantly between defined groups. Likewise, after 1, 3, and 5 years, the creatinine values and eGFR were comparable (Fig. 6).
Fig. 5.
Patient overall survival (a), death-censored graft survival (b) after living donor kidney transplantation for the three groups (heparin, nonheparin, no IA). During the study period, 13 patients died (235 patients with 1,017 patient years at risk). The mortality did not significantly differ between the three treatment groups (log-rank test p = 0.5479; Wilcoxon test p = 0.4450). Twelve patients had a graft failure (235 patients with 1,016 patient years at risk). Graft failure did not significantly differ between the treatment groups (log-rank test p = 0.938; Wilcoxon test p = 0.667).
Fig. 6.
a, b Mean creatinine values and glomerular filtration rate (mL/min/1.73 m2) (CKD-EPI) during follow-up after LDKT at 1 month, 1 year, 3 years, and five years for ABOi recipients treated with heparin, ABOi recipients not treated with heparin, and ABOc recipients of kidney transplants. Dots represent mean values; whiskers represent mean +/− 2 standard deviations. a Creatinine over time. b eGFR over time. All Kruskal-Wallis tests for differences between treatment regimens had p > 0.1.
In the ABOi nonheparin group, two graft failures due to therapy resistant humoral rejection were observed. The cause of the rejection was persisting blood group antibodies. An insufficient accommodation of the donated organ from the elderly mother was suspected. In the ABOc group, graft loss happened in 9 patients (4.9%).
Overall Survival
Overall, 235 patients were included in the analysis and were 116.9 years at risk. Among all patients, 13 patients died (Fig. 4). Overall survival was not significantly different between treatment groups (log-rank test p = 0.548). After a median follow-up of 4 years (range: 0–9), 1 patient died in the ABOi heparin group. Death occurred 3.5 years after kidney transplantation due to heart failure with an intact graft function. In the ABOi nonheparin group, 1 patient died later due to intracranial hemorrhage and 3 patients due to secondary malignancies. In the ABOc group, eight deaths were reported in the same studied period.
Conclusion
Using IA for desensitization in ABOi LDKT has helped to improve outcomes [11, 12, 28]. ACD-A is the almost exclusively used anticoagulant in this setting. Heparin is an established alternative to ACD-A during therapeutic apheresis and allows faster flow rates in plasmapheresis [28]. It is unknown if for patients in need of IA and at risk of volume overload heparin may be advantageous or noninferior to ACD-A. To the best of our knowledge, experiences of heparin anticoagulation during IA in this setting have not been reported so far.
In this retrospective single-center study, we described feasibility and safety of heparin anticoagulation during IA in preparation of ABOi LDKT in thirteen consecutive selected cases.
The adapted procedural anticoagulation scheme was safe and efficient. No major complication, especially no relevant bleeding occurred. Monitoring of anticoagulation was performed continuously and allowed to anticipate procedural complications before their occurrence. Moreover, our experience showed the importance of rigorously checking the filter system for clumping by the operator, allowing immediate intervention (e.g., intravenous heparin bolus) independent of turnaround times for coagulation assays. The applied target for Anti-FXa of 1.0–2.0 IU/mL was in line with recommendations by the Association for the Advancement of Blood & Biotherapies (AABB) [29]. As we did not observe any clotting events in patients with Anti-FXa below the target level, lower Anti-FXa may be sufficient to prevent clotting and may be validated in future patients.
Second, the adapted scheme allowed efficient lowering of A and B antibody titers to enable successful transplant surgery in all cases. During the study period, 1 patient lost his graft on the day of transplantation due to a thrombotic microangiopathy. No association with the IA procedure was found. All other 12 patients had well-functioning grafts, one of them until his death due to an unrelated cardiac cause. The titer threshold of 1:8 was used in line with most centers [12, 30] and is a reasonable basis to define the number of planned procedures [31]. Moreover, patients with isohemagglutinin titers smaller than 1:16 have comparable outcome if pheresis is omitted altogether [14]. Number of procedures per patient pre and post ABOi LDKT were comparable to other centers [32]. This is relevant as postoperative bleeding seems to increase with the number of IA procedures [33]. In addition to our main findings, the outcome of the new heparin protocol was comparable to standard ACD-A protocol in ABOi transplant recipients as well as in recipients of ABOc LDKT with no need for IA at our institution in terms of overall survival, overall graft survival, and kidney function at 1,3, and 5 years.
The study overlooks almost 7 years during which ABOi LDKT numbers were relatively stable. During this period, about 1 third of the patients in need of a therapeutic apheresis procedure were treated with the newly introduced scheme.
The retrospective design of the study and small number of patients limit the generalizability of the observed results. Some adaptations made were tailored to our institution and should be internally validated at each center before introduction. It should be noted that ACD-A is recommended by the manufacturers as preferred anticoagulation option and is not questioned as a standard.
Furthermore, there was no prospective data collection of quality of life to show improvement in the patient's experience. Also, the number of hemodialysis sessions before surgery was not assessed.
In summary, the use of heparin as for anticoagulation in IA could be successfully established at our center and proved to be a valid, safe, and equally efficient procedure for selected patients in our experience. Validation of our single-center experience is needed, but our data may offer other centers an alternative option for anticoagulation during IA in the setting of ABOi LDKT.
Statement of Ethics
The study was performed according to the regulations of the Local Ethics Committee (EKNZ: 2016-01967). Written informed consent from participants was not required in accordance with local/national guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The study was supported by an unrestricted grant from the Blood Transfusion Service, Swiss Red Cross.
Author Contributions
Andreas Holbro, Jörg Halter, and Michael Dickenmann were the initiators and scientific project leaders. Till Junker analyzed data and wrote the manuscript, which was revised and approved by all authors. Till Junker, Beatrice Drexler, Gregor Stehle, Andreas Buser, Laura Infanti, and Stefan Schaub contributed to the data collection. Till Junker, Andreas Holbro, Andreas Buser, Jakob Passweg, Jörg Halter, and Michael Dickenmann participated in the conception and design of the study and contributed methodical expertise. Thomas Volken performed statistical analysis.
Data Availability Statement
The datasets analyzed for this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Cecile Burtscher, Alexandra Plattner, and all members of the apheresis unit who contributed to the data collection.
Michael Dickenmann, Jörg Halter, and Andreas Holbro contributed equally to this work.
Funding Statement
The study was supported by an unrestricted grant from the Blood Transfusion Service, Swiss Red Cross.
References
- 1.Suthanthiran M, Strom TB. Renal transplantation. N Engl J Med. 1994;331((6)):365–376. doi: 10.1056/NEJM199408113310606. [DOI] [PubMed] [Google Scholar]
- 2.Schnuelle P, Lorenz D, Trede M, Van Der Woude FJ. Impact of renal cadaveric transplantation on survival in end-stage renal failure evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J Am Soc Nephrol. 1998;9((11)):2135–2141. doi: 10.1681/ASN.V9112135. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis patients on dialysis awaiting transplantation and recipients of a first cadaveric transplant. N Engl J Med. 1999;341((23)):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 4.Evans RW, Manninen DL, Garrison LP, Hart LG, Blagg CR, Gutman RA, et al. The quality of life of patients with end-stage renal disease. N Engl J Med. 1985;312((9)):553–559. doi: 10.1056/NEJM198502283120905. [DOI] [PubMed] [Google Scholar]
- 5.Curtis R, Robb M. Annual report on kidney transplantation 2020/21 NHS blood and transplant. Available from kidney-annual-report-2020-21.pdf (windows.net) [Google Scholar]
- 6.Colvin RB. Antibody-mediated renal allograft rejection diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18((4)):1046–1056. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 7.Rydberg L. ABO-incompatibility in solid organ transplantation. Transfus Med. 2001;11((4)):325–342. doi: 10.1046/j.1365-3148.2001.00313.x. [DOI] [PubMed] [Google Scholar]
- 8.Hume DM, Merrill JP, Miller BF, Thorn GW. Experiences with renal homotransplantation in the human report of nine cases. J Clin Invest. 1955;34((2)):327–382. doi: 10.1172/JCI103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breimer ME, Mölne J, Nordén G, Rydberg L, Thiel G, Svalander CT. Blood group A and B antigen expression in human kidneys correlated to A1/A2/B and secretor status. Transplantation. 2006;82((4)):479–485. doi: 10.1097/01.tp.0000231697.15817.51. [DOI] [PubMed] [Google Scholar]
- 10.Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA. Kidney paired donation and optimizing the use of live donor organs. JAMA. 2005;293((15)):1883–1890. doi: 10.1001/jama.293.15.1883. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Saito K, Takahara S, Okuyama A, Tanabe K, Toma H, et al. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant. 2004;4((7)):1089–1096. doi: 10.1111/j.1600-6143.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 12.Genberg H, Kumlien G, Wennberg L, Tyden G. The efficacy of antigen-specific immunoadsorption and rebound of anti-A/B antibodies in ABO-incompatible kidney transplantation. Nephrol Dial Transplant. 2011;26((7)):2394–400. doi: 10.1093/ndt/gfr237. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K. Recent findings in ABO-incompatible kidney transplantation classification and therapeutic strategy for acute antibody-mediated rejection due to ABO-blood-group-related antigens during the critical period preceding the establishment of accommodation. Clin Exp Nephrol. 2007;11((2)):128–141. doi: 10.1007/s10157-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 14.Gelpi R, Cid J, Lozano M, Revuelta I, Sanchez-Escuredo A, Blasco M, et al. Desensitization in ABO-incompatible kidney transplantation with low ABO iso-agglutinin titers. Transplant Proc. 2015;47((8)):2340–2343. doi: 10.1016/j.transproceed.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the writing committee of the American society for apheresis the eighth special issue. J Clin Apher. 2019;34((3)):171–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]
- 16.Rostaing L, Allal A, del Bello A, Sallusto F, Esposito L, Doumerc N, et al. Treatment of large plasma volumes using specific immunoadsorption to desensitize ABO-incompatible kidney-transplant candidates. J Nephropathol. 2016;5((3)):90–97. doi: 10.15171/jnp.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee G, Arepally GM. Anticoagulation techniques in apheresis from heparin to citrate and beyond. J Clin Apher. 2012;27((3)):117–125. doi: 10.1002/jca.21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monchi M. Citrate pathophysiology and metabolism. Transfus Apher Sci. 2017;56((1)):28–30. doi: 10.1016/j.transci.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Diaz J, Acosta F, Parrilla P, Sansano T, Bento M, Cura S, et al. Citrate intoxication and blood concentration of ionized calcium in liver transplantation. Transplant Proc. 1994;26((6)):3669–3670. [PubMed] [Google Scholar]
- 20.Weinstein R. Prevention of citrate reactions during therapeutic plasma exchange by constant infusion of calcium gluconate with the return fluid. J Clin Apher. 1996;11((4)):204–210. doi: 10.1002/(SICI)1098-1101(1996)11:4<204::AID-JCA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Baglin T, Barrowcliffe TW, Cohen A, Greaves M, British Committee for Standards in Haematology Guidelines on the use and monitoring of heparin. Br J Haematol. 2006;133((1)):19–34. doi: 10.1111/j.1365-2141.2005.05953.x. [DOI] [PubMed] [Google Scholar]
- 22.Hughes S, Szeki I, Nash MJ, Thachil J. Anticoagulation in chronic kidney disease patients the practical aspects. Clin Kidney J. 2014;7((5)):442–449. doi: 10.1093/ckj/sfu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133((Suppl 6)):141S–159S. doi: 10.1378/chest.08-0689. [DOI] [PubMed] [Google Scholar]
- 24.Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355((8)):809–817. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 25.Dittberner T, Schöttler E, Ranze O, Greinacher A, Knobler R. Heparin-induced thrombocytopenia a complication in extracorporeal photochemotherapy (photopheresis) J Am Acad Dermatol. 2002;47((3)):452–453. doi: 10.1067/mjd.2002.120598. [DOI] [PubMed] [Google Scholar]
- 26.Oettl T, Halter J, Bachmann A, Guerke L, Infanti L, Oertli D, et al. ABO blood group-incompatible living donor kidney transplantation a prospective, single-centre analysis including serial protocol biopsies. Nephrol Dial Transplant. 2009;24((1)):298–303. doi: 10.1093/ndt/gfn478. [DOI] [PubMed] [Google Scholar]
- 27.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51((2)):224–232. [PubMed] [Google Scholar]
- 28.Salvadori M, Tsalouchos A. Therapeutic apheresis in kidney transplantation an updated review. World J Transplant. 2019;9((6)):103–122. doi: 10.5500/wjt.v9.i6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLeod BC, Weinstein R, Winters JL, editors. Apheresis priciples and practice. 3rd ed. AABB press; 2010. [Google Scholar]
- 30.Salvadori M, Tsalouchos A. Current protocols and outcomes of ABO-incompatible kidney transplantation. World J Transpl. 2020;10((7)):191–205. doi: 10.5500/wjt.v10.i7.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connelly-Smith L, Tanhehco YC, Chhibber V, Delaney M, Eichbaum Q, Fernandez C, et al. Choosing wisely for apheresis. J Clin Apher. 2018;33((5)):576–579. doi: 10.1002/jca.21643. [DOI] [PubMed] [Google Scholar]
- 32.Barnett ANR, Manook M, Nagendran M, Kenchayikoppad S, Vaughan R, Dorling A, et al. Tailored desensitization strategies in ABO blood group antibody incompatible renal transplantation. Transpl Int. 2014;27((2)):187–196. doi: 10.1111/tri.12234. [DOI] [PubMed] [Google Scholar]
- 33.Renner FC, Czekalinska B, Kemkes-Matthes B, Feustel A, Stertmann WA, Padberg W, et al. Postoperative bleeding after AB0-incompatible living donor kidney transplantation. Transplant Proc. 2010;42((10)):4164–4166. doi: 10.1016/j.transproceed.2010.09.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed for this study are available from the corresponding author upon reasonable request.