Abstract
Background
C-reactive protein (CRP) is an acute-phase protein and has been found to be a risk factor for acute kidney injury (AKI) and chronic kidney diseases (CKD). However, the role and mechanisms of CRP in AKI and CKD remain largely unclear.
Summary
Clinically, elevated serum CRP is a risk factor or biomarker for patients with AKI and CKD. Interestingly, in critically ill COVID-19 patients, increased serum CRP is also associated with the development of AKI. Functionally, studies using human CRP transgenic mouse models find that CRP is pathogenic and can function as a mediator for AKI and CKD as mice overexpressing human CRP promote AKI and CKD. Mechanistically, CRP can promote AKI and CKD via NF-κB and Smad3-dependent mechanisms. We found that CRP can activate Smad3 signaling directly and cause AKI via the Smad3-p27-dependent G1 cell cycle arrest mechanism. Thus, targeting CRP-Smad3 signaling with a neutralizing antibody or Smad3 inhibitor can inhibit AKI.
Key Messages
CRP acts not only as a biomarker but also as a mediator for AKI and CKD. CRP can activate Smad3 to induce cell death and cause progressive renal fibrosis. Thus, targeting CRP-Smad3 signaling may represent a promising therapy for AKI and CKD.
Keywords: C-reactive protein, CD32, Acute kidney injury, UUO, Diabetic nephropathy
Introduction
C-reactive protein (CRP), a pentameric protein consisting of five identical subunits, is the prototypical acute-phase protein in response to infection and inflammation. Similar to most of the acute-phase reactants, CRP is synthesized in the liver and acts as a reliable biochemical marker for systemic inflammation in clinical practice. Recent studies also demonstrate that CRP can be produced by many inflammatory cells such as inflammatory macrophages [1]. In kidney diseases, CRP is highly expressed by many inflammatory cells, presumably macrophages, and intrinsic kidney cells including tubular cells and endothelial cells [2]. In acute infection or inflammation, CRP can be secreted at the beginning of 4–10 h following inflammatory stimulation, peaking at 48 h with a short half-life of 19 h [3]. Whereas, continuing high levels of CRP may induce chronic inflammation, as reported in patients with chronic kidney disease (CKD) or end-stage renal disease (ESRD) [4]. Therefore, elevated CRP level is considered a biomarker for inflammatory response, tissue injury, and chronic progression of diseases.
Apart from serving as a biomarker for inflammation, CRP also exerts pro-inflammatory actions and anti-inflammatory properties in two conformational isoforms including native pentameric CRP (pCRP) and monomeric CRP (mCRP) [5], which may play a vital role in the pathogenesis of kidney diseases. Native pCRP belongs to the superfamily of pentraxins and has been identified to exhibit pro-inflammatory activities including phagocytosis and clearance as an opsonin [6]. Although pCRP is extremely stable, pCRP may dissociate to mCRP under certain conditions. In the inflammatory microenvironment, both of pCRP and mCRP are found to aggravate inflammation and link to the progression of inflammatory diseases such as atherosclerosis and ischemia-reperfusion injury (IRI) [6]. Meanwhile, an in vitro study demonstrates that mCRP may limit the amplification of tissue injury via inhibiting properdin-mediated renal cell-directed complement activation [4], indicating that the existence of different isoforms of CRP may exhibit diverse functions under different disease conditions. Elevated CRP is associated with development of many diseases, such as cardiovascular diseases, obesity-induced metabolic disorders, and pancreatic and kidney diseases [1]. It is reported that CRP can promote the malignant properties of human pNEN cell lines [7]. Moreover, CRP transgenic mice exhibit endothelial dysfunction and develop perivascular fibrosis and macrophage infiltration [8]. A study by Kaneko et al. [9] has also demonstrate that overexpression of CRP promotes the development of insulin resistance and hepatic steatosis in high-fat diet mice, revealing the pathogenic role of CRP in the development of obesity-induced metabolic disorders. Similarly, CRP also plays a pathogenic role of renal inflammation and fibrosis in various kidney diseases [10]. Thus, this review provides a brief update on the role of CRP in kidney diseases. The possible mechanisms and potential therapy for acute kidney injury (AKI) and CKD by targeting CRP signaling are also discussed.
CRP and AKI
AKI is a clinical syndrome with multiple etiologies and is defined as an acute loss of renal function. The pathogenesis of AKI is multifactorial, involving renal tubular necrosis, inflammation, and vascular dysfunction [10]. As an inflammatory biomarker, elevated CRP levels are associated with worse clinical outcomes and mortality in patients with AKI [11]. It is considered that CRP works as an independent predictor of AKI in ST elevation non-myocardial infarction patients following primary percutaneous coronary intervention [12], as well as in patients undergoing coronary artery bypass graft [13]. Besides, in patients undergoing coronary angiography, CRP is also a risk factor for AKI [14]. Given that a high CRP and a low serum albumin are biomarkers of progressive inflammation, the CRP-to-albumin ratio has been considered an important prognostic indicator in patients with critical illness. Indeed, the CRP/albumin ratio is an independent risk factor for postoperative AKI occurred in elderly cystectomy patients and CRP/albumin ratio ≥0.1 has been shown to be associated with the increased incidence of AKI [15]. Emerging data have also suggested that CRP is an important predictive indicator of sepsis-induced AKI [16]. Moreover, the serum level of CRP is found to be associated with the mortality in older AKI patients and oncology patients with AKI [17, 18]. Our study has also found that elevated serum CRP is associated with deterioration of renal function in patients with AKI, in which the level of CRP is subsequently declined with the recovery of AKI [19].
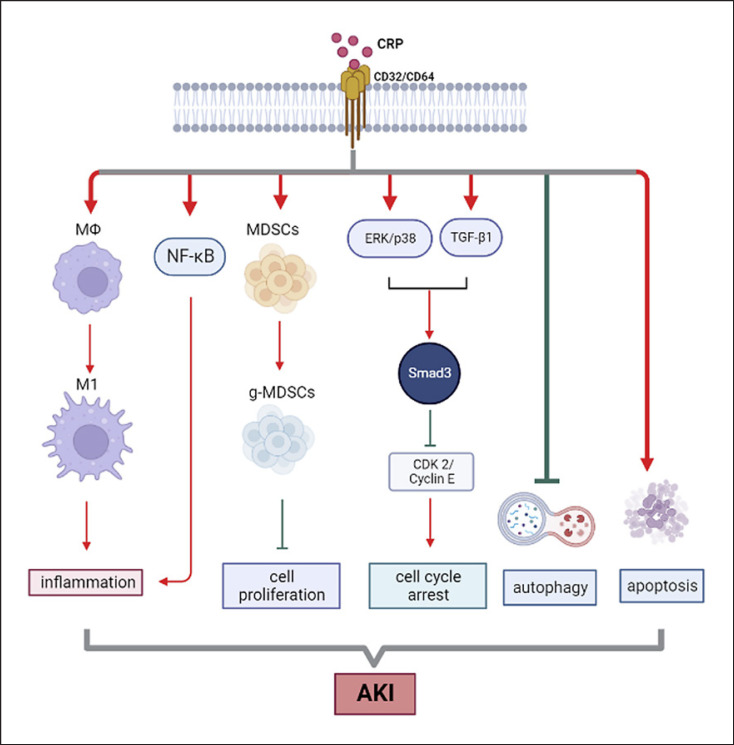
The pathogenic role for CRP in AKI has been demonstrated in a mouse model of ischemic-induced AKI, in which mice overexpressing human CRP are largely enhanced AKI by promoting renal inflammation and tubular necrosis (shown in Fig. 1) [19, 20, 21], which is blocked by using a neutralizing antibody to human CRP [19]. Results from these studies also suggest that targeting CRP may be a novel therapeutic strategy for AKI.
Fig. 1.
Mechanism of CRP in acute kidney injury (AKI). After binding with CD32/CD64, CRP promotes the activation of inflammation and accumulation of myeloid-derived suppressor cells and apoptosis as well as inhibits autophagy and arrests the cell cycle by activating Smad3 via ERK/p38 and TGF-β1 signaling pathways, leading to the progression of AKI. Red arrows represent positive regulation of pathways or biological process, while the green lines represent negative regulation.
CRP and COVID-19-Associated Kidney Diseases
Globally, COVID-19 pandemic is still a serious health threat. COVID-19 may lead to various clinical manifestations including fever, coughing, sweating, and fatigue, although some COVID-19 patients may be asymptomatic [22]. Severe COVID-19 infection may result in acute respiratory metabolic acidosis, coagulation dysfunction, and multiple organ failure [23, 24]. In addition to pulmonary involvement, renal involvement such as AKI has also been identified in critically ill COVID-19 [25]. Moreover, in patients with underlying kidney diseases such as hypertension and diabetes, COVID-19 infection can aggravate the preexisting pathologies in CKD patients. Observational study including 777 COVID-19 patients shows that 45% COVID-19 patients with CKD develop more severe renal injury [26]. A meta-analysis including 344,431 COVID-19 patients also reveal an increased risk of progression and mortality in COVID-19 patients with CKD [27]. In these patients, elevated CRP and cytokines such as IL-6 are found to be the unique risk factor for COVID-19 patients [28]. As reported, high-sensitivity CRP levels of ≥4 mg/L may contribute to the development of severe COVID-19 [29]. Meanwhile, in patients who died from COVID-19, the levels of CRP are much higher when compared to those with survivors [30]. A cohort study by Stringer et al. [31] suggested that levels of CRP predict the mortality in COVID-19 patients. Thus, elevated CRP levels were associated with more severe COVID-19 infection and the disease severity [31, 32, 33, 34, 35, 36]. Notably, a retrospective study including 2,782 patients with COVID-19 has also suggested that the initial high serum level of CRP is strongly associated with the development of AKI [11]. In addition, high CRP level is also an additional risk factor for COVID-19 patients who are required for the renal replacement therapy [37].
To examine the pathogenic role of CRP in COVID-19 AKI, we recently demonstrated that in CRP transgenic mice, kidney-specifically overexpressing SARS-CoV-2 N protein can largely promote AKI (unpublished data). This preliminary observation provides direct evidence for the pathogenic role of CRP in COVID-19 AKI.
CRP and CKD
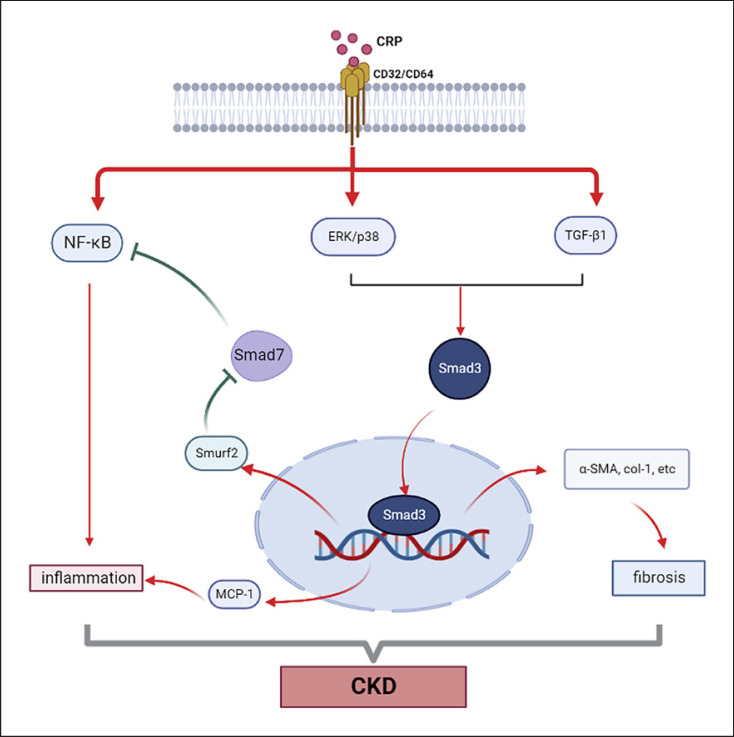
CKD, characterized as a progressive loss of renal function, has become a global public health burden. Inflammation and fibrosis are common pathological features that contribute to the progression of CKD including folic acid nephropathy, obstructed nephropathy, and diabetic nephropathy (DN) [38]. It has been well established that CRP is a risk factor for CKD, and elevated serum levels of CRP are also associated with the mortality and morbidity of CKD [39, 40]. In CKD patients, a high CRP level is found to be a predictor of cardiovascular events [41] and an independent risk factor for all-cause mortality in stage 3 and 4 CKD patients [39]. Besides, increased levels of CRP also correlate with the genetic variants of the CRP locus. According to the data from a large population-based survey, the CRP single nucleotide polymorphism (SNP) rs2808630 is associated with CKD in African Americans and non-Hispanic blacks with hypertensive kidney disease. Meanwhile, the CRP SNP rs2808630 is associated with albuminuria which is a strong risk factor for CKD progression [42]. Hence, genetic predisposition of CRP may predispose patients with a higher risk of CKD progression. It is highly possible that high CRP may promote the infiltration of inflammatory cells and the release of cytokines, chemokines, and TGF-β1 from the diseased kidney, resulting in progressive renal inflammation and fibrosis [43]. Hence, the interplay between inflammation and fibrosis is a major determinant in the progression of CKD in response to CRP. The role of CRP in the pathogenesis of CKD is demonstrated in a mouse model of UUO in which mice overexpressing human CRP develop severe renal inflammation and fibrosis [44], revealing a pathogenic role for CRP in CKD (shown in Fig. 2).
Fig. 2.
Mechanism of CRP in chronic kidney disease. CRP induces the phosphorylation of Smad3 via promoting ERK/p38 and TGF-β1 pathways, which subsequently exacerbate renal inflammation by activating NF-κB pathway via increasing the expression of MCP-1 and decreasing the expression of Smad7 and promote renal fibrosis. Besides, CRP induces renal inflammation via enhancing the activation of NF-κB pathway directly.
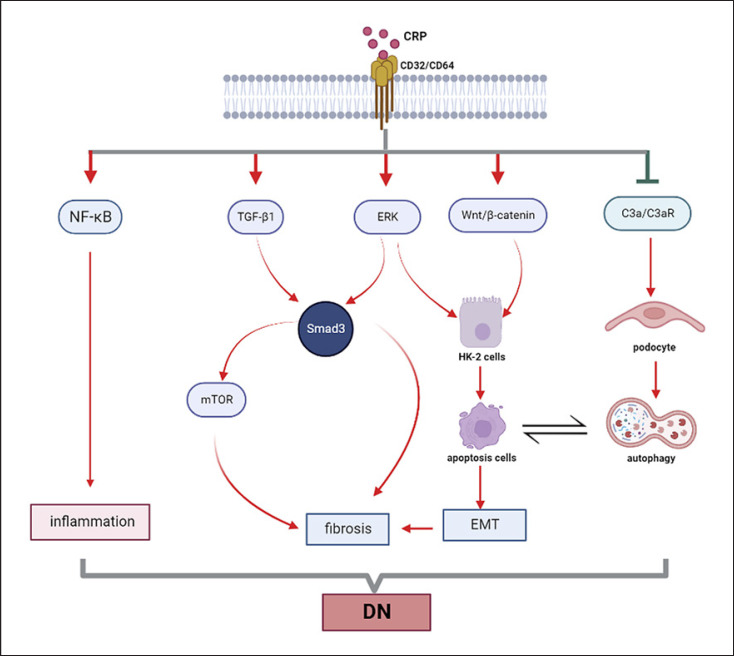
DN is also a severe complication in both type 1 and type 2 diabetes, which is considered a leading cause of end-stage renal disease worldwide. Numerous studies in patients with diabetes have demonstrated that a modest elevation in CRP is associated with an increased risk of type 2 diabetes and its complication such as diabetic retinopathy and DN. For example, a study by Yeo et al. [45, 46] reveals that CRP is an independent risk factor for type 2 DN and is associated with the disease progression. Another study also demonstrates the correlation between elevated levels of CRP and the incidence of cardiovascular events in type 2 DN [47]. Moreover, increased levels of CRP are also associated with the development of microalbuminuria in both types of DN [48, 49]. A meta-analysis containing 1,331 cases and 1,779 controls reveals that high-sensitivity CRP concentration is significantly increased in DN patients and correlated with increased microalbuminuria in different stage of DN [50]. Notably, the detection of mCRP deposition in kidney tubules by immunostaining has found to be associated with the disease severity in DN patients [51], suggesting CRP may be involved in the pathogenesis of DN (shown in Fig. 3). The pathogenic role for CRP in DN is confirmed in a mouse model of type 1 diabetes in which mice overexpressing human CRP are largely promoted STZ-induced DN [49]. In contrast, CRP deficiency inhibits diabetic renal injury in rats [52].
Fig. 3.
Mechanism of CRP in diabetic nephropathy. Under high-glucose condition, CRP induces renal fibrosis by Smad3-mediated mechanism. Meanwhile, the crosstalk between autophagy inhibited by CRP via C3a/C3aR pathway in podocyte, and apoptosis positively regulated by CRP via Wnt/β-catenin and ERK pathways in tubular epithelial cells, is related to the epithelial-mesenchymal transition (EMT) which contribute to renal fibrosis. Similar to the mechanism of CRP in CKD, CRP could promote the activation of NF-κB pathway directly to induce inflammation in diabetic nephropathy (DN).
CRP also plays a role in the pathogenesis of type 2 DN. This is confirmed in CRP transgenic-db/db mice in which db/db mice overexpressing human CRP largely promote diabetic kidney disease by increasing renal inflammation and fibrosis [2].
Mechanisms of CRP Mediate AKI and CKD
The pathogenic role of CRP in AKI and CKD has been demonstrated in different mouse models including AKI, UUO, and db/db mice, in which M1 macrophage activation, NF-κB signaling, and Samd3 signaling are involved as discussed below.
CRP and M1 Macrophage Activation
Macrophages (MФ) which are classified into M1 and M2 macrophages have been found to play an important role in pathogenesis of both AKI and CKD. In the early stage of AKI, M1 macrophages infiltrate into the injury site and release the pro-inflammatory mediator, leading to further damage to the kidney [53]. Although M2 macrophages are considered to play an important role in controlling inflammation and tissue repair in AKI, studies also found that the profibrogenic M2 macrophages can also contribute to the transition from AKI to CKD [54]. Interestingly, a previous study found that CRP can induce polarization of macrophages to M1 phenotype and promote the conversion of macrophages from M2 to M1 phenotype in vitro [55]. This is consistent with an in vivo study that showed that CRP exacerbates IRI-induced AKI by promoting M1 macrophage activation in human CRP transgenic mice [20]. Thus, the activation of M1 phenotype macrophages and expression of FcγR may contribute to IRI-AKI in CRP transgenic mice, as shown in Figure 1.
CRP and NF-kB Signaling
NF-κB signaling pathway is a key inflammatory pathway associated with CRP-mediated AKI and CKD. CRP can activate NF-κB signaling to induce the expression of monocyte chemotactic protein 1 (MCP-1), resulting in macrophage infiltration and renal inflammation [6]. In a mouse model of UUO induced in human CRP transgenic mice, CRP strongly activates NF-κB signaling to promote the early and severe renal inflammation [44]. This is also found in mouse models of both type 2 and type 1 DN in which CRP binds CD32 to activate NF-κB signaling, resulting in the development of renal inflammation [2, 49]. In addition, CRP can also induce expression of integral membrane glycoprotein, DPP4, which regulates the activation of NF-κB, leading to the development of type 2 DN [56]. Thus, CRP may activate NF-κB-dependent mechanism directly or indirectly to mediate renal inflammation in AKI and CKD.
CRP and Smad3 Signaling
Smad3 is not only the vital mediator of TGF-β signaling but can also interact with other signaling pathway such as NF-κB and mTOR signaling to mediate renal inflammation and fibrosis in CKD (Fig. 2). Many studies have revealed the important role of Smad3 in CRP-mediated renal inflammation and fibrosis [2, 19, 57, 58]. By using human CRP transgenic mice, we find that high levels of CRP can promote necrotic renal inflammation and fibrosis via the Smad3-dependent mechanism (shown in Fig. 1 and 2). This is supported by the findings that CRP transgenic mice lacking Smad3 can protect against AKI and CKD in mouse models of ischemic, UUO, and diabetic kidney diseases [2, 57, 58]. Mechanistically, we uncover that after binding to CD32, CRP can activate Smad3 directly and indirectly via both TGF-β1 and/or ERK/p38 MAPK-Smad crosstalk pathways to cause cell death via the Smad3-p27-dependent G1 cell cycle arrest mechanism (shown in Fig. 1). This is confirmed by the findings that genetic deletion or pharmacological inhibition of Smad3 can block or rescue the renal injury in mouse models of ischemic-induced AKI, UUO, and diabetic db/db mice [2, 57, 58]. Similarly, CRP, as a key inflammatory stress molecule, may also play a role in COVID-19-associated AKI [35]. Indeed, SARS-CoV-2 N protein can directly interact with Smad3 and induce tubular epithelial cell death and AKI via the Smad3-p21-dependent G1 cell cycle arrest mechanism [59]. This is also confirmed by genetic deletion and pharmacological inhibition of Smad3 to protect kidneys from SARS-CoV-2-induced AKI [59].
It has been well established that TGF-β/Smad3 signaling is a key pathway leading to renal fibrosis under CKD conditions. CRP can activate Smad3 to mediate renal fibrosis via both TGF-β-dependent and independent mechanisms (shown in Fig. 2). The role of Smad3 in CRP-mediated renal fibrosis is confirmed by the finding that human CRP transgenic mice lacking Smad3 are protected against UUO-induced progressive renal fibrosis [58]. Furthermore, we also find that CRP can induce renal fibrosis through a CD32b-Smad3-mTOR pathway in a mouse model of DN [2]. Compared to diabetic db/db mice, CRP transgenic-db/db mice develop more severe type 2 DN with more progressive renal inflammation and fibrosis, which is associated with over-activation of CRP-CD32b, NF-κB, TGF-β/Smad3, and mTOR signaling [2]. Further studies demonstrate that blockade of mTOR signaling with rapamycin inhibits CRP-induced renal fibrosis, revealing a critical role for CRP-CD32b-Smad3-mTOR signaling in the pathogenesis of DN (shown in Fig. 3).
Others
Other mechanisms such as myeloid-derived suppressor cells and crosstalk between autophagy and apoptosis also participate in the pathogenesis of CRP-induced AKI and CKD. It is reported that CRP can promote AKI by increasing renal accumulation of myeloid-derived suppressor cells [21, 60]. Overexpression of CRP in mice also cause dysregulation of autophagy and activation of apoptosis, resulting in the kidney with more susceptible to IRI-AKI [61]. Furthermore, as shown in Figure 3, CRP can induce apoptosis in HK-2 cells and facilitate epithelium cell to mesenchymal fibroblast transition via the CD32-Wnt/β-catenin and ERK signaling [62]. Emerging evidence also shows that CRP can activate the complement system while inhibiting autophagy via C3a/C3aR signaling under diabetic conditions [52]. Although all these findings are preliminary, they suggest that the regulatory role of CRP in the process of autophagy and apoptosis during AKI to CKD is worthy of further studies.
Therapeutic Strategies by Targeting CRP Signaling
Since CRP is pathogenic in the development of many diseases, it is highly possible that targeting CRP signaling may be a promising therapeutic approach clinically. It has been reported that treatment with CRP antisense oligonucleotide (ASO) can facilitate the degradation of human CRP mRNA to selectively reduce the level of CRP and thus effectively inhibits collagen-induced arthritis in CRP transgenic mice [63, 64]. Importantly, treatment with CRP ASO can also selectively reduce the level of CRP in healthy human male volunteers challenged with endotoxin [65]. In addition, the use of specific CRP inhibitor can protect rats from acute myocardial infarction [66]. It is well established that CRP can bind to Fc receptors such as CD16, CD32, and CD64; however, only the anti-CD32 but not the anti-CD16 or anti-CD64 antibodies can effectively prevent coronary artery disease [67, 68]. Similarly, the neutralizing anti-CD32 antibody can block the CRP-CD32 interaction and thus reverse CRP-induced AKI [19]. Clinically, it is reported the use of selective CRP apheresis can specifically reduce serum levels of CRP and thus has therapeutic effect on “low-risk” COVID-19 patients with respiratory failure, although it fails to show significant benefits to those with “high-risk” COVID-19 patients [69, 70]. Nevertheless, it should be pointed out that the therapeutic effect of anti-CRP treatment on AKI and CKD remains preliminary, and further experimental and clinical studies are warranted.
Conclusion
Increasing evidence shows that CRP is not only a biomarker or risk factor for AKI and CKD, but it may also play a pathogenic role in the development of AKI and CKD. CRP may act via both NF-κB and TGF-β/Smad3 signaling pathways to cause renal inflammation and fibrosis. Thus, targeting CRP signaling may represent a promising therapy for AKI and CKD.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the Research Grants Council of Hong Kong (14117418, 14104019, and 14101121), the Lui Che Woo Institute of Innovative Medicine (CARE program), the National Natural Science Foundation of China (82100723), the President Foundation of the Third Affiliated Hospital of Southern Medical University (YQ2021006), and the Guangdong-Hong Kong-Macao-Joint Labs Program from Guangdong Science and Technology (2019B121205005).
Author Contributions
Jiaxiao Li and Junzhe Chen wrote the manuscript. Ying Tang revised the review. Hui-yao Lan performed the final edits.
Funding Statement
This work was supported by the Research Grants Council of Hong Kong (14117418, 14104019, and 14101121), the Lui Che Woo Institute of Innovative Medicine (CARE program), the National Natural Science Foundation of China (82100723), the President Foundation of the Third Affiliated Hospital of Southern Medical University (YQ2021006), and the Guangdong-Hong Kong-Macao-Joint Labs Program from Guangdong Science and Technology (2019B121205005).
References
- 1.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.You YK, Huang XR, Chen HY, Lyu XF, Liu HF, Lan HY. C-reactive protein promotes diabetic kidney disease in db/db mice via the CD32b-smad3-mTOR signaling pathway. Sci Rep. 2016 May 25;6:26740. doi: 10.1038/srep26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messias BA, Botelho RV, Saad SS, Mocchetti ER, Turke KC, Waisberg J. Serum C-reactive protein is a useful marker to exclude anastomotic leakage after colorectal surgery. Sci Rep. 2020 Feb 3;10((1)):1687. doi: 10.1038/s41598-020-58780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Flynn J, van der Pol P, Dixon KO, Prohaszka Z, Daha MR, van Kooten C. Monomeric C-reactive protein inhibits renal cell-directed complement activation mediated by properdin. Am J Physiol Ren Physiol. 2016 Jun 1;310((11)):F1308–16. doi: 10.1152/ajprenal.00645.2014. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Potempa LA, El Kebir D, Filep JG. C-reactive protein and inflammation: conformational changes affect function. Biol Chem. 2015 Nov;396((11)):1181–1197. doi: 10.1515/hsz-2015-0149. [DOI] [PubMed] [Google Scholar]
- 6.Thiele JR, Zeller J, Bannasch H, Stark GB, Peter K, Eisenhardt SU. Targeting C-reactive protein in inflammatory disease by preventing conformational changes. Mediators Inflamm. 2015;2015:372432. doi: 10.1155/2015/372432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schimmack S, Yang Y, Felix K, Herbst M, Li Y, Schenk M, et al. C-reactive protein (CRP) promotes malignant properties in pancreatic neuroendocrine neoplasms. Endocr Connect. 2019 Jul;8((7)):1007–1019. doi: 10.1530/EC-19-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teoh H, Quan A, Lovren F, Wang G, Tirgari S, Szmitko PE, et al. Impaired endothelial function in C-reactive protein overexpressing mice. Atherosclerosis. 2008 Dec;201((2)):318–325. doi: 10.1016/j.atherosclerosis.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko H, Anzai T, Nagai T, Anzai A, Takahashi T, Mano Y, et al. Human C-reactive protein exacerbates metabolic disorders in association with adipose tissue remodelling. Cardiovasc Res. 2011 Aug 1;91((3)):546–555. doi: 10.1093/cvr/cvr088. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y, Mak SK, Xu AP, Lan HY. Role of C-reactive protein in the pathogenesis of acute kidney injury. Nephrology (Carlton) 2018 Oct;23((Suppl 4)):50–52. doi: 10.1111/nep.13454. [DOI] [PubMed] [Google Scholar]
- 11.Smilowitz NR, Kunichoff D, Garshick M, Shah B, Pillinger M, Hochman JS, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021 Jun 14;42((23)):2270–2279. doi: 10.1093/eurheartj/ehaa1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shacham Y, Leshem-Rubinow E, Steinvil A, Keren G, Roth A, Arbel Y. High sensitive C-reactive protein and the risk of acute kidney injury among ST elevation myocardial infarction patients undergoing primary percutaneous intervention. Clin Exp Nephrol. 2015 Oct;19((5)):838–843. doi: 10.1007/s10157-014-1071-1. [DOI] [PubMed] [Google Scholar]
- 13.Dolapoglu A, Avci E, Kiris T, Bugra O. The predictive value of the prognostic nutritional index for postoperative acute kidney injury in patients undergoing on-pump coronary bypass surgery. J Cardiothorac Surg. 2019 Apr 11;14((1)):74. doi: 10.1186/s13019-019-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim NE, McCarthy CP, Shrestha S, Gaggin HK, Mukai R, Magaret CA, et al. A clinical, proteomics, and artificial intelligence-driven model to predict acute kidney injury in patients undergoing coronary angiography. Clin Cardiol. 2019 Feb;42((2)):292–298. doi: 10.1002/clc.23143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Park JY, Ha S, Hwang JH, Kim YK. C-Reactive protein/albumin ratio and acute kidney injury after radical cystectomy among elderly patients: a propensity score-matched analysis. Dis Markers. 2020;2020:8818445. doi: 10.1155/2020/8818445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ou SM, Lee KH, Tsai MT, Tseng WC, Chu YC, Tarng DC. Artificial intelligence for risk prediction of rehospitalization with acute kidney injury in sepsis survivors. J Pers Med. 2022 Jan 4;12((1)):43. doi: 10.3390/jpm12010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez Valdivieso JR, Bes-Rastrollo M, Monedero P, Olaondo LL, de Irala J, Lavilla FJ. Serum C-reactive protein on the prognosis of oncology patients with acute renal failure: an observational cohort study. Arch Med Res. 2008 Apr;39((3)):326–331. doi: 10.1016/j.arcmed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Kayatas K, Sahin G, Tepe M, Kaya ZE, Apaydin S, Demirtunç R. Acute kidney injury in the elderly hospitalized patients. Ren Fail. 2014 Sep;36((8)):1273–1277. doi: 10.3109/0886022X.2014.934693. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Huang XR, Lv J, Chung AC, Zhang Y, Chen JZ, et al. C-reactive protein promotes acute kidney injury by impairing G1/S-dependent tubular epithelium cell regeneration. Clin Sci (Lond) 2014 May;126((9)):645–659. doi: 10.1042/CS20130471. [DOI] [PubMed] [Google Scholar]
- 20.Pegues MA, McCrory MA, Zarjou A, Szalai AJ. C-reactive protein exacerbates renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2013 Jun 1;304((11)):F1358–65. doi: 10.1152/ajprenal.00476.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jimenez RV, Kuznetsova V, Connelly AN, Hel Z, Szalai AJ. C-reactive protein promotes the expansion of myeloid derived cells with suppressor functions. Front Immunol. 2019;10:2183. doi: 10.3389/fimmu.2019.02183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020 Apr;87((4)):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davoudi A, Ahmadi M, Sharifi A, Hassantabar R, Najafi N, Tayebi A, et al. Studying the effect of taking statins before infection in the severity reduction of COVID-19 with machine learning. Biomed Res Int. 2021;2021:9995073. doi: 10.1155/2021/9995073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong LZ, Shou ZX, Zheng DM, Jin X. The most important biomarker associated with coagulation and inflammation among COVID-19 patients. Mol Cell Biochem. 2021 Jul;476((7)):2877–2885. doi: 10.1007/s11010-021-04122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020 Jun;31((6)):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo E, Esposito P, Taramasso L, Magnasco L, Saio M, Briano F, et al. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J Nephrol. 2021 Feb;34((1)):173–183. doi: 10.1007/s40620-020-00875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Hou J, Ma FZ, Li J, Xue S, Xu ZG. The common risk factors for progression and mortality in COVID-19 patients: a meta-analysis. Arch Virol. 2021 Aug;166((8)):2071–2087. doi: 10.1007/s00705-021-05012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berthelot JM, Lioté F, Maugars Y, Sibilia J. Lymphocyte changes in severe COVID-19: delayed over-activation of STING? Front Immunol. 2020;11:607069. doi: 10.3389/fimmu.2020.607069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Ding X, Xia G, Chen HG, Chen F, Geng Z, et al. Eosinopenia and elevated C-reactive protein facilitate triage of COVID-19 patients in fever clinic: a retrospective case-control study. EClinicalMedicine. 2020 Jun;23:100375. doi: 10.1016/j.eclinm.2020.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020 Feb 15;395((10223)):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stringer D, Braude P, Myint PK, Evans L, Collins JT, Verduri A, et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int J Epidemiol. 2021 May 17;50((2)):420–429. doi: 10.1093/ije/dyab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 May;97((5)):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Zhang T, Li F, Mao Z, Kang H, Tao L, et al. Acute kidney injury can predict in-hospital mortality in elderly patients with COVID-19 in the ICU: a single-center study. Clin Interv Aging. 2020;15:2095–2107. doi: 10.2147/CIA.S273720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020 Jun;50((4)):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Wang W, Tang Y, Huang XR, Yu X, Lan HY. Inflammatory stress in SARS-COV-2 associated acute kidney injury. Int J Biol Sci. 2021;17((6)):1497–1506. doi: 10.7150/ijbs.58791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moledina DG, Simonov M, Yamamoto Y, Alausa J, Arora T, Biswas A, et al. The association of COVID-19 with acute kidney injury independent of severity of illness: a multicenter cohort study. Am J Kidney Dis. 2021 Apr;77((4)):490–9.e1. doi: 10.1053/j.ajkd.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doher MP, Torres de Carvalho FR, Scherer PF, Matsui TN, Ammirati AL, Caldin da Silva B, et al. Acute kidney injury and renal replacement therapy in critically ill COVID-19 patients: risk factors and outcomes: a single-center experience in Brazil. Blood Purif. 2021;50((4–5)):520–530. doi: 10.1159/000513425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang M, Fan J, Qu X, Li S, Nilsson SK, Sun YBY, et al. Combined blockade of Smad3 and JNK pathways ameliorates progressive fibrosis in folic acid nephropathy. Front Pharmacol. 2019;10:880. doi: 10.3389/fphar.2019.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goicoechea M, de Vinuesa SG, Gómez-Campderá F, Aragoncillo I, Verdalles U, Mosse A, et al. Serum fibrinogen levels are an independent predictor of mortality in patients with chronic kidney disease (CKD) stages 3 and 4. Kidney Int Suppl. 2008 Dec;74((111)):S67–70. doi: 10.1038/ki.2008.519. [DOI] [PubMed] [Google Scholar]
- 40.Gao J, Wang A, Li X, Li J, Zhao H, Zhang J, et al. The cumulative exposure to high-sensitivity C-reactive protein predicts the risk of chronic kidney diseases. Kidney Blood Press Res. 2020;45((1)):84–94. doi: 10.1159/000504251. [DOI] [PubMed] [Google Scholar]
- 41.Soriano S, González L, Martín-Malo A, Rodríguez M, Aljama P. C-reactive protein and low albumin are predictors of morbidity and cardiovascular events in chronic kidney disease (CKD) 3-5 patients. Clin Nephrol. 2007 Jun;67((6)):352–357. doi: 10.5414/cnp67352. [DOI] [PubMed] [Google Scholar]
- 42.Hung AM, Ikizler TA, Griffin MR, Glenn K, Greevy RA, Grijalva CG, et al. CRP polymorphisms and chronic kidney disease in the third national health and nutrition examination survey. BMC Med Genet. 2011 May 11;12:65. doi: 10.1186/1471-2350-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanai R, Nakashima A, Doi S, Kimura T, Yoshida K, Maeda S, et al. Interferon-γ enhances the therapeutic effect of mesenchymal stem cells on experimental renal fibrosis. Sci Rep. 2021 Jan 13;11((1)):850. doi: 10.1038/s41598-020-79664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li ZI, Chung AC, Zhou L, Huang XR, Liu F, Fu P, et al. C-reactive protein promotes acute renal inflammation and fibrosis in unilateral ureteral obstructive nephropathy in mice. Lab Invest. 2011 Jun;91((6)):837–851. doi: 10.1038/labinvest.2011.42. [DOI] [PubMed] [Google Scholar]
- 45.Yeo ES, Hwang JY, Park JE, Choi YJ, Huh KB, Kim WY. Tumor necrosis factor (TNF-alpha) and C-reactive protein (CRP) are positively associated with the risk of chronic kidney disease in patients with type 2 diabetes. Yonsei Med J. 2010 Jul;51((4)):519–525. doi: 10.3349/ymj.2010.51.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swift DL, Johannsen NM, Earnest CP, Blair SN, Church TS. Effect of exercise training modality on C-reactive protein in type 2 diabetes. Med Sci Sports Exerc. 2012 Jun;44((6)):1028–1034. doi: 10.1249/MSS.0b013e31824526cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman AN, Hunsicker LG, Selhub J, Bostom AG, Collaborative Study Group C-reactive protein as a predictor of total arteriosclerotic outcomes in type 2 diabetic nephropathy. Kidney Int. 2005 Aug;68((2)):773–778. doi: 10.1111/j.1523-1755.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 48.Schalkwijk CG, Poland DC, van Dijk W, Kok A, Emeis JJ, Dräger AM, et al. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 1999 Mar;42((3)):351–357. doi: 10.1007/s001250051162. [DOI] [PubMed] [Google Scholar]
- 49.Liu F, Chen HY, Huang XR, Chung AC, Zhou L, Fu P, et al. C-reactive protein promotes diabetic kidney disease in a mouse model of type 1 diabetes. Diabetologia. 2011 Oct;54((10)):2713–2723. doi: 10.1007/s00125-011-2237-y. [DOI] [PubMed] [Google Scholar]
- 50.Liu Q, Jiang CY, Chen BX, Zhao W, Meng D. The association between high-sensitivity C-reactive protein concentration and diabetic nephropathy: a meta-analysis. Eur Rev Med Pharmacol Sci. 2015 Dec;19((23)):4558–4568. [PubMed] [Google Scholar]
- 51.Schwedler SB, Guderian F, Dämmrich J, Potempa LA, Wanner C. Tubular staining of modified C-reactive protein in diabetic chronic kidney disease. Nephrol Dial Transplant. 2003 Nov;18((11)):2300–2307. doi: 10.1093/ndt/gfg407. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Li W, Gong M, Zhang Z, Xue X, Mao J, et al. C-reactive protein inhibits C3a/C3aR-dependent podocyte autophagy in favor of diabetic kidney disease. FASEB J. 2022 Jun;36((6)):e22332. doi: 10.1096/fj.202200198R. [DOI] [PubMed] [Google Scholar]
- 53.Juan CX, Mao Y, Cao Q, Chen Y, Zhou LB, Li S, et al. Exosome-mediated pyroptosis of miR-93-TXNIP-NLRP3 leads to functional difference between M1 and M2 macrophages in sepsis-induced acute kidney injury. J Cell Mol Med. 2021 May;25((10)):4786–4799. doi: 10.1111/jcmm.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim MG, Lim K, Lee YJ, Yang J, Oh SW, Cho WY, et al. M2 macrophages predict worse long-term outcomes in human acute tubular necrosis. Sci Rep. 2020 Feb 7;10((1)):2122. doi: 10.1038/s41598-020-58725-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Devaraj S, Jialal I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler Thromb Vasc Biol. 2011 Jun;31((6)):1397–1402. doi: 10.1161/ATVBAHA.111.225508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang PM, Zhang YY, Hung JS, Chung JY, Huang XR, To KF. DPP4/CD32b/NF-κB circuit: a novel druggable target for inhibiting CRP-driven diabetic nephropathy. Mol Ther. 2021 Jan 6;29((1)):365–375. doi: 10.1016/j.ymthe.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai W, Tang Y, Huang XR, Ming-Kuen Tang P, Xu A, Szalai AJ, et al. C-reactive protein promotes acute kidney injury via Smad3-dependent inhibition of CDK2/cyclin E. Kidney Int. 2016 Sep;90((3)):610–626. doi: 10.1016/j.kint.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.You YK, Wu WF, Huang XR, Li HD, Ren YP, Zeng JC, et al. Deletion of Smad3 protects against C-reactive protein-induced renal fibrosis and inflammation in obstructive nephropathy. Int J Biol Sci. 2021;17((14)):3911–3922. doi: 10.7150/ijbs.62929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W, Chen J, Hu D, Pan P, Liang L, Wu W, et al. SARS-CoV-2 N protein induces acute kidney injury via smad3-dependent G1 cell cycle arrest mechanism. Adv Sci. 2022 Jan;9((3)):e2103248. doi: 10.1002/advs.202103248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pegues MA, McWilliams IL, Szalai AJ. C-reactive protein exacerbates renal ischemia-reperfusion injury: are myeloid-derived suppressor cells to blame? Am J Physiol Renal Physiol. 2016 Jul 1;311((1)):F176–81. doi: 10.1152/ajprenal.00107.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bian A, Shi M, Flores B, Gillings N, Li P, Yan SX, et al. Downregulation of autophagy is associated with severe ischemia-reperfusion-induced acute kidney injury in overexpressing C-reactive protein mice. PLoS One. 2017;12((9)):e0181848. doi: 10.1371/journal.pone.0181848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L, Shen ZY, Wang K, Li W, Shi JM, Osoro EK, et al. C-reactive protein exacerbates epithelial-mesenchymal transition through Wnt/β-catenin and ERK signaling in streptozocin-induced diabetic nephropathy. FASEB J. 2019 May;33((5)):6551–6563. doi: 10.1096/fj.201801865RR. [DOI] [PubMed] [Google Scholar]
- 63.Jones NR, Pegues MA, McCrory MA, Singleton W, Bethune C, Baker BF, et al. A selective inhibitor of human C-reactive protein translation is efficacious in vitro and in C-reactive protein transgenic mice and humans. Mol Ther Nucleic Acids. 2012 Nov 13;1((11)):e52. doi: 10.1038/mtna.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szalai AJ, McCrory MA, Xing D, Hage FG, Miller A, Oparil S, et al. Inhibiting C-reactive protein for the treatment of cardiovascular disease: promising evidence from rodent models. Mediators Inflamm. 2014;2014:353614. doi: 10.1155/2014/353614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noveck R, Stroes ES, Flaim JD, Baker BF, Hughes S, Graham MJ, et al. Effects of an antisense oligonucleotide inhibitor of C-reactive protein synthesis on the endotoxin challenge response in healthy human male volunteers. J Am Heart Assoc. 2014 Jul 10;3((4)):e001084. doi: 10.1161/JAHA.114.001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pepys MB, Hirschfield GM, Tennent GA, Ruth Gallimore J, Kahan MC, Bellotti V, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006 Apr 27;440((7088)):1217–1221. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 67.Du Clos TW. Function of C-reactive protein. Ann Med. 2000 May;32((4)):274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 68.El Kebir D, Zhang Y, Potempa LA, Wu Y, Fournier A, Filep JG. C-reactive protein-derived peptide 201-206 inhibits neutrophil adhesion to endothelial cells and platelets through CD32. J Leukoc Biol. 2011 Dec;90((6)):1167–1175. doi: 10.1189/jlb.0111032. [DOI] [PubMed] [Google Scholar]
- 69.Torzewski J, Heigl F, Zimmermann O, Wagner F, Schumann C, Hettich R, et al. First-in-Man: case report of selective C-reactive protein apheresis in a patient with SARS-CoV-2 infection. Am J Case Rep. 2020 Jul 14;21:e925020. doi: 10.12659/AJCR.925020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torzewski J, Zimmermann O, Kayser S, Heigl F, Wagner F, Sheriff A, et al. Successful treatment of a 39-year-old COVID-19 patient with respiratory failure by selective C-reactive protein apheresis. Am J Case Rep. 2021 Aug 5;22:e932964. doi: 10.12659/AJCR.932964. [DOI] [PMC free article] [PubMed] [Google Scholar]