Abstract
Introduction
Golimumab (GLM) is an anti-tumor necrosis factor-alpha antibody therapy for moderately to severely active ulcerative colitis (UC). Endoscopic improvement is considered one of UC treatment's main goals, and earlier prediction of future endoscopic improvement has clinical implications. We retrospectively analyzed data from the PURSUIT-J, a phase III randomized controlled trial evaluating the efficacy of GLM in the maintenance phase, to find predictors for endoscopic improvement after 60 weeks of GLM treatment.
Methods
Ninety-two patients who had completed the maintenance phase of the PURSUIT-J were divided into two groups: those with mucosal healing (MH: Mayo endoscopic subscore of 0 or 1) and those without MH at week 60 (non-MHs). Multivariate logistic regression analysis was conducted using baseline data in the induction phase to determine predictive factors for MHs compared to non-MHs.
Results
Twenty-nine patients were classified as MHs and 63 as non-MHs. The multivariate logistic regression analysis showed that the odds ratio for partial Mayo (pMayo) score was highest in MHs (1.87 [95% CI: 1.18–2.98]) at baseline in the induction phase. The receiver operating characteristic analysis to determine the timing of predictions of MHs using pMayo showed that an area under the curve reached 0.8 at week 14 after the first GLM administration.
Discussion/Conclusion
pMayo scores at week 14 of GLM treatment are associated with MH at week 60. These results suggest the timing when a clinical decision to continue GLM based on the patient-reported outcomes and the physician's general assessment could be considered.
Keywords: Ulcerative colitis, Partial Mayo score, 1-year endoscopic improvement, Predictor, Golimumab
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease that causes long-term inflammation and ulcers in the colon and rectum. UC is characterized by inflammation of the rectum, bleeding, urgency, and tingling, and it significantly reduces quality of life [1]. Anti-tumor necrosis factor-alpha (TNF-α) antibody therapy is used to treat UC patients who have failed to respond adequately to standard therapies such as 5-aminosalicylic acid, corticosteroids, and azathioprine [2]. Although the outcome of UC treatment has improved drastically since the advent of anti-TNF-α antibody treatment, in recent years, it has been recognized that achievement of endoscopic improvement in addition to clinical remission is crucial [1, 3, 4, 5].
Golimumab (GLM) is the third anti-TNF-α antibody approved for the treatment of UC in Japan. In previous phase 3 trials (PURSUIT-SC, PURSUIT-M, and PURSUIT-J), GLM showed significant efficacy in terms of clinical response (CR), clinical remission, and mucosal healing (MH: defined as Mayo endoscopic subscore [MES] of 0 or 1 in the series of PURSUIT studies) in moderate-to-severe UC patients [6, 7, 8]. However, about 40% of randomized patients with UC do not respond to GLM treatment and some patients respond slowly [8]. Therefore, predicting the long-term efficacy of GLM before or early after initiation of GLM treatment is clinically important.
Endoscopic improvement is considered an essential factor for UC patients to maintain long-term remission [5, 9, 10, 11]. Therefore, many researchers are continuously searching for predictors of endoscopic improvement. The usefulness of serum drug level during induction has been reported in predicting future response to GLM [12]; however, its timely assessment may not be easy in clinical practice.
Therefore, in the present study, we conducted a post hoc analysis to identify factors that predict endoscopic improvement 1 year after GLM treatment initiation in a Japanese UC phase 3 study (PURSUIT-J). These analyses focused specifically on routinely collected clinical and laboratory parameters to identify straightforward, accessible, and practical factors to use in a real-world setting.
Materials and Methods
Patients and Study Design
In this study, we conducted a post hoc analysis of the data from PURSUIT-J study to identify predictors of patients achieving MH (defined as MES of 0 or 1 in the series of PURSUIT studies) at 60 weeks, and to use these factors to determine the timing of when to consider the continuation of GLM treatment. Patients enrolled in the PURSUIT-J study have been described in detail previously [8]. The PURSUIT-J study was a phase 3, double-blind, placebo-controlled, parallel-group, randomized withdrawal study conducted to evaluate the efficacy and safety of GLM treatment as maintenance therapy in Japanese UC patients. A total of 144 UC patients with moderately to severely active UC (Mayo score: 6–12) who met the inclusion and exclusion criteria were enrolled. All included patients showed an inadequate response to or failed to tolerate standard treatments (oral 5-aminosalicylates, oral corticosteroids, azathioprine, and/or mercaptopurine) or had corticosteroid dependence. All patients were naïve to anti-TNF-α treatments before GLM treatment.
In the PURSUIT-J study, patients received GLM doses subcutaneously at weeks 0 and 2. A total of 123 UC patients completed the induction phase and entered the maintenance phase. Of these patients, 63 who showed CR at week 6 were randomized (1:1) to the placebo or the GLM group, while 60 who did not show CR at week 6 were included in the open-label group.
The study retrospectively analyzed 92 patients (32 patients from the randomized group and 60 from the open-label group), including 44 patients who completed 60 weeks of GLM every 4 weeks and 48 patients who dropped out of the study. The patients included were classified into two groups. Patients who showed MH (MES of 0 or 1) at week 60 were classified as MHs, while the rest were classified as non-MHs. Patients who dropped out of the study were assumed to have failed to achieve MH and were included in the non-MHs.
Statistical Analysis
Descriptive statistics are displayed as proportions, mean, standard deviation, minimum, median, and maximum values. The last observation carried forward method was applied to the outcome variable such as partial Mayo (pMayo), Mayo, and calprotectin. Multivariate logistic regression analyses were conducted to evaluate the potential predictors using the LOGISTIC procedure of the statistical software SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The potential predictors at baseline in the induction phase included Mayo or pMayo scores, gender, age, body mass index (BMI), disease duration, extent of disease, C-reactive protein (CRP), extraintestinal manifestation, corticosteroid dosage, concentration of calprotectin, and lactoferrin. Mayo and pMayo scores were separately included in the covariate to avoid multicollinearity. The results were presented as odds ratios (ORs) with their 95% confidence intervals (CIs). We estimated the receiver operating characteristic (ROC) curve and the area under the curve (AUC). In this study, AUC > 0.8 was considered as “highly associated.” The Euclidean index method was used to determine the cutoff value corresponding to the point on the ROC curve closest to the left-hand corner of the ROC space [13]. We used this method to determine the cutoff values that optimize the differentiating ability of MHs using the pMayo (or Mayo) score. The positive and negative predictive values were also calculated together with the sensitivity and specificity. All analyses were performed using SAS version 9.4, and the graphics were prepared using R, version 3.4.3 [14].
Results
Study Population and Baseline Characteristics
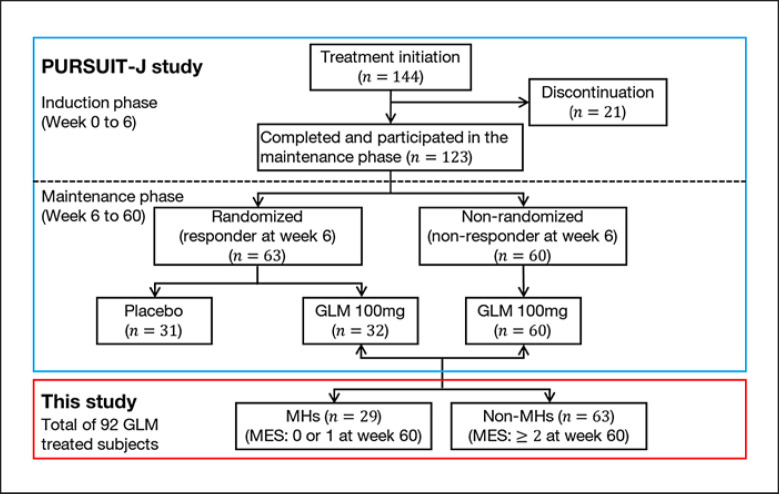
The study population is shown in Figure 1 with 29 patients classified as MHs and 63 as non-MHs. The demographic characteristics of each group (MHs and non-MHs) are summarized in Table 1. There were no apparent differences between the two groups.
Fig. 1.
Study design and patient disposition. The flow diagram of the PURSUIT-J study and the patients who participated are shown in a blue box, and the target patients analyzed in this study from PURSUIT-J are shown in a red box. GLM, golimumab; MH, patients with mucosal healing; non-MH, patients without mucosal healing; MES, Mayo endoscopic subscore.
Table 1.
Patient demographics
| Factor | MHs (n = 29) | Non-MHs (n = 63) |
|---|---|---|
| Gender (male) | 65.5% (19/29) | 66.7% (42/63) |
| Age, years | 37.6 (11.01) | 42.7 (16.12) |
| BMI, kg/m2 | 23.0 (4.15) | 22.4 (3.99) |
| Disease duration, years | 8.1 (7.50) | 6.6 (6.12) |
| Extent of disease, % (n/N) | ||
| Limited to left side colon | 67.7 (21/31) | 59.0 (36/61) |
| Extensive | 31.0 (9/29) | 41.3 (26/63) |
| Mayo scorea (0–12) | 8.3 (6; 11) | 7.8 (6; 11) |
| pMayo scoreb (0–9) | 6.0 (3; 8) | 5.4 (4; 8) |
| Severity of UC disease,c % (n/N) | 96.6 (28/29) | 98.4 (62/63) |
| CRP, mg/L | 5.5 (15.49) | 4.6 (10.84) |
| Corticosteroid dosage, mg/day | 2.7 (6.15) | 3.3 (6.05) |
| Extraintestinal manifestation,d % (n/N) | 75.9 (22/29) | 88.9 (56/63) |
| Calprotectin, mg/kg | 512.6 (387.53) | 439.5 (294.87) |
| Lactoferrin, µg/mL | 174.3 (255.10) | 184.0 (321.07) |
Patient characteristics of the patients showed mucosal healing at week 60 (MHs) and the patients did not show mucosal healing at week 60 (non-MHs).
Median (range)
Median (range)
moderate
absent.
Analysis of Predictors of MH at Week 60 from Patient Demographics
We conducted a multivariate logistic regression analysis using the clinical and laboratory parameters as covariates to identify potential predictor candidates for MH at week 60. Among the 11 factors considered, Mayo scores (especially pMayo score) at baseline in the induction phase (ORs 1.87 [95% CI: 1.18–2.98]) and age (ORs 0.50 [95% CI: 0.30–0.81]) were significantly associated with MH at 60 weeks (Table 2).
Table 2.
Multivariate logistic regression analysis of MHs/non-MHs
| Covariate | MHs versus non-MHs |
|
|---|---|---|
| W/Mayo | W/pMayo | |
| Mayo/pMayo | 1.54 [1.05, 2.27] | 1.87 [1.18, 2.98] |
| Gender (ref: female) | 1.37 [0.45, 4.18] | 1.41 [0.45, 4.37] |
| Age (unit: 10 years) | 0.52 [0.33, 0.83] | 0.50 [0.30, 0.81] |
| BMI | 1.05 [0.93, 1.19] | 1.06 [0.94, 1.20] |
| Disease duration (unit: 5 years) | 1.48 [0.96, 2.27] | 1.50 [0.96, 2.34] |
| Extent of disease (ref: limited) | 0.50 [0.16, 1.57] | 0.52 [0.16, 1.65] |
| CRP, mg/dL | 0.95 [0.65, 1.40] | 0.92 [0.62, 1.36] |
| Extraintestinal manifestation (ref: absent) | 1.55 [0.40, 5.92] | 1.39 [0.36, 5.35] |
| Corticosteroid dosage (unit: 5 mg/day) | 0.83 [0.54, 1.29] | 0.87 [0.56, 1.36] |
| Calprotectin (unit: 100 mg/kg) | 1.06 [0.89, 1.26] | 1.06 [0.89, 1.26] |
| Lactoferrin (unit: 100 µg/mL) | 0.98 [0.78, 1.22] | 0.96 [0.76, 1.22] |
Odds ratio and its 95% confidence interval are presented; the table columns “W/Mayo” and “W/pMayo” designate the multivariate logistic regression using the baseline Mayo score and the baseline pMayo score as covariate, respectively.
Cutoff Value to Predict MH
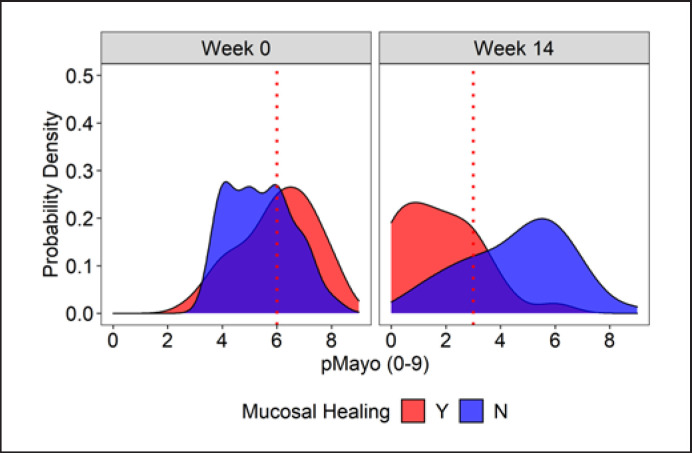
Next, using a ROC analysis, we sought the optimal time point and pMayo score cutoff value to discriminate MHs from non-MHs (Table 3). To distinguish MHs from non-MHs, week 14 was the earliest time point with a pMayo ≤ 3 to reach an AUC > 0.8. The comparison of probability density functions between MHs versus non-MHs confirmed the rationality of the 14-week timing and the cutoff value of pMayo score derived from the AUC (Fig. 2).
Table 3.
Cutoff value that optimizes the differentiating ability of MHs using the pMayo score
| Time point | Cutoff | AUC (95% CI) | Sensitivity/1 – specificity | PPV/NPV, % |
|---|---|---|---|---|
| Week 0 | 6 | 0.644 (0.518, 0.769) | 0.710/0.443 | 20.9/59.2 |
| Week 6 | 2 | 0.711 (0.593, 0.829) | 0.552/0.190 | 66.7/75.3 |
| Week 10 | 3 | 0.775 (0.671, 0.0.879) | 0.793/0.302 | 59.4/83.3 |
| Week 14 | 3 | 0.860 (0.783, 0.937) | 0.897/0.302 | 64.5/85.2 |
| Week 18 | 3 | 0.893 (0.831, 0.955) | 0.931/0.238 | 66.7/88.1 |
| Week 22 | 3 | 0.899 (0.829, 0.969) | 0.931/0/159 | 75.0/87.5 |
The Euclidian index method used to estimate the cutoff value that optimizes the differentiating ability of MHs using the pMayo (or Mayo) score AUC > 0.8 was considered as “highly associated.” The positive and negative predictive values were also calculated together with the sensitivity and specificity. PPV, positive predictive value; NPV, negative predictive value.
Fig. 2.
Comparison of probability density. The distribution of MHs (red) and non-MHs (blue) at week 0 and week 14 is shown. The vertical axis denotes the probability density, while the horizontal axis denotes the pMayo score. The red dotted line denotes the cutoff value of MHs at a crossed point between the red and blue zones. pMayo, partial Mayo score.
Time-Dependent Change of Fecal Calprotectin in MHs and Non-MHs
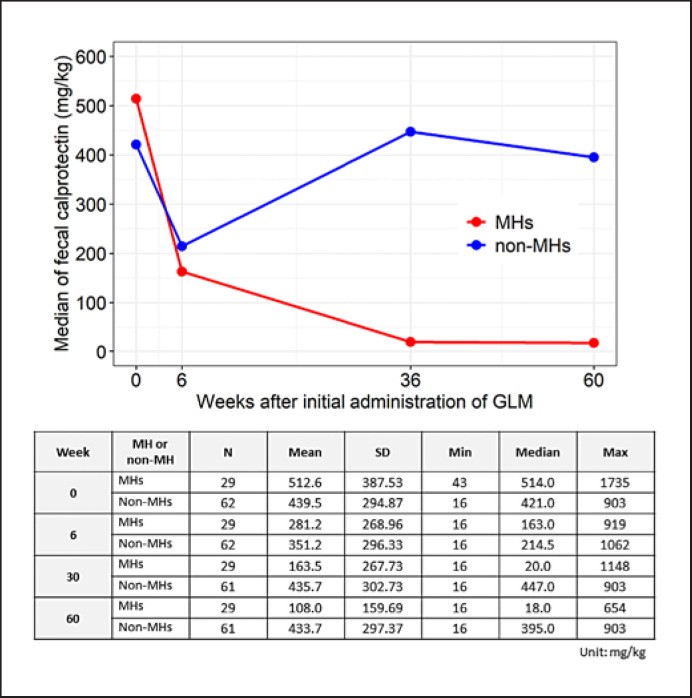
We compared the time-dependent change in fecal calprotectin (FC) concentration in the MHs and non-MHs to evaluate the status of intestinal inflammation (Fig. 3). Compared to non-MHs, the concentration of calprotectin of MHs decreased over time at 6, 36, and 60 weeks. The calprotectin titers in non-MHs showed a tendency to decrease once at 6 weeks but returned to almost baseline values at 36 and 60 weeks.
Fig. 3.
Time-dependent change in the concentration of fecal calprotectin. Median fecal calprotectin concentrations (mg/kg) at week 0 (baseline in the induction phase), 6, 36, and 60 were plotted for MHs (red line) and non-MHs (blue line). GLM, golimumab; MH, patients with mucosal healing; non-MH, patients without MH.
Discussion/Conclusion
In this post hoc analysis of PURSUIT-J data, the pMayo score at week 14 was identified as a potential predictor of endoscopic improvement after 1 year of GLM treatment. Identifying indicators for selecting more effective and economical strategies in UC treatment has become increasingly important in recent years [15]. Therefore, showing the potential of the pMayo score to predict endoscopic improvement with GLM-based therapies is clinically important. Previous studies with various biologics have shown that the rate of MH achievement in UC patients is approximately 25–60% [7, 8, 16, 17]. Similarly, our results show the difficulty of achieving endoscopic improvement after 1 year even with anti-TNF antibody treatment and highlight the importance of monitoring and predicting the effect of treatment at an early stage in order to develop an effective long-term treatment strategy.
We investigated the predictive factors for endoscopic improvement by comparing patients who achieved MH (defined as MES of 0 or 1 in PURSUIT studies) and those who did not after 1 year of GLM administration. In a series of PURSUIT trials, continuous administration of GLM increased the response and remission rates until week 14 after medication initiation [7, 8]. Endoscopic improvement has also recently been suggested as the recommended therapeutic target for achieving long-term maintenance of remission [9, 11]. Therefore, we believe that our findings that the pMayo score at 14 weeks following the first dose of GLM could estimate the group of patients who achieved MH at 1 year will reduce the burden on patients and provide an environment in which patients can consider GLM treatment with improved peace of mind.
In the multivariate logistic regression analysis, age was also extracted as the predictive factor, with ORs of 0.52 (95% CI: 0.33, 0.83) for covariates using Mayo and 0.50 (95% CI: 0.30, 0.81) for pMayo. These findings suggest that it is more difficult to reach MH with increasing age. In fact, when we examined the age distribution of patients who achieved MH at 60 weeks in our study, none of them were over 60 years of age (online suppl. Fig. 1; see www.karger.com/doi/10.1159/000526264 for all online suppl. material). Previous studies have reported that older patients have higher disease activity and more frequent IBD-related hospitalizations and surgeries [18, 19, 20], and our results suggest that older patients may have difficulty in controlling their disease in terms of endoscopic improvement. However, a larger and more detailed analysis is needed in this regard.
In the present study, patients meeting the same definition of MH as PURSUIT-J (MES 0 or 1) were analyzed to predict patients who would show MH at 60 weeks. However, it has recently been reported that patients with a MES of 0 have a significantly lower frequency of subsequent total colorectal resection than those with a subscore of 1 [21], increasing the importance of achieving and predicting endoscopic improvement.
To examine this perspective in detail, we performed an additional logistic regression analysis, defining a MES of 0 as MH. The results suggest that Mayo and pMayo may have the potential to be predictors of MH 60 weeks after the initial administration of GLM (online suppl. Table 1).
In addition to this study, several other reports have explored predictors of GLM treatment efficacy. For example, identification of optimal serum GLM concentration thresholds during induction and maintenance therapy with GLM (GO-LEVEL study) [12] and identification of GLM trough levels to achieve endoscopic remission have been performed [22, 23]. It has also been shown that GLM trough levels at week 6 predict endoscopic remission at week 14 and 1 year [23]. In addition to the measurement of GLM blood level, a new blood marker called suppressor of tumorigenicity 2 may also be useful in predicting early endoscopic remission [24]. However, additional tests are required for these predictors, and in the real-world setting, it is difficult to predict endoscopic improvement by continuing treatment while monitoring these indices. Considering the above, it is clinically useful and important that the pMayo score can predict the therapeutic effect, as suggested by the results in this study.
In this study, we also proposed a cutoff pMayo score value (pMayo ≤ 3 at week 14) for predicting MH at 1 year. In recent years, it has been reported that FC can be an effective alternative biomarker to endoscopy when confirming the presence of intestinal inflammation in UC patients [25, 26, 27]. Furthermore, it has been reported that FC assessment after 8 weeks with biologics is useful in predicting endoscopic improvement after 1 year [28]. In our study, FC showed a decreasing trend at week 6 and decreased to much lower concentrations at 36 and 60 weeks in MHs than non-MHs (Fig. 3). Therefore, in UC patients with a pMayo score close to the cutoff value at week 14, the addition of a FC measurement may enable more accurate prediction.
In line with our results, the recent STRIDE-II consensus conformed that symptom relief is the immediate target and that biomarkers are feasible treatment indicators in the medium term [29]. This sequence of treatment goals has been shown to be appropriate in previous studies [27]. Considering these factors, when the goal is to predict MH at 1 year using indices obtained early in the course of treatment, the pMayo score, which includes items used in the assessment of symptomatic relief such as stool frequency and rectal bleeding, is more appropriate than the FC test which is performed to achieve the intermediate goal.
UC symptoms reduce quality of life (HRQoL) and adversely affect work productivity in most UC patients, and those with moderate-to-severe UC, especially those who have not been able to control their symptoms, will eventually become unable to work [30]. Approximately 45% of patients with UC are reported to receive treatment with the first biologic within 1 year of diagnosis, and nearly 60% of UC patients receive treatment with a 2nd line biologic within 2 months of the last dose of the first biologic [31]. If clinicians can determine at an early stage that the current treatment is ineffective, they can avoid the futile continuation of this treatment and avoid delaying a change to the 2nd line. On the other hand, if early stage predictors indicate that long-term endoscopic improvement is highly likely, then treatment can be continued with confidence. Our results highlighting the short-term improvement are consistent with the recent STRIDE-II consensus statements that identify symptomatic improvement as a short-term target [29]. Nonetheless, regular endoscopy is valuable for the early detection of UC recurrence and colorectal cancer, the necessity of its implementation should be carefully considered, and our results do not negate the usefulness of endoscopy.
Several limitations of our study should be noted. First, this study was based on the retrospective analysis of PURSUIT-J, and only anti-TNF-α naïve UC patients were evaluated. Therefore, we did not evaluate whether the pMayo score might serve as a predictor of MH in patients who had previously received other anti-TNFs or biologics. The use of real-world data in future studies may help clarify this issue. Second, the sample size used to analyze the predictors of MH at 1 year was limited. Hence, larger studies are needed to improve the clinical reliability of our predictor further. Third, since pMayo uses Physician Global Assessment (PGA) as one of its components, which is a general assessment based on physician's subjectivity, our MH prediction method using pMayo also cannot ensure the strict objectivity.
In summary, the present study implies that a pMayo ≤ 3 at 14 weeks is useful to predict the achievement of MH at 1 year with GLM treatment. This predictor is unique in that it can be assessed without additional testing or endoscopy. Furthermore, the combined use of the pMayo score and FC may improve prediction accuracy. The results of this study help make GLM treatment an appropriate and user-friendly treatment option for patients with UC.
Statement of Ethics
The PURSUIT-J study, from which the data analyzed in this study were derived, was conducted in accordance with ethical principles originating from the Declaration of Helsinki, with the protocol approved by the Institutional Review Board of the study site to ensure compliance with good clinical practice and applicable regulatory requirements. This retrospective review of patient data did not require ethical approval in accordance with local/national guidelines. Written informed consent from participants was not required in accordance with informed consent of PURSUIT-J trial. The ClinicalTrials.gov identifier of PURSUIT-J trial is NCT01863771.
Conflict of Interest Statement
Taku Kobayashi has served as a speaker, a consultant, or an advisory board member for AbbVie, Activaid, Ajinomoto Pharma, Asahi Kasei Medical, Astellas, Alfresa Pharma, Bristol Myers Squibb, Celltrion, Covidien, EA Pharma, Eisai, Eli Lilly, Ferring Pharmaceuticals, Gilead Sciences, Janssen, JIMRO, JMDC, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Pfizer, Takeda Pharmaceutical, Thermo Fisher Scientific, and Zeria Pharmaceutical, and has received research funding from AbbVie, Alfresa Pharma, Asahi Kasei Medical, EA Pharma, Kyorin Pharmaceutical, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Sekisui Medical, Thermo Fisher Scientific, and Zeria Pharmaceutical. Yuya Imai, Yoshifumi Ukyo, Katsumasa Nagano, and Seiji Yokoyama are employees of Janssen Pharmaceutical K.K., Japan.
Funding Sources
This study was supported by the funding from Janssen Pharmaceutical K.K. (Tokyo, Japan) and Mitsubishi Tanabe Pharma Corporation.
Author Contributions
Conceptualization, methodology, and writing − review and editing: Yuya Imai, Yoshifumi Ukyo, Katsumasa Nagano, Taku Kobayashi, and Seiji Yokoyama. Formal analysis and visualization: Yoshifumi Ukyo. Funding acquisition, project administration, and writing − original draft: Seiji Yokoyama. Approval of final manuscript: all authors.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical trials/transparency. Requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgments
The researchers greatly acknowledge all study participants and physicians of the PURSUIT-J study for their dedication. The researchers also wish to thank Noriko Sato (Ikuyaku, Integrated Value Development Division, Mitsubishi Tanabe Pharma Corporation) for the contribution to the study design and data interpretation.
Funding Statement
This study was supported by the funding from Janssen Pharmaceutical K.K. (Tokyo, Japan) and Mitsubishi Tanabe Pharma Corporation.
References
- 1.Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020 Sep 10;6((1)):74. doi: 10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 2.Ooi CJ, Hilmi I, Banerjee R, Chuah SW, Ng SC, Wei SC, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn's disease in Asia. Intest Res. 2019 Jul;17((3)):285–310. doi: 10.5217/ir.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015 Sep;110((9)):1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 4.Boal Carvalho P, Cotter J. Mucosal Healing in Ulcerative Colitis: A Comprehensive Review. Drugs. 2017 Feb;77((2)):159–173. doi: 10.1007/s40265-016-0676-y. [DOI] [PubMed] [Google Scholar]
- 5.Danese S, Roda G, Peyrin-Biroulet L. Evolving therapeutic goals in ulcerative colitis: towards disease clearance. Nat Rev Gastroenterol Hepatol. 2020 Jan;17((1)):1–2. doi: 10.1038/s41575-019-0211-1. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014 Jan;146((1)):85–95. doi: 10.1053/j.gastro.2013.05.048. quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 7.Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014 Jan;146((1)):96.e1–109.e1. doi: 10.1053/j.gastro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Hibi T, Imai Y, Senoo A, Ohta K, Ukyo Y. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study-(PURSUIT-J study) J Gastroenterol. 2017 Oct;52((10)):1101–1111. doi: 10.1007/s00535-017-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoyama K, Kobayashi K, Mukae M, Sada M, Koizumi W. Clinical study of the relation between mucosal healing and long-term outcomes in ulcerative colitis. Gastroenterol Res Pract. 2013;2013:192794. doi: 10.1155/2013/192794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zallot C, Peyrin-Biroulet L. Deep remission in inflammatory bowel disease: looking beyond symptoms. Curr Gastroenterol Rep. 2013 Mar;15((3)):315. doi: 10.1007/s11894-013-0315-7. [DOI] [PubMed] [Google Scholar]
- 11.Shah J, Thakur ML, Dutta U. Mucosal healing in inflammatory bowel disease: expanding horizon. Indian J Gastroenterol. 2019 Apr;38((2)):98–109. doi: 10.1007/s12664-019-00950-x. [DOI] [PubMed] [Google Scholar]
- 12.Samaan MA, Cunningham G, Tamilarasan AG, Beltran L, Pavlidis P, Ray S, et al. Therapeutic thresholds for golimumab serum concentrations during induction and maintenance therapy in ulcerative colitis: results from the GO-LEVEL study. Aliment Pharmacol Ther. 2020 Jul;52((2)):292–302. doi: 10.1111/apt.15808. [DOI] [PubMed] [Google Scholar]
- 13.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006 Apr 1;163((7)):670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2018.
- 15.Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. 2015 Oct;149((5)):1275.e2–1285.e2. doi: 10.1053/j.gastro.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012 Feb;142((2)):257.e1–3–265.e1–3. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Furfaro F, Bezzio C, Ardizzone S, Massari A, de Franchis R, Maconi G. Overview of biological therapy in ulcerative colitis: current and future directions. J Gastrointestin Liver Dis. 2015 Jun;24((2)):203–213. doi: 10.15403/jgld.2014.1121.242.bezz. [DOI] [PubMed] [Google Scholar]
- 18.Komoto S, Higashiyama M, Watanabe C, Suzuki Y, Watanabe M, Hibi T, et al. Clinical differences between elderly-onset ulcerative colitis and non-elderly-onset ulcerative colitis: a nationwide survey data in Japan. J Gastroenterol Hepatol. 2018 Nov;33((11)):1839–1843. doi: 10.1111/jgh.14263. [DOI] [PubMed] [Google Scholar]
- 19.Shimodaira Y, Watanabe K, Iijima K. Clinical course of ulcerative colitis associated with an age at diagnosis: a recent Japanese database survey. Tohoku J Exp Med. 2021 Sep;255((1)):33–39. doi: 10.1620/tjem.255.33. [DOI] [PubMed] [Google Scholar]
- 20.Okabayashi S, Yamazaki H, Tominaga K, Miura M, Sagami S, Matsuoka K, et al. Lower effectiveness of intravenous steroid treatment for moderate-to-severe ulcerative colitis in hospitalised patients with older onset: a multicentre cohort study. Aliment Pharmacol Ther. 2022 Mar 11;55((12)):1569–1580. doi: 10.1111/apt.16865. [DOI] [PubMed] [Google Scholar]
- 21.Manginot C, Baumann C, Peyrin-Biroulet L. An endoscopic Mayo score of 0 is associated with a lower risk of colectomy than a score of 1 in ulcerative colitis. Gut. 2015 Jul;64((7)):1181–1182. doi: 10.1136/gutjnl-2014-308839. [DOI] [PubMed] [Google Scholar]
- 22.Boland K, Greener T, Kabakchiev B, Stempak J, Tessolini J, Li R, et al. Identification of target golimumab levels in maintenance therapy of Crohn's disease and ulcerative colitis associated with mucosal healing. Inflamm Bowel Dis. 2020 Apr 11;26((5)):766–773. doi: 10.1093/ibd/izz199. [DOI] [PubMed] [Google Scholar]
- 23.Stefanovic S, Detrez I, Compernolle G, Brouwers E, Sever N, Stabuc B, et al. Endoscopic remission can be predicted by golimumab concentrations in patients with ulcerative colitis treated with the changed label. Eur J Gastroenterol Hepatol. 2021;33((1)):54–61. doi: 10.1097/MEG.0000000000001843. [DOI] [PubMed] [Google Scholar]
- 24.Magro F, Lopes S, Silva M, Coelho R, Portela F, Branquinho D, et al. Soluble human Suppression of Tumorigenicity 2 is associated with endoscopic activity in patients with moderate-to-severe ulcerative colitis treated with golimumab. Therap Adv Gastroenterol. 2019;12:1756284819869141. doi: 10.1177/1756284819869141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoepfer AM, Beglinger C, Straumann A, Safroneeva E, Romero Y, Armstrong D, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013 Feb;19((2)):332–341. doi: 10.1097/MIB.0b013e3182810066. [DOI] [PubMed] [Google Scholar]
- 26.Theede K, Holck S, Ibsen P, Ladelund S, Nordgaard-Lassen I, Nielsen AM. Level of fecal calprotectin correlates with endoscopic and histologic inflammation and identifies patients with mucosal healing in ulcerative colitis. Clin Gastroenterol Hepatol. 2015 Nov;13((11)):1929.e1–1936.e1. doi: 10.1016/j.cgh.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 27.Toyonaga T, Kobayashi T, Nakano M, Saito E, Umeda S, Okabayashi S, et al. Usefulness of fecal calprotectin for the early prediction of short-term outcomes of remission-induction treatments in ulcerative colitis in comparison with two-item patient-reported outcome. PLoS One. 2017;12((9)):e0185131. doi: 10.1371/journal.pone.0185131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertani L, Blandizzi C, Mumolo MG, Ceccarelli L, Albano E, Tapete G, et al. Fecal calprotectin predicts mucosal healing in patients with ulcerative colitis treated with biological therapies: a prospective study. Clin Transl Gastroenterol. 2020 May;11((5)):e00174. doi: 10.14309/ctg.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021 Apr;160((5)):1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Armuzzi A, Tarallo M, Lucas J, Bluff D, Hoskin B, Bargo D, et al. The association between disease activity and patient-reported outcomes in patients with moderate-to-severe ulcerative colitis in the United States and Europe. BMC Gastroenterol. 2020 Jan 21;20((1)):18. doi: 10.1186/s12876-020-1164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alulis S, Vadstrup K, Borsi A, Nielsen A, Rikke Jørgensen T, Qvist N, et al. Treatment patterns for biologics in ulcerative colitis and Crohn's disease: a Danish Nationwide Register Study from 2003 to 2015. Scand J Gastroenterol. 2020 Mar;55((3)):265–271. doi: 10.1080/00365521.2020.1726445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical trials/transparency. Requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.