Abstract
Background
Intestinal fibrosis in Crohn's disease (CD) is considered to be irreversible and induces persistent luminal narrowing and strictures. In the past decades, substantial advances have been made in the understanding of the cellular and molecular mechanisms underlying intestinal fibrosis in inflammatory bowel disease (IBD).
Summary
Intestinal fibrosis is typically associated with mesenchymal cell hyperplasia, tissue disorganization, and deposition of extracellular matrix (ECM). The transient appearance of mesenchymal cells is a feature of normal wound healing, but the persistence of these cells is associated with ECM deposition and fibrosis, leading to loss of normal architecture and function. When homeostatic control of the repair process becomes dysregulated, perpetual activation of profibrotic responses and sustained accumulation of ECM are induced. In the process of intestinal fibrosis, myofibroblasts are considered to be the key effector cells, being responsible for the synthesis of ECM proteins. Activation and accumulation of myofibroblasts in the stricturing lesions of CD patients are mediated by various factors such as growth factors, cytokines, epithelial-to-mesenchymal or endothelial-to-mesenchymal transitions. Despite the identification of many putative targets and target pathways applicable to antifibrotic therapies, no such treatment has yet been successful. Predictive biomarkers and non-invasive diagnostic tools for intestinal fibrosis are still insufficient in IBD.
Key Message
We summarize recent advances in the understanding of the cellular and molecular mechanisms underlying intestinal fibrosis in IBD.
Keywords: Intestinal myofibroblasts, Antifibrotic therapy, Transforming growth factor-β, Epithelial-to-mesenchymal transition
Introduction
Inflammatory bowel diseases (IBD), which include Crohn's disease (CD) and ulcerative colitis (UC), are characterized by chronic intestinal inflammation mediated by dysregulated innate and adaptive immune responses to luminal antigens such as dietary factors and microbiota [1, 2, 3]. The chronic inflammatory process leads to disruption of the epithelial barrier and tissue destruction. Resolution of inflammatory activity is associated with a repair process that facilitates tissue remodelling, which restores normal intestinal architecture. Repair processes in UC patients are often effective in restoring a normal mucosal architecture, but stricture formation associated with excess fibrosis frequently occurs in CD patients [4, 5]. Intestinal fibrosis in CD patients is considered to be irreversible and induces persistent luminal narrowing and strictures [6]. A recent prospective study in Japan reported that approximately 35% of CD patients were classified as stricturing or penetrating phenotype at diagnosis [7, 8]. Cosnes et al. [5] reported that more than 50% of CD patients develop a penetrating or stricturing course of the disease.
Intestinal fibrosis is typically associated with mesenchymal cell hyperplasia, tissue disorganization, and deposition of extracellular matrix (ECM) [9]. The transient appearance of mesenchymal cells is a feature of normal wound healing, but the persistence of these cells is associated with excessive ECM deposition and fibrosis, leading to loss of normal architecture and function. When homeostatic control of the repair process becomes dysregulated, perpetual activation of profibrotic responses and sustained accumulation of ECM are induced. In this review, we focus on recent advances in the understanding of the cellular and molecular mechanisms underlying intestinal fibrosis in IBD.
Myofibroblasts: Key Players in Intestinal Fibrosis
Mesenchymal cells, which play a major role in intestinal fibrosis, include fibroblasts, myofibroblasts, and smooth muscle cells. Proliferation and activation of these cells are induced in response to various bioactive factors such as cytokines and growth factors. These cell types have been classified according to the expression pattern of three immunohistochemical markers: vimentin, α-smooth muscle actin (α-SMA), and desmin [10]. Fibroblasts are positive for vimentin but negative for α-SMA and desmin. In contrast, myofibroblasts are positive for vimentin and α-SMA but negative for desmin. Smooth muscle cells are negative for vimentin but positive for α-SMA and desmin.
In normal intestinal mucosa, myofibroblasts can be detected immediately subjacent to the basement membrane, juxtaposed to the base of the epithelial cells [10]. The location of myofibroblasts below the basement membrane suggests that these cells may play a role in the regulation of a number of epithelial cell functions, such as proliferation and differentiation, and ECM metabolism for maintaining a fresh basement membrane. Previous literature has described the existence of myofibroblasts as a syncytium that extends throughout the lamina propria, merging with the pericytes surrounding the blood vessels [10, 11, 12]. In the region of the crypts, myofibroblasts and muscularis mucosa cells work with epithelial cells to construct the stem cell niche [10].
In the process of intestinal fibrosis, myofibroblasts are considered to be the key effector cells as they are responsible for the synthesis of ECM proteins. Accumulation of myofibroblasts is mainly mediated by the following four mechanisms: proliferation and activation of local fibroblasts or myofibroblasts; induction of epithelial-to-mesenchymal transition (EMT); recruitment and differentiation of bone marrow-derived mesenchymal stem cells; and endothelial-to-mesenchymal transition (Endo-MT) [13]. Alfredsson et al. [14] demonstrated that myofibroblasts accumulate in layers most affected by fibrosis such as the submucosa, subserosa, and peri-cryptal region in the stricturing lesions of CD patients. Zidar et al. [15] have recently reported the major changes in mesenchymal subpopulations in the stricturing lesions of CD patients. In normal gut, CD34-positive fibroblasts/pericytes have been detected in the submucosa and subserosa, particularly around blood vessels [15]. In CD patients however, fibrosis prevailed in the submucosa and subserosa together with proliferation of myofibroblasts and disappearance of CD34-positive fibroblasts/pericytes, suggesting that fibroblasts/pericytes are the most likely source of myofibroblasts in CD [15].
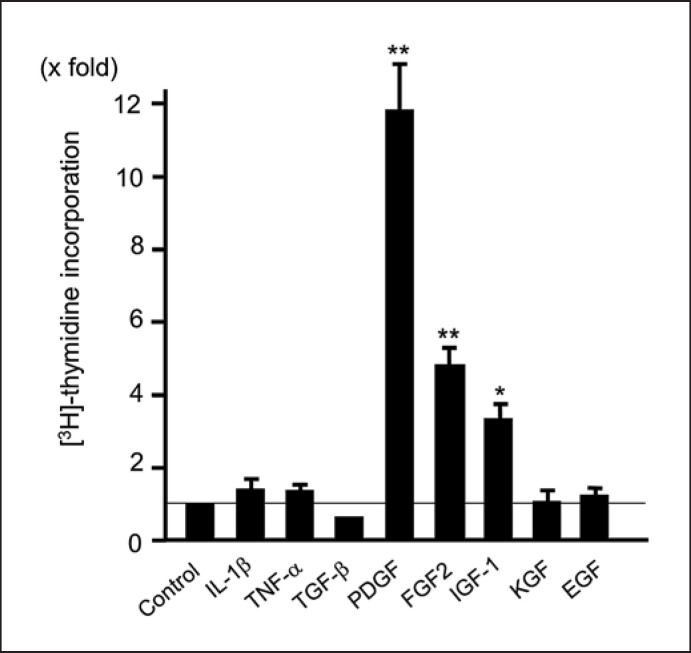
Factors mediating proliferation of myofibroblasts have been identified. Resident myofibroblasts are activated by gradients of autocrine and paracrine growth factors, such as platelet-derived growth factor (PDGF), fibroblast growth factor 2, transforming growth factor (TGF)-β1, insulin-like growth factor-1, and epidermal growth factor [13, 16] (Fig. 1). The most potent mitogen for activated myofibroblasts is PDGF released by macrophages, mesenchymal cells, and endothelial cells. PDGF exerts its action via overexpressed α- and β-receptor subunits (i.e., PDGF-Rα and PDGF-Rβ) of myofibroblasts, and autocrine/paracrine expression of PDGF and upregulation of related receptors sustained by TGF-β1. Migration of activated myofibroblasts to inflammatory sites involves ECM components or adhesion molecules such as fibronectin [17] and N-cadherin [18]. Thus, accumulation of myofibroblasts in the stricturing lesions of CD patients might be mediated by various factors such as growth factor stimuli.
Fig. 1.
Proliferation of intestinal myofibroblasts. Intestinal myofibroblasts were stimulated by various growth factors (100 ng/mL) for 24 h, and the [3H] thymidine incorporation was then determined. Each factor was used at 50 ng/mL. **p < 0.01 and *p < 0.05. IL, interleukin; TNF, tumour necrosis factor; TGF, transforming growth factor; PDGF, platelet-derived growth factor; FGF, fibroblast growth factor; IGF, insulin-like growth factor; KGF, keratinocyte growth factor; EGF, epidermal growth factor. This figure has been reused from Ref. 60 with permission from Springer Nature Group (License Number 5373230819486).
Kinchen et al. [19] have made a very important report about heterogeneity of colonic mesenchymal cells under inflammation using single-cell RNA sequencing analysis (scRNA-Seq). They identified four new populations of mesenchymal cells in addition to classical cell populations, such as myofibroblasts and pericytes, according to gene expression patterns. In UC mucosa, cell population involved in maintenance of stem cell niche was decreased, and inflammatory mesenchymal population characterized by the expression of TNF superfamily member 14, fibroblastic reticular cell-associated genes, IL-33, and Lysyl oxidases was markedly increased [19]. They suggested that changes in mesenchymal cell populations may lead to epithelial barrier dysfunction and contribute to mucosal inflammation in IBD.
Epithelial-to-Mesenchymal Transition and Endothelial-to-Mesenchymal Transition
EMT is a key contributor to the pool of activated myofibroblasts in multiple organ systems [20, 21]. The EMT process is critical for cellular conversion from epithelial cells to mesenchymal phenotypes. Epithelial cells gradually lose their epithelial markers, such as E-cadherin and cytokeratin, translocate β-catenin signals into nuclei, and de novo express some mesenchymal markers, typically α-SMA, vimentin, and fibroblast-specific protein 1 in mesenchymal myofibroblasts [22]. During tissue remodelling or fibrosis, myofibroblasts arise from epithelial lineage cells that have undergone the EMT process [23]. Among the pathways known to induce EMT, TGF-β has been shown to be one of the most common and essential pathways [24]. After being activated by TGF-β, TGF receptor type 1 (TGF-βR1) can act through the canonical Smad-dependent pathway or Smad-independent pathways (e.g., through the PI3K-Akt pathway or the mitogen-activated protein kinase [MAPK] pathway) to induce EMT [24, 25]. Moreover, EMT of polarized epithelial cells into mesenchymal myofibroblast cells is mediated by upregulated MMP-9 induced by TGF-β signalling [22].
A molecular response similar to EMT has been shown to occur in endothelial cells [26]. Endo-MT is a dynamic process in which endothelial cells undergo complex molecular changes through which they lose their endothelial attributes and acquire a mesenchymal cell-like phenotype [26]. This allows the well-ordered endothelial cells to differentiate into spindle-shaped mesenchymal-like cells. Morphological alterations are accompanied by changes in protein expression. In general, loss of endothelial markers (e.g., platelet endothelial cell adhesion molecule-1 [PECAM-1] or cluster of differentiation 31 [CD31]) and simultaneous acquisition of mesenchymal attributes (e.g., vimentin) are observed. TGF-β1 induces endothelial cells to undergo Endo-MT [26], whereas bone morphogenic protein 7 (BMP-7) preserves the endothelial phenotype [27].
The Pathological Background of Strictures of CD
Proliferation of mesenchymal cells induces thickening of the muscularis mucosae and muscularis propria, which are reported to be typical findings of CD strictures [6]. Progressed fibrostenosis results in stiffness and contraction of the entire submucosal layer. This process is mediated not only by excessive submucosal fibrosis but also by hyperplasia of the muscularis mucosae and muscularis propria. Chen et al. [28] reported that the most significant features of stenosis were smooth muscle hyperplasia of submucosa, and hypertrophy of muscularis propria. They also found a high deposition of collagen subtypes I, III, and V around activated mesenchymal cells at the margin of the muscularis mucosae. They concluded that the inflammation-smooth muscle hyperplasia axis may be the most important in the pathogenesis of CD-related strictures [28]. In this context, smooth muscle hyperplasia or hypertrophy, as well as fibrosis, may be the major contributors to increased bowel wall thickness in CD patients.
Extracellular Matrix Metabolism
Replacement of damaged space by ECM accumulation is the final step in restoring continuity of a damaged organ. Activated myofibroblasts become able to increase the synthesis of ECM components, in particular, fibrillar collagen (mainly collagen type I and III), as well as laminin, fibronectin, and α-SMA. The synthesis of these ECM components is stimulated by several profibrogenic growth factors and mediators, in particular, TGF-β1 (mainly released by activated macrophages and myofibroblasts) [29, 30, 31, 32] and oxidative stress-related mediators. ECM has been recognized to be not only a static scaffold holding cells in place and maintaining tissue architecture, but a dynamic and active participant in maintaining gut homeostasis [33]. Furthermore, ECM is considered to act as a reservoir for signalling molecules to surrounding cells of either a homeostatic or a fibrotic response to tissue injury [34]. These signalling molecules include macromolecules (i.e., collagens, fibronectin, laminin, heparan sulphate proteoglycans), proteases and their inhibitors, and growth factors and cytokines. The increased stiffness of the tissue is considered to stimulate the activated cells to deposit additional ECM in fibrotic lesion [35]. Burke et al. [36] have previously demonstrated that in normal intestine, the major collagen subtypes are types I and III and that strictured intestine is characterized by an increase in total collagen and in the relative amount of types III and V collagens. These indicate that certain subtypes of collagen (types I and III) may be associated with the normal repair process, whereas others (type V) may signify a pathological condition, leading to overt fibrosis. ECM regulates the inflammatory response and the tissue repair and fibrosis by promoting adhesion of immune and non-immune cells, such as myofibroblasts [37].
Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases
Histological findings from the inflamed mucosa of IBD patients are characterized by chronic inflammation and aberrant tissue remodelling with excessive accumulation or degradation of ECM (Fig. 2). Matrix metalloproteinases (MMPs) are able to cleave ECM components and are predominant proteases involved in the pathogenesis of IBD. The balanced turnover of ECM is regulated by the opposing functions of MMPs that constantly degrade ECMs and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs) [38, 39] (Fig. 3). Disruption of the MMP-TIMP balance results in a number of pathogenic processes including tumour invasion, angiogenesis, and tissue fibrosis. MMPs are divided into several groups based on their domain structure and substrate specificity: collagenases (MMP-1, −8, and −13), gelatinases (MMP-2 and −9), stromelysins (MMP-3, −10, and −11), matrilysins (MMP-7 and −26), membrane-type MMPs (MT-MMP-1 to −6), and others [40]. Most MMPs are upregulated in response to proinflammatory cytokines, cell-cell, or cell-ECM interactions [41]. MMPs were initially investigated for their capacity to degrade the ECM and basement membrane to induce cell migration, infiltration, and tissue remodelling [42]. They are currently recognized as key regulators of cell function through their ability to cleave many kinds of cytokines, chemokines, receptors, proteases, and adhesion molecules to alter their function [43]. For example, MMP-9 cleaves various substrates involved in the intestinal barrier, such as tight junction components (claudins and occludins), precursor of defensins, actins, cadherins, cytokines (TNF and IL-8), and growth factor VEGF [44]. Based on this, blocking of MMP-9 is considered to be one of the therapeutic targets of IBD [44]. MMPs are regulated at several levels from transcription, translation, secretion, and activation [30, 39]. There are a large number of physiological inhibitors of MMPs, which regulate MMP activity and proteolytic activity [45]. The four TIMPs are specific inhibitors of MMPs that reversibly inhibit the MMPs in a 1:1 stoichiometric fashion [45]. Recent studies suggest that posttranslational modification of MMPs, such as trimerization, glycosylation, and citrullination, influences the affinity of TIMPs to MMPs [46, 47].
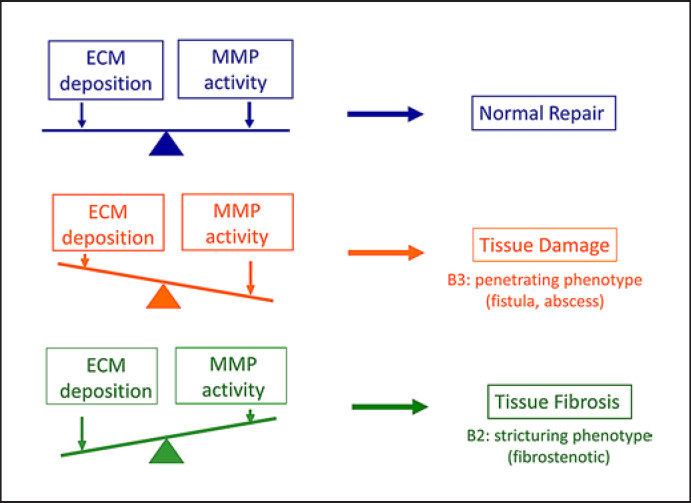
Fig. 2.
Schematic representation of tissue destruction and repair. In the normal repair process, deposition of ECM and activity of MMPs are balanced (upper panel). MMP activity is also determined by the opposing functions of MMPs and their inhibitors, TIMPs. If MMP activity dominates over ECM deposition, tissue damage, such as ulcer and fistula formation, is induced (middle panel). The balance tilted towards ECM accumulation against MMP activity leads to tissue fibrosis (lower panel). B2 and B3 indicate the disease phenotypes of Crohn's disease according to the Montreal classification [7].
Fig. 3.
Expression of MMPs and TIMPs mRNAs in intestinal myofibroblasts. The cells were incubated with each factor (100 ng/mL) for 12 h. The total RNA was extracted, and Northern blotting was performed. IL, interleukin; TNF, tumour necrosis factor; TGF, transforming growth factor; PDGF, platelet-derived growth factor; FGF, fibroblast growth factor; KGF, keratinocyte growth factor; IGF, insulin-like growth factor; EGF, epidermal growth factor. This figure has been reused from Ref. 60 with permission from Springer Nature Group (License Number 5373230819486).
There are many previous reports that describe a role of MMPs and TIMPs in the pathophysiology of IBD [38, 43]. Intestinal myofibroblasts have been shown to secrete MMP-2 and, under inflammatory stimuli, MMP-1, −3, and −9 [29, 48, 49, 50]. Pedersen et al. [51] demonstrated an increased expression of MMP-1, −3, −7, −9, and −10 in colonic epithelial cells of IBD patients. Rath et al. [52] reported that expression of MMP-7 and MMP-13 genes was significantly increased in the inflamed mucosa of UC patients, whereas MMP-28 showed a decreased expression. They identified that endothelial cells and infiltrating leukocytes were the major cellular sources of MMP-7 and MMP-13 [52]. Another study by Koelink et al. [53] has shown that infiltrating neutrophils are the main producing cells of MMP-8, −9, and −10 in the inflamed mucosa of IBD patients. Plasma cells from IBD patients were shown to produce high and sustained amounts of MMP-3 [54]. Thus, a wide range of cell types have been shown to express MMPs in the inflamed mucosa of IBD patients. The accumulated findings have been summarized in a review article by de Bruyn et al. [38]. Biancheri et al. [55] have previously demonstrated that MMP-3 and MMP-12 cleave anti-TNF-α antibodies (infliximab and adalimumab) and that MMP cleavage leads to non-responsiveness of IBD patients to anti-TNF-α antibodies. This suggests that the evaluation of mucosal proteolytic potential through the quantification of serum or mucosal MMP levels may have a potential as a biomarker of non-responsiveness to biologic therapy in IBD.
The mRNA and protein expression of TIMPs have been investigated and reported in many studies. TIMP-1 mRNA was found to be strongly expressed in the granulation tissue of the ulcer in ileal and colonic samples from IBD patients [56]. Serum TIMP-1 levels were significantly elevated in active IBD patients when compared to inactive patients or healthy controls [57], but serum TIMP-4 levels were significantly lower in IBD patients. Moreover, Soomro et al. [58] demonstrated that stool levels of MMP-9, MMP-12, and TIMP-1 were significantly higher in IBD patients than in healthy controls. An mRNA expression study by von Lampe et al. [59] showed a significant increase of MMP-1 and −3 transcripts in the inflamed mucosa of IBD patients, but TIMP-2 mRNA expression was not changed. de Bruyn et al. [38] concluded that although clinical evidence remains scant, TIMP-3 may be protective, whereas TIMP-1 may be disease promoting.
Cytokines and Growth Factors That Contribute to Intestinal Fibrosis
Transforming Growth Factor-β1
The pleomorphic cytokine, TGF-β, especially its TGF-β1 isoform, is the master regulator of fibrosis in both intestine and other organs [60]. TGF-β family signalling is mediated by Smad (canonical) or non-Smad (non-canonical) pathways. Through the binding to TGF-βR1 and 2, TGF-β initiates specific intracellular signalling that is expanded by phosphorylation of Smad 2, 3, and 4 and negatively regulated by Smad 7 [60]. Canonical TGF-β signalling via Smads has a central role in the progression of fibrosis [25]. Di Mola et al. [61] previously demonstrated that expression of all isoforms of TGF-β and TGF-βRs is increased in lymphocytes, epithelial cells, and fibroblasts in the lamina propria of patients with CD. Babyatsky et al. showed that TGF-β expression was increased in the inflamed mucosa of IBD patients and that TGF-β transcripts were detected mostly in inflammatory cells closely located to the luminal surface [62]. Furthermore, di Sabatino et al. [63] reported that myofibroblasts isolated from the mucosa of strictured gut showed higher TGF-β transcripts, a greater quantity of phosphorylated Smad 2 and 3, increased TIMP-1, lower Smad 7, increased collagen production, and reduced migration ability compared with myofibroblasts isolated from the mucosa of non-structured gut.
TGF-β1 is the most potent inducer of collagen synthesis [25]. It also induces the accumulation of myofibroblasts by promoting EMT and Endo-MT through the canonical Smad-dependent or non-canonical Smad-independent pathways and by augmenting the proliferation of myofibroblasts and rendering them resistant to apoptosis [14, 17, 24, 25]. Smooth muscle cells were transformed to myofibroblasts in chronic inflammatory conditions such as IBD [64], and these cells actively promote fibrosis by inducing collagen synthesis and MMPs under the stimulation of TGF-β. In addition, TGF-β1 affects remodelling of the ECM by enhancing tissue expression of TIMP, thus reducing the MMP:TIMP ratio, which inhibits local ECM degradation and sustains fibrosis [65]. TGF-β augments the migration of intestinal myofibroblasts and their collagen-producing capacity [14, 17]. The profibrotic effects of TGF-β were also demonstrated in an intestinal organoid fibrosis model, whereby TGF-β upregulated expression of collagen type I, fibronectin, and α-SMA [32]. In a mouse model of chronic colitis, TGF-β1 peptide-based vaccine, which suppressed excessive TGF-β bioactivity, prevented the development of intestinal fibrosis and associated complications [66], suggesting a therapeutic approach to intestinal fibrosis in IBD.
Platelet-Derived Growth Factor
One factor known to influence tissue fibrosis is platelet-derived growth factor (PDGF). Two subunits of PDGF, PDGF-A and PDGF-B, form either homodimers or heterodimers (PDGF-AA, -AB, -BB), and these bind to the two structurally related tyrosine kinase receptors defined as PDGFR-α and PDGFR-β. Severi et al. reported an increased expression of PDGF in the fibrostenotic lesions of CD patients [61]. We have previously demonstrated that PDGF most strongly stimulated a proliferation of myofibroblasts isolated from human colon [67], although it had a weak effect on collagen synthesis. Previous studies have reported differing results for collagen synthesis. Some reports have shown a dose-dependent decrease in the production of type III collagen in fibroblasts in the presence of PDGF [68], and others have shown an enhanced collagen secretion with PDGF [69]. These suggest that PDGF mainly contributes to intestinal fibrosis by stimulating myofibroblast proliferation.
Proinflammatory Cytokines: IL-1β, TNF-α, and IL-17
IL-1β, TNF-α, and IL-17 are deeply involved in the pathogenesis of IBD and contribute to inflammation and fibrosis through myofibroblast activation [70]. These cytokines induce various inflammatory mediators via activation of NF-κB, AP-1, and MAPK signalling pathways in human colonic myofibroblasts [29, 71]. In addition, these cytokines influence intestinal fibrosis via stimulation of collagen synthesis [29, 72] and induction of MMPs and TIMPs [29, 48, 73] (Fig. 3). The potential role of IL-17 in intestinal fibrosis was investigated by an experiment using anti-IL-17 antibody in TNBS colitis [74]. Treatment with anti-IL-17 antibody significantly alleviated intestinal fibrosis and reduced both mRNA and protein levels of type III collagen, TNF-α, TIMP-1, and MMP-2. The levels of profibrogenic cytokines IL-1β, TGF-β1, and TNF-α were also decreased in mice treated with anti-IL-17 antibody.
IL-33 and IL-36: Newly Identified Fibrogenic Cytokines
We have previously reported that expression of IL-33 and IL-36, members of the IL-1 family, is increased in the inflamed mucosa of IBD patients [75, 76]. IL-33 is associated with Th2 immune responses and exerts profibrotic effects [77]. Masterson et al. [78] showed that eosinophils are activated by IL-33 and IL-33 primes intestinal fibroblasts in the perpetuation of eosinophil recruitment and exacerbated fibrosis via IL-13.
IL-36 consists of three subtypes, IL-36α, IL-36β, and IL-36γ [79, 80, 81], each of which activates NF-κB and MAPK pathways [79, 82] via binding to a heterodimeric receptor consisting of the IL-36R subunit and the IL-1 receptor accessory protein (IL-1RAcP) [82, 83]. Scheibe et al. [84] demonstrated that IL-36R activation of mouse and human fibroblasts resulted in expression of genes that regulate fibrosis and tissue remodelling, as well as expression of collagen type VI. They also showed that injection of anti-IL-36R antibody reduced the severity of colitis and fibrosis in DSS and TNBS colitis models [84]. Recent studies have also implicated IL-36 and IL-36R signalling in fibrogenesis in various organs [85].
Conclusion
In this review, we summarized recent findings on the molecular basis of intestinal fibrosis in IBD. It is well recognized that intestinal myofibroblasts play a crucial role in the induction and perpetuation of fibrotic response via interaction between profibrogenic mediators and pathways (Fig. 4), but recent research using scRNA-Seq identified new mesenchymal cell populations and opened a possibility for further understanding of the molecular mechanisms underlying tissue fibrosis in IBD. On the other hand, accumulated data from preclinical studies have identified many putative targets and target pathways applicable to antifibrotic therapies, but no such treatment has yet been successful. Although MMP-9 has been shown to be a useful biomarker [44], predictive biomarkers and non-invasive diagnostic tools are still insufficient in IBD. Taking full advantage of the latest technologies such as the scRNA-Seq and multi-omics will bring a promising result in the characterization of molecular and cellular processes of intestinal fibrosis in patients with IBD.
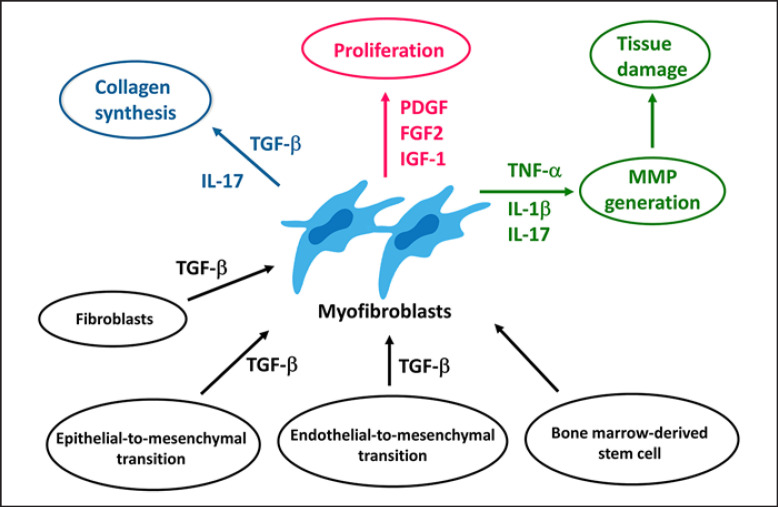
Fig. 4.
Molecular basis of intestinal fibrosis. The molecular milieu that contributes to gut fibrosis includes multiple factors whose upregulation or downregulation might promote excessive deposition of collagens and hyperproliferation of activated myofibroblasts. IL, interleukin; TNF, tumour necrosis factor; TGF, transforming growth factor; PDGF, platelet-derived growth factor; FGF, fibroblast growth factor; IGF, insulin-like growth factor.
Conflict of Interest Statement
The authors have no conflicts of interest to declare about this study.
Funding Sources
This work was supported by the Japan Agency for Medical Research and Development (AMED) under grant number JP20gm1010008h9904 (A.A.), in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan under Grant No. 22K08054 (A.A.), and in part by a Health and Labour Sciences Research Grants for Research on Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan under Grant No. 20FC1037 (A.A.).
Author Contributions
Akira Andoh and Atsushi Nishida equally contributed to this work. Akira Andoh designed the present study. Akira Andoh and Atsushi Nishida split up and wrote the paper.
Funding Statement
This work was supported by the Japan Agency for Medical Research and Development (AMED) under grant number JP20gm1010008h9904 (A.A.), in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan under Grant No. 22K08054 (A.A.), and in part by a Health and Labour Sciences Research Grants for Research on Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan under Grant No. 20FC1037 (A.A.).
References
- 1.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017 Apr 29;389((10080)):1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017 Apr 29;389((10080)):1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018 Jan;15((1)):39–49. doi: 10.1038/nrgastro.2017.136. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein MD, Loftus EV, Sandborn WJ, Tremaine WJ, Feagan BG, Nietert PJ, et al. Clinical course and costs of care for Crohn's disease: markov model analysis of a population-based cohort. Gastroenterology. 1999 Jul;117((1)):49–57. doi: 10.1016/s0016-5085(99)70549-4. [DOI] [PubMed] [Google Scholar]
- 5.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011 May;140((6)):1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 6.Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Ther. 2007 Apr;114((1)):94–106. doi: 10.1016/j.pharmthera.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006 Jun;55((6)):749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuoka K, Fujii T, Okamoto R, Yamada A, Kunisaki R, Matsuura M, et al. Characteristics of adult patients newly diagnosed with Crohn's disease: interim analysis of the nation-wide inception cohort registry study of patients with Crohn's disease in Japan (iCREST-CD) J Gastroenterol. 2022 Aug 5;57((11)):867–878. doi: 10.1007/s00535-022-01907-2. [DOI] [PubMed] [Google Scholar]
- 9.Bamias G, Pizarro TT, Cominelli F. Immunological regulation of intestinal fibrosis in inflammatory bowel disease. Inflamm Bowel Dis. 2022 Mar 2;28((3)):337–349. doi: 10.1093/ibd/izab251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joyce NC, Haire MF, Palade GE. Morphologic and biochemical evidence for a contractile cell network within the rat intestinal mucosa. Gastroenterology. 1987 Jan;92((1)):68–81. doi: 10.1016/0016-5085(87)90841-9. [DOI] [PubMed] [Google Scholar]
- 12.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999 Aug;277((2)):C183–201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- 13.D'Alessio S, Ungaro F, Noviello D, Lovisa S, Peyrin-Biroulet L, Danese S. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat Rev Gastroenterol Hepatol. 2022 Mar;19((3)):169–184. doi: 10.1038/s41575-021-00543-0. [DOI] [PubMed] [Google Scholar]
- 14.Alfredsson J, Wick MJ. Mechanism of fibrosis and stricture formation in Crohn's disease. Scand J Immunol. 2020 Dec;92((6)):e12990. doi: 10.1111/sji.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zidar N, Langner C, Jerala M, Boštjančič E, Drobne D, Tomažič A. Pathology of fibrosis in crohn's disease-contribution to understanding its pathogenesis. Front Med. 2020;7:167. doi: 10.3389/fmed.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andoh A, Fujino S, Okuno T, Fujiyama Y, Bamba T. Intestinal subepithelial myofibroblasts in inflammatory bowel diseases. J Gastroenterol. 2002 Nov;37((Suppl 14)):33–37. doi: 10.1007/BF03326410. [DOI] [PubMed] [Google Scholar]
- 17.Leeb SN, Vogl D, Grossmann J, Falk W, Scholmerich J, Rogler G, et al. Autocrine fibronectin-induced migration of human colonic fibroblasts. Am J Gastroenterol. 2004 Feb;99((2)):335–340. doi: 10.1111/j.1572-0241.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 18.Burke JP, Cunningham MF, Sweeney C, Docherty NG, O'Connell PR. N-cadherin is overexpressed in Crohn's stricture fibroblasts and promotes intestinal fibroblast migration. Inflamm Bowel Dis. 2011 Aug;17((8)):1665–1673. doi: 10.1002/ibd.21543. [DOI] [PubMed] [Google Scholar]
- 19.Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018 Oct 4;175((2)):372.e17–86.e17. doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003 Dec;112((12)):1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010 Jun 25;285((26)):20202–12. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao H, Dong Y, Tian X, Tan TK, Liu Z, Zhao Y, et al. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J Nephrol. 2013 Aug 6;2((3)):84–89. doi: 10.5527/wjn.v2.i3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovisa S. Epithelial-to-Mesenchymal transition in fibrosis: concepts and targeting strategies. Front Pharmacol. 2021;12:737570. doi: 10.3389/fphar.2021.737570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014 Mar;15((3)):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun SM, Kim SH, Kim EH. The molecular mechanism of transforming growth factor-beta signaling for intestinal fibrosis: a mini-review. Front Pharmacol. 2019;10:162. doi: 10.3389/fphar.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimatsu Y, Watabe T. Emerging roles of inflammation-mediated endothelial-mesenchymal transition in health and disease. Inflamm Regener. 2022 Feb 7;42((1)):9. doi: 10.1186/s41232-021-00186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007 Aug;13((8)):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in crohn's fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis. 2017 Jan;11((1)):92–104. doi: 10.1093/ecco-jcc/jjw126. [DOI] [PubMed] [Google Scholar]
- 29.Okuno T, Andoh A, Bamba S, Araki Y, Fujiyama Y, Fujiyama M, et al. Interleukin-1beta and tumor necrosis factor-alpha induce chemokine and matrix metalloproteinase gene expression in human colonic subepithelial myofibroblasts. Scand J Gastroenterol. 2002 Mar;37((3)):317–324. doi: 10.1080/003655202317284228. [DOI] [PubMed] [Google Scholar]
- 30.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012 Jul 6;18((7)):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockey DC, Bell PD, Hill JA. Fibrosis: a common pathway to organ injury and failure. N Engl J Med. 2015 Mar 19;372((12)):1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 32.Rodansky ES, Johnson LA, Huang S, Spence JR, Higgins PDR. Intestinal organoids: a model of intestinal fibrosis for evaluating anti-fibrotic drugs. Exp Mol Pathol. 2015 Jun;98((3)):346–351. doi: 10.1016/j.yexmp.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimshoni E, Yablecovitch D, Baram L, Dotan I, Sagi I. ECM remodelling in IBD: innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut. 2015 Mar;64((3)):367–372. doi: 10.1136/gutjnl-2014-308048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rieder F, Karrasch T, Ben-Horin S, Schirbel A, Ehehalt R, Wehkamp J, et al. Results of the 2nd scientific workshop of the ECCO (III): basic mechanisms of intestinal healing. J Crohns Colitis. 2012 Apr;6((3)):373–385. doi: 10.1016/j.crohns.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007 Dec;293((6)):G1147–54. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 36.Burke JP, Mulsow JJ, O'Keane C, Docherty NG, Watson RWG, O'Connell PR. Fibrogenesis in Crohn's disease. Am J Gastroenterol. 2007 Feb;102((2)):439–448. doi: 10.1111/j.1572-0241.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 37.Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 2009 May;1793((5)):911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 38.de Bruyn M, Vandooren J, Ugarte-Berzal E, Arijs I, Vermeire S, Opdenakker G. The molecular biology of matrix metalloproteinases and tissue inhibitors of metalloproteinases in inflammatory bowel diseases. Crit Rev Biochem Mol Biol. 2016 Sep;51((5)):295–358. doi: 10.1080/10409238.2016.1199535. [DOI] [PubMed] [Google Scholar]
- 39.Cannito S, Novo E, Parola M. Therapeutic pro-fibrogenic signaling pathways in fibroblasts. Adv Drug Deliv Rev. 2017 Nov 1;121:57–84. doi: 10.1016/j.addr.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Qin L, Liu N, Bao CL, Yang DZ, Ma GX, Yi WH, et al. Mesenchymal stem cells in fibrotic diseases-the two sides of the same coin. Acta Pharmacol Sin. 2022 Jul 27;:1–20. doi: 10.1038/s41401-022-00952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007 Jun;6((6)):480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2010 Jan;1803((1)):39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 43.O'Sullivan S, Gilmer JF, Medina C. Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators Inflamm. 2015;2015:964131. doi: 10.1155/2015/964131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Opdenakker G, Vermeire S, Abu El-Asrar A. How to place the duality of specific MMP-9 inhibition for treatment of inflammatory bowel diseases into clinical opportunities? Front Immunol. 2022;13:983964. doi: 10.3389/fimmu.2022.983964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006 Mar;25((1)):99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 46.Serifova X, Ugarte-Berzal E, Opdenakker G, Vandooren J. Homotrimeric MMP-9 is an active hitchhiker on alpha-2-macroglobulin partially escaping protease inhibition and internalization through LRP-1. Cell Mol Life Sci. 2020 Aug;77((15)):3013–3026. doi: 10.1007/s00018-019-03338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boon L, Ugarte-Berzal E, Martens E, Fiten P, Vandooren J, Janssens R, et al. Citrullination as a novel posttranslational modification of matrix metalloproteinases. Matrix Biol. 2021 Jan;95:68–83. doi: 10.1016/j.matbio.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Bamba S, Andoh A, Yasui H, Araki Y, Bamba T, Fujiyama Y. Matrix metalloproteinase-3 secretion from human colonic subepithelial myofibroblasts: role of interleukin-17. J Gastroenterol. 2003;38((6)):548–554. doi: 10.1007/s00535-002-1101-8. [DOI] [PubMed] [Google Scholar]
- 49.Yasui H, Andoh A, Bamba S, Inatomi O, Ishida H, Fujiyama Y. Role of fibroblast growth factor-2 in the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human intestinal myofibroblasts. Digestion. 2004;69((1)):34–44. doi: 10.1159/000076545. [DOI] [PubMed] [Google Scholar]
- 50.Drygiannakis I, Valatas V, Sfakianaki O, Bourikas L, Manousou P, Kambas K, et al. Proinflammatory cytokines induce crosstalk between colonic epithelial cells and subepithelial myofibroblasts: implication in intestinal fibrosis. J Crohns Colitis. 2013 May;7((4)):286–300. doi: 10.1016/j.crohns.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen G, Saermark T, Kirkegaard T, Brynskov J. Spontaneous and cytokine induced expression and activity of matrix metalloproteinases in human colonic epithelium. Clin Exp Immunol. 2009 Feb;155((2)):257–265. doi: 10.1111/j.1365-2249.2008.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rath T, Roderfeld M, Halwe JM, Tschuschner A, Roeb E, Graf J. Cellular sources of MMP-7, MMP-13 and MMP-28 in ulcerative colitis. Scand J Gastroenterol. 2010 Oct;45((10)):1186–1196. doi: 10.3109/00365521.2010.499961. [DOI] [PubMed] [Google Scholar]
- 53.Koelink PJ, Overbeek SA, Braber S, Morgan ME, Henricks PA, Abdul Roda M, et al. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut. 2014 Apr;63((4)):578–587. doi: 10.1136/gutjnl-2012-303252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gordon JN, Pickard KM, Di Sabatino A, Prothero JD, Pender SL, Goggin PM. Matrix metalloproteinase-3 production by gut IgG plasma cells in chronic inflammatory bowel disease. Inflamm Bowel Dis. 2008 Feb;14((2)):195–203. doi: 10.1002/ibd.20302. [DOI] [PubMed] [Google Scholar]
- 55.Biancheri P, Brezski RJ, Di Sabatino A, Greenplate AR, Soring KL, Corazza GR, et al. Proteolytic cleavage and loss of function of biologic agents that neutralize tumor necrosis factor in the mucosa of patients with inflammatory bowel disease. Gastroenterology. 2015 Nov;149((6)):1564.e3–1574.e10. doi: 10.1053/j.gastro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Saarialho-Kere UK, Vaalamo M, Puolakkainen P, Airola K, Parks WC, Karjalainen-Lindsberg ML. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996 Feb;148((2)):519–526. [PMC free article] [PubMed] [Google Scholar]
- 57.Kapsoritakis AN, Kapsoritaki AI, Davidi IP, Lotis VD, Manolakis AC, Mylonis PI, et al. Imbalance of tissue inhibitors of metalloproteinases (TIMP): 1 and − 4 serum levels, in patients with inflammatory bowel disease. BMC Gastroenterol. 2008 Nov 26;8:55. doi: 10.1186/1471-230X-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soomro S, Venkateswaran S, Vanarsa K, Kharboutli M, Nidhi M, Susarla R, et al. Predicting disease course in ulcerative colitis using stool proteins identified through an aptamer-based screen. Nat Commun. 2021 Jun 28;12((1)):3989. doi: 10.1038/s41467-021-24235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000 Jul;47((1)):63–73. doi: 10.1136/gut.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 61.di Mola FF, Friess H, Scheuren A, Di Sebastiano P, Graber H, Egger B, et al. Transforming growth factor-betas and their signaling receptors are coexpressed in Crohn's disease. Ann Surg. 1999 Jan;229((1)):67–75. doi: 10.1097/00000658-199901000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996 Apr;110((4)):975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- 63.Di Sabatino A, Jackson CL, Pickard KM, Buckley M, Rovedatti L, Leakey NA, et al. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn's disease strictures. Gut. 2009 Jun;58((6)):777–789. doi: 10.1136/gut.2008.149096. [DOI] [PubMed] [Google Scholar]
- 64.Rieder F, Fiocchi C. Intestinal fibrosis in IBD: a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009 Apr;6((4)):228–235. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 65.McKaig BC, McWilliams D, Watson SA, Mahida YR. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol. 2003 Apr;162((4)):1355–1360. doi: 10.1016/S0002-9440(10)63931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma Y, Guan Q, Bai A, Weiss CR, Hillman CL, Ma A, et al. Targeting TGF-beta1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis. Inflamm Bowel Dis. 2010 Jun;16((6)):1040–1050. doi: 10.1002/ibd.21167. [DOI] [PubMed] [Google Scholar]
- 67.Andoh A, Bamba S, Fujiyama Y, Brittan M, Wright NA. Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol. 2005 Dec;40((12)):1089–1099. doi: 10.1007/s00535-005-1727-4. [DOI] [PubMed] [Google Scholar]
- 68.Stallmach A, Schuppan D, Riese HH, Matthes H, Riecken EO. Increased collagen type III synthesis by fibroblasts isolated from strictures of patients with Crohn's disease. Gastroenterology. 1992 Jun;102((6)):1920–1929. doi: 10.1016/0016-5085(92)90314-o. [DOI] [PubMed] [Google Scholar]
- 69.Lawrance IC, Maxwell L, Doe W. Inflammation location, but not type, determines the increase in TGF-beta1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis. 2001 Feb;7((1)):16–26. doi: 10.1097/00054725-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Lawrance IC, Rogler G, Bamias G, Breynaert C, Florholmen J, Pellino G, et al. Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis. 2017 Dec 4;11((12)):1491–1503. doi: 10.1016/j.crohns.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002 Jun;282((6)):G1035–44. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 72.Honzawa Y, Nakase H, Shiokawa M, Yoshino T, Imaeda H, Matsuura M, et al. Involvement of interleukin-17A-induced expression of heat shock protein 47 in intestinal fibrosis in Crohn's disease. Gut. 2014 Dec;63((12)):1902–1912. doi: 10.1136/gutjnl-2013-305632. [DOI] [PubMed] [Google Scholar]
- 73.Yagi Y, Andoh A, Inatomi O, Tsujikawa T, Fujiyama Y. Inflammatory responses induced by interleukin-17 family members in human colonic subepithelial myofibroblasts. J Gastroenterol. 2007 Sep;42((9)):746–753. doi: 10.1007/s00535-007-2091-3. [DOI] [PubMed] [Google Scholar]
- 74.Li J, Liu L, Zhao Q, Chen M. Role of interleukin-17 in pathogenesis of intestinal fibrosis in mice. Dig Dis Sci. 2020 Jul;65((7)):1971–1979. doi: 10.1007/s10620-019-05969-w. [DOI] [PubMed] [Google Scholar]
- 75.Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010 Oct;45((10)):999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 76.Nishida A, Hidaka K, Kanda T, Imaeda H, Shioya M, Inatomi O, et al. Increased expression of interleukin-36, a member of the interleukin-1 cytokine family, in inflammatory bowel disease. Inflamm Bowel Dis. 2016 Feb;22((2)):303–314. doi: 10.1097/MIB.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 77.Lopetuso LR, Scaldaferri F, Pizarro TT. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair. 2012 Oct 14;5((1)):18. doi: 10.1186/1755-1536-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Masterson JC, Capocelli KE, Hosford L, Biette K, McNamee EN, de Zoeten EF, et al. Eosinophils and IL-33 perpetuate chronic inflammation and fibrosis in a pediatric population with stricturing crohn's ileitis. Inflamm Bowel Dis. 2015 Oct;21((10)):2429–2440. doi: 10.1097/MIB.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dinarello C, Arend W, Sims J, Smith D, Blumberg H, O'Neill L, et al. IL-1 family nomenclature. Nat Immunol. 2010 Nov;11((11)):973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010 Feb;10((2)):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 81.Tripodi D, Conti F, Rosati M, Maccauro G, Saggini A, Cianchetti E, et al. IL-36 a new member of the IL-1 family cytokines. J Biol Regul Homeost Agents. 2012 Jan-Mar;26((1)):7–14. [PubMed] [Google Scholar]
- 82.Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem. 2004 Apr 2;279((14)):13677–88. doi: 10.1074/jbc.M400117200. [DOI] [PubMed] [Google Scholar]
- 83.Towne JE, Renshaw BR, Douangpanya J, Lipsky BP, Shen M, Gabel CA, et al. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36α, IL-36β, and IL-36γ) or antagonist (IL-36Ra) activity. J Biol Chem. 2011 Dec 9;286((49)):42594–602. doi: 10.1074/jbc.M111.267922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scheibe K, Kersten C, Schmied A, Vieth M, Primbs T, Carle B, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019 Mar;156((4)):1082.e11–1097.e10. doi: 10.1053/j.gastro.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 85.Melton E, Qiu H. Interleukin-36 cytokine/receptor signaling: a new target for tissue fibrosis. Int J Mol Sci. 2020 Sep 4;21((18)):6458. doi: 10.3390/ijms21186458. [DOI] [PMC free article] [PubMed] [Google Scholar]