Abstract
Memories of waking-life events are incorporated into dreams, but their incorporation is not uniform across a night of sleep. This study aimed to elucidate ways in which such memory sources vary by sleep stage and time of night. Twenty healthy participants (11 F; 24.1 ± 5.7 years) spent a night in the laboratory and were awakened for dream collection approximately 12 times spread across early, middle, and late periods of sleep, while covering all stages of sleep (N1, N2, N3, REM). In the morning, participants identified and dated associated memories of waking-life events for each dream report, when possible. The incorporation of recent memory sources in dreams was more frequent in N1 and REM than in other sleep stages. The incorporation of distant memories from over a week ago, semantic memories not traceable to a single event, and anticipated future events remained stable throughout sleep. In contrast, the relative proportions of recent versus distant memory sources changed across the night, independently of sleep stage, with late-night dreams in all stages having relatively less recent and more remote memory sources than dreams earlier in the night. Qualitatively, dreams tended to repeat similar themes across the night and in different sleep stages. The present findings clarify the temporal course of memory incorporations in dreams, highlighting a specific connection between time of night and the temporal remoteness of memories. We discuss how dream content may, at least in part, reflect the mechanisms of sleep-dependent memory consolidation.
Keywords: dreaming, memory sources, serial awakenings, episodic memory, semantic memory, sleep stages, temporal changes, REM sleep, NREM sleep
Graphical Abstract
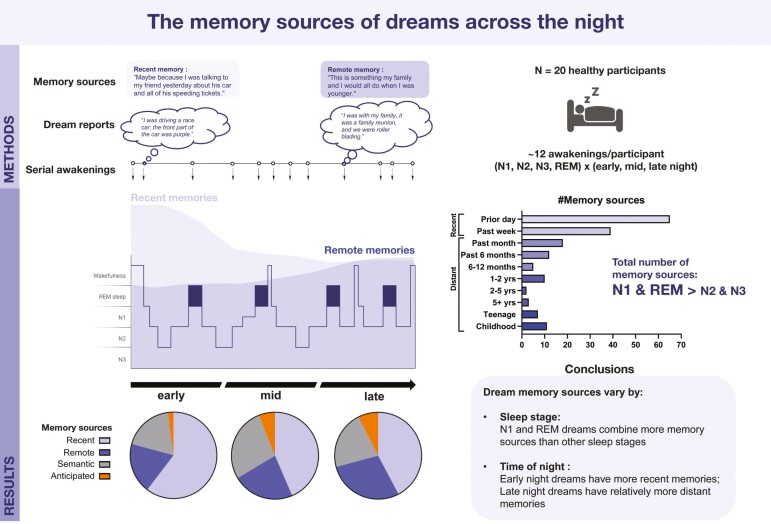
Graphical Abstract.
Statement of Significance.
Using a serial-awakening paradigm, our results show that multiple memory sources can co-exist in dreams, especially in dreams from sleep-onset N1 and REM sleep. Results also show that the memory sources of dreams become relatively less recent and more remote across a night of sleep, and that this effect is independent of sleep stage. Elucidating when different memory types are incorporated into dreams could advance our understanding of sleep-dependent memory processing and inform future research aimed at modulating specific memories during sleep.
Introduction
The content of dreams often reflects elements of waking-life events. Over 80% of dream reports can be associated with at least one identifiable event memory originating in either the recent or remote past, or from a future anticipated event [1–3]. Dreaming about events from the previous day (day residues) are especially prevalent, identified in 60%–75% of reports [4]. However, previous events are rarely replayed in their entirety in dreams [5]. Rather, only fragments of episodic memories seem to be combined into novel scenarios [2, 3, 6, 7].
One view is that this incorporation of memories into dreams reflects sleep-dependent memory consolidation, which involves the reactivation and organization of memory traces into long-term cortical storage during sleep (e.g. [8, 9]). In support of this view, evidence from both rapid eye movement (REM) sleep and non-REM (NREM) sleep shows that dreaming about elements of a recently learned task is associated with improved performance on that task (e.g. [10–14]). Several investigators suggest that the interweaving of recent and distant memories in dream narratives supports a process of integrating novel information into broader semantic and autobiographical memory networks [9, 15, 16].
Beyond merely strengthening individual memories, the creation of novel associations between different waking-life experiences during dreaming may also bolster creativity and help to extract the overall gist of similar experiences [16]. These are functions that have also been attributed to REM sleep more generally (e.g. [17, 18]). Further, the incorporation into dreams of fragments from the past could serve to simulate potential future scenarios and help individuals prepare for upcoming events and challenges [2, 6]. To better understand the central role of memories in dream formation and to shed light on the possible functions of dreaming, we sought to obtain systematic evidence on the types and temporal characteristics of dream memory sources. Specifically, we asked whether different types of memory are incorporated into dreams at different times of the night or in different sleep stages.
Memory sources of dreams across the night
The incorporation of memories into dreams has been shown to be modulated by certain factors, particularly time of night [19]. An early insight about REM dreams was that the temporal recency of a memory source varies across the night [20–22]. The most recent memories tend to be incorporated in early-night dreams, whereas more remote memories tend to appear at the end of the night. This temporal pattern was also found in a case study for sleep-onset (N1) dreams [23]. Nevertheless, this temporal effect has yet to be confirmed or replicated in a laboratory study with an adequate sample size.
Incorporation may also be influenced by sleep stage. Day residues are more frequent in stage 2 (N2) dreams than in REM dreams and remote memories from up to a year ago are more frequent in REM dreams than in N2 dreams [24]. However, because REM awakenings occurred later at night than N2 awakenings in the latter study, the results may reflect an influence of time of night rather than sleep stage.
To understand memory incorporation in dreams, the type of memory (e.g. episodic or semantic) must also be taken into account as mechanisms may differ for different types. Specifically, episodic memories pertain to events that were personally experienced in a certain spatial and temporal context (e.g. “four days ago when I was stung by a scorpion”), whereas semantic memories reflect general knowledge or abstract self-references without a specific spatiotemporal context of learning (e.g. “my fondness for botany”). NREM dream sources are found to be more episodic-like throughout the night than are REM dream sources [25, 26]. REM sources are more semantic, especially later at night, than are NREM sources [27]. NREM dreams also correspond more closely to the original memory source than do REM dreams [28]. In at-home studies, early-night dreams were more clearly related to waking life, while late-night dreams were more hyperassociative, in that they were more bizarre, metaphorical, and remote [29, 30]. However, these effects again have not been disentangled from sleep-stage differences between early and late night.
Finally, the incorporation of specific memory sources, such as the sleep laboratory itself, can be modulated by both sleep stage and time of night. Specifically, dreaming about the lab is more frequent in REM dreams than in NREM dreams, as well as in morning nap dreams than in overnight sleep dreams [6]. Moreover, the probability of dreaming about future scenarios related to the lab (e.g. false awakenings) increases with time of night [6].
Serial awakenings to uncover memory sources
Serial-awakening protocols have been used to efficiently investigate the neural correlates and memory sources of dream content (e.g. [12, 23, 31]). Studies have mainly explored memory sources of dreams that arise either from the same stage of sleep throughout the night [21–23], or from different stages of sleep across multiple nights (e.g. [25–27, 32]). A few studies have performed multiple awakenings in NREM or REM sleep through a single night to assess the dream-lag effect, wherein memory sources re-appear in dreams about a week following the initial experience [33, 34]. These studies show that dream-lag effects occurred mainly in REM sleep dreams, the effect being stronger in early REM periods [33]. However, because of the focus on the dream-lag effect, memory sources in these studies were limited to 10 or 14 days prior to the study, so how these factors relate to more distant memory sources—or to anticipatory cognitions—is still unclear. In a recent study, Wamsley [2] performed up to 10 awakenings at sleep onset and three additional awakenings during the night in NREM sleep, REM sleep, and in the morning. Episodic memory sources were found more frequently in N1 dreams than in REM and NREM dreams that occurred later in the night. In parallel, the probability that a dream would be associated with an episodic memory declined from the first to the fourth quartile of the night, while the probability that a dream would be associated with a semantic memory or an anticipated future event remained stable across the night.
In sum, serial awakenings have been valuable for uncovering variations in memory sources across dreams. Nevertheless, the differential contribution of sleep stages and time of night to which specific memory sources are incorporated remains unclear.
Objectives and hypotheses
Accordingly, our primary objective was to analyze the memory sources of dreams with respect to time of night and sleep stage, while taking into account type of memory (episodic vs. semantic) and temporal period (recent vs. distant past, or anticipated future). We thus planned awakenings during a single night that sampled all stages of sleep (N1, N2, N3, REM) and each of three different periods of the night (early, mid, late). We hypothesized that: (1) REM dreams would have relatively more semantic memory sources, while NREM dreams would have relatively more episodic memory sources, independent of time of night; and (2) remote memory sources would predominate in late-night dreams compared to early-night dreams, while recent memory sources would predominate in early-night dreams.
Methods
Participants
A total of 20 healthy participants (11 females; 9 males; 24.1 ± 5.7 years; range: 18–40 years) were recruited by word of mouth and with ads placed on campus at the University of Rochester and at local businesses. Participants were required to be 18–40 years of age, to self-declare they were mentally and physically healthy, to have no sleep disorders, to not take psychotropic medication and to have recalled at least 3 dreams per week for the last 6 months (mean ± SD: 4.2 ± 1.1; range 3-7 dreams/week). Procedures accorded with the ethical standards of institutional research committees and with the 1964 Helsinki declaration and its later amendments. Ethics approval was obtained from the University of Rochester Research Subjects Review Board. Participants gave written informed consent and received US$120 for their participation as well as compensation for transportation if needed.
Procedures
Participants first came to the University of Rochester Medical Center to receive detailed information on the procedures of the study as well as a brief training on how to complete the at-home dream journal and the Dream-Memory Association task. They gave written informed consent, completed online questionnaires, and completed 5 days of home sleep/dream logs prior to starting laboratory participation, including on the morning of participation (see Questionnaires and Home Dream Journal sections in Supplementary Material for more details). For the in-lab session, participants arrived one hour before their usual bedtime (mean ± SD: 10:01 ± 0:47 PM). Then, a research technician fitted them with a standard montage of polysomnography (PSG) electrodes (see section on Polysomnography). Participants were given a 9-hour window to sleep (~11 PM to 8 AM) during which serial awakenings (n = 12 awakenings) with dream collection were performed. Participants were then allowed to sleep longer (without awakenings) if they wished. In the morning, the PSG electrodes were removed and participants completed the Dream-Memory Association task. They subsequently completed 10 consecutive days of home sleep/dream logs.
Serial awakenings and dream collection
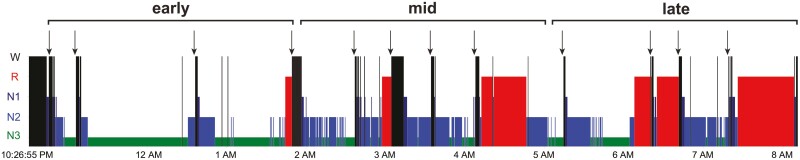
Awakenings were planned to occur in all four stages of sleep (N1, N2, N3, REM) and across three periods of sleep (early, mid, late), resulting in 12 awakenings in total (see Figure 1 for an example). In order to optimize the chance of having awakenings in all stages of sleep for each period of the night, the order of awakenings followed, when possible: N1, N3, N2, then REM sleep for each period.
Figure 1.
Example of a participant’s hypnogram with serial awakenings; each awakening is indicated with an arrow. Wake periods are in black and thirds of the night are indicated with horizontal brackets.
In order to homogenize the awakenings within each sleep stage throughout the night, they were planned to occur after ~5 minutes of stable N2, N3, or REM was detected (we achieved a mean ± SD of 7.8 ± 5.8 minutes, min = 0.5; max = 41 min), or after ~15 seconds of N1 sleep (mean ± SD = 1.2 ± 1.1 minutes). Awakenings were planned to be a minimum of 15 minutes apart, except for N1 awakenings, which could be prompted upon falling back asleep from a previous awakening. Awakenings were also planned to occur a maximum of 90 minutes apart, i.e. if the next expected stage did not come within a full cycle of sleep, the experimenter would provoke an awakening as long as the sleep stage was different from the previous awakening (we achieved a mean ± SD of 51.6 ± 30.6 minutes apart, min = 10; max = 164 minutes). The three sleep periods (early/mid/late) were established based on the participant’s usual sleep duration (divided by 3). The online sleep stage scored for each awakening was later compared to the offline sleep scoring performed over the whole night (see the Polysomnography section). Awakenings were correctly identified online 96.9% of the time (220 out of 227 awakenings). The offline scoring was used for analyses.
The amount of time elapsed in the targeted sleep stage before awakening did not differ significantly between N2 (6.0 ± 3.7 minutes), N3 (5.9 ± 2.6 minutes) and REM (7.8 ± 5.8 minutes), although it was lower in N1 due to our target of only ~15 seconds N1 preceding awakening (1.2 ± 1.1 minutes). Time to fall back asleep after the awakenings, calculated as the time to reach the next stable N1 sleep period (i.e. a sleep onset N1 period which led to N2 sleep rather than to awakening), did not differ between sleep stages (N1: 9.0 ± 8.1 minutes; N2: 8.1 ± 21.2 minutes; N3: 6.2 ± 13.7; REM: 6.3 ± 8.0 minutes; p = .493) or time of night (p = .856). When examining the five minutes preceding each awakening, N2 awakenings contained 91.1 ± 15.1% of N2; N3 awakenings contained 90.2 ± 19.0% of N3; and REM awakenings contained 95.0 ± 11.6% of REM sleep. N1 awakenings were always preceded by at least 15 seconds of N1 sleep.
The experimenter woke participants up by softly calling their names through a loudspeaker in the bedroom. Participants were immediately asked to report in detail “what was going through your mind just before I called your name”. If they reported any dream experience, they were then asked to rate their level of immersion in it (results not reported here). If participants did not recall any dream experience, they were asked whether they had a so-called white dream, with the prompt: “do you still have a strong impression that you were dreaming but forgot the content, or do you feel like you were not experiencing anything?”. Finally, all participants were asked to rate their subjective sleep perceptions (results not reported here; see Supplementary Material for the full script used for In-lab dream collection). They were then told they could go back to sleep. Verbal reports were recorded and were transcribed manually by the experimenters during the night.
Dream-memory association task
Upon awakening each morning, participants were asked to identify and write down associated memories for each at-home and in-lab dream report. For the in-lab session, the transcribed dream reports were displayed one at a time on a computer screen in a random order. For each report, participants were asked to rate how clearly they remembered having had that dream experience on a 1–5 scale (clarity of recall). They were then asked to consider the different dream elements (e.g. characters, places, objects, feelings) and think of any events from their life (past or anticipated) that may have triggered this element and to think of other prominent memories associated with this element. Next, they dated the recalled events as precisely as possible from a list of time periods (past events: yesterday/ 2 days ago/ 3–4 days ago/ 5–6 days ago/ 7–8 days ago/ within the past month/ within the past 6 months/ 6–12 months ago/ 1–2 years ago/ 2–5 years ago/ 5+ years ago (but later than teenage)/ teenage/ childhood; anticipated events: later today/ tomorrow/ in 2–3 days/ in 4–5 days/ in 6–7 days/ in more than a week). Participants also had the option to specify another date or time period not present in the list (‘Other), or to select “Related to waking life events, but not traceable to single event(s)” if the waking life memory could not be traced back to a single event or specific time period (i.e. semantic memory). They were allowed to add more than one memory source to a single dream element, but not to reuse the same memory source for different dream elements. If the memory source could be traced back to a single event, it was considered to be episodic, whereas if a single source was not apparent, it was considered to be semantic. Finally, if the source was an anticipation of a future event, it was considered to be a future source. Past memory sources were further separated into recent past (from yesterday to 7–8 days ago) and distant past (within the past month through childhood). Participants were given detailed instructions on how to report the dream-memory associations and were provided with examples for each type of memory/event (see the full Dream-Memory Association task with instructions in Supplementary Material).
Polysomnography
Participants slept in a private sleep laboratory bedroom with continuous audiovisual surveillance. They were recorded with a standard 10–20 montage of electroencephalography (F3, F4, C3, C4, O1, O2), electrooculography (1 vertical left, 1 horizontal right) and electromyography (3 on the chin) channels; right-hemisphere channels were referenced to A1 and left-hemisphere channels to A2. Biosignals were recorded using Alice 6 Acquisition Systems (-6dB filters with cut-offs at 0.30 and 100 Hz) and controlled by Sleepware G3 software (Philips Inc., United States). Tracings were visually monitored during the night and were later scored by an expert blind to the online-scored sleep stages, using American Academy of Sleep Medicine standards [35]; sleep variables (e.g. REM min, %REM, NREM min, %NREM, Total Sleep Time) were calculated by Sleepware G3 software (Philips Inc., United States).
Qualitative assessment (judge ratings)
Lab incorporation dreams.
The incorporation of the laboratory in dreams was scored based on whether or not (1 or 0) there was any trace of the laboratory, including lab-related people, places, objects, tasks and sleep activities (see step 1 of the SoLID Criteria for more details on the scoring procedure [6]). This scoring procedure was done by one judge familiar with the SoLID criteria and was done independently of the participants’ own identification of lab-related memory sources.
Repetition of dream themes through the night.
Three judges independently identified repeated themes (e.g. a same person or same action repeating through more than one dream) in each participant’s dream reports and then worked to a consensus on these themes. These repeated themes could be lab-related (e.g. electrodes, bed), people (e.g. family members, friends), objects (e.g. cars, food), places (e.g. high school, home), actions (e.g. preparing for something, cleaning) or others (e.g. money, music).
Statistical analyses
Statistical analyses were conducted in R [36]. Results visualizations were performed with R and GraphPad Prism 9.
Generalized linear mixed models (GLMM; glmer function) [37] were used to assess factors that predict the occurrence (1 or 0) of memory sources identified by the participant from the recent past (1-8 days previous), the distant past (within the past month to childhood), the future (later today to more than 1 week in the future) or that were semantic (not traceable to single events); or the occurrence of judge-rated lab incorporations (1 or 0). We entered Sleep stage (N1, N2, N2, REM) and Time of night (early, mid, late) as fixed effects predictors.
Linear mixed models (LMM; lmer function) were used to assess continuous outcomes (proportions of past, semantic or future memory sources; Recent-Distant and Semantic-Episodic relative difference) with Sleep stage and Time of night as fixed factors.
Participant ID# was added as a random effect in all GLMM and LMM models to take into account variable numbers of dreams reported by individual participants. GLMM were fit by maximum likelihood (Laplace Approximation) and LMM were fit by restricted maximum likelihood procedures. Global p-values for fixed factors were calculated with the mixed function (afex package) for GLMM and with the anova function (Satterthwaite’s method) for LMM. Post-hoc tests were calculated with the lsmeans function; degrees of freedom were calculated with the Kenward-Roger method and p-values were adjusted for multiple comparisons with the Tukey method.
Results
Descriptive
Participants were woken up on average 11.40 ± 0.88 times per night (min = 9; max = 12) and recalled 8.20 ± 2.02 dreams (min = 4; max = 12). They spent 8.80 ± 0.86 hours in bed (from lights off to lights on) and slept 7.33 ± 1.08 hours, with a sleep efficiency of 81.69 ± 11.40% (see Supplementary Table S1 for more details on sleep characteristics).
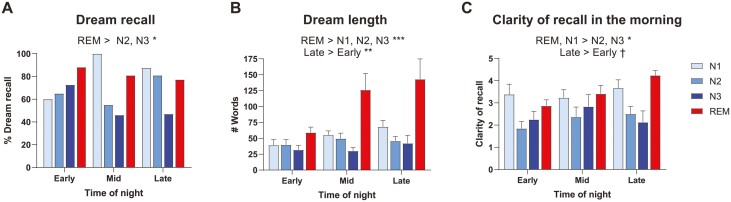
The probability of recalling a dream (excluding white dreams) varied by Sleep stage (p = .002) but not by Time of night (p = .929). Pairwise comparisons show that dreams were more frequently recalled in REM sleep than in N3 sleep (p = .003) and, marginally, in REM sleep than in N2 sleep (p = .056) (Figure 2A and Table 1). The length of dream reports also differed with Sleep stage (p < .001) and Time of night (p = .008), being longer in REM than in all other stages (p < .0001) and shorter in early awakenings than in late awakenings (p = .007) (Figure 2B). A marginal interaction between Sleep stage and Time of night (p = .091) shows that REM dreams were longer than those of other sleep stages during mid and late night (all p < .012), but not during early night (all p > .962), and that only REM dreams increased in length across early to late night (p = .007). Clarity of dream recall was predicted by Sleep stage (p < .001) and marginally by Time of night (p = .075), with no interaction between the two (p = .289): dreams that occurred in N1 or in REM sleep were recalled more clearly in the morning than dreams that occurred in N2 or N3 sleep (all p < .05); and dreams that occurred late in the night tended to be recalled more clearly in the morning compared with earlier dreams (p = .086) (Figure 2C). To control for a potential effect of dream report length and clarity of dream recall on the identification of associated memory sources, these variables were used as additional predictors in the GLMMs and LMMs when indicated.
Figure 2.
Dream recall variation across Sleep stage and Time of night. (A) Dreams were more frequently recalled in REM sleep across the night; (B) dreams were generally longer in REM sleep, especially in later awakenings; (C) dreams were remembered more clearly in the morning when they occurred in REM or in N1 sleep, or when they occurred in later awakenings. Means and SEMs are shown.
Table 1.
Number of dreams recalled and number of awakenings as a function of sleep stage and time of night
| # Recalled dreams/# Awakenings (%) | N1 | N2 | N3 | REM | All stages |
|---|---|---|---|---|---|
| Early | 12/ 20 (60.0%) | 13/20 (65.0%) | 16/ 22 (72.7%) | 15/ 17 (88.2%) | 56/ 79 (70.9%) |
| Mid | 18/ 18 (100.0%) | 11/ 20 (55.0%) | 6/ 13 (46.2%) | 17/ 21 (80.9%) | 52/ 72 (72.2%) |
| Late | 14/ 16 (87.5%) | 17/ 21 (80.9%) | 8/ 17 (47.1%) | 17/ 22 (77.3%) | 56/ 76 (73.7%) |
| All periods | 44/ 58 (75.8%) | 41/ 60 (68.3%) | 30/ 53 (56.6%) | 49/ 56 (87.5%) | 164/ 227 (72.2%) |
Memory sources across sleep stages and time of night
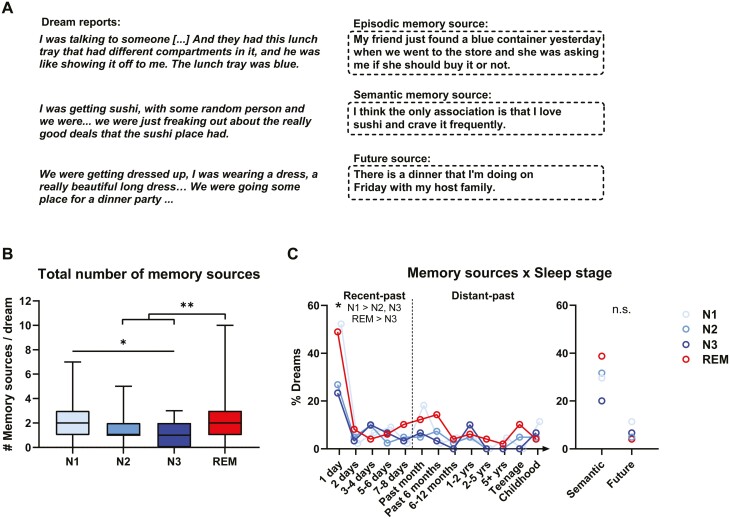
Memory sources were identified for 143 dreams (87.2%) with an average of 1.77 ± 1.39 memory sources per dream (min = 0; max = 10). A total of 91 (55.5%) dreams incorporated memories from the recent past and 49 (29.9%) from the distant past; 51 (31.1%) incorporated semantic sources (not related to one event) and 11 (6.7%) incorporated sources from anticipated future events (see Figure 3A and Supplementary Table S2 for examples). Day-residues were by far the most prevalent time category, occurring in 65 out of 164 dreams (39.6%), and no dream-lag effect (more incorporations from ~5-7 days ago) was observed (Figure 3C). In future memory sources, 6 cases (54.5%) were anticipated events later in the day, 3 cases the following day, 1 case in 2-3 days and 2 cases in more than a week.
Figure 3.
Memory sources of dreams. (A) Examples of episodic, semantic, and future sources associated with dream reports. (B) Total number of memory sources identified within a dream, including episodic, semantic, and future sources (REM>N2, N3; N1>N3). Boxplots with Min to Max point ranges are shown. (C) Percentage of dreams, by Sleep stage, that were associated with memory sources from specific time periods or of specific type (semantic, future). Recent episodic memory sources, and day residues specifically, were more likely to be identified in N1 and REM dreams compared to other sleep stages.
The total number of memory sources identified for each dream was predicted by Sleep stage (p < .001), but not by Time of night (p = .323). Specifically, N1 dreams had more memory sources than N3 sleep (β = 0.818, SE = 0.289, t(145) = 2.830, p = .027) and REM dreams had more memory sources than both N2 (β = −0.856, SE = 0.251, t(142) = −3.413, p = .005) and N3 dreams (β = −1.110, SE = 0.279, t(143) = −3.977, p < .001) (Figure 3B). When added separately to the model, both clarity of recall (p < .001) and dream length (p < .0001) were strong predictors of the total number of memory sources in dreams; the effect of Sleep stage persisted in both cases (p = .030 and p = .029 respectively).
The presence (1 or 0) of recent-past memory sources was also predicted by Sleep stage (p = .012), but not by Time of night (p = .276). Specifically, recent-past memory sources were more frequent in N1 (29/44, 65.9%) than N3 (11/30, 36.7%) dreams (β = 1.620, SD = 0.571, z = 2.839, p = .024) and more frequent in REM (31/49, 63.3%) than N3 dreams (β = −1.464, SD = 0.550, z = −2.664, p = .039) (Figure 3C and Supplementary Table S3). These effects remained significant when excluding dreams incorporating the sleep laboratory (N1 > N3, p = .019; REM > N3, p = .039). When added separately to the model, neither clarity of recall (p = .294) nor dream length (p = .462) predicted the presence of recent-past memory sources in dreams and the effect of Sleep stage persisted in both cases (p = .055 and p = .009, respectively).
When breaking down the Sleep stage effect on recent sources by days, Sleep stage predicted the occurrence of day residues (p = .008), being marginally more frequent in N1 (52.2%) than both N2 (26.8%) (p =.067) and N3 (23.3%) (p = .054), and marginally more frequent in REM (49.0%) than N3 (p = .096) (Figure 3C). When excluding dreams incorporating the sleep laboratory, sleep stage still predicted day residues (p = .013; N1 > N2, p = .083; N1 > N3, p = .057). Sleep stage did not predict the occurrence of memory sources from 2 days (p =.574), 3–4 days (p =.587), 5–6 days (p =.589) or 7–8 days (p = .409) ago.
In contrast, the presence of distant-past memory sources was not predicted by Sleep stage (p = .106) or Time of night (p = .302) and the presence of semantic memory sources was also not predicted by Sleep stage (p = .256) or Time of night (p = .489) (Figure 3C and Supplementary Table S3). However, the total number of semantic memory sources within each dream was marginally predicted by Sleep stage (p = .057), but not by Time of night (p =.302), being higher in REM (mean ± SD = 0.57 ± 0.84) than N3 (mean ± SD = 0.20 ± 0.41) sleep (β = −0.355, SE = 0.149, t(145) = −2.376, p = .086).
The presence of future sources was not predicted by Sleep stage (p =.588) or Time of night (p =.129) (Figure 3C and Supplementary Table S3). We noted, however, that only 1 of the 11 dreams containing future sources occurred in an early awakening. The rest occurred equally in mid (5/11) or late (5/11) awakenings.
Recent memory sources decreased in later awakenings
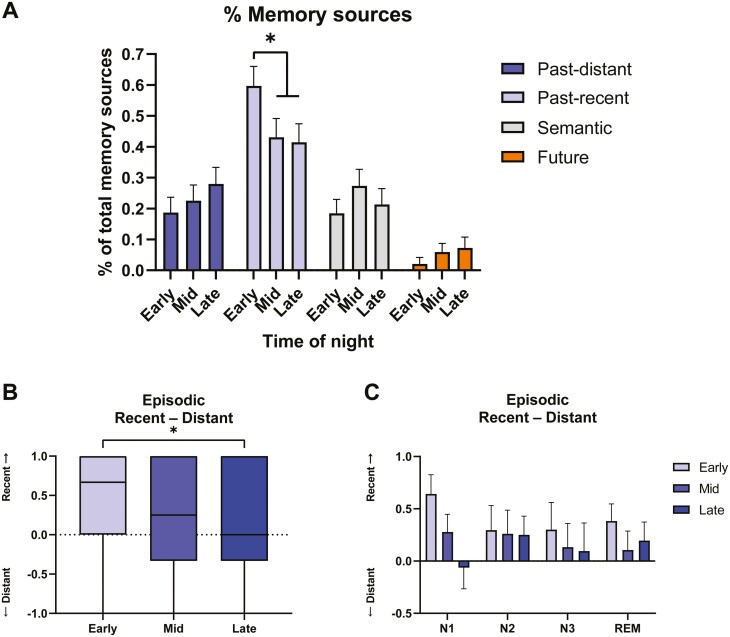
To control for the total number of memory sources in each dream, which was influenced by Sleep stage, we assessed the proportion of each type of memory source in individual dreams (e.g. #recent sources/#total sources). The proportion of recent-past memory sources was predicted by Time of night (p = .023), but not by Sleep stage (p = .394), being more present in dreams from early awakenings than in those from mid (β = 0.199, SE = 0.082, t(123) = 2.429, p = .044) and, marginally, those from late (β = 0.186, SE = 0.079, t(122) = 2.359, p = .052) awakenings (Figure 4A and Supplementary Table S3). The effect of Time of night was still present when excluding lab incorporation dreams (p = .025; early > mid, p =.073; early > late, p = .039).
Figure 4.
Proportion of memory sources in dreams. (A) Proportions in dreams of episodic past-distant, episodic past-recent, semantic and future memory sources across Time of night. (B) Relative difference between the proportions of recent-past and distant-past episodic memory sources by Time of night and (C) by both Sleep stages and Time of night. Means and SEMs are shown in A and C. Boxplots with Min to Max point ranges are shown in B. *p < .05.
In contrast, the proportion of distant-past sources was not predicted by Sleep stage (p = .724) or Time of night (p = .318); nor was the proportion of semantic sources (Sleep stage, p = .304; Time of night, p = .351) or the proportion of future sources (Sleep stage, p = .641; Time of night, p = .352). While numerically there were more distant and future memory sources in later awakenings, this difference was not significant (Figure 4A).
We further assessed the relative difference between recent and distant memory sources (#recent/# total sources – #distant/#total sources) for each dream containing at least one memory source (n = 143 dreams). Recent-Distant relative difference was predicted by Time of night (β = −0.139, SD = 0.063, t(124.9) = −2.210, p = .029), but not by Sleep stage (β = −0.036, SD = 0.043, t(124.9) = −0.842, p = .401), with dreams having relatively more distant memory sources in late awakenings than in early ones (β = 0.288, SE = 0.128, t(122) = 2.256, p = .06) (Figure 4B and Supplementary Table S3). We added a Time of night*Sleep stage interaction to the model, but the interaction was not a significant predictor (p = .141) (Figure 4C). The Time of night effect was slightly reduced when controlling for the clarity (p = .073) and length (p = .114) of dream recall, though neither were significant predictors in the model (all p > .05).
Neither Sleep stage (p = .321) nor Time of night (p = .403) predicted the relative difference between semantic and episodic memory sources (Supplementary Table S3).
Lab incorporations were frequent in REM sleep
A total of 56 (34.2%) dreams contained incorporation of the laboratory (judge-ratings). The presence of lab incorporation dreams was not predicted by Sleep stage (p =.149) or Time of night (p =.490). However, when considering NREM sleep stages (N1, N2, N3) together to better replicate the grouping used in our previous paper [6], Sleep stage became a significant predictor of lab incorporation dreams (β = 0.793, SD = 0.365, z = 2.174, p = 0.029). Specifically, lab incorporation dreams were more frequent in REM (23/49; 46.9%) than NREM (33/115; 28.7%) dreams, but did not differ between early (16/56, 28.6%), mid (21/52, 40.3%), and late (19/56, 33.9%) night dreams.
Co-occurrence of memory sources
Nearly half of the dream reports combined more than one memory source (81 of 164 dreams; 49.4%). A total of 21 (12.8%) dreams contained memory sources from both the recent and distant past; however, this was not predicted by Sleep stage (p = .598) or Time of night (p =.304). Thirteen (7.9%) dreams combined a day residue source with a more remote memory: from a month ago (n = 5 dreams); from 6 months to 2 years ago (n = 5); from teenage years (n = 2) and from childhood (n = 5). For example, one participant had this dream: “I was in a grocery store and it was kind of like sleep study subjects which was a little funny. Because everyone was hooked up to the wiring and stuff and we all had matching pajamas, I guess? And we were in some kind of store or blank space. It was easy to see everyone.”; which they associated with being hooked to electrodes the day before at the lab (day residue) and to a book series they read when they were young where kids were being tested, hooked up to wiring and in matching pajamas (childhood memory). Finally, about half the dreams containing a future waking source (6 of 11 dreams; 54.5%) also contained a past episodic source.
Repetition of themes across the night
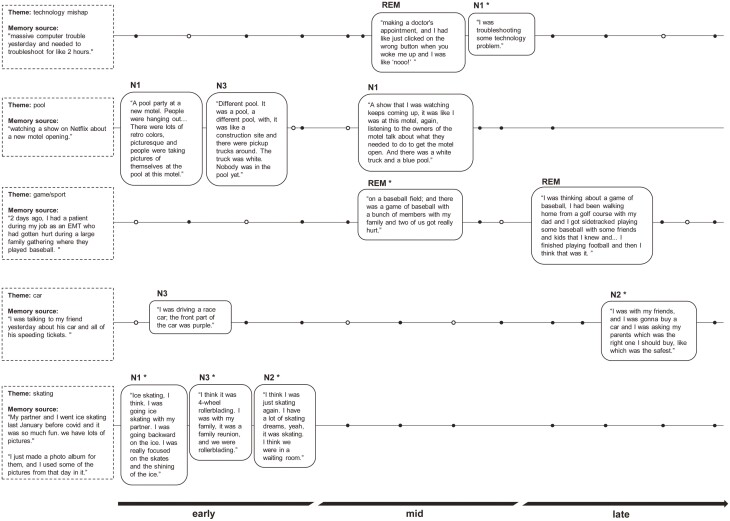
All 20 participants had at least one repeated theme in their dreams across the night (mean ± SD = 5.9 ± 2.7 themes; min = 1; max = 13). On average, an identified theme was repeated in 2.5 ± 0.3 (min = 2; max = 6) dreams per participant. Repetitions occurred on consecutive awakenings in 34.8% of cases, but could be separated by up to 10 awakenings (average distance of 3.3 ± 2.3 awakenings). Themes were repeated through all sleep stages and times of night: when considering all NREM stages together (N1, N2, N3), repetitions most often occurred from NREM→NREM (35.9%), from REM→NREM (27.6%); from NREM→REM (24.9%) and from REM→REM (12.2%). However, when weighting for the number of awakenings with dream recall in each respective stage, and for each participant, the probability was slightly higher for a theme to repeat itself in a different stage of sleep (NREM→REM: 17.9%; REM→NREM: 13.28%) than from repeating again in the same stage (NREM→NREM: 11.4%; REM→REM: 9.9%). No other specific pattern was apparent across the night. Qualitatively, theme repetitions were often related to memory sources (see examples in Figure 5). In some instances, the way the memory source was incorporated in dream content seemed to evolve across the night. For example, one participant who is an emergency medical technician saw a patient who had been hurt while playing baseball at a family gathering. Two days later in the lab, the participant empathetically dreamt (REM dream) about an injury scene where they themselves were “on a baseball field; and there was a game of baseball with a bunch of members with my family and two of us got really hurt”. Later in the same night, they dreamt again that they were playing baseball, this time with some friends, and they were also playing golf with a family member, as well as playing football (REM dream). The memory source, first represented in a dream in a more exact form, was then more loosely represented in contexts that included semantically related activities (football, golf).
Figure 5.
Examples of repeated themes in dreams associated with individual memory sources throughout the night. The dots represent all awakenings performed during the night for dream collection in these participants (black dots = dream recall; empty dots = no dream recall; the positions of the dots are approximate and for illustrative purpose only). Dreams originally associated with the memory sources displayed on the left are marked with (*). Some participants had more than one memory source associated with the repeated theme; only one or two memory sources by theme is shown here.
Discussion
Using a serial-awakening paradigm, we investigated dream memory sources to determine how they are related to sleep stage and time of night. One key finding was that a greater number of memory sources—especially recent memories from the day before—were identified in N1 and REM dreams than in other NREM dreams, an effect that persists after controlling for dream length or clarity of recall. However, we did not find strong evidence that semantic memories are more frequent in REM than in NREM dreams, contrary to expectations.
We also replicated the finding that dream memory sources become relatively less recent and more distant across the night. Prior studies that have reported this finding typically focused either on N1 or on REM sleep [21–23] and thus were unable to clarify whether this change over the night was due to REM sleep predominating in the second half of the night. Our results showed that this effect occurs independently of sleep stage and may instead be a function of time spent asleep or circadian influences.
Finally, we found that dreams tend to repeat similar themes related to memory sources across the night and in different sleep stages. We discuss how these patterns of memory incorporation in dreams may inform our understanding of overnight memory processing during sleep.
N1 and REM dreams combine more memory sources
Memory sources were identified for 87.2% of all dreams, which is similar to what has been found in studies by Wamsley [2] and Vallat et al. [1], i.e. 87.7% and 83.8% respectively. Most dreams (56%) in our sample incorporated episodic memories from the recent past, usually from the day before; 30% incorporated episodic memories from the distant past; 31% incorporated semantic memories; and 7% incorporated anticipated future events. About half of dream reports (49%) were related to multiple memory sources.
Our first hypothesis, that REM dreams would contain more semantic memories and NREM dreams more episodic memories, was not strongly supported by the findings. Although we did see a higher total number of semantic memory sources in REM than in N3 dreams, this difference is conflated by a higher total number of memory sources in REM dreams. Controlling for this factor, there was no sleep-stage difference in proportion of episodic to semantic memory sources. An absence of sleep stage effect on semantic sources was also found by Wamsley [2]. In contrast, we found that recent episodic memories are more likely to be present in both N1 and REM sleep dreams compared to other NREM dreams, an effect mainly driven by day residues. This finding somewhat differs from those of Battaglia et al [24]., who showed that day residues were relatively more frequent than remote memories in early onset N2 but not in REM dreams, and of Wamsley [2], who found that N1 dreams had more past memory sources than either NREM or REM dreams. However, in the latter study, day residues were visibly higher in both N1 and REM dreams than in NREM dreams, similar to what was observed in the present study. We also found that participants generally identified more total memory sources in N1 and REM sleep, partly because reports from these sleep stages were longer and more clearly recalled in the morning—although these dream attributes did not fully explain the effect of sleep stage on the total number of memory sources in dreams.
Our finding that N1 and REM dreams contained more memory sources, including more recent and semantic memories, than do N2 or N3 dreams, may reflect the hyperassociative nature of these sleep stages [15, 16]. Indeed, there is evidence that even brief periods of N1 sleep facilitate insight and creative ways of thinking [38], and N1 dreams seem to form associations between recent and remote waking events [23, 39]. Similarly, widespread associative memory activation during REM sleep may help assimilate recent experiences into broad autobiographical and semantic memory networks. In theory, this associativity subserves a broader function of dreams in adapting to current emotional experiences, which can be better understood through connections to similar experiences from the past [15, 40].
Another possibility is that N1 and REM dreams rely on the activation of numerous memory fragments in order to generate the sensory richness typical of these dreams [41, 42]. In this case, semantic memories and recent waking-life experiences may serve to “fill in” the creation of detailed and complex dream-worlds and, in the case of REM dreams, of longer narratives. In particular, the very recent and unusual laboratory experience might be especially primed for representation in REM dreaming as we showed in a previous study [6] and replicated here.
In sum, we did not find strong evidence that REM dreams incorporate relatively more semantic memories than do NREM dreams. Nonetheless, our findings of a greater number of memories co-existing in N1 and REM dreams may reflect hyperassociative processes that facilitate memory integration, creativity, or emotional processing through the experience of rich dream scenarios.
Time of night dictates the temporal remoteness of dreams
Our second hypothesis, that memory sources would become more remote with later time of night, received strong support. While the occurrence of recent and remote memory sources both remained stable across the night, their relative proportions in dreams changed from early to late night dreams, becoming less recent. The effect was driven by a decrease in the proportion of recent sources after the first third of the night. Importantly, this effect was not influenced by sleep stage. In contrast, future sources did not increase with time of night, conflicting with our previous findings for future-oriented lab-related dreams [6]. Wamsley [2] reported a trend for future sources to become proportionally more common later in the night; a similar trend was also apparent in our current study, but we may have been underpowered to detect the effect given the small number of such dreams. It is also possible that our participants were biased to report past memories moreso than future events, in part due to the framing of the study as investigating memory sources of dreams.
One proposed function of sleep is to organize and integrate new knowledge with existing knowledge (e.g. [43–45]), although it is still not clear how or when this process occurs during sleep. Our finding of relatively more remote memories appearing in dreams across the night suggests that this integration function may be strongest at the end of the night. Circadian rhythms may influence this process. For instance, the fact that the propensity for REM sleep is greater in the morning, i.e. that REM episodes become progressively longer and have increased cortical activation, may enable later-night REM dreams to access progressively broader and more remote memory networks [8, 30]. That NREM dreams similarly incorporate relatively less recent and more remote memories in later sleep cycles may be attributed to a similar circadian effect as for REM sleep, making NREM dreams across the night progressively more “REM-like” and integrative.
Beyond a circadian explanation, the fact that recent memory traces become gradually less apparent over the course of the night may also be attributed to a separate sleep homeostasis mechanism, i.e. a purported synaptic downscaling function by which NREM sleep serves to eliminate weak recent memory traces that are deemed unimportant, and to preserve or reinforce only those select recent memory traces that are worthy of transfer to long-term storage [46]. Over the course of the night, such downscaling could result in a relative dissolution of recent memory traces and preservation of more remote traces. Alternatively, it might be that recent memories are more common earlier in the night because these memory traces have been more recently activated, endowing them with a reduced threshold for reactivation that would progressively increase over time. However, a necessary feature of our experimental design complicates this explanation: repeated exposure to the laboratory after each awakening may have created new, recent, salient waking memories, which could then be primed for incorporation into subsequent dream content. The serial awakenings per se could thus explain why we did not see a relative decrease in lab-related dreams across the night like we did for other recent memory sources more generally. In this case, the decrease in recent memory sources may be considered a function of time spent asleep moreso than circadian time of night.
Memory evolution across the night
Overall, our findings point to the possibility that NREM and REM sleep stages contribute in a complementary or even sequential manner to the processing of increasingly remote memories over the night. On the one hand, N1 and REM dreams contain more memory sources which may facilitate associative integration of memory; on the other hand, relatively more remote memory sources appear in all stages across the night, suggesting that the assimilation of recent experience into autobiographical memory networks is a cumulative function of a night of sleep. Of relevance, sequential theories of memory consolidation suggest that the same memory traces are processed in different sleep stages across the night or over multiple nights [45, 47–50]. In this explanation, salient experiences from waking life may be tagged for reactivation and preservation from downscaling in NREM sleep [51]; and these memory traces may then be primed for further hyperassociative integration in REM dreams [8, 18]. As a second NREM/REM cycle begins, we speculate that some of the broader and more remote memories that were experienced in the rich and immersive REM dream scenarios may in turn be tagged—e.g. via experiencing a meaningful emotion within the dream—to take part in further NREM sleep processing where downscaling or reinforcement of selected memories occurs. This scenario would allow a reweighting of older memories in light of both recent waking and dreamed experiences. As the night progresses through these cycles, relatively more remote memories would thus be accessed and processed in both NREM and REM dreams, and broad autobiographical memory networks would be maintained and updated.
In this view, dreams would not only draw from various waking life events (recent, remote, semantic, anticipatory), but also from recently dreamed events, or “night residues” [52], to allow continuous or sequential processing of current concerns or salient episodic experiences. Previous investigations have shown that dreams from the same night tend to share similar themes that persist or evolve across the night, although without being perfectly continuous with one another [52–56]. Similarly, a memory task performed before sleep can be dreamed about repeatedly within the same night of sleep in different sleep stages [14] or over several nights [57]. When we looked at dreams thematically, we observed that repeating dream themes were often related to memory sources, and indeed, these themes could repeat in several different sleep stages across the night. In fact, all participants had at least one theme that repeated across two or more dreams, and repetitions were especially likely to be from one stage to another (e.g. REM→NREM or NREM→REM), but could also repeat again in the same stage (REM→REM or NREM→NREM). Qualitatively, the original memory source could be incorporated in a variety of ways in different dreams and, in some cases, seemed to evolve across the night within more hyperassociative dream contexts. Nevertheless, given the nature of the serial awakenings paradigm, it is unclear whether the act of repeatedly recalling and reporting dreams throughout the night influences recurrence of their contents later in the night, an issue that was raised nearly five decades ago by Dement and Wolpert [53].
In general, uncovering patterns of memory source incorporation into dreaming is of importance to the broader field focusing on sleep-dependent memory consolidation. While an extensive literature suggests that particular sleep stages affect memory consolidation processes (e.g. [58, 59]), our findings further point to the importance of time of night in understanding the evolution of memory traces over a night of sleep. Whether this effect is mainly driven by circadian influences affecting all stages of sleep, or by time spent asleep and the succession of sleep cycles, or both, remains unclear. Finally, while our interpretations rest on the assumption that memory incorporation in dreaming is directly related to underlying memory consolidation, further evidence could lend additional support to this assumption by correlating dream-memory sources with neural memory reactivation and post-sleep memory performance.
Limitations
One major limitation of this study is that the sleep (and dream) architecture of participants may have been disrupted by the awakenings performed during the night. It remains possible that the memory sources of dreams would be different in the absence of awakenings and dream reports. For example, repeated awakenings may explain why we did not fully replicate some previous findings, such as a greater presence of semantic memories in REM dreams compared with NREM dreams. Nevertheless, even with serial awakenings we replicated prior findings of patterns of dream recall and length being modulated by sleep stage and time of night, such as high recall rates for N1 and REM awakenings (Table 1), and increasing dream report length across the night for REM dreams. Also, participants in our study had an average total sleep time of 7.3 hours and sleep efficiency of over 80%, which means that in spite of the awakenings, they were able to fall back asleep quickly enough to maintain near normal sleep efficiency. Moreover, one study found that serial awakenings did not alter pre- to post-sleep performance changes on a declarative learning task compared to a night of sleep without awakenings [12], which suggests that overnight memory processing may be at least partially preserved.
Another limitation of our method is that it depends on the ability of participants to accurately identify memory sources associated with their dreams. While a majority of dream reports (87%) were associated with at least one memory source, our average of ~1.8 memory sources identified per dream is a bit lower than what was observed in a case study of a highly trained participant who averaged 2.6 memory sources per dream [23]. It is also possible that some episodic sources were falsely considered to be semantic sources simply because participants could not recall when the specific episodes took place. Age differences among participants may also have impacted the range of memories that could potentially have been incorporated in dreams, as well as a participant’s ability to recall these memories (e.g. teenage years are much further away for a 40-year-old than for an 18-year-old participant). While we did not have enough age variability in our cohort to study such effects, future studies could assess whether different age groups differ on how and when memories are incorporated in dreams. Also, because we were interested in memory sources ranging from childhood to future upcoming events, we used a free association method (as in [24]), which prevented us from verifying the accuracy of the memory sources, as was done in studies where participants kept a daily diary of the main events from prior days or weeks (e.g. [3, 33, 60]). The absence of such verifiable waking events in our study could have led to both false positives and false negatives in the dream-memory association task. Future studies using a similar free association method would benefit from asking participants how strongly the identified waking-life events are related to their dreams and how confident they are about these associations. In addition, the identification of semantic memories may have been especially prone to false negatives, as it is difficult to recognize associations between dream elements and such a broad range of self-knowledge. Moreover, it is possible that remote, semantic, or future sources could at times originate from recent thoughts or planning, e.g. if a participant recently thought about a childhood memory or an anticipated event.
A recall bias for recent events (e.g. day residues) or for future events that are imminent (e.g. tomorrow’s events) is also almost inevitable, especially when there is no daily diary available to remind participants of more remote events, and could explain the large majority of day residues found in our sample and other studies. Even so, it is unlikely that all of the memory sources identified by participants would be recorded in a daily diary, e.g. a minor part of a discussion they had with someone, or the features of an object they glanced at in a store. Moreover, presuming a recall bias for recent events was equally present for all dreams, which were presented in a random order to the participants in the morning, this bias should not have distorted the observed decline of recent memory sources across the night.
Finally, a strict cut-off between recent memories (within 8 days ago) and remote memories (within the past month through childhood) was used to facilitate and simplify the analyses. However, such a cut-off is arbitrary and the use of a different time point, such as in Battaglia et al. [24], which referred to memories up to a year ago as “recent residues,” could have led to different results. Framing recent and remote memories in different ways, such as with different cut-offs or the use of a continuous time scale, could be important in future research.
Conclusions
Our serial-awakenings protocol across the night revealed that (1) N1 and REM dreams combine more memory sources than other sleep stages and that (2) recent memories populate early-night dreams, while relatively more remote memories populate late-night dreams, independently of sleep stages. Our interpretation is that NREM and REM sleep contribute to memory processing in a complementary and sequential fashion, enabling integration of recent experience into broad autobiographical memory networks across the night. Future studies would benefit from studying the memory sources of dreams in expert participants trained to clearly recall their dreams and find associations with their waking life, or in special populations, such as those with highly superior autobiographical memory who can remember a large number of past experiences in great detail [61].
Supplementary Material
Contributor Information
Claudia Picard-Deland, Department of Neuroscience, University of Montreal, Montreal, Quebec, Canada.
Karen Konkoly, Department of Psychology, Northwestern University, Evanston, IL, USA.
Rachel Raider, Department of Psychiatry, University of Rochester Medical Center, Rochester, NY, USA.
Ken A Paller, Department of Psychology, Northwestern University, Evanston, IL, USA.
Tore Nielsen, Department of Psychiatry and Addictology, University of Montreal, Montreal, Quebec, Canada.
Wilfred R Pigeon, Department of Psychiatry, University of Rochester Medical Center, Rochester, NY, USA.
Michelle Carr, Department of Psychiatry, University of Rochester Medical Center, Rochester, NY, USA.
Funding
Dream Science Foundation/International Association for the Study of Dreams; National Institute of General Medical Sciences grant: K12 GM106997 (MC).
Disclosure Statement
None declared.
Data Availability
The data underlying this article cannot be shared publicly due to ethical/privacy reasons. Sharing of the data was not included in the informed consent form signed by the research participants. The data will be shared on reasonable request to the corresponding author if approved by our local ethics review board.
References
- 1. Vallat R, et al. Characteristics of the memory sources of dreams: A new version of the content-matching paradigm to take mundane and remote memories into account. PLoS One. 2017;12(10):e0185262. doi: 10.1371/journal.pone.0185262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wamsley EJ. Constructive episodic simulation in dreams. PLoS One. 2022;17(3):e0264574. doi: 10.1371/journal.pone.0264574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malinowski JE, et al. Memory sources of dreams: the incorporation of autobiographical rather than episodic experiences. J Sleep Res. 2014;23(4):441–447. doi: 10.1111/jsr.12134. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen T, et al. The day-residue and dream-lag effects: A literature review and limited replication of two temporal effects in dream formation. Dreaming 1992;2(2):67–77. doi: 10.1037/h0094348. [DOI] [Google Scholar]
- 5. Fosse MJ, et al. Dreaming and episodic memory: a functional dissociation? J Cogn Neurosci. 2003;15(1):1–9. doi: 10.1162/089892903321107774. [DOI] [PubMed] [Google Scholar]
- 6. Picard-Deland C, et al. Dreaming of the sleep lab. PLoS One. 2021;16(10):e0257738e0257738. doi: 10.1371/journal.pone.0257738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hartmann E. The dream always makes new connections: the dream is a creation, not a replay. Sleep Med Clin 2010;5(2):241–248. doi: 10.1016/j.jsmc.2010.01.009. [DOI] [Google Scholar]
- 8. Horton CL, et al. Autobiographical memory and hyperassociativity in the dreaming brain: implications for memory consolidation in sleep. Hypothesis and Theory. Front Psychol. 2015;6:874. doi: 10.3389/fpsyg.2015.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wamsley EJ, et al. Dreaming and offline memory processing. Curr Biol. 2010;20(23):R1010–R1013. doi: 10.1016/j.cub.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Picard-Deland C, et al. Whole-body procedural learning benefits from targeted memory reactivation in REM sleep and task-related dreaming. Neurobiol Learn Mem. 2021;183:107460. doi: 10.1016/j.nlm.2021.107460. [DOI] [PubMed] [Google Scholar]
- 11. Plailly J, et al. Incorporation of fragmented visuo-olfactory episodic memory into dreams and its association with memory performance. Sci Rep. 2019;9(1):15687. doi: 10.1038/s41598-019-51497-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schoch SF, et al. The effect of dream report collection and dream incorporation on memory consolidation during sleep. J Sleep Res. 2019;28(1):e12754. doi: 10.1111/jsr.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wamsley EJ, et al. Dreaming of a learning task is associated with enhanced sleep-dependent memory consolidation. Curr Biol. 2010;20(9):850–855. doi: 10.1016/j.cub.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wamsley EJ, et al. Dreaming of a learning task is associated with enhanced memory consolidation: Replication in an overnight sleep study. J Sleep Res. 2019;28(1):e12749. doi: 10.1111/jsr.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hartmann E. The nature and functions of dreaming. In: Barrett D, McNamara P, eds. The new science of dreaming: Content, recall and personality correlates. Praeger Publishers/Greenwood Publishing Group; 2007:171–192. [Google Scholar]
- 16. Malinowski JE, et al. Metaphor and hyperassociativity: the imagination mechanisms behind emotion assimilation in sleep and dreaming. Review. Front Psychol. 2015;6:1132. doi: 10.3389/fpsyg.2015.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walker MP, et al. Overnight alchemy: sleep-dependent memory evolution. Nat Rev Neurosci. 2010;11(3):218; author reply 218. doi: 10.1038/nrn2762-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis PA, et al. How Memory Replay in Sleep Boosts Creative Problem-Solving. Trends Cogn Sci. 2018;22(6):491–503. doi: 10.1016/j.tics.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nielsen T. Chronobiological features of dream production. Sleep Med Rev. 2004;8(5):403–424. doi: 10.1016/j.smrv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 20. Offenkrantz W, et al. Clinical studies of sequential dreams. I.A patient in psychotherapy. Arch Gen Psychiatry. 1963;8:497–508. doi: 10.1001/archpsyc.1963.01720110073009. [DOI] [PubMed] [Google Scholar]
- 21. Verdone P. Temporal reference of manifest dream content. Percept Mot Skills. 1965;20:1253–1268. doi: 10.2466/pms.1965.20.3c.1253. [DOI] [PubMed] [Google Scholar]
- 22. Roffwarg H, et al. The effects of sustained alterations of waking visual input on dream content. In: Arking A, Antrobus J, and Ellman S, eds. The mind in sleep: Psychology and Psychophysiology. Lawrence Erlbaum; 1978:295–349. [Google Scholar]
- 23. Stenstrom P, et al. Mentation during sleep onset theta bursts in a trained participant: A role for NREM stage 1 sleep in memory processing? Int J Dream Res. 2012;5(1):37–46. doi: 10.11588/ijodr.2012.1.9135. [DOI] [Google Scholar]
- 24. Battaglia D, et al. Temporal reference of the mnemonic sources of dreams. Percept Mot Skills. 1987;64(3, Pt 1):979–983E. doi: 10.2466/pms.1987.64.3.979. [DOI] [Google Scholar]
- 25. Baylor GW, et al. Memory sources associated with REM and NREM dream reports throughout the night: a new look at the data. Sleep 2001;24(2):165–170. doi: 10.1093/sleep/24.2.165. [DOI] [PubMed] [Google Scholar]
- 26. Cicogna P, et al. Cognitive aspects of mental activity during sleep. Am J Psychol. 1991;104(3):413–425. doi: 10.2307/1423247. [DOI] [PubMed] [Google Scholar]
- 27. Cavallero C, et al. Memory sources of REM and NREM dreams. Sleep 1990;13(5):449–455. doi: 10.1093/sleep/13.5.449. [DOI] [PubMed] [Google Scholar]
- 28. Foulkes D, et al. Processing of memories and knowledge in REM and NREM dreams. Percept Mot Skills. 1989;68(2):365–366. doi: 10.2466/pms.1989.68.2.365. [DOI] [PubMed] [Google Scholar]
- 29. Malinowski JE, et al. The effect of time of night on wake–dream continuity. Dreaming 2014;24(4):253–269. doi: 10.1037/a0037817. [DOI] [Google Scholar]
- 30. Malinowski JE, et al. Dreams reflect nocturnal cognitive processes: Early-night dreams are more continuous with waking life, and late-night dreams are more emotional and hyperassociative. Conscious Cogn. 2021;88:103071. doi: 10.1016/j.concog.2020.103071. [DOI] [PubMed] [Google Scholar]
- 31. Siclari F, et al. The neural correlates of dreaming. Nat Neurosci. 2017;20(6):872–878. doi: 10.1038/nn.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cavallero C, et al. Slow wave sleep dreaming. Sleep 1992;15(6):562–566. doi: 10.1093/sleep/15.6.562. [DOI] [PubMed] [Google Scholar]
- 33. Blagrove M, et al. Assessing the dream-lag effect for REM and NREM stage 2 dreams. PLoS One. 2011;6(10):e26708e26708. doi: 10.1371/journal.pone.0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Rijn E, et al. The dream-lag effect: Selective processing of personally significant events during Rapid Eye Movement sleep, but not during Slow Wave Sleep. Neurobiol Learn Mem. 2015;122:98–109. doi: 10.1016/j.nlm.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Berry RB, et al. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Darien, IL: American Academy of Sleep Medicine; 2012.
- 36. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. http://www.R-project.org/ [Google Scholar]
- 37. Bates D, et al. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 38. Lacaux C, et al. Sleep onset is a creative sweet spot. Sci Adv. 2021;7(50):eabj5866. doi: 10.1126/sciadv.abj5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nielsen T. Microdream neurophenomenology. Neurosci Conscious 2017;2017(1):nix001–nix001. doi: 10.1093/nc/nix001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cartwright RD. The twenty-four hour mind: The role of sleep and dreaming in our emotional lives. Oxford University Press; 2010:224. [Google Scholar]
- 41. Nielsen T. A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci. 2000;23(6):851–66; discussion 904-1121. doi: 10.1017/s0140525x0000399x. [DOI] [PubMed] [Google Scholar]
- 42. Hobson JA, et al. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci. 2000;23(6):793–842; discussion 904. doi: 10.1017/s0140525x00003976. [DOI] [PubMed] [Google Scholar]
- 43. Dudai Y, et al. The Consolidation and Transformation of Memory. Neuron 2015;88(1):20–32. doi: 10.1016/j.neuron.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 44. Landmann N, et al. The reorganisation of memory during sleep. Sleep Med Rev. 2014;18(6):531–541. doi: 10.1016/j.smrv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 45. Stickgold R, et al. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013;16(2):139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tononi G, et al. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 47. Ficca G, et al. What in sleep is for memory. Sleep Med. 2004;5(3):225–230. doi: 10.1016/j.sleep.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 48. Fogel SM, et al. Evidence for 2-stage models of sleep and memory: learning-dependent changes in spindles and theta in rats. Brain Res Bull. 2009;79(6):445–451. doi: 10.1016/j.brainresbull.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 49. Pereira SIR, et al. The differing roles of NREM and REM sleep in the slow enhancement of skills and schemas. Curr Opin Physiol 2020;15:82–88. doi: 10.1016/j.cophys.2019.12.005. [DOI] [Google Scholar]
- 50. Giuditta A, et al. The sequential hypothesis of the function of sleep. Behav Brain Res. 1995;69(1-2):157–166. doi: 10.1016/0166-4328(95)00012-i. [DOI] [PubMed] [Google Scholar]
- 51. Seibt J, et al. Primed to sleep: the dynamics of synaptic plasticity across brain states. Front Syst Neurosci. 2019;13:2. doi: 10.3389/fnsys.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kramer M, et al. Patterns of dreaming: the interrelationship of the dreams of a night. J Nerv Ment Dis. 1964;139(5):426–439. doi: 10.1097/00005053-196411000-00003. [DOI] [PubMed] [Google Scholar]
- 53. Dement W, et al. Relationships in the manifest content of dreams occurring on the same night. J Nerv Ment Dis. 1958;126:568–578. doi: 10.1097/00005053-195806000-00009. [DOI] [PubMed] [Google Scholar]
- 54. Cipolli C, et al. The thematic continuity of mental experiences in REM and NREM sleep. Int J Psychophysiol. 1988;6(4):307–313. doi: 10.1016/0167-8760(88)90018-9. [DOI] [PubMed] [Google Scholar]
- 55. Rechtschaffen A, et al. Interrelatedness of mental activity during sleep. Arch Gen Psychiatry. 1963;9(6):536–547. doi: 10.1001/archpsyc.1963.01720180008002. [DOI] [PubMed] [Google Scholar]
- 56. Trosman H, et al. Studies in psychophysiology of dreams: IV. Relations among dreams in sequence. Arch Gen Psychiatry. 1960;3(6):602–607. doi: 10.1001/archpsyc.1960.01710060034006. [DOI] [PubMed] [Google Scholar]
- 57. Picard-Deland C, et al. Targeted memory reactivation has a sleep stage-specific delayed effect on dream content. J Sleep Res. 2021;31(1):e13391. doi: 10.1111/jsr.13391. [DOI] [PubMed] [Google Scholar]
- 58. Plihal W, et al. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9(4):534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 59. Smith C. Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Med Rev. 2001;5(6):491–506. doi: 10.1053/smrv.2001.0164. [DOI] [PubMed] [Google Scholar]
- 60. Eichenlaub JB, et al. Incorporation of recent waking-life experiences in dreams correlates with frontal theta activity in REM sleep. Soc Cogn Affect Neurosci 2018;13(6):637–647. doi: 10.1093/scan/nsy041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Konkoly K, et al. Dreaming in individuals with Highly Superior Autobiographical Memory [Conference presentation abstract]. Presented at: International Association for the Study of Dreams; July 17–21, 2022; Tucson, AZ. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to ethical/privacy reasons. Sharing of the data was not included in the informed consent form signed by the research participants. The data will be shared on reasonable request to the corresponding author if approved by our local ethics review board.