Abstract
Squamous cell carcinoma is the main subtype of esophageal cancer in East Asia. The effect of the number of lymph nodes (LNs) removed to treat middle and lower thoracic esophageal squamous cell carcinoma (ESCC) in China remains controversial. Therefore, the present study aimed to investigate the impact of the number of LNs removed during lymphadenectomy on the survival of patients with middle and lower thoracic ESCC. Data were obtained from the Sichuan Cancer Hospital and Institute Esophageal Cancer Case Management Database from January 2010 to April 2020. Either three-field systematic lymphadenectomy (3F group) or two-field systematic lymphadenectomy (2F group) was performed for ESCC cases with or without suspicious tumor-positive cervical LNs, respectively. Subgroups were designed for further analysis based on the quartile number of resected LNs. After 50.7 months of median follow-up, 1,659 patients who underwent esophagectomy were enrolled. The median overall survival (OS) of the 2F and 3F groups was 50.0 months and 58.5 months, respectively. The OS rates at 1, 3 and 5 years were 86, 57 and 47%, respectively, in the 2F group, and 83, 52 and 47%, respectively, in the 3F group (P=0.732). The average OS of the 3F B and D groups was 57.7 months and 30.2 months, respectively (P=0.006). In the 2F group, the OS between subgroups was not significantly different. In conclusion, resection of >15 LNs during two-field dissection in patients with ESCC undergoing esophagectomy did not affect their survival outcomes. In three-field lymphadenectomy, the extent of LNs removed could lead to different survival outcomes.
Keywords: esophageal cancer, squamous cell carcinoma, lymph node, two-field lymphadenectomy, three-field lymphadenectomy
Introduction
Globally, more than 50% of the esophageal cancer cases in occur in China (1). Squamous cell carcinoma (SCC) is a major subtype of esophageal carcinoma in China, Japan, and other East Asian countries (2). According to a study in 2021, the 5-year overall survival (OS) rate of patients with esophageal cancer after esophagectomy was 59.3% in Japan (3). In Japan, Europe, and America, esophageal cancer is treated in much the same method as in China. In addition to differences in tumor characteristics across these countries and regions, the largest difference is the extent of lymph node (LN) dissection. Professor Akiyama's study showed that three-field lymphadenectomy increased 5-year OS in patients with esophageal cancer compared to systematic thoracoabdominal two-field lymphadenectomy (55.0% vs. 38.3%) (4). Thus, LN metastasis is an important factor affecting OS in esophageal cancer; this has been confirmed in an increasing number of studies (5–9). According to the literature, at least 15 LNs should be removed during esophageal cancer surgery. Similar findings have been confirmed by the National Comprehensive Cancer Network. However, studies encouraging the removal of more than 15 LNs are still controversial (10–12). A series of studies carried out by Professor Liu, involving two-field and three-field lymphadenectomy, has sparked widespread discussion among Chinese surgeons (13,14). While a large number of studies have indicated that LN dissection can reduce the likelihood of recurrence and increase OS, some have also shown that extensive LN dissection incurs greater surgical trauma and increases the incidence and mortality of postoperative complications (15).
The appropriate extent of LN removal necessary to limit surgical trauma and postoperative complications remains a problem that must be resolved. In February 2021, a Chinese single-center randomized controlled study showed that three-field LN dissection could allow more precise staging of middle and lower thoracic SCC than two-field LN dissection, but there was no improvement in OS and disease-free survival (DFS) (16). However, the choice of procedure is usually individualized based on the case and patient preferences. For patients without suspicious tumor-positive cervical LNs, two-field systematic LN dissection for esophageal SCC (ESCC) is sufficient, while for patients with suspicious tumor-positive cervical LNs, three-field systematic LN dissection for ESCC is necessary. In both cases, the number of LNs resected during surgery should be adjusted. To this end, this study was designed to elucidate the impact of the number of resected LNs (RLNs) on the OS of patients with middle and lower thoracic ESCC.
Materials and methods
Data were obtained from the Sichuan Cancer Hospital & Institute Esophageal Cancer Case Management Database. We retrospectively evaluated middle and lower thoracic esophageal cancer patients who underwent esophagectomy from January 2010 to April 2020. Patient records were reviewed for clinicopathologic findings and outcomes. Approximate esophagectomy was performed through a right transthoracic procedure with two-field or three-field lymphadenectomy. Surgical approaches depended on patient characteristics and surgeon preferences; for example, patients with enlargement of specific cervical LN groups underwent three-field lymphadenectomy. Disease stage was presented according to the American Joint Committee on Cancer 8th edition tumor-node-metastasis (TNM) system. There were three inclusion criteria: (1) patients who underwent esophagectomy, (2) tumor located in the thoracic esophagus, and (3) tumor with pathological evidence of SCC. The following exclusion criteria were also considered: (1) less than 15 RLNs, (2) presence of other malignant tumors, (3) pathological T stage=Tis/Ia/IVb, and (4) absence of any required data.
Patients were followed up once every 3 months for the first 2 years and once every 6 months for the next 3–5 years. OS was estimated from the month and year of surgery to death or last follow-up in April 2022. The study was approved by the Ethics Committee for Medical Research and New Medical Technology of Sichuan Cancer Hospital (SCCHEC-02-2022-050). All patients were fully informed before providing verbal consent as per approved ethics protocol.
Patients were grouped depending on the presence or absence of suspicious tumor-positive cervical LNs. The reference standard was LN short diameter ≥1 cm observed on contrast-enhanced computed tomography (17,18). Patients were divided into two groups based on the presence of suspicious tumor-positive cervical LNs. In cases without suspicious tumor-positive cervical LNs, two-field systematic LN dissection for ESCC was performed and patients were categorized into the ‘2F group’. For patients with suspicious tumor-positive cervical LNs, three-field systematic LN dissection for ESCC was performed and the patients were categorized into the ‘3F group’.
According to the quartile number of RLNs, four subgroups from both groups were designed for analysis depending on the extent of lymphadenectomy. In the 2F group, patients with 15–18, 19–22, 23–29, and more than 30 RLNs were divided into the ‘A group’, ‘B group’, ‘C group’, and ‘D group’, respectively. In the 3F group, patients with 17–30, 31–37, 38–50, and more than 51 RLNs were divided into the ‘A group’, ‘B group’, ‘C group’, and ‘D group’, respectively.
Statistical analysis
Categorical variables are reported as percentages. They were investigated using the chi-square or Fisher's exact test. Univariate and multivariate logistic regression analyses were used to calculate the independent risk factors associated with LN metastasis, and the hazard ratio and 95% confidence interval (CI) are used to describe each variable. The Cox proportional hazards regression model was used to evaluate the impact of all baseline covariates on the outcome. A graphical representation of the model was developed using GraphPad Prism (GraphPad Software, Boston, MA, USA). OS was evaluated by plotting Kaplan-Meier curves, compared using the log-rank test, and described as the median rate and value at specific time points with 95% CIs. Statistical significance was defined as a P-value less than 0.05. All analyses were conducted using SPSS version 23.0 software (SPSS Inc., Chicago, IL, USA).
Results
A total of 1659 patients were included in the analysis. A flowchart describing the study enrollment is shown in Fig. 1. The clinicopathological and pathological characteristics of the 2F and 3F groups are shown in Tables I and II. Two-field systematic LN dissection was performed in 1562 of 1659 patients (94.2%) and categorized as the 2F group; 97 of 1659 patients (5.8%) underwent three-field systematic lymphadenectomy and were categorized as the 3F group. Additionally, postoperative pathological examination showed that 20 of the 97 patients (20.6%) in the 3F group had tumor-positive cervical LNs.
Figure 1.
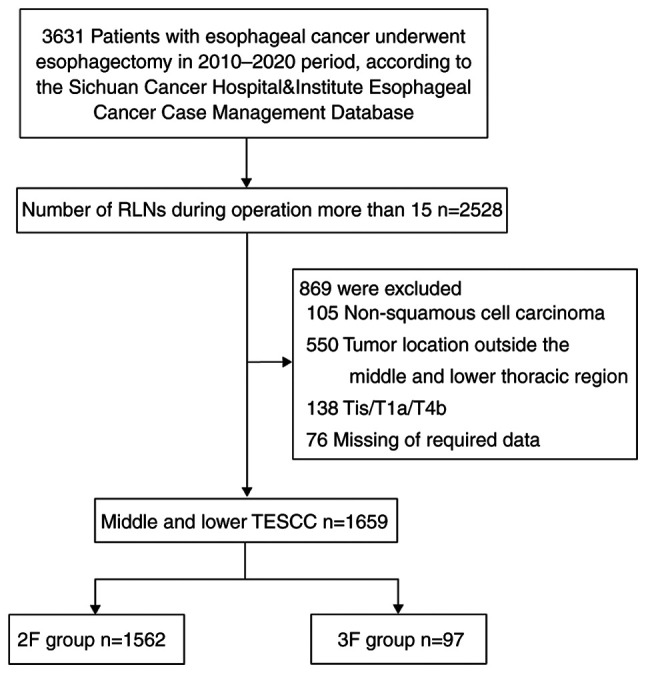
Consolidated Standards of Reporting Trials diagram of patient selection. RLN, resected lymph node; TESCC, thoracic esophageal squamous cell carcinoma; 3F, three-field systematic lymphadenectomy; 2F, two-field systematic lymphadenectomy.
Table I.
Baseline demographic and patient characteristics of the 2F (n=1,562) group.
| Variables | Group A (n=393) | Group B (n=373) | Group C (n=421) | Group D (n=375) |
|---|---|---|---|---|
| Sex | ||||
| Male | 337 (85.8%) | 317 (85.0%) | 375 (89.1%) | 343 (91.5%) |
| Female | 56 (14.2%) | 56 (15.0%) | 46 (10.9%) | 32 (8.5%) |
| Age, years | ||||
| Median (range) | 62 (39–84) | 63 (36–81) | 62 (37–85) | 62 (39–84) |
| <75 | 365 (92.9%) | 355 (95.2%) | 403 (95.7%) | 359 (95.7%) |
| ≥75 | 28 (7.1%) | 18 (4.8%) | 18 (4.3%) | 16 (4.3%) |
| Pathological differentiation grade | ||||
| Moderate or Well G1-2 | 240 (61.1%) | 241 (64.6%) | 267 (63.4%) | 248 (66.1%) |
| Poor or undifferentiated G3 | 153 (38.9%) | 132 (35.4%) | 154 (36.6%) | 127 (33.9%) |
| Tumor location | ||||
| Middle | 269 (68.4%) | 258 (69.2%) | 287 (68.2%) | 240 (64.0%) |
| Lower | 124 (31.6%) | 115 (30.8%) | 134 (31.8%) | 135 (36.0%) |
| Pathological T stage | ||||
| T1b | 45 (11.5%) | 42 (11.3%) | 39 (9.3%) | 32 (8.5%) |
| T2 | 120 (30.5%) | 103 (27.6%) | 116 (27.6%) | 94 (25.1%) |
| T3 | 207 (52.7%) | 198 (53.1%) | 238 (56.5%) | 218 (58.1%) |
| T4 | 21 (5.3%) | 30 (8.0%) | 28 (6.7%) | 31 (8.3%) |
| N stage | ||||
| N0 | 180 (45.8%) | 170 (45.6%) | 140 (33.2%) | 138 (36.8%) |
| N1 | 124 (31.5%) | 104 (27.9%) | 138 (32.8%) | 106 (28.3%) |
| N2 | 69 (17.6%) | 64 (17.1%) | 95 (22.6%) | 71 (18.9%) |
| N3 | 20 (5.1%) | 35 (9.4%) | 48 (11.4%) | 60 (16.0%) |
| 8th TNM Stage | ||||
| I | 36 (9.2%) | 39 (10.5%) | 28 (6.7%) | 12 (3.2%) |
| II | 145 (36.9%) | 128 (34.3%) | 113 (26.8%) | 125 (33.3%) |
| III | 186 (47.3%) | 167 (44.7%) | 224 (53.2%) | 170 (45.4%) |
| IV | 26 (6.6%) | 39 (10.5%) | 56 (13.3%) | 68 (18.1%) |
| Radical resection rate | ||||
| R0 | 387 (98.5%) | 358 (96.0%) | 413 (98.1%) | 365 (97.3%) |
| R1/2 | 6 (1.5%) | 15 (4.0%) | 8 (1.9%) | 10 (2.7%) |
| Thoracic surgery | ||||
| MIE | 226 (57.5%) | 207 (55.5%) | 224 (53.2%) | 179 (47.7%) |
| OE | 167 (42.5%) | 166 (44.5%) | 197 (46.8%) | 196 (52.3%) |
| Abdominal surgery | ||||
| MIE | 194 (49.4%) | 178 (47.7%) | 210 (49.9%) | 160 (42.7%) |
| OE | 199 (50.6%) | 195 (52.3%) | 211 (50.1%) | 215 (57.3%) |
Group A, 15–18 RLNs; group B, 19–22 RLNs; group C, 23–29 RLNs; and group D, >29 RLNs. MIE, minimally invasive esophagectomy; OE, open esophagectomy; RLN, resected lymph node; TNM, tumor-node-metastasis; 2F, two-field systematic lymphadenectomy.
Table II.
Baseline demographic and patient characteristics of the 3F (n=97) group.
| Variables | Group A (n=24) | Group B (n=25) | Group C (n=24) | Group D (n=24) |
|---|---|---|---|---|
| Sex | ||||
| Male | 18 (75.0%) | 18 (72.0%) | 20 (83.3%) | 23 (95.8%) |
| Female | 6 (25.0%) | 7 (28.0%) | 4 (16.7%) | 1 (4.2%) |
| Age (years) | ||||
| Median (range) | 61 (44–71) | 61 (39–70) | 61.5 (46–67) | 61 (40–75) |
| <75 | 24 (100%) | 25 (100%) | 24 (100%) | 22 (91.7%) |
| ≥75 | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8.3%) |
| Pathological differentiation grade | ||||
| Moderate or Well G1-2 | 12 (50.0%) | 18 (72.0%) | 16 (66.7%) | 13 (54.2%) |
| Poor or undifferentiated G3 | 12 (50.0%) | 7 (28.0%) | 8 (33.3%) | 11 (45.8%) |
| Tumor location | ||||
| Middle | 18 (75.0%) | 21 (84.0%) | 19 (79.2%) | 17 (70.8%) |
| Lower | 6 (25.0%) | 4 (16.0%) | 5 (20.8%) | 7 (29.2%) |
| Pathological T stage | ||||
| T1b | 3 (12.5%) | 2 (8.0%) | 1 (4.2%) | 0 (0%) |
| T2 | 9 (37.5%) | 8 (32.0%) | 9 (37.5%) | 6 (25.0%) |
| T3 | 12 (50.0%) | 15 (60.0%) | 12 (50.0%) | 16 (66.7%) |
| T4 | 0 (0%) | 0 (0%) | 2 (8.3%) | 2 (8.3%) |
| N stage | ||||
| N0 | 8 (33.3%) | 8 (32.0%) | 7 (29.2%) | 7 (29.2%) |
| N1 | 7 (29.2%) | 10 (40.0%) | 6 (25.0%) | 9 (37.5%) |
| N2 | 8 (33.3%) | 4 (16.0%) | 6 (25.0%) | 4 (16.7%) |
| N3 | 1 (4.2%) | 3 (12.0%) | 5 (20.8%) | 4 (16.7%) |
| 8th TNM Stage | ||||
| I | 2 (8.3%) | 2 (8.0%) | 2 (8.3%) | 0 (0%) |
| II | 5 (20.8%) | 6 (24.0%) | 5 (20.8%) | 6 (25.0%) |
| III | 16 (66.7%) | 13 (52.0%) | 10 (41.7%) | 13 (54.2%) |
| IV | 1 (4.2%) | 4 (16.0%) | 7 (29.2%) | 5 (20.8%) |
| Radical resection rate | ||||
| R0 | 24 (100.0%) | 25 (100.0%) | 24 (100.0%) | 24 (100.0%) |
| Thoracic surgery | ||||
| MIE | 20 (83.3%) | 18 (72.0%) | 19 (79.2%) | 15 (62.5%) |
| OE | 4 (16.7%) | 7 (28.0%) | 5 (20.8%) | 9 (37.5%) |
| Abdominal surgery | ||||
| MIE | 20 (83.3%) | 17 (68.0%) | 19 (79.2%) | 15 (62.5%) |
| OE | 4 (16.7%) | 8 (32.0%) | 5 (20.8%) | 9 (37.5%) |
Group A, 17–30 RLNs; group B, 31–37 RLNs; group C, 38–50 RLNs; and group D, >50 RLNs. MIE, minimally invasive esophagectomy; OE, open esophagectomy; RLN, resected lymph node; TNM, tumor-node-metastasis; 3F, three-field systematic lymphadenectomy.
After 50.7 months (95% CI, 47.2-54.1) of median follow-up, 1659 patients who underwent esophagectomy were enrolled. The median OS of the 2F group was 50.0 months (95% CI, 46.6-53.5) and that of the 3F group was 58.5 months (95% CI, 45.5-71.5). The OS rates at 1, 3, and 5 years were 86, 57, and 47%, respectively, in the 2F group (Fig. 2). In the 3F group, the OS rates at 1, 3, and 5 years were 83, 52, and 47%, respectively (P=0.732). However, the 3F B group did not reach the median OS time, and the average OS of the 3F B and 3F D groups was 57.7 months (95% CI, 47.3-68.0) and 30.2 months (95% CI, 22.5-37.9), respectively. The OS rates at 1, 3, and 5 years were 92, 71, and 65%, respectively, in the 3F B group. In the 3F D group, the OS rates at 1, 3, and 5 years were 79, 28, and 28%, respectively (hazard ratio, 3.095; 95% CI, 1.318-7.273; P=0.01). In the 2F subgroups, the OS was not significantly different between the A, B, C, and D groups (Fig. 3).
Figure 2.
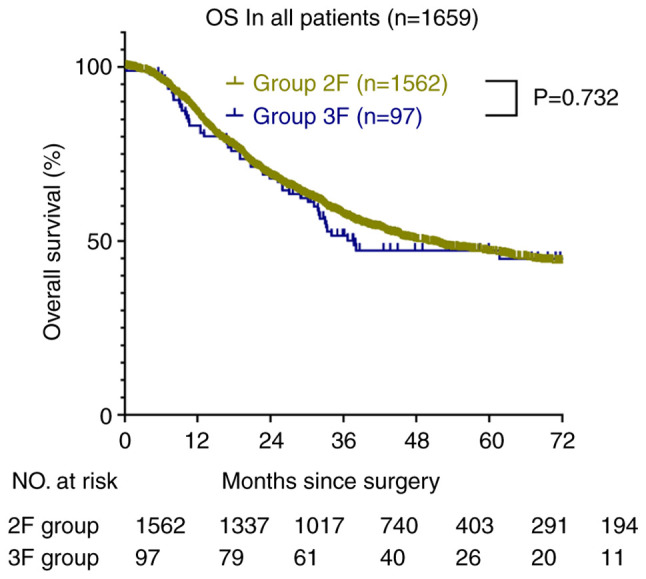
Overall survival of the 2F and 3F groups. OS, overall survival; 3F, three-field systematic lymphadenectomy; 2F, two-field systematic lymphadenectomy.
Figure 3.
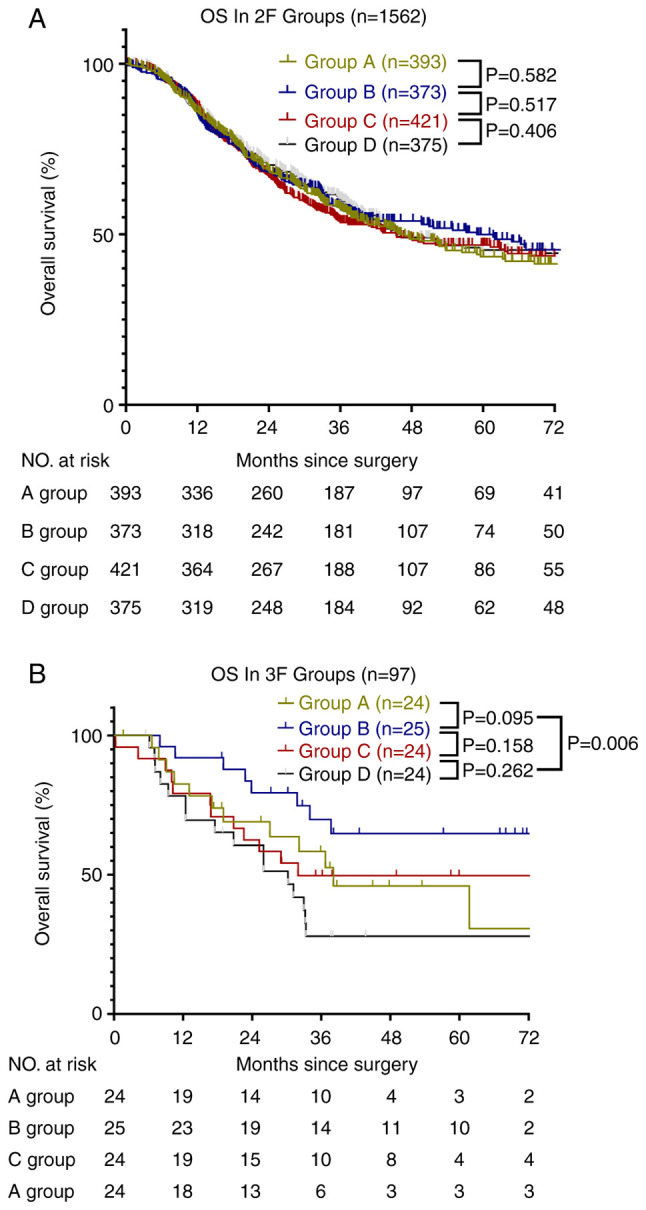
Study cohort. (A) Overall survival of four subgroups in the 2F group; A group: 15–18 RLNs; B group: 19–22 RLNs; C group: 23–29 RLNs; D group: >29 RLNs. (B) Overall survival of the four subgroups in the 3F group; A group: 17–30 RLNs; B group: 31–37 RLNs; C group: 38–50 RLNs; D group >50 RLNs. RLN, resected lymph node; OS, overall survival; 3F, three-field systematic lymphadenectomy; 2F, two-field systematic lymphadenectomy.
Kaplan-Meier curves showed that the OS of the four subgroups of the 2F group were all similar. However, the OS in the four subgroups of the 3F group were all significantly different, particularly between groups B and D (P<0.05). This result suggested that the best strategy for lymphadenectomy in patients with enlarged specific cervical LNs was the resection of 31 to 37 LNs. Therefore, we compared the clinical and pathological characteristics of the patients. However, there were no significant differences between the 3F B and D groups, as shown in Table III.
Table III.
Summary of pathological characteristics compared between the 2F/3F and 3F B/D subgroups.
| Total esophageal cancer (n=1659) | Esophageal cancer 3F (n=97) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variables | 2F Group (n=1562) | 3F Group (n=97) | P-value | Group B (n=25) | Group D (n=24) | P-value |
| Sex | 0.065 | 0.024 | ||||
| Male | 1372 (87.8%) | 79 (81.4%) | 18 (72.0%) | 23 (95.8%) | ||
| Female | 190 (12.2%) | 7 (18.6%) | 7 (28.0%) | 1 (4.2%) | ||
| Age (years) | 0.177 | 0.141 | ||||
| <75 | 1482 (94.9%) | 95 (97.9%) | 25 (100%) | 22 (91.7%) | ||
| ≥75 | 80 (5.1%) | 2 (2.1%) | 0 (0%) | 2 (8.3%) | ||
| Pathological differentiation grade | 0.559 | 0.196 | ||||
| Moderate or Well G1-2 | 996 (63.8%) | 59 (60.8%) | 18 (72.0%) | 13 (54.2%) | ||
| Poor or undifferentiated G3 | 566 (36.2%) | 38 (39.2%) | 7 (28.0%) | 11 (45.8%) | ||
| Tumor location | 0.044 | 0.269 | ||||
| Middle | 1054 (67.5%) | 75 (77.3%) | 21 (84.0%) | 17 (70.8%) | ||
| Lower | 508 (32.5%) | 22 (22.7%) | 4 (16.0%) | 7 (29.2%) | ||
| Pathological T stage | 0.32 | 0.231 | ||||
| T1b | 158 (10.1%) | 6 (6.2%) | 2 (8.0%) | 0 (0%) | ||
| T2 | 433 (27.7%) | 32 (33.0%) | 8 (32.0%) | 6 (25.0%) | ||
| T3 | 861 (55.1%) | 55 (56.7%) | 15 (60.0%) | 16 (66.7%) | ||
| T4 | 110 (7.1%) | 30 (30.9%) | 0 (0%) | 2 (8.3%) | ||
| N stage | 0.313 | 0.971 | ||||
| N0 | 628 (40.2%) | 30 (30.9%) | 8 (32.0%) | 7 (29.2%) | ||
| N1 | 472 (30.2%) | 32 (33.0%) | 10 (40.0%) | 9 (37.5%) | ||
| N2 | 299 (19.1%) | 22 (22.7%) | 4 (16.0%) | 4 (16.7%) | ||
| N3 | 163 (10.4%) | 13 (13.4%) | 3 (12.0%) | 4 (16.7%) | ||
| 8th TNM Stage | 0.12 | 0.554 | ||||
| I | 115 (7.4%) | 6 (6.2%) | 2 (8.0%) | 0 (0%) | ||
| II | 511 (32.7%) | 22 (22.7%) | 6 (24.0%) | 6 (25.0%) | ||
| III | 747 (47.8%) | 52 (53.6%) | 13 (52.0%) | 13 (54.2%) | ||
| IV | 189 (12.1%) | 17 (17.5%) | 4 (16.0%) | 5 (20.8%) | ||
| Radical resection rate | 0.115 | |||||
| R0 | 1523 (97.5%) | 97 (100.0%) | 25 (100.0%) | 24 (100.0%) | ||
| R1/2 | 39 (2.5%) | 0 | 0 | 0 | ||
| Thoracic surgery | <0.001 | 0.478 | ||||
| MIE | 1523 (97.5%) | 72 (74.2%) | 18 (72.0%) | 15 (62.5%) | ||
| OE | 39 (2.5%) | 25 (25.8%) | 7 (28.0%) | 9 (37.5%) | ||
| Abdominal surgery | <0.001 | 0.686 | ||||
| MIE | 742 (47.5%) | 71 (73.2%) | 17 (68.0%) | 15 (62.5%) | ||
| OE | 820 (52.5%) | 26 (26.8%) | 8 (32.0%) | 9 (37.5%) | ||
MIE, minimally invasive esophagectomy; OE, open esophagectomy; TNM, tumor-node-metastasis; 2F, two-field systematic lymphadenectomy; 3F, three-field systematic lymphadenectomy.
Univariate analysis of patients without suspicious tumor-positive cervical LNs indicated that sex (P=0.001), age (P=0.045), tumor grade G3 (P<0.001), pathological T1b/T2/T3/T4 stage, pathological N0/N1/N2/N3 stage, 8th TNM stage I/II/III/IV, radical resection (P<0.001), thoracic surgery (P<0.001), and abdominal surgery (P<0.001) significantly influenced the 5-year OS after esophagectomy (Table IV). Further analysis using multivariate methods indicated that sex (P=0.008), age (P<0.001), tumor grade G3 (P=0.007), pathological T1b/T3/T4 stage, N0/N2/N3 stage, and radical resection (P=0.026) affected the 5-year OS after esophagectomy (Table IV).
Table IV.
Univariate and multivariate Cox regression analysis factors affecting survival in the 2F group.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Sex | 0.001 | 0.008 | ||||
| Male | 1.49 | 1.171-1.897 | 1.394 | 1.090-1.783 | ||
| Female | 1 | 0 | 1 | 0 | ||
| Age (years), | 0.045 | <0.001 | ||||
| <75 | 1 | 0 | 1 | 0 | ||
| ≥75 | 1.371 | 1.008-1.865 | 2.006 | 1.465-2.747 | ||
| Pathological differentiation grade | <0.001 | 0.007 | ||||
| Moderate or Well G1-2 | 1 | 0 | 1 | 0 | ||
| Poor or undifferentiated G3 | 1.415 | 1.226-1.634 | 1.226 | 1.058-1.420 | ||
| Tumor location | 0.879 | |||||
| Middle | 1.012 | 0.869-1.178 | ||||
| Lower | 1 | 0 | ||||
| Pathological T stage | ||||||
| T1b | 1 | 0 | <0.001 | 1 | 0 | <0.001 |
| T2 | 1.761 | 1.242-2.495 | 0.001 | 1.289 | 0.864-1.923 | 0.213 |
| T3 | 2.675 | 1.930-3.707 | <0.001 | 1.777 | 1.196-2.641 | 0.004 |
| T4 | 4.505 | 3.061-6.631 | <0.001 | 2.388 | 1.404-4.062 | 0.001 |
| N stage | ||||||
| N0 | 1 | 0 | <0.001 | 1 | 0 | <0.001 |
| N1 | 1.869 | 1.538-2.271 | <0.001 | 1.523 | 0.957-2.424 | 0.076 |
| N2 | 3.855 | 3.167-4.693 | <0.001 | 2.877 | 1.768-4.682 | <0.001 |
| N3 | 5.391 | 4.307-6.747 | <0.001 | 3.5 | 1.674-7.318 | 0.001 |
| 8th TNM Stage | ||||||
| I | 1 | 0 | <0.001 | 1 | 0 | 0.83 |
| II | 1.692 | 1.083-2.643 | <0.001 | 1.128 | 0.684-1.861 | 0.636 |
| III | 4.033 | 2.627-6.193 | <0.001 | 1.383 | 0.672-2.844 | 0.379 |
| IV | 8.814 | 5.633-13.791 | <0.001 | 1.412 | 0.549-3.631 | 0.474 |
| Radical resection rate | <0.001 | 0.026 | ||||
| R0 | 1 | 0 | 1 | 0 | ||
| R1/2 | 2.272 | 1.586-3.256 | 1.527 | 1.051-2.219 | ||
| Thoracic surgery | <0.001 | 0.683 | ||||
| MIE | 1 | 0 | 1 | 0 | ||
| OE | 1.43 | 1.238-1.650 | 0.941 | 0.703-1.259 | ||
| Abdominal surgery | <0.001 | 0.366 | ||||
| MIE | 1 | 0 | 1 | 0 | ||
| OE | 1.404 | 1.212-1.626 | 1.146 | 0.853-1.539 | ||
HR, hazard ratio; CI, confidence interval; MIE, minimally invasive esophagectomy; OE, open esophagectomy; TNM, tumor-node-metastasis; 2F, two-field systematic lymphadenectomy.
In the 3F group, univariate analysis indicated that age (P=0.018), pathological N0/N2/N3 stage, and 8th TNM stage I (P=0.006) significantly influenced the 5-year OS after esophagectomy (Table V). Further analysis using multivariate methods indicated that pathological N0 stage (P=0.003) significantly affected the 5-year OS after esophagectomy (Table V).
Table V.
Univariate and multivariate Cox regression analysis of factors affecting survival among patients in the 3F group.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Sex | ||||||
| Male | 1.085 | 0.525-2.241 | 0.826 | |||
| Female | 1 | |||||
| Age (years) | ||||||
| <75 | 1 | |||||
| ≥75 | 5.744 | 1.353-24.384 | 0.018 | 3.68 | 0.800-16.939 | 0.094 |
| Pathological differentiation grade | ||||||
| Moderate or Well G1-2 | 1 | |||||
| Poor or undifferentiated G3 | 1.556 | 0.881-2.749 | 0.128 | |||
| Tumor location | ||||||
| Middle | 1.573 | 0.831-2.979 | 0.164 | |||
| Lower | 1 | |||||
| Pathological T stage | 0.457 | |||||
| T1b | 1 | |||||
| T2 | 1.334 | 0.306-5.804 | 0.701 | |||
| T3 | 1.298 | 0.308-5.465 | 0.722 | |||
| T4 | 3.306 | 0.551-19.822 | 0.191 | |||
| N stage | <0.001 | 0.003 | ||||
| N0 | 1 | 1 | ||||
| N1 | 1.974 | 0.827-4.715 | 0.126 | 0.604 | 0.150-2.432 | 0.479 |
| N2 | 8.438 | 3.495-20.371 | <0.001 | 2.716 | 0.645-11.445 | 0.173 |
| N3 | 4.833 | 1.840-12.692 | 0.001 | 2.003 | 0.284-14.150 | 0.486 |
| 8th TNM Stage | 0.006 | 0.198 | ||||
| I | 1 | 1 | ||||
| II | 0.526 | 0.096-2.873 | 0.458 | 0.543 | 0.099-2.969 | 0.481 |
| III | 2.694 | 0.642-11.302 | 0.176 | 2.82 | 0.421-18.879 | 0.285 |
| IV | 3.464 | 0.771-15.559 | 0.105 | 1.751 | 0.182-16.884 | 0.628 |
| Thoracic surgery | ||||||
| MIE | 1 | |||||
| OE | 1.005 | 0.522-1.933 | 0.989 | |||
| Abdominal surgery | ||||||
| MIE | 1 | |||||
| OE | 1.056 | 0.548-2.032 | 0.871 | |||
HR, hazard ratio; CI, confidence interval; MIE, minimally invasive esophagectomy; OE, open esophagectomy; TNM, tumor-node-metastasis; 3F, three-field systematic lymphadenectomy.
Discussion
This study clarified the influence of the number of LNs resected during lymphadenectomy on long-term survival. Patients who underwent two-field lymphadenectomy exhibited no improvement in OS as the number of RLNs increased, but the OS of patients who underwent three-field lymphadenectomy was significantly different in the B and D groups. In addition, there were no statistically significant differences in the characteristics of patients between the B and D groups, except sex.
From the study findings, it can be seen that the most reasonable number of RLNs is between 31 and 37; deviations greater or less than this are both detrimental to the OS of patients with suspicious tumor-positive cervical LNs. There was a significant reduction in OS when the number of RLNs was greater than 50. However, this was not observed in patients without suspicious tumor-positive cervical LNs who underwent two-field lymphadenectomy. Consistent with these results, research by the Cleveland Clinic in Cleveland show that up to 25 to 30 RLNs can improve the OS of patients (19), while more than 30 RLNs reduce the OS. This suggests that surgical strategies should be individualized to optimize OS in different patients.
Few studies have been conducted on the extent of LN dissection by grouping according to this scheme recently. Although articles examining LN dissection have been published, the conclusions are not comparable due to different strategies being examined (20). As more studies focus on comprehensive treatment options for esophageal cancer, the debate now centers around how and when chemotherapy and radiation should be administered, rather than the details of lymphadenectomy (21–23).
In the Chemoradiotherapy for Oesophageal Cancer followed by Surgery Study (CROSS), the median OS of the neoadjuvant chemoradiotherapy plus surgery group was 48.6 months and the 5-year OS rate was 47% whereas the median OS of the surgery alone group was 24.0 months and the 5-year OS rate was 33% (16). In the CheckMate 577 study, the median DFS of the neoadjuvant chemoradiotherapy plus surgery and surgery alone groups was 22.4 months and 11.0 months, respectively, and the 5-year OS rates were both lower than at our center (22). However, histological classification may be an important factor that influences survival, along with strategies of lymphadenectomy. In the NEOCRTEC5010 Clinical Trial, the median OS was 66.5 months (21), which is better than the median OS achieved at our center and may have been influenced by lymphadenectomy (13,21–23). According to research from Fudan University Shanghai Cancer Center, the differences in OS and DFS were not significant between three-field and two-field lymphadenectomy for middle and lower thoracic esophageal cancer; a 5-year OS of 63% was observed in both groups (23). In our study, the 5-year OS in the 2F group was 41%, and there were no significant differences based on the number of RLNs. This may be due to various differences in case characteristics such as pathological T/N stage.
Theoretically, on the one hand, if the intensity of lymphadenectomy is too low, the metastatic LNs would not be removed and the risk of recurrence and metastasis would remain very high (24). On the other hand, if the intensity is too high, the lymphatic system may be damaged, which is not conducive to immune function. Consequently, OS will not improve significantly (25,26).
Therefore, it is crucial to find an appropriate lymphadenectomy intensity in the treatment of esophageal cancer. The Japan Esophageal Society guidelines state that three-field systematic LN dissection is indispensable in the treatment of upper thoracic ESCC. As for middle and lower thoracic ESCC, the condition of the cervical LN needs to be checked carefully. The international current consensus is that two-field systematic LN dissection is enough to treat patients without suspicious tumor-positive cervical LNs, whereas three-field systematic lymphadenectomy is required to treat patients with suspicious tumor-positive cervical LNs (27). Consequently, different cases will require removal of the LN station in different zones as well as different number of RLNs. Thus, the treatment of middle and lower thoracic ESCC should involve lymphadenectomy of different numbers and zones of RLNs based on whether tumor-positive cervical LNs are present.
According to the Chinese Society of Clinical Oncology guidelines for the diagnosis and treatment of malignant LNs published in 2021, the number of RLNs during surgery should be ≥15 for ESCC, consistent with the 8th Union for International Cancer Control edition standards (27,28). However, variations by case, such as tumor location and LN condition, and the uniform standard for ≥15 RLNs during esophagectomy must be discussed further.
Some limitations in this study may have affected the results. Esophagectomy was performed by 12 surgeons in our center from January 2010 to April 2017. The main surgical types were McKeown esophagectomy and Ivor-Lewis esophagectomy with two-field lymphadenectomy or three-field lymphadenectomy, and there was heterogeneity in lymph node dissection at each station. And there was a difference in the rates of minimally invasive surgery between the 2F group and the 3F group, but MIE showed no significant statistical difference according to the multivariate analysis. Thus, there may be subjective selection bias in the results. Additionally, because we retrospectively analyzed data from a single center, the representativeness of the results needs to be considered. The clinical value and efficacy of different LN stations were different, and the results may vary based on tumor location (29). These aspects can be examined further in future studies.
Based on a 5-year follow-up period in this study, the OS of patients without tumor-positive cervical LNs does not improve when more than 15 LNs are dissected. However, patients with suspected tumor-positive cervical LNs should undergo three-field lymphadenectomy and the number of RLNs should range from 31 to 37. Therefore, optimal surgical strategies must be selected based on the patient and case characteristics.
Acknowledgements
The authors would like to thank Professor Lihua Chen (Division of Thoracic Surgery, Sichuan Cancer Research Center for Cancer, Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China) for their instruction.
Glossary
Abbreviations
- CI
confidence interval
- DFS
disease-free survival
- ESCC
esophageal squamous cell carcinoma
- LN
lymph node
- OS
overall survival
- RLN
resected lymph node
- SCC
squamous cell carcinoma
- TNM
tumor-node-metastasis
- 2F group
patients who underwent two-field systematic lymph node dissection
- 3F group
patients who underwent three-field systematic lymph node dissection
Funding Statement
This work was supported by the Wu Jieping Medical Foundation (grant no. 320.6750.2020-15-3, Bethune Charitable Foundation (grant no. HZB-20190528-19), the Science and Technology Department of Sichuan Province (grant nos. 2020YFH0169, 2021YJ0118, 23QYCX0261 and 23ZDYF2430) and Sichuan Province Clinical Key Specialty Construction Project.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KXL, KYD, KL, XN, CDL, WWH, KZL, CHW, ZYL, KZ, TQM, LLJ, HJL, YM, QX, QF, YTH, XFL and LP contributed to the study concept and design; acquisition, analysis, or interpretation of data; and critical revision of the article for important intellectual content. KXL drafted the article. KYD performed statistical analysis. LP obtained funding. YTH and LP provided administrative, technical, or material support and supervised the study. KXL and KYD confirm the authenticity of all the raw data. KXL and XFL had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethical Committee of the Sichuan Cancer Hospital (approval no. NCC2014ZC-01), and informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. 2017;14:33–41. doi: 10.20892/j.issn.2095-3941.2016.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe M, Tachimori Y, Oyama T, Toh Y, Matsubara H, Ueno M, Kono K, Uno T, Ishihara R, Muro K, et al. Comprehensive registry of esophageal cancer in Japan, 2013. Esophagus. 2021;18:1–24. doi: 10.1007/s10388-020-00785-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220:364–373. doi: 10.1097/00000658-199409000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236:177–183. doi: 10.1097/00000658-200208000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koenig AM, Prenzel KL, Bogoevski D, Yekebas EF, Bubenheim M, Faithova L, Vashist YK, Gawad KA, Baldus SE, Pantel K, et al. Strong impact of micrometastatic tumor cell load in patients with esophageal carcinoma. Ann Surg Oncol. 2009;16:454–462. doi: 10.1245/s10434-008-0169-7. [DOI] [PubMed] [Google Scholar]
- 7.Okholm C, Svendsen LB, Achiam MP. Status and prognosis of lymph node metastasis in patients with cardia cancer-a systematic review. Surg Oncol. 2014;23:140–146. doi: 10.1016/j.suronc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Stein HJ, Feith M, Bruecher BL, Naehrig J, Sarbia M, Siewert JR. Early esophageal cancer: Pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566–575. doi: 10.1097/01.sla.0000184211.75970.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M, Li Z, Ma Y, Zhu G, Zhang H, Xue Y. Prognostic predictors of patients with carcinoma of the gastric cardia. Hepatogastroenterology. 2012;59:930–933. doi: 10.5754/hge09356. [DOI] [PubMed] [Google Scholar]
- 10.Xia W, Liu S, Mao Q, Chen B, Ma W, Dong G, Xu L, Jiang F. Effect of lymph node examined count on accurate staging and survival of resected esophageal cancer. Thorac Cancer. 2019;10:1149–1157. doi: 10.1111/1759-7714.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Zhang X, Zhang X, Liu-Helmersson J, Zhang L, Xiao W, Jiang Y, Liu K, Sang S. Prognostic value of the extent of lymphadenectomy for esophageal cancer-specific survival among T1 patients. BMC Cancer. 2021;21:403. doi: 10.1186/s12885-021-08080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizk NP, Ishwaran H, Rice TW, Chen LQ, Schipper PH, Kesler KA, Law S, Lerut TE, Reed CE, Salo JA, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010;251:46–50. doi: 10.1097/SLA.0b013e3181b2f6ee. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Wang Z, Wang F. Optimal lymphadenectomy for thoracic esophageal cancer: Three-field or modified two-field lymphadenectomy. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19:975–978. (In Chinese) [PubMed] [Google Scholar]
- 14.Zheng Y, Wang Z, Wang F, Huang Q, Liu S. Proposed modifications of supraclavicular lymph node metastasis in the esophageal squamous cell carcinoma staging system for improved survival stratification. Oncotarget. 2017;8:41563–41571. doi: 10.18632/oncotarget.14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y, Hu C, Zhang H, Ping Y, Chen LQ. How does the number of resected lymph nodes influence TNM staging and prognosis for esophageal carcinoma? Ann Surg Oncol. 2010;17:784–790. doi: 10.1245/s10434-009-0818-5. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 17.Choi J, Kim SG, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg Endosc. 2010;24:1380–1386. doi: 10.1007/s00464-009-0783-x. [DOI] [PubMed] [Google Scholar]
- 18.Onbaş O, Eroglu A, Kantarci M, Polat P, Alper F, Karaoglanoglu N, Okur A. Preoperative staging of esophageal carcinoma with multidetector CT and virtual endoscopy. Eur J Radiol. 2006;57:90–95. doi: 10.1016/j.ejrad.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Raja S, Rice TW, Murthy SC, Ahmad U, Semple ME, Blackstone EH, Ishwaran H, Worldwide Esophageal Cancer Collaboration Investigators Value of lymphadenectomy in patients receiving neoadjuvant therapy for esophageal adenocarcinoma. Ann Surg. 2021;274:e320–e327. doi: 10.1097/SLA.0000000000004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng YZ, Li XQ, Wang JY, Yang H, Wen J, Zhai WY, Yuan LX, Fu SS, Liao HY, Fu JH. Impact of examined lymph node count for esophageal squamous cell carcinoma in patients who underwent right transthoracic esophagectomy. Ann Surg Oncol. 2021;28:3025–3033. doi: 10.1245/s10434-020-09217-8. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36:2796–2803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384:1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 23.Li B, Zhang Y, Miao L, Ma L, Luo X, Zhang Y, Ye T, Li H, Zhang J, Li Y, et al. Esophagectomy with three-field versus two-field lymphadenectomy for middle and lower thoracic esophageal cancer: Long-term outcomes of a randomized clinical trial. J Thorac Oncol. 2021;16:310–317. doi: 10.1016/j.jtho.2020.10.157. [DOI] [PubMed] [Google Scholar]
- 24.Yu L, Zhang XT, Guan SH, Cheng YF, Li LX. The number of negative lymph nodes is positively associated with survival in esophageal squamous cell carcinoma patients in China. Open Med (wars) 2020;15:152–159. doi: 10.1515/med-2020-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo X, Wang Z, Yang H, Mao T, Chen Y, Zhu C, Yu Z, Han Y, Mao W, Xiang J, et al. Impact of lymph node dissection on survival after neoadjuvant chemoradiotherapy for locally advanced esophageal squamous cell carcinoma: From the results of NEOCRTEC5010, a randomized multicenter study. Ann Surg. 2023;227:259–266. doi: 10.1097/SLA.0000000000004798. [DOI] [PubMed] [Google Scholar]
- 26.Chao YK, Liu HP, Hsieh MJ, Wu YC, Liu YH, Yeh CH, Chang HK, Tseng CK. Lymph node dissection after chemoradiation in esophageal cancer: A subgroup analysis of patients with and without pathological response. Ann Surg Oncol. 2012;19:3500–3505. doi: 10.1245/s10434-012-2402-7. [DOI] [PubMed] [Google Scholar]
- 27.Chinese society of clinical oncology (CSCO), corp-author Guidelines of the Chinese Society of Clinical Oncology (CSCO) Esophageal Cancer. 2022 (In Chinese) [Google Scholar]
- 28.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, et al., editors. 8th edition. Springer; New York: 2017. AJCC cancer staging manual. [DOI] [Google Scholar]
- 29.Tachimori Y, Ozawa S, Numasaki H, Matsubara H, Shinoda M, Toh Y, Udagawa H, Fujishiro M, Oyama T, Uno T, Registration Committee for Esophageal Cancer of the Japan Esophageal Society Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus. 2016;13:1–7. doi: 10.1007/s10388-015-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


