Abstract
Objective
The study objective was to evaluate the surgical outcomes of mitral valve repair in the era of percutaneous technology.
Methods
We retrospectively reviewed 452 patients who underwent mitral valve repair for degenerative disease between 2010 and 2021. Survival, mitral valve reoperation, and mitral regurgitation recurrence were assessed using Cox regression, dichotomized for those aged more than or less than 60 years.
Results
Median age in years (interquartile range) was 52 (47-57) in the younger cohort and 67 (63-73) in the older cohort (P < .0001). Preoperative comorbidities and leaflet pathology were comparable between groups. After adjustment for sex, prior sternotomy, diabetes, atrial fibrillation, and type of leaflet repair, age 60 years or more was not associated with increased mortality (hazard ratio, 6.96, 95% confidence interval, 0.85-56.8, P = .07). Considering death as a competing outcome, cumulative incidence of mitral valve reoperation at 1, 3, and 5 years was 0.9%, 1.4%, and 1.8% in the younger cohort, respectively, and 2.7%, 4.0%, and 5.1% in the older cohort, respectively (subhazard ratio, 2.95, 95% confidence interval, 0.84-10.4, P = .09). Cumulative incidence of mitral regurgitation recurrence with moderate-severe or greater mitral regurgitation at 1, 3, and 5 years was 1.4%, 3.6%, and 5.1%, and 2.7%, 3.5%, and 4.7% in the younger and older cohorts, respectively (subhazard ratio, 0.85, 95% confidence interval, 0.29-2.50, P = .76). Subgroup analysis focusing on isolated mitral valve repairs (n = 388) showed equivalent results with respect to mortality (hazard ratio, 5.31, 95% confidence interval, 0.64-44.0, P = .12), mitral valve reoperation (subhazard ratio, 4.04, 95% confidence interval, 0.89-18.4, P = .07), and mitral regurgitation recurrence (subhazard ratio, 0.98, 95% confidence interval, 0.30-3.15, P = .97).
Conclusions
Mitral valve repair outcomes continue to be excellent, even in low-risk patients aged more than 60 years.
Key Words: degenerative mitral valve disease, mitral valve, mitral valve repair, transcatheter edge-to-edge repair
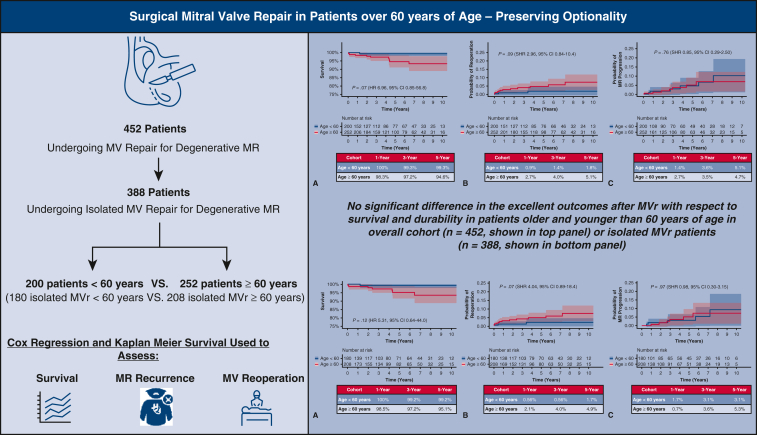
Graphical abstract
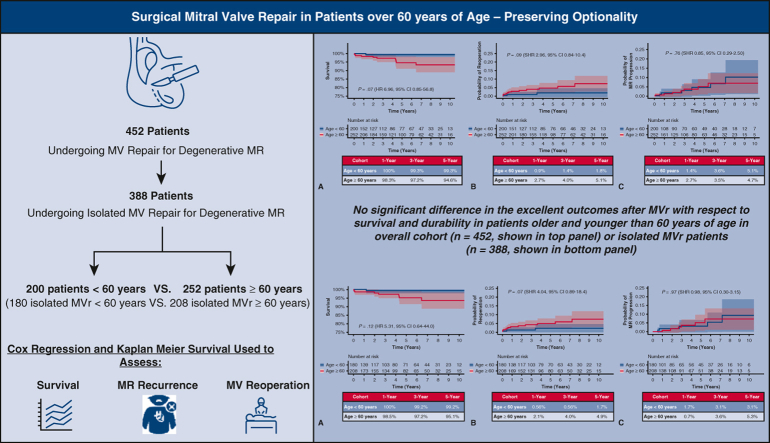
Surgical MV repair is associated with excellent outcomes even in older patients. Ultimately, pursuing surgical MV repair preserves optionality.
Survival, reoperation, and MR recurrence after MV repair for DMVD in those <60 years or ≥60 years old.
Central Message.
Surgical MV repair results are excellent, even in patients over the age of 60 years. MV repair is associated with low mortality and low recurrence, and the need for MV reoperation, even in older patients, is uncommon.
Perspective.
MV repair for DMVD is a nuanced operation aimed at normalizing valve function, but it cannot halt the degenerative process. Because durability outcomes are not captured by national registries, we report our institutional outcomes of MV repair and conclude there is no significant difference in the excellent outcomes after MV repair with respect to survival and durability in patients aged more than or less than 60 years.
Valvular heart disease affects more than 2.5% of the US population, with mitral regurgitation (MR) being the most frequent etiology.1 As the population has aged, the incidence of degenerative mitral valve disease (DMVD) has increased correspondingly over the last 30 years.2 Currently, 6.4% of all Americans age 65 to 74 years old have DMVD, and this prevalence increases to 9.3% of Americans 75 years or older.1,3
Mitral valve (MV) repair repeatedly has been demonstrated to improve survival and quality of life for those with symptomatic, degenerative MR, and thus has been widely accepted as superior to replacement.4, 5, 6, 7, 8, 9 Over the last 10 to 15 years, not only has surgical technique for MV repair improved but also institutional and surgeon MV operative volumes have increased. This has resulted in an increasing rate of successful, durable MV repairs for DMVD.7 In the modern era, operative mortality of MV repair is less than 1%, whereas the 10-year Kaplan–Meier event rate for mitral reoperation using Centers for Medicare and Medicaid Services and Society of Thoracic Surgeons (STS) data is reported to be 6.2%.10,11 Recurrence of MR and need for reoperation are particularly low in patients with isolated posterior repair and annuloplasty ring.10,12,13
Even with excellent results, there remains a subset of patients with high or prohibitive surgical risk who are not candidates for surgical MV repair. In 2007, the percutaneous MV repair via the MitraClip system (Abbott Vascular, Menlo Park, Calif) was introduced into clinical use for high-risk surgical patients, thus broadening options for patients with severe MR.
In light of this, we sought to analyze our contemporary outcomes of surgical MV repair, particularly comparing a cohort of older patients to their younger counterparts with respect to mortality, recurrence of MR, and need for reoperation to help guide future therapeutic comparisons.
Materials and Methods
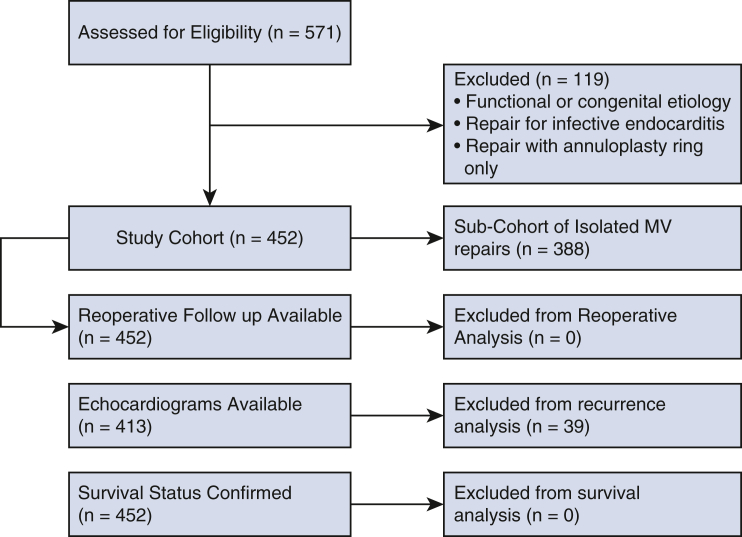
This was a systematic, retrospective cohort study of 452 consecutive patients undergoing MV repair for degenerative disease at the Keck Hospital of the University of Southern California (USC) between January 2010 and August 2021. The follow-up period closed October 2021. Isolated MV repair was defined as MV reconstruction, with or without an annuloplasty ring, with or without tricuspid valve repair or replacement, and with or without Maze. Patients who had functional, ischemic, or congenital MR were excluded (Figure 1). Additionally, we excluded patients who underwent isolated MV annuloplasty for the treatment of MR. Degenerative disease was defined on the basis of valve pathology as described in the operative report. Patients undergoing other concomitant cardiac procedures were included in the overall cohort. Surgical approach was conventional sternotomy or via a minimally invasive, right thoracotomy approach with peripheral cannulation. The MV was accessed through Sondergaard's groove and less commonly trans-septally or via the left atrial dome.
Figure 1.
Consolidated standards of reporting trials type flow diagram. MV, Mitral valve.
The Institutional Review Board of the USC Health Sciences Campus approved this study (HS-15-00509, continued review amendment approved August 30, 2021) and waived the requirement for individual patient consent.
The senior author performed 82.7% of the procedures. Patients, baseline demographics, operative characteristics, and perioperative outcomes were identified through the USC Cardiac Surgery Research Database and our STS Adult Cardiac Database. Subsequent outcomes (follow-up transthoracic echocardiograms or transesophageal echocardiograms, need for reoperation, and mortality) were requested, collected, and reviewed from our electronic medical record, the patients' referring providers, or outside cardiologist. Recurrent MR was assessed from the report of our institutional or outside facility echocardiogram. Reoperation was defined as a repeat intervention to the MV, that is, second MV repair, MV replacement, or transcatheter MV intervention such as transcatheter edge-to-edge repair (TEER). Mortality was confirmed through direct patient, family, or provider contact.
For the purposes of this study, patients were divided into 2 cohorts: those aged less than 60 years (n = 200, cohort 1) and those aged 60 years or more (n = 252, cohort 2). Primary end points were survival, need for MV reoperation, and MR recurrence. Based on the echocardiogram reports received, MR severity at follow-up was coded 0 to 4 (0 = no MR, 1 = trace MR, 1.5 = trace to mild MR, 2 = mild MR, 2.5 = mild to moderate MR, 3 = moderate MR, 3.5 = moderate-severe MR, 4 = severe MR). Progression of MR was defined as the presence of moderate-severe MR (echocardiography grade 3.5) or greater. Follow-up echocardiograms were not obtained at fixed time points but at the discretion of the patient's primary cardiologist. Patients with partial follow-up were included in the appropriate analysis given the data obtained. Once reoperated on, patients were censored from recurrence analysis.
A subset analysis, with the same primary end points, was conducted on patients taken from the overall cohort who underwent isolated MV repair (n = 388). These patients were also subdivided for purpose of analysis into 2 cohorts: those patients aged less than 60 years (n = 180, subcohort 1) and those aged 60 years or more (n = 208, subcohort 2). Isolated MV repair was defined using the STS definition, which includes patients who underwent concomitant tricuspid valve interventions, atrial septal defect closures, and Maze procedures.
Statistical Analysis
Patient demographics, preoperative, and operative characteristics were summarized. For mortality, Cox proportional hazards regression was used to estimate and test associations of variables with time-to-event; Kaplan–Meier survival curves were used and comparisons between our 2 cohorts were made by log-rank tests. Statistical analysis of time to reoperation and MR recurrence considered mortality as a competing risk event. Survival regression used competing risks analysis with the Fine-Gray model; results are presented as subhazard ratios (SHRs) and 95% confidence intervals (CIs). Data were collected with Microsoft Excel spreadsheets (Microsoft Corp) and further analyzed with STATA Version 14 (Statistical Software).
Results
Characteristics of the Cohorts
During this time period, 571 MV repairs were performed. A total of 452 MV repairs (79.2% of repairs) were performed for DMVD. The remaining 119 repairs did not meet inclusion criteria because they were performed for functional MR, congenital MR (typically previous partial, transitional, or complete atrioventricular canal defects), and infective endocarditis, or the repair involved only placement of an isolated annuloplasty ring. A subset of 388 patients (85.8%) underwent isolated MV repair. Preoperative and operative characteristics of the overall cohort and the isolated MV repair subcohort are shown in Tables 1 and 2, respectively.
Table 1.
Preoperative and operative characteristics of the entire cohort
| Entire cohort, N = 452 | Cohort 1: Age < 60 y, N = 200 | Cohort 2: Age ≥ 60 y, N = 252 | P value | |
|---|---|---|---|---|
| Preoperative characteristics | ||||
| Age, y | 60.4 ± 11.7 | 50.3 ± 8.2 | 68.4 ± 6.7 | <.0001 |
| Male sex | 296 (65.5) | 139 (69.5) | 157 (62.3) | .110 |
| Race, non-White | 107 (23.7) | 60 (30) | 47 (18.7) | .005 |
| Ethnicity, Hispanic | 34 (7.5) | 18 (9%) | 16 (6.4) | .289 |
| Body mass index | 25.9 ± 4.5 | 25.8 ± 4.4 | 25.9 ± 4.6 | .767 |
| Diabetes | 32 (7.1) | 14 (7.2) | 18 (7.1) | .784 |
| Hypertension | 208 (46) | 75 (37.5) | 133 (52.8) | .005 |
| Atrial fibrillation | 113 (25) | 44 (22) | 69 (27.4) | .299 |
| Prior CVA | 12 (2.7) | 5 (2.5) | 7 (2.8) | .974 |
| Congestive heart failure | 82 (18.1) | 35 (17.5) | 47 (18.7) | .758 |
| COPD | 35 (7.7) | 15 (7.5) | 20 (7.9) | .739 |
| Previous myocardial infarction | 18 (4) | 4 (2) | 14 (5.6) | .129 |
| Hyperlipidemia | 121 (26.8) | 40 (20) | 81 (31.1) | .015 |
| Chronic kidney disease | 17 (3.8) | 7 (3.5) | 10 (4) | .757 |
| Renal failure requiring dialysis | 8 (1.8) | 4 (2) | 4 (1.6) | .741 |
| Ejection fraction, % | 61.1 ± 8.3 | 61.1 ± 7.5 | 61.2 ± 8.9 | .891 |
| Previous cardiac surgery | ||||
| Previous sternotomy | 14 (3.1) | 5 (2.5) | 9 (3.6) | .514 |
| Previous CABG | 1 (0.2) | 0 | 1 (0.4%) | .372 |
| Previous MV surgery | 7 (1.6) | 3 (1.5) | 4 (1.6) | .940 |
| Previous any valve | 13 (2.9) | 4 (2) | 9 (3.6) | .321 |
| Preoperative MV pathology (as assessed on echocardiogram) | ||||
| Anterior leaflet | 46 (10.2) | 22 (11) | 24 (9.5) | .67 |
| Posterior leaflet | 337 (74.6) | 145 (72.5) | 192 (76.2) | |
| Bileaflet | 69 (15.3) | 33 (16.5) | 36 (14.3) | |
| Operative characteristics | ||||
| Cardiopulmonary bypass time, min | 84.2 ± 38.4 | 85.6 ± 36.5 | 85.5 ± 39.9 | .424 |
| Crossclamp time, min | 61.8 ± 31.1 | 60.7 ± 30.6 | 62.7 ± 31.5 | .497 |
| Need for second CPB run | 10 (2.2) | 5 (2.5) | 5 (2) | .711 |
| Isolated MV repair | 388 (85.8) | 180 (90) | 208 (82.5) | .024 |
| Minimally invasive MV repair | 301 (66.6) | 144 (72) | 157 (62.3) | .030 |
| Conversion from minimally invasive to sternotomy | 4 (0.9) | 0 | 4 (1.6) | .074 |
| Concomitant procedures | 140 (31) | 52 (26) | 88 (35) | .042 |
| Maze | 76 (16.8) | 27 (13.5) | 49 (19.4) | .093 |
| Aortic valve | 21 (4.7) | 8 (4) | 13 (5.2) | .561 |
| Tricuspid valve | 50 (11.1) | 18 (9) | 32 (12.7) | .213 |
| Pulmonary valve | 3 (0.7) | 1 (0.5) | 2 (0.8) | .703 |
| CABG | 20 (4.4) | 4 (2) | 16 (6.4) | .026 |
| Aortic procedure | 20 (4.4) | 5 (2.5) | 15 (6) | .076 |
| IABP | 6 (1.3) | 2 (15) | 4 (1.6) | .588 |
| Atrial septal defect repair | 12 (2.7) | 4 (2) | 8 (3.2) | .440 |
| Septal myectomy | 7 (1.6) | 5 (2.5) | 2 (0.8) | .145 |
| Size of mitral annuloplasty ring, mm | 32 (30, 38) | 32 (30, 34) | 30 (30, 32) | .051∗ |
| Type of MV repair | ||||
| Any anterior leaflet repair | 127 (28.1) | 64 (32) | 63 (25) | .100 |
| Any posterior leaflet repair | 408 (90.3) | 178 (89) | 230 (91.2) | .419 |
| Isolated anterior leaflet repair | 44 (9.7) | 22 (11) | 22 (8.7) | .419 |
| Isolated posterior leaflet repair | 325 (71.9) | 136 (68) | 189 (75) | .100 |
| Bileaflet repair | 83 (18.4) | 42 (21) | 41 (16.3) | .197 |
| Anterior cords | 86 (19) | 41 (20.5) | 45 (17.9) | .477 |
| Posterior cords | 40 (8.9) | 15 (7.5) | 25 (9.9) | .368 |
Continuous variables are expressed as mean ± standard deviation; categorical variables are expressed as frequency (%). Bolded P-values are statistically significant at an alpha level of 0.05. CVA, Cerebral vascular accident; COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass graft; MV, mitral valve; CPB, cardiopulmonary bypass; IABP, intra-aortic balloon pump.
Size of mitral annuloplasty ring expressed as median (25th, 75th percentiles); group differences tested with Wilcoxon rank sum. Concomitant procedures excludes tricuspid valve interventions.
Table 2.
Preoperative and operative characteristics of isolated mitral repairs
| Entire cohort, N = 388 | Cohort 1: Age < 60 y, N = 180 | Cohort 2: Age ≥ 60 y, N = 208 | P value | |
|---|---|---|---|---|
| Preoperative characteristics | ||||
| Age, y | 59.9 ± 11.6 | 50.3 ± 8.2 | 68.2 ± 6.6 | <.001 |
| Male sex | 244 (62.9) | 121 (67.2) | 123 (59.1) | .100 |
| Race, non-White | 90 (23.2) | 54 (30) | 36 (17.3) | .003 |
| Ethnicity, Hispanic | 31 (8) | 17 (9.4) | 14 (6.7) | .326 |
| Body mass index | 25.9 ± 4.6 | 25.8 ± 4.4 | 26 ± 4.8 | .689 |
| Diabetes | 25 (6.4) | 12 (6.7) | 13 (6.3) | .844 |
| Hypertension | 174 (44.9) | 65 (36.1) | 109 (52.4) | .005 |
| Atrial fibrillation | 92 (23.7) | 37 (20.6) | 55 (26.4) | .330 |
| Prior CVA | 10 (2.6) | 4 (2.2) | 6 (2.9) | .911 |
| Congestive heart failure | 71 (18.3) | 33 (18.3) | 38 (18.3) | .858 |
| COPD | 30 (7.7) | 14 (7.8) | 16 (7.7) | .728 |
| Previous myocardial infarction | 11 (2.8) | 3 (1.7) | 8 (3.9) | .382 |
| Hyperlipidemia | 98 (25.3) | 33 (18.3) | 65 (31.3) | .014 |
| Chronic kidney disease | 13 (3.4) | 7 (3.9) | 6 (2.9) | .732 |
| Renal failure requiring dialysis | 7 (1.8) | 4 (2.2) | 3 (1.4) | .565 |
| STS PROM, % | 0.44 (0.25, 0.71) | 0.25 (0.19, 0.44) | 0.63 (0.4, 1.2) | <.001 |
| Ejection fraction, % | 61.3 ± 8.0 | 61.2 ± 7.4 | 61.4 ± 8.5 | .821 |
| Previous cardiac surgery | ||||
| Previous sternotomy | 9 (2.3) | 4 (2.2) | 5 (2.4) | .906 |
| Previous CABG | 1 (0.3) | 0 | 1 (0.5) | .352 |
| Previous MV surgery | 5 (1.3) | 2 (1.1) | 3 (1.4) | .773 |
| Previous any valve | 7 (1.8) | 3 (1.7) | 4 (1.9) | .850 |
| Preoperative MV pathology (as assessed on echocardiogram) | ||||
| Anterior leaflet | 28 (7.2) | 15 (8.3) | 13 (6.3) | .658 |
| Posterior leaflet | 296 (76.3) | 134 (74.4) | 162 (77.9) | |
| Bileaflet | 64 (16.5) | 31 (17.2) | 33 (15.9) | |
| Operative characteristics | ||||
| Cardiopulmonary bypass time, min | 78.8 ± 33.5 | 80.2 ± 34.3 | 77.6 ± 32.8 | .440 |
| Crossclamp time, min | 56.9 ± 26.6 | 58.1 ± 27.7 | 55.8 ± 25.7 | .403 |
| Need for second CPB run | 10 (2.6) | 5 (2.8) | 5 (2.4) | .817 |
| Minimally invasive MV repair | 292 (75.3) | 140 (77.8) | 152 (73.1) | .285 |
| Conversion from minimally invasive to sternotomy | 4 (1) | 0 | 4 (1.9) | .061 |
| Additional procedures | ||||
| Maze | 16 (15.7) | 26 (14.4) | 35 (16.8) | .520 |
| Tricuspid valve | 36 (9.3) | 13 (7.2) | 23 (11.1) | .194 |
| IABP | 6 (1.6) | 2 (1.1) | 4 (1.9) | .518 |
| Atrial septal defect repair | 0 | 0 | 0 | |
| Size of mitral annuloplasty ring, mm | 32 (26, 38) | 32 (30, 34) | 32 (30, 34) | .187∗ |
| Type of MV repair | ||||
| Any anterior leaflet repair | 102 (26.3) | 54 (30) | 48 (23.1) | .122 |
| Any posterior leaflet repair | 360 (92.8) | 164 (91.1) | 196 (94.2) | .236 |
| Isolated anterior leaflet repair | 28 (7.2) | 16 (8.9) | 12 (5.8) | .236 |
| Isolated posterior leaflet repair | 286 (73.7) | 126 (70) | 160 (76.9) | .122 |
| Bileaflet repair | 74 (19.1) | 38 (21.1) | 36 (17.3) | .342 |
| Anterior cords | 70 (18) | 34 (18.9) | 36 (17.3) | .686 |
| Posterior cords | 38 (9.8) | 15 (8.3) | 23 (11.1) | .368 |
Continuous variables are expressed as mean ± standard deviation; categorical variables are expressed as frequency (percent). Bolded P-values are statistically significant at an alpha level of 0.05. CVA, Cerebral vascular accident; COPD, chronic obstructive pulmonary disease; STS PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; CABG, coronary artery bypass grafting; MV, mitral valve; CPB, cardiopulmonary bypass; IABP, intra-aortic balloon pump; MR, mitral regurgitation.
Size of mitral annuloplasty ring and STS PROM expressed as median (25th, 75th percentiles); group differences tested with Wilcoxon rank sum. Additional procedures (Maze and tricuspid valve intervention) are included in the STS definition of “isolated mitral repair”.
Mitral Valve Repairs for Degenerative Mitral Valve Disease
Average age in years ± standard deviation was 61 ± 11.7 in the total cohort, 50.3 ± 8.2 in the younger cohort, and 68.4 ± 6.7 in the older cohort (P < .0001). Preoperative ejection fraction did not differ between the younger and the older cohorts (P = .89). Aside from a higher prevalence of hypertension and hyperlipidemia in the older cohort, the younger and older cohorts were comparable with respect to comorbidities. There was no difference in rates of prior cardiac surgery between the groups or preoperative leaflet pathology on echocardiogram (Table 1).
Patients in the younger cohort were more likely to undergo an isolated MV repair (P = .024) via a right-anterolateral, minithoracotomy (P = .03), while patients in the older cohort were more likely to undergo concomitant cardiac procedures (P = .042), particularly CABG (P = .026). Despite the difference in rates of concomitant procedures, cardiopulmonary bypass time, crossclamp time, and need for second bypass run were equivalent in the older and younger cohorts.
There was no difference in the type of MV repair performed or the size of the annuloplasty ring used. The majority of patients in each cohort underwent an isolated posterior leaflet repair (n = 136, 68% and n = 189, 75%) or a bileaflet repair (n = 42, 21% and n = 41, 16.3%).
The most common pathology was P2 prolapse (80% of cohort). Typical strategy for repair was quadrangular resection, folding valvuloplasty of P1 and P3, followed by reconstruction of the posterior leaflet. If there is anterior leaflet prolapse, a neochord is placed. This is secured after placement of a partial annuloplasty ring (Medtronic Colvin Galloway Future Band with half of our patients receiving size 30 or 32 bands) and distension of left ventricle to ensure proper neochordal height.
Isolated Mitral Valve Repairs for Degenerative Mitral Valve Disease
Within the subset of patients who underwent isolated MV repair (n = 388), the STS preoperative mortality risk was less than 1%. The average age of this younger subcohort was 50.3 ± 8.2 years and 68.2 ± 6.6 years in this older subcohort (P < .001). Similar preoperative and operative characteristics were observed in the isolated MV repair group as the overall cohort described earlier. Ejection fraction, rates of previous cardiac surgery, and preoperative MV pathology were not different between the subcohorts (Table 2).
Cardiopulmonary bypass time, crossclamp time, and need for second bypass run were equivalent in the 2 subcohorts receiving an isolated MV repair. Four patients in the older subcohort (1.9%) required conversion from minithoracotomy to sternotomy, either for better exposure or control of bleeding, whereas none in the younger cohort required conversion. However, this did not reach statistical significance (P = .061). Once again, the majority of patients (70% of the younger subcohort and 76.9% of the older subcohort) received isolated posterior leaflet repairs. Overall, the type of MV repair performed and the size of the annuloplasty ring used were not different between the 2 subcohorts.
Survival
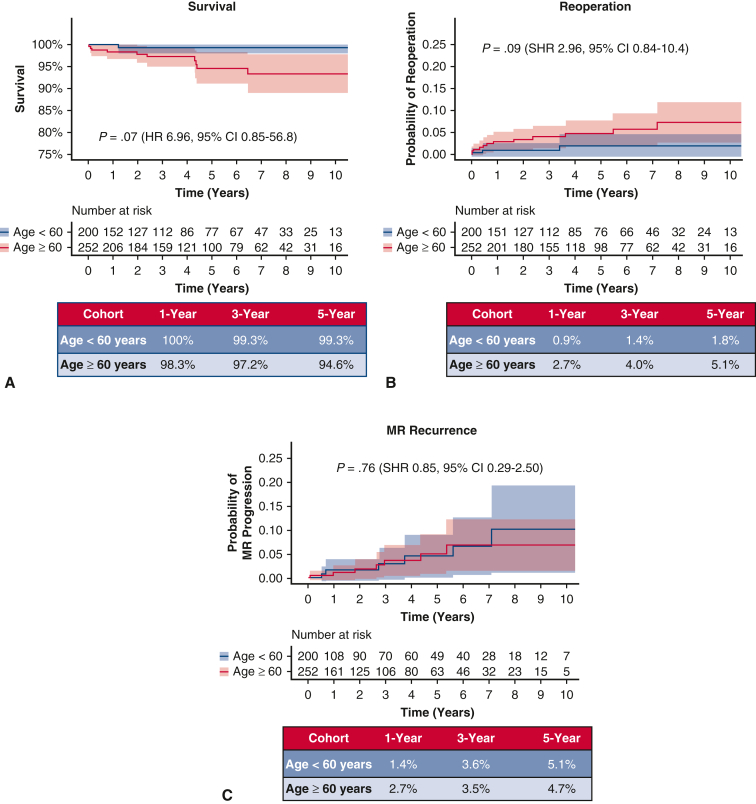
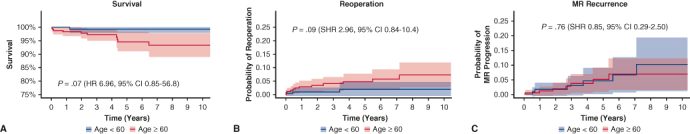
In the overall cohort, median follow-up was 3.6 years (interquartile range, 1.3-6.8) and did not differ between cohorts (P = .37). Two mortalities (0.4%) occurred within 30 days of the index MV operation, both of which were in patients aged 60 years or more (P = .207, Table 3). Overall mortality occurred in 11 patients (2.3%) during the study period, 1 patient in the younger cohort and 10 patients in the older cohort (P = .017). Kaplan–Meier survival at 1, 3, and 5 years was 100%, 99.3%, and 99.3% in cohort 1 and 98.3%, 97.2%, and 94.6% in cohort 2, respectively (log-rank P = .02, Figure 2, A). After adjustment for sex, prior sternotomy, diabetes, atrial fibrillation, and location of leaflet repair (anterior, posterior, or bileaflet), age 60 years or more was not associated with increased mortality (hazard ratio, 6.96, 95% CI, 0.85-56.8, P = .07).
Table 3.
End points in entire cohort
| Variable | Entire cohort, N = 452 | Cohort 1: Age < 60 y, N = 200 | Cohort 2: Age ≥ 60 y, N = 252 | P value |
|---|---|---|---|---|
| Postoperative ejection fraction, % | 56.9 ± 8.5 | 57.6 ± 6.7 | 56.3 ± 9.6 | .124 |
| MV reoperation | 15 (3.3) | 3 (1.5) | 12 (4.8) | .054 |
| MR recurrence | 13 (2.9) | 6 (3) | 7 (2.3) | .984 |
| 30-d mortality | 2 (0.4) | 0 | 2 (0.8) | .207 |
| Overall mortality (assessed at last follow-up) | 11 (2.3) | 1 (0.5) | 10 (3.9) | .017 |
Continuous variables are expressed as mean ± standard deviation; categorical variables are expressed as frequency (percent). MR recurrence defined as progression to moderate-severe or severe MR (3.5-4) as assessed at latest echocardiogram. Bolded P-values are statistically significant at an alpha level of 0.05. MV, Mitral valve; MR, mitral regurgitation.
Figure 2.
A, Kaplan–Meier survival for the entire cohort undergoing MV repair. B, Need for MV reoperation with death as a competing outcome in overall cohort. C, Rate of MR recurrence in overall cohort. MR, Mitral regurgitation; SHR, subhazard ratio; CI, confidence interval; HR, hazard ratio.
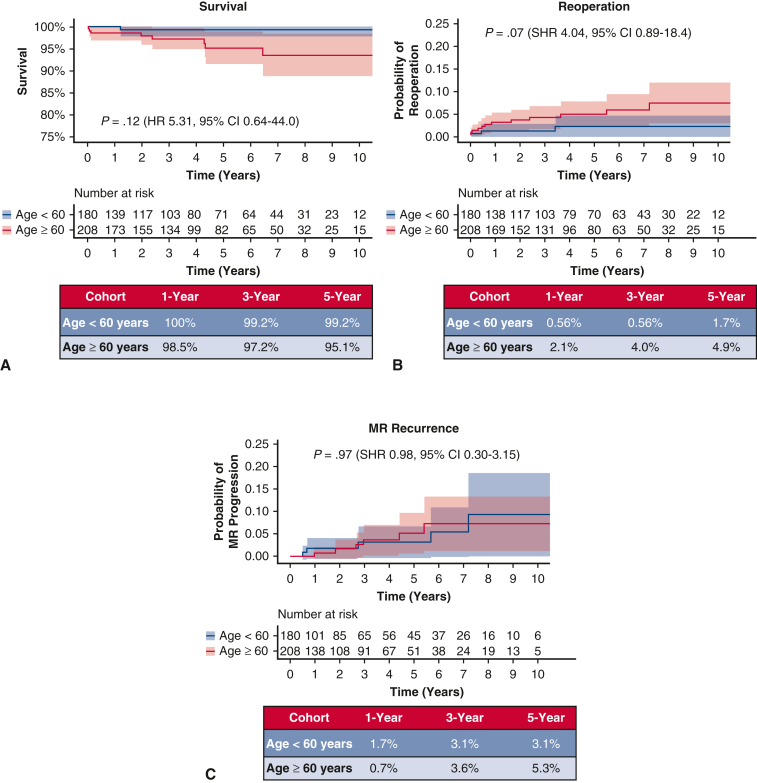
In the subset of patients who underwent isolated MV repair, 2 mortalities (0.5%) occurred within 30 days of the index MV operation, both of which were in patients aged 60 years or more (P = .187, Table 4). Overall mortality occurred in 9 patients, 1 in the younger subcohort and 8 in the older subcohort (P = .032). Similar results were found in the subgroup who underwent isolated MV repair, as age greater than 60 years was not associated with increased mortality with Kaplan–Meier survival at 1, 3, and 5 years of 100%, 99.2%, and 99.2% in the younger subcohort and 98.5%, 97.2%, and 95.1% in the older subcohort, respectively (adjusted hazard ratio, 5.31, 95% CI, 0.64-44.0, P = .12, Figure 3, A).
Table 4.
End points in isolated mitral valve cohort
| Variable | Entire cohort, N = 388 | Cohort 1: Age < 60 y, N = 180 | Cohort 2: Age ≥ 60 y, N = 208 | P value |
|---|---|---|---|---|
| Postoperative ejection fraction, % | 57.2 ± 7.9 | 57.9 ± 6.7 | 56.6 ± 8.3 | .138 |
| MV reoperation | 12 (3.1) | 2 (1.1) | 10 (4.8) | .036 |
| MR recurrence | 11 (2.8) | 5 (2.8) | 6 (2.8) | .725 |
| 30-d mortality | 2 (0.5) | 0 | 2 (1) | .187 |
| Overall mortality (assessed at last follow-up) | 9 (2.3) | 1 (0.6) | 8 (3.9) | .032 |
Continuous variables are expressed as mean ± standard deviation; categorical variables are expressed as frequency (percent). MR recurrence defined as progression to moderate-severe or severe MR (3.5-4) as assessed at latest echocardiogram. Bolded P-values are statistically significant at an alpha level of 0.05. MV, Mitral valve; MR, mitral regurgitation.
Figure 3.
A, Kaplan–Meier survival for the subcohort undergoing isolated MV repair. B, Need for MV reoperation with death as a competing outcome in isolated MV repairs. C, Rate of MR recurrence in isolated MV repairs. MR, Mitral regurgitation; SHR, subhazard ratio; CI, confidence interval; HR, hazard ratio.
Need for Mitral Valve Reoperation
MV reoperation was required in 15 patients (3.3%) in the overall cohort (3 in the younger cohort 1; 12 in the older cohort 2, P = .054). Cumulative incidence of need for MV reoperation with death as a competing outcome at 1, 3, and 5 years was 0.9%, 1.4%, and 1.8% in cohort 1 and 2.7%, 4.0%, and 5.1% in cohort 2, respectively (SHR, 2.95, 95% CI, 0.84-10.4, P = .09, Figure 2, B).
Among those undergoing an isolated MV repair, MV reoperation was required in 12 patients (3.1%), with 2 in the younger subcohort and 10 in the older subcohort (P = .036). Cumulative incidence of need for MV reoperation with death as a competing outcome at 1, 3, and 5 years was 0.56%, 0.56%, and 1.7% in subcohort 1 and 2.1%, 4.0%, and 4.9% in subcohort 2, respectively (SHR, 4.04, 95% CI, 0.89-18.4, P = .07, Figure 3, B).
Progression of Mitral Regurgitation
Follow-up echocardiograms were available in 413 patients (91%) at a median of 2.1 (0.4-5.2) years. Thirteen patients in the overall cohort progressed to moderate-severe MR or greater (6 in the younger cohort; 7 in the older cohort, P = .88). Considering competing risk due to mortality, the cumulative incidence of MR progression to moderate to severe or greater at 1, 3, and 5 years was 1.4%, 3.6%, and 5.1% in cohort 1 and 2.7%, 3.5%, and 4.7% in cohort 2, respectively (SHR, 0.85, 95% CI, 0.29-2.50, P = .76, Figure 2, C).
In the isolated MV repair subcohort, 11 patients progressed to moderate to severe MR or greater (5 in the younger subcohort, 6 in the older subcohort, P = .725). Considering competing risk due to mortality, cumulative incidence of MR progression to moderate to severe or greater at 1, 3, and 5 years was 1.7%, 3.1%, and 3.1% in subcohort 1, and 0.7%, 3.6%, and 5.3% in subcohort 2, respectively (SHR, 0.98, 95% CI, 0.30-3.15, P = .97, Figure 3, C).
Discussion
This study analyzed the outcomes of surgical MV repair, with a special focus on isolated MV repair, particularly comparing a cohort of older patients with their younger counterparts. Results of surgical MV repair are excellent, even in low-risk patients over the age of 60. MV repair with or without concomitant cardiac procedures is associated with low mortality and low recurrence, and the need for MV reoperation, even in an older cohort, is uncommon.
Previous studies have demonstrated both the feasibility and durability of MV repair for DMVD. The value of mitral repair in elderly patients with more complex cardiac pathology or more advanced myxomatous degeneration, however, has been called into question,14 especially with the advances being made in mitral TEER technology. In fact, by mid-2018 the use of TEER had significantly surpassed the use of surgical MV repair in Medicare beneficiaries.15 Previous studies, including one from our institution, found that increasing age was associated with not only an increased risk of mortality but also an increased risk of MR recurrence.12,13,16 This analysis demonstrates that age above 60 years is not associated with an increased mortality or an increased risk of MR recurrence or MV reoperation, when death is used as a competing outcome.
It bears remembering that MR recurrence in and of itself is associated with increased morbidity and mortality.12,13 Although the need for MV reoperation and progression of MR are often used in tandem to indicate the durability of the surgical repair as well as the adequacy of long-term MR correction, one must remember that surgical MV repair attempts to normalize valvular function but is unable to halt the degenerative process. It is for this reason that many long-term studies on MV repair outcomes for DMVD report MR recurrence rates that seem high, certainly higher than rates of reoperation. Braunberger and colleagues17 reported a 20-year freedom from reoperation between 83% and 92%, depending on the presence of anterior leaflet involvement. Freedom from recurrent MR is more variable across studies. For example, reported rates of freedom from recurrent moderate or severe MR vary from 77% at 5 years18 to 71% at 7 years19 and 81% at 10 years.20
For purposes of our study, we elected to report rates of recurrence of moderate-to-severe MR or greater (grade 3.5 or 4), because the presence of moderate MR alone is unlikely to require reoperation. Although our study follow-up time was not as long as those mentioned above, our 5-year recurrence rates in both the overall cohort and isolated MV repairs are low and slightly less than the 2% to 3% per year rates reported in the literature.21,22 Finally, although predicting who will develop recurrent MR and who will require reoperation is important, we did not perform multivariable regression analysis in this study because our institution has previously published on such risk factors.13
Although recurrent MR was the most common reason for reoperation in our study, the need for MV reoperation was similarly uncommon in both the overall cohort and the subgroup of isolated MV repairs. More important, age more than 60 years was not associated with increased need for MV reoperation.
To examine whether age as a continuous variable was associated with MR recurrence, several univariate logistic regressions were performed, examining the association between MR recurrence and age, MV reoperation, and age, and finally a composite end point of MR recurrence plus MV reoperation and age. None of the models demonstrated a significant association between age and MR recurrence, MV reoperation, or the composite of the 2 (P values of .594, .464, and .787, respectively).
It certainly can be argued that age 60 years or more is neither particularly advanced nor is it the cutoff being used in TEER clinical trials. We selected this cutoff for 2 reasons. The first reason being that it allowed us to dichotomize by the average age of patient cohort, as well as the average age of patients undergoing isolated MV surgery as reported by STS data. Thus, it created a cohort of patients younger than average and a cohort of patients older than average. Second, we aimed to create older and younger cohorts whose comorbidities were not significantly different and whose surgical risk profile was also neither different nor significantly increased by more advanced age, particularly with respect to the older cohort. Dichotomizing at 70 years of age resulted in cohorts with a sample size mismatch (373 in the younger cohort and 79 in the older cohort). Additionally, there were significant differences in baseline and operative characteristics with dichotomization schema at 65 and 70 years. We know that certain comorbid conditions including diabetes, renal failure, and heart failure are associated with increased rates of MR recurrence and reoperation. Furthermore, certain operative factors, including anterior leaflet intervention, are associated with increased rates of MR recurrence and reoperation.
Study Limitations
Our study has the limitations inherent to all single-center, retrospective cohort analyses. Additionally, although we were able to obtain follow-up echocardiograms in 91% of patients, it should be acknowledged firstly that median echocardiographic follow-up was relatively short at 2.1 (0.4-5.2) years; secondly, echocardiograms were not obtained at designated time intervals; thirdly, not all patients had a postoperative echocardiogram before the one demonstrating recurrence; and fourthly, the grade of MR was determined by the cardiologist who was reading echocardiograms at the facility the patient was sent to by their primary cardiologist and thus was not standardized. In this way, it is possible for rates of MR recurrence to be overestimated or underestimated in this series.
We believe these results to be of particularly timely import because we will soon be enrolling even low and moderate surgical risk patients with DMVD in 2 new clinical trials comparing surgical MV repair with TEER. Although the emergence of transcatheter approaches has certainly broadened options for patients with severe MR, it must be remembered that the durability of such interventions has yet to be firmly established. The EVEREST II trial, which randomized patients without high surgical risk with an average age of 67 years to transcatheter or surgical MV repair, found that although there was no significant difference in mortality between surgery and percutaneous repair at 5 years (20.8% vs 26.8%; P = .36), MV surgery or reintervention was significantly more frequent with percutaneous repair (27.9% vs 8.9%; P = .003) as was recurrence of 3+ or 4+ MR (12.3% vs 1.8%; P = .02).23 Although data from the STS/American College of Cardiology Transcatheter Valve Therapy Registry have demonstrated acute procedural success of TEER in 92% of patients (of whom > 80% have been classified as having DMVD), mortality occurs in approximately 25% of patients at 1 year and approximately one-fifth of patients are rehospitalized for heart failure.24 Additionally, 30.5% of patients have 2+ MR or greater remaining immediately after the procedure.25 Furthermore, surgeons must also carefully consider that all 20 published clinical studies reporting on surgical intervention for a failed MitraClip procedure (Abbott Vascular) found that most patients (63.5%) require MV replacement and that surgery for a failed MitraClip is burdened by a high in-hospital mortality rate of 15% and a high rate of death at 1 year of 26.5%.26
Although we make no attempts to compare our results with transcatheter outcomes, we believed it crucial to consider the excellent surgical repair results in low-risk patients, particularly those undergoing isolated MV repair, because these are the patients these upcoming trials will focus on. As applications broaden, the onus will be on the surgeon to decide whether an otherwise suitable surgical candidate should receive a transcatheter repair instead. This evaluation must be thoughtful given the potential for MR progression after repair because the biological degeneration of the valve cannot be reversed or halted. Ultimately, pursuing surgical MV repair preserves optionality for low-risk patients with severe degenerative MR who are referred early. Although a percentage of these patients will not require a reintervention, performing surgical repair first provides those who do with the option of a re-repair, a TEER, or a replacement if repair is not feasible. On the other hand, performing a TEER first will commit the majority of patients whose MR progresses to the point of requiring reintervention down a pathway that ends in MV replacement, which we know to be inferior.
Conclusions
There is no significant difference in the excellent outcomes after MV repair with respect to survival and durability as they relate to the need for reoperation and MR progression in patients age less than 60 years and those age 60 years or more (Figure 4). As broader application of transcatheter mitral repair techniques looms on the horizon, we need to consider surgical repair results, especially in older populations, very carefully.
Figure 4.
Surgical MV repair is associated with excellent outcomes even in older patients. Ultimately, pursuing surgical MV repair preserves optionality. SHR, Subhazard ratio; CI, confidence interval; HR, hazard ratio; MV, mitral valve; MR, mitral regurgitation.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank Melody Malig for assistance with data collection and Mark Barr, MD, and Sanjeet Patel, MD, PhD, for assistance with manuscript edits and revisions.
Footnotes
Institutional Review Board Approval: HS-15-00509 (continued review amendment approved 8/30/2021). Individual patient informed consent was waived by the Institutional Review Board.
Read at the 48th Annual Meeting of the Western Thoracic Surgical Association, Koloa, Hawaii, June 22-25, 2022.
References
- 1.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Chen J., Li W., Xiang M. Burden of valvular heart disease, 1990-2017: results from the Global Burden of Disease Study 2017. J Glob Health. 2020;10:020404. doi: 10.7189/jogh.10.020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 4.Shuhaiber J., Anderson R.J. Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur J Cardio Thorac Surg. 2007;31:267–275. doi: 10.1016/j.ejcts.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., Gentile F., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Thorac Cardiovasc Surg. 2021;162:e183–e353. doi: 10.1016/j.jtcvs.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Gillinov A.M., Blackstone E.H., Nowicki E.R., Slisatkorn W., Al-Dossari G., Johnston D.R., et al. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg. 2008;135:885–893. doi: 10.1016/j.jtcvs.2007.11.039. 893.e1-893. [DOI] [PubMed] [Google Scholar]
- 7.Suri R.M., Schaff H.V., Dearani J.A., Sundt T.M., Daly R.C., Mullany C.J., et al. Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg. 2006;82:819–826. doi: 10.1016/j.athoracsur.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 8.Lazam S., Vanoverschelde J.L., Tribouilloy C., Grigioni F., Suri R.M., Avierinos J.F., et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: analysis of a large, prospective, multicenter, international registry. Circulation. 2017;135:410–422. doi: 10.1161/CIRCULATIONAHA.116.023340. [DOI] [PubMed] [Google Scholar]
- 9.McNeely C.A., Vassileva C.M. Long-term outcomes of mitral valve repair versus replacement for degenerative disease: a systematic review. Curr Cardiol Rev. 2015;11:157–162. doi: 10.2174/1573403X10666140827093650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaff H.V., Nguyen A. Contemporary techniques for mitral valve repair-the Mayo Clinic experience. Indian J Thorac Cardiovasc Surg. 2020;36(Suppl 1):18–26. doi: 10.1007/s12055-019-00801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badhwar V., Peterson E.D., Jacobs J.P., He X., Brennan J.M., O’Brien S.M., et al. Longitudinal outcome of isolated mitral repair in older patients: results from 14,604 procedures performed from 1991 to 2007. Ann Thorac Surg. 2012;94:1870–1877. doi: 10.1016/j.athoracsur.2012.05.105. discussion 1877-9. [DOI] [PubMed] [Google Scholar]
- 12.Suri R.M., Clavel M.A., Schaff H.V., Michelena H.I., Huebner M., Nishimura R.A., et al. Effect of recurrent mitral regurgitation following degenerative mitral valve repair: long-term analysis of competing outcomes. J Am Coll Cardiol. 2016;67:488–498. doi: 10.1016/j.jacc.2015.10.098. [DOI] [PubMed] [Google Scholar]
- 13.Tatum J.M., Bowdish M.E., Mack W.J., Quinn A.M., Cohen R.G., Hackmann A.E., et al. Outcomes after mitral valve repair: a single-center 16-year experience. J Thorac Cardiovasc Surg. 2017;154:822–830.e2. doi: 10.1016/j.jtcvs.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 14.Gillinov A.M., Faber C., Houghtaling P.L., Blackstone E.H., Lam B.K., Diaz R., et al. Repair versus replacement for degenerative mitral valve disease with coexisting ischemic heart disease. J Thorac Cardiovasc Surg. 2003;125:1350–1362. doi: 10.1016/s0022-5223(02)73274-1. [DOI] [PubMed] [Google Scholar]
- 15.Young M.N., Kearing S., Albaghdadi M.A., Latib A., Iribarne A. Trends in transcatheter versus surgical mitral valve repair among Medicare beneficiaries, 2012 to 2019. JAMA Cardiol. 2022;7:770–772. doi: 10.1001/jamacardio.2022.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David T.E., Armstrong S., McCrindle B.W., Manlhiot C. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation. 2013;127:1485–1492. doi: 10.1161/CIRCULATIONAHA.112.000699. [DOI] [PubMed] [Google Scholar]
- 17.Braunberger E., Deloche A., Berrebi A., Abdallah F., Celestin J.A., Meimoun P., et al. Very long-term results (more than 20 years) of valve repair with Carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation. 2001;104(12 Suppl 1):I8–I11. [PubMed] [Google Scholar]
- 18.Stevens L.M., Basmadjian A.J., Bouchard D., El-Hamamsy I., Demers P., Carrier M., et al. Late echocardiographic and clinical outcomes after mitral valve repair for degenerative disease. J Card Surg. 2010;25:9–15. doi: 10.1111/j.1540-8191.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- 19.Flameng W., Herijgers P., Bogaerts K. Recurrence of mitral valve regurgitation after mitral valve repair in degenerative valve disease. Circulation. 2003;107:1609–1613. doi: 10.1161/01.CIR.0000058703.26715.9D. [DOI] [PubMed] [Google Scholar]
- 20.Shimokawa T., Kasegawa H., Katayama Y., Matsuyama S., Manabe S., Tabata M., et al. Mechanisms of recurrent regurgitation after valve repair for prolapsed mitral valve disease. Ann Thorac Surg. 2011;91:1433–1438. doi: 10.1016/j.athoracsur.2011.01.015. discussion 1438-9. [DOI] [PubMed] [Google Scholar]
- 21.Flameng W., Meuris B., Herijgers P., Herregods M.C. Durability of mitral valve repair in Barlow disease versus fibroelastic deficiency. J Thorac Cardiovasc Surg. 2008;135:274–282. doi: 10.1016/j.jtcvs.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 22.Tomšič A., Hiemstra Y.L., van Hout F.M.A., van Brakel T.J., Versteegh M.I.M., Marsan N.A., et al. Long-term results of mitral valve repair for severe mitral regurgitation in asymptomatic patients. J Cardiol. 2018;72:473–479. doi: 10.1016/j.jjcc.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Feldman T., Kar S., Elmariah S., Smart S.C., Trento A., Siegel R.J., et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. 2015;66:2844–2854. doi: 10.1016/j.jacc.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Sorajja P., Vemulapalli S., Feldman T., Mack M., Holmes D.R., Stebbins A., et al. Outcomes with transcatheter mitral valve repair in the United States: An STS/ACC TVT Registry report. J Am Coll Cardiol. 2017;70:2315–2327. doi: 10.1016/j.jacc.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Chhatriwalla A.K., Vemulapalli S., Szerlip M., Kodali S., Hahn R.T., Saxon J.T., et al. Operator experience and outcomes of transcatheter mitral valve repair in the United States. J Am Coll Cardiol. 2019;74:2955–2965. doi: 10.1016/j.jacc.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Melillo F., Baldetti L., Beneduce A., Agricola E., Margonato A., Godino C., et al. Mitral valve surgery after a failed MitraClip procedure. Interact Cardiovasc Thorac Surg. 2021;32:380–385. doi: 10.1093/icvts/ivaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]