Abstract
Background:
The auditory N100 is an event related potential (ERP) that is reduced in schizophrenia, but its status in individuals at clinical high risk for psychosis (CHR) and its ability to predict conversion to psychosis remains unclear. We examined whether N100 amplitudes are reduced in CHR subjects relative to healthy controls (HC), and this reduction predicts conversion to psychosis in CHR.
Methods:
Subjects included CHR individuals (n = 552) and demographically similar HC subjects (n = 236) from the North American Prodrome Longitudinal Study. Follow-up assessments identified CHR individuals who converted to psychosis (CHR—C; n = 73) and those who did not (CHR-NC; n = 225) over 24 months. Electroencephalography data were collected during an auditory oddball task containing Standard, Novel, and Target stimuli. N100 peak amplitudes following each stimulus were measured at electrodes Cz and Fz.
Results:
The CHR subjects had smaller N100 absolute amplitudes than HC subjects at Fz (F(1,786) = 4.00, p 0.046). A model comparing three groups (CHR—C, CHR-NC, HC) was significant for Group at the Cz electrode (F(2,531) = 3.58, p = 0.029). Both Standard (p = 0.019) and Novel (p = 0.017) stimuli showed N100 absolute amplitude reductions in CHR-C relative to HC. A smaller N100 amplitude at Cz predicted conversion to psychosis in the CHR cohort (Standard: p = 0.009; Novel: p = 0.001) and predicted shorter time to conversion (Standard: p = 0.013; Novel: p = 0.001).
Conclusion:
N100 amplitudes are reduced in CHR individuals which precedes the onset of psychosis. N100 deficits in CHR individuals predict a greater likelihood of conversion to psychosis. Our results highlight N100’s utility as a biomarker of psychosis risk.
Keywords: Event-related potential (ERP), Auditory processing, Schizophrenia, Clinical high risk, N100
1. Introduction
Schizophrenia (SCZ) typically emerges in late adolescence with prodromal symptoms characterized by a dysfunction in thinking, mood and/or cognition. A greater understanding of brain changes in the prodrome could potentially lead to improved early treatment and course of SCZ later in life.
The auditory N100 is an event related potential (ERP) evoked by acoustic stimuli (Naatanen and Picton, 1987; Rosburg et al., 2008). Auditory N100 generators have been localized to the auditory cortex, with additional contributions from frontal and parietal sources (Naatanen and Picton, 1987). N100 abnormalities have been found in many studies of patients with SCZ ((Brockhaus-Dumke et al., 2008) and see review (Rosburg et al., 2008)). Furthermore, N100 reduction is a heritable trait and considered to be an endophenotype of SCZ (Turetsky et al., 2008). Thus, N100 can be used as an electrophysiological indicator of brain dysfunction associated with psychosis.
While N100 amplitude reduction has been repeatedly observed in SCZ, its status in individuals at clinical high risk for psychosis (CHR) remains unclear. One study reported that in CHR individuals the absolute amplitude of the N100 decreases as psychotic symptomatology increases (Gonzalez-Heydrich et al., 2015). One prior study found reduced N100 amplitude in CHR compared to control subjects (del Re et al., 2015), but another study found no group difference in N100 amplitude (Bramon et al., 2008). Further, a prior study reported reduced baseline N100 amplitudes in subjects at risk for psychosis who went on to develop psychosis three years after baseline compared to controls or those who did not develop psychosis (van Tricht et al., 2015). However, two other studies did not detect a significant difference in baseline N100 amplitudes that distinguished subjects who converted to psychosis compared to controls or those who did not convert (Brockhaus-Dumke et al., 2008; Hsieh et al., 2012). It should be noted, however, that the sample sizes of those converting to psychosis in these studies were quite modest (18, 11, and 21 converters respectively in these aforementioned papers).
Thus, there is a lack of definitive evidence to date on the ability of N100 amplitude to predict development of psychosis in those at risk. The National Institutes of Health-funded 8-site North American Prodrome Longitudinal Study (NAPLS-2) tested a large dataset of CHR subjects (n = 552) vs. healthy controls (HC; n = 236) at baseline and at 24 months of follow up with rigorous criteria for determining conversion to psychosis at endpoint. The goal of this analysis was to use this large dataset to determine whether auditory N100 amplitude is reduced in CHR individuals relative to HCs, and, if so, whether it predicts conversion to psychosis in CHR individuals during a 24-month follow-up period. A secondary goal was to examine whether the CHR group would demonstrate more impaired N100 responses to Target and Novel stimuli than to Standard stimuli when compared to the HC group, particularly since N100 responses are affected by attention (Rosburg et al., 2008). This goal was based on the finding that first episode schizophrenia subjects had reduced N100 amplitudes to attended tones in an oddball task consisting of standard stimuli and oddball target stimuli (Ren et al., 2021).
2. Methods
2.1. Subjects
This study included 788 individuals from the 8-site NAPLS-2 who had analyzable baseline N100 data. Recruitment and inclusion criteria are outlined in detail in a prior publication from the group (Addington et al., 2012; Hamilton et al., 2019). In summary, subjects were recruited for this study in two subject groups. The CHR group included subjects who met the Criteria of Psychosis-Risk Syndromes (n = 552) as determined by the Structured Interview for Psychosis-Risk (SIPS) (McGlashan et al., 2010; Miller et al., 2003). Their symptoms were rated with the Scale of Psychosis-Risk Symptoms (SOPS) (Woods et al., 2019). Potential subjects in this group were excluded if they met current or past diagnostic criteria for a psychotic disorder, had an IQ below 70, had substance dependence in the past 6 months, or had a significant neurological disorder. A group of demographically matched healthy controls (HC; n = 236) was recruited with the additional exclusion criteria of having any first-degree relatives with a psychotic disorder or taking any psychotropic medication. Additional details of inclusion and exclusion criteria for both subject groups have been previously published (Addington et al., 2012; Hamilton et al., 2019).
The study protocol called for subjects to be followed every six months for up to 24 months or until the onset of psychosis. Of the CHR individuals who had sufficient follow up for classification, 73 converted to psychosis (converters, CHR—C) and 225 did not convert to psychosis (non-converters, CHR-NC) by the end of the 24-month follow up period. CHR subjects lost to follow up were not included in analyses predicting conversion to psychosis.
2.2. EEG and N100 acquisition
Prior to psychophysiological testing, subjects were screened for intact auditory acuity. N100 amplitudes were collected during an auditory oddball task as described in Hamilton et al. (2019). The task consisted of auditory stimuli presented through insert headphones (ER1-A Etymotic; Etymotic Research Inc., Elk Grove Village, IL). The task included three blocks with a total of 450 trials, each with an auditory stimulus. Trials had a stimulus onset asynchrony of 1250 milliseconds (ms). The stimuli were of three types presented in a pseudorandom order: frequent (80 %) Standard tones, infrequent (10 %) Target tones requiring a button press, and infrequent (10 %) Novel distractor stimuli. The Standard and Target stimuli were 50 ms pure tones with a 5 ms rise and fall time. The Standard tones were 500 Hertz (Hz), and the Target tones were 1000 Hz. The Novel sounds were a variety of natural and human-made sounds with an average duration of ~250 ms. Prior to the start of the session, subjects were presented with a brief instructional video telling them to press a button on a button box when they heard the Target tones.
During the paradigm, the subjects were seated in front of a computer screen wearing the insert headphones. Electroencephalography (EEG) data were collected by means of a Biosemi ActiveTwo system (Biosemi, Amsterdam, Netherlands). Subjects wore a cap fitted with either 32 or 64 scalp electrodes placed at standard sites. Only data from the Cz and Fz electrodes were included for the N100 analysis. This decision was made based on the known robust detection of the N100 response in central and frontal regions captured by Cz and Fz respectively (Naatanen and Picton, 1987), and the robust differences in N100 between CHR and HCs at these electrode sites (del Re et al., 2015). Reference electrodes were affixed to the mastoids, and electrodes were placed above and below the right eye and just outside the outer canthus of each eye to record eye movements and blinks. As has been reported from our group previously (Hamilton et al., 2019), the EEG data were referenced to the average of the signals from the two mastoid electrodes, and all data were subjected to a high band-pass filter at 0.1 Hz. A complete description of offline de-noising and artifact rejection routines has been described previously (Hamilton et al., 2019). For each trial type, N100 was characterized as the most negative peak between 70 ms and 130 ms after the onset of the stimulus in the ERP average.
2.3. Statistical methods
After pre-processing and data cleaning as outlined above, N100 peak amplitudes in response to each stimulus type for each trial were measured at midline scalp electrodes Cz and Fz where N100 is largest. These were subsequently transformed to age- and site-corrected z-scores, reflecting deviations in standard units from the age-specific estimates provided by the HCs at each site. These transformed N100 amplitudes were then averaged across each stimulus type for each subject. Latency data were similarly transformed to age- and site-corrected z-scores for each subject. Means ± SD of the amplitude and latency z-scores were computed for each stimulus type and subject group.
Statistics were completed using SPSS version 27 (Armonk, NY). N100 z-scores were compared between the CHR and HC groups using Group × Stimulus (Standard, Novel, Target) repeated measures ANOVAs for electrodes Cz and Fz separately with follow up post hoc tests for each stimulus type. To assess N100 z-scores between the three groups (CHR—C, CHR-NC, HC), similarly structured Group × Stimulus (Standard, Novel, Target) repeated measures ANOVAs were conducted for electrodes Cz and Fz separately with follow up post hoc tests for each stimulus type. For N100 latency, similarly structured repeated measures ANOVAs were used. Logistic regression was completed to determine whether the z-score of the N100 amplitude to the Novel, Target or Standard stimuli at either electrode (Cz or Fz) was associated with conversion to psychosis in the CHR group. These logistic regressions were computed first without SOPS scores and then with the addition of SOPS positive and negative symptom scores. The significance of these regressions was corrected for multiple comparisons by means of the Benjamini-Hochberg procedure. These models were then followed by Cox Proportional Hazard regression models to predict the time to conversion to psychosis. For both the logistic regression and Cox Proportional Hazard regression models, each N100 amplitude was run individually because of collinearity amongst the N100 amplitudes. To confirm results of the Cox Proportional Hazard models, these were rerun on a holdout set of a randomly selected 90 % of the subjects. For graphical display purposes, the z-scores of N100 amplitudes were divided into quartiles.
Separate regression models split by electrode and stimulus type were run to determine whether N100 amplitude and latency individually associated with symptom ratings (both positive and negative) from the SOPS. These were run as separate models due to the collinearity of the predictor variables. Each model included symptom scores as the dependent variable, a N100 amplitude or latency z-score for a given stimulus type (Novel, Standard, or Target) as a predictor variable, and dummy variables to control for site-to-site variability in SOPS scores. The unstandardized B values and the respective p values reported herein are based upon the full model. A correction for multiple comparisons was done for these regressions by means of the Benjamini-Hochberg procedure.
3. Results
3.1. Demographic and clinical variables
The subjects included 552 CHR individuals who met criteria for the psychosis risk syndrome based on a SIPS interview and a demographically similar group of 236 HCs. Based on clinical follow-up assessments, CHR individuals were further classified as converters to full psychosis (CHR—C; n = 73) and non-converters who completed 24 months of follow-up without converting to psychosis (CHR-NC; n = 225). Those in the CHR-C group met full diagnostic criteria for the presence of psychotic symptoms as measured by the SOPS (Miller et al., 2003). Of those who converted to psychosis by these criteria, diagnosis was ascertained at the time of conversion or at 24 months’ follow up by SCID-V interview (First et al., 2017). The diagnoses for this group were as follows: 26 had a diagnosis of schizophrenia, 22 had a diagnosis of psychosis not otherwise specified, seven had a diagnosis of bipolar disorder, five had a diagnosis of major depression, two had a diagnosis of delusional disorder, one had a diagnosis of posttraumatic stress disorder, and ten did not satisfy criteria for a specific diagnosis. Table 1 shows demographic and clinical information on these subject groups.
Table 1.
Demographic and clinical information by group.
| HC (n = 236) |
CHR (n = 552) |
CHR | ||
|---|---|---|---|---|
| CHR-NC (n = 225) |
CHR-C (n = 73) |
|||
| Age (years, mean ± SD)a | 20.44 ± 4.72 | 19.21 ± 4.38 | 19.39 ± 4.59 | 18.49 ± 3.64 |
| Sex, N (percentage) | ||||
| Female | 111 (47.0) | 236 (42.8) | 104 (46.2) | 27 (37.0) |
| Male | 125 (53.0) | 316 (57.2) | 121 (53.8) | 46 (63.0) |
| Education (years, mean ± SD)b | 12.8(3.6) | 11.3(2.8) | 11.3(2.7) | 11.0(2.7) |
| Income bracket (mean ± SD) | 4.1(1.7) | 4.1(1.7) | 4.1(1.6) | 4.2(1.7) |
| SOPS (mean ± SD) | ||||
| Positive symptomsc | 0.90 ± 1.50 | 11.42 ± 4.16 | 10.88 ± 4.41 | 13.19 ± 4.02 |
| Negative symptomsd | 1.53 ± 2.47 | 11.49 ± 6.16 | 11.14 ± 6.30 | 12.27 ± 6.33 |
| Disorganizatione | 0.65 ± 1.18 | 4.98 ± 3.06 | 4.70 ± 3.10 | 6.12 ± 3.97 |
| General symptomsf | 1.31 ± 2.12 | 8.77 ± 4.35 | 7.99 ± 4.34 | 9.53 ± 4.33 |
HC = Healthy control subjects; CHR = Clinical High Risk subjects (at Baseline).
CHR-NC = Subjects who did not converted to psychosis by 2 years.
CHR-C = Subjects who converted to psychosis by 2 years; SOPS = Scale of Prodromal Symptoms.
HC > CHR (p < 0.001); HC > CHR_NC (p = 0.013); HC > CHR_C (p = 0.001).
HC > CHR (p < 0.001); HC > CHR_NC (p < 0.001); HC > CHR_C (p < 0.001).
HC < CHR (p < 0.001); HC < CHR_NC < CHR_C (p < 0.001).
HC < CHR (p < 0.001); HC < CHR_NC (p < 0.001); HC < CHR_C (p < 0.001).
HC < CHR (p < 0.001); HC < CHR_NC < CHR_C (p < 0.001).
HC < CHR (p < 0.001); HC < CHR_NC < CHR_C (p ≤ 0.001).
3.2. N100 amplitude
In comparing results, we will refer to N100 amplitudes by their absolute values. The N100 is a negative wave, so blunted amplitudes are larger (less negative) numbers with smaller absolute values. “Smaller” N100 responses are therefore those with smaller absolute values (Table 2).
Table 2.
N100 peak amplitude and latency by group.
| HC (n = 236) |
CHR (n = 552) |
CHR | ||
|---|---|---|---|---|
| CHR-NC (n =225) |
CHR-C (n = 73) |
|||
| Cz electrode | ||||
| N100 amplitude, μV (mean ± SD) | ||||
| Standard stimuli | −4.50 ± 2.40 | −4.16 ± 2.44 | −4.26 ± 2.64 | −3.66 ± 2.16 |
| Novel stimuli | −6.12 ± 4.31 | −5.80 ± 4.45 | −6.28 ± 4.64 | −4.43 ± 3.80 |
| Target stimuli | −6.59 ± 3.58 | −6.32 ± 3.77 | −6.56 ± 3.96 | −6.24 ± 3.76 |
| N100 latency, ms (mean ± SD) | ||||
| Standard stimuli | 97.85 ± 9.77 | 98.85 ± 10.82 | 97.98 ± 11.44 | 98.62 ± 9.39 |
| Novel stimuli | 105.41 ± 15.45 | 105.44 ± 15.55 | 106.06 ± 15.89 | 103.33 ± 14.13 |
| Target stimuli | 97.10 ± 12.01 | 97.76 ± 13.17 | 98.39 ± 14.12 | 98.33 ± 12.83 |
| Fz electrode | ||||
| N100 amplitude, μV (mean ± SD) | ||||
| Standard stimuli | −4.55 ± 2.47 | −4.24 ± 2.56 | −4.35 ± 2.84 | −3.73 ± 2.25 |
| Novel stimuli | −5.27 ± 4.04 | −4.68 ± 4.08 | −4.98 ± 4.18 | −4.19 ± 4.10 |
| Target stimuli | −7.06 ± 3.89 | −7.08 ± 3.85 | −7.42 ± 4.04 | −7.09 ± 4.20 |
| N100 latency, ms (mean ± SD) | ||||
| Standard stimuli | 100.47 ± 10.43 | 102.24 ± 11.96 | 101.42 ± 12.36 | 101.68 ± 10.82 |
| Novel stimuli | 104.45 ± 14.77 | 103.03 ± 14.42 | 102.76 ± 14.61 | 103.81 ± 14.83 |
| Target stimuli | 98.58 ± 12.00 | 100.92 ± 13.89 | 101.58 ± 14.57 | 99.64 ± 15.28 |
HC = Healthy control subjects; CHR = Clinical High Risk subjects (at Baseline).
CHR-NC = Subjects who did not converted to psychosis by 2 years.
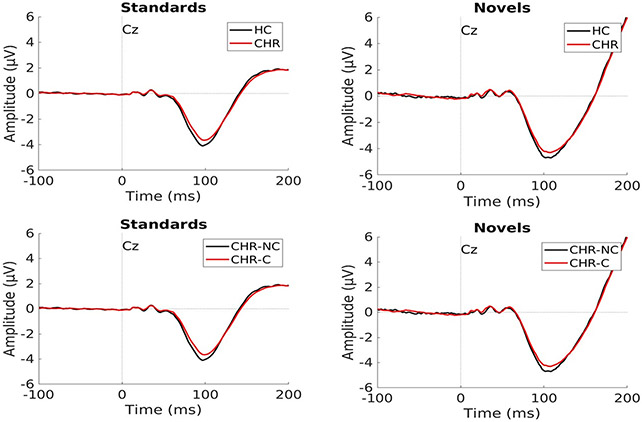
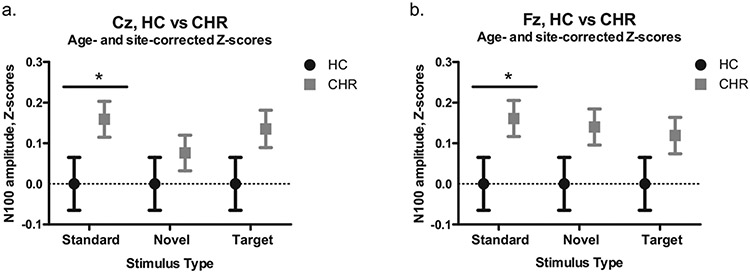
A separate repeated measures Group × Stimulus ANOVA was run for each electrode. At the Cz electrode, neither the Group effect comparing HC to CHR subjects (F(1,786) = 3.05, p = 0.081), nor the Stimulus effect (F(2,1572) = 0.79, p = 0.454) was significant. Because we had hypothesized that different between group effects could emerge for different stimulus types, we proceeded with post hoc testing for this model and found that the CHR group had reduced N100 amplitudes compared to HC for the Standard stimuli (p = 0.049, Cohen’s d = 0.154). Post hoc tests were not significant for the Novel (p = 0.40, Cohen’s d = 0.066) or Target stimuli (p = 0.11, Cohen’s d = 0.125). Fig. 1 shows averaged N100 waveforms for this comparison for Standard and Novel stimuli between HC and CHR, as well as between CHR-C and CHR-NC. Fig. 2a shows means of N100 amplitude z-scores at the Cz electrode.
Fig. 1.
Examples of average N100 waves for Standard and Novel trial types for HC vs. CHR (top) and CHR-C vs. CHR-NC (bottom). Note: blunting of N100 amplitude is evidenced by a larger number, although smaller absolute number, because the N100 is a negative wave.
Fig. 2.
N100 amplitude z-scores for HC and CHR subjects. (a.) Results for the Cz electrode. (b.) Results for the Fz electrode. *p < 0.05.
A repeated measures Group × Stimulus ANOVA run for the Fz electrode indicated that the Group effect was significant such that the CHR subjects had smaller N100 absolute amplitudes than HC subjects (F(1,786) = 4.00, p = 0.046). In this model, Stimulus was not significant (F(2,1572) = 0.42, p = 0.657). Post hoc testing similarly indicated that the CHR group had reduced N100 amplitudes compared to the HC group for the Standard stimuli (p = 0.050, Cohen’s d = 0.153). Post hoc tests were not significant for the Novel (p = 0.057, Cohen’s d = 0.148) or the Target stimuli (p = 0.231, Cohen’s d = 0.093). Fig. 2b shows means of N100 amplitude z-scores at the Fz electrode.
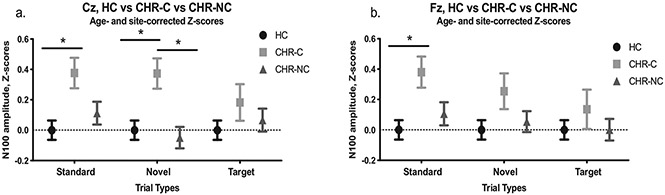
In similarly structured analyses, repeated measures Group × Stimulus ANOVAs comparing three groups (CHR—C, CHR-NC, and HC) were tested at each electrode. The model was significant for Group at the Cz electrode (F(2,531) = 3.58, p = 0.029). Stimulus was not significant in this model (F(2,1062) = 1.56, p = 0. 210). At Cz, Group effects were tested for each stimulus type and found to be significant for Standard (F(2,531) = 3.77, p = 0.024) and Novel (F(2,531) = 5.11, p = 0.006) stimuli, but not for Targets (F(2,531) = 0.87, p = 0.420). For Standard stimuli, post hoc tests showed N100 absolute amplitude to be significantly reduced (i.e., less negative) in CHR-C relative to HC (p = 0.019, Cohen’s d = 0.395) but not significantly different relative to CHR-NC (p = 0.171, Cohen’s d = 0.248). There was no difference between CHR-NC and HC (p = 0.728, Cohen’s d = 0.107; Fig. 3a). For Novel stimuli, post hoc tests showed N100 absolute amplitude to be significantly reduced (i. e., less negative) in CHR-C relative to both HC (p = 0.017, Cohen’s d = 0.392) and CHR-NC (p = 0.005, Cohen’s d = 0.416). These, in turn, did not significantly differ from each other (p = 1.00, Cohen’s d = −0.048; Fig. 3a). For Target stimuli at Cz, no comparisons were significant across the three subject groups (CHR—C, CHR-NC, and HC).
Fig. 3.
N100 amplitude z-scores for HC, CHR—C, and CHR-NC subjects. (a.) Results for the Cz electrode. (b.) Results for the Fz electrode. *p < 0.05; **p < 0.01.
A repeated measures Group × Stimulus ANOVA comparing three groups (CHR—C, CHR-NC, and HC) was tested at Fz. The effect of Group was not significant at the Fz electrode (F(2,531) = 2.42, p = 0.090), but the effect of Stimulus was (F(2,1062) = 3.12, p = 0.045). Group effects were then tested for each stimulus type at Fz and found to be significant for Standard stimuli (F(2,531) = 3.75, p = 0.024). Neither Novel (F(2,531) = 1.79, p = 0.168) nor Target stimuli reached significance (F(2,531) = 0.539, p = 0.584). For Standard stimuli, post hoc tests showed N100 absolute amplitude to be significantly reduced (i.e., less negative) in CHR-C relative to HC (p = 0.019, Cohen’s d = 0.397) but not significantly different relative to CHR-NC (p = 0.152, Cohen’s d = 0.253). There was no difference between CHR-NC and HC (p = 0.818, Cohen’s d = 0.100; Fig. 3b). For Novel and for Target stimuli, post hoc tests did not reveal any significant between-group differences across the three subject groups (CHR—C, CHR-NC, and HC).
3.3. N100 latency
To compare N100 latency z-scores between CHR and HC subjects, we conducted repeated measures Group × Stimulus ANOVAs for each electrode (Table 2). For the Cz model, there was no significant main effect of Group (F(1,786) = 0.07, p = 0.797). Likewise, there was no significant effect of Stimulus (F(2,1572) = 0.08, p = 0.927) or Group × Stimulus (F(1,1572) = 0.08, p = 0.927). Post hoc testing revealed no significant differences between the two groups for any of the stimulus types at the Cz electrode (p > 0.600). For the Fz electrode in a similarly structured ANOVA, there was no significant main effect of Group (F(1,786) = 0.07, p = 0.791). However, there was a significant effect of Stimulus (F(2,1572) = 3.88, p = 0.021) and for Group × Stimulus (F(2,1572) = 3.88, p = 0.021). Post hoc testing revealed a significant difference such that latency for CHR subjects was faster than for the HC subjects to Novel stimuli (p = 0.034, Cohen’s d = −0.165). Latency did not differ between HC and CHR subjects in response to Standard or Target stimuli (P > 0.40).
To compare N100 latency z-scores amongst CHR—C, CHR-NC, and HC subjects at electrode Cz, we conducted a repeated measures Group × Stimulus ANOVA. The main effect of Group was not significant (F(2,531) = 0.30, p = 0.739). Likewise, there was no significant effect of Stimulus (F(2,1062) = 0.25, p = 0.776) or for Group × Stimulus (F(4,1062) = 0.50, p = 0.733). Follow-up univariate models were conducted on CHR-C versus CHR-NC versus HC regarding N100 latency at Cz for each of the three stimulus types, and there were no significant findings.
To compare N100 latency z-scores between CHR—C, CHR-NC, and HC subjects at electrode Fz, we conducted a similarly structured repeated measures Group × Stimulus ANOVA. The main effect of Group was not significant (F(2,531) = 0.44, p = 0.644). Likewise, there was no significant effect of Stimulus (F(2,1062) = 1.59, p = 0.205), Group × Stimulus (F(4,1062) = 1.73, p = 0.141). Follow-up univariate models were conducted on CHR-C versus CHR-NC versus HC regarding N100 latency at Fz for each of the three stimulus types, and there were no significant findings.
3.4. N100 amplitude predicting conversion to psychosis
Logistic regressions were run using N100 amplitude z-scores at electrode Cz and Fz to predict the likelihood of conversion to psychosis in the entire CHR sample. These models found that a unit increase in Cz N100 amplitude increased the likelihood of conversion to psychosis in response to the Standard stimuli by 45 % (OR = 1.45, 95 % CI [1.10, 1.93], p = 0.009) and in response to the Novel stimuli by 64 % (OR = 1.64, 95 % CI [1.22, 2.21], p = 0.001). Cz N100 amplitude to the Target stimuli did not successfully predict conversion (OR = 1.10, 95 % CI [0.86, 1.41], p = 0.451). At the Fz electrode, a one unit increase in Fz N100 amplitude increased the likelihood of conversion to psychosis in response to Standard stimuli by 43 % (OR = 1.43, 95 % CI [1.09, 1.89], p = 0.011). N100 amplitude at the Fz electrode did not predict conversion in response to either Novel stimuli (OR = 1.25, 95 % CI [0.96, 1.64], p = 0.104) or Target stimuli (OR = 1.09, 95 % CI [0.85, 1.41], p = 0.499).
These models were rerun with the addition of SOPS positive scores and SOPS negative scores to examine the effect of these variables on prediction of conversion. The results for N100 amplitude z-scores predicting conversion were virtually identical to the models without SOPS scores. A one unit increase in Cz N100 amplitude increased the likelihood of conversion to psychosis in response to the Standard stimuli by 45 % (OR = 1.45, 95 % CI [1.10, 1.93], p = 0.013) and in response to the Novel stimuli by 63 % (OR = 1.63, 95 % CI [1.20, 2.21], p = 0.002). Cz N100 amplitude to the Target stimuli did not successfully predict conversion (OR = 1.10, 95 % CI [0.85, 1.41], p = 0.492). At the Fz electrode, a one unit increase in Fz N100 amplitude increased the likelihood of conversion to psychosis in response to Standard stimuli by 41 % (OR = 1.41, 95 % CI [1.06, 1.88], p = 0.017). N100 amplitude at the Fz electrode did not predict conversion in response to either Novel stimuli (OR = 1.25, 95 % CI [0.95, 1.65], p = 0.109) or Target stimuli (OR = 1.07, 95 % CI [0.82, 1.40], p = 0.627). For each of these models, greater SOPS positive scores predicted conversion to psychosis (Cz Standard: OR =1.14, 95 % CI [1.07, 1.23], p < 0.001; Cz Novel and Cz Target: OR = 1.15, 95 % CI [1.07, 1.23], p < 0.001; Fz Standard OR = 1.14, 95 % CI [1.07, 1.23], p < 0.001; Fz Novel and Fz Target OR = 1.15, 95 % CI [1.07, 1.23], p < 0.001). In none of these models did SOPS negative scores predict conversion.
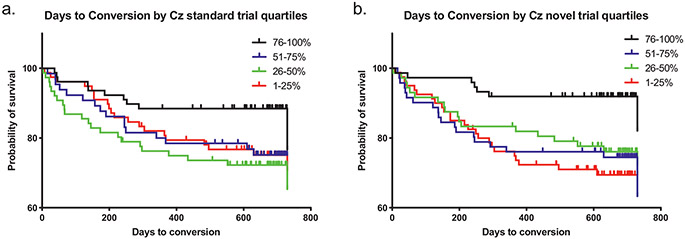
Cox regressions predicting time to conversion to psychosis in the entire CHR sample from N100 amplitude z-scores at electrode Cz and Fz were run separately for each stimulus type, with significance levels confirmed by the Benjamini-Hochberg procedure to correct for multiple comparisons. An earlier conversion to psychosis was predicted by smaller N100 amplitude responses to Standard stimuli (Wald = 6.21, p = 0.013, Exp(B) = 1.39) and to Novel stimuli (Wald = 10.74, p = 0.001, Exp(B) = 1.59) at the Cz electrode. At the Fz electrode, smaller N100 amplitude responses also predicted earlier conversion to psychosis based upon responses to the Novel stimuli at a trend significance (Wald = 3.10, p = 0.079, Exp(B) = 1.26), and significantly to the Standard stimuli (Wald = 5.73, p = 0.017, Exp(B) = 1.36). The regression models for N100 amplitude response to Target stimuli both at the Cz and Fz electrodes were not significant (Cz: Wald = 0.35, p = 0.552, Exp(B) = 1.07; Fz: Wald = 0.367, p = 0.545, Exp(B) = 1.08). Fig. 4 shows the results of these models for Novel (Fig. 4a) and Standard (Fig. 4b) stimuli at the Cz electrode. A greater N100 deficit, a one unit increase in amplitude z-scores, to Standard stimuli decreased the time until conversion to psychosis by 38 %, and to Novel stimuli decreased the time until conversion to psychosis by 57 %. To confirm these results, a holdout analysis was done by repeating these Cox regressions on a randomly selected 90 % of the sample. The results were not changed materially except that prediction by N100 at the Fz electrode to Novel stimuli emerged as significant (Cz Novel: Wald = 10.78, p = 0.001, Exp(B) = 1.65; Cz Standard: Wald = 5.52, p = 0.019, Exp(B) = 1.39; Cz Target: Wald = 1.02, p = 0.313, Exp(B) = 1.14; Fz Novel: Wald = 4.13, p = 0.042, Exp(B) = 1.33; Fz Standard: Wald = 5.06, p = 0.025, Exp(B) = 1.36; Fz Target: Wald = p = 0.298, Exp(B) = 1.15).
Fig. 4.
Cox regressions predicting time to conversion to psychosis in the entire CHR sample from N100 amplitude z-scores (electrode Cz) shown separately for each stimulus type. Quartiles are for z-scores of magnitude at Cz. Note: the smallest quartile is the smallest absolute amplitude of N100 wave. (a.) An earlier conversion to psychosis was predicted by smaller N100 absolute amplitudes to Standard stimuli (p = 0.013). (b.) An earlier conversion to psychosis was predicted by smaller N100 absolute amplitudes to Novel stimuli (p = 0.001).
3.5. N100 variables predicting symptoms
A series of linear regressions split by electrode and by stimulus type were performed with the SOPS positive and SOPS negative symptom subscale scores as the dependent variables and N100 amplitude or latency z-scores as predictor variables to determine whether N100 responses were predictive of symptoms in the CHR group. Dummy variables were included in each model to account for potential effects of site on SOPS scores. Table 3 shows the results of these regressions with N100 amplitude. To clarify these results, because the N100 is a negative wave, blunting of N100 amplitude is evidenced by a larger number, although a smaller absolute value. Regressions with a positive B mean that a smaller N100 absolute amplitude is associated with higher symptom scores. After Benjamini-Hochberg correction for multiple comparisons, higher SOPS positive symptoms were predicted by smaller absolute N100 amplitudes to Standard stimuli at Fz (B = 0.469, p = 0.025) but not at Cz (B = 0.429, p = 0.041). Other regressions on SOPS positive symptoms were not significant. Regressions indicated that lower absolute N100 amplitudes in the Cz electrode in response to all stimuli types were associated with greater SOPS negative symptom scores (Standard: B = 0.740, p = 0.003; Novel: B = 0.598, p = 0.017; Target: B = 0.548, p = 0.023). At the Fz electrode, lower absolute N100 amplitude responses to Standard (B = 0.721, p = 0.003) and Novel (B = 0.822, p = 0.001) stimuli were also significantly associated with greater SOPS negative symptoms, but N100 responses to Target stimuli did not predict SOPS negative symptoms (B = 0.383, p = 0.124). No regressions for N100 latency predicting SOPS symptoms were significant.
Table 3.
Symptoms associated with N100 amplitude.
| SOPS positive |
SOPS negative |
|||||
|---|---|---|---|---|---|---|
| Ba | R- squared |
p Value |
Ba | R- squared |
p Value |
|
| Cz-N100 amplitude | ||||||
| Standard stimuli | 0.429 | 0.034 | 0.041 | 0.740 | 0.011 | 0.003b |
| Novel stimuli | 0.306 | 0.031 | 0.150 | 0.598 | 0.007 | 0.017b |
| Target stimuli | 0.272 | 0.031 | 0.187 | 0.548 | 0.007 | 0.023b |
| Fz-N100 amplitude | ||||||
| Standard stimuli | 0.469 | 0.035 | 0.025b | 0.721 | 0.011 | 0.003b |
| Novel stimuli | 0.438 | 0.034 | 0.040 | 0.822 | 0.014 | 0.001b |
| Target stimuli | 0.232 | 0.030 | 0.280 | 0.383 | 0.003 | 0.124 |
Values are unstandardized B in regression models.
Significant after Benjamini-Hochberg correction for multiple comparisons.
4. Discussion
In this large multi-site cohort of subjects at clinical high risk for psychosis, auditory N100 amplitude, an established biomarker and candidate genetic endophenotype for schizophrenia (Brockhaus-Dumke et al., 2008; Rosburg et al., 2008; Turetsky et al., 2008), is reduced (i.e. has a smaller absolute amplitude) in CHR individuals compared to HC. This indicates that N100 amplitude reduction is present in at-risk individuals who have yet to develop schizophrenia. Amongst the CHR cohort, those who converted to psychosis had reduced N100 amplitudes compared to HCs and to those CHR subjects who did not convert to a psychotic disorder during the two years of study follow up. Furthermore, greater N100 amplitude deficits in CHR individuals predicted both a greater likelihood of subsequent conversion to psychosis and reduced time until psychosis onset. Specifically, a greater N100 deficit to stimuli decreased the time until conversion to psychosis by 38 % for Standard stimuli and 57 % for Novel stimuli. Finally, in the CHR group, smaller N100 amplitudes predicted greater positive and negative symptom severity.
The auditory N100 is an ERP evoked by acoustic stimuli (Naatanen and Picton, 1987; Rosburg et al., 2008). Auditory N100 generators are widely distributed, with a major source in the auditory cortex bilaterally and additional contributions from frontal and parietal motor areas (Naatanen and Picton, 1987; Rosburg et al., 2008). Our results cannot determine if the impairment in CHR and CHR-C responses is due to a specific network component or more generalized dysfunction within this large, distributed network.
Our results concerning the association of N100 amplitudes with symptoms should be viewed in the context of inconsistent results in prior publications regarding SCZ as reviewed in Rosburg et al. (2008). As per this review, the majority of studies did not detect significant associations between N100 amplitude and symptoms, although a small number of studies reported modest associations of smaller N100 absolute amplitudes with greater symptom severity (Ford et al., 1999; Gallinat et al., 2002; Valkonen-Korhonen et al., 2003; Sumich et al., 2006). One study in a CHR cohort found that the absolute amplitude of the N100 decreased as psychotic symptomatology increased (Gonzalez-Heydrich et al., 2015). Our results on symptom associations with N100 amplitudes differed for the two electrodes: specifically, lower absolute amplitudes in response to Standard stimuli at Fz but not at Cz predicted worse positive symptoms on the SOPS. The signal at Fz would be expected to reflect more frontal EEG activity than the signal at Cz. We speculate that this difference in results between Fz and Cz could reflect differentially impaired frontal circuitry in those with more severe positive symptoms. On the other hand, worse SOPS negative symptoms were predicted by lower N100 amplitudes to all stimuli at Cz, but only lower amplitude responses to Standard and Novel stimuli predicted negative symptoms at Fz. This electrode difference is rather counterintuitive given that frontal pathology is associated with negative symptoms in SCZ (Wolkin et al., 1992).
Ours is the largest cohort to date using follow up period to determine the ability of N100 amplitude to predict conversion to psychosis. The current finding complements a quite consistent literature that indicates impaired N100 amplitude in SCZ compared to healthy controls, although there have been some negative studies (Brockhaus-Dumke et al., 2008; see review in Rosburg et al., 2008). A smaller number of prior studies have studied subjects at high risk for SCZ. Between-group comparisons are less definitive than studies that include baseline N100 measures and a later clinical outcome determination. In a study of subjects at high risk for psychosis, baseline N100 amplitude was smaller in those who developed psychosis (n = 18) than in controls (n = 28) or those who did not develop psychosis (n = 43) three years after baseline, although the number of subjects in the first group was rather modest (van Tricht et al., 2015). Hsieh et al. examined N100 amplitude in high-risk subjects but did not find a significant difference in baseline N100 between converters (n = 11) and nonconverters (n = 19) to psychosis at two years (Hsieh et al., 2012). Similarly, Brockhaus-Dumke et al. did not find a difference in N100 amplitude in prodromal SCZ subjects who later transitioned to psychosis (n = 21) or in those high-risk subjects who did not transition to psychosis (n = 18) compared to controls (n = 6) (Brockhaus-Dumke et al., 2008).
This work adds to a growing literature regarding predictors of psychosis in at-risk subjects. Mismatch negativity is an event-related potential to oddball stimuli that is well known to be impaired in SCZ (Erickson et al., 2016; Umbricht and Krljes, 2005) and predict conversion to psychosis (Bodatsch et al., 2011; Naatanen et al., 2015; Perez et al., 2014). The P300 wave amplitude is another ERP reduced in SCZ (Bramon et al., 2004; Ford et al., 1999; Jeon and Polich, 2003), reductions of which predicted conversion to psychosis in a high-risk sample (Bramon et al., 2008). Conversely, in NAPLS CHR subjects who also met criteria for autism spectrum disorder (Foss-Feig et al., 2021), larger P300 amplitudes predicted conversion. Prepulse inhibition of the acoustic startle response has been extensively studied and found to be heritable (Greenwood et al., 2007; Hasenkamp et al., 2010) and impaired in SCZ (Braff et al., 2001; Swerdlow et al., 2006). This measure did not predict conversion to psychosis, but latency of startle, which is slower in SCZ subjects than controls (Fargotstein et al., 2018) and heritable (Hasenkamp et al., 2010), was another significant predictor of conversion to psychosis in our NAPLS cohort (Cadenhead et al., 2020). Other significant predictors in our NAPLS cohort included reduced processing speed, impaired verbal memory (Seidman et al., 2010), attention and working memory (Seidman et al., 2016), and brain volume reduction (Chung et al., 2019). Additionally, fifteen analytes associated with immune function, hypothalamic-pituitary-adrenal axis (HPA) function, or oxidative stress also predicted psychosis in our cohort (Perkins et al., 2015). Finally, a risk calculator was developed that incorporates several symptom, course, and cognitive variables to predict conversion to psychosis in this cohort (Cannon et al., 2016). These varied findings indicate that a spectrum of abnormalities in brain functioning, spanning from structural to psychophysiological to cognitive measures, are present in those at risk before the onset of frank psychosis (Cannon et al., 2016). A question for future research in this area is whether a common pathway of molecular aberrations may underlie these disparate abnormalities.
Strengths of this study include the large cohort size, extensive phenotyping of the subjects, a concurrent attention task imbedded within the paradigm (known to enhance N100 amplitude (Hillyard et al., 1973; Rosburg et al., 2008)), and two year follow up to determine whether conversion to psychosis occurred during this time frame. A limitation of the study is that a fixed 1250 ms stimulus onset asynchrony between trials is less than the duration thought to maximize N100 responses (Davis et al., 1966).
Our results indicate that pathophysiological alterations in auditory cortical circuitry subserving processing of sounds predict future conversion to psychosis in CHR individuals. The fact that this reduction precedes conversion to psychosis highlights the utility of N100 amplitude as a biomarker of psychosis risk and an endophenotype of SCZ. A future direction of this work will be to evaluate the relationship of N100 amplitude with cognitive function in this cohort.
Acknowledgements
Supported by the National Institute of Mental Health (grant U01MH081984 to Dr. Addington; grants U01 MH081928; P50 MH080272; Commonwealth of Massachusetts SCDMH82101008006 to Dr. Seidman; grants R01 MH60720, U01 MH082022 and K24 MH76191 to Dr. Cadenhead; grant MH081902 and U01MH081902 to Dr. Cannon; grant P50 MH066286 and support from the Staglin Family Music Festival for Mental Health to Dr. Bearden; grant U01MH076989 to Dr. Mathalon; grant U01MH082004-01A1 to Dr. Perkins; grant U01MH081988 to Dr. Walker; grant U01MH082022 to Dr. Woods; and U01 MH081857-05 grant to Dr. Cornblatt). Additional NIH funding to Dr. Duncan supported these analyses (grant 1R01MH117315-01A1).
Footnotes
Credit authorship contribution statement
BJR, HKH, PMB, AB, REC, JKJ, GAL, MAN, JA, CEB, KSC, TDC, BAC, THM, DOP, MTT, EFW, SWW, and DHM designed the study and obtained funding to conduct the study. Data were collected by BJR, HKH, PMB, AB, REC, JKJ, GAL, MAN, JA, CEB, KSC, TDC, BAC, THM, DOP, MTT, EFW, SWW, and DHM. Secondary analyses of N100 data were designed by ED, NM, HKH, and DHM. The data were analyzed and interpreted by ED, NM, HKH, and DHM. The manuscript was written by ED and NM. All authors reviewed and approved the final manuscript.
Declaration of competing interest
Infrastructure support was provided by the Office of Research and Development, the Mental Health Service Lines, and the Center of Visual and Neurocognitive Rehabilitation at the Atlanta Veterans Affairs Medical Center, Decatur, GA. Additional infrastructure support was provided by the Department of Psychiatry and Behavioral Sciences of the Emory University School of Medicine, Atlanta, GA. ED has received research support for work unrelated to this project from Auspex Pharmaceuticals, Inc. and Teva Pharmaceuticals, Inc. DHM is a consultant for Boehringer Ingelheim and Cadent Therapeutics. Other authors have nothing to disclose. ED is a full-time attending psychiatrist in the Mental Health Service Line at the Atlanta Veterans Affairs Medical Center, Decatur, GA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs.
References
- Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Addington JA, Cannon TD, 2012. North american prodrome longitudinal study (NAPLS 2): overview and recruitment. Schizophr. Res 142 (1–3), 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodatsch M, Ruhrmann S, Wagner M, Muller R, Schultze-Lutter F, Frommann I, Brinkmeyer J, Gaebel W, Maier W, Klosterkotter J, Brockhaus-Dumke A, 2011. Prediction of psychosis by mismatch negativity. Biol. Psychiatry 69 (10), 959–966. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR, 2001. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacol 156, 234–258. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S, 2004. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr. Res 70 (2–3), 315–329. [DOI] [PubMed] [Google Scholar]
- Bramon E, Shaikh M, Broome M, Lappin J, Berge D, Day F, Woolley J, Tabraham P, Madre M, Johns L, Howes O, Valmaggia L, Perez V, Sham P, Murray RM, McGuire P, 2008. Abnormal P300 in people with high risk of developing psychosis. NeuroImage 41 (2), 553–560. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, Klosterkoetter J, Ruhrmann S, 2008. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol. Psychiatry 64 (5), 376–384. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Duncan E, Addington J, Bearden C, Cannon TD, Cornblatt BA, Mathalon D, McGlashan TH, Perkins DO, Seidman LJ, Tsuang M, Walker EF, Woods SW, Bauchman P, Belger A, Carrion RE, Donkers F, Johannesen J, Light G, Niznikiewicz M, Nunag J, Roach B, 2020. Evidence of slow neural processing, developmental differences and sensitivity to cannabis effects in a sample at clinical high risk for psychosis from the NAPLS consortium assessed with the human startle paradigm. Front. Psychiatry 11, 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, Heinssen R, Jeffries CD, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Kattan MW, 2016. An individualized risk calculator for research in prodromal psychosis. Am. J. Psychiatry 173 (10), 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Allswede D, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Seidman LJ, Tsuang M, Walker E, Woods SW, McEwen S, van Erp TGM, Cannon TD, North American Prodrome Longitudinal Study C., 2019. Cortical abnormalities in youth at clinical high-risk for psychosis: findings from the NAPLS2 cohort. Neuroimage Clin. 23, 101862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H, Mast T, Yoshie N, Zerlin S, 1966. The slow response of the human cortex to auditory stimuli: recovery process. Electroencephalogr. Clin. Neurophysiol 21 (2), 105–113. [DOI] [PubMed] [Google Scholar]
- del Re EC, Spencer KM, Oribe N, Mesholam-Gately RI, Goldstein J, Shenton ME, Petryshen T, Seidman LJ, McCarley RW, Niznikiewicz MA, 2015. Clinical high risk and first episode schizophrenia: auditory event-related potentials. Psychiatry Res. 231 (2), 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Ruffle A, Gold JM, 2016. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol. Psychiatry 79 (12), 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargotstein M, Hasenkamp W, Gross R, Cuthbert B, Green A, Swails L, Lewison B, Boshoven W, Keyes M, Duncan E, 2018. The effect of antipsychotic medications on acoustic startle latency in schizophrenia. Schizophr. Res 198, 28–35. [DOI] [PubMed] [Google Scholar]
- First M, Williams J, Karg R, Spitzer R, 2017. Structured Clinical Interview for DSM-5 (SCID-5 for DSM-5). American Psychiatric Association, Arlington, VA. [Google Scholar]
- Ford JM, Mathalon DH, Marsh L, Faustman WO, Harris D, Hoff AL, Beal M, Pfefferbaum A, 1999. P300 amplitude is related to clinical state in severely and moderately ill patients with schizophrenia. Biol. Psychiatry 46 (1), 94–101. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Guillory SB, Roach BJ, Velthorst E, Hamilton H, Bachman P, Belger A, Carrion R, Duncan E, Johannesen J, Light GA, Niznikiewicz M, Addington JM, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan T, Perkins D, Seidman LJ, Stone WS, Tsuang M, Walker EF, Woods S, Bearden CE, Mathalon DH, 2021. Abnormally large baseline p300 amplitude is associated with conversion to psychosis in clinical high risk individuals with a history of autism: a pilot study. Front Psychiatry 12, 591127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Mulert C, Bajbouj M, Herrmann WM, Schunter J, Senkowski D, Moukhtieva R, Kronfeldt D, Winterer G, 2002. Frontal and temporal dysfunction of auditory stimulus processing in schizophrenia. NeuroImage 17 (1), 110–127. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Heydrich J, Bosquet Enlow M, D’Angelo E, Seidman LJ, Gumlak S, Kim A, Woodberry KA, Rober A, Tembulkar S, Graber K, O’Donnell K, Hamoda HM, Kimball K, Rotenberg A, Oberman LM, Pascual-Leone A, Keshavan MS, Duffy FH, 2015. Early auditory processing evoked potentials (N100) show a continuum of blunting from clinical high risk to psychosis in a pediatric sample. Schizophr. Res 169 (1–3), 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ, 2007. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch. Gen. Psychiatry 64 (11), 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton HK, Roach BJ, Bachman PM, Belger A, Carrion RE, Duncan E, Johannesen JK, Light GA, Niznikiewicz MA, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Cannon TD, Mathalon DH, 2019. Association between P300 responses to auditory oddball stimuli and clinical outcomes in the psychosis risk syndrome. JAMA Psychiatry 76 (11), 1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Epstein MP, Green A, Wilcox L, Boshoven W, Lewison B, Duncan E, 2010. Heritability of acoustic startle magnitude, prepulse inhibition, and startle latency in schizophrenia and control families. Psychiatry Res. 178 (2), 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW, 1973. Electrical signs of selective attention in the human brain. Science 182 (4108), 177–180. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Shan JC, Huang WL, Cheng WC, Chiu MJ, Jaw FS, Hwu HG, Liu CC, 2012. Auditory event-related potential of subjects with suspected prepsychotic state and first-episode psychosis. Schizophr. Res 140 (1–3), 243–249. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J, 2003. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiol 40 (5), 684–701. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Walsh BC, Woods SW, 2010. The Psychosis-risk Syndrome: Handbook for Diagnosis and Follow-up. Oxford University Press, New York. [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW, 2003. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull 29 (4), 703–715. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T, 1987. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiol 24 (4), 375–425. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Shiga T, Asano S, Yabe H, 2015. Mismatch negativity (MMN) deficiency: a break-through biomarker in predicting psychosis onset. Int. J. Psychophysiol 95 (3), 338–344. [DOI] [PubMed] [Google Scholar]
- Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, Mathalon DH, 2014. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol. Psychiatry 75 (6), 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, Mathalon DH, McGlashan TH, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Heinssen R, 2015. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr. Bull 41 (2), 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Fribance SN, Coffman BA, Salisbury DF, 2021. Deficits in attentional modulation of auditory N100 in first-episode schizophrenia. Eur. J. Neurosci 53 (8), 2629–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN, Ford JM, 2008. Reduced auditory evoked potential component N100 in schizophrenia–a critical review. Psychiatry Res. 161 (3), 259–274. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RS, Heinssen R, Cornblatt BA, North American Prodrome Longitudinal Study G., 2010. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry 67 (6), 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Mathalon DH, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, 2016. Association of neurocognition with transition to psychosis: baseline functioning in the second phase of the north american prodrome longitudinal study. JAMA Psychiatry 73 (12), 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumich A, Harris A, Flynn G, Whitford T, Tunstall N, Kumari V, Brammer M, Gordon E, Williams LM, 2006. Event-related potential correlates of depression, insight and negative symptoms in males with recent-onset psychosis. Clin. Neurophysiol 117 (8), 1715–1727. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL, 2006. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch. Gen. Psychiatry 63 (12), 1325–1335. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Calkins ME, 2008. Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol. Psychiatry 64 (12), 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Krljes S, 2005. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr. Res 76 (1), 1–23. [DOI] [PubMed] [Google Scholar]
- Valkonen-Korhonen M, Purhonen M, Tarkka IM, Sipila P, Partanen J, Karhu J, Lehtonen J, 2003. Altered auditory processing in acutely psychotic never-medicated first-episode patients. Brain Res. Cogn. Brain Res 17 (3), 747–758. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, Koelman JTM, Mensink AJM, Bour LJ, van der Meer JN, van Amelsvoort TA, Linszen DH, de Haan L, 2015. Sensory gating in subjects at ultra high risk for developing a psychosis before and after a first psychotic episode. World J. Biol. Psychiatry 16 (1), 12–21. [DOI] [PubMed] [Google Scholar]
- Wolkin A, Sanfilipo M, Wolf AP, Angrist B, Brodie JD, Rotrosen J, 1992. Negative symptoms and hypofrontality in chronic schizophrenia. Arch. Gen. Psychiatry 49 (12), 959–965. [DOI] [PubMed] [Google Scholar]
- Woods SW, Walsh BC, Powers AR, McGlashan TH, 2019. Reliability, validity, epidemiology, and cultural variation of the structured interview for psychosis-risk syndromes (SIPS) and the scale of psychosis-risk symptoms (SOPS). In: Li H, Shapiro D, Seidman L (Eds.), Handbook of Attenuated Psychosis Syndrome Across Cultures. Springer Nature Switzerland, ChamSwitzerland. [Google Scholar]