Abstract
Background
There is a need for a targeted, comprehensive, minimally invasive myocardial restoration treatment aimed at patients with chronic postinfarction heart failure that can provide a sustained effect and be conveniently adopted with transcatheter techniques. Here we evaluated the effectiveness of a platelet-rich plasma hydrogel-based, cell-free therapeutic compound delivered with the aid of a 3-dimensional electromechanical mapping and catheter-based technique (NOGA) in a porcine translational model.
Methods
We assessed the feasibility of targeted, minimally invasive transcatheter NOGA-guided injections of the therapeutic compound in myocardial infarction (MI) survivors at 8 weeks post-MI.
Results
Animals undergoing NOGA-guided hydrogel injections at 8 weeks post-MI demonstrated a significant improvement of the selected left ventricular parameters at a 12-week follow-up. Compared to nonintervention, the hydrogel-based therapy provided significant improvements in end-diastolic volume (−11.0% ± 11.1% vs 6.3% ± 15.2%; P = .008) and ejection fraction (−9.1% ± 16% vs 12.7% ± 18.6%; P = .009). In the slice closest to the apex, significant differences in scar area were observed; the treatment group demonstrated a smaller mean scar area in the infarcted zone compared with the control group (47.1% ± 7.0% vs 59% ± 8.2%; P = .013) and a smaller mean scar area in the border zone compared with the saline group (31.4% ± 8.3% vs 42.6% ± 9.0%; P = .016).
Conclusions
The study implies a translational potential of the hydrogel-based therapy and should trigger clinical trials focused on establishing a restoration therapy that can be integrated into a clinical protocol.
Key Words: myocardial infarction, transcatheter, hydrogel, NOGA, myocardial restoration

A method of evaluation of a platelet-rich plasma hydrogel-based compound in postinfarction heart failure.
Central Message.
Percutaneous guided injections of the hydrogel-based cell-free therapeutic compound are safe and feasible and may translate into improved ejection fraction in subjects suffering from postinfarction heart failure.
Perspective.
Transcatheter-targeted myocardial restoration implies a translational potential of hydrogel-based therapy. Compared to nonintervention, the hydrogel-based therapy provided significant improvement and demonstrated a smaller scar area in the infarcted zone, triggering the need for clinical trials focused on establishing a restoration therapy.
Heart failure (HF) following myocardial infarction (MI) constitutes a growing burden for individual patients, societies, and public health systems, with high mortality and morbidity rates.1, 2, 3, 4 Currently available therapies fail to reverse the loss of functional cardiac tissue, and thus the fundamental pathology of the clinical entity remains unaddressed. The search for an alternative treatment for myocardial ischemia and its sequelae has seen an array of attempts to restore the myocardium by cell and compound injections of all types, mostly without robust effect and without wide clinical uptake.5
We previously addressed the significance of structural support to the injured myocardium beyond cellular interventions and codeveloped injectable and hence clinically more adoptable compounds.6,7 To date, studies by us and others have demonstrated that such compounds can trigger certain improvements in myocardial viability and function in small animals, as well as in large animal models.7,8 Available data indicate that hydrogels are promising, nontoxic vehicles for sustained engraftment, at least during the critical phase of the ischemic cycle.7 However, we should take into account that many patients present late after the acute event of an infarction, which may vary from 6 to 8 weeks based on the clinical scenario. A late percutaneous intervention, introduced long after the acute injury (8 weeks), has yielded positive results.8, 9, 10 Early revascularization interventions during the acute infarct may add hazards.11
Ischemic HF is a complex clinical condition, and an effective myocardial restoration therapy needs to be based on a multifactorial approach rather than on monotherapy.6 HF involves acute and chronic phases, which unfold through cell death, ischemic and nonischemic expansion of the infarct, release of free radicals, autophagy, and apoptosis. As a result, scar tissue forms, and microscopic and macroscopic reorganization of the left ventricular (LV) wall may result in an aneurysm.12 This adverse remodeling can progress over several months after acute MI; thus, there is value in a restoration treatment that can offer structural benefits even when introduced as late as several weeks after the acute stage of MI.
The Laplace law applies here, aggravating circumferential wall stress, ventricular dysfunction, and progressive adverse ventricular remodeling that ultimately may lead to HF. This underscores the need to comprehensively address both architectural and paracrine events in the failing heart. Stimulation of paracrine pathways, crucial for restorative effect, can be achieved by delivery of platelet-rich plasma (PRP).13, 14, 15 As a rich source of autologous growth factors stimulating the survival and recovery of damaged tissue, PRP promotes angiogenesis and attenuates adverse cardiac remodeling.
Following the promising results of use of an autologous PRP hyaluronic acid (HA)-based hydrogel in the acute stage of MI in a porcine model, we studied the effectiveness of the compound in improving cardiac function and attenuating adverse cardiac remodeling in the chronic phase of the disease, replicating the chronic coronary patient following ischemic heart injury, with the aim of developing a clinically practical procedure.
Methods
This study was approved by the Institutional Animal Care and Use Committee of the National University of Singapore (Protocol R17-1228; approved January 31, 2018), and carried out in accordance with established guiding principles for animal research. The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Female Yorkshire pigs were obtained for the Department of Comparative Medicine from the National Large Animal Facility of Singapore. Informed consent was not necessary. All studies were conducted at the Cardiac Surgery Research Laboratory between January 2018 and January 2021. According to Office of Safety, Health & Environment guidelines, all data were collected prospectively and in compliance with standard biological hazards tissue handling procedures at a Biosafety Level 3 Laboratory facility.
Study Groups
Out of 38 female Yorkshire pigs (Sus scrofa) enrolled, 24 animals survived MI induction; 2 were sacrificed 2 weeks after saline injection, 1 died at 8 weeks during NOGA (NOGA XP Cardiac Navigation System; Biologics Delivery Systems, a Johnson & Johnson company) mapping evaluation, and the remaining 21 animals survived for 12 weeks following MI induction and were included in the final analysis. Seven animals were assigned to the control (sham) group (no intramyocardial injections), 6 to the saline (placebo) group (NOGA-guided injections of normal saline), and 8 to the treatment group (NOGA-guided injections of the therapeutic compound). The NOGA (Biologics Delivery Systems)-guided injections were performed at 8 weeks post-MI. At 12 weeks post-MI, all the animals were reevaluated and then euthanized. The distribution of the study animals is shown in Figure E1.
Figure E1.
Study flow diagram showing the evaluation and investigation of the study animals over 12 weeks. MI, Myocardial infarction; LCX, left circumflex branch; LAD, left anterior descending artery.
Animal Model of MI
MI was induced by intracoronary coil implantation in the mid-left anterior descending artery (LAD). The procedure was performed under general anesthesia using midazolam, ketamine, and isoflurane at recommended doses and in accordance with Institutional Animal Care and Use Committee protocol. Vascular access was obtained via the femoral approach using the Seldinger technique. Intravenous heparin at 100 U/kg was administered at the beginning of the procedure. The left coronary ostium was selectively cannulated with a 6 Fr Amplatz Right 1.0 guiding catheter (Medtronic), and angiography was performed to determine the mid LAD site for coil implantation. After a Whisper MS coronary guidewire (Abbott) was passed into the distal LAD, a Cantata microcatheter (Cook Medical) was introduced into the mid-LAD. Subsequently, a Nester or Tornado embolization coil (Cook Medical) was deployed into the target site via the microcatheter.
The procedure was terminated after complete occlusion of the mid LAD was achieved (Thrombosis in Myocardial Infarction risk score 0), as confirmed by coronary angiography (Figure E2). Oral amiodarone was administered periprocedurally to reduce arrhythmic complications. Additional intravenous amiodarone and/or metoprolol were administered to treat intraprocedural malignant arrhythmias on an as-needed basis. To maximize survival and reduce early arrhythmic mortality, the animals were monitored postprocedurally with hemodynamic monitoring and continuous telemetric electrocardiographic monitoring under general anesthesia for 6 hours after MI. Furthermore, 125 mg of carprofen (a nonsteroidal anti-inflammatory drug) and 1000 mg of antibiotic (amoxycillin with clavulanic acid) were given orally for 7 days after the procedure.
Figure E2.
Fluoroscopy of the experimental large animal in left anterior oblique cranial view showing engagement of the left anterior descending artery (LAD) with a 6 Fr Amplatz right 1.0 guiding catheter (A1), initiation of deployment of a Nester or Tornado embolization coil (Cook Medical) into the mid-LAD region (A2), and completion view of the successful coil embolization and complete occlusion of the LAD (A3).
NOGA-Guided Mapping and Injections
The 3-dimensional (3D) NOGA mapping system simultaneously analyzes the electrical and mechanical activities of the left ventricle, assesses myocardial viability, and precisely localizes infarct and peri-infarct regions.16 Based on the generated 3D reconstruction of the left ventricle and using the NOGA-Myostar injection catheter, precise injections of therapeutic compounds into the myocardium can be performed.17
The NOGA mapping and intramyocardial injections were performed at 8 weeks following induction of MI. The animals were placed under general anesthesia, and prior to the NOGA-guided procedure, cardiac MRI and echocardiography were performed. After gaining femoral arterial access with the use of the Seldinger technique, a 7 Fr NOGA mapping catheter was advanced across the aortic valve into the left ventricle under fluoroscopic guidance. Then mapping points were obtained until all endocardial segments were sampled, and a complete 3D silhouette of the chamber was produced. The electromechanical map visually demonstrated infarct zones, peri-infarct areas (border zones) as well as healthy heart tissue (Figure 1, A).
Figure 1.
A, Three-dimensional dynamic visualization of the scar area (<0.5 mV) (red) and viable tissue (>1.5 mV) (purple). B, Electromechanical assessment showing viability in the left column. The dense scar is visible at the apex and the anteroseptal wall; surrounding areas show a wall motion deficit (red in linear local shortening [LLS]): B1, unipolar voltage map; B2, bullseye view of unipolar voltage map; B3, wall movement map (LLS%); B4, LLS% bullseye view. C1, Intramyocardial injections; 10 injections within the infarct zone and the remaining 10 within the peri-infarct zone, identified as brown dots. C2 to C4, Electrical conduction propagation showing the delayed activation in the scar area (left anterior descending artery territory). D, Visualization of dyskinetic zones: D1, volume–time graph showing synchronous left ventricular (LV) volume (yellow line) and local wall movement (white line); D2, dyssynchronous LV volume (yellow line) and local wall movement (white line).
Intramyocardial injections were performed with an 8 Fr NOGA-Myostar injection catheter, comprising a 27-gauge needle and a core lumen inside the catheter. The needle extension length was adjusted at 0 to 90-degrees flex, guided by the thickness of the infarcted myocardium measured on preprocedural echocardiography and MRI. The NOGA-Myostar injection catheter was advanced to the target areas of the left ventricle, and an average of 20 injection target points were selected based on the 3D electromechanical map (Figure 1, B1-B4). Ten injections were performed within the infarct zone, and 10 injections were performed within the peri-infarct zone. The areas of interest were identified based on the NOGA electromechanical mapping (Figure 1, C1-C4). The needle was advanced into the myocardium once the catheter tip demonstrated stable electrical and fluoroscopic contact with the LV endocardium. This was followed by injection of 0.3 mL of the therapeutic or control agent. The procedure was terminated after all intramyocardial injections were completed (20 injections with a total volume of 6 mL).
After the procedure, the animals received 125 mg of carprofen (a nonsteroidal anti-inflammatory drug) and 1000 mg of antibiotic (amoxycillin with clavulanic acid) orally for 7 days. Following the procedure, the NOGA map enabled analysis of the local wall motion, and the dyssynchronous LV volume and wall motion was identified using the volume–time graph. The scar area and total dyskinetic area were extrapolated from the NOGA mapping evaluation in the bullseye view (Figure 1, B2, B4, D1, and D2).
Therapeutic Compound Preparation
Hydrogel preparation
The HA-based hydrogel for intramyocardial injection was prepared using an Extracel-HP Kit (Glycosan BioSystems). This HA hydrogel kit consists of 4 separate components: Heprasil (thiol-modified sodium hyaluronate with thiol-modified heparin), Gelin-S (thiol-modified gelatin), Extralink (polyethyleneglycol diacrylate), and degassed water. The pure hydrogel was prepared according to the manufacturer's instructions. In brief, all components were resuspended in predetermined volumes of degassed water. The Heprasil and Gelin-S solutions were mixed at a 1:1 volume ratio at room temperature to form an HA:gelatin solution, which was then cross-linked by adding the Extralink solution as a cross-linker. Importantly, the hydrogel was enriched with ascorbic acid (50 mg/L), as described previously.7
PRP Preparation
Autologous PRP was collected from the Yorkshire pigs on the day of treatment. A blood volume of 140 mL was withdrawn from the venous line and dispensed into disposable tubes (RegenKit THT tubes; RegenLab). These tubes feature a gel layer for the separation of blood components and contain citrate as an anticoagulant. All the tubes were centrifuged at 1200 × g (corresponding to 2500 rpm) for 7 minutes at room temperature, using PRP-Centri centrifuge (RegenLab). After the first centrifugation, red blood cells were separated from plasma and a pellet of platelets by the gel layer. To collect platelets out of the RegenKit THT tubes, the platelet pellet was resuspended in the supernatant. The plasma containing platelets was then transferred to clean 15-mL Falcon tubes and centrifuged again at 3500 rpm for 15 minutes to separate platelets from plasma, which then became platelet-poor plasma. The separated PRP was sent for a complete blood count analysis at the Veterinary Diagnostic Laboratory, Department of Comparative Medicine, National University of Singapore, using a CELL-DYN3700 multiparameter automated hematology analyzer (Abbott). For intramyocardial injection purposes, the pellet of platelets was then resuspended in platelet-poor plasma to form PRP at a concentration of 3 × 106 platelets/μL. Platelets were activated with bovine thrombin before injection. For PRP loaded in a hydrogel, bovine thrombin and the pellet of platelets were resuspended in the HA:gelatin solution and titrated carefully to reach a final concentration of 3 × 106 platelets/μL before being mixed with Extralink. A volume of 6 mL per animal was used for intramyocardial injection.
Echocardiography
LV function and geometry were evaluated under general anesthesia by transthoracic echocardiography at baseline, 8 weeks post-MI, and 12 weeks post-MI, using the Vivid S6 system with a 3.5-MHz probe (GE Healthcare). Parasternal long- and short-axis views were obtained with both M mode and 2-dimensional mode (Figure E3). LV end-diastolic volume (EDV), end-systolic volume (ESV), and ejection fraction (EF) were calculated using the method of Simpson.18 Offline analyses were performed using EchoPac version BT06 (GE VingMed), and data from 3 consecutive cardiac cycles were averaged.
Figure E3.
A, Periprocedural transthoracic echocardiography at 8 weeks after myocardial infarction (MI): A1, parasternal long-axis view; A2, pulsed-wave (PW) Doppler measurement; A3, short-axis view. B, Periprocedural transthoracic echocardiography at 12 weeks post-MI: B1, parasternal long-axis view; B2, PW Doppler measurement; B3, short-axis view. C, Statistical comparison of the changes from 8 weeks to 12 weeks post-MI in the study group: C1, end-diastolic volume (EDV) (P = .173); C2, end-systolic volume (ESV) (P = .292); C3, ejection fraction (EF) (P = .920).
Cardiac MRI
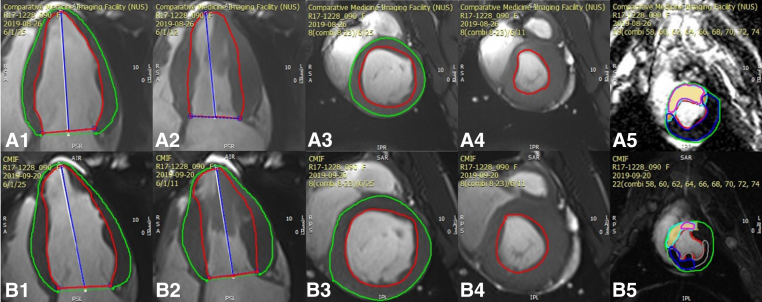
Cardiac MRI was performed at 8 weeks and 12 weeks post-MI using a 3-T MRI scanner (MAGNETOM Skyra; Siemens Healthineers AG). For the saline and therapeutic groups, the imaging modality was used prior to the NOGA-guided injections (Figure 2, A1-A4). Several measurement methods were used to evaluate LV function; however, indices obtained in short-axis view were chosen because of better data comparability.19 The standard MRI protocol included cine imaging, namely global and regional LV systolic function, including volume, mass, EF, and T1-weighted mapping (Figure 2, B1-B4). Late gadolinium enhancement was performed (Figure 2, A5 and B5) to evaluate scar volume within the affected myocardium.20
Figure 2.
A, Cardiac magnetic resonance imaging (MRI) at 8 weeks after myocardial infarction (MI): A1, long axis (diastole); A2, long axis (systole), A3, short axis (diastole); A4, short axis (systole); A5, late gadolinium enhancement (LGE) showing the quantified area of scar tissue in the left ventricle. B, Cardiac MRI at 12 weeks after MI: B1, long axis (diastole); B2, long axis (systole); B3, short axis (diastole); B4, short axis (systole); B5, LGE.
Histologic Analysis
After 12 weeks, the animals were euthanized, and their hearts were harvested and infused with 0.9% cold saline solution via the aortic root. The harvested organs were then prepared for histologic studies. The left ventricle was sectioned into 1-cm-thick slices perpendicular to the LAD from the apex to the base (Figure 3, A1 and A2). LV tissues from the infarct zone, border zone, and remote zone (healthy cardiac tissue) were obtained and fixed in 10% formalin at 4 °C for 24 hours, then embedded in paraffin (Figure 3, A3, B1-B3). Staining with Masson's trichrome and hematoxylin and eosin were performed to evaluate morphology and collagen content, as described previously.7
Figure 3.
Histologic assessment of the explanted myocardium. A1, The left ventricle was sectioned and sliced. A2, Transverse sections of the left ventricle. A3, Schematic of the 3 zones. B, Hematoxylin and eosin staining of slides from the 3 zones: B1, infarct; B2, remote; B3, border.
Statistical Analysis
All data are presented as mean ± SD. One-way analysis of variance followed by Tukey's post hoc test or the independent-samples Student t-test was used as appropriate. Statistical analyses were performed with SPSS version 17 for Windows (IBM). P values < .05 were considered statistically significant.
Results
We assessed the feasibility of targeted, minimally invasive transcatheter NOGA-guided injections of the therapeutic compound in all post-MI survivors at 8 weeks. The primary outcome comprised LVEF, EDV, and infarct area, and the secondary outcome comprised troponin-I and serum creatinine levels, scar segment, and total dyskinetic area determination.
Primary Outcomes
LVEF was determined by 2-dimensional echocardiography, performed at week 8 (prior to intervention) and at week 12 after MI. Echocardiographic measurements calculated using FAC in 3 planes, with LV length, using the modified Simpson rule,21 showed EF changes from week 8 to week 12 of 5.70% ± 11.3%, 6.30% ± 7.7%, and 7.66% ± 8.9% in the control, saline, and treatment groups, respectively (P = .92).
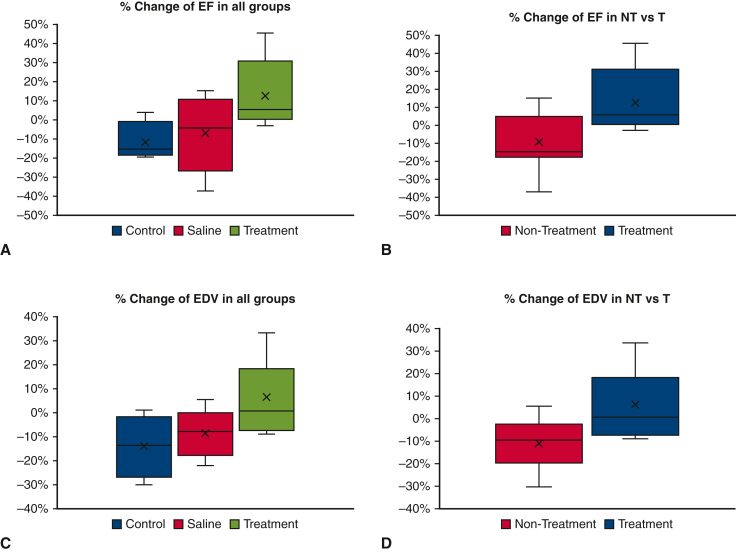
Out of the 21 study animals, 17 had complete MRI data available (control group, n = 4; saline group, n = 5; treatment group, n = 8) for analysis. Three initial control group animals did not follow standardized MRI according to the study protocol, and there was an outlier in the saline group, and these 4 animals were excluded from the final analysis (Table E1). Cardiac MRI–derived EF also was calculated in short-axis view. The percentage change in EF (EF%) from week 8 to week 12 was −11.5% ± 1.1% in the control group, −7.2% ± 2.0% in the saline group, and 12.7% ± 6.6% in the treatment group (P = .068) (Figure 4, A). Compared with nonintervention, the hydrogel-based therapy provided significant improvement in EF (−9.1% ± 16% vs 12.7% ± 18.6%; P = .009) (Figure 4, B).
Table E1.
Left ventricular measurements evaluated by cardiac MRI
| Pig ID | Group | Short-axis view |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EDV wk 8, mL | EDV wk 12, mL | EDV change, mL | EDV change, % | ESV wk 8, mL | ESV wk 12, mL | ESV change, mL | ESV change, % | SV wk 8, mL | SV wk 12, mL | SV change, mL | SV change, % | EF wk 8, % | EF wk 12, % | EF change, mL | EF change, % | ||
| 039 | C | 166.81 | 168.37 | 1.56 | 0.94 | 112.29 | 111.24 | −1.05 | −0.94 | 54.52 | 57.14 | 2.62 | 4.81 | 32.68 | 33.93 | 1.25 | 3.82 |
| 073 | C | 173.77 | 143.30 | −30.47 | −17.53 | 125.67 | 109.44 | −16.23 | −12.91 | 48.10 | 33.85 | −14.25 | −29.63 | 27.68 | 23.62 | −4.06 | −14.67 |
| 077 | C | 158.73 | 111.10 | −47.63 | −30.01 | 82.67 | 66.41 | −16.26 | −19.67 | 76.06 | 44.70 | −31.36 | −41.23 | 47.92 | 40.23 | −7.69 | −16.05 |
| 078 | C | 194.15 | 175.73 | −18.42 | −9.49 | 111.76 | 115.58 | 3.82 | 3.42 | 82.39 | 60.16 | −22.23 | −26.98 | 42.44 | 34.23 | −8.21 | −19.34 |
| 042 | S | 155.09 | 163.74 | 8.65 | 5.58 | 102.36 | 99.54 | −2.82 | −2.75 | 52.74 | 64.19 | 11.45 | 21.71 | 34.00 | 39.21 | 5.21 | 15.32 |
| 075 | S | 168.32 | 155.33 | −12.99 | −7.72 | 104.04 | 105.50 | 1.46 | 1.40 | 64.28 | 49.83 | −14.45 | −22.48 | 38.19 | 32.08 | −6.11 | −16.00 |
| 076 | S | 193.77 | 151.44 | −42.33 | −21.85 | 116.59 | 113.51 | −3.08 | −2.64 | 77.18 | 37.93 | −39.25 | −50.86 | 39.83 | 25.05 | −14.78 | −37.11 |
| 082 | S | 157.99 | 229.36 | 71.37 | 45.17 | 114.80 | 148.62 | 33.82 | 29.46 | 43.19 | 80.74 | 37.55 | 86.94 | 27.34 | 35.20 | 7.86 | 28.75 |
| 084 | S | 187.07 | 177.05 | −10.02 | −5.36 | 133.95 | 123.71 | −10.24 | −7.64 | 53.12 | 53.34 | 0.22 | 0.41 | 28.39 | 30.13 | 1.74 | 6.13 |
| 085 | S | 159.10 | 137.54 | −21.56 | −13.55 | 107.99 | 95.20 | −12.79 | −11.84 | 51.11 | 42.34 | −8.77 | −17.16 | 32.13 | 30.78 | −1.35 | −4.20 |
| 004 | T | 221.15 | 212.99 | −8.16 | −3.69 | 145.60 | 134.03 | −11.57 | −7.95 | 75.55 | 78.97 | 3.42 | 4.53 | 34.16 | 37.07 | 2.91 | 8.52 |
| 006 | T | 145.51 | 132.66 | −12.85 | −8.83 | 104.23 | 96.09 | −8.14 | −7.81 | 41.28 | 36.57 | −4.71 | −11.41 | 28.37 | 27.57 | −0.8 | −2.82 |
| 007 | T | 131.91 | 176.18 | 44.27 | 33.56 | 92.70 | 100.08 | 7.38 | 7.96 | 39.21 | 76.10 | 36.89 | 94.08 | 29.72 | 43.20 | 13.48 | 45.36 |
| 008 | T | 134.37 | 123.05 | −11.32 | −8.42 | 85.94 | 77.61 | −8.33 | −9.69 | 48.43 | 45.45 | −2.98 | −6.15 | 36.04 | 36.93 | 0.89 | 2.47 |
| 036 | T | 156.41 | 185.68 | 29.27 | 18.71 | 104.49 | 123.84 | 19.35 | 18.52 | 51.92 | 61.84 | 9.92 | 19.11 | 33.20 | 33.30 | 0.1 | 0.30 |
| 044 | T | 170.24 | 199.93 | 29.69 | 17.44 | 136.56 | 145.22 | 8.66 | 6.34 | 33.68 | 54.72 | 21.04 | 62.47 | 19.78 | 27.37 | 7.59 | 38.37 |
| 049 | T | 164.43 | 166.85 | 2.42 | 1.47 | 107.45 | 103.88 | −3.57 | −3.32 | 56.98 | 62.97 | 5.99 | 10.51 | 34.65 | 37.74 | 3.09 | 8.92 |
| 090 | T | 136.97 | 137.62 | 0.65 | 0.47 | 74.83 | 74.93 | 0.1 | 0.13 | 62.13 | 62.69 | 0.56 | 0.90 | 45.36 | 45.55 | 0.19 | 0.42 |
EDV, End-diastolic volume; ESV, end-systolic volume; SV, stroke volume; EF, ejection fraction; C, control; S, saline; T, treatment; MRI, magnetic resonance imaging.
Figure 4.
Comparison of changes in primary endpoints from 8 weeks to 12 weeks in the study groups. A, Percentage change in ejection fraction (EF) measured by magnetic resonance imaging (MRI) in all groups. B, Percentage change in EF measured by MRI in the nontreatment (control + saline) group and the treatment group. C, Percentage change in end-diastolic volume (EDV) measured by MRI in all groups. D, Percentage change in EDV measured by MRI in the nontreatment and treatment groups. In all the box-and-whisker plots, the lower and upper borders of the box represent the 25th and 75th percentiles, respectively, and the middle horizontal line represents the median. The lower and upper whiskers represent the minimum and maximum values of nonoutliers, and outliers were excluded.
EDV was the primary index measured by both echocardiography and cardiac MRI. Echocardiography-derived EDV changes from week 8 to week 12 were −4.75 ± 22.2 mL in the control group, −31.2 ± 33.8 mL in the saline group, and −2.81 ± 30.2 mL in the treatment group (P = .173). Similarly, across all 3 groups, the percentage change in EDV on cardiac MRI from week 8 to week 12 was worse in the control group and saline group (excluding outliers) compared with the treatment group (−14% ± 13.1% vs −8.6% ± 10.2% vs 6.4% ± 15.2%; P = .052) (Figure 4, C). Overall, the hydrogel-based treatment group showed a significant improvement compared with the nontreatment groups (−11.0% ± 11.1% vs 6.3% ± 15.2%; P = .008) (Figure 4, D).
The mean percentage of fibrotic tissue (myocardial scar) over each area in all the histology slices was calculated for the 3 study groups (Figure 3). The treatment group had consistently smaller scar areas in all 3 zones: infarct was 55.1% ± 8.0% in the control group, 52.3% ± 7.6% in the saline group and 51.7% ± 6.4% (P = .747); remote, 25.8% ± 12.2% versus 27% ± 8.6% versus 18.7% ± 13.3% (P = .393); border zone, 39.8% ± 15.3% versus 43.5% ± 10.7% versus 35.4% ± 9.1% versus (P = .418). In the slice closest to the apex, significant differences in scar area were observed; the treatment group had a smaller mean scar area in the infarcted zone compared with the control group (47.1% ± 7.0% vs 59% ± 8.2%; P = .013) and a smaller mean scar area in the border zone compared with the saline group (31.4% ± 8.3% vs 42.6% ± 9.0%; P = .016).
Secondary Outcomes
At 8 weeks post-MI, the NOGA mapping system was used to determine the scar area (Figure 1, A-C) and dyskinetic area of the left ventricle in both the saline and treatment groups before the injections. The mean changes in calculated total dyskinetic area from week 8 to week 12 were −7.60 ± 2.7 cm2 in the control group, −24.74 ± 0 cm2 in the saline group, and −11.44 ± 14.3 cm2 in the treatment group (P = .576). The mean number of scar segments in the 3 groups changed from week 8 to week 12, identified by NOGA 3D dynamic mapping as −2.50 ± 0.7, −2.00 ± 0, and −1.40 ± 2.1, respectively (P = .785). The treatment group had a lower calculated total dyskinetic area and fewer scar segments, but the differences were not statistically significant. However, dyskinesia disappeared in 3 of the compound-treated animals (Table E2).
Serum troponin levels were measured before occlusion of the mid-LAD, 24 hours after occlusion, and every 2 weeks until sacrifice. Within the first 24 hours, all animals showed a marked elevation in troponin level, which returned to baseline in a logarithmic pattern (Table 1). This troponin trend is consistent with that observed in MI across the study groups. Serum creatinine levels were measured before occlusion of the mid-LAD and every 2 weeks after until sacrifice at 12 weeks post-MI. There was a subtle increase in mean plasma creatinine level from baseline to 8 weeks post-MI (1.86 ± 0.28 vs 1.87 ± 0.30 mg/dL; P = .032).
Table 1.
Troponin-I and serum creatinine levels in the study animals
| Pig ID | Group | Troponin-I, ng/mL |
Serum creatinine, mmol/L |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 24 h | 2 wk post-MI | 4 wk post-MI | 8 wk pre-NOGA | 8 wk post-NOGA | 12 wk post-MI | Baseline | 2 wk post-MI | 4 wk post-MI | 8 wk post-MI | 12 wk post-MI | ||
| 027 | C | 957 | >400,000 | 279 | 56 | 46 | - | 20 | 2.0 | 1.8 | 2. | 2.2 | 2.0 |
| 038 | C | 144 | 145,568 | 1404 | 232 | 32 | - | 7 | 1.6 | 1.6 | 1.7 | 1.8 | 2.0 |
| 039 | C | 205 | 94,292 | 4169 | 199 | 52 | - | 11 | 1.6 | 1.4 | 1.7 | 1.9 | 2.0 |
| 041 | C | 133 | 266,660 | 443 | 143 | 16 | - | 8 | 1.9 | 1.7 | 1.9 | 2.0 | 1.8 |
| 073 | C | <2 | 46,843 | 152 | 70 | 16.2 | 12.2 | - | 1.8 | 1.8 | 2.3 | 2.4 | 2.1 |
| 077 | C | 242 | >160,000 | 477 | 47 | 22 | - | 8 | 1.3 | 1.3 | 1.3 | 1.4 | 1.6 |
| 078 | C | <2 | >400,000 | 346 | 117.9 | 85.2 | 30.1 | 4.7 | 1.7 | 1.9 | 1.9 | 2.3 | 2.2 |
| 042 | S | 1144 | 146,928 | 528 | 131 | 374 | 37 | 15 | 2.5 | 2.4 | 2.4 | 2.2 | 2.1 |
| 075 | S | 138 | 98,506 | 747 | 91 | 23 | 1021 | 18 | 1.7 | 1.6 | 1.7 | 1.9 | 1.7 |
| 076 | S | 259 | 88,839 | 1098 | 136 | 22 | 422 | 36 | 1.2 | 1.3 | 1.4 | 1.5 | 1.8 |
| 082 | S | <2 | 36,649 | 383.9 | 39.6 | 59.7 | - | 2.3 | 1.7 | 1.8 | 1.8 | 2.0 | 1.6 |
| 084 | S | 66 | 138,172 | 1009 | 119 | - | - | 9 | 1.6 | 1.5 | 1.6 | 1.9 | 1.7 |
| 085 | S | 45 | 255,791 | 1059 | 148 | 17 | 216 | 13 | 1.7 | 1.5 | 1.7 | 1.9 | 1.8 |
| 004 | T | 14 | - | 899 | 202 | 48 | - | 15 | 1.7 | 1.8 | 1.8 | 1.9 | 1.9 |
| 006 | T | 31/27 | 124,941 | 782.7 | - | 14 | - | 5 | 1.9 | 1.7 | 1.6 | 1.8 | 1.4 |
| 007 | T | 14 | 106,550 | 4061 | 512 | 63 | - | 43 | 1.6 | 1.8 | 1.8 | 2.1 | 2.1 |
| 008 | T | 6 | 214,512 | 1038 | 97.5 | <2 | 5443/182 | 6 | 2.4 | 2.1 | 2.3 | 2.3 | 1.9 |
| 036 | T | 69 | 106,777 | 453 | 152 | 14 | 4671 | 16 | 1.4 | 1.0 | 1.3 | 1.4 | 1.8 |
| 044 | T | - | 206,822 | 2239 | 187 | 19 | 4084 | 12 | 1.6 | 1.5 | 1.8 | 1.5 | 1.7 |
| 049 | T | 65 | 188,186 | 5211 | 541 | 73 | 32,750 | 241 | 1.6 | 1.5 | 1.8 | 1.8 | 2.0 |
| 090 | T | 13 | 232,570 | 530 | 69 | 34 | - | 24 | 1.5 | 1.2 | 1.4 | 1.6 | 1.6 |
MI, Myocardial infarction; C, control; S, saline; T, treatment.
Discussion
Our findings suggest that transcatheter myocardial restoration using hydrogel-based, cell-free compounds is feasible in a large animal model. The less invasiveness of the procedure and the cell-free (autologous) nature of the plasma used, coupled with hydrogel as a carrier, may translate into an applicable clinical protocol without ethical impediments. Similar to the majority of the ‘restorative photocopy,' we did not observe consistent behavior of all hemodynamic and morphologic parameters; nonetheless, based on our previous studies, we do not anticipate a regenerative impact of any of the existing compounds, including our own.
However, we have postulated that there is a crucial structural and architectural component to similar, compound-based interventions beyond the therapeutic effect of the stem cells. The myocardial helix features complex intercalations and interplays of muscle fiber bands with distinct physical properties, explaining the disproportionate increase in contractile force on only a minor increase in muscle fiber diameter.22, 23, 24, 25 Furthermore, we have previously recognized the impact of an ischemic insult on the so-called “ischemic vicious cycle” of the myocardium, including oxidative stress, inflammation, and cell loss.26
The results from this preclinical study of hydrogel in HF following MI has revealed specific beneficial outcomes. The overall trends in EF, EDV, and infarct area suggest a therapeutic advantage following the hydrogel injection. We postulate that this could be driven predominantly by the reduction in dyskinesia and improvement in LV structural stability after hydrogel administration, allowing for a more efficient contraction of the left ventricle during systole contributed by the remaining noninfarcted myocardial segments. This idea is further supported by the lack of change in the extent of LV scarring calculated in all the tissue slices obtained from our study cohort. However, myocardial restoration is a part of the polytherapy, which should include a comprehensive combination of pharmacologic therapy and surgical and interventional procedures.27
Echocardiographic indices trend toward a benefit from the hydrogel injection, but owing to known technical difficulties in performing a proper scan in a porcine model and its interpretation, we would caution against their overemphasis. Therefore, we decided to use cardiac MRI evaluation to overcome such challenges and provide high diagnostic accuracy.28 Notably, cardiac MRI was reported by a blinded cardiologist with cardiac MRI certification to reduce potential bias. Of note, despite the small number of animals inherent to this type of study, there was a statistically significant improvement in cardiac MRI-derived EF in the group that received the hydrogel injection compared with those that did not. One of the animals in the control arm showed an unexpected improvement in the EDV and EF; we suspect that there may have been spontaneous recanalization of the occluded mid LAD and/or recruitment and compensatory hyperkinesia of the surviving myocardial segments.
In our series, no animal demonstrated signs of hemiplegic stroke after the hydrogel injection, which implies a low risk of clinically relevant embolic complications. The NOGA-guided hydrogel-based therapeutic compound delivered into the myocardium can be considered safe, as also demonstrated by several other studies.29, 30, 31, 32 From a clinical standpoint, it would be contraindicated to proceed with the LV NOGA injection if echocardiography or cardiac MRI showed the presence of LV thrombus at 8 weeks post-MI.33 In the present study, all mortalities were a result of early complications following anterior MI (most likely arrhythmic death) and were not associated with the NOGA procedure per se. This supports the idea that NOGA-guided injections of the hydrogel-based therapeutic compound are safe and may be integrated into a viable clinical protocol.
Some limitations of the study need to be acknowledged. First, we compared a relatively small number of animals in each group, which might have translated into trends in several parameters with no statistically significant differences. However, the abundance of data from NOGA mapping, image analysis, and biochemical and histological analyses argues for further development of this therapy. Second, there was a short follow-up period in our study protocol. Although an improvement in EF was observed, we cannot verify the durability of this improvement. It can be postulated that if this improvement were sustained, it would theoretically translate into improved morbidity and mortality rates for patients with HF. Conversely, in the event of a transient effect, given the minimally invasive transcatheter protocol not requiring open-heart surgery, the injections could be repeated to achieve a sustained effect.
Conclusions
The present study suggests that percutaneous guided injections of the hydrogel-based cell-free therapeutic compound are safe and feasible and may translate into improved EF in patients suffering from post-MI HF. A larger-scale clinical trial using our translational protocol is warranted. Our results imply a translational potential of the hydrogel-based therapy and should be an impetus for conducting clinical trials focused on establishing a restoration therapy that can be integrated into a viable clinical protocol.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
We appreciate Mr Hanspeter Fischer, EMEA Field Manager, Biologics Delivery Systems, for his valuable contributions to this work. Ms Nurdiyana Binte Ja'afar and Mr Wellington Wu made significant contributions to this project at various points, for which we are grateful.
Footnotes
This research was funded by the National Medical Research Council of Singapore's Centre Grant program (CGAug16M008 [SCEPTRE grant]).
Data Availability Statement: Data are available on demand.
Appendix 1
Table E2.
Measurement of scar area and dyskinetic segments by NOGA mapping
| Pig ID | Group | Scar area (%) | Bipolar <1.5 mV bullseye area, wk 8 | Bipolar <1.5 mV bullseye area, wk 12 | Injections |
Dyskinetic segments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unipolar, wk 8 | Unipolar, wk 12 | Bipolar, wk 8 | Bipolar, wk 12 | Total | LLS, wk 8, % | LLS, wk 12, % | Calculated total dyskinetic area (cm2, bullseye area), wk 8 | Calculated total dyskinetic area (cm2, bullseye area), wk 12 | ||||
| 073 | C | 44.44 | 22.22 | 11.11 | 11.11 | 20.25 | 15.73 | 0 | 1 | 1 | 21.35 | 11.81 |
| 078 | C | 66.67 | 33.33 | 33.33 | 22.22 | 43.76 | 30 | 0 | 0 | 0 | 7.78 | 2.12 |
| 042 | S | 22.22 | - | 11.11 | - | 4.3 | - | 22 | 1 | - | 7.47 | - |
| 075 | S | 44.44 | - | 22.22 | - | 15.26 | - | 20 | 2 | - | 17.43 | - |
| 076 | S | 55.56 | - | 22.22 | - | 12.03 | - | 20 | 0 | - | 19.6 | - |
| 082 | S | 66.67 | 44.44 | 22.22 | 22.22 | 24.24 | 40.65 | 20 | 0 | 0 | 26.95 | 2.21 |
| 084 | S | 100 | - | 55.56 | - | 62.69 | - | 20 | 2 | - | 30.76 | - |
| 085 | S | 88.89 | - | 33.33 | - | 28.87 | - | 20 | 0 | - | 0 | - |
| 004 | T | 77.78 | - | 44.44 | - | 39.4 | - | 20 | 0 | - | 17.5 | - |
| 006 | T | 22.22 | - | 22.22 | - | 9.18 | - | 18 | 0 | - | 0 | - |
| 007 | T | 66.67 | 77.78 | 22.22 | 22.22 | 18.75 | 21.65 | 21 | 0 | 1 | 1.83 | 12.65 |
| 008 | T | 22.22 | 11.11 | 22.22 | 0 | 17.74 | 8.52 | 21 | 1 | 0 | 19.21 | 0 |
| 036 | T | 55.56 | 55.56 | 55.56 | 11.11 | 14.01 | 11.22 | 19 | 2 | 0 | 20.8 | 0 |
| 044 | T | 100 | 66.67 | 66.67 | 66.67 | 47.58 | 55.84 | 20 | 1 | 0 | 35.55 | 12.72 |
| 049 | T | 88.89 | 44.44 | 44.44 | 22.22 | 24.96 | 33.71 | 20 | 0 | 0 | 5.18 | 0 |
| 090 | T | 0 | - | 0 | - | 3.29 | - | 19 | 0 | - | 0 | - |
LLS, Linear local shortening; C, control; S, saline; T, treatment.
References
- 1.Joseph P., Leong D., McKee M., Anand S.S., Schwalm J.D., Teo K., et al. Reducing the global burden of cardiovascular disease, Part 1: the epidemiology and risk factors. Circ Res. 2017;121:677–694. doi: 10.1161/CIRCRESAHA.117.308903. [DOI] [PubMed] [Google Scholar]
- 2.Bansilal S., Castellano J.M., Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol. 2015;201(Suppl 1):S1–S7. doi: 10.1016/S0167-5273(15)31026-3. [DOI] [PubMed] [Google Scholar]
- 3.Lesyuk W., Kriza C., Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004-2016. BMC Cardiovasc Disord. 2018;18:74. doi: 10.1186/s12872-018-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessup M. The heart failure paradox: an epidemic of scientific success. Presidential address at the American Heart Association 2013 scientific sessions. Circulation. 2014;129:2717–2722. doi: 10.1161/CIR.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 5.Sazzad F., Kuzemczak M., Loh E., Wu W., Kofidis T. Targeted myocardial restoration with injectable hydrogels-in search of the holy grail in regenerating damaged heart tissue. Biomedicines. 2021;9:595. doi: 10.3390/biomedicines9060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vu D.T., Martinez E.C., Kofidis T. Myocardial restoration: is it the cell or the architecture or both? Cardiol Res Pract. 2012;2012 doi: 10.1155/2012/240497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vu T.D., Pal S.N., Ti L.K., Martinez E.C., Rufaihah A.J., Ling L.H., et al. An autologous platelet-rich plasma hydrogel compound restores left ventricular structure, function and ameliorates adverse remodeling in a minimally invasive large animal myocardial restoration model: a translational approach: Vu and Pal "myocardial repair: PRP, hydrogel and supplements". Biomaterials. 2015;45:27–35. doi: 10.1016/j.biomaterials.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Kofidis T., Lebl D.R., Martinez E.C., Hoyt G., Tanaka M., Robbins R.C. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112(9 Suppl):I173–I177. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 9.Dong H., Li X., Xiao D., Tang Y. Late percutaneous coronary intervention is associated with better prognosis of patients with acute myocardial infarction. Int J Gen Med. 2022;15:2621–2627. doi: 10.2147/IJGM.S357330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkhalil M., Choudhury R.P. Reperfusion treatment in late presentation acute myocardial infarction. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.118.007287. [DOI] [PubMed] [Google Scholar]
- 11.Glancy D.L. Late presentation of acute myocardial infarction due to ramus intermedius disease. Proc (Bayl Univ Med Cent) 2017;31:70–71. doi: 10.1080/08998280.2017.1391044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah A.I., Alabaster A., Dontsi M., Rana J.S., Solomon M.D., Krishnaswami A. Comparison of coronary revascularization strategies in older adults presenting with acute coronary syndromes. J Am Geriatr Soc. 2022;70:2235–2245. doi: 10.1111/jgs.17794. [DOI] [PubMed] [Google Scholar]
- 13.Li X.H., Zhou X., Zeng S., Ye F., Yun J.L., Huang T.G., et al. Effects of intramyocardial injection of platelet-rich plasma on the healing process after myocardial infarction. Coron Artery Dis. 2008;19:363–370. doi: 10.1097/MCA.0b013e3282fc6165. [DOI] [PubMed] [Google Scholar]
- 14.Mishra A., Velotta J., Brinton T.J., Wang X., Chang S., Palmer O., et al. RevaTen platelet-rich plasma improves cardiac function after myocardial injury. Cardiovasc Revasc Med. 2011;12:158–163. doi: 10.1016/j.carrev.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Kofidis T., de Bruin J.L., Yamane T., Tanaka M., Lebl D.R., Swijnenburg R.J., et al. Stimulation of paracrine pathways with growth factors enhances embryonic stem cell engraftment and host-specific differentiation in the heart after ischemic myocardial injury. Circulation. 2005;111:2486–2493. doi: 10.1161/01.CIR.0000165063.09283.A8. [DOI] [PubMed] [Google Scholar]
- 16.Gyöngyösi M., Dib N. Diagnostic and prognostic value of 3D NOGA mapping in ischemic heart disease. Nat Rev Cardiol. 2011;8:393–404. doi: 10.1038/nrcardio.2011.64. [DOI] [PubMed] [Google Scholar]
- 17.Li K., Wagner L., Moctezuma-Ramirez A., Vela D., Perin E. A robust percutaneous myocardial infarction model in pigs and its effect on left ventricular function. J Cardiovasc Transl Res. 2021;14:1075–1084. doi: 10.1007/s12265-021-10123-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu X., Fan Y., Li S., Chen M., Li M., Hau W.K., et al. Deep learning-based automated left ventricular ejection fraction assessment using 2-D echocardiography. Am J Physiol Heart Circ Physiol. 2021;321:H390–H399. doi: 10.1152/ajpheart.00416.2020. [DOI] [PubMed] [Google Scholar]
- 19.Nanni S., Westenberg J.J., Bax J.J., Siebelink H.M., de Roos A., Kroft L.J. Biplane versus short-axis measures of the left atrium and ventricle in patients with systolic dysfunction assessed by magnetic resonance. Clin Imag. 2016;40:907–912. doi: 10.1016/j.clinimag.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Doltra A., Amundsen B.H., Gebker R., Fleck E., Kelle S. Emerging concepts for myocardial late gadolinium enhancement MRI. Curr Cardiol Rev. 2013;9:185–190. doi: 10.2174/1573403x113099990030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folland E.D., Parisi A.F., Moynihan P.F., Jones D.R., Feldman C.L., Tow D.E. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. A comparison of cineangiographic and radionuclide techniques. Circulation. 1979;60:760–766. doi: 10.1161/01.cir.60.4.760. [DOI] [PubMed] [Google Scholar]
- 22.Buckberg G.D., Nanda N.C., Nguyen C., Kocica M.J. What is the heart? Anatomy, function, pathophysiology, and misconceptions. J Cardiovasc Dev Dis. 2018;5:33. doi: 10.3390/jcdd5020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckberg G.D. Rethinking the cardiac helix--a structure/function journey: overview. Eur J Cardio Thorac Surg. 2006;29(Suppl 1):S2–S3. doi: 10.1016/j.ejcts.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Marcé-Nogué J., Fortuny G., Ballester-Rodés M., Carreras F., Roure F. Computational modeling of electromechanical propagation in the helical ventricular anatomy of the heart. Comput Biol Med. 2013;43:1698–1703. doi: 10.1016/j.compbiomed.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Kofidis T., Balsam L.B., Robbins R.C. A few critical aspects--and Achilles heels--of tissue engineering approaches to restore injured myocardium. J Thorac Cardiovasc Surg. 2003;126:2113–2116. doi: 10.1016/j.jtcvs.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Opie L.H. The metabolic vicious cycle in heart failure. Lancet. 2004;364:1733–1734. doi: 10.1016/S0140-6736(04)17412-6. [DOI] [PubMed] [Google Scholar]
- 27.Birks E.J., Drakos S.G., Patel S.R., Lowes B.D., Selzman C.H., Starling R.C., et al. Prospective multicenter study of myocardial recovery using left ventricular assist devices (RESTAGE-HF [remission from stage D heart failure]): medium-term and primary end point results. Circulation. 2020;142:2016–2028. doi: 10.1161/CIRCULATIONAHA.120.046415. [DOI] [PubMed] [Google Scholar]
- 28.Saeed M., Hetts S.W., Jablonowski R., Wilson M.W. Magnetic resonance imaging and multi-detector computed tomography assessment of extracellular compartment in ischemic and non-ischemic myocardial pathologies. World J Cardiol. 2014;6:1192–1208. doi: 10.4330/wjc.v6.i11.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banovic M., Ostojic M.C., Bartunek J., Nedeljkovic M., Beleslin B., Terzic A. Brachial approach to NOGA-guided procedures: electromechanical mapping and transendocardial stem-cell injections. Tex Heart Inst J. 2011;38:179–182. [PMC free article] [PubMed] [Google Scholar]
- 30.Bassetti B., Carbucicchio C., Catto V., Gambini E., Rurali E., Bestetti A., et al. Linking cell function with perfusion: insights from the transcatheter delivery of bone marrow-derived CD133+ cells in ischemic refractory cardiomyopathy trial (RECARDIO) Stem Cell Res Ther. 2018;9:235. doi: 10.1186/s13287-018-0969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garbayo E., Gavira J.J., de Yebenes M.G., Pelacho B., Abizanda G., Lana H., et al. Catheter-based intramyocardial injection of FGF1 or NRG1-loaded MPs improves cardiac function in a preclinical model of ischemia-reperfusion. Sci Rep. 2016;6 doi: 10.1038/srep25932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singelyn J.M., Sundaramurthy P., Johnson T.D., Schup-Magoffin P.J., Hu D.P., Faulk D.M., et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59:751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habash F., Vallurupalli S. Challenges in management of left ventricular thrombus. Ther Adv Cardiovasc Dis. 2017;11:203–213. doi: 10.1177/1753944717711139. [DOI] [PMC free article] [PubMed] [Google Scholar]