Abstract
Objectives
Safety-net hospitals (SNHs) provide essential services to predominantly underserved patients regardless of their ability to pay. We hypothesized that patients who underwent coronary artery bypass grafting (CABG) would have inferior observed outcomes at SNHs compared with non-SNHs but that matched cohorts would have comparable outcomes.
Methods
We queried the Nationwide Readmissions Database for patients who underwent isolated CABG from 2016 to 2018. We ranked hospitals by the percentage of all admissions in which the patient was uninsured or insured with Medicaid; hospitals in the top quartile were designated as SNHs. We used propensity-score matching to mitigate the effect of confounding factors and compare outcomes between SNHs and non-SNHs.
Results
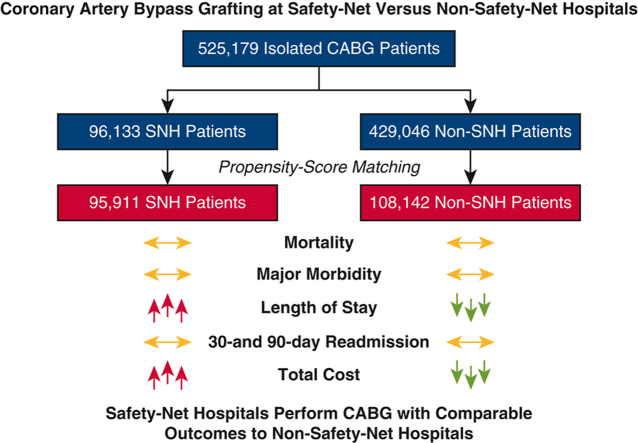
A total of 525,179 patients underwent CABG, including 96,133 (18.3%) at SNHs, who had a greater burden of baseline comorbidities (median Elixhauser score 8 vs 7; P = .04) and more frequently required urgent surgery (57.1% vs 52.8%; P < .001). Observed in-hospital mortality (2.1% vs 1.8%; P = .004) and major morbidity, length of stay (9 vs 8 days; P < .001), cost ($46,999 vs $38,417; P < .001), and readmission rate at 30 (12.4% vs 11.3%) and 90 days (19.0% vs 17.7%) were greater at SNHs (both P < .001). After matching, none of these differences persisted except length of stay (9 vs 8 days) and cost ($46,977 vs $39,343) (both P < .001).
Conclusions
After matching, early outcomes after CABG were comparable at SNHs and non-SNHs. Improved discharge resources could reduce length of stay and curtail cost, improving the value of CABG at SNHs.
Key Words: coronary artery bypass grafting, outcomes, cost, safety-net burden, socioeconomic status, health care disparities
Graphical abstract
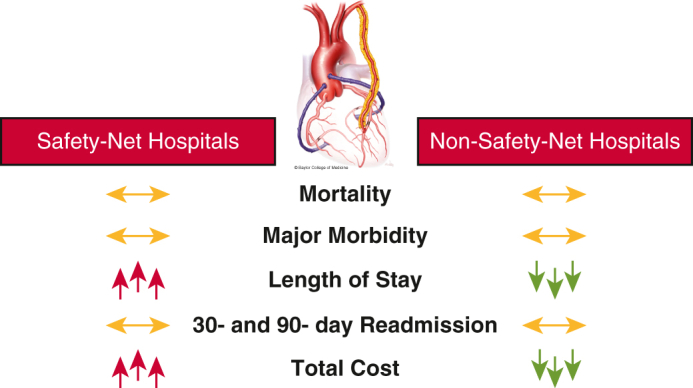
Early outcomes after CABG are comparable at safety-net and non–safety-net hospitals.
Central Message.
Safety-net hospitals perform coronary artery bypass grafting with comparable early outcomes to those of propensity-matched non-safety-net hospitals but with longer stays and greater costs.
Perspective.
Although previous studies have suggested that the quality of complex surgical care at safety-net hospitals lags behind nonsafety-net hospitals, we found that safety-net hospitals perform coronary artery bypass grafting with comparable propensity-score matched outcomes to non-safety-net hospitals, albeit with longer length of stay and greater costs.
Safety-net hospitals (SNHs) are defined as those that, by mission or mandate, provide care to a substantial share of vulnerable patients regardless of their ability to pay.1 Important disparities exist for these patients in terms of both the burden of cardiovascular risk factors2 and access to cardiac surgical interventions.3, 4, 5, 6 Nevertheless, SNHs have been the subject of scrutiny with respect to delivery of complex surgical care, including cardiac surgery, with several studies suggesting that the quality of surgical care at SNHs lags behind that at non-SNHs.7, 8, 9 Given these disparities, along with a growing emphasis on health care equity, the quality of cardiac surgical care at SNHs versus non-SNHs is an area worthy of examination. In addition, identifying specific areas in which SNHs may fall short of non-SNHs can provide policymakers and cardiothoracic surgical societies with areas to target for improvement.
Coronary artery bypass grafting (CABG) is the most common cardiac operation performed in the United States.10 Although previous studies have examined the effect of hospital safety-net burden on outcomes after CABG,7,9,11, 12, 13 few have mitigated the effect of confounding variables by comparing matched patient cohorts. We hypothesized that patients who undergo CABG would have inferior outcomes and greater cost at SNHs than at non-SNHs but that matched cohorts drawn from a nationwide sample would have comparable outcomes and cost.
Patients and Methods
Study Population and Data Collection
The Nationwide Readmissions Database (NRD) is the largest publicly available all-payer inpatient database that provides linked admissions for a patient within a given calendar year. It is maintained by the Agency for Healthcare Research and Quality's Healthcare Cost and Utilization Project. The NRD provides demographic, clinical, and cost data from more than 90% of adult discharges from 28 states, accounting for approximately 60% of all patients and hospitals whose data are recorded in the American Hospital Association Annual Survey Database, and uses a complex survey design to allow for national estimates of patient outcomes.
We ensured that our data were coded consistently by abstracting admissions data between January 1, 2016, and December 31, 2018, during which time all data were coded according to the 10th Revision of the International Classification of Diseases and Related Health Problems (ICD-10). First, we used a combination of ICD-10-Clinical Modification and ICD-10-Procedure Coding System codes to identify all patients who underwent CABG during the study period. Next, we excluded patients who underwent concomitant percutaneous coronary intervention, valve repair or replacement, or open or endovascular aortic intervention to generate a cohort of patients who underwent isolated CABG (Table E1). Given that only deidentified data were used, this study was deemed exempt from institutional review board approval.
Study Definitions
To facilitate a direct comparison, and in line with previous studies, we defined safety-net burden as the percentage of all admissions with the patient's primary payer designated as uninsured or insured with Medicaid.7,9,12, 13, 14, 15, 16, 17 We defined hospitals in the top quartile of safety-net burden as SNHs and the remaining hospitals as non-SNHs.12,16, 17, 18
We abstracted patient characteristics, including age, sex, payer, and median household income, directly from the NRD. Comorbidities were abstracted by using ICD-10-Clinical Modification codes. The Elixhauser Comorbidity Index,19 a composite score of 30 chronic conditions, was used to quantify the burden of baseline comorbidities. Elixhauser score was calculated by using a Python (Python Software Foundation. Python Language Reference, version 3.7. http://www.python.org) implementation of Healthcare Cost and Utilization Project Software and Tools (hcuppy package, version 0.0.7).
Outcomes
Our primary outcome was in-hospital mortality, and our secondary outcomes were major morbidity (acute kidney injury, stroke, respiratory failure, infections), length of stay (LOS), cost, and 30- and 90-day readmission. Admissions during the month of December were excluded from 30-day readmission calculations, and admissions during the months of October through December were excluded from 90-day readmission calculations. The total hospitalization cost was calculated by multiplying the total hospital charge by cost-to-charge ratios provided by the NRD.
Statistical Analysis
Analyses were performed in R 4.1 (The R Project for Statistical Computing). In all patient-level analyses, we accounted for the complex survey design of the NRD, including clustering, stratification, and sample weighting. Data are reported as weighted national estimates, as is standard in studies using the NRD, unless otherwise noted. All hospital-level analyses are based on data directly abstracted from the NRD and were performed by traditional statistical methods, as these data are not weighted to provide national estimates. Of note, hospitals are given a unique identifier each year in the NRD and cannot be tracked across years; therefore, we stratified these data by year.
Categorical variables are presented as number (%), and continuous variables are presented as median (interquartile range). Univariate comparisons were performed with the χ2 test with Rao and Scott correction or Wilcoxon rank-sum test adjusted for complex survey design, as appropriate. National estimates were generated by using sampling weights assigned through the NRD.
A survey-adjusted multivariable logistic regression was performed on the complete cohort to determine which baseline patient characteristics were associated with in-hospital mortality. All significant variables in the univariate analysis, along with hospital safety-net status, were included in the initial model. All data were reduced to binary variables, and missing data cells were replaced by the mode of that column. We divided the data into a training set (80%) and a testing set (20%) with a partitioning tool. Logistic regressions were carried out on the training set, and predictions were made for the testing set, which were used to calculate the area under the curve (C-statistic) of the receiver operator characteristic curve. Next, we used the C-statistic to guide independent variable selection, and the variables in the final model were presented as adjusted odds ratio (OR) with 95% confidence interval (CI).
Propensity Score–Matched Analysis
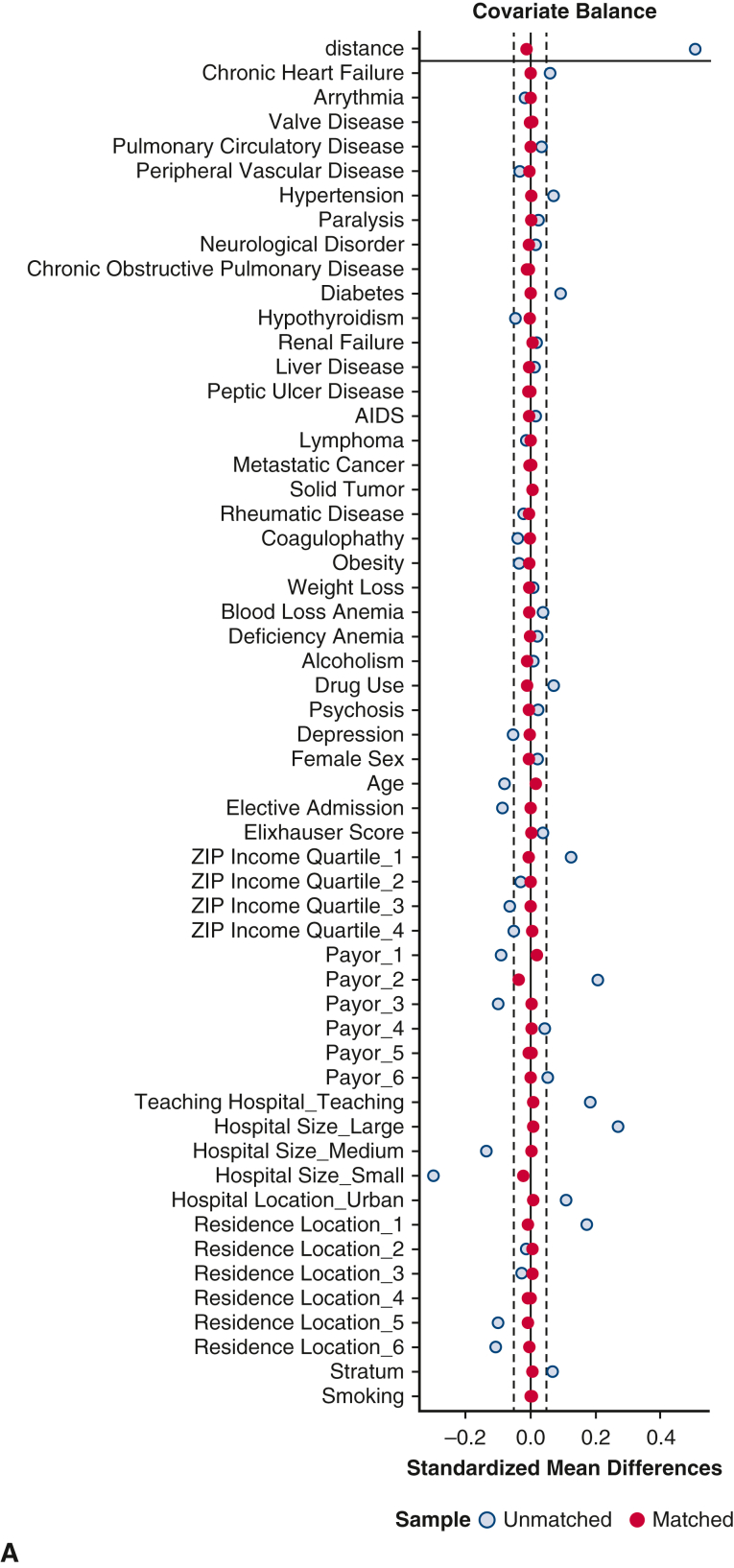
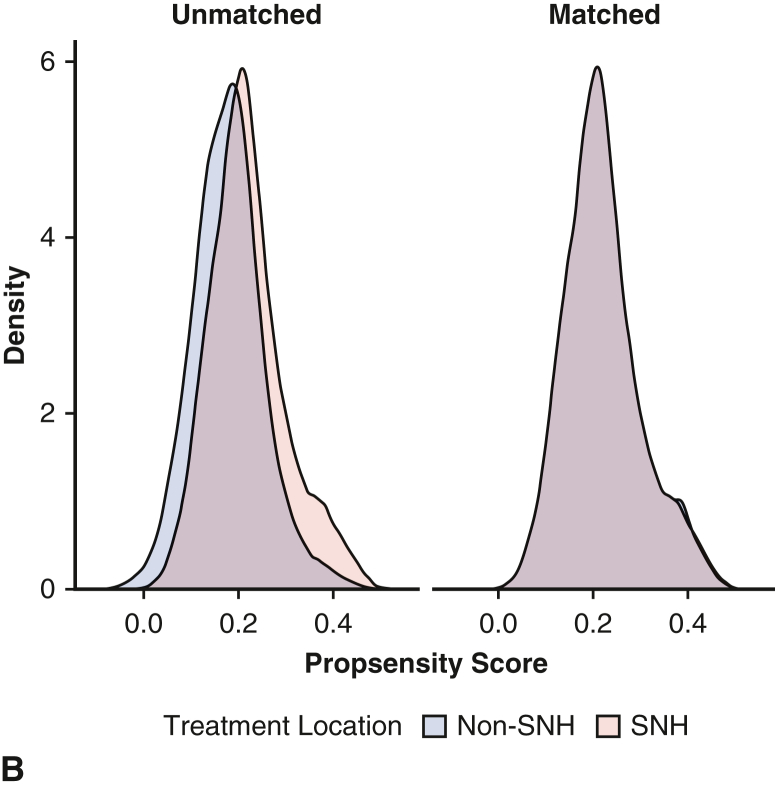
A propensity score–matched analysis was performed to compare outcomes between matched populations at SNHs versus non-SNHs. Cohorts were matched according to the following covariates: age, sex, insurance status, area of residence, household income quartile, elective admission status, hospital characteristics, and 28 comorbid conditions included in the composite Elixhauser comorbidities (Figure E1). Greedy nearest neighbor matching through the MatchIt package was used to create pairs in a 1:1 ratio between SNHs and non-SNHs with a caliper of 0.05 standard deviation of the logit. Of note, matching yielded a modest difference in weighted sample sizes between groups due to post hoc sample weighting calculations. Quality of matching was determined with balance diagnostics, including computing standardized mean differences and visually inspecting covariate distribution. An average standard mean difference of 0.10 was considered acceptable. After matching, univariate comparisons were performed with the tests described previously.
Figure E1.
Assessment of covariate balance before and after propensity-score matching. A, Love plot of standardized mean differences before and after matching. B, Density plot of propensity scores before and after matching. SNH, Safety-net hospital.
Results
Preoperative Characteristics
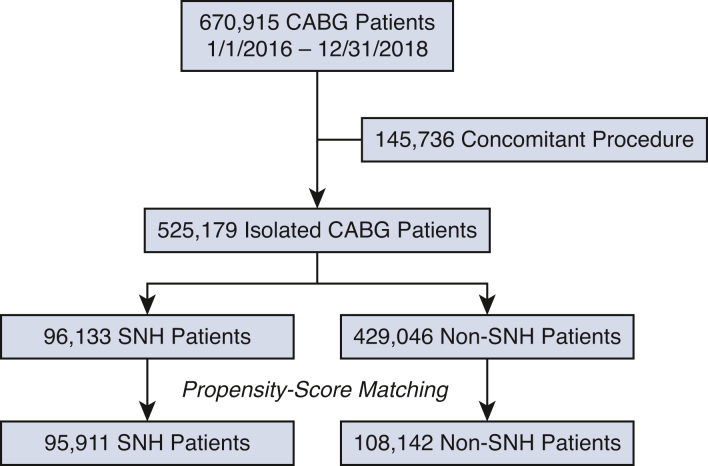
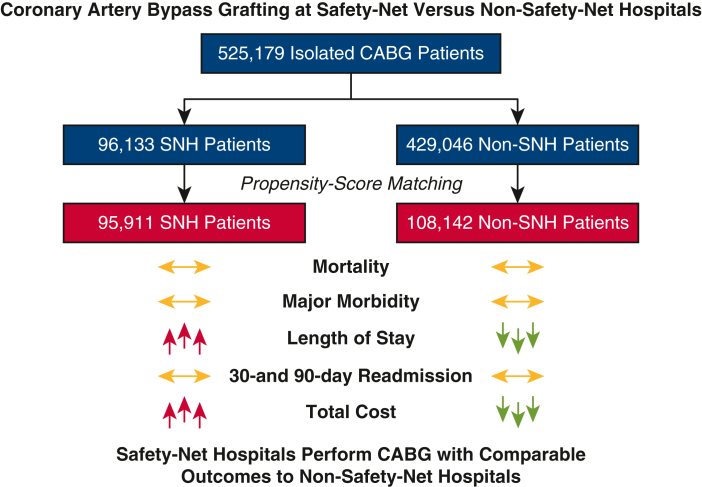
A total of 525,179 patients (75.5% male) underwent isolated CABG in the United States between 2016 and 2018 (96,133 [18.3%] at SNHs and 429,046 [81.7%] at non-SNHs) (Figure 1). There were several important baseline differences between the 2 cohorts. Patients at SNHs were younger (median age 65 vs 67 years) and more frequently in the lowest income quartile (32.5% vs 26.6%) than patients at non-SNHs (both P < .001). In addition, patients at SNHs had a greater burden of baseline comorbidities (median Elixhauser score 8 vs 7; P = .04), including several chronic conditions known to portend adverse outcomes after CABG: congestive heart failure (37.2% vs 34.3%), diabetes mellitus (52.2% vs 47.5%), and hypertension (89.7% vs 87.5%) (all P < .001). In contrast, patients at non-SNHs had greater rates of obesity (29.7% vs 28.1%; P = .04) and peripheral arterial disease (15.2% vs 14.1%; P = .003) than patients at SNHs. Tobacco use was similar between groups. Last, patients at SNHs more frequently required urgent surgery (57.1% vs 52.8%; P < .001) (Table 1).
Figure 1.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram. CABG, Coronary artery bypass grafting; SNH, safety-net hospital.
Table 1.
Preoperative characteristics of patients who underwent coronary artery bypass grafting at SNHs versus non-SNHs
| Variable | Unmatched (n = 525,179) |
Matched (n = 204,053) |
||||
|---|---|---|---|---|---|---|
| SNHs (n = 96,133) | Non-SNHs (n = 429,046) | P value | SNHs (n = 95,911) | Non-SNHs (n = 108,142) | SMD | |
| Age, y | 65 (58-72) | 67 (59-73) | <.001 | 66 (58-72) | 65 (58-72) | 0.017 |
| Male | 71,862 (74.8%) | 324,890 (75.7%) | <.001 | 71,708 (74.8%) | 80,650 (74.6%) | 0.004 |
| Elixhauser score | 8 (0-18) | 7 (0-17) | .04 | 8 (0-18) | 8 (0-18) | 0.003 |
| Anemia | 4165 (4.3%) | 14,878 (3.5%) | <.001 | 4131 (4.3%) | 4712 (4.4%) | −0.001 |
| Arrhythmia | 44,237 (46.0%) | 201,017 (46.9%) | .17 | 44,154 (46.0%) | 49,755 (46.0%) | 0.001 |
| Chronic kidney disease | 20,893 (21.7%) | 89,821 (20.9%) | .08 | 20,849 (21.7%) | 23,220 (21.5%) | 0.007 |
| Chronic obstructive pulmonary disease | 21,549 (22.4%) | 97,780 (22.8%) | .40 | 21,511 (22.4%) | 24,434 (22.6%) | −0.004 |
| Coagulopathy | 18,988 (19.8%) | 91,428 (21.3%) | .13 | 18,961 (19.8%) | 21,401 (19.8%) | −0.001 |
| Congestive heart failure | 35,772 (37.2%) | 147,031 (34.3%) | <.001 | 35,658 (37.2%) | 40,109 (37.1%) | 0.002 |
| Diabetes mellitus | 50,158 (52.2%) | 203,821 (47.5%) | <.001 | 49,987 (52.1%) | 56,303 (52.1%) | 0.001 |
| Drug abuse | 3663 (3.8%) | 10,361 (2.4%) | <.001 | 3608 (3.8%) | 4270 (3.9%) | −0.010 |
| Hypertension | 86,261 (89.7%) | 375,518 (87.5%) | <.001 | 86,047 (89.7%) | 96,915 (89.6%) | 0.003 |
| Liver disease | 3560 (3.7%) | 14,692 (3.4%) | .03 | 3551 (3.7%) | 4075 (3.8%) | −0.004 |
| Obesity | 27,032 (28.1%) | 127,223 (29.7%) | .04 | 26,985 (28.1%) | 30,553 (28.3%) | −0.003 |
| Peripheral arterial disease | 13,560 (14.1%) | 65,279 (15.2%) | .003 | 13,536 (14.1%) | 15,325 (14.2%) | −0.002 |
| Tobacco use | 49,846 (51.9%) | 222,316 (51.9%) | .96 | 49,732 (51.9%) | 55,874 (51.7%) | 0.007 |
| Valvular heart disease | 15,496 (16.1%) | 69,386 (16.2%) | .92 | 15,464 (16.1%) | 17,199 (15.9%) | 0.006 |
| Household income∗ | <.001 | |||||
| Quartile 1 | 31,201 (32.5%) | 114,173 (26.6%) | 30,997 (32.3%) | 35,201 (32.6%) | −0.005 | |
| Quartile 2 | 27,808 (28.9%) | 129,780 (30.2%) | 27,795 (29.0%) | 31,272 (28.9%) | 0.001 | |
| Quartile 3 | 21,849 (22.7%) | 108,768 (25.4%) | 21,845 (22.8%) | 24,648 (22.8%) | −0.001 | |
| Quartile 4 | 15,275 (15.9%) | 76,325 (17.8%) | 15,274 (15.9%) | 17,021 (15.7%) | 0.005 | |
| Nonelective | 54,871 (57.1%) | 226,658 (52.8%) | <.001 | 54,696 (57.0%) | 61,675 (57.0%) | −0.001 |
Categorical variables are presented as number (%), and continuous variables are presented as median (interquartile range). SNHs, Safety-net hospitals; SMD, standardized mean difference.
Indexed to patient's ZIP code.
Hospital Characteristics
An average of 783 hospitals performed CABG each year. In general, SNHs were larger, more likely to be in an urban location, and more likely to be designated as a teaching hospital, whereas non-SNHs were smaller and more likely to be in a rural location. Median annual CABG volume was 111 at SNHs and 158 at non-SNHs (P < .001) (Table 2).
Table 2.
Characteristics of SNH versus non-SNHs performing CABG, stratified by year
| Characteristic | 2016 |
2017 |
2018 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-SNH (n = 601) | SNH (n = 187) | P value | Non-SNH (n = 616) | SNH (n = 192) | P value | Non-SNH (n = 568) | SNH (n = 185) | P value | |
| Teaching hospital∗ | 320 (53.2%) | 120 (64.2%) | .01 | 347 (56.3%) | 140 (72.9%) | <.001 | 361 (63.6%) | 132 (71.4%) | .053 |
| Hospital size∗ | <.001 | .001 | <.001 | ||||||
| Large | 293 (48.8%) | 120 (64.2%) | 301 (48.9%) | 115 (59.9%) | 272 (47.9%) | 117 (63.2%) | |||
| Medium | 184 (30.6%) | 46 (24.6%) | 177 (28.7%) | 56 (29.2%) | 169 (29.8%) | 46 (24.9%) | |||
| Small | 124 (20.6%) | 21 (11.2%) | 138 (22.4%) | 21 (10.9%) | 127 (22.4%) | 22 (11.9%) | |||
| Urban hospital∗ | 527 (87.7%) | 174 (93.0%) | .04 | 543 (88.1%) | 183 (95.3%) | .004 | 520 (91.5%) | 170 (91.9%) | .88 |
| Total volume† | 21,693 (12,276, 36,685) | 27,993 (17,608, 49,555) | <.001 | 21,875 (12,682, 36,257) | 27,899 (17,924, 43,894) | <.001 | 22,932 (14,390, 38,160) | 27,140 (17,069, 45,732) | .02 |
| Medicaid or self-pay admissions† | 3605 (1778, 6838) | 11,637 (6870, 18,474) | <.001 | 3714 (1761, 6969) | 11,445 (7411, 17,027) | <.001 | 4024 (2080, 7581) | 11,621 (7004, 17,672) | <.001 |
| Percent Medicaid or self-pay admissions† | 18 (13, 23) | 38 (35, 45) | <.001 | 18 (12, 23) | 38 (34, 45) | <.001 | 18 (13, 23) | 38 (34, 45) | <.001 |
| CABG cases† | 145 (29, 335) | 115 (34, 245) | .050 | 155 (38, 340) | 110 (37, 257) | .01 | 174 (73, 348) | 101 (31, 270) | < .001 |
Categorical variables are presented as number (%), and continuous variables are presented as median (interquartile range). SNH, Safety-net hospital; CABG, coronary artery bypass grafting.
Observed data derived from unweighted hospital-level data.
National estimates derived from weighted patient-level data.
Postoperative Outcomes
Observed rates of in-hospital mortality (2.1% vs 1.8%; P = .004), acute kidney injury (21.6% vs 19.9%; P < .001), stroke (2.0% vs 1.7%; P < .001), pneumonia (6.2% vs 4.6%; P < .001), sepsis (2.7% vs 1.9%; P < .001), 30-day readmission (12.4% vs 11.3%; P < .001), and 90-day readmission (19.0% vs 17.7%; P < .001), the median LOS (9 vs 8 days; P < .001), and the median total cost ($46,999 vs $38,417; P < .001) were greater at SNHs than at non-SNHs. In addition, patients at SNHs were less likely to be discharged with home health care (35.1% vs 43.7%; P < .001) (Table 3).
Table 3.
Postoperative outcomes of patients who underwent coronary artery bypass grafting at SNH versus non-SNHs
| Variable | Unmatched (n = 525,179) |
Matched (n = 204,053) |
||||
|---|---|---|---|---|---|---|
| SNHs (n = 96,133) | Non-SNHs (n = 429,046) | P Value | SNHs (n = 95,911) | Non-SNHs (n = 108,142) | P Value | |
| In-hospital mortality | 2018 (2.1%) | 7865 (1.8%) | .004 | 2017 (2.1%) | 2076 (1.9%) | .11 |
| Acute kidney injury | 20,773 (21.6%) | 85,339 (19.9%) | <.001 | 20,704 (21.6%) | 22,983 (21.3%) | .54 |
| Stroke | 1926 (2.0%) | 7140 (1.7%) | <.001 | 1915 (2.0%) | 2174 (2.0%) | .90 |
| Respiratory failure | 19,383 (20.2%) | 80,746 (18.8%) | .10 | 19,335 (20.2%) | 21,981 (20.3%) | .85 |
| Infection | ||||||
| Surgical site infection | 371 (0.4%) | 1348 (0.3%) | .07 | 368 (0.4%) | 376 (0.3%) | .42 |
| Deep sternal wound infection | 235 (0.2%) | 991 (0.2%) | .63 | 236 (0.2%) | 256 (0.2%) | .80 |
| Pneumonia | 5971 (6.2%) | 19,722 (4.6%) | <.001 | 5941 (6.2%) | 5435 (5.0%) | <.001 |
| Sepsis | 2580 (2.7%) | 8278 (1.9%) | <.001 | 2569 (2.7%) | 2397 (2.2%) | <.001 |
| LOS, days | 9 (6-13) | 8 (6-11) | <.001 | 9 (6-13) | 8 (6-12) | <.001 |
| Total cost, $ | $46,999 (35,677-62,800) | $38,417 (29,623-52,025) | <.001 | $46,977 (35,670-62,773) | $39,343 (30,501-53,160) | <.001 |
| Discharge disposition | <.001 | <.001 | ||||
| Routine | 43,707 (46.4%) | 158,968 (37.7%) | 43,611 (46.4%) | 38,187 (36.0%) | ||
| Home health care | 32,991 (35.1%) | 184,169 (43.7%) | 32,904 (35.0%) | 48,556 (45.8%) | ||
| SNF or LTACH | 16,514 (17.5%) | 75,401 (17.9%) | 16,482 (17.6%) | 18,592 (17.5%) | ||
| Other | 872 (1.0%) | 2488 (0.7%) | 868 (1.0%) | 674 (0.7%) | ||
| Readmission within 30 d∗ | 10,643 (12.4%) (n = 86,171) | 43,408 (11.3%) (n = 385,166) | <.001 | 10,607 (12.3%) (n = 85,969) | 11,541 (11.9%) (n = 97,118) | .13 |
| Readmission within 90 d† | 13,476 (19.0%) (n = 70,859) | 55,814 (17.7%) (n = 315,631) | <.001 | 13,427 (19.0%) (n = 70,688) | 14,713 (18.6%) (n = 79,307) | .27 |
SNHs, Safety-net hospitals; LOS, length of stay; SNF, skilled nursing facility; LTACH, long-term acute care hospital.
Admissions in the month of December excluded.
Admissions in the months of October through December excluded. Categorical variables are presented as number (%) and continuous variables are presented as median (interquartile range).
Our multivariable model of the complete cohort identified several factors associated with in-hospital mortality: congestive heart failure (OR, 3.22; 95% CI, 2.96-3.49), chronic kidney disease (OR, 2.00; 95% CI, 1.85-2.16), age >65 years (OR, 1.99; 95% CI, 1.84-2.14), need for urgent surgery (OR, 1.69; 95% CI, 1.56-1.84), female sex (OR, 1.60; 95% CI, 1.49-1.72), peripheral arterial disease (OR, 1.55; 95% CI, 1.43-1.68), lowest household income quartile (OR, 1.17; 95% CI, 1.08-1.27), and Medicaid/uninsured (OR, 1.17; 95% CI, 1.03-1.34). Of note, hospital safety-net status was not significantly associated with in-hospital mortality (OR, 1.09; 95% CI, 0.98-1.20) (Table 4).
Table 4.
Multivariable logistic regression model for in-hospital mortality
| Variable | Adjusted OR (95% CI) | P value |
|---|---|---|
| Congestive heart failure | 3.22 (2.96-3.49) | <.001 |
| Chronic kidney disease | 2.00 (1.85-2.16) | <.001 |
| Age >65 y | 1.99 (1.84-2.14) | <.001 |
| Non-elective surgery | 1.69 (1.56-1.84) | <.001 |
| Female sex | 1.60 (1.49-1.72) | <.001 |
| Peripheral arterial disease | 1.55 (1.43-1.68) | <.001 |
| Lowest household income quartile | 1.17 (1.08-1.27) | <.001 |
| Uninsured or insured with Medicaid | 1.17 (1.03-1.34) | .02 |
| Safety-net hospital | 1.09 (0.98-1.20) | .11 |
| Obesity | 0.80 (0.73-0.87) | <.001 |
| Diabetes mellitus | 0.80 (0.74-0.86) | <.001 |
| Hypertension | 0.56 (0.51-0.62) | <.001 |
C-statistic = 0.754. Categorical variables are presented as number (%), and continuous variables are presented as median (interquartile range). OR, Odds ratio; CI, confidence interval.
After propensity-score matching, 204,053 patients were included (95,911 at SNHs and 108,142 at non-SNHs). Comparable rates of in-hospital mortality (2.1% vs 1.9%; P = .11), acute kidney injury (21.6% vs 21.3%; P = .54), stroke (2.0% vs 2.0%; P = .90), respiratory failure (20.2% vs 20.3%; P = .85), 30-day readmission (12.3% vs 12.0%; P = .33), and 90-day readmission (19.0% vs 18.7%; P = .52) were observed at SNHs and non-SNHs; however, the rates of pneumonia (6.2% vs 5.0%) and sepsis (2.7% vs 2.0%) were greater at SNHs than at non-SNHs (both P < .001). In addition, the longer median LOS (9 vs 8 days) and greater median total cost ($46,977 vs $39,343) observed at SNHs persisted after matching (both P < .001). After matching, patients at SNHs also remained less likely to be discharged with home health care (35.0% vs 45.8%; P < .001) (Table 3).
In a subanalysis of Medicaid-insured and -uninsured patients, we found that differences in preoperative characteristics between groups were generally similar to those observed in the original cohort (Table E2). Of note, SNHs had more Medicaid patients (81.3% vs 72.9%) whereas non-SNHs had more uninsured/self-pay patients (27.1% vs 18.7%) (both P < .001). Observed and propensity-score matched in-hospital mortality, major morbidity, and 30- and 90-day readmission for this cohort of patients were comparable at SNHs and non-SNHs. Again, after matching, these patients had greater total cost ($50,110 vs $40,318) and were less likely to be discharged with home health care (32.8% vs 39.7%) at SNHs than at non-SNHs (both P < .001) (Table E3).
Discussion
In this retrospective cohort study of patients who underwent isolated CABG in the NRD, as expected, patients at SNHs were more medically and socioeconomically complex and more often required urgent surgery than those at non-SNHs, which is in accordance with previous studies.7,12,14,16 Consistent with our hypothesis, observed outcomes were generally inferior at SNHs compared with non-SNHs; however, after matching, we found that most outcomes after CABG were comparable at SNHs and non-SNHs. Interestingly, patients who underwent CABG at SNHs had marginally longer LOS and greater total cost, even after propensity-score matching. Of note, previous studies have identified postoperative complications as a primary driver of variation in cost after CABG.20,21 Although there were comparable rates of most postoperative complications, patients at SNHs had greater rates of pneumonia and sepsis; pneumonia is often cited as a source of prolonged LOS and greater total cost after cardiac surgery.22 In part, the differences in LOS and cost may also be attributable to differences in discharge disposition between groups. We found that patients at SNHs were less likely to be discharged with home health care than patients at non-SNHs. Given that outcomes were generally similar between groups, this finding is more likely to reflect disparities in socioeconomic factors and access to skilled care, rather than a difference in patient condition. Consequently, patients at SNHs may have required additional time in the hospital to facilitate safe discharge. Given the substantial overall cost of CABG, targeted strategies to reduce LOS are warranted for SNHs to facilitate cost containment. In addition, readmission rates were comparable at SNHs and non-SNHs in our matched analysis; this is encouraging, given that previous studies have highlighted readmission rates as a metric of concern at SNHs.7,13
The existing literature comparing outcomes after cardiac surgery at SNHs versus non-SNHs has yielded conflicting results.7,9,11, 12, 13, 14,17 In a study by Hoyler and colleagues12 that examined the effect of hospital safety-net burden on the outcomes of 304,080 patients who underwent CABG in the State Inpatient Database, an initial association between safety-net burden and observed in-hospital mortality did not persist after adjustment for baseline patient and hospital characteristics by multivariable regression. Our study extends these findings by providing a matched comparison and more rigorously isolating the effect of hospital safety-net status on outcomes after CABG. Although additional studies have shown comparable early outcomes after cardiac surgery at SNHs versus non-SNHs after adjusting for patient and hospital characteristics,7,11,14,17 others have identified persistent differences in specific domains, such as failure to rescue.9 It is worth noting, however, that failure to rescue may be particularly sensitive to the disproportionate financial pressures and limited hospital resources at SNHs.23
In addition, patient- and hospital-level socioeconomic factors are inherently interconnected, rendering studies of these factors complex. Patients at SNHs, many of whom are uninsured, may be more likely to forgo necessary care and screening24 and, as a result, present with more advanced disease and greater acuity. Indeed, lower socioeconomic status25, 26, 27, 28, 29, 30 and lack of insurance31,32 are associated with greater morbidity and mortality after cardiac surgery, and they probably exert a synergistic effect on access to cardiac surgical care.3 Additional studies are needed to elucidate the complex relationship between patient- and hospital-level socioeconomic factors, their relative effects on outcomes after cardiac surgery, and where the greatest return on investment may lie for policymakers and other stakeholders aiming to improve the quality of cardiac surgical care for vulnerable populations. In general, SNHs are especially vulnerable due to their public funding, low operating margins, and relatively high rate of uncompensated care.33 One potential strategy that has been proposed to reduce the rate of uncompensated care and financial strain at SNHs is Medicare/Medicaid expansion, with efforts currently underway.34
In designing this study, the primary aims were 2-fold: first, to provide a snapshot of outcomes of CABG at SNHs in reference to non-SNHs, and second, to identify specific areas in which SNHs may fall short of non-SNHs, to provide policymakers and cardiac surgical societies areas to target for improvement. After propensity-score matching to mitigate the effect of confounding baseline covariates, we found that the rates of in-hospital mortality, major morbidity, and readmission at SNHs were comparable with those at non-SNHs. Given the practical barriers to comparing hospital safety-net status in a randomized clinical trial, this propensity-score matched study provides important insight into the controversy as to whether SNHs can provide routine cardiac surgical care with comparable outcomes to those of non-SNHs. In addition, the NRD provides a nationwide sample of all-payer admissions, giving the results of our study strong external validity and generalizability. Although the United States Department of Health and Human Services launched a large, federal action plan aimed at improving health care equity in 2011,35 significant disparities remain,5,6 and renewed initiatives to mitigate disparities are warranted. Ultimately, the results of our study are encouraging because they show that patients can obtain similar outcomes whether they undergo CABG at an SNH or a non-SNH. Although beyond the direct scope of the present study, it was also encouraging to find that these hospitals were more likely to be teaching hospitals. In turn, these results support continued funding of cardiac surgical care at SNHs, along with further investigation regarding potential cost-containment strategies such as enhanced care coordination and disposition planning to reduce LOS and minimize unplanned readmissions at SNHs.36
Limitations
Our findings should be interpreted in the context of the inherent limitations associated with retrospective studies and administrative databases. Although the NRD provides a large sample size capable of powering a robust propensity-score matched analysis, administrative data can be incomplete, and they rely on accurate coding. Consequently, patients with limited access to or use of routine health care services before surgery may be “undercoded” with respect to comorbidities. In addition, we attempted to minimize the influence of baseline covariates by using established comorbidity codes; however, the influence of residual confounding factors cannot be fully excluded. Several potential unmeasured confounders influencing the findings of this study include race and ethnicity, time from diagnosis to surgery, preoperative risk modification, frailty, left ventricular ejection fraction, lesion complexity (ie, SYNTAX score), and variation in surgical technique (eg, on- vs off-pump, number of grafts, arterial vs venous grafts). Additional commonly used risk scores, including the Society of Thoracic Surgeons Postoperative Risk of Mortality and European System for Cardiac Operative Risk Evaluation II, were not available within the NRD, precluding an analysis of observed versus expected outcomes between groups. The Society of Thoracic Surgeons Adult Cardiac Surgery Database captures several of the aforementioned data points, which are unavailable in the NRD, and could therefore provide an important extension of the present study. Nevertheless, we expect that if differences in the aforementioned factors did exist, this would bias the results against our hypothesis. In addition, we were not able to account for differences in postoperative resources or failure to rescue, key factors that have been identified as potential areas for improvement at SNHs,23 although again, we would expect that these differences would bias the results against our hypothesis. Other important limitations of the NRD are the lack of granularity with respect to intensive care unit versus overall hospital LOS and the availability of intermediate care or “step-down” units, both of which can influence discharge planning and the total cost of admission. Cost was also calculated indirectly by using cost-to-charge ratios provided by the NRD. Last, there is no consensus definition of “safety-net hospital,” and although we used a definition previously described in the literature to facilitate a 1:1 propensity-score matched analysis, the definition varies across studies in this space.37
Conclusions
After propensity-score matching, we found that early outcomes after isolated CABG were comparable at SNHs and non-SNHs (Figure 2). Initiatives to reduce LOS through improved discharge planning and resources could curtail cost and improve the value of CABG for vulnerable patients at SNHs.
Figure 2.
In the present study, we found that early outcomes after coronary artery bypass grafting (CABG) were similar at safety-net hospitals (SNHs) compared with nonsafety-net hospitals (non-SNH) after propensity-score matching.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank Scott A. Weldon, MA, CMI, FMI, for creating the illustrations and Stephen N. Palmer, PhD, ELS, for editorial support.
Appendix E1
Table E1.
ICD-10 code library
| Codes used for inclusion | |
| ICD-10-PCS 0210∗ | Bypass, coronary artery, one artery |
| ICD-10-PCS 0211∗ | Bypass, coronary artery, two arteries |
| ICD-10-PCS 0212∗ | Bypass, coronary artery, three arteries |
| ICD-10-PCS 0213∗ | Bypass, coronary artery, four or more arteries |
| Codes used for exclusion | |
| ICD-10-CM I25.42 | Coronary artery dissection |
| ICD-10-PCS 02QF∗ | Repair, aortic valve |
| ICD-10-PCS 02QG∗ | Repair, mitral valve |
| ICD-10-PCS 02QH∗ | Repair, pulmonary valve |
| ICD-10-PCS 02QJ∗ | Repair, tricuspid valve |
| ICD-10-PCS 02RF∗ | Replacement, aortic valve |
| ICD-10-PCS 02RG∗ | Replacement, mitral valve |
| ICD-10-PCS 02RH∗ | Replacement, pulmonary valve |
| ICD-10-PCS 02RJ∗ | Replacement, tricuspid valve |
| ICD-10-PCS 02H0∗ | Insertion of device, coronary artery, one artery |
| ICD-10-PCS 02H1∗ | Insertion of device, coronary artery, two arteries |
| ICD-10-PCS 02H2∗ | Insertion of device, coronary artery, three arteries |
| ICD-10-PCS 02H3∗ | Insertion of device, coronary artery, four or more arteries |
| ICD-10-PCS 02RX∗ | Replacement of thoracic aorta, ascending/arch |
| ICD-10-PCS 02RW∗ | Replacement of thoracic aorta, descending |
| ICD-10-PCS 02QX∗ | Repair of thoracic aorta, ascending/arch |
| ICD-10-PCS 02QW∗ | Repair of thoracic aorta, descending |
| ICD-10-PCS 02VX∗ | Resection of thoracic aorta, ascending/arch |
| ICD-10-PCS 02VW∗ | Resection of thoracic aorta, descending |
| ICD-10-PCS 02HX∗ | Insertion of device, thoracic aorta, ascending/arch |
| ICD-10-PCS 02HW∗ | Insertion of device, thoracic aorta, descending |
| ICD-10-PCS 04R0∗ | Replacement of abdominal aorta |
| ICD-10-PCS 04Q0∗ | Repair of abdominal aorta |
| ICD-10-PCS 04V0∗ | Resection of abdominal aorta |
| ICD-10-PCS 04H0∗ | Insertion of device, abdominal aorta |
| Codes used for tobacco use | |
| ICD-10-CM Z72.0 | Tobacco use |
| ICD-10-CM Z87.891 | Personal history of nicotine dependence |
| ICD-10-CM F17∗ | Nicotine dependence |
| Codes used for acute kidney injury | |
| ICD-10-CM N17∗ | Acute kidney failure |
| ICD-10-CM N99.0 | Postprocedural (acute) (chronic) kidney failure |
| Codes used for stroke | |
| ICD-10-CM I63∗ | Cerebral infarction |
| ICD-10-CM I97.81∗ | Intraoperative cerebrovascular infarction |
| ICD-10-CM I97.82∗ | Postprocedural cerebrovascular infarction |
| Codes used for respiratory failure | |
| ICD-10-CM J95.1 | Acute pulmonary insufficiency following thoracic surgery |
| ICD-10-CM J95.3 | Chronic pulmonary insufficiency following surgery |
| ICD-10-CM J95.82∗ | Postprocedural respiratory failure |
| ICD-10-CM J96∗ | Respiratory failure, not elsewhere classified |
| Codes used for surgical-site infection | |
| ICD-10-CM T81.31∗ | Disruption of external operation (surgical) wound, not elsewhere classified |
| ICD-10-CM T81.41∗ | Infection following a procedure, superficial incisional surgical site |
| ICD-10-CM T81.49∗ | Infection following a procedure, other surgical site |
| Codes used for deep sternal wound infection | |
| ICD-10-CM T81.32∗ | Disruption of internal operation (surgical) wound, not elsewhere classified |
| ICD-10-CM T81.42∗ | Infection following a procedure, deep incisional surgical site |
| ICD-10-CM T81.43∗ | Infection following a procedure, organ and space surgical site |
| Codes used for pneumonia | |
| ICD-10-CM J13∗ | Pneumonia due to Streptococcus pneumoniae |
| ICD-10-CM J14∗ | Pneumonia due to Hemophilus influenzae |
| ICD-10-CM J15∗ | Bacterial pneumonia, not elsewhere classified |
| ICD-10-CM J16∗ | Pneumonia due to other infectious organisms, not elsewhere classified |
| ICD-10-CM J17∗ | Pneumonia in diseases classified elsewhere |
| ICD-10-CM J18∗ | Pneumonia, unspecified organism |
| ICD-10-CM J95.851 | Ventilator associated pneumonia |
| Codes used for sepsis | |
| ICD-10-CM T81.12∗ | Postprocedural septic shock |
| ICD-10-CM T81.44∗ | Sepsis following a procedure |
| ICD-10-CM A41∗ | Other sepsis |
PCS, Procedure Coding System; CM, Clinical Modification.
All combinations of characters following the listed prefix were included.
Table E2.
Preoperative characteristics of Medicaid/uninsured patients who underwent coronary artery bypass grafting at SNH versus non-SNHs
| Variable | Unmatched (n = 51,116) |
Matched (n = 33,675) |
||||
|---|---|---|---|---|---|---|
| SNHs (n = 15,461) | Non-SNHs (n = 35,655) | P value | SNHs (n = 15,239) | Non-SNHs (n = 18,436) | SMD | |
| Age, y | 58 (52-62) | 57 (51-61) | <.001 | 58 (52-62) | 57 (51-61) | −0.140 |
| Male | 11,085 (71.7%) | 25,619 (71.9%) | .82 | 10,930 (71.7%) | 12,982 (70.4%) | −0.029 |
| Elixhauser score | 7 (−1-17) | 7 (−1-17) | .61 | 7 (−1-17) | 8 (−1-18) | 0.038 |
| Anemia | 683 (4.4%) | 1236 (3.5%) | <.001 | 650 (4.3%) | 813 (4.4%) | 0.010 |
| Arrhythmia | 5552 (35.9%) | 12,806 (35.9%) | .99 | 5470 (35.9%) | 6702 (36.4%) | 0.010 |
| Chronic kidney disease | 2746 (17.8%) | 5887 (16.5%) | .05 | 2702 (17.7%) | 3368 (18.3%) | 0.014 |
| Chronic obstructive pulmonary disease | 3790 (24.5%) | 10,634 (29.8%) | <.001 | 3751 (24.6%) | 5285 (28.7%) | 0.092 |
| Coagulopathy | 2548 (16.5%) | 6301 (17.7%) | .23 | 2521 (16.5%) | 3169 (17.2%) | 0.017 |
| Congestive heart failure | 6676 (43.2%) | 15,047 (42.2%) | .29 | 6562 (43.1%) | 8085 (43.9%) | 0.016 |
| Diabetes mellitus | 8704 (56.3%) | 18,508 (51.9%) | <.001 | 8532 (56.0%) | 10,248 (55.6%) | −0.008 |
| Drug abuse | 1544 (10.0%) | 3198 (9.0%) | .04 | 1490 (9.8%) | 1987 (10.8%) | 0.033 |
| Hypertension | 13,820 (89.4%) | 30,729 (86.2%) | <.001 | 13,606 (89.3%) | 16,253 (88.2%) | −0.035 |
| Liver disease | 707 (4.6%) | 1721 (4.8%) | .40 | 698 (4.6%) | 946 (5.1%) | 0.026 |
| Obesity | 4558 (29.5%) | 11,429 (32.1%) | .01 | 4511 (29.6%) | 5581 (30.3%) | 0.015 |
| Peripheral arterial disease | 1912 (12.4%) | 4816 (13.5%) | .03 | 1888 (12.4%) | 2415 (13.1%) | 0.021 |
| Tobacco use | 9272 (60.0%) | 23,109 (64.8%) | <.001 | 9157 (60.1%) | 11,704 (63.5%) | 0.070 |
| Valvular heart disease | 2282 (14.8%) | 5068 (14.2%) | .46 | 2251 (14.8%) | 2634 (14.3%) | −0.014 |
| Household income∗ | .04 | |||||
| Quartile 1 | 6107 (39.5%) | 12,761 (35.8%) | 5903 (38.7%) | 7608 (41.3%) | 0.052 | |
| Quartile 2 | 4379 (28.3%) | 11,262 (31.6%) | 4366 (28.7%) | 5469 (29.7%) | 0.022 | |
| Quartile 3 | 3242 (21.0%) | 7528 (21.1%) | 3238 (21.3%) | 3483 (18.9%) | −0.059 | |
| Quartile 4 | 1733 (11.2%) | 4105 (11.5%) | 1732 (11.4%) | 1876 (10.2%) | −0.038 | |
| Nonelective | 10,740 (69.5%) | 24,076 (67.5%) | .12 | 10,526 (69.2%) | 12,681 (69.2%) | −0.007 |
Categorical variables are presented as number (%), and continuous variables are presented as median (interquartile range). SNH, Safety-net hospital. SMD, standardized mean difference.
Indexed to patient's ZIP code.
Table E3.
Postoperative outcomes of Medicaid/uninsured patients who underwent coronary artery bypass grafting at SNHs versus non-SNHs
| Variable | Unmatched (n = 51,116) |
Matched (n = 33,675) |
||||
|---|---|---|---|---|---|---|
| SNHs (n = 15,461) | Non-SNHs (n = 35,655) | P value | SNHs (n = 15,239) | Non-SNHs (n = 18,436) | P value | |
| In-hospital mortality | 285 (1.8%) | 600 (1.7%) | .41 | 284 (1.9%) | 293 (1.6%) | .21 |
| Acute kidney injury | 3257 (21.1%) | 6902 (19.4%) | .02 | 3188 (20.9%) | 3803 (20.6%) | .71 |
| Stroke | 326 (2.1%) | 758 (2.1%) | .94 | 315 (2.1%) | 465 (2.5%) | .05 |
| Respiratory failure | 3201 (20.7%) | 7876 (22.1%) | .18 | 3154 (20.7%) | 4256 (23.1%) | .04 |
| Infection | ||||||
| Surgical-site infection | 60 (0.4%) | 148 (0.4%) | .78 | 58 (0.4%) | 66 (0.4%) | .83 |
| Deep sternal wound infection | 26 (0.2%) | 117 (0.3%) | .02 | 26 (0.2%) | 64 (0.3%) | .06 |
| Pneumonia | 1101 (7.1%) | 2181 (6.1%) | .02 | 1071 (7.0%) | 1198 (6.5%) | .25 |
| Sepsis | 433 (2.8%) | 868 (2.4%) | .11 | 422 (2.8%) | 495 (2.7%) | .76 |
| LOS, d | 9 (7-13) | 9 (6-12) | <.001 | 9 (7-13) | 9 (6-13) | .002 |
| Total cost, $ | $50,110 (37,655-66,456) | $40,318 (31,265-54,638) | <.001 | $49,987 (37,601-66,358) | $41,352 (31,983-56,331) | <.001 |
| Discharge disposition | <.001 | <.001 | ||||
| Routine | 8498 (56.0%) | 17,850 (50.9%) | 8402 (56.2%) | 8416 (46.4%) | ||
| Home health care | 4980 (32.8%) | 13,919 (39.7%) | 4893 (32.7%) | 7844 (43.2%) | ||
| SNF or LTACH | 1524 (10.0%) | 3013 (8.6%) | 1491 (10.0%) | 1751 (9.7%) | ||
| Other | 459 (1.2%) | 873 (2.4%) | 169 (1.1%) | 132 (0.7%) | ||
| Readmission within 30 d∗ | 1893 (13.6%) (n = 13,879) | 4086 (12.7%) (n = 32,145) | .10 | 1857 (13.6%) (n = 13,677) | 2224 (13.4%) (n = 16,606) | .77 |
| Readmission within 90 d† | 2408 (21.0%) (n = 11,443) | 5319 (20.2%) (n = 26,353) | .22 | 2359 (20.9%) (n = 11,271) | 2907 (21.5%) (n = 13,508) | .46 |
SNH, Safety-net hospital; LOS, length of stay; SNF, skilled nursing facility; LTACH, long-term acute care hospital.
Excluding admissions in the month of December.
Excluding admissions in the months of October through December. Categorical variables are presented as number (%), and continuous variables are presented as median (interquartile range).
References
- 1.Ein Lewin M., Altman S. National Academies Press (US); 2000. Americas's Health Care Safety Net: Intact but Endangered. [DOI] [PubMed] [Google Scholar]
- 2.Patel S.A., Ali M.K., Narayan K.M., Mehta N.K. County-level variation in cardiovascular disease mortality in the United States in 2009-2013: comparative assessment of contributing factors. Am J Epidemiol. 2016;184:933–942. doi: 10.1093/aje/kww081. [DOI] [PubMed] [Google Scholar]
- 3.Whittle J., Conigliaro J., Good C.B., Lofgren R.P. Racial differences in the use of invasive cardiovascular procedures in the Department of Veterans Affairs medical system. N Engl J Med. 1993;329:621–627. doi: 10.1056/NEJM199308263290907. [DOI] [PubMed] [Google Scholar]
- 4.Peterson E.D., Shaw L.K., DeLong E.R., Pryor D.B., Califf R.M., Mark D.B. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? N Engl J Med. 1997;336:480–486. doi: 10.1056/NEJM199702133360706. [DOI] [PubMed] [Google Scholar]
- 5.Schoenfeld A.J., Sturgeon D.J., Dimick J.B., Bono C.M., Blucher J.A., Barton L.B., et al. Disparities in rates of surgical intervention among racial and ethnic minorities in Medicare accountable care organizations. Ann Surg. 2019;269:459–464. doi: 10.1097/SLA.0000000000002695. [DOI] [PubMed] [Google Scholar]
- 6.Best M.J., McFarland E.G., Thakkar S.C., Srikumaran U. Racial disparities in the use of surgical procedures in the US. JAMA Surg. 2021;156:274–281. doi: 10.1001/jamasurg.2020.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoehn R.S., Wima K., Vestal M.A., Weilage D.J., Hanseman D.J., Abbott D.E., et al. Effect of hospital safety-net burden on cost and outcomes after surgery. JAMA Surg. 2016;151:120–128. doi: 10.1001/jamasurg.2015.3209. [DOI] [PubMed] [Google Scholar]
- 8.Mouch C.A., Regenbogen S.E., Revels S.L., Wong S.L., Lemak C.H., Morris A.M. The quality of surgical care in safety net hospitals: a systematic review. Surgery. 2014;155:826–838. doi: 10.1016/j.surg.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Wakeam E., Hevelone N.D., Maine R., Swain J., Lipsitz S.A., Finlayson S.R., et al. Failure to rescue in safety-net hospitals: availability of hospital resources and differences in performance. JAMA Surg. 2014;149:229–235. doi: 10.1001/jamasurg.2013.3566. [DOI] [PubMed] [Google Scholar]
- 10.Bowdish M.E., D’Agostino R.S., Thourani V.H., Desai N., Shahian D.M., Fernandez F.G., et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2020 update on outcomes and research. Ann Thorac Surg. 2020;109:1646–1655. doi: 10.1016/j.athoracsur.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Glance L.G., Kellermann A.L., Osler T.M., Li Y., Li W., Dick A.W. Impact of risk adjustment for socioeconomic status on risk-adjusted surgical readmission rates. Ann Surg. 2016;263:698–704. doi: 10.1097/SLA.0000000000001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyler M.M., Tam C.W., Thalappillil R., Jiang S., Ma X., Lui B., et al. The impact of hospital safety-net burden on mortality and readmission after CABG surgery. J Card Surg. 2020;35:2232–2241. doi: 10.1111/jocs.14738. [DOI] [PubMed] [Google Scholar]
- 13.Talutis S.D., Chen Q., Wang N., Rosen A.K. Comparison of risk-standardized readmission rates of surgical patients at safety-net and non-safety-net hospitals using Agency for Healthcare Research and Quality and American Hospital Association data. JAMA Surg. 2019;154:391–400. doi: 10.1001/jamasurg.2018.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ando T., Adegbala O., Akintoye E., Briasoulis A., Takagi H. The impact of safety-net burden on in-hospital outcomes after surgical aortic valve replacement. J Card Surg. 2019;34:1178–1184. doi: 10.1111/jocs.14187. [DOI] [PubMed] [Google Scholar]
- 15.Ando T., Akintoye E., Adegbala O., Ashraf S., Shokr M., Takagi H., et al. In-hospital outcomes of ST-segment elevation myocardial infarction complicated with cardiogenic shock at safety-net hospitals in the United States (from the Nationwide Inpatient Sample) Am J Cardiol. 2019;124:485–490. doi: 10.1016/j.amjcard.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Sridhar P., Bhatt M., Qureshi M.M., Asokan S., Truong M.T., Suzuki K., et al. Esophageal cancer presentation, treatment, and outcomes vary with hospital safety-net burden. Ann Thorac Surg. 2019;107:1472–1479. doi: 10.1016/j.athoracsur.2018.11.065. [DOI] [PubMed] [Google Scholar]
- 17.Frankel W.C., Sylvester C.B., Asokan S., Ryan C.T., Zea-Vera R., Zhang Q., et al. Outcomes, cost, and readmission after surgical aortic or mitral valve replacement at safety-net and non-safety-net hospitals. Ann Thorac Surg. 2022;114:703–709. doi: 10.1016/j.athoracsur.2022.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakowitz S., Verma A., Mabeza R.M., Cho N.Y., Hadaya J., Toste P., et al. Clinical and financial outcomes of pulmonary resection for lung cancer in safety-net hospitals. J Thorac Cardiovasc Surg. 2022 doi: 10.1016/j.jtcvs.2022.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Mehaffey J.H., Hawkins R.B., Byler M., Charles E.J., Fonner C., Kron I., et al. Cost of individual complications following coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2018;155:875–882.e1. doi: 10.1016/j.jtcvs.2017.08.144. [DOI] [PubMed] [Google Scholar]
- 21.Salenger R., Etchill E.W., Fonner C.E., Alejo D., Matthew T.L., Whitman G.J.R., et al. Hospital variability in modifiable factors driving coronary artery bypass charges. J Thorac Cardiovasc Surg. 2023;165:764–772.e2. doi: 10.1016/j.jtcvs.2021.02.094. [DOI] [PubMed] [Google Scholar]
- 22.Ailawadi G., Chang H.L., O’Gara P.T., O’Sullivan K., Woo Y.J., DeRose J.J., Jr., et al. Pneumonia after cardiac surgery: experience of the National Institutes of Health/Canadian Institutes of Health Research Cardiothoracic Surgical Trials Network. J Thorac Cardiovasc Surg. 2017;153:1384–1391.e3. doi: 10.1016/j.jtcvs.2016.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewan K.C., Zhou G., Koroukian S.M., Gillinov A.M., Roselli E.E., Svensson L.G., et al. Failure to rescue after cardiac surgery at minority-serving hospitals: room for improvement. Ann Thorac Surg. 2022;114:2180–2187. doi: 10.1016/j.athoracsur.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Ayanian J.Z., Weissman J.S., Schneider E.C., Ginsburg J.A., Zaslavsky A.M. Unmet health needs of uninsured adults in the United States. JAMA. 2000;284:2061–2069. doi: 10.1001/jama.284.16.2061. [DOI] [PubMed] [Google Scholar]
- 25.Koch C.G., Li L., Shishehbor M., Nissen S., Sabik J., Starr N.J., et al. Socioeconomic status and comorbidity as predictors of preoperative quality of life in cardiac surgery. J Thorac Cardiovasc Surg. 2008;136:665–672.e1. doi: 10.1016/j.jtcvs.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Koch C.G., Li L., Kaplan G.A., Wachterman J., Shishehbor M.H., Sabik J., et al. Socioeconomic position, not race, is linked to death after cardiac surgery. Circ Cardiovasc Qual Outcomes. 2010;3:267–276. doi: 10.1161/CIRCOUTCOMES.109.880377. [DOI] [PubMed] [Google Scholar]
- 27.Charles E.J., Mehaffey J.H., Hawkins R.B., Fonner C.E., Yarboro L.T., Quader M.A., et al. Socioeconomic distressed communities index predicts risk-adjusted mortality after cardiac surgery. Ann Thorac Surg. 2019;107:1706–1712. doi: 10.1016/j.athoracsur.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehaffey J.H., Hawkins R.B., Charles E.J., Thibault D., Williams M.L., Brennan M., et al. Distressed communities are associated with worse outcomes after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2020;160:425–432.e9. doi: 10.1016/j.jtcvs.2019.06.104. [DOI] [PubMed] [Google Scholar]
- 29.Coyan G.N., Okoye A., Shah A., Wang Y., Thoma F., Sciortino C., et al. Effect of neighborhood socioeconomic factors on readmissions and mortality after coronary artery bypass grafting. Ann Thorac Surg. 2021;111:561–567. doi: 10.1016/j.athoracsur.2020.05.102. [DOI] [PubMed] [Google Scholar]
- 30.Patrick W.L., Bojko M., Han J.J., Kelly J.J., Iyengar A., Helmers M., et al. Neighborhood socioeconomic status is associated with differences in operative management and long-term survival after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2022;164:92–102.e8. doi: 10.1016/j.jtcvs.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaPar D.J., Bhamidipati C.M., Walters D.M., Stukenborg G.J., Lau C.L., Kron I.L., et al. Primary payer status affects outcomes for cardiac valve operations. J Am Coll Surg. 2011;212:759–767. doi: 10.1016/j.jamcollsurg.2010.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaPar D.J., Stukenborg G.J., Guyer R.A., Stone M.L., Bhamidipati C.M., Lau C.L., et al. Primary payer status is associated with mortality and resource utilization for coronary artery bypass grafting. Circulation. 2012;126:S132–S139. doi: 10.1161/CIRCULATIONAHA.111.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popescu I., Fingar K.R., Cutler E., Guo J., Jiang H.J. Comparison of 3 safety-net hospital definitions and association with hospital characteristics. JAMA Netw Open. 2019;2:e198577. doi: 10.1001/jamanetworkopen.2019.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khullar D., Song Z., Chokshi D.A. Safety-net health systems at risk: who bears the burden of uncompensated care? Health Aff. May 10, 2018. https://www.healthaffairs.org/do/10.1377/forefront.20180503.138516/full/
- 35.U.S. Department of Health and Human Services HHS action plan to reduce racial and ethnic health disparities: a nation free of disparities in health and health care. https://www.minorityhealth.hhs.gov/assets/pdf/hhs/HHS_Plan_complete.pdf
- 36.Chudgar N.P., Zhu R., Gray K.D., Chiu R., Carrera A.D., Lang S.J., et al. Implementing a high-value care discharge protocol in patients undergoing CABG reduces readmission. Ann Thorac Surg. 2022;113:1112–1118. doi: 10.1016/j.athoracsur.2021.07.036. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee P., Sommers B.D., Joynt Maddox K.E. Essential but undefined - reimagining how policymakers identify safety-net hospitals. N Engl J Med. 2020;383:2593–2595. doi: 10.1056/NEJMp2030228. [DOI] [PubMed] [Google Scholar]