Abstract
A cell line was established from Culex tarsalis Coquillett embryonated eggs and designated as CxTr. The cell line is heterogeneous, composed predominantly of small, round cells, and spindle-shaped cells with a doubling time of approximately 52–60 h. The identity of the cell line was verified as Cx. tarsalis by sequencing of cytochrome oxidase I and the cells were found to be free of contaminating cells, bacteria, fungi, and mycoplasma. The permissiveness of CxTr cells to arbovirus infection was investigated with vaccine and wildtype arboviruses from four viral families: Flaviviridae (Japanese encephalitis virus), Phenuiviridae (Rift Valley fever phlebovirus), Rhabdoviridae (vesicular stomatitis virus), and Togaviridae (Mayaro virus). All viruses were able to infect and replicate within CxTr cells.
Keywords: cell line, mosquito, arbovirus, Culex tarsalis
Culex tarsalis Coquillett mosquitoes are known vectors of arthropod-borne viruses (arboviruses) such as West Nile virus, western equine encephalitis virus, and St. Louis encephalitis virus that impact human and animal health (Goddard et al. 2002, Brault 2009, Swetman et al. 2020). They are also known to be competent vectors for Japanese encephalitis virus (JEV), Rift Valley fever phlebovirus (RVFV), and Cache Valley virus by experimental infection (Reeves et al. 1946, Turell et al. 2010, Ayers et al. 2018). The availability of cell lines from natural and competent vector species is critical research tool for understanding vector–virus relationships of endemic pathogens and to assess the risk of introduced arboviruses (Wechsler et al. 1989, Tan et al. 2001, Andrade et al. 2016, Ruckert et al. 2019). Although methods for the development of mosquito cells have improved substantially, there are still a limited number of cell lines available to address specific virus–vector interactions (Kuwata et al. 2012, Walker et al. 2014, Hoshino et al. 2015). For Culex species five cell lines have been developed and used for viral research (Walker et al. 2014). In the 1970s, a Cx. tarsalis cell line was described by Chao and Ball (1976) but the use of these cells is not widespread.
Here we describe the development of a new Cx. tarsalis embryonic cell line (CxTr) that supports infection and amplification of several arboviruses. This cell line provides a useful resource for the isolation of and experimentation with viruses that are or can be transmitted Cx. tarsalis mosquitoes.
Materials and Methods
Mosquito Colony
The Cx. tarsalis mosquito colony in Manhattan, KS was established in 2017 from egg rafts from the Kern National Wildlife Refuge colony established at University of California, Davis by William Reisen in 2002. Larvae were reared at 26°C, 14:8 h light cycle, and fed a 1:1 mixture of ground TetraMin tropical fish flakes (Tetra, Blacksburg, VA) and liver powder three times/week. Pupae were collected and allowed to emerge at 26°C, 70–80% RH, and a photoperiod of 14:8 (L:D) h. Adults were provided 10% sucrose solution and deionized water ad libitum. Sheep blood (Lampire Biological Laboratories, Pipersville, PA) was provided twice/week. Open cups of deionized water were offered for oviposition four days postfeeding. Egg rafts were collected after 24 h and stored at 4°C overnight.
Establishment of CxTr Cells
Approximately 10,000 fresh Cx. tarsalis eggs were incubated at 27°C for 6 hours for embryonation. Eggs were disinfected with sodium hypochlorite (0.5% active chlorine) for 5 min with periodic vortexing, washed with 70% ethanol for 2 min with periodic vortexing, and then washed twice with sterile Glucose–Potassium–Sodium buffer (Supp Data S1 [online only]) to remove any residual bleach or ethanol. Eggs were resuspended in 5 ml of CxTr growth medium (Supp Data S1 [online only]) and homogenized in a glass Ten Broeck homogenizer to release the embryos. After supernatant removal, the homogenizer was rinsed twice with 5 ml of CxTr growth medium + antibiotics (Supp Data S1 [online only]). The rinses were added to the supernatant and split into two 25 cm2 tissue culture-treated (TC) flasks (Thermo Fisher Scientific, Waltham, MA), filled with CxTr growth medium + antibiotics and incubated at 27°C without CO2. Cultures were observed using a Leica DMIL inverted microscope to track cell attachment and growth.
Growth Kinetics of CxTr Cells
The growth kinetics of the CxTr cell line was determined by seeding nine 25 cm2 TC flasks at three different seeding densities: 4,000 cells/cm2, 12,000 cells/cm2, and 40,000 cells/cm2. On days 1–8 and day 11, cells were removed from one flask of each seeding density, diluted 1:5 in Trypan Blue (0.04% w/v; Thermo Fisher Scientific, Waltham, MA), counted on a hemocytometer and cell density (cells per cm2) was calculated. Cell density at each timepoint was log2 transformed and a simple linear regression line was fit to the points in Graphpad Prism 9.0 (www.graphpad.com) to determine doubling time and identify times of exponential growth at each seeding density.
Viral Permissiveness of CxTr Cells
Vaccine and/or wildtype arboviruses in the families Flaviviridae (JEV), Phenuiviridae (RVFV), Rhabdoviridae (vesicular stomatitis virus, VSV), and Togaviridae (Mayaro virus, MAYV) were used to assess the permissiveness of the newly developed CxTr cell line (see Table 1 for strains and passage history). TC flasks (25 cm2) were seeded at 40,000 cells/cm2 with CxTr cells and incubated at 27°C without CO2 until cells reached 80–90% confluency. Each virus was diluted in CxTr growth medium to achieve MOI = 1 and added to flasks in triplicate. Cells were incubated at 27°C (except RVFV Kenya 128b-15 which was incubated at 30°C because a 27°C incubator was not available at BSL-3) without CO2 for 1 h rocking every 15 min. After inoculum removal, cells were washed twice with 1X PBS pH 7.4 (Millepore Sigma, St. Louis, MO) and 5 ml of CxTr growth medium was added to the flask. A 1 ml sample of media was immediately removed from each flask as time zero, and replaced with 1 ml of fresh CxTr growth medium. Samples were centrifuged for 10 min at 500 × g at 4°C, supernatants were aliquoted and stored at −80°C until titration by plaque assay. This procedure was repeated every 24 h for 120 h. Samples were titrated in duplicate in 24-well plates by inoculation of serial 10-fold dilutions onto 80% confluent Vero-MARU (RVFV, MAYV, VSV) or BHK-21 (JEV) cells. Plates were incubated for 1 h at 37°C, 5% CO2 in a humidified incubator, rocking every 15 min. The inoculum was removed and 1 ml of methylcellulose overlay (Supp Data S1 [online only]) was added to each well. After 2 d (VSV), 3 d (JEV, MAYV), or 6 d (RVFV) at 37°C, 5% CO2 the plates were stained with crystal violet fixative (Supp Data S1 [online only]). Titers were calculated and reported as log10 plaque forming units per ml (pfu/ml). Viral growth curves were graphed in GraphPad Prism 9.0.
Table 1.
Virus strains used to assess the permissiveness of Culex tarsalis cells to arbovirus infection
| Family | Virus/strain | Passage history |
|---|---|---|
| Phenuiviridae | Rift Valley fever phlebovirus/MP-12 (Attenuated) |
a MRC-5 p2 |
| Phenuiviridae | *Rift Valley fever phlebovirus/Kenya 2006 128b-15 (Wildtype) |
b Vero p1 |
| Flaviviridae | **Japanese encephalitis virus/SA 14-14-2 (Vaccine strain) |
c
BHK-21 p6, dC6/36 p1, bVero p5, eBHK-21 p1 |
| Togaviridae | ***Mayaro virus/TRVL 15537 (Wildtype) |
f Vero-MARU p2 |
| Rhabdoviridae | ****Vesicular stomatitis virus/New Jersey (Wildtype) |
e
BHK-21 p1, gAG08113 p1 |
*Isolate was received from R. Bowen, Colorado State University through B. Miller, Centers for Disease Control, Fort Collins, CO. Passage history prior to receipt of the virus is unknown.
**World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) through the University of Texas Medical Branch at Galveston, TX, USA.
***American Tissue Culture Collection (ATCC), VA, USA.
****USDA APHIS National Veterinary Services Laboratory, IA, USA.
a MRC-5—Human lung fibroblasts, CCL-171, ATCC, VA, USA.
b Vero—African green monkey kidney cells, unknown source.
c BHK-21—Baby hamster kidney cells, unknown source.
d C6/36—Aedes albopictus clone C6/36 larval cells, unknown source.
e BHK-21—Baby hamster kidney fibroblast cells, CCL-10, ATCC, VA, USA.
f Vero-MARU—African green monkey kidney cells from ARS Middle America Research Unit, Panama.
g AG08113—Porcine dermal cells, Coreille Institute, NJ, USA.
Results
Establishment of CxTr Cells
Cells from the Cx. tarsalis embryos began to attach to the TC flasks after 4 d in culture. After 8 d, the attached cells, which consisted of a variety of cell morphologies, were removed from the surface of the flask by forceful pipetting and split 1:2 into new flasks. During the first week in culture, some cells were seen contracting when viewed under a light microscope. This ceased after the first passage. Over the next several weeks small, rounded cells, spindle-shaped cells, and giant cells became dominant in the culture. Passage 6 cells were submitted to IDEXX BioAnalytics (Columbia, MO) for cell type verification and sterility testing. PCR and sequencing of the cytochrome oxidase I gene verified that the cells were Cx. tarsalis, and PCR detecting multiple mycoplasma species and plating of cell culture supernatant on microbiological media were negative for microbial contaminants.
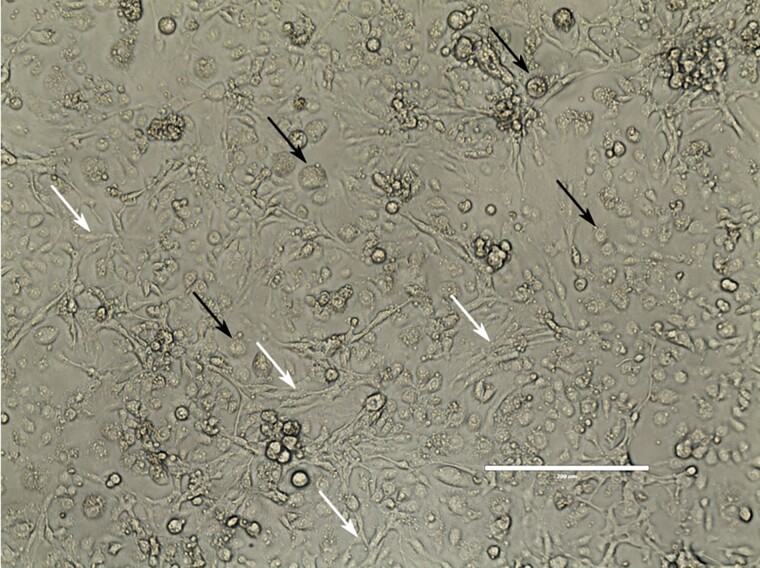
Cells remained healthy when maintained at 27°C and were subcultured at 1:10 approximately every 7 d (current passage #87). Cultures are heterogeneous with two predominant cell types: round and spindle-shaped (Fig. 1). Aliquots of 1 × 108 CxTr cells in 95% insect-tested fetal bovine serum, 5% dimethyl sulfoxide stored in liquid nitrogen regularly showed greater than 80% survival after thawing and achieved a confluent monolayer 1−2 d after inoculation into a closed 75 cm2 TC flask.
Fig. 1.
The embryonic CxTr cell line is composed of two predominant cell morphologies: round (black arrows) and spindle (white arrows). Image was taken using a Leica DMIL inverted microscope with phase contrast, total magnification 20×. Bar is 200 um.
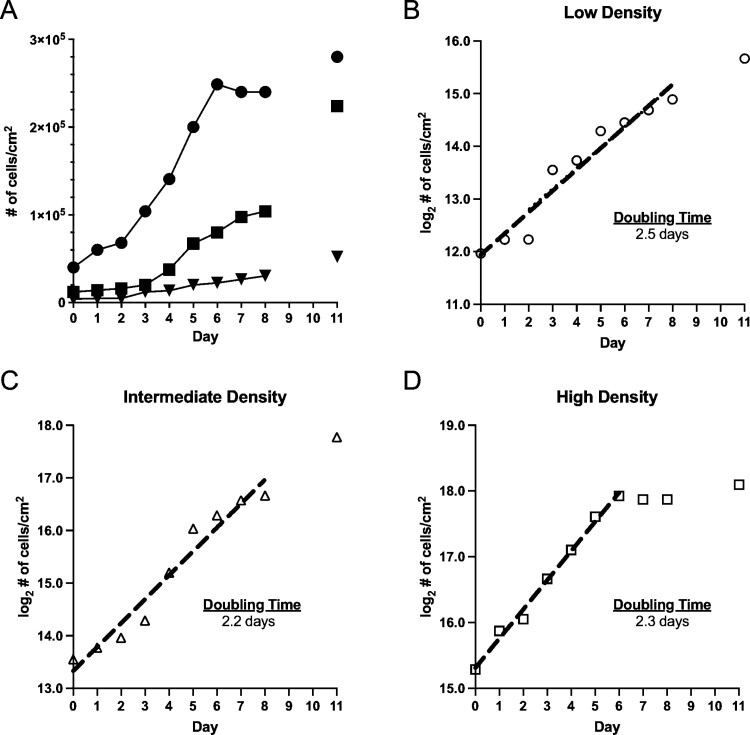
Investigation of CxTr growth kinetics at three initial densities (4,000, 12,000, and 40,000 cells/cm2) showed that the cells survived and began to increase in number, although at different rates (Fig. 2A). At the lowest initial cell density (4,000 cells/cm2), cell numbers increased slowly throughout the sampling period with a doubling time of ~2.5 d (Fig. 2B). After 3 d of minimal growth, the intermediate density cells doubled every 24 hours until day 5 and then growth slowed through day 8 (Fig. 2C). The doubling time of the intermediate density cells was ~2.2 d. When cells were seeded at high density (40,000 cells/cm2) the doubling time was ~2.3 d until day 6 and then growth ceased through day 8 (Fig. 2D).
Fig. 2.
Growth kinetics of CxTr cells seeded at different initial densities. (A) Cell growth reported as cell density cells/cm2 for: Filled inverted triangles represent low initial density—4,000 cells/cm2 data points. Filled squares represent intermediate initial density—12,000 cells/cm2 data points. Filled circles represent high initial density—40,000 cells/cm2 data points. Log2 transformations of cell count data from seeding densities over the 8-day period. Dashed lines were fitted by simple linear regression and indicate exponential growth. Doubling times are indicated in the graphs. (B) Low density, (C) Intermediate density, (D) High density. The line fitted excludes the points after Day 5, when growth began to decline. Analysis and graphs were made with GraphPad Prism 9.0. See Supp Data S2 (online only) for details of the linear regressions.
Viral Susceptibility of CxTr Cells
The permissiveness of CxTr cells to arbovirus infection was assessed by growth curves with viruses from four virus families (Table 1). All viruses were able to infect CxTr cells and initiate a productive infection as shown by increases in viral titers across the sampling period (Fig. 3). No cytopathic effect was seen in CxTr cells during infection with any of the viruses tested. The peak titer of RVFV MP-12, an attenuated vaccine strain was nearly 2 log10 pfu/ml lower (Fig. 3A) than RVFV Kenya 2006 128b-15 (Fig. 3B) (5.8 pfu/ml vs 7.6 pfu/ml respectively). The two strains of RVFV showed a continued increase in viral titer throughout the sampling period, with peak titer occurring at 120 hpi. MAYV produced a modest increase in viral titer during the sampling period (~2.5 log10 pfu/ml), most of which occurred during the first 24 hpi (Fig. 3C). The vaccine strain of JEV quickly grew to high titer in CxTr cells (~6 log10 pful/ml at 24 hpi) and remained at this level through 120 hpi (Fig. 3D). VSV showed the lowest total increase in viral titer of all the viruses (~1.4 log10 pful/ml at 120 hpi). The majority of this increase (~1.2 log10 pful/ml) occurred within 48 hpi (Fig. 3E).
Fig. 3.
Assessment of CxTr cells for infection and amplification of viruses in Phenuiviridae, Flaviviridae, Togaviridae, and Rhabdoviridae families by growth curve. The three replicates of each growth curve are graphed to show the variability between replicates. (A) Rift Valley fever phlebovirus MP-12, (B) Rift Valley fever phlebovirus Kenya 2006 128b-15, (C) Japanese encephalitis virus SA 14-14-2, (D) Mayaro virus TRVL 15537, (E) Vesicular stomatitis virus New Jersey, and (F) all viruses.
Discussion
In this study, we describe the establishment of an embryonic Cx. tarsalis cell line, designated CxTr. Growth kinetics of CxTr cells seeded at low, intermediate, and high densities show a doubling time of 2.2–2.5 d and simple linear regression of the log2 cell density values over the 8-day sampling period indicated that CxTr cells achieved exponential growth rates. Growth at low and intermediate initial densities was limited for several days prior to the cells achieving exponential growth, suggesting that to maintain exponential growth CxTr cells should be split at densities greater than 12,000 cells/cm2. At the highest initial density, cell growth plateaued at 5 d which indicated that the cells may display contact inhibition as confluency approached 100%. Although no testing was done to determine cell type, we speculate that the round cells may be epithelial-like cells, similar to C6/36 [Aedes albopictus (Skuse)] cells, whereas the spindle-shaped cells may be fibroblasts. Similar cellular morphologies have also been found in cell lines from Aedes triseriatus (Say) and Aedes fluviatilis (Lutz) (Charpentier et al. 1995, Lynn 1996, Conceição et al. 2021).
The ability of the viruses studied to infect and replicate in CxTr cells correlates well with the viruses’ natural vectors. JEV and RVFV are both known to be transmitted by Culex spp mosquitoes in Asia and Africa, respectively. Although Cx. tarsalis are not found in JEV and RVFV endemic areas, these mosquitoes are known to be competent vectors for both viruses by experimental infection (Reeves et al. 1946, Gargan et al. 1988, Turell et al. 2010). The high titers achieved by these viruses during infection of CxTr cells likely reflected the shared genetics within the Culex genus. The two RVFV strains showed a similar pattern of increasing virus titer over the sampling period, which was consistent with the pattern of RVFV replication described by Oelofsen et al. (1990) upon infection of a newly established Culex theileri Theobald cell line (a natural vector of RVFV). In our study, the RVFV MP-12 and wildtype growth curves were separated by nearly 2 log10 pfu/ml. This result was likely due to the higher incubation temperature for the wildtype virus. Turell and Rossi (1991) showed that RVFV MP-12 and ZH501 reached nearly identical titers after 5 d when grown in Vero cells at 35°C. Because the plateau phase was not reached during the sampling period, it indicated that despite an MOI of 1, only a fraction of CxTr cells were initially infected and the number of infected CxTr cells increased throughout the sampling period. Although, this hypothesis was not confirmed in this study, it was supported by the work of Main et al. (1977). In their study, Chao and Ball Cx. tarsalis cells were infected with two togaviruses and a bunyavirus at high MOIs and when the cells were labeled with a virus-specific antibody only 5–40% of cells were positive for viral antigen (Main et al. 1977). MAYV is transmitted by canopy-dwelling Hemagogus spp mosquitoes and VSV has been isolated from Aedes spp mosquitoes, sand flies, black flies, and midges (Rozo-Lopez et al. 2018). The phylogenetic distance between Hemagogus spp vectors or the many vectors of VSV and Cx. tarsalis mosquitoes may account for the low titers produced by MAYV and VSV in this study.
Supplementary Material
Acknowledgments
We thank Christopher Barker and Olivia Winokur of the University of California, Davis who generously provided egg rafts from the Kern National Wildlife Refuge Cx. tarsalis mosquito colony. This work was supported by United States Department of Agriculture, Agricultural Research Service, National Program 103 and National Program 104, Project #3020-32000-019-00D and #3020-32000-020-00D and, Project #3020-32000-018-00D. This work was also partially funded through grants from The National Bio- and Agro-Defense Facility Transition Fund (JAR), the AMP Core of the Center of Emerging Zoonotic Infectious Diseases under award number P20GM130448 (JAR), and The NIAID supported Center of Excellence for Influenza Research and Response, contract number 75N93021C00016 to JAR).
Disclaimer: Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The conclusions in this report are those of the authors and do not necessarily represent the views of the USDA. USDA is an equal opportunity provider and employer. Raw data for figures available through Ag Data Commons within 30 months of publication.
Contributor Information
Erin E Schirtzinger, Arthropod-borne Animal Diseases Research Unit, USDA, ARS, Manhattan, KS, USA; Department of Diagnostic Medicine/Pathology, College of Veterinary Medicine, Kansas State University, Manhattan, KS, USA.
Dane C Jasperson, Arthropod-borne Animal Diseases Research Unit, USDA, ARS, Manhattan, KS, USA.
Dustin A Swanson, Arthropod-borne Animal Diseases Research Unit, USDA, ARS, Manhattan, KS, USA.
Dana Mitzel, Foreign Arthropod-borne Animal Diseases Research Unit, National Bio- and Agro-Defense Facility, USDA, ARS, Manhattan, KS, USA.
Barbara S Drolet, Arthropod-borne Animal Diseases Research Unit, USDA, ARS, Manhattan, KS, USA.
Juergen A Richt, Department of Diagnostic Medicine/Pathology, College of Veterinary Medicine, Kansas State University, Manhattan, KS, USA.
William C Wilson, Foreign Arthropod-borne Animal Diseases Research Unit, National Bio- and Agro-Defense Facility, USDA, ARS, Manhattan, KS, USA.
References Cited
- Andrade, C. C., Young K. I., Johnson W. L., Villa M. E., Buraczyk C. A., Messer W. B., and Hanley K. A.. 2016. Rise and fall of vector infectivity during sequential strain displacements by mosquito-borne dengue virus. J. Evol. Biol. 29: 2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers, V. B., Huang Y. S., Lyons A. C., Park S. L., Higgs S., Dunlop J. I., Kohl A., Alto B. W., Unlu I., Blitvich J., . et al. 2018. Culex tarsalis is a competent vector species for Cache Valley virus. Parasit. Vector. 11: 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault, A. 2009. Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility. Vet. Res. 40: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, L., and Ball G. H.. 1976. A comparison of amino acid utlilization by cell lines of Culex tarsalis and Culex pipiens., pp. 263–266. In Kunstak E. and Maramorosch K. (eds.), Invertebrate tissue culture: applications in medicine, biology and agriculture. Academic Press, New York. [Google Scholar]
- Charpentier, G., Belloncik S., Ducros G., Fontenille D., Tian L., and Quiot J. M.. 1995. Establishment and characterization of three cell lines from Aedes triseriatus (Diptera: Culicidae). J. Med. Entomol. 32: 793–800. [DOI] [PubMed] [Google Scholar]
- Conceição, C. C., de Silva J. N., Arcanjo A., Nogueira C. L., de Abreu L. A., de Oliveira P. L., Gondim K. C., Moraes B., de Carvalho S. S., da Silva R. M., . et al. 2021. Aedes fluviatilis cell lines as new tools to study metabolic and immune interactions in mosquito-Wolbachia symbiosis. Sci. Rep. 11: 19202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargan, T. P., 2nd, Clark G. G., Dohm D. J., Turell M. J., and Bailey C. L.. 1988. Vector potential of selected North American mosquito species for Rift Valley fever virus. Am. J. Trop. Med. Hyg. 38: 440–446. [DOI] [PubMed] [Google Scholar]
- Goddard, L. B., Roth A. E., Reisen W. K., and Scott T. W.. 2002. Vector competence of California mosquitoes for West Nile virus. Emerg. Infect. Dis. 8: 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino, K., Isawa H., Kuwata R., Tajima S., Tomohiko T., Iwabuchi K., Sawabe K., Kobayashi M., and Sasaki T.. 2015. Establishment and characterization of two new cell lines from the mosquito Armigeres subalbatus (Coquillett) (Diptera: Culicidae). In Vitro Cell. Dev. Biol. Anim. 51: 672–679. [DOI] [PubMed] [Google Scholar]
- Kuwata, R., Hoshino K., Isawa H., Tsuda Y., Tajima S., Sasaki T., Takasaki T., Kobayashi M., and Sawabe K.. 2012. Establishment and characterization of a cell line from the mosquito Culex tritaneniorhynchus (Diptera: Culicidae). In Vitro Cell. Dev. Biol. Anim. 48: 369–376. [DOI] [PubMed] [Google Scholar]
- Lynn, D. E. 1996. Development and characterization of insect cell lines. Cytotechnology 20: 3–11. [DOI] [PubMed] [Google Scholar]
- Main, O., Hardy J. L., and Reeves W. C.. 1977. Growth of arboviruses and other viruses in a continuous line of Culex tarsalis cells. J. Med. Entomol. 14: 107–112. [DOI] [PubMed] [Google Scholar]
- Oelofsen, M. J., Gericke A., Smith M. S., and Van Der Linde T. C. De K.. 1990. Establishment and characterization of a cell line from the mosquito Culex (Culex) theileri (Diptera: Culicidae) and its susceptibility to infection with arboviruses. J. Med. Entomol. 27: 939–944. [DOI] [PubMed] [Google Scholar]
- Reeves, W. C., Hammon W. M., Wolf G. G., and Espana C.. 1946. Laboratory transmission of Japanese B encephalitis virus by seven species (three genera) of North American mosquitoes. J. Exp. Med. 83: 185–194. [PubMed] [Google Scholar]
- Rozo-Lopez, P., Drolet B. S., and Londono-Renteria B.. 2018. Vesicular stomatitis virus transmission: a comparison of incriminated vectors. Insects. 9: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckert, C., Prasad A. N., Garcia-Luna S. M., Robison A., Grubaugh N. D., Weger-Lucarelli J., and Ebel G. D.. 2019. Small RNA responses of Culex mosquitoes and cell lines during acute and persistent virus infection. Insect Biochem Molec 109: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swetman, D. M., Stuart J. B., Young K., Maharaj P. D., Fang Y., Garcia S., Barker C. M., Smith K., Godsey M. S., Savage H. M., . et al. 2020. Movement of St. Louis encephalitis virus in the Western United States, 2014-2018. PLoS Negl. Trop. Dis. 14: e0008343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, B. H., Nason E., Staeuber N., Jiang W., Monastryrskaya K., and Roy P.. 2001. RGD tripeptide of bluetongue virus VP7 protein is responsible for core attachment to Culicoides cells. J. Virol. 75: 3937–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell, M. J., and Rossi C. A.. 1991. Potential for mosquito transmission of attenuated strains of Rift Valley fever virus. Am. J. Trop. Med. 44: 278–282. [DOI] [PubMed] [Google Scholar]
- Turell, M. J., Wilson W. C., and Bennett K. E.. 2010. Potential for North American mosquitoes (Diptera: Culicidae) to transmit Rift Valley fever virus. J. Med. Entomol. 47: 884–889. [DOI] [PubMed] [Google Scholar]
- Walker, T., Jeffries C. L., Mansfield K. L., and Johnson N.. 2014. Mosquito cell lines: history, isolation, availability and application to assess the threat of arbovirus transmission in the United Kingdom. Parasit. Vector. 7: 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, S. J., McHolland L. E., and Tabachnick W. J.. 1989. Cell lines from Culicoides variipennis (Diptera: Ceratopogonidae) support replication of Bluetongue virus. J. Invertebr. Pathol. 54: 385–393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.