Abstract
Context
Long-term follow-up has been recommended for patients with pheochromocytoma or paraganglioma (PPGL) due to potential for recurrent disease. However, the need to follow patients with sporadic PPGL has recently become controversial.
Objective
To investigate the prevalence of recurrence among patients with sporadic compared with hereditary PPGL and to identify predictors of recurrence for sporadic disease.
Methods
This multicenter study included retrospective data from 1127 patients with PPGL. In addition to sex and age at primary tumor diagnosis, clinical information included location, size, and catecholamine phenotype of primary tumors, genetic test results, and subsequent development of recurrent and/or metastatic disease. Patients with sporadic PPGL were defined as those with negative genetic test results.
Results
Prevalence of recurrence among patients with sporadic PPGL (14.7%) was lower (P < 0.001) than for patients with pathogenic variants that activate pseudohypoxia pathways (47.5%), but similar to those with variants that activate kinase pathways (14.9%). Among patients with sporadic recurrent PPGL, 29.1% and 17.7% were respectively diagnosed at least 10 and 15 years after first diagnosis. Multivariable regression analysis showed that a noradrenergic/dopaminergic phenotype (HR 2.73; 95% CI, 1.553-4.802; P < 0.001), larger size (HR 1.82; 95% CI, 1.113-2.962; P = 0.017) and extra-adrenal location (HR 1.79; 95% CI, 1.002-3.187; P = 0.049) of primary tumors were independent predictors of recurrence in sporadic PPGL.
Conclusion
Patients with sporadic PPGL require long-term follow-up, as supported by the 14.7% prevalence of recurrent disease, including recurrences at more than 10 years after first diagnosis. The nature of follow-up could be individualized according to tumor size, location, and biochemical phenotype.
Keywords: pheochromocytoma, paraganglioma, sporadic, metastases, recurrence, disease free period
Pheochromocytomas and paragangliomas (PPGLs) are neuroendocrine tumors derived from chromaffin cells of the adrenal medulla or extra-adrenal paraganglionic tissue (1). PPGLs exhibit a 15% to 20% prevalence of recurrence (2–4), which may only become apparent decades after primary tumor resection (5). Recurrence among patients with PPGL mainly involves metastatic disease (2, 6, 7), and as expected is associated with heightened morbidity and mortality (8, 9). There remain no histopathological methods to reliably assess potential recurrence after resection of the primary tumor (10, 11). Therefore, follow-up is recommended for all patients (12).
Findings on outcomes including propensity to recurrence are quite heterogeneous. The variance depends mainly on factors such as hereditary background (13). Close to 35% of patients with PPGL have hereditary disease, reflecting pathogenic variants in at least 20 tumor susceptibility genes (14, 15). Patients with PPGL due to pathogenic variants in cluster 1 genes that cause stabilization of hypoxia-inducible factors and activation of hypoxia signaling pathways have high rates of recurrent disease (16, 17). Thus, lifelong follow-up is recommended for all patients with PPGL due to cluster 1 pathogenic variants (12). Recurrence rates are significantly lower in patients with PPGL due to pathogenic variants in cluster 2 genes, which are associated with activation of kinase signaling pathways (18). Nevertheless, lifelong follow-up is still considered essential (12).
Although the need for follow-up is clearly established for patients with hereditary PPGL, the need for follow-up on patients with sporadic tumors remains unclear. A systematic review of 38 studies indicated a 5-year probability of recurrence among patients with sporadic PPGL of 4.7% (13). Similarly, in the systematic meta-analysis of Holscher et al (19), prevalence of recurrence among patients with sporadic pheochromocytoma did not exceed 3%, again raising questions concerning need for follow-up. However, heterogeneity of methodological design and lack of exhaustive follow-up in the aforementioned studies, preclude any firm conclusions concerning the need for follow-up among patients with sporadic PPGL.
Several studies focused on the identification of prognostic markers to elucidate potential of recurrent disease among patients with PPGL. Apart from hereditary predisposition, younger age at initial tumor diagnosis (4, 20), extra-adrenal tumor location, larger tumor size (6, 20, 21), and higher levels of dopamine and its metabolite, methoxytyramine (6), are reported to be associated with a heightened risk of recurrence. However, it remains unclear how these factors might contribute to recurrence in patients specifically with sporadic PPGL. This is important, since should follow-up be indicated among such patients, it could be useful to tailor this according to specific clinical and biochemical predictors of recurrence.
With the above considerations in mind, the aim of this study was to investigate the prevalence of recurrent disease among patients with sporadic PPGL. A second objective was to identify predictors of recurrence that might be useful to triage any need for follow-up. For perspective, we also compared the prevalence of recurrence in patients with sporadic PPGL with that in patients with hereditary disease due to pathogenic variants in cluster 1 and cluster 2 genes.
Methods
Study Design
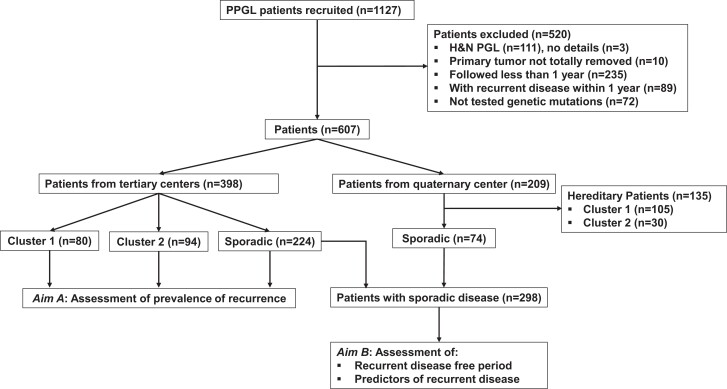
This study included retrospective data from 1127 patients with PPGL enrolled at 1 quaternary center and 7 tertiary clinical centers as detailed in the online supplement (22). Among all patients, 520 were excluded from the analysis due to one or more of the following reasons: (1) presence of only head and neck paragangliomas, as tumors in these locations were treated with different surgical approach; (2) lack of data regarding the exact location of primary tumors; (3) incomplete resected/unresected primary tumors; (4) insufficient follow-up (<12 months); (5) presence of recurrent disease within 12 months from primary tumor diagnosis; and/or (6) incomplete genetic test results (Fig. 1). Among the remaining 607 patients, 398 were enrolled at the 7 tertiary clinical centers and 209 at a quaternary referral center. Since the quaternary referral center specializes in patients with recurrent and metastatic PPGL, prevalence of recurrent disease was only assessed for patients enrolled at the tertiary clinical centers. On the other hand, data to assess recurrence-free period and identify predictors of recurrent disease were from all patients with sporadic PPGL (Fig. 1).
Figure 1.
Flow chart of patients enrolled in the study.
Clinical Data Collection
In addition to sex and age at primary tumor diagnosis, other clinical information included locations and size of primary tumors, genetic test results, and tumor catecholamine biochemical phenotypes as assessed from measurements of plasma free metanephrines and methoxytyramine. Assessments of recurrent disease took into account dates of first diagnosis of primary tumors and follow-up time in relation to any local recurrence or development of new tumor and/or metastatic disease. Diagnosis of primary tumors was based on histopathological examination of surgically resected specimens. Patients with multifocal tumors located both within and outside of adrenal glands were classified as having extra-adrenal PPGL. Location and dimensions of tumors were determined based on imaging studies and/or pathological records. Tumor diameter was determined according to at least 2 recorded dimensions as described elsewhere (23). Recurrence was defined as the presence of locoregional and/or a new PPGL and/or metastatic disease, at least 1 year after complete surgical resection of primary tumors. In our cohort all patients presented with functional PPGLs, and recurrence was diagnosed in conventional and occasionally functional imaging studies after a positive biochemical test during follow-up. Confirmation of recurrent disease required histopathological examination of surgically resected or biopsied tissue or diagnosis of inoperable metastases according to combinations of conventional and functional imaging studies.
Biochemical Measurements
Measurements of plasma free normetanephrine, metanephrine, and methoxytyramine were performed using liquid chromatography with electrochemical detection (24) or tandem mass spectrometry (25). Designation of an adrenergic biochemical phenotype required an elevated plasma concentration of metanephrine above 62 pg/mL (0.31 nmol/L) and a tumor-derived increase of metanephrine of more than 5% of the combined increases of normetanephrine, metanephrine, and methoxytyramine (23). All other tumors with positive biochemical test results, including those with predominantly increased plasma methoxytyramine, were defined as noradrenergic/dopaminergic.
Genetic Testing
Genetic testing for germline pathogenic variants of SDHx, VHL, FH, MDH2, EPAS1, TMEM127, MAX, and RET was performed using next-generation sequencing and/or Sanger sequencing as described before (26). Multiplex ligation-dependent probe amplification or custom array based comparative genomic hybridization were used for detecting large-scale deletions (26). Testing of patients also included next-generation sequencing directed primarily to test tumor tissue for somatic pathogenic variants of VHL, RET, SDHB, SDHC, SDHD, MAX, and TMEM127, NF1, HRAS, and HIF2a genes. For NF1, the diagnosis was based mainly on genetic testing and on clinical manifestations according to established criteria (27). Germline pathogenic variants in SDHA, SDHB, SDHC, SDHD, SDHAF2, VHL, FH, and MDH2, as well as mosaic variants of EPAS1 were classified as cluster 1 pathogenic variants, while germline variants in TMEM127, MAX, RET, and NF1 were classified as cluster 2 pathogenic variants (26). All other patients with negative germline genetic test results, with or without somatic pathogenic variants, were defined to have sporadic disease.
Statistical Analysis
Continuous data were expressed as medians and interquartile ranges (IQR). Mann–Whitney and Kruskal–Wallis tests were used for non-normally distributed continuous parameters. Chi-square or the Fisher exact test were used to compare categorical parameters. Bonferroni corrections were used for pairwise comparisons between subgroups. Cox proportional hazards regression models with hazard ratios (HR) were evaluated to study the association of clinical parameters with recurrent disease. Cutoffs for continuous parameters were determined using receiver operating characteristic (ROC) curve analysis and the derived Youden index. IBM SPSS statistics 25 (Systat Software GmbH, Erkarth, Germany) was used for data analysis. P < 0.05 was considered statistically significant.
Results
Clinical Characteristics
Among 398 patients enrolled at the tertiary clinical centers, 56.3% (224/398) had sporadic disease, 20.1% (80/398) had PPGL due to cluster 1 pathogenic variants, and 23.6% (94/398) PPGL due to cluster 2 pathogenic variants (Table 1). Patients with sporadic PPGL were older than those with hereditary tumors (P < 0.001), whereas sex distributions did not differ among the groups (P = 0.713). Patients with sporadic PPGL presented more often with adrenal tumors than those with PPGL due to cluster 1 pathogenic variants (94.2% vs 53.8%, P < 0.001), but not to those with tumors due to cluster 2 pathogenic variants (94.2% vs 98.9%). Finally, patients with sporadic PPGL presented more often (62.8% vs 2.9%, P < 0.001) with adrenergic tumors than those with cluster 1 pathogenic variants, but less often (62.8% vs 91.4% P < 0.001) than those with cluster 2 pathogenic variants. Finally, 17 patients presented with multifocal primary tumors, all due to cluster 1 pathogenic variants.
Table 1.
Clinical characteristics of patients enrolled at the tertiary clinical centers
| Cluster 1 PPGL | Cluster 2 PPGL | Sporadic PPGL | |
|---|---|---|---|
| Number of patients | 80 | 94 | 224 |
| Age (years) at first diagnosis, median (IQR) | 28.7 (20.5-43.8) | 46.8 (31.9-59)a | 52.9 (42.5-62.4)a,b |
| Males | 47.5% (38/80) | 41.5% (39/94) | 44.6% (109/224) |
| Location | |||
| ȃAdrenal | 53.8% (43/80) | 98.9% (93/94)a | 94.2% (211/224)a |
| ȃExtra-adrenal | 46.3% (37/80) | 1.1% (1/94)a | 5.8% (13/224)a |
| Biochemical phenotype | |||
| ȃAdrenergic | 2.9% (2/68) | 91.4% (74/81)a | 62.8% (113/180)a,b |
| ȃNoradrenergic/dopaminergic | 97.1% (66/68) | 8.6% (7/81)a | 37.2% (67/180)a,b |
| Size of primary tumor (cm), medians (IQR) | 3.6 (2.6-4.8) | 3.5 (2.4-5.0) | 4.1 (2.8-5.8) |
| With recurrent disease | 47.5% (38/80) | 14.9% (14/94)a | 14.7% (33/224)a |
| ȃLocal recurrence/new tumor only | 22.5% (18/80) | 13.8% (13/94) | 6.7% (15/224)a |
| ȃWith metastasis | 25% (20/80) | 1.1% (1/94)a | 8% (18/224)a,b |
| Follow-up (years), median (IQR) | 8.7 (5.2-16.3) | 5.3 (3.1-9.8)a | 7 (4.6-10)a |
Abbreviations: IQR, interquartile range; PPGL, pheochromocytoma and paraganglioma.
Significantly different from cluster 1.
Significantly different from cluster 2.
Prevalence of Recurrent Disease Among Patients With PPGL
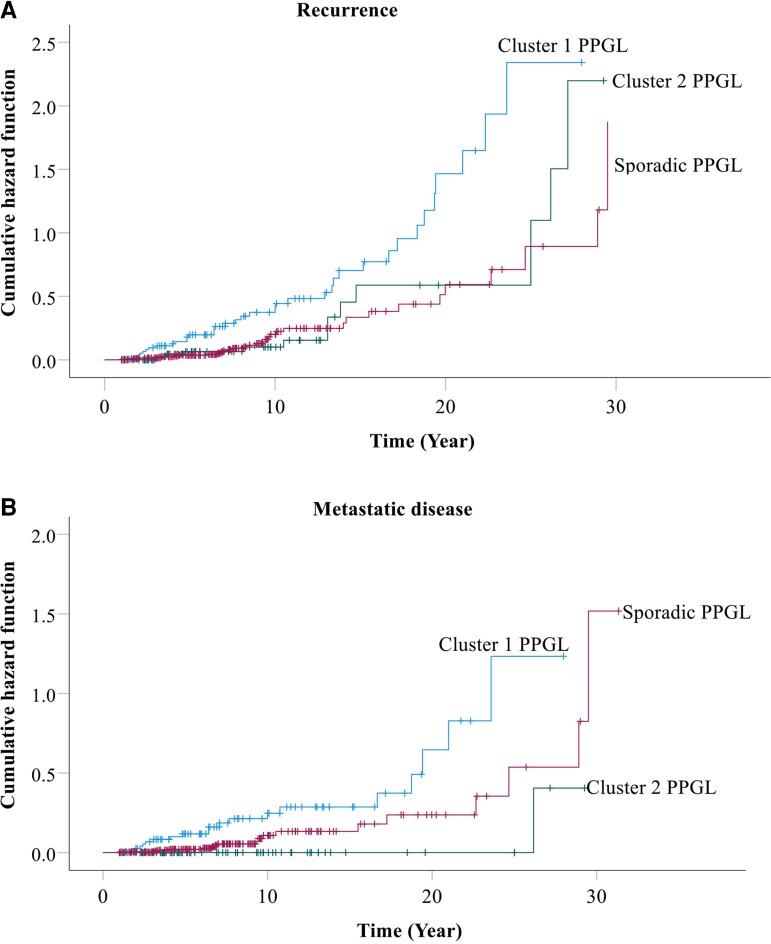
Prevalence of recurrence was analyzed based only on patients recruited at tertiary centers in order to avoid potential overestimation associated with a higher proportion of patients with recurrent disease from the quaternary center. Among patients with sporadic PPGL enrolled at the tertiary clinical centers, 14.7% (33/224) were diagnosed with recurrence, predominantly due to metastatic (8%) disease (Table 1). Prevalence of recurrence among patients with sporadic PPGL was significantly lower (14.7% vs 47.5%, P < 0.001) compared to those with PPGL due to cluster 1 pathogenic variants, but similar to those with cluster 2 pathogenic variants (14.7% vs 14.9%). This translated to a 2.45-fold higher risk of recurrence in patients with PPGL due to cluster 1 pathogenic variants compared to those with sporadic PPGL (P < 0.001), but similar risks of recurrence (P = 0.777) in patients with sporadic tumors and those with tumors due to cluster 2 pathogenic variants (Fig. 2A). The higher risk of recurrence was mainly related to metastatic disease, as patients with PPGL due to cluster 1 pathogenic variants presented with a 2.48-fold higher risk of metastasis than those with sporadic PPGL (Fig. 2B). After excluding 13 cases of patients with sporadic paraganglioma, prevalence of recurrence among patients with sporadic pheochromocytoma was only slightly reduced to 14.2% [Supplementary Table S1, (22)].
Figure 2.
Cumulative hazard of recurrence (A) and only metastatic disease (B) among patients with sporadic vs hereditary PPGL. Patients with cluster 1 PPGL presented with a 2.45-fold higher risk of recurrence compared with those with sporadic tumors, whereas risk of recurrence was similar between patients with cluster 2 and sporadic PPGL (A). Similarly, patients with cluster 1 PPGL presented with a 2.48-fold higher risk of metastasis compared with those with sporadic tumors, whereas risk of recurrence was similar between patients with cluster 2 and sporadic PPGL (B).
Among patients with sporadic PPGL, those with an adrenergic biochemical phenotype presented with lower prevalence of recurrent disease (9.7% vs 26.9%, P < 0.001) than those with a noradrenergic/dopaminergic biochemical phenotype. Importantly, the prevalence of recurrent disease was reduced to 3.2% for patients with small (< 3 cm) sporadic adrenergic PPGL.
Among the 224 patients with sporadic PPGL enrolled at the 7 tertiary clinical centers (Table 1), 37% had a previous history of tumors at recruitment into the study. In order to avoid overestimation of recurrence due to referral bias, we then analyzed the prevalence of recurrence only among patients without a previous history of PPGL and who had a follow-up period of at least 5 years. According to the above criteria, 149 patients were included in the subanalysis, among whom 64.4% (96/149) had sporadic PPGL, 17.4% (26/149) had PPGL due to cluster 1 pathogenic variants, and 18.1% (27/149) due to cluster 2 pathogenic variants (Table 2). In this subanalysis, 6 out of the 96 patients (6.3%) with sporadic PPGL were diagnosed with recurrence; all had pheochromocytoma. The aforementioned recurrence rate for patients with sporadic PPGL was significantly lower compared to the rate among patients with PPGL due to cluster 1 pathogenic variants (6.3% vs 23.1%, P < 0.001), while not significantly different from the rate in patients with PPGL due to cluster 2 pathogenic variants (6.3% vs 11.1%, Table 2).
Table 2.
Clinical characteristics of a subgroup of patients enrolled at the tertiary clinical centers, as defined by the following 2 criteria: absence of previous history of PPGL and a follow-up duration of more than 5 years
| Cluster 1 PPGL | Cluster 2 PPGL | Sporadic PPGL | |
|---|---|---|---|
| Number of patients | 26 | 27 | 96 |
| Age (years) at first diagnosis, median (IQR) | 27.2 (16.5-45.9) | 48.8 (28.2-58.9)a | 53.4 (44.3-62.7)a |
| Males | 42.3% (11/26) | 33.3% (9/27) | 39.6% (38/96) |
| Location | |||
| ȃAdrenal | 65.4% (17/26) | 96.3% (26/27)a | 95.8% (92/96)a |
| ȃExtra-adrenal | 34.6% (9/26) | 3.7% (1/27)a | 4.2% (4/96)a |
| Biochemical phenotype | |||
| ȃAdrenergic | 0 | 88.9% (24/27)a | 65.6% (63/96)a |
| ȃNoradrenergic/dopaminergic | 100% (26/26) | 11.1% (3/27)a | 34.4% (33/96)a |
| Size of primary tumor (cm), median (IQR) | 3.7 (2.5-4.3) | 3.6 (2-4.5) | 4 (2.9-5.7) |
| With recurrent disease | 23.1% (6/26) | 11.1% (3/27) | 6.3% (6/96)a |
| ȃLocal recurrence/new tumor only | 11.5% (3/26) | 11.1% (3/27) | 5.2% (5/96) |
| ȃWith metastasis | 11.5% (3/26) | 0 | 1.0% (1/96)a |
| Follow-up (years), median (IQR) | 8.2 (6.2-10.4) | 8.2 (5.6-11.4) | 7.3 (6.2-9.5) |
Abbreviations: IQR, interquartile range; PPGL, pheochromocytoma and paraganglioma.
Significantly different from cluster 1.
Time to Detected Recurrence
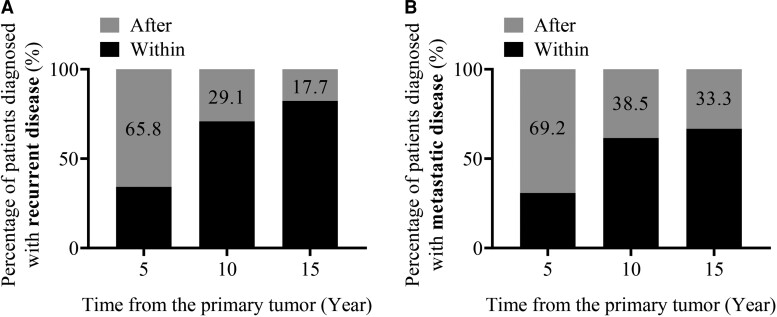
Among all 298 patients with sporadic PPGL in the entire cohort (Fig. 1), 46 from the quaternary center and 33 from the tertiary centers developed recurrent disease (Supplementary Table S3 (22)). Among those 79 patients with sporadic recurrent PPGL, only 34.2% (27/79) were diagnosed with recurrence within the first 5 years of diagnosis of the primary tumor, whereas 29.1% and 17.7% were respectively diagnosed with recurrence after 10 and 15 years of follow-up (Fig. 3A). Similarly, only 30.8% of patients with sporadic PPGL were diagnosed with metastatic disease within the first 5 years of diagnosis of the primary tumor, whereas 38.5% and 33.3% were respectively diagnosed with metastasis after 10 and 15 years of follow-up (Fig. 3B).
Figure 3.
Recurrence- (A) and metastasis- (B) free period of patients with sporadic PPGL and recurrent disease. Percentage of patients that developed recurrence (A) or only metastatic disease (B) within the specific time periods (5, 10, 15 years) corresponds to the black part of each bar.
We then examined the recurrence-free period among different subgroups of patients with sporadic recurrent PPGL. Compared to those with noradrenergic/dopaminergic PPGL, patients with adrenergic tumors were diagnosed with recurrent disease at a later time point (P < 0.001) [Supplementary Fig. S1A and S1B, (22)]. Similarly, for patients with PPGL smaller than 5 cm, the recurrence-free period was significantly longer than for those with tumors larger than 5 cm [P < 0.001, Supplementary Fig. S1C and S1D, (22)]. Finally, patients with sporadic pheochromocytoma were diagnosed with recurrences at later time points than those with sporadic paraganglioma [P = 0.022, Supplementary Fig. E and S1F, (22)].
Predictors of Recurrent Disease
Among all 298 patients with sporadic PPGL enrolled at the quaternary center and the 7 tertiary centers (Fig. 1), those with recurrent disease were younger at diagnosis of primary tumors (P < 0.001), and they presented with larger (P = 0.002) tumors and more often at extra-adrenal (P < 0.001) locations than those without recurrent disease (Supplementary Table S3, (22)). In addition, patients with recurrent disease presented more often (67.1% vs 32.8%, P < 0.001) with noradrenergic/dopaminergic tumors than those without recurrence.
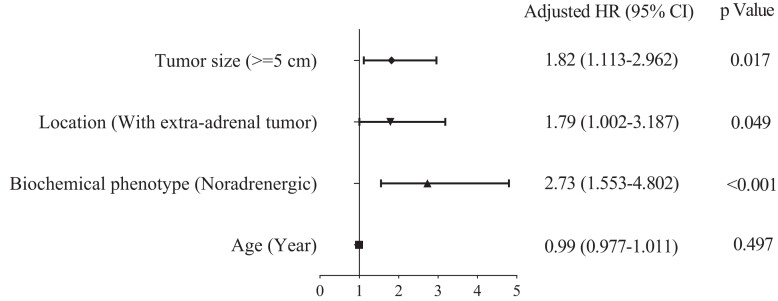
Univariable analysis revealed that among patients with sporadic PPGL, a noradrenergic/dopaminergic biochemical phenotype was the most important predictor of recurrent disease (HR 2.81; 95% CI, 1.711-4.594; P < 0.001), followed by extra-adrenal tumor location (HR 2.21; 95% CI, 1.314-3.721; P = 0.003), larger (> 5 cm) tumor size (HR 1.67; CI, 1.040-2.668; P = 0.034) and younger age at diagnosis of primary tumors (HR 0.98; 95% CI, 0.970-0.999; P = 0.031). Multivariable analysis showed that a noradrenergic/dopaminergic biochemical phenotype (HR 2.73; 95% CI, 1.553-4.802; P < 0.001), larger tumor size (≥ 5 cm) (HR 1.82; 95% CI, 1.113-2.962; P = 0.017) and extra-adrenal tumor location (HR 1.79; 95% CI, 1.002-3.187; P = 0.049) remained independent predictors of recurrence for patients with sporadic PPGL (Fig. 4).
Figure 4.
Multivariable Cox regression analysis for predictors of recurrent disease for patients with sporadic PPGL.
Discussion
It is well established that patients with hereditary PPGL require long-term follow-up (13, 16–18), whereas the need to follow patients with sporadic tumors remains unclear (4, 13). Our study is the first to focus on sporadic PPGL separately from hereditary tumors in one of the largest patient cohorts to date. Our findings of a 14.7% prevalence of recurrence in patients with sporadic PPGL, including a significant proportion diagnosed more than 10 years after initial tumor diagnosis, indicate that these patients also require long-term follow-up.
The above conclusion and our results contrast with those of a recent systematic review by Holscher et al (19), where the prevalence of recurrent disease among patients with sporadic pheochromocytoma was calculated to be 3%, compared to 14.2% for sporadic pheochromocytoma in our study. This discrepancy can be largely explained by the shorter duration of follow-up in that meta-analysis, which likely led to underestimation of the prevalence of recurrent disease. In particular, in our study, most (65.8%) patients with sporadic PPGL were diagnosed with recurrence at least 5 years after diagnosis of primary tumors. Taking this finding into consideration, the prevalence of recurrent disease might have reached 8.7% if the studies in the meta-analysis of Holscher et al (19) had involved a longer duration of follow-up.
On the other hand, the population of patients with a previous history of sporadic PPGL included in our study may have led to overestimation of recurrence. In order to control for this confounder, we performed a subanalysis, excluding patients with previous history of PPGL. We then further minimized the possibility of misclassifying patients with potential of recurrence by including in the subanalysis only those with at least 5 years of follow-up. According to the above criteria, we found a 6.3% prevalence of recurrence among patients with sporadic PPGL, which constitutes our lowest estimate. Thus, we assume that the true prevalence of recurrence among patients with sporadic PPGL is between 6.3% and 14.7%. Prospective clinical trials are required to confirm this. Apart from the meta-analysis of Holscher et al (19), another meta-analysis of 38 studies showed 5-year probabilities of recurrence between 4.7% and 7% for patients with sporadic PPGL (13). In a more recent study of Parasiliti-Caprino et al (4) that involved a limited number of patients with sporadic tumor, recurrence rates were 12% and close to the findings of our study. Besides, a recent published study by Salle et al (28) indicated a prevalence of recurrent disease of 18.2% in patients with sporadic PPGL. Highly heterogeneous methodological designs and lack of adequate follow-up preclude reliable interpretation in most previous studies.
In our study, patients with PPGL due to cluster 1 germline pathogenic variants presented with the highest rate of recurrence, mainly metastatic disease. This finding is not surprising and likely reflects different developmental origins of cluster 1 compared with cluster 2 or sporadic tumors. These origins are associated with a more undifferentiated nature of the former tumors, and activation of hypoxia signaling pathways (29) that in turn impacts the invasion-metastases cascade, leading to higher rates of metastatic disease (30, 31). In contrast to patients with cluster 1 PPGL, prevalence of recurrent disease among patients with sporadic disease was similar to that in patients with cluster 2 germline pathogenic variants. However, recurrent disease among patients with sporadic PPGL was mainly due to metastases (54.5%), whereas metastatic disease was rare among patients with tumors due to cluster 2 germline pathogenic variants (7.1%). These findings support follow-up programs for patients with sporadic PPGL in addition to already established need in patients with cluster 1 germline pathogenic variants. Follow-up for patients with PPGL due to cluster 2 germline pathogenic variants, as in most patients with cluster 1 variants, is anyhow necessary due to other clinical disorders associated with those variants (12, 32).
The majority of patients with PPGL seem to develop recurrence after 5 years from primary tumor diagnosis (13). However, the recurrence-free period for patients with sporadic recurrent PPGL remains unclear. In our study, 29% of all patients with recurrent sporadic PPGL, were diagnosed with recurrence at least 10 years after primary tumor diagnosis. Interestingly, 17.7% of patients with sporadic recurrent tumors were diagnosed with recurrence only after 15 years of follow-up, findings that might support long-term follow-up beyond 15 years. The long interval between diagnosis of first tumors and recurrent disease in many patients may reflect either or both the slow growing nature of PPGLs, with tumor volume doubling times of over 5 years (5, 33, 34), and delayed or intermittent follow-up in many patients.
Despite the implications of our study that patients with sporadic PPGL should receive long-term follow-up, the nature of follow-up programs could be tailored according to specific patient characteristics. In our study, larger tumor size and a noradrenergic/dopaminergic phenotype were associated with a higher risk of recurrence for patients with sporadic PPGL, whereas those with small (< 3 cm) adrenergic tumors had a low (3.2%) prevalence of recurrence. For the latter patients, the nature of follow-up may thus not be as exhaustive as for the former group.
As in patients with PPGL due to cluster 1 germline pathogenic variants, it is possible that specific characteristics of sporadic tumors, such as large tumor size and a noradrenergic/dopaminergic tumor phenotype, may be related to different developmental origins. Also, some patients with sporadic tumors may harbor rare (35, 36) or unknown germline pathogenic variants or somatic genomic alterations related to metastatic disease. The latter might involve genes that impact telomere maintenance, such as NOP10, ATRX, and TERT, or MAML3 gene translocations (14, 37). Larger tumors might also harbor a higher rate of genomic alterations and have a higher probability of being locally advanced, with capsular, vascular, or surrounding tissue invasion, conditions related to high rates of recurrence (4, 7, 38). The above possibilities might also explain recurrences in more than 50% of sporadic PPGL that involved metastatic disease.
Our study has limitations. First is the retrospective nature of our data and the possible referral bias that may have led to overestimation of the prevalence of recurrent disease. Also, despite standardized follow-up programs at most centers, some patients were not regularly followed and were likely to have had their recurrent disease diagnosed at more advanced stages than with regular follow-up. In this context, follow-up varied among patients of different subgroups. Despite the above limitations, our study has unparalleled strengths. We retrieved comprehensive and complete clinical, genetic, and biochemical data from one of the largest cohorts of patients with sporadic PPGL to date. All patients included in the analysis had their initial tumors completely resected. Finally, the long duration of follow-up represents a study strength that minimized the possibility of misclassifying patients with potential of recurrence and therefore underestimation of recurrence rates.
Conclusion
Patients with sporadic PPGL require long-term follow-up as they are at significant risk of recurrent disease, even 10 years after initial tumor diagnosis. Specific characteristics, such as tumor size and biochemical tumor phenotype could be used to guide individualized follow-up strategies for patients with sporadic PPGL.
Abbreviations
- HR
hazard ratio
- IQR
interquartile range
- PPGL
pheochromocytoma/paraganglioma
Contributor Information
Minghao Li, Department of Medicine ΙΙΙ, University Hospital Carl Gustav Carus at the TU Dresden, Dresden 01307, Germany.
Tamara Prodanov, Section on Medical Neuroendocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), Bethesda 20892, USA.
Leah Meuter, Section on Medical Neuroendocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), Bethesda 20892, USA.
Michiel N Kerstens, Department of Endocrinology, University of Groningen, University Medical Center Groningen, Groningen 9700, The Netherlands.
Nicole Bechmann, Institute of Clinical Chemistry and Laboratory Medicine, University Hospital Carl Gustav Carus at the TU Dresden, Dresden 01307, Germany.
Aleksander Prejbisz, Department of Hypertension, Institute of Cardiology, Warsaw 04-628, Poland.
Hanna Remde, Department of Medicine, Division of Endocrinology and Diabetes, University Hospital, University of Würzburg, Würzburg 97080, Germany.
Henri J L M Timmers, Department of Internal Medicine, Radboud University Hospital, Nijmegen 6500, The Netherlands.
Svenja Nölting, Department of Internal Medicine, University Hospital of Munich, Munich 80539, Germany; Department of Endocrinology, Diabetology, and Clinical Nutrition, University Hospital, Zurich 8091, Switzerland.
Sara Talvacchio, Section on Medical Neuroendocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), Bethesda 20892, USA.
Annika M A Berends, Department of Endocrinology, University of Groningen, University Medical Center Groningen, Groningen 9700, The Netherlands.
Stephanie Fliedner, Department of Medicine, University Medical Center Schleswig-Holstein, Luebeck 23538, Germany.
Mercedes Robledo, Hereditary Endocrine Cancer Group, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid 28029, Spain; Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER), Instituto de Salud Carlos III, Madrid 28029, Spain.
Jacques W M Lenders, Department of Medicine ΙΙΙ, University Hospital Carl Gustav Carus at the TU Dresden, Dresden 01307, Germany; Department of Internal Medicine, Radboud University Hospital, Nijmegen 6500, The Netherlands.
Karel Pacak, Section on Medical Neuroendocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), Bethesda 20892, USA.
Graeme Eisenhofer, Department of Medicine ΙΙΙ, University Hospital Carl Gustav Carus at the TU Dresden, Dresden 01307, Germany; Institute of Clinical Chemistry and Laboratory Medicine, University Hospital Carl Gustav Carus at the TU Dresden, Dresden 01307, Germany.
Christina Pamporaki, Department of Medicine ΙΙΙ, University Hospital Carl Gustav Carus at the TU Dresden, Dresden 01307, Germany.
Funding
M.L. is funded by the China Scholarship Council (No. 201906370033). This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG]) (No. 314061271-TRR/CRR 205-1/2) within the CRC/Transregio 205/2 “The Adrenal: Central Relay in Health and Disease” to N.B., H.R., S.N., J.L., G.E., and C.P. and the Intramural Research Program of the National Institutes of Health, Bethesda, USA to T.P., L.M., S.T., and K.P.
Disclosures
All of the authors have disclosed no conflicts of interest.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Tischler AS. Pheochromocytoma and extra-adrenal paraganglioma: updates. Arch Pathol Lab Med. 2008;132(8):1272–1284. [DOI] [PubMed] [Google Scholar]
- 2. Plouin PF, Chatellier G, Fofol I, Corvol P. Tumor recurrence and hypertension persistence after successful pheochromocytoma operation. Hypertension. 1997;29(5):1133–1139. [DOI] [PubMed] [Google Scholar]
- 3. Amar L, Fassnacht M, Gimenez-Roqueplo AP, et al. . Long-term postoperative follow-up in patients with apparently benign pheochromocytoma and paraganglioma. Horm Metab Res. 2012;44(05):385–389. [DOI] [PubMed] [Google Scholar]
- 4. Parasiliti-Caprino M, Lucatello B, Lopez C, et al. . Predictors of recurrence of pheochromocytoma and paraganglioma: a multicenter study in Piedmont, Italy. Hypertens Res. 2020;43(6):500–510. [DOI] [PubMed] [Google Scholar]
- 5. Olson SW, Yoon S, Baker T, Prince LK, Oliver D, Abbott KC. Longitudinal plasma metanephrines preceding pheochromocytoma diagnosis: a retrospective case–control serum repository study. Eur J Endocrinol. 2016;174(3):289–295. [DOI] [PubMed] [Google Scholar]
- 6. Eisenhofer G, Lenders JW, Siegert G, et al. . Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48(11):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Venugopal S, Chhabria M, Quartuccio M. Recurrence of pheochromocytoma with metastases after resection of primary tumor. Cureus. 2020;12(5):e8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamidi O, YoungWF, Jr., Iniguez-Ariza NM, et al. . Malignant pheochromocytoma and paraganglioma: 272 patients over 55 years. J Clin Endocrinol Metab. 2017;102(9):3296–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pamporaki C, Prodanov T, Meuter L, et al. . Determinants of disease-specific survival in patients with and without metastatic pheochromocytoma and paraganglioma. Eur J Cancer. 2022;169:32–41. [DOI] [PubMed] [Google Scholar]
- 10. Lam AK. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28(3):213–227. [DOI] [PubMed] [Google Scholar]
- 11. Mete O, Asa SL, Gill AJ, Kimura N, de Krijger RR, Tischler A. Overview of the 2022 WHO classification of paragangliomas and pheochromocytomas. Endocr Pathol. 2022;33(1):90–114. [DOI] [PubMed] [Google Scholar]
- 12. Plouin PF, Amar L, Dekkers OM, et al. . European Society of Endocrinology Clinical Practice guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur J Endocrinol. 2016;174(5):G1–G10. [DOI] [PubMed] [Google Scholar]
- 13. Amar L, Lussey-Lepoutre C, Lenders JW, Djadi-Prat J, Plouin PF, Steichen O. Management of endocrine disease: recurrence or new tumors after complete resection of pheochromocytomas and paragangliomas: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(4):R135–R145. [DOI] [PubMed] [Google Scholar]
- 14. Fishbein L, Leshchiner I, Walter V, et al. . Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flores SK, Estrada-Zuniga CM, Thallapureddy K, Armaiz-Peña G, Dahia PLM. Insights into mechanisms of pheochromocytomas and paragangliomas driven by known or new genetic drivers. Cancers (Basel). 2021;13(18):4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brouwers FM, Eisenhofer G, Tao JJ, et al. . High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. J Clin Endocrinol Metab. 2006;91(11):4505–4509. [DOI] [PubMed] [Google Scholar]
- 17. Amar L, Baudin E, Burnichon N, et al. . Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822–3828. [DOI] [PubMed] [Google Scholar]
- 18. Asari R, Scheuba C, Kaczirek K, Niederle B. Estimated risk of pheochromocytoma recurrence after adrenal-sparing surgery in patients with multiple endocrine neoplasia type 2A. Arch Surg. 2006;141(12):1199–1205. discussion 1205. [DOI] [PubMed] [Google Scholar]
- 19. Holscher I, van den Berg TJ, Dreijerink KMA, Engelsman AF, Nieveen van Dijkum EJM. Recurrence rate of sporadic pheochromocytomas after curative adrenalectomy: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2021;106(2):588–597. [DOI] [PubMed] [Google Scholar]
- 20. Schovanek J, Martucci V, Wesley R, et al. . The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB-related pheochromocytoma and paraganglioma: a retrospective cohort study. BMC Cancer. 2014;14(1):523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ayala-Ramirez M, Feng L, Johnson MM, et al. . Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96(3):717–725. [DOI] [PubMed] [Google Scholar]
- 22. Li M, Prodanov T, Meuter L, et al. . Recurrent disease in patients with sporadic pheochromocytoma and paraganglioma. zenodo. 2022. 10.5281/zenodo.7007064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eisenhofer G, Deutschbein T, Constantinescu G, et al. . Plasma metanephrines and prospective prediction of tumor location, size and mutation type in patients with pheochromocytoma and paraganglioma. Clin Chem Lab Med. 2020;59(2):353–363. [DOI] [PubMed] [Google Scholar]
- 24. Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ. Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Clin Chem. 1993;39(1):97–103. [PubMed] [Google Scholar]
- 25. Peitzsch M, Prejbisz A, Kroiss M, et al. . Analysis of plasma 3-methoxytyramine, normetanephrine and metanephrine by ultraperformance liquid chromatography-tandem mass spectrometry: utility for diagnosis of dopamine-producing metastatic phaeochromocytoma. Ann Clin Biochem. 2013;50(2):147–155. [DOI] [PubMed] [Google Scholar]
- 26. Jiang J, Zhang J, Pang Y, et al. . Sino-European differences in the genetic landscape and clinical presentation of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2020:105(10):dgaa502. [DOI] [PubMed] [Google Scholar]
- 27. Tonsgard JH. Clinical manifestations and management of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13(1):2–7. [DOI] [PubMed] [Google Scholar]
- 28. Salle S P-L, Chbat J, Lacroix A, et al. . Postoperative recurrences in patients operated for pheochromocytomas and paragangliomas: new data supporting lifelong surveillance. Cancers (Basel). 2022:14(12):2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qin N, de Cubas AA, Garcia-Martin R, et al. . Opposing effects of HIF1α and HIF2α on chromaffin cell phenotypic features and tumor cell proliferation: insights from MYC-associated factor X. Int J Cancer. 2014;135(9):2054–2064. [DOI] [PubMed] [Google Scholar]
- 30. Favier J, Plouin PF, Corvol P, Gasc JM. Angiogenesis and vascular architecture in pheochromocytomas: distinctive traits in malignant tumors. Am J Pathol. 2002;161(4):1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bechmann N, Moskopp ML, Ullrich M, et al. . HIF2alpha Supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocr Relat Cancer. 2020;27(11):625–640. [DOI] [PubMed] [Google Scholar]
- 32. DeLellis RA, Wolfe HJ, Gagel RF, et al. . Adrenal medullary hyperplasia. A morphometric analysis in patients with familial medullary thyroid carcinoma. Am J Pathol. 1976;83(1):177–196. [PMC free article] [PubMed] [Google Scholar]
- 33. Baysal BE, Rubinstein WS, Taschner PE. Phenotypic dichotomy in mitochondrial complex II genetic disorders. J Mol Med (Berl). 2001;79(9):495–503. [DOI] [PubMed] [Google Scholar]
- 34. Michalowska I, Cwikla JB, Michalski W, et al. . Growth rate of paragangliomas related to germline mutations of the SDHX genes. Endocr Pract. 2017;23(3):342–352. [DOI] [PubMed] [Google Scholar]
- 35. Remacha L, Pirman D, Mahoney CE, et al. . Recurrent germline DLST mutations in individuals with multiple pheochromocytomas and paragangliomas. Am J Hum Genet. 2019;104(4):651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hadrava Vanova K, Pang Y, Krobova L, et al. . Germline SUCLG2 variants in patients with pheochromocytoma and paraganglioma. J Natl Cancer Inst. 2022;114(1):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Monteagudo M, Martínez P, Leandro-García LJ, et al. . Analysis of telomere maintenance related genes reveals NOP10 as a new metastatic-risk marker in pheochromocytoma/paraganglioma. Cancers (Basel). 2021;13(19):4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cui Y, Ma X, Gao Y, et al. . Local-regional recurrence of pheochromocytoma/paraganglioma: characteristics, risk factors and outcomes. Front Endocrinol (Lausanne). 2021;12:762548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.