Abstract
OBJECTIVE
We conducted a Mendelian randomization (MR) study to examine the associations of type 2 diabetes and glycemic traits with gastrointestinal diseases (GDs).
RESEARCH DESIGN AND METHODS
Uncorrelated genetic variants associated with type 2 diabetes (n = 231), fasting insulin (n = 38), fasting glucose (n = 71), and hemoglobin A1c (n = 75) at the genome-wide significance were selected as instrument variables. Genetic associations with 23 common GDs were obtained from the FinnGen and UK Biobank studies and other large consortia.
RESULTS
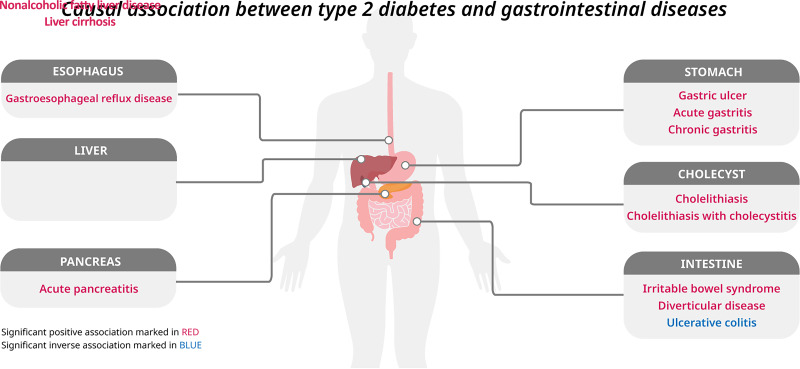
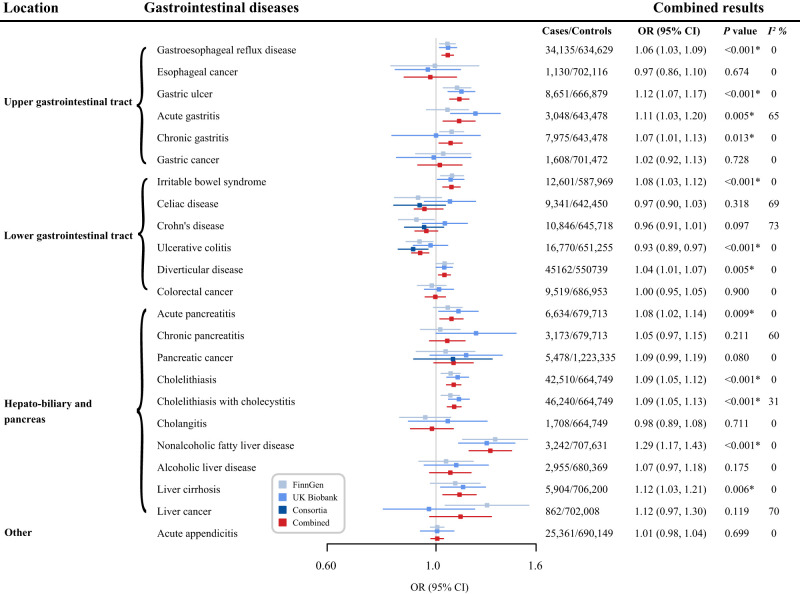
Genetic liability to type 2 diabetes was associated with the risk of 12 GDs. Per 1-unit increase in the log-transformed odds ratio (OR) of type 2 diabetes, the OR was 1.06 (95% CI, 1.03–1.09) for gastroesophageal reflux disease, 1.12 (95% CI, 1.07–1.17) for gastric ulcer, 1.11 (95% CI, 1.03–1.20) for acute gastritis, 1.07 (95% CI, 1.01–1.13) for chronic gastritis, 1.08 (95% CI, 1.03–1.12) for irritable bowel syndrome, 1.04 (95% CI, 1.01–1.07) for diverticular disease, 1.08 (95% CI, 1.02–1.14) for acute pancreatitis, 1.09 (95% CI, 1.05–1.12) for cholelithiasis, 1.09 (95% CI, 1.05–1.13) for cholelithiasis with cholecystitis, 1.29 (95% CI, 1.17–1.43) for nonalcoholic fatty liver disease, 1.12 (95% CI, 1.03–1.21) for liver cirrhosis, and 0.93 (95% CI, 0.89–0.97) for ulcerative colitis. Genetically predicted higher levels of fasting insulin and glucose were associated with six and one GDs, respectively.
CONCLUSIONS
Associations were found between genetic liability to type 2 diabetes and an increased risk of a broad range of GDs, highlighting the importance of GD prevention in patients with type 2 diabetes.
Graphical Abstract
Introduction
Type 2 diabetes is a global health issue with an increasing prevalence and disease burden (1). Type 2 diabetes, in which there is impaired glycemic homeostasis, has been identified as an important risk factor for cardiovascular disease (2) and certain cancers (3). Population-based epidemiological studies have also linked type 2 diabetes to increased risk of gastrointestinal diseases, such as gastroesophageal reflux disease (4), cholelithiasis (5), nonalcoholic fatty liver disease (6), and certain gastrointestinal cancers (3). However, the evidence for these associations was inconsistent (3,7,8). Limited data are available on the associations of type 2 diabetes with risk of gastritis and Crohn’s disease, both of which cause a relatively large disease burden. In addition, these findings from observational studies may be biased by possible limitations, including residual confounding, misclassification, and reverse causation, because type 2 diabetes is a chronic metabolic disorder with a long-term course and early insidious symptoms. A clear appraisal of the causal associations between type 2 diabetes and gastrointestinal diseases is of great importance in gastrointestinal disease prevention and management among patients with type 2 diabetes.
Mendelian randomization (MR) analysis is an epidemiological design that can strengthen causal inference by using genetic variants as instrumental variables for an exposure (9). Compared with observational studies, MR analysis can diminish confounding bias because genetic alleles are randomly assorted at conception and, therefore, have no correlations with environmental and self-adopted factors. The MR design can also prevent reverse causality because germline genotype cannot be modified by disease (10). Previous MR studies identified causal associations of type 2 diabetes with risk of individual gastrointestinal disease, including gastroesophageal reflux disease (11), gastrointestinal cancer (12), diverticular disease (13), pancreatitis (14), gallstones (15,16), and nonalcoholic fatty liver disease (17,18), but there does not appear to be an overview available of its gastrointestinal consequences, which would be informative for promoting gastrointestinal health of patients with type 2 diabetes. Here, we conducted an updated, two-sample MR study to comprehensively examine the associations of genetic liability to type 2 diabetes with 23 gastrointestinal diseases. To further explore underlying mechanisms related to impaired glycemic homeostasis, we examined the associations of three genetically predicted glycemic traits (fasting insulin, fasting glucose, and hemoglobin A1c [HbA1c]) with gastrointestinal diseases as supplementary analyses.
Research Design and Methods
Study Design
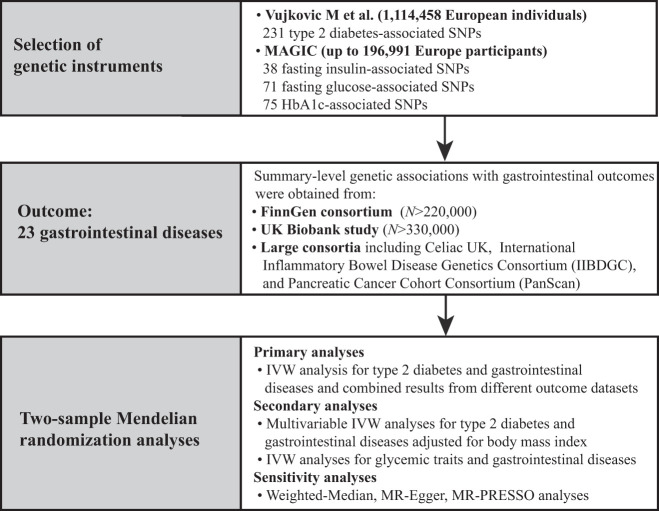
This two-sample MR study was designed to explore the causal effect of type 2 diabetes and glycemic traits on the risk of 23 gastrointestinal diseases, as shown in Fig. 1. The study was based on publicly available, summary-level data of genome-wide association studies (GWAS), the FinnGen study, the UK Biobank study, and other large consortia, without overlap of study populations (Supplementary Table 1). We estimated the associations of genetic liability to type 2 diabetes and three glycemic traits with each gastrointestinal disease in the FinnGen, UK Biobank, and other large consortia individually, and the association estimates were then combined using meta-analysis of a fixed-effects model. Included studies had been approved by a relevant ethical review board and participants had given informed consent.
Figure 1.
Study design. MAGIC, Meta-Analyses of Glucose and Insulin-Related Traits Consortium.
Genetic Instrument Selection
Single-nucleotide polymorphisms (SNPs) associated with type 2 diabetes and glycemic traits (namely, fasting insulin, fasting glucose, and HbA1c) at the genome-wide significance level (P < 5 × 10−8) were selected from a GWAS meta-analysis including 1,114,458 European individuals (n = 148,726 case patients and 965,732 control participants) (19) and from the Meta-Analyses of Glucose and Insulin-Related Traits Consortium, with up to 196,991 participants of European ancestry without diabetes (20) (Supplementary Table 1). Linkage disequilibrium among SNPs for each exposure was calculated on the basis of the 1000 Genomes European panel using the PLINK clumping method, and SNPs with linkage disequilibrium (defined as r2 > 0.001 and clump distance <10,000 kb) were excluded. SNPs in or near the FTO gene with pleiotropic effects were excluded (21), leaving 231 SNPs for type 2 diabetes, 38 for fasting insulin, 71 for fasting glucose, and 75 for HbA1c. For glycemic traits, the used genetic instruments explained approximately 0.5%, 3.6%, and 5.0% of variance in fasting insulin, fasting glucose, and HbA1c, respectively. Detailed information on SNPs used in this study is presented in Supplementary Tables 2 and 3.
Gastrointestinal Disease Data Sources
This study included 23 gastrointestinal diseases, including six upper gastrointestinal tract diseases (gastroesophageal reflux disease, esophageal cancer, gastric ulcer, acute gastritis, chronic gastritis, gastric cancer), six lower gastrointestinal tract diseases (irritable bowel syndrome, celiac disease, Crohn’s disease, ulcerative colitis, colorectal cancer, diverticular disease), six biliary or pancreas diseases (acute pancreatitis, chronic pancreatitis, pancreatic cancer, cholelithiasis, cholelithiasis with cholecystitis, cholangitis), four liver diseases (nonalcoholic fatty liver disease, alcoholic liver disease, liver cirrhosis, liver cancer), and acute appendicitis, with numbers of cases ranging from 862 (liver cancer) to 46,240 (cholelithiasis with cholecystitis). Summary-level genetic associations with these outcomes were obtained from the FinnGen consortium (n = ≤220,000) and UK Biobank study (n = ≤330,000), and additional GWAS consortia data were derived from the Celiac UK (n = 4,533 case patients and 10,750 control individuals for celiac disease) (22), Inflammatory Bowel Disease Genetics Consortium (n = 5,956 case patients and 14,927 control individuals for Crohn’s disease; n = 6,968 case patients and 20,464 control individuals for ulcerative colitis) (23), and Pancreatic Cancer Cohort Consortium (n = 3,835 case patients and 521,863 control individuals for pancreatic cancer) (24).
The FinnGen consortium is a study collecting health and genetic data based on Finnish health registries. We used the R7 data release of FinnGen, and genome-wide association analyses for each trait adjusted for sex, age, genotyping batch, and the first 10 genetic principal components. Gastrointestinal outcomes were ascertained by codes from the ICD-8, ICD-9, and ICD-10 and surgery and medicine purchase codes.
The UK Biobank study is a large prospective cohort study with more than 500,000 people aged >40 years recruited between 2006 and 2010. Gastrointestinal cases were diagnosed by codes from the ICD-9 and ICD-10, surgery, and self-reported information. GWAS data used in the present MR study were collected by Lee Laboratory for Statistical Genetics and Data Science (Seoul National University, Seoul, Republic of Korea; https://www.leelabsg.org/resources), adjusting for sex, birth year, genotyping batch, and first four principal components. Detailed information on the outcome data sources and outcome definition are shown in Supplementary Tables 1 and 4.
Data on BMI
Summary-level data on BMI were obtained from a GWAS meta-analysis of 694,649 individuals of European ancestry from the UK Biobank study and Genetic Investigation of Anthropometric Traits consortium (25). Genetic associations were adjusted for sex, age at assessment, the square of age at assessment, and assessment center.
Statistical Analysis
The random-effects multiplicative inverse-variance weighted (IVW) method was used as the primary MR method to estimate the associations of genetic liability to type 2 diabetes and genetically predicted glycemic trait levels (fasting insulin, fasting glucose, and HbA1c) with risk of gastrointestinal diseases. MR estimates for each outcome from different sources were combined by the fixed-effects meta-analysis method. The I2 statistic was calculated to assess the heterogeneity of each outcome from different data sources, and the I2 values <25%, 25–75%, and >75% were considered to indicate low, moderate, and high heterogeneity, respectively. We performed several sensitivity analyses, including the weighted median, MR-Egger, and MR pleiotropy Residual Sum and Outlier (MR-PRESSO), to examine the robustness of the results and identify possible horizontal pleiotropy. The weighted median method can provide valid MR estimates assuming that more than 50% of the weight comes from valid SNPs. MR-Egger regression can detect horizontal pleiotropy by its embedded intercept test and provide estimates after correction for pleiotropic effects. The MR-PRESSO method can detect and correct for possible outliers, and the MR-PRESSO global test can be used to evaluate horizontal pleiotropy caused by heterogeneity among SNPs’ estimates. We also used the Cochran Q test and modified Cochran Q test (26) to examine heterogeneity of SNPs’ estimates in each MR association. An imbalanced horizontal pleiotropy that distorts causal inference was assessed by all three heterogeneity tests, as well as the MR-Egger intercept test. To minimize the pleiotropy from obesity, we performed a sensitivity analysis after removing five SNPs associated with BMI at the loci significance level and conducted a multivariable MR analysis with adjustment for genetically predicted BMI. We additionally conducted two sensitivity analyses: 1) excluding SNPs associated with gastrointestinal diseases at the loci significance level, and 2) using SNPs for type 2 diabetes extracted from the Diabetes Genetics Replication and Meta-analysis (DIAGRAM) consortium (27) (n = 74,124 case patients and 824,006 control participants), which has no sample overlap with UK Biobank, to further test the robustness of the primary findings.
The odds ratios (ORs) and corresponding 95% CIs of the associations were scaled to a 1-unit increase in the log-transformed OR of type 2 diabetes and a 1-unit increase in circulating levels of fasting insulin (log-transformed pmol/L), fasting glucose (mmol/L), and HbA1c (%). The F statistic was calculated for each SNP associated with the glycemic traits and was found to be greater than the empirical threshold of 10, suggesting a good strength of the SNPs we used. The false discovery rate, based on the Benjamini-Hochberg method, was used to correct for multiple testing of the 23 gastrointestinal diseases. The association with a nominal P value <0.05 but a Benjamini-Hochberg adjusted P value >0.05 was considered suggestive and the association with a Benjamini-Hochberg adjusted P value <0.05 was deemed significant. All analyses were two-sided and performed using R packages TwoSampleMR and MRPRESSO in R 4.1.3.
Data and Resource Availability
All data used in this study were obtained from GWAS summary statistics, which were publicly released by genetic consortia.
Results
Type 2 Diabetes and Gastrointestinal Diseases
Genetic liability to type 2 diabetes was associated with increased risk of 11 gastrointestinal diseases and a decreased risk of ulcerative colitis in the meta-analysis of all outcome sources (Fig. 2). All these associations survived after multiple testing correction (Supplementary Table 5). For a 1-unit increase in log-transformed odds of type 2 diabetes, the combined OR of estimates from FinnGen, UK Biobank, and large consortia was 1.06 (95% CI, 1.03–1.09; P < 0.001) for gastroesophageal reflux disease, 1.12 (95% CI, 1.07–1.17; P < 0.001) for gastric ulcer, 1.11 (95% CI, 1.03–1.20; P = 0.005), 1.07 (95% CI, 1.01–1.13; P = 0.013) for acute and chronic gastritis, 1.08 (95% CI, 1.03–1.12; P < 0.001) for irritable bowel syndrome, 1.04 (95% CI, 1.01–1.07; P = 0.005) for diverticular disease, 1.08 (95% CI, 1.02–1.14; P = 0.009) for acute pancreatitis, 1.09 (95% CI, 1.05–1.12; P < 0.001) for cholelithiasis, 1.09 (95% CI, 1.05–1.13; P < 0.001) for cholelithiasis with cholecystitis, 1.29 (95% CI, 1.17–1.43; P < 0.001) for nonalcoholic fatty liver disease, 1.12 (95% CI, 1.03–1.21; P = 0.006) for liver cirrhosis, and 0.93 (95% CI, 0.89–0.97; P < 0.001) for ulcerative colitis. Genetic liability to type 2 diabetes was not strongly associated with risk of esophageal cancer, gastric cancer, celiac disease, Crohn’s disease, colorectal cancer, chronic pancreatitis, pancreatic cancer, cholangitis, alcoholic liver disease, liver cancer, or acute appendicitis (Fig. 2). We observed low to moderate heterogeneity across MR estimates from individual studies of most gastrointestinal diseases (Fig. 2).
Figure 2.
Associations of genetic liability to type 2 diabetes mellitus with gastrointestinal diseases. The ORs were scaled to a 1-unit increase in log-transformed OR of type 2 diabetes. P values are for ORs (95% CIs). *P < 0.05 after multiple testing correction. I2 values <25%, 25–75%, and >75% were considered to indicate low, moderate, and high heterogeneity, respectively.
Results for genetic liability to type 2 diabetes and risk of gastrointestinal outcomes were directionally consistent in sensitivity analyses (Supplementary Table 6). We detected heterogeneity in the analysis of most gastrointestinal end points across SNPs (Supplementary Table 6). No horizontal pleiotropy was observed in MR-Egger intercept analysis except for that for diverticular disease in the FinnGen and UK Biobank data, and gastroesophageal reflux disease, nonalcoholic fatty liver disease, and liver cirrhosis in the UK Biobank data (Supplementary Table 6). The MR-PRESSO analysis identified one to seven outliers; however, the associations remained stable but with smaller estimates and narrower CIs for most outcomes after the removal of outliers (Supplementary Table 6). The observed associations, except for that for ulcerative colitis, were stable in the sensitivity analysis excluding BMI-related SNPs and in the multivariable MR analysis adjusting for genetically predicted BMI (Supplementary Tables 7 and 8). Results were consistent overall with the primary findings in the analyses where SNPs strongly associated with gastrointestinal diseases were removed and SNPs for type 2 diabetes from the DIAGRAM consortium were used as genetic instruments (Supplementary Table 9).
Glycemic Traits and Gastrointestinal Diseases
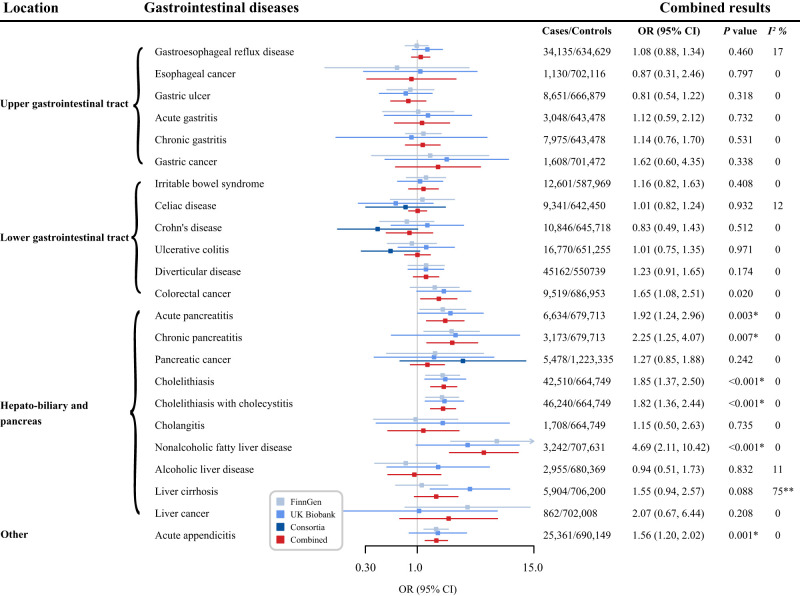
Genetically predicted levels of fasting insulin, fasting glucose, and HbA1c were associated with risk of 7, 5, and 1, respectively, of the 23 gastrointestinal outcomes (Supplementary Tables 5 and 10). After multiple testing correction, genetically predicted, per-unit in log-transformed pmol/L increases in fasting insulin levels were associated with increased risk of acute pancreatitis (OR, 1.92; 95% CI, 1.24–2.96; P = 0.003), chronic pancreatitis (OR, 2.25; 95% CI, 1.25–4.07; P = 0.007), cholelithiasis (OR, 1.85, 95% CI 1.37–2.50; P < 0.001), cholelithiasis with cholecystitis (OR, 1.82; 95% CI, 1.36–2.44; P < 0.001), nonalcoholic fatty liver disease (OR, 4.69; 95% CI, 2.11–10.42; P < 0.001), and acute appendicitis (OR, 1.56; 95% CI, 1.20–2.02; P = 0.001). There were suggestive associations for colorectal cancer (OR, 1.65; 95% CI, 1.08–2.51; P = 0.020) (Fig. 3). Moreover, a genetically predicted 1 mmol/L increase in fasting glucose levels was associated with decreased risk of Crohn’s disease (OR, 0.65; 95% CI, 0.51–0.83; P = 0.001) and diverticular disease (OR, 0.87; 95% CI, 0.78–0.98; P = 0.017), and increased risk of acute pancreatitis (OR, 1.29; 95% CI, 1.03–1.62; P = 0.028), chronic pancreatitis (OR, 1.56; 95% CI, 1.12–2.18; P = 0.009), and cholelithiasis with cholecystitis (OR, 1.17; 95% CI, 1.00–1.37; P = 0.043). The associations, except for Crohn’s disease, became suggestive after multiple testing. For HbA1c, there was a suggestive association between genetically predicted HbA1c levels and decreased risk of nonalcoholic fatty liver disease (per 2-unit in 2 percentage increase OR, 0.54; 95% CI, 0.33–0.90; P = 0.018). The associations remained stable in sensitivity analyses and no indication of heterogeneity and horizontal pleiotropy was detected in most outcomes (Supplementary Tables 11–13).
Figure 3.
Associations of genetically predicted fasting insulin levels with gastrointestinal diseases. The ORs were scaled to a 1-unit increase in log-transformed pmol/L. P values are for ORs (95% CIs). *P < 0.05 after multiple testing correction; **P < 0.05 for heterogeneity test. I2 values <25%, 25–75%, and >75% were considered to indicate low, moderate, and high heterogeneity, respectively.
Conclusions
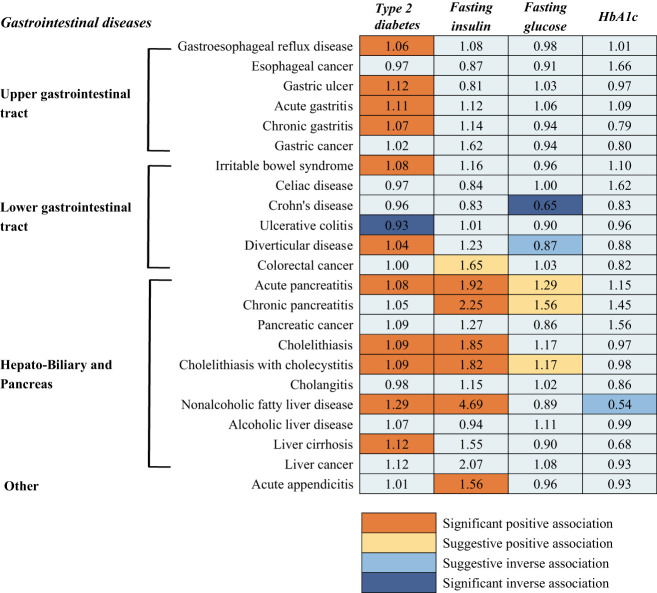
The findings of this study are summarized in Fig. 4. In this two-sample MR study, we systematically assessed the associations of genetic liability to type 2 diabetes and genetically predicted levels of three glycemic traits with the risk of 23 gastrointestinal diseases. In line with findings from previous studies (11,13–17), we found that genetic liability to type 2 diabetes was associated with increased risk of gastroesophageal reflux disease, diverticular disease, acute pancreatitis, cholelithiasis, and nonalcoholic fatty liver disease. We identified several novel associations of genetic liability to type 2 diabetes with increased risk of gastric ulcer, gastritis, irritable bowel diseases, and liver cirrhosis, and a decreased risk of ulcerative colitis. All associations with the exception of ulcerative colitis, were independent of genetically predicted BMI. We also found significant or suggestive associations of genetically predicted levels of fasting insulin, glucose, and HbA1c with some gastrointestinal diseases.
Figure 4.
Summary of associations of genetically predicted type 2 diabetes mellitus and glycemic traits with gastrointestinal diseases. Numbers in the boxes are ORs for associations of exposure and each gastrointestinal outcome. The reported ORs are scaled to a 1-unit increase in log odds of genetic liability to type 2 diabetes and a 1-unit increase of circulating levels of fasting insulin (log-transformed pmol/L), fasting glucose (mmol/L), and HbA1c (%). The association with a P value <0.05, but Benjamini-Hochberg adjusted P values >0.05, was considered suggestive, and adjusted P values <0.05 were deemed significant.
The observational associations of type 2 diabetes with higher risk of gastroesophageal reflux disease (4), diverticular diseases (28), acute pancreatitis (29), cholelithiasis (5), nonalcoholic fatty liver disease, and liver cirrhosis (6), reported in previous observational studies, were supported by the genetic evidence from MR studies (11,13–18). Although associations between type 2 diabetes and liver cirrhosis have not been widely studied, MR studies provided evidence for causal associations between genetic liability to type 2 diabetes and increased risk of nonalcoholic fatty liver disease (17,18). In this study, we found a positive association between type 2 diabetes and liver cirrhosis, which might be likely to occur through driving nonalcoholic fatty liver disease, which can progress to liver cirrhosis. Moreover, we found positive associations between genetically predicted levels of fasting insulin and fasting glucose with risk of pancreatitis, cholelithiasis, and nonalcoholic fatty liver disease, indicating the associations between type 2 diabetes and these gastrointestinal diseases may be driven by impaired glucose and insulin resistance, substantiated by basic research (30). The findings between impaired glycemic homeostasis and gastrointestinal diseases could also be of clinical importance and suggest that besides type 2 diabetes, pharmacological or lifestyle interventions that lower circulating glucose levels, and thus also fasting insulin levels, may be beneficial in preventing gastrointestinal diseases.
In this study, we found evidence of novel causal associations of genetic liability to type 2 diabetes with increased risk of gastric ulcer, acute and chronic gastritis, and irritable bowel syndrome, but lower risk of ulcerative colitis, and genetically predicted levels of fasting glucose were significantly associated with lower risk of Crohn’s disease. Although epidemiological evidence for these gastrointestinal diseases in type 2 diabetes is scarce, cross-sectional studies showed an increased prevalence of peptic ulcer, severe acute gastritis, and irritable bowel syndrome characterized by symptoms such as abdominal pain, constipation, and diarrhea in type 2 diabetes (31,32). As for the inverse association between genetic liability to type 2 diabetes and ulcerative colitis, authors of a cross-sectional study observed reduced colonic inflammation in patients with metabolic syndrome (33), which possibly occurred by shifting the local inflammatory response to enhance the involvement of immunosuppressive cells and mediators, providing a potential explanation the inverse causal association of type 2 diabetes and fasting glucose with inflammatory bowel diseases in this study. Moreover, the associations between genetic liability to type 2 diabetes and risk of ulcerative colitis did not remain when accounting for BMI, which indicated that the association might be biased by the pleiotropic effects of the variants on obesity traits. Our findings for the novel associations between type 2 diabetes and the aforementioned gastrointestinal diseases provide evidence for support of early screening of gastric ulcer, gastritis, and irritable bowel syndrome in patients with type 2 diabetes.
We did not observe any association between genetic liability to type 2 diabetes and gastrointestinal cancers, although previous studies provided inconclusive evidence. An umbrella review of meta-analyses (8) based on observational studies found that type 2 diabetes was associated with increased risk of developing colorectal cancer and cholangiocarcinoma. Another umbrella review (3) found the association of type 2 diabetes with colorectal, hepatocellular, and pancreatic cancers. A previous MR study showed that genetic liability to type 2 diabetes was associated with increased risk of esophageal and pancreatic cancers but not stomach or liver cancers (12). Previous MR findings on colorectal and pancreatic cancers were inconsistent (12,34–36). Although we did not detect significant associations between genetic liability to type 2 diabetes and gastrointestinal cancers, the point estimates were above 1 for gastric cancer (OR = 1.02), pancreatic cancer (OR = 1.09), and liver cancer (OR = 1.12). Given inconsistent results, the nonsignificant associations in the present study between genetic liability to type 2 diabetes and gastrointestinal cancers need further examination. Moreover, we found genetically predicted fasting insulin levels were associated with an increased risk of colorectal cancer, which was in line with findings of a recent MR study based on large-scaled consortium data (37). Notably, type 2 diabetes has been robustly associated with colorectal cancer in observational studies (3,8) but not in MR studies (37). A possible explanation may be that diabetes cases predicted by high genetic liability predominate in β-cell depletion instead of insulin resistance. However, type 2 diabetes in observational studies reflects both, and the association with colorectal cancer mainly is driven by insulin resistance. The risk of colorectal cancer appeared to be substantially increased among patients with diabetes at the early stage and attenuated over time (37), which supports our hypothesis.
Several potential mechanisms support the associations between type 2 diabetes and gastrointestinal diseases. Individuals with type 2 diabetes have altered glucose and insulin metabolism as well as accelerated vascular endothelium damage and chronic inflammation (6), which may contribute to the observed associations with gastrointestinal diseases. Especially, type 2 diabetes–associated insulin resistance was shown to promote the formation of cholesterol gallstones (30). In the present study, we found associations of fasting insulin and fasting glucose with gastrointestinal outcomes, especially hepato-biliary and pancreatic diseases, further substantiating the essential roles of insulin and glucose in the associations of type 2 diabetes with gastrointestinal diseases. In addition to the disturbed insulin and glucose metabolism, neuropathy, and esophageal and intestinal dysmotility (4,31) also played critical roles in gastroesophageal reflux disease, irritable bowel syndrome, and diverticular disease. Downstream factors of diabetes diagnosis, like medication, may also play a role in the development of gastrointestinal diseases in type 2 diabetes. For example, glyburide prescribed to patients with type 2 diabetes was associated with an increased risk of acute pancreatitis (38). Some studies speculated that the link between type 2 diabetes and gastrointestinal diseases might be caused by shared risk factors such as obesity. However, the associations between type 2 diabetes and gastrointestinal disease remained consistent in our sensitivity analysis excluding BMI-related SNPs and multivariable MR analysis with adjustment for genetically predicted BMI.
This study has several strengths. A chief strength is the MR study design, which mitigated confounding and reverse causality bias. We obtained summary-level data from large genetic studies in the European populations, so the results are unlikely to be biased by the population structure bias. However, this confined the generalizability of our findings to other populations. In addition, the associations were estimated in independent data sources and combined by the meta-analysis, which ensured an adequate statistical power and the robustness of findings.
Limitations should be considered. First, we could not completely rule out the possibility that the type 2 diabetes–related SNPs affect gastrointestinal diseases through other causal pathways (i.e., horizontal pleiotropy). To minimize this bias, we excluded SNPs in the FTO gene that were strongly associated with obesity, excluded BMI-related SNPs, and conducted multivariable MR with adjustment for BMI that possible exerted pleiotropic effects. In addition, several sensitivity analyses were conducted to examine the consistency of the results. Although the observed associations in the primary analysis were no longer statistically significant in certain sensitivity analyses (e.g., the weighted Median and MR-Egger analyses), possibly due to reduced power, the direction of these associations was consistent with the primary findings, supporting the consistency of our results. The consistent associations obtained from these analyses indicated that our findings were somehow minimally influenced by horizontal pleiotropy. Second, even though we combined data from different outcome sources, which increased the power to some extent, the weak associations might still be overlooked because of a few cases of uncommon outcomes, like liver cancer and cholangitis. Given phenotypical variance explained by SNPs for type 2 diabetes could not be precisely estimated on the basis of the summary-level data, the power calculation was confined. Third, the nonlinearity of the associations for glycemic traits could not be assessed on the basis of the summary-level data. The MR analysis for type 2 diabetes (a binary phenotype used as the exposure) might be biased by the exclusion restriction assumption if type 2 diabetes was majorly defined by a dichotomization of a continuous risk factor (39). However, the exposure (i.e., type 2 diabetes) was not merely defined by a cutoff of a continuous biomarkers but defined by ICD codes, medication use, self-report, or cutoff of HbA1c and blood glucose levels in this study (27). In addition, a few associations were identified between genetically predicted levels of fasting glucose and HbA1c and the risk of gastrointestinal diseases. Thus, the observed associations between genetic liability to type 2 diabetes and gastrointestinal diseases were unlikely to be a consequence of violation of the exclusion restriction assumption. Still, the OR of the outcomes could not be interpreted in a direct unit of the exposure in MR analysis for type 2 diabetes (39,40), which might confine the comparison of our findings with observational studies regarding the magnitude. Fourth, SNPs for type 2 diabetes were obtained from the general population, whereas SNPs for glycemic traits were obtained from the population without diabetes diagnosis. Thus, whether the findings of glycemic traits can be generalized to the whole population or to the patients with diabetes needs to be confirmed in future studies even though patients with diabetes were included in the outcome data. Additionally, This study only included European populations, which limits the generalizability of the findings to other populations, such as East Asian, African, and others. Fifth, there might be case misclassification due to discrepancy between diabetes proxied by genetic score and clinical diagnosis. However, the participants with the highest polygenic risk had more than five times the risk of being diagnosed as type 2 diabetes compared with the reference group (20), which showed a good predictive ability of genetic score.
In conclusion, we found that genetic liability to type 2 diabetes was associated with increased risk of a broad range of gastrointestinal diseases. Impaired glycemic homeostasis, featuring high insulin levels, was also associated with increased risk of several gastrointestinal diseases. These findings highlight the potential role of early screening and prevention for gastrointestinal diseases in patients with type 2 diabetes.
Article Information
Funding. S.C.L. is funded by the Swedish Cancer Society (Cancerfonden), the Swedish Heart-Lung Foundation (Hjärt-Lungfonden; grant 20210351), the Karolinska Institutet Research Foundation (grant 2020-01842), the Swedish Research Council for Health, Working Life and Welfare (Forte; grant 2018-00123), and the Swedish Research Council (Vetenskapsrådet; grants 2016-01042 and 2019-00977). D.G. is funded by the Wellcome 4i Clinical PhD Program (203928/Z/16/Z) and the British Heart Foundation Centre of Research Excellence (RE/18/4/34215) at Imperial College London. S.B. is supported by the Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant 204623/Z/16/Z). X.L. is supported by the Natural Science Fund for Distinguished Young Scholars of Zhejiang Province (LR22H260001). X.Y.W. is supported by the National Natural Science Foundation of China (81970494) and Key Project of Research and Development Plan of Hunan Province (2019SK2041).
Author Contributions. J.C., S.Y., X.W., X.L., D.G., S.B., E.L.G., and S.C.L. designed the study and initial analysis plan. J.C., S.Y., T.F., and X.R. contributed to the statistical analysis, performed the data analysis, and wrote the draft of the manuscript. T.F., X.R., and J.Q. contributed to discussion and revision of the manuscript. D.G., S.B., E.L.G., and S.C.L. critically reviewed the manuscript. J.C., S.Y., T.F., and X.R. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21922890.
J.C. and S.Y. contributed equally to this study.
References
- 1. Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol 2016;12:616–622 [DOI] [PubMed] [Google Scholar]
- 2. Sattar N, Rawshani A, Franzén S, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 2019;139:2228–2237 [DOI] [PubMed] [Google Scholar]
- 3. Pearson-Stuttard J, Papadimitriou N, Markozannes G, et al. Type 2 diabetes and cancer: an umbrella review of observational and Mendelian randomization studies. Cancer Epidemiol Biomarkers Prev 2021;30:1218–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Punjabi P, Hira A, Prasad S, Wang X, Chokhavatia S. Review of gastroesophageal reflux disease (GERD) in the diabetic patient. J Diabetes 2015;7:599–609 [DOI] [PubMed] [Google Scholar]
- 5. Wirth J, Joshi AD, Song M, et al. A healthy lifestyle pattern and the risk of symptomatic gallstone disease: results from 2 prospective cohort studies. Am J Clin Nutr 2020;112:586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dewidar B, Kahl S, Pafili K, Roden M. Metabolic liver disease in diabetes - from mechanisms to clinical trials. Metabolism 2020;111S:154299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zawada AE, Moszak M, Skrzypczak D, Grzymisławski M. Gastrointestinal complications in patients with diabetes mellitus. Adv Clin Exp Med 2018;27:567–572 [DOI] [PubMed] [Google Scholar]
- 8. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 9. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pingault JB, O’Reilly PF, Schoeler T, Ploubidis GB, Rijsdijk F, Dudbridge F. Using genetic data to strengthen causal inference in observational research. Nat Rev Genet 2018;19:566–580 [DOI] [PubMed] [Google Scholar]
- 11. Yuan S, Larsson SC. Adiposity, diabetes, lifestyle factors and risk of gastroesophageal reflux disease: a Mendelian randomization study. Eur J Epidemiol 2022;37:747–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yuan S, Kar S, Carter P, et al. Is type 2 diabetes causally associated with cancer risk? Evidence from a two-sample Mendelian randomization study. Diabetes 2020;69:1588–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yuan S, Larsson SC. Genetically predicted adiposity, diabetes, and lifestyle factors in relation to diverticular disease. Clin Gastroenterol Hepatol 2022;20:1077–1084 [DOI] [PubMed] [Google Scholar]
- 14. Yuan S, Giovannucci EL, Larsson SC. Gallstone disease, diabetes, calcium, triglycerides, smoking and alcohol consumption and pancreatitis risk: Mendelian randomization study. NPJ Genom Med 2021;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yuan S, Gill D, Giovannucci EL, Larsson SC. Obesity, type 2 diabetes, lifestyle factors, and risk of gallstone disease: a Mendelian randomization investigation. Clin Gastroenterol Hepatol 2022;20:e529–e537 [DOI] [PubMed] [Google Scholar]
- 16. Chen L, Yang H, Li H, He C, Yang L, Lv G. Insights into modifiable risk factors of cholelithiasis: a Mendelian randomization study. Hepatology 2022;75:785–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuan S, Chen J, Li X, et al. Lifestyle and metabolic factors for nonalcoholic fatty liver disease: Mendelian randomization study. Eur J Epidemiol 2022;37:723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Z, Zhang Y, Graham S, et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J Hepatol 2020;73:263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vujkovic M, Keaton JM, Lynch JA, et al.; HPAP Consortium; Regeneron Genetics Center; VA Million Veteran Program . Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet 2020;52:680–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen J, Spracklen CN, Marenne G, et al.; Lifelines Cohort Study; Meta-Analysis of Glucose and Insulin-related Traits Consortium (MAGIC) . The trans-ancestral genomic architecture of glycemic traits. Nat Genet 2021;53:840–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubois PC, Trynka G, Franke L, et al. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet 2010;42:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu JZ, van Sommeren S, Huang H, et al.; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium . Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015;47:979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet 2009;41:986–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pulit SL, Stoneman C, Morris AP, et al.; GIANT Consortium . Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet 2019;28:166–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016;40:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 2018;50:1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin X, Li J, Ying M, Wei F, Xie X. Diabetes increases morbidities of colonic diverticular disease and colonic diverticular hemorrhage: a systematic review and meta-analysis. Am J Ther 2017;24:e213–e221 [DOI] [PubMed] [Google Scholar]
- 29. Yang L, He Z, Tang X, Liu J. Type 2 diabetes mellitus and the risk of acute pancreatitis: a meta-analysis. Eur J Gastroenterol Hepatol 2013;25:225–231 [DOI] [PubMed] [Google Scholar]
- 30. Biddinger SB, Haas JT, Yu BB, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med 2008;14:778–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodrigues ML, Motta ME. Mechanisms and factors associated with gastrointestinal symptoms in patients with diabetes mellitus. J Pediatr (Rio J) 2012;88:17–24 [DOI] [PubMed] [Google Scholar]
- 32. Boehme MW, Autschbach F, Ell C, Raeth U. Prevalence of silent gastric ulcer, erosions or severe acute gastritis in patients with type 2 diabetes mellitus--a cross-sectional study. Hepatogastroenterology 2007;54:643–648 [PubMed] [Google Scholar]
- 33. Jovanovic M, Simovic Markovic B, Gajovic N, et al. Metabolic syndrome attenuates ulcerative colitis: correlation with interleukin-10 and galectin-3 expression. World J Gastroenterol 2019;25:6465–6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murphy N, Song M, Papadimitriou N, et al. Associations between glycemic traits and colorectal cancer: a Mendelian randomization analysis. J Natl Cancer Inst 2022;114:740–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carreras-Torres R, Johansson M, Gaborieau V, et al. The role of obesity, type 2 diabetes, and metabolic factors in pancreatic cancer: a Mendelian randomization study. J Natl Cancer Inst 2017;109:djx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu Y, Gentiluomo M, Lorenzo-Bermejo J, et al. Mendelian randomisation study of the effects of known and putative risk factors on pancreatic cancer. J Med Genet 2020;57:820–828 [DOI] [PubMed] [Google Scholar]
- 37. Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia 2011;54:2263–2271 [DOI] [PubMed] [Google Scholar]
- 38. Blomgren KB, Sundström A, Steineck G, Wiholm BE. Obesity and treatment of diabetes with glyburide may both be risk factors for acute pancreatitis. Diabetes Care 2002;25:298–302 [DOI] [PubMed] [Google Scholar]
- 39. Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol 2018;33:947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howe LJ, Tudball M, Davey Smith G, Davies NM. Interpreting Mendelian-randomization estimates of the effects of categorical exposures such as disease status and educational attainment. Int J Epidemiol 2022;51:948–957 [DOI] [PMC free article] [PubMed] [Google Scholar]